ABSTRACT
Toll-like receptor 3 (TLR3) agonists such as polyinosinic:polycytidylic acid (poly(I:C)) have immunostimulatory effects that can be taken advantage of to induce anticancer immune responses in preclinical models. In addition, poly(I:C) has been introduced into clinical trials to demonstrate its efficacy as an adjuvant and to enhance the immunogenicity of locally injected tumors, thus reverting resistance to PD-L1 blockade in melanoma patients. Here, we report the pharmacokinetic, pharmacodynamic, mechanistic and toxicological profile of a novel TLR3 agonist, TL-532, a chemically synthesized double-stranded RNA that is composed by blocks of poly(I:C) and poly(A:U) (polyadenylic – polyuridylic acid). In preclinical models, we show that TL-532 is bioavailable after parenteral injection, has an acceptable toxicological profile, and stimulates the production of multiple chemokines and interleukins that constitute pharmacodynamic markers of its immunostimulatory action. When given at a high dose, TL-532 monotherapy reduced the growth of bladder cancers growing on mice. In addition, in immunodeficient mice lacking formylpeptide receptor-1 (FPR1), TL-532 was able to restore the response of orthotopic subcutaneous fibrosarcoma to immunogenic chemotherapy. Altogether, these findings may encourage further development of TL-532 as an immunotherapeutic anticancer agent.
Keywords: Cancer immunotherapy, dendritic cells, formyl peptide receptor 1, immmunogenic cell death
Introduction
Toll-like receptor 3 (TLR3) agonists, most prominently, polyinosinic:polycytidylic acid (poly(I:C)), have been extensively characterized in preclinical cancer models to understand their antineoplastic potential. Thus, poly(I:C) has been found to induce potent anticancer effects if injected locally into tumors or systemically, usually in combination with other treatments such as CpG-containing oligodeoxynucleotides (which stimulates TLR9)1, tumor vaccines2,3, type-1 interferon4, Bacillus Calmette-Guérin immunotherapy5, blockade of the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) interaction6,7, immunogenic chemotherapy8 and stimulator of interferon genes (STING) agonists9. Such poly(I:C) effects have been attributed to TLR3-mediated killing of cancer cells10 or the activation of immune effectors including dendritic cells (DC)11, natural killer (NK) cells12 and macrophages13 or the depletion of myeloid-derived suppressor cells14. Of note, direct effects of poly(I:C) on TLR3-expressing cancer cells may indirectly enhance anticancer immune responses, for instance by eliciting the secretion of immunostimulatory type-1 interferons15 and chemokines16, as well as by inducing immunogenic cell death7. Indeed, the knockout of TLR3 in cancer cells may facilitate their evasion from immunosurveillance17,18. Of note, retrospective analyses suggest that polymorphisms in the human TLR3 gene may affect risk, prognosis and therapeutic outcome of patients with various malignancies including mammary carcinoma19, colorectal cancer20, stomach adenocarcinoma21 and hepatitis C-induced liver cancer22,23.
Spurred by these preclinical results, poly(I:C) has been introduced into clinical trials as an adjuvant for eliciting anticancer immune responses by vaccination24–26. In addition, poly(I:C) has been evaluated for its capacity to treat solid tumors by intra-tumor injections, alone27 or in combination with recombinant fms-like tyrosine kinase 3 (FLT3) protein and radiotherapy28. Encouraging results have been obtained in anti-PD-1 refractory melanoma patients, in which the intralesional injection of BO-112, a nanoplexed form of poly(I:C), reverted anti-PD-1 resistance29. Moreover, multiple clinical trials are underway to evaluate poly(I:C) (which is provided by some companies in specific galenic formulations) or other TLR3 agonists for its anticancer activity30,31.
Encouraged by these findings, attempts are on the way to create new TLR3 ligands that are chemically distinct from poly(I:C). Similar to poly(I:C) (high molecular weight (HMW) 1500–8000 bp), TL-532 is a fully synthetic double stranded RNA (HMW 70 bp; Kd <5 nM)32. However, at difference with poly(I:C), it is composed by blocks of poly(A:U) (polyadenylic – polyuridylic acid) and poly(I:C) thus limiting off-target effects by overcoming the structural homogeneity of other TLR3 agonists. Here, we report the preclinical characterization of TL-532 at the pharmacokinetic, pharmacodynamic, mechanistic and toxicological levels. In addition, we provide evidence that TL-532 has anticancer effect in mouse models.
Results
Pharmacokinetic characteristics of TL-532
To determine the pharmacokinetic characteristics of TL-532, we parenterally administered the drug once into male swiss mice via different routes (i.e., intraperitoneal [i.p.], intravenous [i.v.] or subcutaneous [s.c.] injection at 5 mg/kg, 2 mg/kg, and 10 mg/kg, respectively) and then determined TL-532 plasma concentrations by high-performance liquid chromatography (HPLC) coupled to UV absorbance measurements (Figure 1A–C). Plasma TL-532 peaked 0, ~30 and ~60 min post i.v., s.c. and i.p. injection, respectively, and exhibited a rapid decline, suggesting that the half-life of the product is in the range of 1–2 h (Figure 1A–C, Table S1). Next, we determined the biodistribution of TL-532 in BALB/c mice bearing clearly discernible subcutaneous MBT2 bladder carcinomas by i.v. injecting them with TL-532 conjugated to the fluorochrome cyanine-5 (TL-532-Cy5). We then determined the abundance of TL-532-Cy5 in different tissues including liver, tumor and the tumor-draining lymph node by fluorescence-based tomography (Figure 1D, Fig. S1). The distribution of TL-532-Cy5 at the site of the tumor represents ~ 4% of the injected dose and ~ 10% compared to the whole body. When reported on a per/weight basis as shown in Figure 1E, the amount of TL-532-Cy5 slightly undercut the one found in the liver (by a factor of ~ 2). The intratumoral peak of TL-532-Cy5 was attained ~60 min post-injection and declined with a half-life of ~1 h. In conclusion, TL-532 has an in vivo half-life in the range of 1–2 h in organs and tumor, as well as in plasma (post s.c. and i.p. injection).
Figure 1.
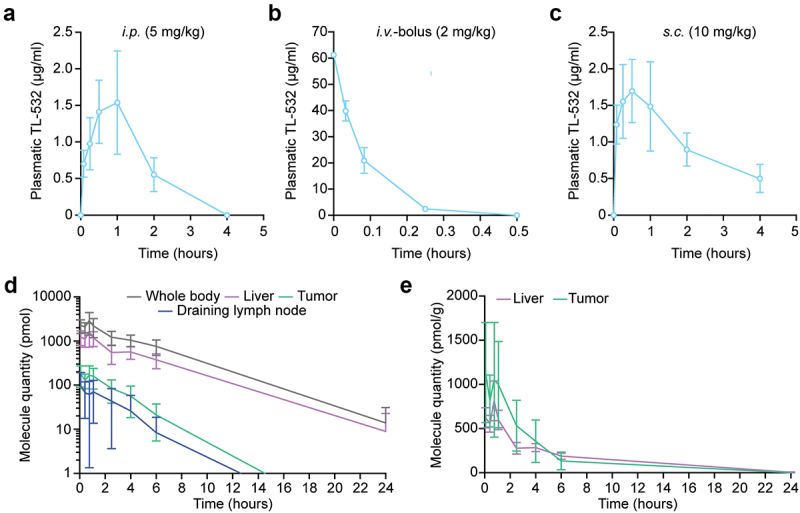
Pharmacokinetics and biodistribution after TL-532 administration in mice. TL-532 pharmacokinetics were evaluated in 12 male swiss mice by i.P. (a), i.V. bolus (b) and s.C. (c) injection at the following concentrations of 5 mg/kg, 2 mg/kg, and 10 mg/kg, respectively. Biodistribution of Cyanine-5 conjugated TL-532 injected intravenously in four BALB/c-nude mice bearing MBT2 tumors was performed using quantitative fluorescent molecular tomography (d and e). Global quantifications over time are shown for the whole-body, liver, tumor and draining lymph-nodes (d), and expressed relatively to the organ weight for both liver and the tumor (e).
Immunological and anticancer effects of monotherapy with high-dose TL-532
We next determined some pharmacodynamic aspects of TL-532. After 10 repeated daily i.p. injections into tumor-free mice, high-dose TL-532 (380 mg/kg/day) induced several chemokines (CCL2, CCL3, CCL4, CCL5, CXCL1, CXCL10/IP10) and interleukins (IL2, IL6, IL10, IL17a, TNF), as determined by multiplexed ELISA (Figure 2A). Of note, this regimen did not cause any increase in C-reactive protein (CRP), which is a well-established marker of systemic inflammation (Figure 2B). Repeated s.c. injections of TL-532 at the same dose and frequency induced a similar pattern of cytokines as did i.p. injection, though with a more important induction of CXCL9 (Figure 2C, Fig. S2A). In contrast, administration via the i.v. route was less effective in inducing chemokines and interleukins than i.p. or s.c. injections (Figure 2C, Fig. S2B), correlating with the half-life of TL-532 and perhaps reflecting the accelerated clearance of TL-532 (Figure 1A–C). Neither s.c. nor i.v. administration of TL-532 (380 mg/kg/day for 10 days) caused an increase in CRP (Figure 2B).
Figure 2.
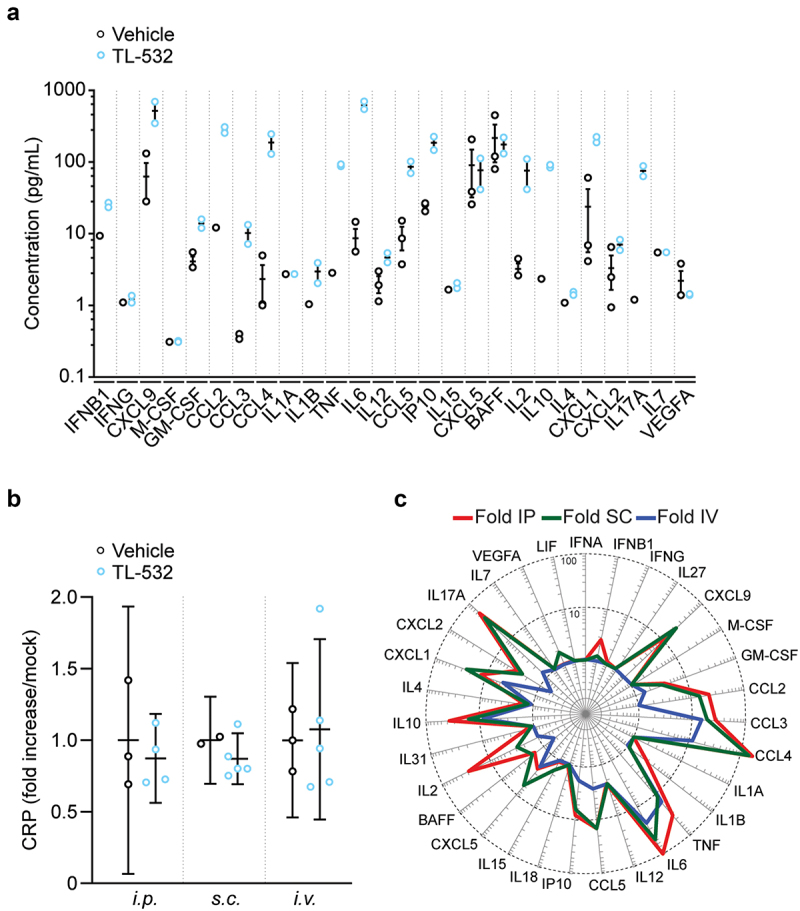
Cytokine, chemokine and C reactive protein (CRP) production after i.P. administration of TL-532. After 10 treatments with either TL-532 (380 mg/kg) or vehicle (0.9% NaCl), cytokine/chemokine (a) and CRP (b) production were evaluated by ELISA 3 h after the tenth i.P. administration of TL-532 (380 mg/kg) or vehicle (0.9% NaCl). Plasmatic soluble factor secretion signature for each route of administration (i.P. i.V. or s.C.) is shown as the fold change of median values normalized to the vehicle controls (c) (n = 2–5).
In the next experiment, we administered high-dose TL-532 to BALB/c mice that were inoculated s.c. with syngeneic MBT-2 bladder carcinomas. TL-532 was either injected three times per week up to 10 times (380 mg/kg; i.p. or s.c) or provided as a continuous i.v. infusion (47.3 g/L; 200 μL/h for 1 h). Compared to vehicle-treated controls, TL-532 led to a significant inhibition of tumor growth, irrespective of the route of administration (i.p. in Figure 3A, Fig. S3A, s.c. in Figure 3C, Fig. S3C and i.v. in Figure 3E, Fig. S3E). The weight of the animals was monitored as well. While i.p.-injected mice did manifest a minor weight loss (Figure 3B, Fig. S3B), the s.c. and i.v. administration of TL-532 did not cause any significant weight loss (Figure 3D,F, Fig. S3D,F).
Figure 3.
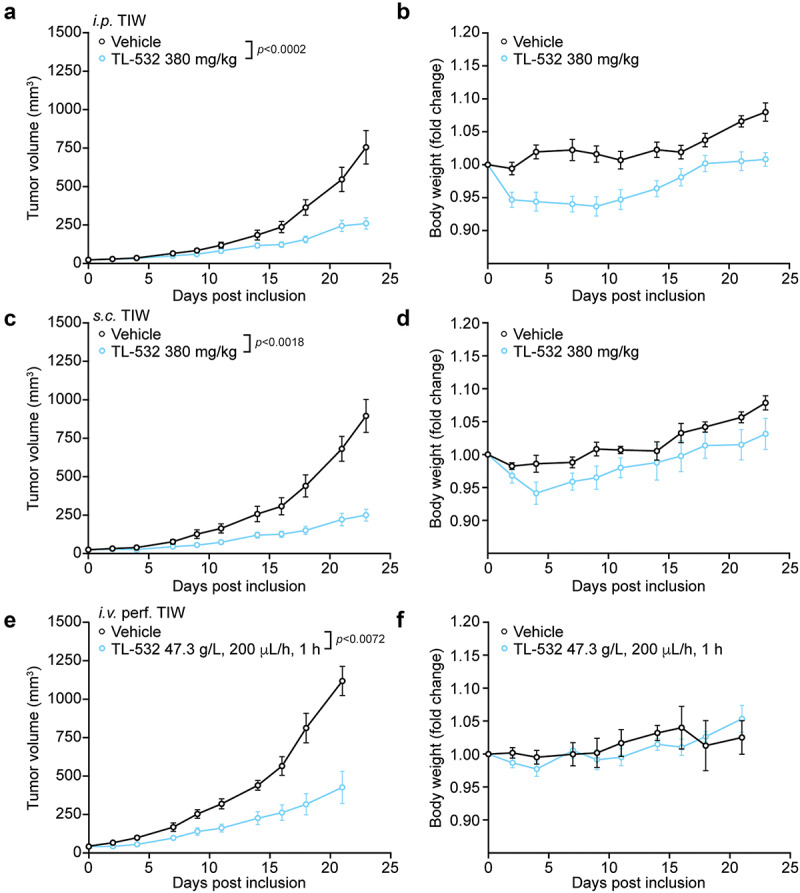
Effects of i.P., s.C. and i.V. TL-532 in MBT2-bearing mice. Murine MBT-2 bladder cancer cells were inoculated s.c. into immunocompetent C3H/HeN mice. TL-532 was administered i.P. (380 mg/kg) (a and b), s.c. (380 mg/kg) (c and d) and i.V. (47.3 g/L; 200 µL/h for 1 h) (e and f), thrice a week, up to 10 treatments. Tumor volume (a, c and e) and body weight loss (b, c and f) were routinely assessed.
We conclude that repeated administration of TL-532 induces tonic activation of immune system, as reflected by an increase in the circulating levels of multiple chemokines and interleukins. In addition, monotherapy with high-dose TL-532 has anticancer effects.
On-target effects of TL-532 on mouse dendritic cells in vitro via TLR3
We determined whether TL-532 would act on-target (via TLR3) or might also have off-target effects in a sort of mini-immune system composed by professional antigen-presenting cells and antigen-specific major histocompatibility complex class I (MHC-I) T cells. For this, we took advantage of a peculiar dendritic cell preparation that is generated from immortalized precursors in the form of de-inhibited/de-immortalized DCs (de-iniDCs), as explained in the Materials & Methods. When pulsed with ovalbumin (OVA) protein, such de-iniDCs can present the OVA-derived SIINFEKL peptide bound to Kb MHC-I to SIINFEKL/Kb-specific B3Z T cell hybridoma cells, stimulating their IL2 secretion33. The de-iniDCs were either wild type (WT) or had been subjected to the CRISP/Cas9-mediated knockout of Fpr1 and Tlr3, alone or in combination8. Poly(I:C), poly(A:U) and TL-532 indistinguishably enhanced antigen presentation by WT (genotype: Fpr1+/+ Tlr3+/+) and DCs lacking Fpr1 only (Fpr1−/− Tlr3+/+), but failed to act on Fpr1+/+ Tlr3−/− or Fpr1−/− Tlr3−/− DCs (Figure 4).
Figure 4.
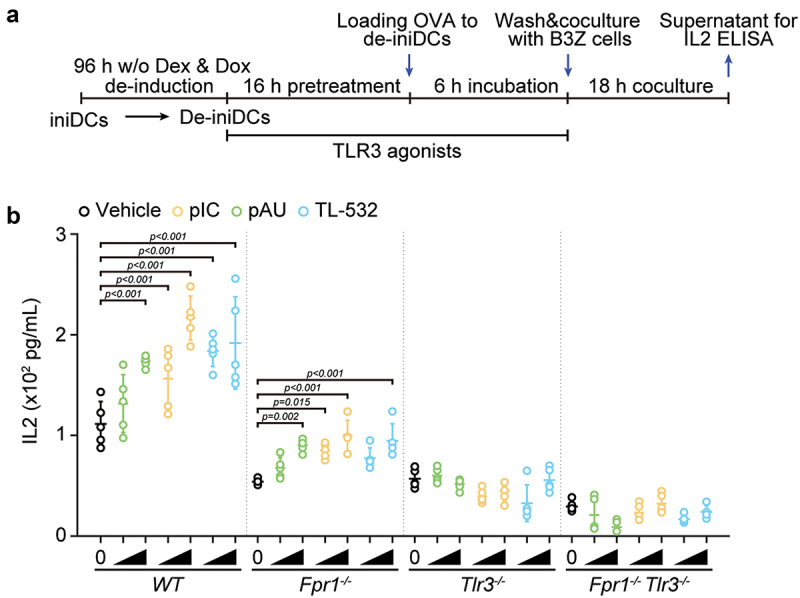
In vitro effects of TLR3 agonists on the antigen cross-presentation by de-iniDcs of different genotypes. (a) Scheme of the in vitro antigen cross-presentation assay. Inducible immortalized dendritic cell (iniDC) precursors were differentiated to DCs (de-iniDC) in the absence of dexamethasone or doxycycline (Dex/Dox) for 3 days. De-iniDcs were pretreated with the indicated TLR3 agonists (poly(I:C) (pIC) and poly(A:U) (pAU) at 2 and 20 µg/mL, and TL-532 at 20 and 200 µg/mL.) for 16 h. For measuring antigen cross-presentation, soluble ovalbumin (OVA) was added to the de-iniDC culture and the de-iniDcs were incubated for additional 6 h before wash and co-culture with B3Z hybridoma T cells. Eighteen hours later, the co-culture supernatants were collected for the quantification of IL2 by ELISA. (b) IL2 concentrations were calculated based on standard curves and normalized to untreated wild-type controls. One representative experiment out of three replicates is reported as aligned dot plots (mean ± SD, n = 5). Statistical significance was calculated by means of two-way ANOVA with Dunnett’s multiple comparisons test.
These findings indicate that, similarly to poly(I:C) and poly(A:U), TL-532 acts on-target, on TLR3, to stimulate immune responses.
TL-532 compensates deficient immunosurveillance in Fpr1−/− mice
Formyl peptide receptor 1 (FPR1) is necessary for DC homing on cancer cells that release the FPR1 ligand annexin A1 (ANXA1), which allows further cellular engagement and cross-presentation of tumor-associated antigens8,34. Fpr1 deficiency entails an immune defect that leads to the early manifestation of some cancers (such as luminal B breast cancer in patients and hormone-induced breast cancer in mice)8, as well as to resistance to immunogenic chemotherapy34. The TLR3 ligand poly(I:C) is able to overcome this immune defect when injected into tumor-bearing Fpr1−/− mice8. We therefore decided to perform a side-by-side comparison of TL-532, poly(I:C) and poly(A:U) with respect to their capacity to reestablish deficient chemotherapy responses in Fpr1−/− mice. For this, we designed in experiment (Figure 5A) in which WT C57BL/6 mice or isogenic Fpr1−/− mice were s.c. inoculated with syngeneic MCA205 cutaneous fibrosarcomas. In addition, WT C57BL/6 mice were inoculated with genetically modified MCA205 cells lacking the FPR1 ligand annexin A1 (genotype: Anxa1−/−). Then, tumor-bearing mice (i.e., WT mice with WT MCA205 tumors, left panels in Figure 5B), (WT mice with Anxa1−/− MCA205 tumors, middle panels in Figure 5B) and Fpr1−/− mice with WT tumors, right panels in Fig. 5B) were treated with chemotherapy (via i.p. injection of the immunogenic cell death inducers mitoxantrone, MTX, and cyclophosphamide, CTX) or vehicle (PBS), followed by i.p. injections of TL-532 (used at two doses: dose 1:200 µg/mouse or dose 2:2 mg/mouse), TL-532, poly(I:C), poly(A:U) or vehicle (Figure 5A). In mice without prior chemotherapy, TL-532, poly(I:C) and poly(A:U) failed to reduce tumor growth (upper panels in Figure 5B). Chemotherapy with MTX/CTX reduced tumor growth significantly in WT mice bearing WT tumors, but failed to yield significant effects when either FPR1 (on the host) or its ligand ANXA1 (on tumor cells) were absent from the system (compare black and red lines in the middle and lower panels in Figure 5B). TL-532, poly(I:C) and poly(A:U) did not improve outcome of MTX/CTC chemotherapy against WT tumors growing on WT hosts. However, if combined with MTX/CTX chemotherapy, TL-532, poly(I:C) or poly(A:U) conferred significant tumor growth reduction, even if the tumors lacked ANXA1 or the host was deficient for FPR1 (middle and lower panels in Figure 5B, Fig. S4).
Figure 5.
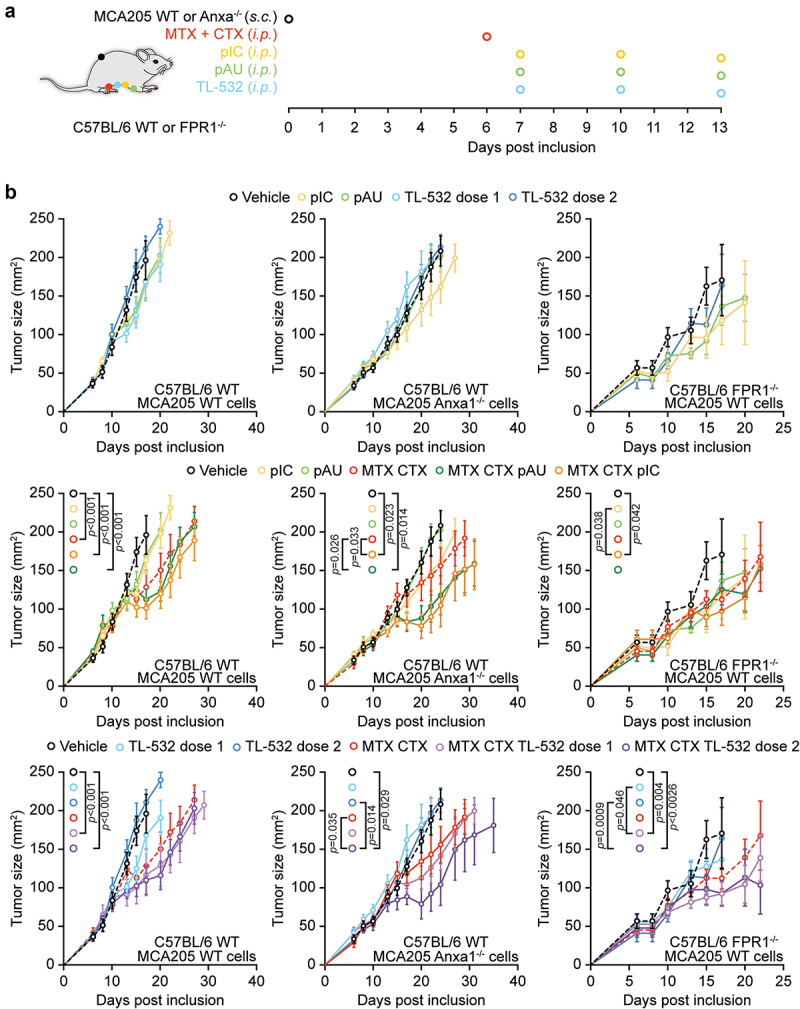
TLR3 agonists restore the efficacy of chemotherapy in defective Anxa1/Fpr1 signaling in fibrosarcoma-bearing mice. (a) Scheme of the in vivo tumor growth experiment. (b) Murine MCA205 cells WT or Anxa1−/− were inoculated s.c. into immunocompetent C57BL/6 WT or Fpr1−/− mice. When tumor became palpable (with a size inferior to 80 mm2) mice received 5.17 mg/kg i.P. mitoxantrone (MTX), 50 mg/kg i.P. cyclophosphamide (CTX), 50 μg/mouse, i.P. poly(I:C) (pIC), 150 µg/mouse i.P. poly(A:U) pAU, and 200 µg/mouse or 2 mg/mouse i.P. TL-532 (injected at days 1, 4, and 7 post treatment), or an equivalent volume of NaCl 0.9%. Tumor growth was routinely assessed using a digital caliper. Of note, data from the right panel represent results of two experiments yielding similar results.
In conclusion, it appears that, like poly(I:C) (and poly(A:U)), TL-532 is able to restore chemotherapy-induced immunosurveillance in spite of defective FPR1/ANXA1 signaling.
Toxicity and dose finding in cynomolgus monkeys
TL-532 was i.v. injected in cynomolgus monkeys (one group of one male and one female) on days 1, 7, 13 and 19 at ascending concentrations of 35, 70, 140 and 280 mg/kg (dose volume of 2 mL/kg into the cephalic vein). Apart from erythema at the injection site, there were no major signs of toxiciy apart from an increase in alanine aminotransferase and aspartate aminotransferase activity at day 20, for the male only. The potential toxicity of TL-532 was then evaluated in three groups of monkeys (one male and one female) following three repeated i.v. (days 1, 5 and 9) injections of 70, 140 and 280 mg/kg in a dose volume of 2 mL/kg. TL-532 administered at 70 mg/kg induced no signs of toxicity in the male and female cynomolgus monkeys. TL-532 administered three times i.v. at 280 mg/kg, 4 days apart, induced changes in hematology parameters and bilateral pyelonephritis in the male and female cynomolgus monkeys (determined on day 10). This dose level was considered to be above the maximum tolerated dose. When administered three times at 140 mg/kg, changes induced were limited to minor clinical signs in females (piloerection, abnormal appearance of fur and/or alopecia) after the last dosing associated with body weight losses (−8%) and changes in hematology parameters in both genders (increase in total circulating leukocytes neutrophils, decrease in red blood cell count, hemoglobin and hematocrit).
Hence, repeated dosing of 70 mg/kg TL-532 can be considered as safe in this non-human primate.
Discussion
In this paper, we report on the pharmacokinetic, pharmacodynamic, mechanistic and toxicological characterization of TL-532, which, in molecular terms, is a hybrid molecule encompassing features of two widely used TLR3 ligands, poly(I:C) and poly(A:U). Unlike poly(A:U) as well as poly(I:C) and its derivatives which have an enzymatic manufacturing process leading to a wide range in product size thus inducing batch-dependent variations in activity, TL-532 is a defined chemically synthetized dsRNA sequence that can be further bio-conjugated to improve target delivery32.
We found that TL-532 can be easily detected post-injection as a native molecule by HPLC. Pharmacokinetic measurement of the tissue redistribution of native TL-532 and its fluorescent derivative TL-532-Cy5 revealed similar half-lives, suggesting that labeling with cyanine-5 did not distort the pharmacokinetic features of TL-532. The use of TL-532-Cy5 allowed to demonstrate that the molecule effectively distributes into cancers, though with a relatively short half-life (in organs, tumors and plasma after s.c. and i.p. injection) of approximately 1–2 h, with an increased clearance in plasma after i.v. injection. We found that administration of TL-532 by i.p. or s.c. injection (but less as an i.v. bolus injection) led to an increase of multiple chemokines and interleukins in the plasma, reflecting a systemic immune activation. High-dose administration of TL-532 (by i.p. or s.c. injection as well as by continuous i.v. infusion) inhibited the growth of tumors in syngeneic mice as a monotherapy. Moreover, low-dose administration of TL-532, which failed to affect the growth of tumors as a monotherapy, synergized with chemotherapy to reduce the growth of cancers in mice in which the ANXA1-FPR1 signaling pathway was interrupted due to knockout of Anxa1 in cancer cells or that of Fpr1 in the host. In this respect, TL-532 exhibited similar effects as established TLR3 agonists such as poly(I:C) and poly(A:U). Reassuringly, we found that the immunostimulatory effects of TL-532 on an in vitro mini-immune system composed by DCs and MHC-I-restricted T cells were lost when TLR3 was knocked out in DCs, meaning that this agent acts on-target. Finally, we obtained evidence in favor of the notion that TL-532 can be safely administered to non-human primates.
Altogether, the aforementioned results support the continued development of TL-532. Pending further toxicological examination of a GMP product, it will be important to study the possibility to select patients that have a high chance to respond to TLR3 agonists based on biomarkers. Not surprisingly, the clinical effects of poly(A:U) against mammary carcinoma turned out to be more pronounced against breast cancers expressing TLR3 than against TLR3-negative tumors35. Hence, the presence of TLR3 on tumor cells or on other cells in the tumor microenvironment might guide the inclusion of patients in clinical trials. In addition, based on the preclinical experiments involving Fpr1-/- mice, a specific segment of immunodeficient patients bearing the FPR1 rs867228 loss-of-function allele might constitute an ideal target population for testing TLR3 agonists in the context of chemotherapy. Although it can be argued that human FPR1 and mouse Fpr1 share limited homology36, it appears that the migration-deficient phenotype of human dendritic cells expressing FPR1 rs867228 and mouse dendritic cells lacking Fpr1 is similar34,37, supporting the idea that Fpr1-/- mice constitute a valid model for the immunodeficiency associated with FPR1 rs867228.
Currently, several trials are evaluating the antineoplastic effects of several TLR3 agonists. Such trials use ampligene (which is a modified poly(I:C) into which uridylic acid molecules have been introduced at specific intervals) against breast or ovarian cancer (NCT03734692, NCT04081389), BO-112 (which is poly(I:C) stabilized with poly-L-lysine) against melanoma (NCT04570332) and poly-ICLC (which is poly(I:C) acid stabilized with poly-L-lysine carboxymethyl cellulose) against melanoma (NCT04364230), mostly in combination with a range of distinct immunotherapies (inhibition of PD-1, vaccination against tumor antigens, administration of recombinant IFN-α2b2) or together with chemotherapy31. However, none of these trials targets a patient subpopulation that would be selected based on biomarkers rather than on purely clinical criteria. Nonetheless, future retrospective analyses of these clinical trials might confirm the potential utility of candidate biomarkers such as TLR3 expression in the tumor and polymorphisms affecting TLR3 or FPR1.
In sum, the present paper provides information on the preclinical characterization of TL-532 as a novel TLR3 agonist endowed with anticancer activity. Future studies must determine the clinical utility of TL-532 or similar TLR3 agonists.
Material and methods
Chemicals
Unless otherwise indicated, media and supplements for cell culture were obtained from Gibco-Invitrogen Life Technologies Inc. (Carlsbad, CA, USA). Plasticware was purchased from Corning B.V. Life Sciences (Amsterdam, The Netherlands). Mitoxantrone dihydrochloride (MTX), cyclophosphamide (CTX), sodium chloride 0.9%, polyadenylic:polyuridylic acid (poly(A:U)) and polyinosinic:polycytidylic acid (poly(I:C)) were provided by Sigma Aldrich (St. Louis, MO, USA). Synthesis of TL-532 and TL-532-Cy5 was performed by Horizon Discovery (Waterbeach, UK) and NittoAVECIA (Milford, MA, USA), respectively. Lyophilized powders were resuspended in apyrogenic, nuclease-free, sterile 0.9% NaCl (InvivoGen, San Diego, CA, USA). 0.9% NaCl was used as a mock condition for all experiments unless otherwise stated.
Mouse experiments
Mice were bred and maintained in the animal facilities of the Centre de Recherche des Cordeliers, Centre Leon Berard (P-PAC, Plateforme du Petit Animal du CRCL – Agreement n° D 69 388 0202) and Université Claude Bernard Lyon-I (SCAR, Service Commun des Animaleries de Rockefeller – Agreement n° C69388 10 01), in specific pathogen – free conditions in a temperature-controlled environment with 12 h light/12 h dark cycles and received food and water ad libitum. Animal experiments followed the Federation of European Laboratory Animal Science Association (FELASA) guidelines and followed EU Directive 63/2010. Protocol #34928–2022012015416724-v7 was approved by the Ethical Committee of the CRC (registered C2EA–05 at the French Ministry of Research). Protocols #37310–2022041314222803-V2 was approved by ACCeS (registered C2EA–10 at the French Ministry of Research), and Projects #23845–201906281645171-V10 and #31779–2021022015165226-V4 were approved by the ethical committee CECCAPP (registered C2EA–15 at the French Ministry of Research). Six- to 7-week-old female wild-type C57Bl/6 mice were obtained from Envigo (Indianapolis, IN, USA). Eight-week-old Balbc-nude-mice, eight-week-old C3H/HeN mice and 11-week-old C3H/HeN jugular vein catheterized mice were obtained from Charles River (Wilmington, MA, USA). Pharmacokinetics were evaluated in 12 male swiss mice from Janvier Labs, Le Genest-Saint-Isle, France, with a weight ≥35 g.
Pharmacokinetic experiments
TL-532 pharmacokinetics were evaluated by Eurofins Scientific (Luxembourg), in 9 weeks male Swiss mice from Janvier Labs with a weight at least equal to 35 g, for i.v. bolus, i.p. and s.c. administration routes at the respective doses of 2 mg/kg, 5 mg/kg and 10 mg/kg. Three mice per administration routes were used. Plasma samples were collected at indicated time before TL-532 quantification by HPLC-UV method from Eurofins BIOANALYSES.
Dose ranging and maximum-tolerated dose
Dose range finding (DRF) and maximum-tolerated dose (MTD) studies were established in cynomolgus monkeys (Macaca fascicularis) by the European Research Biology Center (ERBC, Baugy, France). To determine the MTD, one male and one female received four increasing doses (35, 70, 140 and 280 mg/kg) of TL-532 by bolus i.v. injection at a dose volume of 2 mL. Each dose was given every 6 days at day 1, 7, 13 and 19, and animal was sacrificed at day 20. To establish suitable DRF, three groups of one male and one female received three repeated doses of TL-532 at 70, 140 and 280 mg/kg, respectively. TL-532 was administered every 4 days on day 1, 5 and day 9 by bolus i.v. injection at a dose volume of 2 mL/kg and animals were sacrificed at day 10. For both MTD and DRF studies, parameters monitored included daily mortality checks, clinical examination, changes in body weight and blood samples were taken for hematology, coagulation and serum biochemical tests before first dosing and the day after last administration at day 20 and day 10, respectively.
TL-532 biodistribution
TL-532 biodistribution was determined on BALB/c-nude mice bearing ectopic tumor. Briefly, mice were grafted ectopically by subcutaneous injection of 1 × 106 MBT2 cells (in 50 μL sterile RPMI medium without FBS or antibiotic) into the right flank. When tumor reached about 150 mm3, tumor vascularization was both determined by echo-doppler and contrast echography using the Vevo LAZR Photoacoustic Imaging System (FUJIFILM Visualsonics, Amsterdam, The Netherlands). The four most representative mice in terms of tumor volume and vascularization were injected into the caudal vein with 5 nmol of TL-532 conjugated with cyanine-5 (TL-532-Cy5), before being analyzed by using FMT-4000 Quantitative Fluorescent Molecular Tomography (Perkin Elmer, Waltham, MA, USA), at the time points: 5 min, 25 min, 45 min, 1 h 5 min, 2 h 25 min, 4 h, 6 h and 24 h. TL-532-Cy5 quantities, expressed in pmol, were determined in the whole body, liver, tumor and draining lymph-nodes as region of interest (ROI).
Multiparameter ELISA
Mouse tumor interstitial fluids and plasma were analyzed using multiplexed ELISA mouse ProcartaPlex 31-Plex Immunoassays (Thermo Fischer Scientific, Waltham, MA, USA, #PPX-31-MXD5Y2R). Beads corresponding to kit analytes were read on a dual-laser flow-based detection instrument (Luminex Bioplex MAGPIX, Bio-Rad, Hercules, CA, USA). Data were analyzed using xPONENT (Bio-Rad), Bio-Plex Manager 6.1 (Biorad), and ProcartaPlex Analysis App (Thermo Fischer Scientific) software. C-reactive protein (CRP) ELISA (Thermo Fischer Scientific, EM20RB) was performed on plasma and analyzed using SparkControl Magellan 3.1 (Tecan, Männedorf, Switzerland) software.
Cell lines and culture conditions
Bladder cancer cell-line MBT2 cells, syngeneic to C3H/HeN strain mice, kindly provided by Pr. Yves Fradet’s laboratory (Laboratoire d’Uro-Oncologie Expérimentale, Université Laval, Québec, QC, Canada) and cultured in RPMI-1640 (Gibco, Thermo Fischer Scientific, #21875–034) supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco, #15140–122). Murine fibrosarcoma MCA205 cells (class I MHC haplotype H-2b, syngeneic for C57BL/6 mice) were cultured in Roswell Park Memorial Institute medium (RPMI)-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 IU/mL, penicillin G sodium salt, 100 µg/mL streptomycin sulfate and 1 mM HEPES buffer.
TL-532 used as monotherapy by systemic administration
Eight-week-old C3H/HeN mice were grafted ectopically by subcutaneous injection of 1 × 106 MBT2 cells (in 50 μL sterile RPMI medium without FBS or antibiotic) into the right flank. Mice were treated when the tumors reached a mean volume of 30 mm3, using TL-532 at 380 mg/kg, thrice a week, up to 10 treatments, administered either by i.p., s.c., or i.v. infusion route at 8 mL/kg/h flow rate. Vehicle (0.9%-NaCl) was administered to control mice at the same volume and frequency. Tumor development was monitored three times a week, with tumor volume determined by the formula (L × W × H) x 3,14159/6 where L is tumor length, W is tumor width and H is tumor height. Mice were sacrificed 3 h after the last treatment at day-23 for plasma and tumor interstitial fluids soluble factors quantification.
IniDC cell culture
The murine inducible immortalized dendritic cell (iniDC) precursors and murine B3Z hybridoma T cells were maintained as previously described33. Both cell lines were cultured in basic DC medium (RPMI 1640 medium supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 100 U/mL pen/strep, and 50 μM beta-mercaptoethanol). B3Z T cells do not need any supplementation of cytokines. To maintain the expression of the large T antigen for the immortalization and to repress activation of iniDCs, basic DC medium was complemented with 100 μM of dexamethasone plus 1 μg/mL of doxycycline (Dex/Dox) and 10 ng/mL recombinant GM-CSF, respectively. Dex/Dox supplement removal and an increase in the concentration of recombinant GM-CSF for 3 ~ 4 days let to the de-immortalize and differentiation of iniDCs into de-iniDCs with the characteristic immunophenotype of primary dendritic cells.
In vitro antigen cross-presentation assay
De-iniDCs were seeded at a concentration of 1 × 106 cells/mL in basic DC medium containing 20 ng/mL recombinant GM-CSF in 96-well tissue culture U-bottom plates. The cells were pretreated with TLR3 agonists for 16 h. Then, OVA was added at a final concentration of 1 µg/mL, gently agitated and then incubated for 6 h in the cell culture incubator. Then, the plates were centrifuged at 500 g for 5 min and the supernatant containing OVA was removed. The cells were washed with 200 μL of RPMI 1640 medium before centrifugation at 500 g for 5 min. This washing step was repeated once more before adding the 1 × 105 B3Z cells/well in basic DC medium containing 20 ng/mL recombinant GM-CSF. The coculture was then incubated in an incubator for 18 h before collecting the supernatant for ELISA. IL2 concentration was measured with the ELISA MAX™ Deluxe Set Mouse IL-2 (Biolegend, Ref 431,004) following the manufacturer's protocol.
Tumor chemotherapy model
For the establishment of syngeneic solid tumors, 3 × 105 wildtype (WT) or Anxa1-/- murine fibrosarcoma MCA205 cells were inoculated s.c. (near the thigh) into WT, Formyl peptide receptor 1 mutated homozygous (Fpr1-/-) or Fpr1 heterozygous (Fpr1±) C57BL/6 (H-2b) mice, and tumor surface (longest dimension × perpendicular dimension) was routinely monitored using a digital caliper. When the tumor surface reached 35–60 mm2, mice received 5.17 mg/kg i.p. MTX in 200 μl PBS and 50 mg/kg i.p. CTX in 200 μl PBS or an equivalent volume of vehicle (PBS). When appropriate, mice also received 50 µg/mice i.p. poly(I:C) or 150 µg/mice i.p. poly(A:U) or 200 µg/mice i.p. TL-532 or 2 mg/mice i.p. TL-532 in 50 µl of PBS (injected at day 1, 4 and 7 after the other treatments). All experiments contained at least five mice per group.
Statistical analysis of in vivo experiments
Longitudinal analysis of tumor growth data was performed over the entire time course by linear mixed-effect modeling. Wald test was used to compute p values by testing jointly that both tumor growth slopes and intercepts (on a log scale) were the same between treatment groups of interest (https://kroemerlab.shinyapps.io/TumGrowth/”)38. Tumor growth curves are represented either as: individual curves from all measurements of each mouse and group-averaged tumor size alongside its SEM computed at each time point. For mice euthanized before the selected sampling point, the last measure was retained.
Supplementary Material
Funding Statement
O.K. received funding from the Institut National du Cancer (INCa) and the DIM ELICIT initiative of the Ile de France. GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; European Research Council Advanced Investigator Grand “ICD-Cancer”, Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council (ICD-Cancer), European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Disclosure statement
O.K is a scientific co-founder of Samsara Therapeutics. G.K. received research support by Tollys. S.T., M. B-J., M.B., C.P. C.R., B.M. and B.W. were full-time employees of Tollys. Outside of this work, GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. GK’wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim.
Abbreviations
- CTX
Cyclophosphamide
- DC
dendritic cell
- iniDC
inducible immortalized dendritic cell
- i.p.
intraperitoneally
- i.t.
intratumorally
- i.v.
intravenously
- MHC-I
major histocompatibility complex class I
- MTX
mitoxantrone
- OVA
ovalbumin
- pAU
polyadenylic:polyuridylic acid
- pIC
polyinosinic:polycytidylic acid
- ROI
region of interest
- s.c.
subcutaneously
- WT
wildtype
Author contributions
J.L.N, S.T., S.A.S, M. B-J., P.L, M.B., Y.P., C.P., L.Z., M.M., C.R., M. P-L, and B.M. performed experiments; O.K., G.K. and B.W. conceived the study and wrote the paper.
Data availability statement
All data are available from the corresponding authors upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2023.2227510
References
- 1.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR.. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64(16):5850–11. doi: 10.1158/0008-5472.CAN-04-0063. [DOI] [PubMed] [Google Scholar]
- 2.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I: c)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28(3):220–228. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 3.Tormo D, Ferrer A, Bosch P, Gaffal E, Basner-Tschakarjan E, Wenzel J, Tuting T. Therapeutic efficacy of antigen-specific vaccination and toll-like receptor stimulation against established transplanted and autochthonous melanoma in mice. Cancer Res. 2006;66(10):5427–5435. doi: 10.1158/0008-5472.CAN-06-0399. [DOI] [PubMed] [Google Scholar]
- 4.Muthuswamy R, Wang L, Pitteroff J, Gingrich JR, Kalinski P. Combination of IFNα and poly-I: c reprograms bladder cancer microenvironment for enhanced CTL attraction. J ImmunoTher Cancer. 2015;3(1):6. doi: 10.1186/s40425-015-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayari C, Besancon M, Bergeron A, LaRue H, Bussieres V, Fradet Y. Poly(i: c) potentiates bacillus calmette-guerin immunotherapy for bladder cancer. Cancer Immunol Immunother. 2016;65(2):223–234. doi: 10.1007/s00262-015-1789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Waele J, Marcq E, Van Audenaerde JR, Van Loenhout J, Deben C, Zwaenepoel K, Van de Kelft E, Van der Planken D, Menovsky T, Van den Bergh JM, et al. Poly(i: c) primes primary human glioblastoma cells for an immune response invigorated by PD-L1 blockade. Oncoimmunology. 2018;7(3):e1407899. doi: 10.1080/2162402X.2017.1407899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aznar MA, Planelles L, Perez-Olivares M, Molina C, Garasa S, Etxeberria I, Perez G, Rodriguez I, Bolanos E, Lopez-Casas P, et al. Immunotherapeutic effects of intratumoral nanoplexed poly I: c. J ImmunoTher Cancer. 2019;7(1):116. doi: 10.1186/s40425-019-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Naour J, Liu P, Zhao L, Adjemian S, Sztupinszki Z, Taieb J, Mulot C, Silvin A, Dutertre CA, Ginhoux F, et al. A TLR3 ligand reestablishes chemotherapeutic responses in the context of FPR1 deficiency. Cancer Discov. 2020;11(2):408–423. doi: 10.1158/2159-8290.CD-20-0465. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez M, Molina C, De Andrea CE, Fernandez-Sendin M, Villalba M, Gonzalez-Gomariz J, Ochoa MC, Teijeira A, Glez-Vaz J, Aranda F, et al. Intratumoral co-injection of the poly I: c-derivative BO-112 and a STING agonist synergize to achieve local and distant anti-tumor efficacy. J ImmunoTher Cancer. 2021;9(11):e002953. doi: 10.1136/jitc-2021-002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Q, Wei H, Tian Z. Poly I: c enhances cycloheximide-induced apoptosis of tumor cells through TLR3 pathway. Bmc Cancer. 2008;8:12. doi: 10.1186/1471-2407-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez-Ribas D, Schreibelt G, van de Rakt MW, Scharenborg NM, de Boer A, et al. Maturation of monocyte-derived dendritic cells with toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immunother. 2008;57(11):1589–1597. doi: 10.1007/s00262-008-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J Immunol. 2007;179(6):3472–3479. doi: 10.4049/jimmunol.179.6.3472. [DOI] [PubMed] [Google Scholar]
- 13.Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y, Tahara H, Inoue N, Seya T. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci U S A. 2012;109(6):2066–2071. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forghani P, Waller EK. Poly (I: c) modulates the immunosuppressive activity of myeloid-derived suppressor cells in a murine model of breast cancer. Breast Cancer Res Treat. 2015;153(1):21–30. doi: 10.1007/s10549-015-3508-y. [DOI] [PubMed] [Google Scholar]
- 15.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo B, Dempsey PW, Cheng G. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70(7):2595–2603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli R, Starace D, Busa R, Angelini DF, Paone A, De Cesaris P, Filippini A, Sette C, Battistini L, Ziparo E, et al. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J Immunol. 2010;184(12):6658–6669. doi: 10.4049/jimmunol.0902401. [DOI] [PubMed] [Google Scholar]
- 17.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 18.Vacchelli E, Sistigu A, Yamazaki T, Vitale I, Zitvogel L, Kroemer G. Autocrine signaling of type 1 interferons in successful anticancer chemotherapy. Oncoimmunol. 2015;4:e988042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan L, Zhou P, Chen AX, Liu GY, Yu KD, Shao ZM. Toll-like receptor 3 -926T>A increased the risk of breast cancer through decreased transcriptional activity. Oncoimmunol. 2019;8(12):e1673126. doi: 10.1080/2162402X.2019.1673126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro FA, Forsti A, Buch S, Kalthoff H, Krauss C, Bauer M, Egberts J, Schniewind B, Broering DC, Schreiber S, et al. TLR-3 polymorphism is an independent prognostic marker for stage II colorectal cancer. Eur J Cancer. 2011;47(8):1203–1210. doi: 10.1016/j.ejca.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, He A, Wang J, Lu H, Xu X, Zhang R, Liao W, Feng Q, Wu L. Toll-like receptor 3 is a potential prognosis marker and associated with immune infiltration in stomach adenocarcinoma. Cancer Biomark. 2022;34(1):77–93. doi: 10.3233/CBM-210354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosaad YM, Metwally SS, Farag RE, Lotfy ZF, AbdelTwab HE. Association between toll-like receptor 3 (TLR3) rs3775290, TLR7 rs179008, TLR9 rs352140 and chronic HCV. Immunol Invest. 2019;48(3):321–332. doi: 10.1080/08820139.2018.1527851. [DOI] [PubMed] [Google Scholar]
- 23.Sghaier I, Zidi S, Mouelhi L, Ghazoueni E, Brochot E, Almawi WY, Loueslati BY. TLR3 and TLR4 SNP variants in the liver disease resulting from hepatitis B virus and hepatitis C virus infection. Br J Biomed Sci. 2019;76(1):35–41. doi: 10.1080/09674845.2018.1547179. [DOI] [PubMed] [Google Scholar]
- 24.Melssen MM, Petroni GR, Chianese-Bullock KA, Wages NA, Grosh WW, Varhegyi N, Smolkin ME, Smith KT, Galeassi NV, Deacon DH, et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete freund’s adjuvant in melanoma patients. J ImmunoTher Cancer. 2019;7(1):163. doi: 10.1186/s40425-019-0625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlick A, Blazquez AB, Meseck M, Lattanzi M, Ott PA, Marron TU, Holman RM, Mandeli J, Salazar AM, McClain CB, et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol Res. 2020;8(1):70–80. doi: 10.1158/2326-6066.CIR-19-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slingluff CL Jr., Petroni GR, Chianese-Bullock KA, Wages NA, Olson WC, Smith KT, Haden K, Dengel LT, Dickinson A, Reed C, et al. Trial to evaluate the immunogenicity and safety of a melanoma helper peptide vaccine plus incomplete freund’s adjuvant, cyclophosphamide, and polyICLC (Mel63). J ImmunoTher Cancer. 2021;9(1):e000934. doi: 10.1136/jitc-2020-000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyi C, Roudko V, Sabado R, Saenger Y, Loging W, Mandeli J, Thin TH, Lehrer D, Donovan M, Posner M, et al. Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: a pilot trial. Clin Cancer Res. 2018;24(20):4937–4948. doi: 10.1158/1078-0432.CCR-17-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med. 2019;25(5):814–824. doi: 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 29.Marquez-Rodas I, Longo F, Rodriguez-Ruiz ME, Calles A, Ponce S, Jove M, Rubio-Viqueira B, Perez-Gracia JL, Gomez-Rueda A, Lopez-Tarruella S, et al. Intratumoral nanoplexed poly I: c BO-112 in combination with systemic anti-PD-1 for patients with anti-PD-1-refractory tumors. Sci Transl Med. 2020;12(565). doi: 10.1126/scitranslmed.abb0391. [DOI] [PubMed] [Google Scholar]
- 30.Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: tLR3 agonists in cancer therapy. Oncoimmunol. 2020;9(1):1771143. doi: 10.1080/2162402X.2020.1771143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Naour J, Kroemer G. Trial watch: toll-like receptor ligands in cancer therapy. Oncoimmunol. 2023;12(1):2180237. doi: 10.1080/2162402X.2023.2180237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thierry S, Maadadi S, Berton A, Dimier L, Perret C, Vey N, Ourfali S, Saccas M, Caron S, Boucard-Jourdin M, et al. TL-532, a novel specific toll-like receptor 3 agonist rationally designed for targeting cancers: discovery process and biological characterization. Microbial Cell. 2023;10(6):117–132. in press. doi: 10.15698/mic2023.06.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Liu P, Xie W, Zhang S, Thieme S, Zitvogel L, Kroemer G, Kepp O. A genotype-phenotype screening system using conditionally immortalized immature dendritic cells. STAR Protoc. 2021;2(3):100732. doi: 10.1016/j.xpro.2021.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–978. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- 35.Salaun B, Zitvogel L, Asselin-Paturel C, Morel Y, Chemin K, Dubois C, Massacrier C, Conforti R, Chenard MP, Sabourin JC, et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 2011;71(5):1607–1614. doi: 10.1158/0008-5472.CAN-10-3490. [DOI] [PubMed] [Google Scholar]
- 36.Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17(6):501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Biselli E, Agliari E, Barra A, Bertani FR, Gerardino A, De Ninno A, Mencattini A, Di Giuseppe D, Mattei F, Schiavoni G, et al. Organs on chip approach: a tool to evaluate cancer -immune cells interactions. Sci Rep. 2017;7(1):12737. doi: 10.1038/s41598-017-13070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enot DP, Vacchelli E, Jacquelot N, Zitvogel L, Kroemer G. TumGrowth: an open-access web tool for the statistical analysis of tumor growth curves. Oncoimmunol. 2018;7(9):e1462431. doi: 10.1080/2162402X.2018.1462431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding authors upon reasonable request.


