Abstract
Objective
The aim of this study isto assess the efficacy of a very low-calorie ketogenic diet (VLCKD) method vs a Mediterranean low-calorie diet (LCD) in obese polycystic ovary syndrome (PCOS) women of a reproductive age.
Design
Randomized controlled open-label trial was performed in this study. The treatment period was 16 weeks; VLCKD for 8 weeks then LCD for 8 weeks, according to the Pronokal® method (experimental group; n = 15) vs Mediterranean LCD for 16 weeks (control group; n = 15). Ovulation monitoring was carried out at baseline and after 16 weeks, while a clinical exam, bioelectrical impedance analysis (BIA), anthropometry, and biochemical analyses were performed at baseline, at week 8, and at week 16.
Results
BMI decreased significantly in both groups and to a major extent in the experimental group (−13.7% vs −5.1%, P = 0.0003). Significant differences between the experimental and the control groups were also observed in the reduction of waist circumference (−11.4% vs −2.9%), BIA-measured body fat (−24.0% vs −8.1%), and free testosterone (−30.4% vs −12.6%) after 16 weeks (P = 0.0008, P = 0.0176, and P = 0.0009, respectively). Homeostatic model assessment for insulin resistance significantly decreased only in the experimental group (P = 0.0238) but without significant differences with respect to the control group (−23% vs −13.2%, P > 0.05). At baseline, 38.5% of participants in the experimental group and 14.3% of participants in the control group had ovulation, which increased to 84.6% (P = 0.031) and 35.7% (P > 0.05) at the end of the study, respectively.
Conclusion
In obese PCOS patients, 16 weeks of VLCKD protocol with the Pronokal® method was more effective than Mediterranean LCD in reducing total and visceral fat, and in ameliorating hyperandrogenism and ovulatory dysfunction.
Significance statements
To the best of our knowledge, this is the first randomized controlled trial on the use of the VLCKD method in obese PCOS. It demonstrates the superiority of VLCKD with respect to Mediterranean LCD in reducing BMI with an almost selective reduction of fat mass and a unique effect of VLCKD in reducing visceral adiposity, insulin resistance, and in increasing SHBG with a consequent reduction of free testosterone. Interestingly, this study also demonstrates the superiority of the VLCKD protocol in improving ovulation, whose occurrence increased by 46.1% in the group treated by the VLCKD method against a rise of 21.4% in the group treated by Mediterranean LCD. This study extends the therapeutic approach possibilities in obese PCOS women.
Keywords: VLCKD, PCOS, ovulation, hyperandrogenism, weight loss, abdominal obesity
Introduction
Polycystic ovary syndrome (PCOS) is a frequent hyperandrogenic disorder characterized by pathophysiological heterogeneity that translates into the heterogeneity of the phenotype, thus complicating condition management (1). In obese PCOS women, visceral adiposity, insulin resistance (IR), and compensatory hyperinsulinemia are usually the triggering pathogenetic factors, whereas androgen excess is frequently mild and sometimes secondary to visceral obesity and IR (2). Therefore, in obese PCOS, the main treatment target is usually obesity and IR. These two conditions maintain and aggravate each other and should thus be treated together to obtain the best outcome (3, 4).
Accordingly, in obese PCOS, weight loss results in a significant attenuation of clinical and metabolic manifestations for most patients, and, in some cases, in complete remission of the syndrome (5, 6, 7). In order to obtain beneficial effects on hormones, metabolism, and clinical outcomes, current guidelines recommend a 510% weight loss in obese women with PCOS (8, 9, 10).
Therefore, dietary interventions that will lead to effective weight loss are the first-line management strategy for obese women with PCOS (8, 11). However, IR, visceral adiposity, hyperandrogenism (12, 13), low basal metabolic rate (14), low postprandial thermogenesis (15), and, frequently, low physical activity (16), as well as psychological distress (17, 18) are major obstacles for weight loss in PCOS treated with standard lifestyle intervention programs.
A very low-calorie ketogenic diet (VLCKD) is a dietary strategy that appears to be effective in populations with obesity in terms of rapid weight loss, total and visceral fat reduction, and marked improvement of IR and hyperinsulinemia (19, 20, 21, 22). To our knowledge, no published randomized controlled studies exploring the use of a VLCKD in obese PCOS women are available.
We thus performed a 16-week open-label randomized controlled trial (RCT) in a population of obese women with PCOS at a reproductive age in order to analyze the efficacy of the VLCKD on body weight and body composition (primary outcomes) and on abdominal adipose tissue, IR, hyperandrogenemia, hirsutism, ovulation, ovarian morphology, and psychological well-being and distress (secondary outcomes).
Material and methods
Subjects
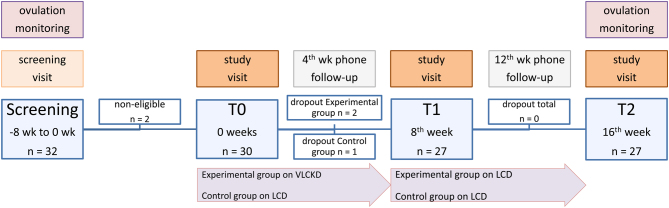
Thirty-two consecutive women of a reproductive age (18–45 years) and with a BMI between 28 and 40 kg/m2 attending the Division of Endocrinology of Azienda Ospedaliero-Universitaria of Bologna for PCOS were invited to participate in the study and, after informed consent signature, were screened. During the screening, a diagnosis of PCOS was confirmed according to the National Institutes of Health (NIH) criteria in 30 women, who were all part of the study as they matched the inclusion criteria (Fig. 1). The diagnosis of PCOS according to the NIH criteria included the presence of oligo/amenorrhea with hirsutism (modified Ferriman-Gallwey-mFG ≥ 8) and/or hyperandrogenemia (free androgen index (FAI) ≥ 4), after excluding other causes of hyperandrogenism) (23, 24).
Figure 1.
Flow chart of the study. There were three drop-outs during the study, two in the experimental group, and one in the control group, all during the first 8 weeks of the study. The reasons for dropping out were kidney stones and gastrointestinal intolerance in the experimental group and scarce compliance with the diet in the control group.
Exclusion criteria (Fig. 1) were any of the following: type 1 diabetes mellitus, type 2 diabetes mellitus treated with insulin, obesity caused by endocrine diseases unrelated to PCOS or drug-induced obesity, any weight loss diet or estro-progestin therapy up to 3 months preceding the start of the trial, severe depression or other psychiatric disorder, alcohol and drug abuse, severe hepatic insufficiency, any level of renal insufficiency or calculosis of the urinary tract, gout, malignancy, previous cardio- or cerebrovascular events, uncontrolled hypertension, electrolytic disturbances, any drug capable of interfering with glucose or steroid metabolism, menopause, pregnancy, breastfeeding, and refusal to sign the informed consent form.
The study was approved by the local Ethics Committee Area Vasta Emilia Centro-AVEC (26/2020/Sper/AOUBo) and preregistered at the ClinicalTrials.gov Protocol Registration and Results System of the U.S. National Library of Medicine (NCT04801173).
Study protocol
At baseline, blood samples were drawn between 08:00 and 09:00 h, after overnight fasting. Samples used for measurement of biochemistry, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and sex hormone-binding globulin (SHBG) were immediately processed at a laboratory in Bologna (Laboratorio Unico Metropolitano). Additionally, serum was obtained by a 5 mL blood sample and stored at −20°C for later measurement of androgens (testosterone, androstenedione, DHEA, 17OH-progesterone, and progesterone) by liquid chromatography-tandem mass spectrometry (LC-MS/MS), at the Center for Applied Biomedical Research of the University of Bologna, S. Orsola Hospital.
The same day, a medical examination was made, during which arterial blood pressure, heart rate, and anthropometric parameters (height, weight, waist, and hip circumference) were measured. In addition, upper and lower extremity bioimpedance (BIA 101, Akern s.r.l, Italy) was performed in order to measure fat mass and fat-free mass (25). Hirsutism was also assessed using the mFG score and digital videodermoscopy by quantifying terminal hair growth in three androgen-sensitive skin areas of the body – chin, thorax, and lower abdomen, covering 8 cm2 of chin and 38 cm2 of median line thorax and lower abdomen skin (Leviacam 13 megapixel dermatoscope, FotoFinder Systems GmbH, Germany, through TrichoLAB epilens35 body hair lens, TrichoLAB GmbH, Germany). Images obtained by videodermoscopy were analyzed using TrichoLAB Suite software (TrichoLAB GmbH). Three measurements were obtained for each skin area: total hair count, hair thickness, and hair density (26).
An estimate of psychological distress and well-being was performed using validated self-report psychometric questionnaires. Psychological distress was operationalized with anxiety symptoms, depressive symptoms, somatization, and hostility-irritability (27) of the Kellner’s Symptom Questionnaire (SQ) (28). This instrument is composed of 92 items which evaluate the levels of anxiety, depression, somatization, and hostility-irritability during the past week. Items are dichotomous (0 = no or false, 1 = yes or true). Each symptoms subscale has a score ranging from 0 (reporting no symptoms) to 23 (endorsing all symptoms). A total distress score is calculated by summing the four symptoms subscale (range 0–92) with higher scores representing higher psychological distress. The instrument significantly discriminates between clinical and non-clinical settings and between medical or psychiatric patients and healthy controls (29). Psychological well-being was evaluated with the Italian-validated version (30) of the Psychological Wellbeing Scale (31). The questionnaire is composed of 84 items divided into 6 subcategories: autonomy (self-determination and independence), environmental mastery (a sense of competence to manage the environment and everyday activities), personal growth (the extent to which an individual feels that they are engaging in continued development), positive relations (the ability to maintain warm and trusting interpersonal relationships), purpose in life (being guided by a sense of direction in life), and self-acceptance (having a positive attitude toward the self). Items are rated on a 7-point scale (1 = strongly disagree to 6 = strongly agree). Responses to negatively formulated items were reversed and a total score was calculated by summing up the corresponding items (range 84–504) with higher scores corresponding to higher psychological well-being. Previous studies have reported good psychometric properties of such self-reporting (30, 32).
The following day, participants were placed on diet therapy depending on whether they had been allocated to the experimental or the control study group (15 women in each group). The experimental group was placed on the VLCKD for 8 weeks, followed by the low-calorie diet (LCD) for another 8 weeks, according to the PronoKal® method (Pronokal Group, pronokal.com). The VLCKD phase consisted of three steps of 600–800 kcal/day with high-biological-value protein preparations obtained from cow’s milk, soy, eggs, green peas, and cereals, <50 g daily of carbohydrates from vegetables, and 10 g of olive oil per day. In step 1, which lasted for 6 weeks, the patients ate high biological value protein preparations five times a day and vegetables with a low glycemic index. In step 2 (1 week), one of the protein servings was substituted with a natural protein (e.g. meat or fish) either at lunch or at dinner. In the step 3 (1 week), a second serving of a low-fat natural protein replaced the second serving of a biological protein preparation.
Throughout these steps, supplements of vitamins and minerals were provided, such as K, Na, Mg, Ca, and omega-3 fatty acids. The LCD phase consisted of a gradual incorporation of different food groups with a daily calorie intake rising progressively from 1050 to 1400 kcal/day. In this phase, carbohydrates were gradually reintroduced in the following order: foods with the lowest glycemic index (fruit and dairy products), followed by foods with a moderate glycemic index (legumes), and finally by foods with a high glycemic index (bread, pasta, and cereals).
The control group was placed on a Mediterranean LCD for 16 weeks. The caloric intake in the control group ranged from 1200 to 1420 kcal/day, with 15, 30, and 55% contributions from proteins, lipids, and carbohydrates, respectively. The dietary energy intake was calculated by subtracting 500 kcal from the usual individual energy intake, according to the LARN guidelines (33). Habitual energy intake was estimated based on the diet history method and a 3-day recall questionnaire.
All diets were prescribed by the same dietician from the Division of Endocrinology of Azienda Ospedaliero-Universitaria of Bologna. Allocation to the two study arms was randomized per recruitment order before the recruitment phase, ensuring comparable age and BMI at baseline.
The experimental group was instructed to do muscle tone exercises during the 8 weeks of VLCKD, while both groups were instructed to do aerobic and muscle tone exercises during the time on LCD, following the exercise tables of the Pronokal® method (available on pronokal.com).
At the end of week 8 (T1) and week 16 (T2) of the trial, all the measurements and examinations performed at baseline were repeated.
Furthermore, a 4-week ovulation monitoring was performed during screening period and at the end of the trial. Monitoring was done by pelvic ultrasound (US) and measurements of serum LH, FSH, E2, and progesterone on days 7, 1, 21, and 28 after the start of menstrual bleeding. Ovulation was assumed to have occurred if a dominant follicle of diameter above 12 mm or corpus luteum was seen on ultrasound, with progesterone and E2 concentrations above 8 and 100 pg/mL, respectively (34).
Pelvic US performed on day 7 was used to obtain data on ovarian morphology. We measured ovarian volume, as well as the stroma to total area ratio, which is a US parameter related to circulating androgen levels in PCOS and polycystic ovary morphology (35). The ovarian area was calculated by outlining the edges of the ovary in the maximal plane section, and the stromal area was calculated in the same section by outlining the outer profile of the relatively hyperechoic stroma (35). We measured both ovaries and used the values of the largest one.
Diet and exercise regime compliance were evaluated by the same dietician from the Division of Endocrinology of Azienda Ospedaliero-Universitaria of Bologna at each scheduled visit (T1 and T2), as well as by phone between the visits through self-reports and food records. Onset and maintenance of ketosis in the experimental group were controlled with self-administered acetoacetic acid urine indicator strips (Ketostix, Ascensia Diabetes Care Holdings AG, Switzerland). Participants were given six strips and instructed to perform the test every 7 days, until the end of the VLCKD.
Tolerance and side effects
Tolerance to the diet and incidence of side effects was assessed during scheduled visits after 8 (T1) and 16 weeks (T2), as well as by phone after 4 and 12 weeks, through a structured interview on adherence to the diet, exercise regime, water intake, and specific adverse effects (excessive hunger, headache, asthenia, constipation, and nausea, as well as stool regularity and consistency), followed by free-form questioning on changes in health status and life habits.
Assays
Sodium, potassium, phosphorus, calcium, creatinine, albumin, total protein, alanine aminotransferase (ALT), aspartate aminotransferase, γ-glutamyl transferase, total cholesterol, high-density lipoproteins (HDL), triglycerides, urea, bilirubin, and glucose were measured using a combination of colorimetric, photometric, kinetic, and potentiometric methods, as specified by the manufacturer, on a Beckman Coulter AU5800 analyzer (Beckman Coulter Inc., Brea, CA, USA). Insulin, LH, FSH, E2, and SHBG were measured by immunoenzymatic chemiluminescence on a Beckman Coulter DXI analyzer. Glycated hemoglobin was measured using high-performance liquid chromatography. Blood cell count was done using flow cytometry.
Testosterone, androstenedione, DHEA, 17OH-progesterone, and progesterone were measured by LC-MS/MS, following a previous methodology (36). Intra- and interassay coefficients of variation were <10 and <11%, respectively, and accuracy ranged between 83.7 and 104.4% for all the analytes. The sensitivity in serum was 0.019, 0.039, 0.781, 0.078, and 0.049 ng/mL for testosterone, androstenedione, DHEA, 17OH-progesterone, and progesterone, respectively.
IR was estimated using the homeostasis model assessment-IR (HOMA-IR) index, using the formula: fasting insulin (microU/L) × fasting glucose (mmol/L)/22.5. Low-density lipoprotein fraction was calculated using the formula proposed by Friedewald et al. (37). Free testosterone was estimated from values of testosterone measured by LC-MS, albumin, and SHBG, using the Vermeulen formula (38).
Statistical analysis
Sample size was a priori calculated by estimating that BMI after 4 months of LCD would be 31.0 ± 2.2 kg/m2, and after 4 months of VLCKD by Pronokal® method 27.9 ± 1.8 kg/m2 (40). The two-tailed test of α = 0.05 and β = 0.80 thus required groups of 11 patients each for a statistically significant result. Expecting an approximately 20% dropout rate per group, we enrolled a total of 15 patients per group (http://riskcalc.org:3838/samplesize/).
Study data were entered anonymously into a dedicated database and analyzed using the statistical package IBM SPSS Statistics (v. 26 64-bit for Windows, IBM Corporation). Continuous variables were presented as mean ± s.d. or s.e.m., and frequencies as proportions and percentages of the total. The normality of distribution was tested for all continuous variables with the Kolmogorov–Smirnov test. Variables with a skewed distribution were normalized using log10 transformation. Independent-samples t-test was used to test differences between groups at baseline.
Changes in variables in relation to time (baseline, 8-, and 16-week follow-up) and group (experimental and control) were analyzed using the general linear model (GLM) for repeated measures. The sphericity of the variables was tested using Mauchly's test, and variables that violated the assumption of sphericity were corrected with the Huynh–Feldt formula. Differences between each timepoint by the group were analyzed using pairwise post hoc multiple comparisons, Bonferroni-corrected. Differences by the group between baseline and final follow-up in ovulation and cut-off frequencies of pelvic US parameters were tested using McNemar’s test, while between-group differences were tested using Fisher’s exact test. Comparison differences were considered significant for respective two-tailed P-values < 0.05.
Results
Treatment compliance, tolerance, and side effects
There were three drop-outs during the study, two in the experimental group and one in the control group, all during the first 8 weeks of the study (Fig. 1). The reasons for dropping out were kidney stones and gastrointestinal intolerance, as a consequence of the diet, in the experimental group, and scarce compliance to the diet in the control group. All patients completing the study had excellent compliance with both dietary treatments and physical activity. VLCKD was well tolerated by all of the women.
Anthropometry, blood pressure, and fat distribution
There was no significant difference in baseline parameters between the two groups (Table 1). BMI decreased in both groups but significantly more in the experimental than in the control group (P = 0.0003). The experimental group, but not the control group, showed a significantly reduced waist circumference, above all during the first 8 weeks of the study, as well as fat mass and fat-free mass, and systolic and diastolic blood pressure (Table 1). However, only changes in waist circumference and fat mass were significantly higher in the experimental compared to the control group (P = 0.0008 and P = 0.0176, respectively; Table 1).
Table 1.
Age and anthropometric parameters at baseline (T0), 8 weeks (T1), and 16 weeks (T2) of the study in the experimental group and in the control group.
| Group | Mean ± s.d. | ANOVA P-valuea | GLM P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 (baseline) | T1 (8 weeks) | T2 (16 weeks) | T0 vs T1 | T1 vs T2 | T0 vs T2 | Time | Time × treatment | ||
| Age (years) | Experimental | 28.1 ± 5.8 | |||||||
| Control | 24.7 ± 6.0 | ||||||||
| Systolic blood pressure (mmHg) | Experimental | 133.08 ± 13.31 | 133.31 ± 15.54 | 124.31 ± 9.38 | 1.00000 | 0.05575 | 0.08438 | 0.03247 | 0.55625 |
| Control | 128.21 ± 9.92 | 125.71 ± 11.91 | 122.50 ± 8.59 | 1.00000 | 1.00000 | 0.24275 | 0.24435 | ||
| Diastolic blood pressure (mmHg) | Experimental | 86.15 ± 6.50 | 84.85 ± 6.79 | 80.69 ± 8.84 | 1.00000 | 0.03253 | 0.15786 | 0.02889 | 0.20266 |
| Control | 83.93 ± 9.03 | 82.50 ± 8.03 | 82.93 ± 6.45 | 1.00000 | 1.00000 | 1.00000 | 0.60578 | ||
| Heart rate (min−1) | Experimental | 78.31 ± 8.48 | 80.69 ± 10.29 | 75.23 ± 11.12 | 1.00000 | 0.43568 | 1.00000 | 0.30027 | 0.50043 |
| Control | 78.43 ± 8.49 | 80.36 ± 7.84 | 79.50 ± 8.92 | 1.00000 | 1.00000 | 1.00000 | 0.76275 | ||
| BMI (kg/m2) | Experimental | 33.9 ± 3.8 | 30.3 ± 3.4 | 29.3 ± 3.9 | <0.00001 | 0.00642 | <0.00001 | <0.00001b | 0.00027b |
| Control | 33.7 ± 4.3 | 32.6 ± 4.3 | 32.0 ± 4.9 | 0.00030 | 0.61618 | 0.02960 | 0.01024b | ||
| Waist circumference (cm) | Experimental | 98.2 ± 5.6 | 90.2 ± 5.7 | 87.0 ± 9.2 | 0.00005 | 0.09257 | 0.00034 | 0.00001b | 0.00072 |
| Control | 97.3 ± 9.1 | 95.2 ± 8.5 | 94.5 ± 11.9 | 0.13528 | 1.00000 | 0.35636 | 0.15965 | ||
| BIA fat mass (kg) | Experimental | 38.9 ± 10.4 | 33.0 ± 9.3 | 29.6 ± 8.8 | 0.00139 | 0.00323 | <0.00001 | <0.00001 | 0.01764 |
| Control | 38.4 ± 8.7 | 36.9 ± 9.6 | 35.3 ± 10.7 | 1.00000 | 0.76136 | 0.43545 | 0.23935 | ||
| BIA fat-free mass (kg) | Experimental | 51.1 ± 3.9 | 49.4 ± 4.0 | 49.1 ± 3.9 | 0.07506 | 1.00000 | 0.01464 | 0.00311 | 0.86444 |
| Control | 52.0 ± 7.9 | 50.6 ± 5.1 | 50.8 ± 5.8 | 1.00000 | 1.00000 | 1.00000 | 0.63257 | ||
aP-values adjusted by Bonferroni correction; bP-values of variables violating the sphericity assumption were adjusted using the Huynh–Feldt correction.
ANOVA, analysis of variance; BMI, body mass index; BIA, bioelectrical impedance analysis; GLM, general linear model.
Metabolic parameters
No significant between-group differences in baseline metabolic parameters were observed (Table 2). Fasting insulin, HOMA-IR, and total and HDL cholesterol significantly decreased in the experimental group only. Reductions in fasting insulin, HOMA-IR, and total cholesterol were mainly observed in the first 8 weeks of the study. Triglycerides and AST showed a decreasing trend in the experimental group but not in the control group. However, significance was not achieved (P = 0.0799 and P = 0.0527, respectively; Table 2). When comparing changes in these parameters between the two groups, no significant differences were found.
Table 2.
Metabolic parameters at baseline (T0), 8 weeks (T1), and 16 weeks (T2) of the study in the experimental group and in the control group.
| Mean ± s.d. | ANOVA P-value | GLM P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | T0 (baseline) | T1 (8 weeks) | T2 (16 weeks) | T0 vs T1 | T1 vs T2 | T0 vs T2 | Time | Time × treatment | |
| Glucose, fasting (mg/dL) | Experimental | 82.2 ± 6.7 | 81.4 ± 7.5 | 84.9 ± 7.9 | 1.00000 | 1.00000 | 1.00000 | 0.53591 | 0.98798 |
| Control | 88.0 ± 16.9 | 85.2 ± 11.5 | 88.6 ± 14.2 | 1.00000 | 0.67136 | 1.00000 | 0.56461 | ||
| Insulin, fasting (μU/mL) | Experimental | 13.4 ± 7.0 | 8.6 ± 3.9 | 9.9 ± 5.6 | 0.03898 | 0.98329 | 0.08481 | 0.01294 | 0.95010 |
| Control | 17.6 ± 9.9 | 12.9 ± 5.5 | 15.2 ± 8.4 | 0.12356 | 0.89186 | 1.00000 | 0.14258 | ||
| HOMA-IR index | Experimental | 2.74 ± 1.48 | 1.75 ± 0.89 | 2.11 ± 1.26 | 0.06118 | 1.00000 | 0.15121 | 0.02379 | 0.81895 |
| Control | 3.96 ± 2.73 | 2.79 ± 1.48 | 3.43 ± 2.28 | 0.15988 | 0.53596 | 1.00000 | 0.15080 | ||
| Total cholesterol (mg/dL) | Experimental | 194 ± 34 | 165 ± 26 | 174 ± 32 | 0.00433 | 0.64525 | 0.03723 | 0.00056 | 0.14060a |
| Control | 195 ± 41 | 190 ± 41 | 188 ± 39 | 1.00000 | 1.00000 | 1.00000 | 0.86452a | ||
| HDL cholesterol (mg/dL) | Experimental | 51.8 ± 7.3 | 44.8 ± 6.4 | 49.4 ± 6.4 | 0.04802 | 0.06807 | 0.88822 | 0.01721 | 0.34995 |
| Control | 49.1 ± 7.7 | 45.4 ± 7.1 | 48.3 ± 11.2 | 0.36801 | 0.55491 | 1.00000 | 0.31895 | ||
| Triglycerides (mg/dL) | Experimental | 126 ± 79 | 84 ± 19 | 78 ± 23 | 0.39306 | 1.00000 | 0.15047 | 0.07991a | 0.58854 |
| Control | 123 ± 69 | 111 ± 62 | 102 ± 57 | 0.22285 | 1.00000 | 0.34885 | 0.15210 | ||
| LDL cholesterol (mg/dL) | Experimental | 117 ± 25 | 103 ± 25 | 109 ± 29 | 0.16966 | 0.66260 | 0.93629 | 0.11683 | 0.25302 |
| Control | 122 ± 31 | 123 ± 34 | 123 ± 32 | 1.00000 | 1.00000 | 1.00000 | 0.98158 | ||
| AST (IU/L) | Experimental | 24.9 ± 9.7 | 22.7 ± 8.4 | 18.0 ± 4.7 | 1.00000 | 0.30839 | 0.09454 | 0.05266 | 0.42454 |
| Control | 26.0 ± 13.0 | 24.6 ± 13.9 | 24.1 ± 11.3 | 1.00000 | 1.00000 | 0.93344 | 0.53769 | ||
| ALT (IU/L) | Experimental | 36.7 ± 35.3 | 28.1 ± 27.2 | 20.9 ± 12.9 | 0.56761 | 1.00000 | 0.51606 | 0.20348 | 0.75997 |
| Control | 37.6 ± 36.8 | 32.3 ± 38.9 | 29.9 ± 31.4 | 1.00000 | 1.00000 | 0.42616 | 0.32149 | ||
aP-values of variables violating the sphericity assumption were adjusted using the Huynh–Feldt correction.
ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; GLM, general linear model, HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein.
Testosterone, SHBG, and hirsutism
At baseline, total testosterone was significantly higher in the experimental than in the control group, whereas SHBG and free testosterone concentrations were comparable between the two groups (Fig. 2). No treatment significantly changed serum levels of total testosterone, whereas SHBG significantly increased and free testosterone significantly decreased in the experimental group only. These modifications were significant in the first 8 weeks of the study (Fig. 2). Overall changes in SHBG and free testosterone were significantly higher in the experimental group when compared with controls (P = 0.0004 for SHBG and P = 0.0009 for free testosterone).
Figure 2.
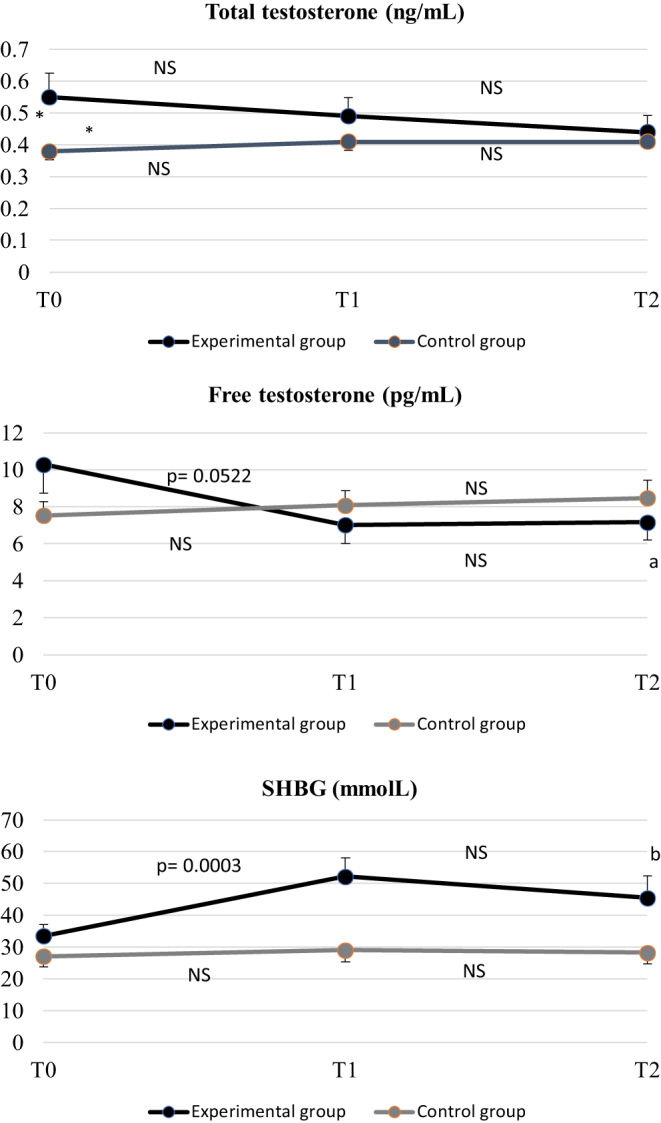
Total and free testosterone and SHBG at baseline (T0), 8 weeks (T1), and 16 weeks (T2) of the study in the experimental and control groups. Free testosterone was calculated by Vermeulen method. Data are expressed as mean and s.e.m.. *P = 0.0491 in the comparison between the two groups at baseline, and aP = 0.0234 and bP = 0.0036 in the comparison between T0 and T2 within the experimental group by ANOVA (no statistical differences were observed between T0 and T2 within the control group). P values shown in the figures represent the comparison between T0 and T1 and T1 and T2 within each group by ANOVA. The overall modification of free testosterone and SHBG during the study, estimated by the GLM, was significant only in the experimental group (P = 0.035 and P < 0.0001, respectively). Moreover, the overall modification of free testosterone and SHBG in the experimental group was significantly different with respect to the overall modification in the control group (P = 0.0009 and P = 0.0004, respectively).
No significant between-group differences in baseline hirsutism evaluated by videodermoscopy (Table 3) or by mFG score (P = 0.3480) were observed. Using videodermoscopy, we observed a significant reduction in chin hair density and hair thickness of the thorax and lower abdomen in the experimental group only (Table 3). Overall changes in chin hair density and hair thickness of the lower abdomen were significantly higher in the experimental group than the control group (P = 0.0062 and P = 0.0489, respectively) (Table 3). No treatment significantly modified the mFG score (P = 0.1654 in the experimental group and P = 0.4104 in the control group).
Table 3.
Hirsutism by videodermoscopy at baseline (T0), 8 weeks (T1), and 16 weeks (T2) of the study in the experimental group and in the control group.
| Mean ± s.d. | ANOVA P-value | GLM P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | T0 (baseline) | T1 (8 weeks) | T2 (16 weeks) | T0 vs T1 | T1 vs T2 | T0 vs T2 | Time | Time × treatment | |
| Total hair count, chin | Experimental | 16.0 ± 11.2 | 11.5 ± 12.9 | 4.27 ± 4.02 | 1.00000 | 0.43262 | 0.07478 | 0.06575 | 0.06420 |
| Control | 4.33 ± 1.67 | 3.94 ± 1.87 | 8.93 ± 10.6 | 0.92820 | 0.85678 | 1.00000 | 0.40256 | ||
| Hair thickness, chin (µm) | Experimental | 56.1 ± 21.3 | 53.5 ± 27.8 | 65.5 ± 24.7 | 1.00000 | 0.52591 | 0.52531 | 0.21920 | 0.03542 |
| Control | 64.9 ± 17.1 | 64.4 ± 14.0 | 48.9 ± 28.7 | 1.00000 | 0.52518 | 0.50826 | 0.17064 | ||
| Hair density, chin (n./cm2) | Experimental | 15.7 ± 11.3 | 11.4 ± 13.1 | 3.73 ± 4.00 | 0.77069 | 0.24495 | 0.01812 | 0.01333 | 0.00624 |
| Control | 3.68 ± 1.10 | 2.76 ± 0.84 | 7.88 ± 9.49 | 0.19585 | 0.40583 | 0.64536 | 0.16630 | ||
| Cumulative hair index, chin | Experimental | 0.90 ± 0.78 | 0.72 ± 1.02 | 0.25 ± 0.16 | 1.00000 | 0.37979 | 0.06969 | 0.05804 | 0.17502 |
| Control | 0.28 ± 0.13 | 0.25 ± 0.13 | 0.37 ± 0.32 | 1.00000 | 1.00000 | 1.00000 | 0.98086a | ||
| Total hair count, thorax | Experimental | 9.36 ± 6.85 | 8.54 ± 5.46 | 6.45 ± 4.41 | 1.00000 | 0.87314 | 0.63053 | 0.34316 | 0.10484 |
| Control | 4.99 ± 3.91 | 3.68 ± 2.74 | 6.83 ± 2.27 | 1.00000 | 0.07120 | 0.57889 | 0.07612 | ||
| Hair thickness, thorax (µm) | Experimental | 61.2 ± 17.4 | 48.2 ± 20.2 | 44.9 ± 14.1 | 1.00000 | 0.37283 | 0.03696 | 0.09066 | 0.32250 |
| Control | 59.1 ± 12.9 | 54.1 ± 23.8 | 59.5 ± 14.5 | 1.00000 | 1.00000 | 1.00000 | 0.64207 | ||
| Hair density, thorax (n./cm2) | Experimental | 7.91 ± 5.96 | 6.27 ± 3.87 | 5.27 ± 3.22 | 1.00000 | 1.00000 | 0.47717 | 0.27890 | 0.12742 |
| Control | 3.81 ± 3.11 | 2.49 ± 1.91 | 5.25 ± 2.14 | 0.82655 | 0.05794 | 0.93002 | 0.08379 | ||
| Cumulative hair index, thorax | Experimental | 0.45 ± 0.33 | 0.42 ± 0.25 | 0.39 ± 0.28 | 1.00000 | 1.00000 | 1.00000 | 0.85228 | 0.23417 |
| Control | 0.28 ± 0.19 | 0.23 ± 0.21 | 0.42 ± 0.17 | 1.00000 | 0.12350 | 0.29759 | 0.05746 | ||
| Total hair count, lower abdomen | Experimental | 7.45 ± 4.30 | 8.36 ± 6.90 | 9.18 ± 5.67 | 1.00000 | 1.00000 | 1.00000 | 0.67773a | 0.68181a |
| Control | 7.09 ± 4.22 | 7.88 ± 5.94 | 6.30 ± 5.42 | 1.00000 | 1.00000 | 1.00000 | 0.83317 | ||
| Hair thickness, lower abdomen (µm) | Experimental | 54.4 ± 16.5 | 53.4 ± 13.7 | 43.9 ± 14.9 | 1.00000 | 0.19679 | 0.05138 | 0.04059 | 0.04896 |
| Control | 43.4 ± 17.7 | 42.1 ± 18.4 | 53.3 ± 26.7 | 1.00000 | 1.00000 | 1.00000 | 0.39775a | ||
| Hair density, lower abdomen (n./cm2) | Experimental | 5.54 ± 3.32 | 6.64 ± 5.86 | 7.54 ± 4.19 | 1.00000 | 1.00000 | 0.64026 | 0.54554a | 0.69277 |
| Control | 5.91 ± 3.24 | 6.30 ± 4.18 | 5.51 ± 4.46 | 1.00000 | 1.00000 | 1.00000 | 0.92515 | ||
| Cumulative hair index, lower abdomen (mm/cm2) | Experimental | 0.37 ± 0.17 | 0.43 ± 0.32 | 0.43 ± 0.26 | 1.00000 | 1.00000 | 1.00000 | 0.72460a | 0.87003 |
| Control | 0.35 ± 0.21 | 0.35 ± 0.25 | 0.33 ± 0.26 | 1.00000 | 1.00000 | 1.00000 | 0.97684 | ||
Statistical significance set to P < 0.05.
aP-values of variables violating the sphericity assumption were adjusted using the Huynh–Feldt correction.
ANOVA, analysis of variance; GLM, general linear model.
Ovulation and ovarian morphology
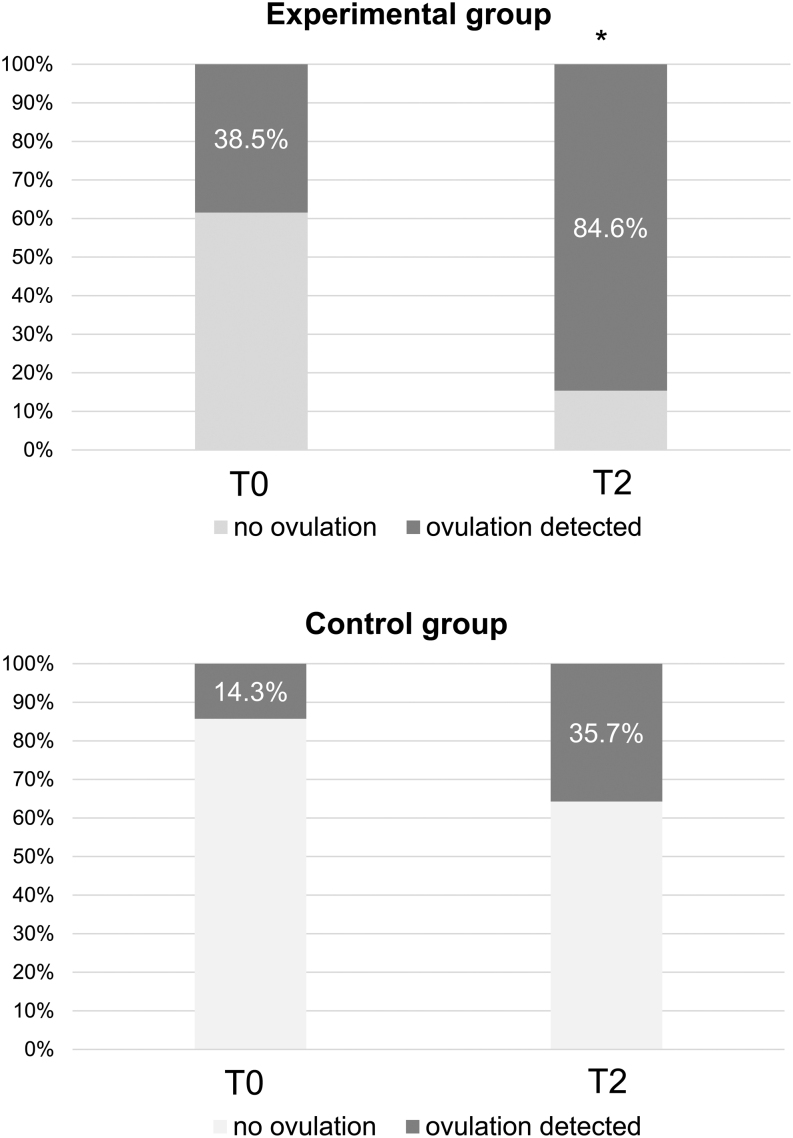
Ovulation occurrence at baseline was similar in the two groups, but it differed significantly by the end of the study (P = 0.018). At the end of the study, ovulation occurrence improved significantly in the experimental group (+46.1%, P = 0.031), while the change observed in the control group was not significant (+21.4%, P > 0.05; Fig. 3).
Figure 3.
Percentage of ovulation at baseline (T0) and 16 weeks (T2) of the study in the experimental and control groups. No statistical difference was observed between the two groups at baseline, but at the end of the study, it was significantly different between the two groups (P = 0.018) (χ2 test). *P = 0.031 by McNemar's test in the comparison between T0 and T2 within the experimental group. The comparison between T0 and T2 within the control group was not significant.
Both ovarian volume and stromal to total area ratio of the largest ovary were similar in the two groups at baseline. The volume of the largest ovary did not significantly change in either group by the end of the study (experimental group, from 14.5 ± 4.2 to 12.6 ± 4.8 cm3, P = 0.1754; control group, from 12.5 ± 3.3 to 11.8 ± 2.5 cm3, P = 0.4008). Stromal to total area ratio of the largest ovary decreased with a borderline significance in the experimental (from 0.32 ± 0.07 to 0.27 ± 0.05, P = 0.0594), but not in the control group (from 0.26 ± 0.05 to 0.28 ± 0.05, P = 0.5564). However, overall changes in this parameter did not differ between the two groups (P = 0.1411).
Psychological assessment
Psychological distress as well as psychological well-being were similar in the two groups at baseline and did not significantly change in either group by the end of the study (Table 4).
Table 4.
Psychological assessment at baseline (T0), 8 weeks (T1), and 16 weeks (T2) of the study in the experimental group and in the control group.
| Group | Mean ± s.d. | ANOVA P-value | GLM P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 (baseline) | T1 (8 weeks) | T2 (16 weeks) | T0 vs T1 | T1 vs T2 | T0 vs T2 | Time | Time × treatment | ||
| SQ, anxiety | Experimental | 6.77 ± 6.13 | 4.31 ± 4.99 | 6.08 ± 6.24 | 1.00000 | 0.59639 | 1.00000 | 0.52468 | 0.15170 |
| Control | 6.78 ± 5.02 | 6.89 ± 5.18 | 5.44 ± 3.64 | 0.43419 | 0.54053 | 1.00000 | 0.20967 | ||
| SQ, depression | Experimental | 4.54 ± 4.27 | 4.00 ± 4.28 | 4.85 ± 5.30 | 1.00000 | 1.00000 | 1.00000 | 0.90665 | 0.75243 |
| Control | 4.44 ± 4.30 | 4.00 ± 3.74 | 3.56 ± 2.88 | 1.00000 | 0.97065 | 1.00000 | 0.70797a | ||
| SQ, somatization | Experimental | 7.77 ± 4.48 | 4.54 ± 5.21 | 4.38 ± 5.64 | 1.00000 | 1.00000 | 1.00000 | 0.81809 | 0.61737a |
| Control | 6.33 ± 6.42 | 5.11 ± 5.75 | 5.00 ± 4.66 | 1.00000 | 1.00000 | 1.00000 | 0.67381a | ||
| SQ, hostility-irritability | Experimental | 3.85 ± 3.95 | 3.15 ± 3.76 | 3.92 ± 4.82 | 1.00000 | 1.00000 | 1.00000 | 0.82331 | 0.56217 |
| Control | 3.22 ± 3.07 | 2.89 ± 2.47 | 1.78 ± 1.99 | 1.00000 | 0.44801 | 1.00000 | 0.60900 | ||
| SQ, total distress | Experimental | 22.9 ± 16.9 | 16.0 ± 17.0 | 19.2 ± 20.6 | 1.00000 | 1.00000 | 1.00000 | 0.82984 | 0.58273 |
| Control | 20.8 ± 15.6 | 18.9 ± 12.7 | 15.8 ± 9.7 | 1.00000 | 0.37779 | 1.00000 | 0.57890a | ||
| PWB, total | Experimental | 368 ± 60 | 378 ± 67 | 368 ± 69 | 1.00000 | 1.00000 | 1.00000 | 0.85184a | 0.68232 |
| Control | 383 ± 42 | 391 ± 33 | 381 ± 53 | 1.00000 | 1.00000 | 1.00000 | 0.74842 | ||
Statistical significance set to P < 0.05.
aP-values of variables violating the sphericity assumption were adjusted using the Huynh–Feldt correction.
ANOVA, analysis of variance; GLM, general linear model; PWB, psychological well-being scale; SQ, Kellner’s symptom questionnaire.
Discussion
This monocentric open-label RCT performed in a population of obese reproductive-aged PCOS women demonstrated the superiority of the VLCKD Pronokal® method with respect to the Mediterranean LCD in reducing BMI with an almost selective reduction of impedentiometry-measured fat mass and a unique effect of VLCKD in reducing waist circumference, fasting insulin, HOMA-IR, total cholesterol, and in increasing SHBG with a consequent reduction of free testosterone. This study also demonstrated the superiority of the VLCKD method in improving ovulation, whose occurrence increased by 46.1% in the experimental group against a rise of 21.4% in the control group. A selective effect of the VLCKD method in improving hirsutism was also observed, but only when estimated by videodermoscopy and not by the mFG score. No changes in psychological well-being or distress were observed.
To the best of our knowledge, this is the RCT on the use of the VLCKD method in obese PCOS. The previous study was an uncontrolled study, where a mixed ketogenic diet of 600 kcal/day composed of 10–20 g/day of carbohydrates, 30 g/day of lipids and 1.1–1.2 g/kg/day of proteins was administered to 17 obese PCOS women for 45 days (39). In that study, significant improvements in anthropometric and body composition parameters, metabolic and hormonal variables, and in gynecological clinical outcomes, that is, menses abnormalities and the pregnancy rate, were observed.
In RCTs in obese patients of both sexes, the VLCKD has consistently exhibited high weight loss and low incidence of weight regain, at least in the short-medium term (40, 41, 42). In this study we confirmed the beneficial effect of VLCKD in reducing body weight in obese PCOS women, with a 13.7% average decrease in BMI after 16 weeks. The short-term duration of this study means that no information could be provided on the maintenance of obtained weight loss in the context of the study. However, at the end of the study, all the patients in the experimental group were given an eating plan balanced in carbohydrates, protein, and fat, a target to maintain the lost weight and promote a healthy lifestyle, which they followed regularly. None of the patients regained weight up to baseline values.
Other anthropometric parameters evaluated in this study, such as fat mass and waist circumference, indicate improved body composition and reduced visceral fat. The latter is a major metabolic disease risk factor and a trigger of pathological pathways in PCOS women with obesity that induces and/or aggravates hyperandrogenism and IR (43, 44, 45).
Remarkably, there is evidence that the effect of increased BMI on IR is proportionally more evident in PCOS women than in controls (46). This could possibly be due to the presence of dysfunctional adipocytes and/or a preferential visceral distribution of fat in PCOS (46, 47, 48). An intrinsic dysfunction of adipose tissue in PCOS has been demonstrated with consequent production of several mediators involved in developing/aggravating IR (49, 50). Among these, free fatty acids (FFA), androgens, cortisol, cytokines, some adipokines, and oxidative stress have been described (49, 50). Interestingly, although both the body weight and body fat decreased during the study period in the experimental group, waist circumference significantly decreased at the end of the VLCKD phase and was maintained during the LCD phase of the PronoKal® method, thus suggesting a direct beneficial effect of the ketogenic diet in decreasing visceral adiposity.
Along with the decrease in visceral fat, the ability of the VLCKD to decrease FFA production by adipose tissue, and of the ketogenic diet in general to decrease pro-inflammatory cytokines and oxidative stress, could cumulatively dampen IR in obese PCOS women (19, 51). In fact, in this study, we demonstrated a reduction of HOMA-IR, a surrogate index of IR, and of fasting insulin, in the experimental group at the end of the VLCKD phase, an effect that was maintained during the LCD phase.
A similar, but more pronounced behavior, was observed for SHBG and free testosterone, above all at the end of the VLCKD phase, where a marked increase in SHBG and a decrease in free testosterone were observed. The increase in SHBG could be due to many factors, such as the decrease in insulin and in proinflammatory cytokines, but also due to the marked decrease in monosaccharides ingested during the VLCKD phase of the protocol (52).
Another notable effect of the VLCKD method that we observed was on ovulation: comprehensive 4-week monitoring of ovulation before and after treatment revealed a large increase in ovulatory cycles detected in the experimental group. To our knowledge, this is the first RCT to test the efficacy of a ketogenic diet for potential fertility treatment in a robust way, using both US and biochemical monitoring of ovulation. It is possible that a reduction of ovarian lipotoxicity, along with a reduction of local inflammation and oxidative stress, boosted the effect of the reduction in visceral adiposity, IR, and hyperandrogenism, and thus improved ovulatory function at the end of the VLCKD protocol (53).
In contrast to the VLCKD studies in obese patients of the general population, where VLCKD therapy was able to reduce perceived stress and improve general well-being (19), in our study we did not observe any impact of VLCKD on psychological distress or psychological well-being. This result may be explained by the lack of improvement in hirsutism evaluated by the mFG score at the end of the study, which usually takes at least 6 months to change significantly (54). Hirsutism and changes in its magnitude are acutely perceptible by the patients, and studies have shown that hirsutism is correlated with psychological outcomes more than other PCOS manifestations (55, 56).
To the best of our knowledge, there have been no studies on the impact of VLCKD on distress or quality of life in PCOS. However, previous studies that highlighted the psychological benefits of short-term LCD in obese PCOS even without an amelioration in hirsutism, used other psychological instruments, particularly PCOSQ, which has a broader focus and emphasis on the symptomatology of PCOS (57).
This study has some limitations. First, the study sample is small. Also, the follow-up period reported here is too short to evaluate certain effects. However, since finishing the study, we have continued to monitor the participants and are planning to collect sufficient data for long-term evaluation of the treatment, including the occurrence of pregnancy. IR and visceral adiposity were approximated using surrogate indices HOMA-IR and waist circumference; although these have a lower resolution than some more direct methods, they have also been extensively field-tested and their properties are well known.
The study also has important strengths. The study protocol allowed for effective compliance monitoring. In addition, androgens and progestogens hormones were measured by the LC-MS/MS method (36, 58, 59), as well as videodermoscopy for precise monitoring of terminal hair growth, and the described four-week ovulation monitoring. Furthermore, the dropout rate was low in both trial arms, and this is probably due to the population of patients enrolled and the short-term period of treatment. The main issue for PCOS women of fertile age, particularly when menses abnormalities are present, as in our study population, is not being able to have children. These patients were informed at the beginning of the study how obesity can negatively impact fertility in PCOS, and this knowledge likely increased their motivation, and therefore, adherence to the dietary treatments.
To conclude, this RCT demonstrates that VLCKD is a valuable strategy for reducing total and abdominal fat, and for rapidly ameliorating hyperandrogenism, through an important impact on SHBG, as well as ovulatory dysfunction in obese PCOS women.
Declaration of interest
IS is the Medical Director of Pronokal, Spain. The other authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Funding
This work was supported by the Pronokal Group®. The funder was not involved in acquisition and analysis of data or in the statistical evaluation and interpretation of the results.
References
- 1.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews. Endocrinology 201814270–284. ( 10.1038/nrendo.2018.24) [DOI] [PubMed] [Google Scholar]
- 2.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R, et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. European Journal of Endocrinology 20141711–29. ( 10.1530/EJE-14-0253) [DOI] [PubMed] [Google Scholar]
- 3.Pasquali R Diamanti-Kandarakis E & Gambineri A. Management of endocrine disease: secondary polycystic ovary syndrome: theoretical and practical aspects. European Journal of Endocrinology 2016175R157–R169. ( 10.1530/EJE-16-0374) [DOI] [PubMed] [Google Scholar]
- 4.Moran L & Norman RJ. Understanding and managing disturbances in insulin metabolism and body weight in women with polycystic ovary syndrome. Best Practice and Research. Clinical Obstetrics and Gynaecology 200418719–736. ( 10.1016/j.bpobgyn.2004.05.003) [DOI] [PubMed] [Google Scholar]
- 5.Pasquali R Gambineri A Cavazza C Gasparini DI Ciampaglia W Cognigni GE & Pagotto U. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. European Journal of Endocrinology 201116453–60. ( 10.1530/EJE-10-0692) [DOI] [PubMed] [Google Scholar]
- 6.Li YJ Han Y & He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surgery for Obesity and Related Diseases 201915942–950. ( 10.1016/j.soard.2019.03.032) [DOI] [PubMed] [Google Scholar]
- 7.Christ JP & Falcone T. Bariatric surgery improves hyperandrogenism, menstrual irregularities, and metabolic dysfunction among women with polycystic ovary syndrome (PCOS). Obesity Surgery 2018282171–2177. ( 10.1007/s11695-018-3155-6) [DOI] [PubMed] [Google Scholar]
- 8.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ. & International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clinical Endocrinology 201889251–268. ( 10.1111/cen.13795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambineri A Pelusi C Vicennati V Pagotto U & Pasquali R. Obesity and the polycystic ovary syndrome. International Journal of Obesity and Related Metabolic Disorders 200226883–896. ( 10.1038/sj.ijo.0801994) [DOI] [PubMed] [Google Scholar]
- 10.Faghfoori Z Fazelian S Shadnoush M & Goodarzi R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes and Metabolic Syndrome 201711(Supplement 1) S429–S432. ( 10.1016/j.dsx.2017.03.030) [DOI] [PubMed] [Google Scholar]
- 11.Barber TM Hanson P Weickert MO & Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clinical Medicine Insights. Reproductive Health 2019131179558119874042. ( 10.1177/1179558119874042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbosa-Desongles A Hernández C Simó R & Selva DM. Testosterone induces cell proliferation and cell cycle gene overexpression in human visceral preadipocytes. American Journal of Physiology. Cell Physiology 2013305C355–C359. ( 10.1152/ajpcell.00019.2013) [DOI] [PubMed] [Google Scholar]
- 13.Blouin K Nadeau M Perreault M Veilleux A Drolet R Marceau P Mailloux J Luu-The V & Tchernof A. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clinical Endocrinology 201072176–188. ( 10.1111/j.1365-2265.2009.03645.x) [DOI] [PubMed] [Google Scholar]
- 14.Georgopoulos NA Saltamavros AD Vervita V Karkoulias K Adonakis G Decavalas G Kourounis G Markou KB & Kyriazopoulou V. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertility and Sterility 200992250–255. ( 10.1016/j.fertnstert.2008.04.067) [DOI] [PubMed] [Google Scholar]
- 15.Robinson S Chan SP Spacey S Anyaoku V Johnston DG & Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clinical Endocrinology 199236537–543. ( 10.1111/j.1365-2265.1992.tb02262.x) [DOI] [PubMed] [Google Scholar]
- 16.Banting LK Gibson-Helm M Polman R Teede HJ & Stepto NK. Physical activity and mental health in women with polycystic ovary syndrome. BMC Women’s Health 20141451. ( 10.1186/1472-6874-14-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emeksiz HC Bideci A Nalbantoğlu B Nalbantoğlu A Çelik C Yulaf Y Çamurdan MO & Cinaz P. Anxiety and depression states of adolescents with polycystic ovary syndrome. Turkish Journal of Medical Sciences 201848531–536. ( 10.3906/sag-1708-131) [DOI] [PubMed] [Google Scholar]
- 18.Hussain A Chandel RK Ganie MA Dar MA Rather YH Wani ZA Shiekh JA & Shah MS. Prevalence of psychiatric disorders in patients with a diagnosis of polycystic ovary syndrome in Kashmir. Indian Journal of Psychological Medicine 20153766–70. ( 10.4103/0253-7176.150822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, Mariani S, Lubrano C, Poggiogalle E, Migliaccio S, et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). Journal of Endocrinological Investigation 2019421365–1386. ( 10.1007/s40618-019-01061-2) [DOI] [PubMed] [Google Scholar]
- 20.de Luis D Domingo JC Izaola O Casanueva FF Bellido D & Sajoux I. Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: a randomized clinical trial. Endocrine 201654111–122. ( 10.1007/s12020-016-0964-z) [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Arbelaez D Bellido D Castro AI Ordoñez-Mayan L Carreira J Galban C Martinez-Olmos MA Crujeiras AB Sajoux I & Casanueva FF. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. Journal of Clinical Endocrinology and Metabolism 2017102488–498. ( 10.1210/jc.2016-2385) [DOI] [PubMed] [Google Scholar]
- 22.Moreno B Crujeiras AB Bellido D Sajoux I & Casanueva FF. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine 201654681–690. ( 10.1007/s12020-016-1050-2) [DOI] [PubMed] [Google Scholar]
- 23.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK. & Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2013984565–4592. ( 10.1210/jc.2013-2350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, Hart R, Johnson L, Jordan C, Legro RS, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Human Reproduction Open 20192019hoy021. ( 10.1093/hropen/hoy021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun SS Chumlea WC Heymsfield SB Lukaski HC Schoeller D Friedl K Kuczmarski RJ Flegal KM Johnson CL & Hubbard VS. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. American Journal of Clinical Nutrition 200377331–340. ( 10.1093/ajcn/77.2.331) [DOI] [PubMed] [Google Scholar]
- 26.Moretti C Guccione L Di P Simonelli I Exacoustos C Toscano V Motta C De Leo V Petraglia F & Lenzi A. Combined oral contraception and bicalutamide in polycystic ovary syndrome and severe hirsutism: a double-blind randomized controlled trial. Journal of Clinical Endocrinology and Metabolism 2018103824–838. ( 10.1210/jc.2017-01186) [DOI] [PubMed] [Google Scholar]
- 27.Fava GA Kellner R Perini GI Fava M Michelacci L Munari F Evangelisti LP Grandi S Bernardi M & Mastrogiacomo I. Italian validation of the symptom rating test (SRT) and symptom questionnaire (SQ). Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 198328117–123. ( 10.1177/070674378302800208) [DOI] [PubMed] [Google Scholar]
- 28.Kellner R. A symptom questionnaire. Journal of Clinical Psychiatry 198748268–274. [PubMed] [Google Scholar]
- 29.Benasi G Fava GA & Rafanelli C. Kellner’s symptom questionnaire, a highly sensitive patient-reported outcome measure: systematic review of clinimetric properties. Psychotherapy and Psychosomatics 20208974–89. ( 10.1159/000506110) [DOI] [PubMed] [Google Scholar]
- 30.Ruini C Ottolini F Rafanelli C Ryff C & Fava GA. La validazione italiana delle Psychological Well-being Scales (PWB). [Italian validation of Psychological Well-being Scales (PWB)]. Rivista di Psichiatria 200338117–130. [Google Scholar]
- 31.Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology 1989571069–1081. ( 10.1037/0022-3514.57.6.1069) [DOI] [Google Scholar]
- 32.Burns RA & Machin MA. Investigating the structural validity of Ryff’s psychological well-being scales across two samples. Social Indicators Research 200993359–375. ( 10.1007/s11205-008-9329-1) [DOI] [Google Scholar]
- 33.Società Italiana di Nutrizione Umana (SINU). LARN: Livelli di Assunzione di Riferimento di Nutrienti ed energia. 4th ed. Milan, Italy. [Google Scholar]
- 34.Gambineri A Patton L De Iasio R Cantelli B Cognini GE Filicori M Barreca A Diamanti-Kandarakis E Pagotto U & Pasquali R. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. Obstetrical and Gynecological Survey 20066131–32. ( 10.1097/01.ogx.0000193857.39332.d8) [DOI] [PubMed] [Google Scholar]
- 35.Fulghesu AM Canu E Casula L Melis F & Gambineri A. Polycystic ovarian morphology in Normocyclic non-hyperandrogenic adolescents. Journal of Pediatric and Adolescent Gynecology 202134610–616. ( 10.1016/j.jpag.2021.02.004) [DOI] [PubMed] [Google Scholar]
- 36.Fanelli F, Belluomo I, Di Lallo VD, Cuomo G, De Iasio R, Baccini M, Casadio E, Casetta B, Vicennati V, Gambineri A, et al. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids 201176244–253. ( 10.1016/j.steroids.2010.11.005) [DOI] [PubMed] [Google Scholar]
- 37.Friedewald WT Levy RI & Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry 197218499–502. ( 10.1093/clinchem/18.6.499) [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen A Verdonck L & Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. Journal of Clinical Endocrinology and Metabolism 1999843666–3672. ( 10.1210/jcem.84.10.6079) [DOI] [PubMed] [Google Scholar]
- 39.Cincione RI Losavio F Ciolli F Valenzano A Cibelli G Messina G & Polito R. Effects of mixed of a ketogenic diet in overweight and obese women with polycystic ovary syndrome. International Journal of Environmental Research and Public Health 202118 12490. ( 10.3390/ijerph182312490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goday A Bellido D Sajoux I Crujeiras AB Burguera B García-Luna PP Oleaga A Moreno B & Casanueva FF. Short-Term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutrition and Diabetes 20166 e230. ( 10.1038/nutd.2016.36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunha GM Guzman G Correa De Mello LL Trein B Spina L Bussade I Prata JM Sajoux I & Countinho W. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Frontiers in Endocrinology (Lausanne) 202011607. ( 10.3389/fendo.2020.00607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno B Bellido D Sajoux I Goday A Saavedra D Crujeiras AB & Casanueva FF. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 201447793–805. ( 10.1007/s12020-014-0192-3) [DOI] [PubMed] [Google Scholar]
- 43.Escobar-Morreale HF & Millán JLS. Abdominal adiposity and the polycystic ovary syndrome. Trends in Endocrinology and Metabolism 200718266–272. ( 10.1016/j.tem.2007.07.003) [DOI] [PubMed] [Google Scholar]
- 44.Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Reviews in Molecular Medicine 200810e3. ( 10.1017/S1462399408000598) [DOI] [PubMed] [Google Scholar]
- 45.Glueck CJ & Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism: Clinical and Experimental 201992108–120. ( 10.1016/j.metabol.2018.11.002) [DOI] [PubMed] [Google Scholar]
- 46.Moghetti P & Tosi F. Insulin resistance and PCOS: chicken or egg? Journal of Endocrinological Investigation 202144233–244. ( 10.1007/s40618-020-01351-0) [DOI] [PubMed] [Google Scholar]
- 47.Tosi F Bonora E & Moghetti P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Human Reproduction 2017322515–2521. ( 10.1093/humrep/dex308) [DOI] [PubMed] [Google Scholar]
- 48.Carmina E Bucchieri S Esposito A Del Puente A Mansueto P Orio F Di Fede G & Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. Journal of Clinical Endocrinology and Metabolism 2007922500–2505. ( 10.1210/jc.2006-2725) [DOI] [PubMed] [Google Scholar]
- 49.Repaci A Gambineri A & Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Molecular and Cellular Endocrinology 201133530–41. ( 10.1016/j.mce.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 50.Spritzer PM Lecke SB Satler F & Morsch DM. Adipose tissue dysfunction, adipokines and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 2015149R219–R227. ( 10.1530/REP-14-0435) [DOI] [PubMed] [Google Scholar]
- 51.Milder J & Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Research 2012100295–303. ( 10.1016/j.eplepsyres.2011.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simó R Sáez-López C Barbosa-Desongles A Hernández C & Selva DM. Novel insights in SHBG regulation and clinical implications. Trends in Endocrinology and Metabolism 201526376–383. ( 10.1016/j.tem.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 53.Robker RL Wu LLY & Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. Journal of Reproductive Immunology 201188142–148. ( 10.1016/j.jri.2011.01.008) [DOI] [PubMed] [Google Scholar]
- 54.Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, Pugeat M, Qiao J, Wijeyaratne CN, Witchel SF, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the androgen excess and polycystic ovary syndrome society. Human Reproduction Update 201218146–170. ( 10.1093/humupd/dmr042) [DOI] [PubMed] [Google Scholar]
- 55.Mezzullo M, Fanelli F, Di Dalmazi G, Fazzini A, Ibarra-Gasparini D, Mastroroberto M, Guidi J, Morselli-Labate AM, Pasquali R, Pagotto U, et al. Salivary cortisol and cortisone responses to short-term psychological stress challenge in late adolescent and young women with different hyperandrogenic states. Psychoneuroendocrinology 20189131–40. ( 10.1016/j.psyneuen.2018.02.022) [DOI] [PubMed] [Google Scholar]
- 56.Guidi J Gambineri A Zanotti L Fanelli F Fava GA & Pasquali R. Psychological aspects of hyperandrogenic states in late adolescent and young women. Clinical Endocrinology 201583872–878. ( 10.1111/cen.12783) [DOI] [PubMed] [Google Scholar]
- 57.Thomson RL Buckley JD Lim SS Noakes M Clifton PM Norman RJ & Brinkworth GD. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertility and Sterility 2010941812–1816. ( 10.1016/j.fertnstert.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 58.Büttler RM, Martens F, Fanelli F, Pham HT, Kushnir MM, Janssen MJW, Owen L, Taylor AE, Soeborg T, Blankenstein MA, et al. Comparison of 7 published LC-MS/MS methods for the simultaneous measurement of testosterone, androstenedione, and dehydroepiandrosterone in serum. Clinical Chemistry 2015611475–1483. ( 10.1373/clinchem.2015.242859) [DOI] [PubMed] [Google Scholar]
- 59.Fanelli F, Cantù M, Temchenko A, Mezzullo M, Lindner JM, Peitzsch M, Hawley JM, Bruce S, Binz PA, Ackermans MT, et al. Report from the HarmoSter study: impact of calibration on comparability of LC-MS/MS measurement of circulating cortisol, 17OH-progesterone and aldosterone. Clinical Chemistry and Laboratory Medicine 202260726–739. ( 10.1515/cclm-2021-1028) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a