ABSTRACT
Filamentous cyanobacteria exhibit some of the greatest developmental complexity observed in the prokaryotic domain. This includes the ability to differentiate nitrogen-fixing cells known as heterocysts, spore-like akinetes, and hormogonia, which are specialized motile filaments capable of gliding on solid surfaces. Hormogonia and motility play critical roles in several aspects of the biology of filamentous cyanobacteria, including dispersal, phototaxis, the formation of supracellular structures, and the establishment of nitrogen-fixing symbioses with plants. While heterocyst development has been investigated extensively at the molecular level, much less is known about akinete or hormogonium development and motility. This is due, in part, to the loss of developmental complexity during prolonged laboratory culture in commonly employed model filamentous cyanobacteria. In this review, recent progress in understanding the molecular level regulation of hormogonium development and motility in filamentous cyanobacteria is discussed, with a focus on experiments performed using the genetically tractable model filamentous cyanobacterium Nostoc punctiforme, which retains the developmental complexity of field isolates.
KEYWORDS: cyanobacteria, motility, type IV pili, polysaccharide secretion, hormogonia
INTRODUCTION
Cyanobacteria are a phylum of Gram-negative bacteria defined by their capacity for oxygenic photosynthesis (1). Many strains are also capable of nitrogen fixation, and thus cyanobacteria play a major role in providing reduced carbon and nitrogen to global ecosystems (2). Given the photosynthetic capacity and ease of genetic manipulation, cyanobacteria have also become an attractive platform for biotechnological applications such as biofuel production (3) and the mining of new optogenetic tools (4) and natural products (5).
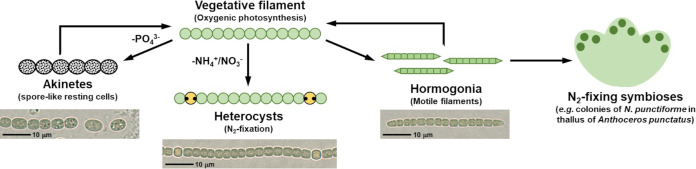
While cyanobacteria belonging to taxonomic subsections I and II are unicellular, those in subsections III-V are filamentous. Subsections III and IV possess linear trichomes while subsection V displays branched trichomes (1). Subsections IV and V have developmentally complex life cycles with an array of filaments and cell types (Fig. 1) (1). Under optimal growth conditions the filaments are comprised of photosynthetic, actively growing and dividing vegetative cells. Depletion of fixed nitrogen leads to the development of a patterned array of heterocysts interspersed among the vegetative cells (6). Heterocysts are terminally differentiated cells that cease oxygenic photosynthesis, increase aerobic respiration, and accumulate an outer layer of glycolipids and polysaccharides to provide the microoxic environment necessary for nitrogen fixation. The heterocysts then provide fixed nitrogen to adjacent vegetative cells and in turn receive fixed carbon to power nitrogen fixation. In some strains, depletion of other nutrients, such as phosphate, and changes to light and temperature can stimulate the differentiation of akinetes (Fig. 1), spore-like resting cells that are more resistant to environmental stress (7). Akinetes will germinate upon the return of more favorable conditions, giving rise to new vegetative trichomes.
FIG 1.
The life cycle of N. punctiforme. Vegetative filaments contain actively growing and dividing cells that perform oxygenic photosynthesis and have the capacity to differentiate into alternative cell or filament types. Depletion of nutrients such as phosphate can lead to the formation of spore-like akinetes, while depletion of fixed nitrogen stimulates the formation of heterocysts. The differentiation of hormogonia is constitutive under standard laboratory conditions but is also influenced by various environmental signals. Hormogonia are capable of surface motility and are the infective unit for establishing nitrogen-fixing symbioses with numerous plants.
Finally, most subsection IV and V strains can differentiate motile trichomes known as hormogonia (1). Hormogonia differentiate from vegetative filaments, resulting in fragmentation and release of heterocysts, if present, to produce multiple, shorter motile filaments that glide on solid surfaces (Fig. 1; see Movie S1 in the supplemental material). The absence of heterocysts in hormogonia even when fixed nitrogen is unavailable indicates there is complex genetic regulation between these two differentiated states. Hormogonium development involves synchronous, reductive cell division (uncoupled from cell growth) and a transition in the cell architecture resulting in smaller, rod-shaped cells with tapered filament termini. The production of gas vesicles, which enhance buoyancy, are also expressed specifically in hormogonia (8, 9). Once differentiated, hormogonia are temporarily senescent, ceasing cell growth and division as well as synthesis of most macromolecules (10), and are thus dedicated exclusively to motility. In many strains, hormogonium development is constitutive, with vegetative filaments and hormogonia somewhat analogous to the stalk and swarmer cells of Caulobacter crescentus (11). However, a wide range of environmental factors can influence the differentiation of hormogonium as well. While filamentous cyanobacteria in subsection III are also motile, the motile filaments do not typically display dramatic changes in morphology and are therefore not traditionally considered to be hormogonia (1).
Once differentiated, hormogonia and motility play several critical roles in the life cycle of filamentous cyanobacteria. They facilitate dispersal and phototaxis, allowing migration toward optimal light environments. Motility is also essential for the formation of supracellular structures such as the colonial aggregates observed for Nostoc and Trichodesmium spp. (12, 13) and the reticulate mats formed by some Pseudoanabaena spp. (14). Finally, hormogonium development and motility are prerequisites for the establishment of nitrogen-fixing symbioses with eukaryotic partners (Fig. 1), such as the hornwort Anthoceros punctatus (13), and the water fern Azolla (15).
This article provides an overview of the genetic and molecular regulation of hormogonium development and motility in filamentous cyanobacteria, based primarily on recent progress made using the model filamentous cyanobacterium Nostoc punctiforme ATCC 29133/PCC73102. Laboratory cultures of Nostoc sp. strain PCC 7120, the most widely adopted model filamentous cyanobacterium, have lost the ability to differentiate hormogonia, likely due to prolonged laboratory domestication (16). Thus, N. punctiforme has been developed into a critical model system for the study of hormogonium development and motility, as it is amenable to genetic manipulation and laboratory cultures retain the developmental complexity of field isolates. Although this review is not intended to be a comprehensive synthesis of motility in cyanobacteria, where appropriate, literature on motility in unicellular cyanobacteria will be included given that most cyanobacteria appear to utilize a common surface motility system.
SIGNALS INFLUENCING HORMOGONIUM DEVELOPMENT
In laboratory cultures of wild-type N. punctiforme, hormogonium development is constitutive, with sequential rounds of differentiation and return to the vegetative state observed. However, several environmental factors are also known to influence hormogonium development, including light quality, as discussed further in a subsequent section (Fig. 2). N. punctiforme was initially isolated from the coralloid roots of a cycad (17) and several studies have indicated that symbiotic plant partners produce signals stimulating the production of hormogonia. Both the hornwort Anthoceros punctatus (10) and the cycad Cycas revoluta (18) produce hormogonium-inducing factors (HIF). In C. revoluta, one of these chemicals was identified as 1-palmitoyl-2-linoleoyl-sn-glycerol (18), while in A. punctatus, the chemical has not been identified. These signals are thought to promote hormogonium development to recruit the cyanobacterium to the plant, but the molecular mechanism by which N. punctiforme perceives these signals is currently unknown (Fig. 2).
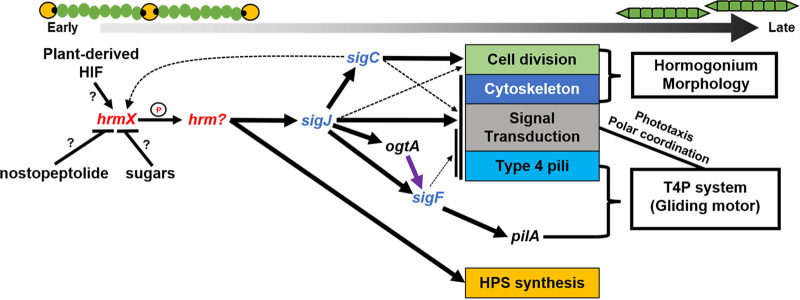
FIG 2.
The hormogonium gene regulatory network. Various chemical signals influence the initiation of hormogonium development through unknown means. The hybrid histidine kinase HrmX is the earliest known actor in the GRN and is presumed to regulate the phosphorylation state of an unknown response regulator which in turn directly activates expression of some hormogonium-specific genes while indirectly activating others via the initiation of the sigma-factor cascade, leading to the development of motile hormogonia. Black arrows indicate positive transcriptional regulation. Black lines with bars indicate negative transcriptional regulation. Purple arrow indicates posttranscriptional regulation. Thickness of lines represents an approximation of the strength of regulation, or the number of genes regulated within a functional category (e.g., Cell Division).
In contrast, various sugars have been shown to repress hormogonium development (Fig. 2), most notably sucrose (19), and the nonmetabolizable sucrose analog sucralose (20). Sucrose, glucose, and fructose were found to be abundant in the symbiotic stem glands of the angiosperm Gunnera manicata (19), and it has been suggested that they function as a hormogonium-repressing factor to keep the symbiotic Nostoc in the vegetative state following colonization. In laboratory cultures, incorporation of sucralose can be utilized to suppress hormogonium development, producing homogenous cultures of vegetative filaments (20). Subsequent removal of sucralose can synchronously induce hormogonium formation of the entire culture and is thus a useful tool for studying hormogonium development in the lab. The exact mechanism by which sugars influence hormogonium development is poorly understood but may be related to a genetic locus which contains genes encoding predicted proteins involved in hexuronic acid metabolism as well as a LacI/GalR family transcriptional repressor, HrmR (21, 22). The DNA-binding activity of HrmR is repressed by galacturonate, resulting in increased transcription of other genes within the hrm locus, which in turn are thought to be involved in the synthesis of a hormogonium-repressing signal (22). Notably, a sugar transport system is encoded adjacent to this locus that is also regulated by HrmR, and the sugar transporter has been shown to be essential for symbiotic competence with A. punctatus (23).
In addition to signals from symbiotic partners, there is evidence for the regulation of hormogonium development by autogenic repressors (Fig. 2). The secondary metabolite nostopeptolide has been shown to repress hormogonium development at high concentrations while also acting as a chemoattractant at lower concentrations (24). Mutation of a gene cluster encoding a polyketide synthase system, pks2, reduced nostopeptolide production and lead to an increase in hormogonium development (24, 25). However, the pks2 gene cluster is not thought to be directly responsible for nostopeptolide production, and therefore the effect of pks2 on nostopeptolide levels and hormogonium development appears to be indirect. As with the other chemical signals, the sensory system(s) that respond to nostopeptolide and in turn influence hormogonium development and motility are undefined.
THE HORMOGONIUM GENE REGULATORY NETWORK
While it is unclear how the various chemical signals described above influence the gene regulatory network promoting hormogonium development, several key regulators of this network have been identified (Fig. 2). The earliest acting factor identified thus far is a hybrid histidine kinase encoded by locus tag Npun_R3825 (26). This gene was originally designated hrmK, however, upon preparing this review it became apparent that a prior study used the hrmK designation for the putative hexokinase encoded by Npun_R5331. Therefore, Npun_R3825 has been renamed hrmX to avoid confusion. HrmX is presumed to regulate the phosphorylation state of a yet unidentified response regulator which then promotes transcription of sigJ, initiating a hierarchical sigma factor cascade, as well as a subset of hormogonium-specific genes that are regulated independently from this cascade (Fig. 2), including several involved in the production of hormogonium polysaccharide (HPS) (26).
SigJ, in turn, directly promotes transcription of sigC and indirectly sigF, as well as many other hormogonium-specific genes (Fig. 2) (16). SigC also promotes the transcription of a substantial number of hormogonium-specific genes, including many known to be involved in cell division, implying that SigC plays a key role in reductive cell division (16). This is corroborated by the fact that a sigC mutant fails to display typical hormogonium morphology (16). Additionally, one of the two transcriptional start sites identified in the hrmX promoter region is dependent on SigC, creating a positive feedback loop between hrmX, sigJ, and sigC (Fig. 2) (27). It should also be noted that both SigJ and SigC often regulate the expression of distinct subsets of genes that are involved in the same cellular process, such as those involved in the assembly of the T4P system, and even within a putative operon encoding the Hmp chemotaxis-like system (16, 27). This coregulation could be related to coordinating the timing of cell-division and initiation of motility.
SigF appears to have a remarkably limited regulon, with pilA, which encodes the major pilin for the type IV pilus system, the only gene stringently dependent on SigF for expression (Fig. 2) (16). However, several SigJ-dependent genes also show a minor decrease in transcription in a sigF mutant, implying some overlap in the SigJ and SigF regulons (16). Moreover, sigJ and sigF are thought to have arisen via gene duplication, and while many cyanobacteria contain orthologs of both SigJ and SigF, others contain only SigF, where it is likely to simultaneously perform the function of both sigma factors (16). In addition to sigF, expression of pilA also requires the activity of a putative β-O-linked N-acetylglucosamine transferase (OGT) encoded by ogtA (28). In eukaryotes, OGTs are known to play key regulatory roles by modulating the activity of target proteins via glycosylation (29). The ogtA gene is found immediately downstream of pilA but is directly regulated by SigJ and influences PilA accumulation through an undefined posttranscriptional mechanism (Fig. 2) (28).
A combination of RNAseq and Cappable-seq experiments have identified putative consensus sequences for SigJ- and SigF-dependent promoters (16, 27), both of which contain an extended −10 region, with a consensus sequence of GGGAATACT and GGGTAAGTT for SigJ- and SigF-dependent promoters, respectively. It is likely that the similarity in these sequences explains the partial overlap in SigJ and SigF promoter recognition. No strong consensus sequence has been identified for SigC-dependent promoters. Aside from SigJ- and SigF-dependent promoters, the −10 region for most promoters analyzed in N. punctiforme contain a consensus motif TANNNT, but promoters that are most stringently dependent on SigC appear to have a greater tolerance for other nucleotides in the position of the T most proximal to the transcriptional start site (27).
PARTNER-SWITCHING SYSTEMS
Two partner-switching systems have been implicated in regulating hormogonium development and motility (Fig. 3). The components of these systems are homologous to those from the canonical Rsb partner-switching system which regulates the availability of SigB in Bacillus subtilus (30). The Hmp partner-switching system, comprised of the Sulfate Transporter and Anti-Sigma factor antagonist (STAS) domain protein HmpV, the anti-sigma factor/serine kinase HmpW and phosphatase HmpU, is transcriptionally upregulated in developing hormogonia by the sigma factor cascade (16). Deletion of hmpV or hmpU reduces motility, the accumulation of surface PilA and HPS, and the length of time spent in the hormogonium state (31). In contrast, deletion of hmpW has the opposite effect, producing hyper-motile filaments that spend extended periods of time in the hormogonium state (31). Epistasis analysis between hmpV and hmpW indicates that this system probably does not regulate a sigma factor, but rather works in a noncanonical manner where unphosphorylated HmpV functions as the output of the system, positively influencing hormogonium development (Fig. 3) (31). In this model, phosphorylation of HmpV by HmpW would limit HmpV activity, and the phosphorylation state of HmpV is ultimately controlled by the phosphatase HmpU. HmpU contains a sensory domain with both an REC and GAF domain, which could indicate that its phosphatase activity is regulated by some combination of light, cyclic nucleotides, and phosphorylation by histidine kinases (32, 33), but the exact signals influencing HmpU are currently unknown. Expression of several hormogonium-specific genes is diminished in an hmpV mutant, implying that the system exerts transcriptional control of hormogonium development (31). It is possible that this system may reinforce the hormogonium GRN by enhancing expression of one or more early acting genes in the network, creating a positive feedback loop (Fig. 3). This system is conserved in nearly all filamentous cyanobacteria and absent in nearly all unicellular strains, indicating that it performs a function specific to motility in filamentous cyanobacteria (31).
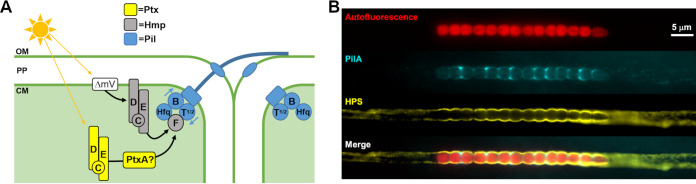
FIG 3.
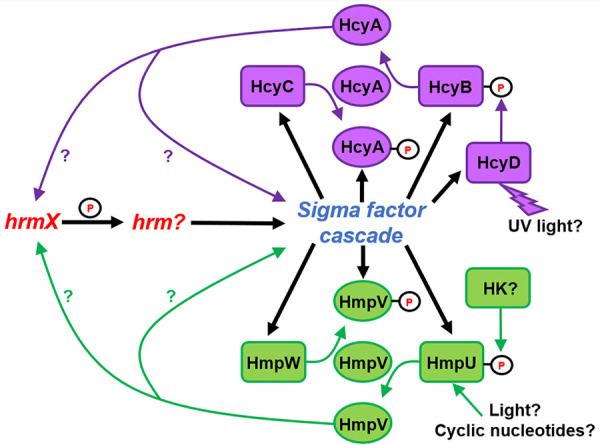
Partner-switching systems regulating hormogonium development. The Hcy system regulates hormogonium development in response to UV light, while the inputs to the Hmp system are unclear but may involve light or cyclic nucleotide sensing as well as inputs from histidine kinases (HK). The output of either system is currently unknown, but evidence suggests the unphosphorylated forms of the STAS proteins HmpV and HcyA may positively influence early stages of the hormogonium gene regulatory network.
The second partner-switching system, the Hcy system, is comprised of the STAS domain protein HcyA, the anti-sigma factor/serine kinase HcyC and phosphatase HcyB, and is also upregulated in hormogonia by the sigma factor cascade (Fig. 3) (16). Mutation of genes in this system affect the development of hormogonia under exposure to UV radiation, indicating that this system regulates the decision to differentiate hormogonia under UV stress (34). The sensory domain of HcyB contains a REC domain, indicating that phosphatase activity may be controlled by a histidine kinase. Intriguingly, a fourth gene found at this locus, hcyD, is upregulated in hormogonia and encodes a histidine kinase with a GAF domain predicted to bind a bilin and thus be capable of directly sensing light, leading to speculation that HcyD may phosphorylate HcyB in a UV-light dependent manner to regulate the activity of the system (34). Further genetic analysis will be needed to verify this hypothesis. As with the Hmp partner-switching system, it is unclear how the Hcy system influences hormogonium development. Protein-protein interaction studies failed to detect any interaction between HcyC and SigJ, SigF, or SigC, implying that the Hcy system may also function in a noncanonical manner (34). Epistasis analysis with hcyA and hcyC would provide further support for this hypothesis.
THE GLIDING MOTOR
Historically, several theories have been proposed to explain the gliding motility observed by various strains of filamentous cyanobacteria. These include the generation of waves propagated by contractile fibrils on the surface of filaments, and the extrusion of polysaccharide (35, 36). However, genetic analyses have clearly demonstrated that motility in N. punctiforme is driven by type IV pilus motors (37, 38), and comparative genomics strongly implies that this mechanism of motility is employed by all filamentous and unicellular cyanobacteria that display surface motility (37). Moreover, a recent study demonstrated that T4P genes are essential for gliding motility in a member of the Phormidium genus (39). T4P-dependent surface motility is widespread in bacteria, and the T4P systems in cyanobacteria are highly similar to those that have been characterized in much greater detail in other organisms (40). This form of motility relies on cycles of pilus extension, followed by adherence to the substratum and subsequent retraction, pulling the cell forward. The pilus is composed primarily of the major pilin PilA, and extension of the pilus is driven by the motor ATPase PilB, which interacts with the innermembrane protein PilC to facilitate incorporation of new PilA monomers into the pilus. Retraction is subsequently driven by the motor ATPase PilT, which also interacts with PilC to facilitate the removal of PilA monomers from the pilus. Most cyanobacteria encode two PilT homologs, PilT1 and PilT2, with PilT1 serving as the primary retraction ATPase (37).
However, there are at least two proteins that appear to be unique to cyanobacterial T4P systems, Hfq and EbsA. In E. coli and other bacteria, Hfq functions as an RNA chaperone that assists small regulatory RNAs in associating with their targets (41). In contrast, in both unicellular and filamentous cyanobacteria, Hfq has been shown to associate with PilB and appears to be required for PilB function (Fig. 4A), given that mutation of hfq abolishes T4P extension and motility (42–44). It is unlikely cyanobacterial Hfq also functions as an RNA chaperone (45). EbsA is a small protein that is highly conserved in many cyanobacteria but absent outside the cyanobacterial clade (46). Studies in unicellular cyanobacteria demonstrated that EbsA, like Hfq and PilB, is essential for assembly of pili and motility, and that EbsA directly interacts with either Hfq, PilB, or both (46). The EbsA homolog in N. punctiforme has yet to be characterized, but it is likely that it plays a similar role in filamentous cyanobacteria given its ubiquity in these organisms and the fact that it is expressed specifically in developing hormogonia via direct regulation by SigJ (16, 27).
FIG 4.
Regulation of the T4P motors and directional motility. (A) A model depicting regulation of the T4P motors. The Hmp chemotaxis-like system establishes polarity of T4P activation by modulating the interaction of HmpF with the motors at one side of each cell and is also indirectly sensitive to light via alterations in membrane potential. The Ptx system contains a broad-spectrum power sensing cyanobacteriochrome MCP and is required for positive phototaxis. The influence of the Ptx system on T4P activity may be modulated by the PatA-type response regulator PtxA. (B) Fluorescence micrographs depicting cellular autofluorescence, PilA immunofluorescence, lectin staining of HPS, and a merged image of all color channels (as indicated) for a hormogonium filament.
In many bacteria where T4P-dependent motility is observed, directional regulation of movement is achieved by altering the cellular localization of the motor ATPases PilB and PilT (47). This appears to be the case for at least some unicellular cyanobacteria. In the model unicellular cyanobacterium Synechocystis sp. PCC 6803, pili can extend from any region of the spherical cells and PilB dynamically localizes to the leading side of motile cells where it presumably activates cycles of pilus extension (48). However, in N. punctiforme, the T4P systems, including the motor ATPases, are found in bipolar, static arrays that ring the circumference of the cell immediately adjacent to the cell-cell junctions (Fig. 4A) (37). Immunofluorescence of PilA in motile hormogonia indicate that pili are found exclusively at the cell junctions and display a directional bias, implying that at any given time the T4P motors are only active on one side of each cell along the filament in a coordinated manner (Fig. 4B) (37, 49). Electron microscopy studies on numerous filamentous cyanobacteria have reported the presence of ringed arrays of pores in the outer membrane adjacent to the cell septa (50). While these were initially termed junctional pore complexes and thought to be involved in the secretion of motility associated polysaccharides, they have now been shown to be formed by the secretin PilQ, which allows pili to pass through the outer membrane (51).
Notably, the absence of obvious pili in electron microscopy studies has been cited as evidence that T4P do not power motility in several filamentous cyanobacteria. However, to the author’s knowledge, T4P have never been observed specifically at the cell junctions in motile hormogonia of N. punctiforme or any other motile filamentous cyanobacteria using electron microscopy, despite ample evidence that the junctions are the site of the T4P systems. In fact, it was the failure to visualize pili by electron microscopy in motile hormogonia that prompted the author’s lab to take an immunofluorescence approach to detect pili on the cell surface. It should be noted that in several subsection IV filamentous cyanobacteria, including N. punctiforme, peritrichous pili have been observed via electron microscopy (38, 52). However, these filaments are most likely old hormogonia that have ceased motility and are transitioning to the vegetative state, as immunofluorescence studies of PilA have demonstrated that pili transition from polar accumulation to a peritrichous arrangement upon the cessation of motility (37). The absence of obvious T4P in motile filamentous cyanobacteria could be explained by the fact that the pili adhere tightly to the surface of the adjacent cells along the filament and are likely to be encased in a layer of motility-associated polysaccharide (Fig. 4B).
CHEMOTAXIS-LIKE SYSTEMS AND REGULATION OF THE T4P MOTORS
Both filamentous and unicellular cyanobacteria are known to exhibit phototaxis in response to light (53), and reports have indicated that symbiotically competent filamentous cyanobacteria exhibit chemotaxis toward plant-derived compounds (18, 54). While phototaxis in some unicellular cyanobacteria has been shown to result from perception of directional light and orientation of motility toward or away from this light (55), in N. punctiforme, phototaxis is at least in part facilitated by a random walk where temporal changes in light intensity trigger filament reversals (49). This is likely to be the case in other filamentous cyanobacteria, given that movement is typically restricted to the long axis of the filament, and spontaneous reversals have been observed in many filamentous strains, although some reports indicate that filamentous cyanobacteria might be capable of orienting their movement in response to directional light sources as well (56, 57).
Presumably, biased directional movement requires signal transduction systems that perceive these signals and subsequently interface with and regulate the T4P motors. Like many bacteria, cyanobacteria harbor chemotaxis-like systems homologous to those originally described in E. coli for regulation of flagellar motility (58), and it is likely that these systems regulate the T4P motors to achieve directed motility. The genome of N. punctiforme harbors 5 loci with contiguous genes encoding chemotaxis-like system proteins (59). Four of these loci exhibit a similar genetic arrangement, encoding a PatA-type response regulator, a CheY-type response regulator, a CheW adaptor, methyl-accepting chemotaxis proteins (MCP), and CheA histidine kinase, in the 5′–3′ orientation, respectively. All four of these gene clusters are transcriptionally upregulated in hormogonia, and two of them have been associated with specific biological functions.
The hmp locus is essential for motility (13, 59, 60). Deletion of any gene in this locus, except for the PatA-type response regulator hmpA, completely abolishes motility (13). The mutants are still capable of assembling pili, but lose the directional bias observed in the wild-type strain, implying that this system is critical for coordinating the polar bias of T4P activity along the filament that leads to directional movement (13). Components of the Hmp system localize in static, bipolar rings like those observed for T4P-systems, indicating that these complexes exist in proximity and may directly interact with one another (13). Mutations in the hmp locus also influence transcription of hormogonium-specific genes, such as those in the hpsA-K locus, implying that the system plays a regulatory role in the hormogonium GRN in addition to directly influencing the T4P motors (60). Furthermore, certain hmp mutants produce lower molecular weight isoforms of PilA, perhaps due to decreased transcription of genes involved in the posttranslational modification of the major pilin (13).
More recently, a sixth protein, HmpF, was shown to participate in the Hmp system. HmpF does not resemble canonical chemotaxis proteins and is predicted to be composed primarily of coiled-coil domains (49). HmpF is confined to the cyanobacterial lineage and is typically encoded immediately upstream and divergent from the rest of the hmp locus (49). Unlike the other Hmp-system components, HmpF is essential for accumulation of surface pili, exhibits dynamic, unipolar localization at the leading poles of motile filaments, and interacts directly with the T4P proteins Hfq, PilT1 and PilT2, implying that it is involved in activation of the T4P motors to establish directional movement (Fig. 4A) (44, 49). Deletion of other hmp genes results in the static, bipolar accumulation of HmpF, demonstrating that the hmp system modulates HmpF localization to establish coordinated polarity of motor activation and motility, and HmpF has been found to interact directly with the CheA-like HmpE protein (44) (Fig. 4).
The sensory capacity of the Hmp chemotaxis system is not entirely clear. HmpD, the MCP of this system, is predicted to be anchored to the cytoplasmic membrane with both an N-terminal sensory domain and C-terminal signaling domain found within the cytoplasm. HmpD lacks any of the obvious features known to be involved in directly sensing light by other cyanobacterial MCPs (see below for details), but the Hmp system has been shown to alter HmpF localization in response to both light and treatment with the proton ionophore CCCP (44), leading to speculation that this system may perceive light indirectly through alteration of proton motive force, or membrane potential more generally, and in turn influence directional motility (Fig. 4A).
Both the pix and ptx locus encode chemotaxis-like systems with cyanobacteriochrome MCPs (59). Cyanobacteriochomes contain GAF domains which bind light-absorbing bilins capable of directly sensing various wavelengths of light, and homologous systems have been shown to regulate phototaxis in unicellular cyanobacteria (55). The pix and ptx system are highly similar and appear to have arisen via a gene duplication event. However, only the ptx locus is essential for positive phototaxis. Strains with null mutations in the ptx locus retain motility but fail to exhibit biased movement toward a directional light source, while those in the pix system have no obvious phenotype (59). PtxD, the MCP of the Pix system contains multiple GAF domains and appears to function as a broad-spectrum light power sensor (61). It is currently unclear how this system interfaces with the T4P motors to achieve directional motility. Aside from the hmp system, the other chemotaxis loci do not encode homologs of HmpF. It is possible that the ptx system also directly influences localization of HmpF. However, it has recently been shown that the PatA-type response regulators in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 interact directly with the T4P motors (62), and it is possible that this may be the mechanism of regulation for the ptx locus as well (Fig. 4A).
The fourth locus, spanning locus tags Npun_F5636 to Npun_F5640, has not been assigned a gene designation and its function is poorly understood. Mutation of the gene encoding the MCP from this locus resulted in curved, rather than straight hormogonia, which displayed a reduced rate of gliding but retained phototaxis (59). This MCP is predicted to be membrane bound and contains a GAF domain that lacks the conserved cysteines typically required for binding bilins and is therefore not expected to function as a cyanobacteriochrome capable of sensing light.
The fifth locus appears to have arisen via horizontal gene transfer and lacks a PatA-type response regulator but includes the methyltransferase and methylesterase CheR and CheB, respectively, which are typically involved in sensory adaptation (59). Unlike the other four chemotaxis loci, transcription of this locus is not upregulated in hormogonia and the overall transcript abundance from RNAseq experiments indicate that this locus is not substantially transcribed under laboratory conditions that have been investigated (16). Deletion of the entire locus did not produce any obvious phenotype, as this mutant strain retained wild-type levels of motility and phototaxis and was still capable of infecting the symbiotic plant partner Anthoceros punctatus (59). However, another report indicated that insertional mutation of several genes in this locus reduced motility and infectivity with the symbiotic plant partner Blasia pusilla (63). Thus, more work is needed to determine what role, if any, this locus plays in motility and symbiotic competency.
HORMOGONIUM POLYSACCHARIDE
The deposition of motility-associated polysaccharides is commonly observed in bacteria exhibiting T4P-dependent motility (40). This polysaccharide is thought to serve as an interface between the substratum or adjacent cells and the T4P, providing an anchor point so that pilus retraction results in productive movement, and possibly as a mechanism to reduce friction. This is the case for filamentous cyanobacteria as well, with the association between polysaccharide production and motility cited as evidence that polysaccharide secretion may provide the propulsive force for motility (35). N. punctiforme produces a motility-associated polysaccharide, termed hormogonium polysaccharide (HPS). Lectin-based analyses indicate that HPS contains fucose and galactose, but the precise chemical composition has not been determined by other methods (37). Many genes encoding glycosyl transferases involved in HPS synthesis have been identified (60, 64) (Fig. 5). Inactivation of these genes abolishes motility and HPS production, and in some cases, also reduces the accumulation of surface PilA, indicating a regulatory link between HPS synthesis and T4P activity via an unknown mechanism (64) (Fig. 5). In mutant strains where T4P activity does not appear to be affected by the loss of HPS, exogenous HPS addition is sufficient to restore motility, indicating that HPS plays a passive, rather than active role in powering motility (37, 64).
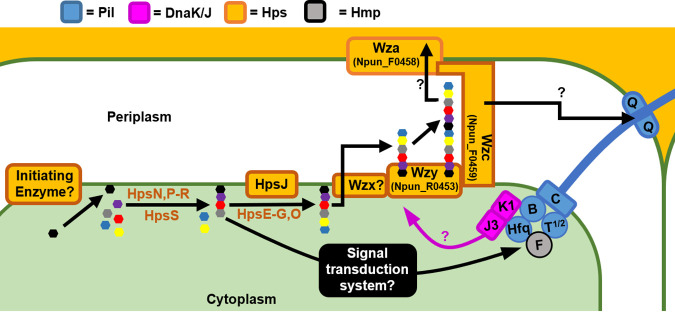
FIG 5.
Hormogonium polysaccharide (HPS) synthesis, export, and regulation. Assembly of the repeat units of HPS is facilitated by several glycosyl transferases and influences T4P motor activity via an unidentified signal transduction system. Repeat units are subsequently exported to the periplasm and polymerized. Export across the outer membrane is not well defined and may occur via a canonical Wza-type outer membrane channel, or possibly through the secretin channel formed by PilQ via the activity of the T4P system. The DnaK1/J3 chaperone system influences HPS synthesis/secretion in response to T4P activity.
How HPS is exported out of the cell has not been definitively determined. As previously mentioned, the outer membrane secretin PilQ, which also allows the passage of T4P through the outer membrane, has been posited as the site of HPS extrusion (Fig. 5). Several studies, including those in N. punctiforme, have noted that polysaccharide appears to emanate from the cell junctions (35, 60), which are also the site of the T4P complexes (37), and mutation of either pilB or pilQ abolishes HPS production, while mutation of pilT1 or pilA reduces, but does not eliminate accumulation of HPS outside the cells (37). Moreover, one of the genetic loci encoding glycosyl transferases involved in HPS synthesis also contains several genes encoding minor pilins (60). However, while these results are consistent with the idea that the T4P systems themselves may be involved in HPS secretion, the accumulation and release of HPS from the cell junctions may be a result of a concentrating effect of T4P retraction on HPS at these sites, and the loss of HPS in T4P mutants could also be due to regulatory feedback between T4P activity and HPS production, as discussed further below.
Alternatively, HPS may be secreted through a more canonical Wzx/Wzy-type pathway (65) (Fig. 5). A recent study identified a gene cluster encoding several components homologous to Wzx/Wzy-type polysaccharide export systems (Npun_R0453 to Npun_F0459), which based on genomic cooccurrence with other hps genes, and enhanced expression in hormogonia, has been suggested to function in HPS export (64). However, genetic studies are still needed to determine what role, if any, this gene cluster has in HPS synthesis and secretion.
Complicating efforts to determine the route of export for HPS is the fact that HPS production may be inextricably linked to the activity of the T4P systems, and thus mutations that disrupt the T4P may result in indirect, rather than direct effects on HPS secretion. A recent study identified a DnaK(Hsp70)-type chaperone system comprised of DnaK1, DnaJ3, and GrpE, which appears to sense the status of the T4P motors, and in turn influence HPS production (66) (Fig. 5). Mutation of either dnaK1 or dnaJ3 drastically reduces motility and HPS accumulation but does not affect other aspects of hormogonium development (66). DnaK1 associates with Hfq and PilB of active motor complexes and is thought to in turn modulate the folding state of an unknown protein which subsequently regulates HPS production (66). The influence of this system on HPS production does not appear to occur at the level of transcription, at least for currently identified hps genes, implying a posttranscriptional regulatory mechanism (66). Given that disruption of the DnaK system does not affect the accumulation of surface PilA, it is likely to work downstream of the Hps proteins that are also required for T4P assembly. Thus, it appears that there is a complex interplay between HPS production and T4P activity, with each system influencing the activity of the other.
EVOLUTIONARY CONSERVATION AND IMPLICATIONS
T4P have been demonstrated to power motility in phylogenetically diverse cyanobacteria, and the genes encoding the T4P-system are almost universally conserved in these organisms (37), providing strong evidence that most cyanobacteria share a conserved T4P-dependent motility system. Both the chemotaxis-like systems and the DnaK1/J3 chaperone system are also broadly conserved (49, 66), as well as the hormogonium-specific sigma factors (16). Thus, it appears that fundamental aspects of T4P regulation and transcription of motility-associated genes are shared by most cyanobacteria.
In contrast, there are certain systems that appear to be conserved primarily in filamentous cyanobacteria. Orthologs of the genes involved in HPS synthesis are much more common in filamentous cyanobacteria and are conspicuously absent from the motile unicellular cyanobacteria Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 7942, indicating that filamentous cyanobacteria employ motility-associated polysaccharides that are distinct from most unicellular strains (64). The Hmp partner-switching system is also found almost exclusively in filamentous cyanobacteria (31), implying that it provides a key function for the regulation of motility specifically in filamentous strains, lending further support to the idea that filamentous cyanobacteria share a mechanism of motility. Finally, HrmX, which functions at the earliest stages of hormogonium development, appears to be exclusively found in heterocyst-forming cyanobacteria (26). Thus, it appears that cyanobacteria in subsections IV and V have evolved additional levels of regulation to coordinate gene expression during the process of hormogonium development.
FUTURE DIRECTION
While substantial progress in understanding the mechanisms of hormogonium development and motility in filamentous cyanobacteria has been made in recent years, several outstanding questions remain. It is likely that several genes involved in these processes have yet to be identified, such as the gene encoding the putative response regulator that is phosphorylated by HrmX. The signal transduction systems that sense environmental signals such as plant-derived HIF, sucrose, and nostopeptolide are also currently unknown. Continued forward genetic approaches, especially high-throughput approaches such as randomly barcoded transposon sequencing (67), have the potential to shed new light on these questions.
In many cases where genes have been identified, it is not entirely clear how the disparate protein products coordinate with one another to regulate hormogonium development and motility. This is particularly the case for the partner-switching and DnaK chaperone systems. Given that regulation by these types of systems typically involve transient protein-protein interactions, experimental approaches to identify novel interaction partners, such as coimmunoprecipitation or proximity labeling (68) may be able to close some of these gaps. These future experiments have the potential to further refine our understanding of hormogonium development and motility in filamentous cyanobacteria and shed new light on related processes such as the establishment of plant-cyanobacterial symbioses.
Biography

Douglas D. Risser joined Sean M. Callahan’s research group at the University of Hawaii at Manoa as a PhD student in 2003, where his research focused on heterocyst development in filamentous cyanobacteria. He completed his PhD in 2009 and subsequently joined John C. Meeks’ research group at the University of California, Davis, as a postdoctoral researcher where he continued investigating heterocysts as well as developing a new line of inquiry focused on genetic and molecular regulation of motile hormogonia in filamentous cyanobacteria. In 2013, he joined the Department of Biological Sciences at the University of the Pacific and in 2022 moved to the Department of Biology at University of Colorado, Colorado Springs. His research group is focused on the continued development of the filamentous cyanobacterium Nostoc punctiforme as a model organism for studying hormogonium differentiation and motility, as well as associated processes such as nitrogen-fixing symbioses with plants.
Footnotes
Supplemental material is available online only.
Contributor Information
Douglas D. Risser, Email: drisser@uccs.edu.
Gladys Alexandre, University of Tennessee at Knoxville.
REFERENCES
- 1.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61. doi: 10.1099/00221287-111-1-1. [DOI] [Google Scholar]
- 2.Issa AA. 2014. Nitrogen fixing cyanobacteria: future prospect, p Ch. 2. In Mohamed Hemida Abd-Alla (ed), Advances in Biology and Ecology of Nitrogen Fixation. IntechOpen, Rijeka. [Google Scholar]
- 3.Ducat DC, Way JC, Silver PA. 2011. Engineering cyanobacteria to generate high-value products. Trends Biotechnol 29:95–103. doi: 10.1016/j.tibtech.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Wiltbank LB, Kehoe DM. 2019. Diverse light responses of cyanobacteria mediated by phytochrome superfamily photoreceptors. Nat Rev Microbiol 17:37–50. doi: 10.1038/s41579-018-0110-4. [DOI] [PubMed] [Google Scholar]
- 5.Demay J, Bernard C, Reinhardt A, Marie B. 2019. Natural products from cyanobacteria: focus on beneficial activities. Mar Drugs 17:320. doi: 10.3390/md17060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng X, Zhang CC. 2022. The making of a heterocyst in cyanobacteria. Annu Rev Microbiol 76:597–618. doi: 10.1146/annurev-micro-041320-093442. [DOI] [PubMed] [Google Scholar]
- 7.Garg R, Maldener I. 2021. The formation of spore-like akinetes: a survival strategy of filamentous cyanobacteria. Microb Physiol 31:296–305. doi: 10.1159/000517443. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong RE, Hayes PK, Walsby AE. 1983. Gas vacuole formation in hormogonia of Nostoc muscorum. Microbiology 129:263–270. doi: 10.1099/00221287-129-2-263. [DOI] [Google Scholar]
- 9.Damerval T, Houmard J, Guglielmi G, Csiszar K, Tandeau de Marsac N. 1987. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene 54:83–92. doi: 10.1016/0378-1119(87)90350-7. [DOI] [PubMed] [Google Scholar]
- 10.Campbell EL, Meeks JC. 1989. Characteristics of hormogonia formation by symbiotic nostoc spp. in response to the presence of Anthoceros punctatus or its extracellular products. Appl Environ Microbiol 55:125–131. doi: 10.1128/aem.55.1.125-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkpatrick CL, Viollier PH. 2012. Decoding Caulobacter development. FEMS Microbiol Rev 36:193–205. doi: 10.1111/j.1574-6976.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 12.Tzubari Y, Magnezi L, Be’er A, Berman-Frank I. 2018. Iron and phosphorus deprivation induce sociality in the marine bloom-forming cyanobacterium Trichodesmium. ISME J 12:1682–1693. doi: 10.1038/s41396-018-0073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risser DD, Chew WG, Meeks JC. 2014. Genetic characterization of the hmp locus, a chemotaxis-like gene cluster that regulates hormogonium development and motility in Nostoc punctiforme. Mol Microbiol 92:222–233. doi: 10.1111/mmi.12552. [DOI] [PubMed] [Google Scholar]
- 14.Shepard RN, Sumner DY. 2010. Undirected motility of filamentous cyanobacteria produces reticulate mats. Geobiology 8:179–190. doi: 10.1111/j.1472-4669.2010.00235.x. [DOI] [PubMed] [Google Scholar]
- 15.Pratte BS, Thiel T. 2021. Comparative genomic insights into culturable symbiotic cyanobacteria from the water fern Azolla. Microb Genom 7. doi: 10.1099/mgen.0.000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez A, Riley KW, Harwood TV, Zuniga EG, Risser DD. 2019. A tripartite, hierarchical sigma factor cascade promotes hormogonium development in the filamentous cyanobacterium Nostoc punctiforme. mSphere 4. doi: 10.1128/mSphere.00231-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeks JC, Elhai J, Thiel T, Potts M, Larimer F, Lamerdin J, Predki P, Atlas R. 2001. An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium. Photosynth Res 70:85–106. doi: 10.1023/A:1013840025518. [DOI] [PubMed] [Google Scholar]
- 18.Hashidoko Y, Nishizuka H, Tanaka M, Murata K, Murai Y, Hashimoto M. 2019. Isolation and characterization of 1-palmitoyl-2-linoleoyl-sn-glycerol as a hormogonium-inducing factor (HIF) from the coralloid roots of Cycas revoluta (Cycadaceae). Sci Rep 9:4751–4758. doi: 10.1038/s41598-019-39647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khamar HJ, Breathwaite EK, Prasse CE, Fraley ER, Secor CR, Chibane FL, Elhai J, Chiu WL. 2010. Multiple roles of soluble sugars in the establishment of Gunnera-Nostoc endosymbiosis. Plant Physiol 154:1381–1389. doi: 10.1104/pp.110.162529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Splitt S, Risser D. 2016. The non-metabolizable sucrose analog sucralose is a potent inhibitor of hormogonium differentiation in the filamentous cyanobacterium Nostoc punctiforme. Arch Microbiol 198:137–147. doi: 10.1007/s00203-015-1171-7. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MF, Meeks JC. 1997. A hormogonium regulating locus, hrmUA, of the cyanobacterium Nostoc punctiforme strain ATCC 29133 and its response to an extract of a symbiotic plant partner Anthoceros punctatus. Mol Plant Microbe Interact 10:280–289. doi: 10.1094/MPMI.1997.10.2.280. [DOI] [PubMed] [Google Scholar]
- 22.Campbell EL, Wong FC, Meeks JC. 2003. DNA binding properties of the HrmR protein of Nostoc punctiforme responsible for transcriptional regulation of genes involved in the differentiation of hormogonia. Mol Microbiol 47:573–582. doi: 10.1046/j.1365-2958.2003.03320.x. [DOI] [PubMed] [Google Scholar]
- 23.Ekman M, Picossi S, Campbell EL, Meeks JC, Flores E. 2013. A Nostoc punctiforme sugar transporter necessary to establish a Cyanobacterium-plant symbiosis. Plant Physiol 161:1984–1992. doi: 10.1104/pp.112.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liaimer A, Helfrich EJN, Hinrichs K, Guljamow A, Ishida K, Hertweck C, Dittmann E. 2015. Nostopeptolide plays a governing role during cellular differentiation of the symbiotic cyanobacterium Nostoc punctiforme. Proc Natl Acad Sci USA 112:1862–1867. doi: 10.1073/pnas.1419543112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liaimer A, Jenke-Kodama H, Ishida K, Hinrichs K, Stangeland J, Hertweck C, Dittmann E. 2011. A polyketide interferes with cellular differentiation in the symbiotic cyanobacterium Nostoc punctiforme. Environ Microbiol Rep 3:550–558. doi: 10.1111/j.1758-2229.2011.00258.x. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga EG, Figueroa NM, Gonzalez A, Pantoja AP, Risser DD. 2020. The hybrid histidine kinase HrmK is an early-acting factor in the hormogonium gene regulatory network. J Bacteriol 202. doi: 10.1128/JB.00675-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harwood TV, Risser DD. 2021. The primary transcriptome of hormogonia from a filamentous cyanobacterium defined by cappable-seq. Microbiology (Society for General Microbiology) 167. doi: 10.1099/mic.0.001111. [DOI] [PubMed] [Google Scholar]
- 28.Khayatan B, Bains DK, Cheng MH, Cho YW, Huynh J, Kim R, Omoruyi OH, Pantoja AP, Park JS, Peng JK, Splitt SD, Tian MY, Risser DD. 2017. A putative o-linked beta-n-acetylglucosamine transferase is essential for hormogonium development and motility in the filamentous cyanobacterium Nostoc punctiforme. J Bacteriol 199:e00075-17. doi: 10.1128/JB.00075-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janetzko J, Walker S. 2014. The making of a sweet modification: structure and function of O-GlcNAc transferase. J Biol Chem 289:34424–34432. doi: 10.1074/jbc.R114.604405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paget MS. 2015. Bacterial sigma factors and anti-sigma factors: structure, function and distribution. Biomolecules 5:1245–1265. doi: 10.3390/biom5031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley KW, Gonzalez A, Risser DD. 2018. A partner-switching regulatory system controls hormogonium development in the filamentous cyanobacterium Nostoc punctiforme. Mol Microbiol 109:555–569. doi: 10.1111/mmi.14061. [DOI] [PubMed] [Google Scholar]
- 32.Hurley JH. 2003. GAF domains: cyclic nucleotides come full circle. Sci STKE 2003:pe1. doi: 10.1126/stke.2003.164.pe1. [DOI] [PubMed] [Google Scholar]
- 33.Rumyantsev KA, Shcherbakova DM, Zakharova NI, Emelyanov AV, Turoverov KK, Verkhusha VV. 2015. Minimal domain of bacterial phytochrome required for chromophore binding and fluorescence. Sci Rep 5:18348. doi: 10.1038/srep18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klicki K, Ferreira D, Risser D, Garcia-Pichel F. 2022. A regulatory linkage between scytonemin production and hormogonia differentiation in Nostoc punctiforme. iScience 25:104361. doi: 10.1016/j.isci.2022.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoiczyk E, Baumeister W. 1998. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr Biol 8:1161–1168. doi: 10.1016/s0960-9822(07)00487-3. [DOI] [PubMed] [Google Scholar]
- 36.Halfen LN, Castenholz RW. 1970. Gliding in a blue-green alga: a possible mechanism. Nature 225:1163–1165. doi: 10.1038/2251163a0. [DOI] [PubMed] [Google Scholar]
- 37.Khayatan B, Meeks JC, Risser DD. 2015. Evidence that a modified type IV pilus-like system powers gliding motility and polysaccharide secretion in filamentous cyanobacteria. Mol Microbiol 98:1021–1036. doi: 10.1111/mmi.13205. [DOI] [PubMed] [Google Scholar]
- 38.Duggan PS, Gottardello P, Adams DG. 2007. Molecular analysis of genes in Nostoc punctiforme involved in pilus biogenesis and plant infection. J Bacteriol 189:4547–4551. doi: 10.1128/JB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamparter T, Babian J, Fröhlich K, Mielke M, Weber N, Wunsch N, Zais F, Schulz K, Aschmann V, Spohrer N, Krauß N. 2022. The involvement of type IV pili and the phytochrome CphA in gliding motility, lateral motility and photophobotaxis of the cyanobacterium Phormidium lacuna. PLoS One 17:e0249509. doi: 10.1371/journal.pone.0249509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier B, Wong GCL. 2015. How bacteria use type IV pili machinery on surfaces. Trends Microbiol 23:775–788. doi: 10.1016/j.tim.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Kavita K, de Mets F, Gottesman S. 2018. New aspects of RNA-based regulation by Hfq and its partner sRNAs. Curr Opin Microbiol 42:53–61. doi: 10.1016/j.mib.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuergers N, Ruppert U, Watanabe S, Nurnberg DJ, Lochnit G, Dienst D, Mullineaux CW, Wilde A. 2014. Binding of the RNA chaperone Hfq to the type IV pilus base is crucial for its function in Synechocystis sp. PCC 6803. Mol Microbiol 92:840–852. doi: 10.1111/mmi.12595. [DOI] [PubMed] [Google Scholar]
- 43.Dienst D, Duhring U, Mollenkopf HJ, Vogel J, Golecki J, Hess WR, Wilde A. 2008. The cyanobacterial homologue of the RNA chaperone Hfq is essential for motility of Synechocystis sp. PCC 6803. Microbiology (Reading) 154:3134–3143. doi: 10.1099/mic.0.2008/020222-0. [DOI] [PubMed] [Google Scholar]
- 44.Harwood TV, Zuniga EG, Kweon H, Risser DD. 2021. The cyanobacterial taxis protein HmpF regulates type IV pilus activity in response to light. Proc Natl Acad Sci USA 118. doi: 10.1073/pnas.2023988118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Hess WR, Zhang C. 2022. “Life is short, and art is long”: RNA degradation in cyanobacteria and model bacteria. mLife 1:21–39. doi: 10.1002/mlf2.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yegorov Y, Sendersky E, Zilberman S, Nagar E, Waldman Ben-Asher H, Shimoni E, Simkovsky R, Golden SS, LiWang A, Schwarz R. 2021. A cyanobacterial component required for pilus biogenesis affects the exoproteome. mBio 12. doi: 10.1128/mBio.03674-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bulyha I, Schmidt C, Lenz P, Jakovljevic V, Hone A, Maier B, Hoppert M, Sogaard-Andersen L. 2009. Regulation of the type IV pili molecular machine by dynamic localization of two motor proteins. Mol Microbiol 74:691–706. doi: 10.1111/j.1365-2958.2009.06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuergers N, Nurnberg DJ, Wallner T, Mullineaux CW, Wilde A. 2015. PilB localization correlates with the direction of twitching motility in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology (Reading) 161:960–966. doi: 10.1099/mic.0.000064. [DOI] [PubMed] [Google Scholar]
- 49.Cho YW, Gonzales A, Harwood TV, Huynh J, Hwang Y, Park JS, Trieu AQ, Italia P, Pallipuram VK, Risser DD. 2017. Dynamic localization of HmpF regulates type IV pilus activity and directional motility in the filamentous cyanobacterium Nostoc punctiforme. Mol Microbiol 106:252–265. doi: 10.1111/mmi.13761. [DOI] [PubMed] [Google Scholar]
- 50.Hoiczyk E, Baumeister W. 1995. Envelope structure of four gliding filamentous cyanobacteria. J Bacteriol 177:2387–2395. doi: 10.1128/jb.177.9.2387-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuckerman DM, Man To So J, Hoiczyk E. 2022. Type two secretion systems secretins are necessary for exopolymeric slime secretion in cyanobacteria and myxobacteria. bioRxiv. doi: 10.1101/2022.03.08.483542. [DOI]
- 52.Damerval T, Guglielmi G, Houmard J, De Marsac NT. 1991. Hormogonium differentiation in the cyanobacterium Calothrix: a photoregulated developmental process. Plant Cell 3:191–201. doi: 10.1105/tpc.3.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castenholz RW. 1982. Motility and taxis, p 413–419. In Carr NG, Whitton BA (ed), The Biology of Cyanobacteria. Blackwell scientific publications. Hoboken, NJ. [Google Scholar]
- 54.Nilsson M, Rasmussen U, Bergman B. 2006. Cyanobacterial chemotaxis to extracts of host and nonhost plants. FEMS Microbiol Ecol 55:382–390. doi: 10.1111/j.1574-6941.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 55.Schuergers N, Mullineaux CW, Wilde A. 2017. Cyanobacteria in motion. Curr Opin Plant Biol 37:109–115. doi: 10.1016/j.pbi.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Nultsch W, Schuchart H, HHl M. 1979. Investigations on the phototactic orientation of Anabaena variabilis. Arch Microbiol 122:85–91. doi: 10.1007/BF00408050. [DOI] [Google Scholar]
- 57.Nultsch W, Wenderoth K. 1983. Partial irradiation experiments with Anabaena variabilis (Kütz). Zeitschrift Für Pflanzenphysiologie 111:1–7. doi: 10.1016/S0044-328X(83)80067-1. [DOI] [Google Scholar]
- 58.Sourjik V, Wingreen NS. 2012. Responding to chemical gradients: bacterial chemotaxis. Curr Opin Cell Biol 24:262–268. doi: 10.1016/j.ceb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell EL, Hagen KD, Chen R, Risser DD, Ferreira DP, Meeks JC. 2015. Genetic analysis reveals the identity of the photoreceptor for phototaxis in hormogonium filaments of Nostoc punctiforme. J Bacteriol 197:782–791. doi: 10.1128/JB.02374-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Risser DD, Meeks JC. 2013. Comparative transcriptomics with a motility-deficient mutant leads to identification of a novel polysaccharide secretion system in Nostoc punctiforme. Mol Microbiol 87:884–893. doi: 10.1111/mmi.12138. [DOI] [PubMed] [Google Scholar]
- 61.Kirpich JS, Chang CW, Madsen D, Gottlieb SM, Martin SS, Rockwell NC, Lagarias JC, Larsen DS. 2018. Noncanonical photodynamics of the orange/green cyanobacteriochrome power sensor NpF2164g7 from the PtxD phototaxis regulator of Nostoc punctiforme. Biochemistry 57 57:2636–2648. doi: 10.1021/acs.biochem.7b01252. [DOI] [PubMed] [Google Scholar]
- 62.Jakob A, Nakamura H, Kobayashi A, Sugimoto Y, Wilde A, Masuda S. 2020. The (PATAN)-CheY-like response regulator PixE Interacts with the motor ATPase PilB1 to control negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 61:296–307. doi: 10.1093/pcp/pcz194. [DOI] [PubMed] [Google Scholar]
- 63.Duggan PS, Thiel T, Adams DG. 2013. Symbiosis between the cyanobacterium Nostoc and the liverwort Blasia requires a CheR-type MCP methyltransferase. Symbiosis 59:111–120. doi: 10.1007/s13199-012-0216-9. [DOI] [Google Scholar]
- 64.Zuniga EG, Boateng KKA, Bui NU, Kurnfuli S, Muthana SM, Risser DD. 2020. Identification of a hormogonium polysaccharide-specific gene set conserved in filamentous cyanobacteria. Mol Microbiol 114:597–608. doi: 10.1111/mmi.14566. [DOI] [PubMed] [Google Scholar]
- 65.Woodward L, Naismith JH. 2016. Bacterial polysaccharide synthesis and export. Curr Opin Struct Biol 40:81–88. doi: 10.1016/j.sbi.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 66.McDonald HJ, Kweon H, Kurnfuli S, Risser DD. 2022. A DnaK(Hsp70) chaperone system connects type IV pilus activity to polysaccharide secretion in cyanobacteria. mBio 13:e0051422-22. doi: 10.1128/mbio.00514-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wetmore KM, Price MN, Waters RJ, Lamson JS, He J, Hoover CA, Blow MJ, Bristow J, Butland G, Arkin AP, Deutschbauer A. 2015. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. mBio 6:306. doi: 10.1128/mBio.00306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, Roux KJ. 2016. An improved smaller biotin ligase for BioID proximity labeling. Mol Biol Cell 27:1188–1196. doi: 10.1091/mbc.E15-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.00392-23-s0001.docx, DOCX file, 0.01 MB (11.9KB, docx)
Supplemental material. Download aem.00392-23-s0002.mp4, MP4 file, 8.2 MB (8.2MB, mp4)