ABSTRACT
During the rapid deployment of COVID-19 vaccines in 2021, safety concerns may have led some pregnant individuals to postpone vaccination until after giving birth. This study aimed to describe temporal patterns and factors associated with COVID-19 vaccine series initiation after recent pregnancy in Ontario, Canada. Using the provincial birth registry linked with the COVID-19 vaccine database, we identified all individuals who gave birth between January 1 and December 31, 2021, and had not yet been vaccinated by the end of pregnancy, and followed them to June 30, 2022 (follow-up ranged from 6 to 18 months). We used cumulative incidence curves to describe COVID-19 vaccine initiation after pregnancy and assessed associations with sociodemographic, pregnancy-related, and health behavioral factors using Cox proportional hazards regression to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI). Among 137,198 individuals who gave birth in 2021, 87,376 (63.7%) remained unvaccinated at the end of pregnancy; of these, 65.0% initiated COVID-19 vaccination by June 30, 2022. Lower maternal age (<25 vs. 30–34 y aHR: 0.73, 95%CI: 0.70–0.77), smoking during pregnancy (vs. nonsmoking aHR: 0.68, 95%CI: 0.65–0.72), lower neighborhood income (lowest quintile vs. highest aHR: 0.79, 95%CI: 0.76–0.83), higher material deprivation (highest quintile vs. lowest aHR: 0.74, 95%CI: 0.70–0.79), and exclusive breastfeeding (vs. other feeding aHR: 0.81, 95%CI: 0.79–0.84) were associated with lower likelihood of vaccine initiation. Among unvaccinated individuals who gave birth in 2021, COVID-19 vaccine initiation after pregnancy reached 65% by June 30, 2022, suggesting persistent issues with vaccine hesitancy and/or access to vaccination in this population.
KEYWORDS: COVID-19, COVID-19 vaccination, COVID-19 vaccine series initiation, pregnancy, pregnant, birth
Introduction
COVID-19 disease during pregnancy is a risk factor for severe maternal illness and adverse birth outcomes;1–7 COVID-19 vaccination has, thus, been recommended for pregnant individuals in many countries.8 Although COVID-19 vaccination during pregnancy has been found to be effective against SARS-CoV-2 infection in pregnant individuals,9,10 has been associated with a lower incidence of SARS-CoV-2 infection in newborns,11–13 and has not been associated with adverse pregnancy or birth outcomes (e.g., preterm birth, small-for-gestational-age at birth, and stillbirth),14–19 vaccine coverage in the pregnant population in 2021 was lower than in the general population of women of reproductive age in many settings,20,21 including in Ontario, Canada.22 Lower uptake of the COVID-19 vaccine primary series by pregnant people in 2021 may have reflected safety concerns at that time,23–25 potentially leading some people to postpone vaccination until after giving birth. Similar to influenza vaccination during pregnancy, COVID-19 vaccine hesitancy among pregnant people is likely influenced by a variety of factors such as low perceived effectiveness of the vaccine, lack of confidence in vaccine safety, and misconceptions about the disease or vaccine.26
A survey conducted in the United Kingdom in late 2020 of over 1,500 participants with a current or recent pregnancy found that a higher proportion of individuals was ‘definitely’ or ‘leaning toward’ accepting a future COVID-19 vaccine once no longer pregnant (81.2%), rather than while pregnant (62.1%).24 Thus, some individuals who were reluctant to get vaccinated against COVID-19 during pregnancy, may have been more willing to get vaccinated after giving birth. The objectives of this study, therefore, were to describe COVID-19 vaccine series initiation after pregnancy and to assess sociodemographic, pregnancy-related, and health behavioral determinants of COVID-19 vaccine series initiation after pregnancy in a population-based study of people who gave birth in 2021 and remained unvaccinated against COVID-19 by the time of delivery.
Methods
Study design, setting, and data sources
We conducted this population-based retrospective cohort study in Ontario, Canada’s most populous province with approximately 140,000 births each year27 and a publicly funded healthcare system. The COVID-19 vaccination program in Ontario began in December 14, 2020. Initially, pregnant individuals could only access COVID-19 vaccines if they were part of an early eligibility group (e.g., healthcare workers); however, pregnancy became prioritized in the vaccination program on April 23, 2021, as a high-risk population for COVID-19 complications.28 From May 18, 2021, all individuals over 18 y of age became eligible for COVID-19 vaccination.29
We used data from the Better Outcomes Registry & Network (BORN)30—Ontario’s birth registry – to identify the study population. BORN Ontario receives pregnancy and birth information from all hospitals with birthing units, all midwifery practice groups, birth centers, and prenatal screening laboratories in the province. The registry collects data on maternal demographics, health behaviors, preexisting medical health conditions, obstetric complications, pregnancy history, and birth outcomes. The registry data have been shown to be of high quality in validation studies.30,31 Using unique health card numbers, birth registry records during the study period were deterministically linked with the provincial COVID-19 immunization database, COVaxON, which contains immunization records for all COVID-19 vaccines administered in the province. Maternal residential postal code was used to link the study population to Statistics Canada’s 2016 Census to obtain information on rural/urban residence and neighborhood income, and to the Ontario Marginalization Index, which provides four area-based measures reflecting social and economic marginalization.32 Additional details on data sources are provided in Table S1.
Study population
The source population included all Ontario residents who gave birth in the province between January 1 and December 31, 2021 (i.e., the first year of the province’s COVID-19 vaccination program). We excluded individuals who had a surgical or medical termination of pregnancy, or had a documented gestational age <20 weeks’ and birth weight <500 grams, because these events are not systematically captured in the birth registry. We then excluded individuals who had already received one or more doses of COVID-19 vaccine before or during pregnancy (up to and including the date of delivery). To assess the determinants of COVID-19 vaccine series initiation after pregnancy, we further restricted the study population to individuals who gave birth on or after May 1, 2021, when COVID-19 vaccines were widely available to the general population of adults in Ontario.29
Measures
The study outcome was COVID-19 vaccine series initiation after pregnancy, defined as receiving at least one dose of COVID-19 vaccine from 1 d after the date of delivery until the end of follow-up on June 30, 2022; follow-up time ranged from 6 to 18 months depending on calendar month of delivery (i.e., all individuals had a minimum of 6 months of follow-up after delivery).
The following variables were evaluated as potential determinants of COVID-19 vaccine series initiation after pregnancy: maternal age at delivery (<25, 25–29, 30–34, 35–39, ≥40 y); month of delivery; parity; multiple birth; preexisting maternal health conditions (composite of asthma, chronic hypertension, diabetes, heart disease, thyroid disease); exclusive breastfeeding at discharge from hospital/birth center after delivery (yes/no); healthcare provider at delivery (midwife, registered nurse or clinical nurse specialist/nurse practitioner, family physician, obstetrician, other, unattended); self-reported maternal smoking or substance use during pregnancy (yes/no); pregnancy complications (composite of gestational hypertension, eclampsia, pre-eclampsia, gestational diabetes, pulmonary embolism); preterm birth (delivery at <37 weeks’ gestation; yes/no); pre-pregnancy body mass index (kilograms/meter2 [kg/m2]); provincial public health unit region (seven geographic regions); rural/urban residence; neighborhood-level income quintiles; and neighborhood-level marginalization quintiles (four dimensions: residential instability, material deprivation, dependency, ethnic concentration).32 Additional details on variables are available in Table S1.
Statistical analysis
Follow-up time began 1 d after the date of delivery and continued until either the date that dose 1 was received or until follow-up ended on June 30, 2022. We first plotted descriptive cumulative incidence curves displaying time-to-COVID-19 vaccine series initiation by 6 months (24 weeks), 12 months (48 weeks), and by the end of follow-up, overall and stratified by calendar month of delivery. Plots were also stratified by maternal age group (<30 vs. ≥30 y) and newborn feeding status at discharge from hospital/birth center.
Among the subgroup of individuals who gave birth between May 1 and December 31, 2021, when COVID-19 vaccines were widely available,29 we calculated unadjusted incidence rates of COVID-19 vaccine series initiation per 1,000 person-days of follow-up by sociodemographic, pregnancy-related, and health behavioral factors. Cox proportional hazards regression was used to assess associations between these factors with COVID-19 vaccine series initiation after pregnancy; we computed unadjusted hazard ratios (HR) and 95% confidence intervals (CI) as well as hazard ratios adjusted for a core set of important sociodemographic variables: maternal age, neighborhood income quintile, rural residence, and public health unit region. We assessed the proportional hazards assumptions by examining Schoenfeld residual plots and Wald tests for interaction between the variables in each multivariable model and time. In a subgroup analysis, we explored whether there was any modification of associations by calendar time by stratifying according to timing of delivery (i.e., from May 1 to August 31, 2021, and from September 1 to December 31, 2021). In a sensitivity analysis, we also specifically explored COVID-19 vaccine series initiation during the first 6 months after pregnancy descriptively (through cumulative incidence plots and a Lexis diagram) and by repeating the analyses of determinants of series initiation, limited to 6 months of follow-up after pregnancy.
Multiple imputation was used to address variables with missing values using a fully conditional specification to generate five multiple-imputation datasets using the ‘MI’ procedure (SAS Version 9.4, SAS Institute Inc, Cary, NC). The crude and imputed frequencies of baseline characteristics for variables with missing information are shown in Table S2. We used SAS Version 9.4 (SAS Institute Inc, Cary, NC) for data imputation and all analyses and R 4.2.0 and RStudio 2022.02.1 (RStudio Boston, MA) for cumulative incidence curves and the Lexis diagram. The study was approved by the Children’s Hospital of Eastern Ontario (CHEO) Research Ethics Board (REB protocol number 21/05PE).
Results
Study population
Among 137,198 individuals who gave birth between January 1 and December 31, 2021, 49,822 (36.3%) had already received one or more doses of COVID-19 vaccine before or during pregnancy, leaving a study population of 87,376 unvaccinated individuals (Figure 1). The monthly percentage of individuals who remained unvaccinated at the time of delivery decreased from 99.9% in January 2021 to 36.1% in December 2021 (Table S3). In addition to the decreasing number of individuals remaining unvaccinated at the time of delivery each month, the distribution of sociodemographic characteristics of these individuals also changed over time – compared to January 2021, a higher proportion of those who gave birth in December 2021 was under 25 y of age and living in neighborhoods with lower household incomes and higher material deprivation (Figure S1). Between January and December 2021, there were also small increases in the proportion of individuals who smoked during pregnancy, had a BMI ≥30 kg/m2, or resided in a rural setting, while the proportion of individuals with a preexisting medical condition or who were exclusively breastfeeding their newborn at the time of discharge from hospital/birth center after delivery decreased slightly (Figure S1).
Figure 1.
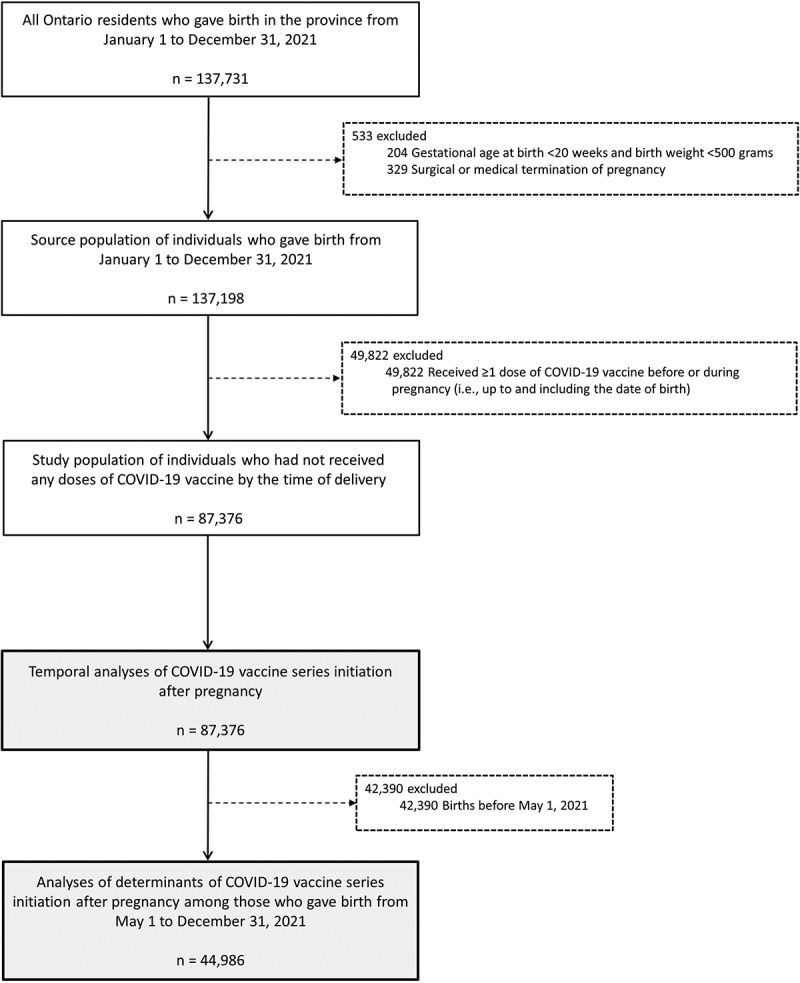
Study flow diagram.
Temporal trends in COVID-19 vaccine series initiation after pregnancy
Overall, the cumulative incidence of COVID-19 vaccine series initiation after pregnancy increased most rapidly in the first several months after delivery, reaching 56.3% by 6 months (24 weeks) and 65.0% by the end of follow-up (Figure 2 and Table S4). When stratified by month of delivery, 6-month cumulative incidences of series initiation ranged from a low of 26.0% among individuals who gave birth in December 2021 to a high of 69.6% among those who gave birth in April 2021 (Figure 3 and Table S4). Among individuals who had a minimum of 12 months of follow-up (i.e., those who gave birth in January through June 2021), 12-month cumulative incidences of COVID-19 series initiation ranged from a low of 59.5% among individuals who gave birth in July 2021 to a high of 77.1% among those who gave birth in March 2021 (Figure 3; Table S4).
Figure 2.
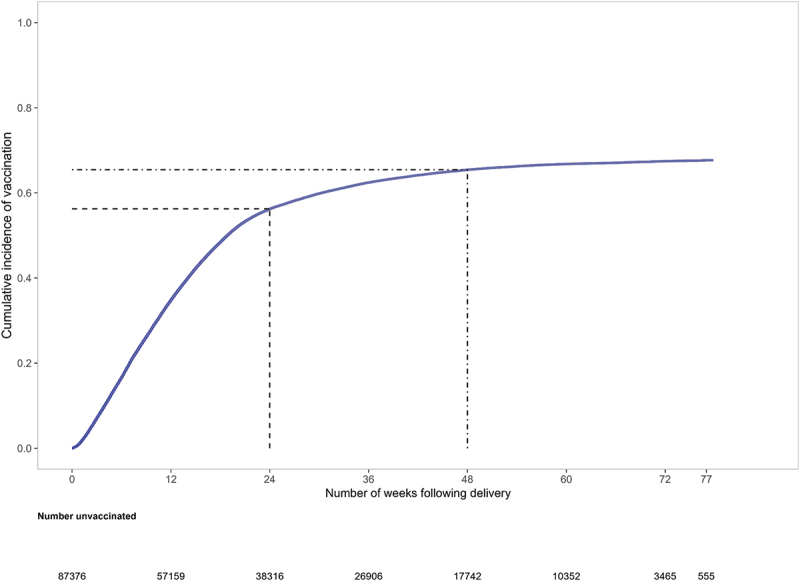
Cumulative incidence plot of time-to-COVID-19 vaccine series initiation after pregnancy among individuals who gave birth between January 1 and December 31, 2021 a,b.
a Dashed line displays COVID-19 vaccine series initiation by 6 months (24 weeks).
b Dash-dotted line displays COVID-19 vaccine series initiation by 12 months (48 weeks).
Figure 3.
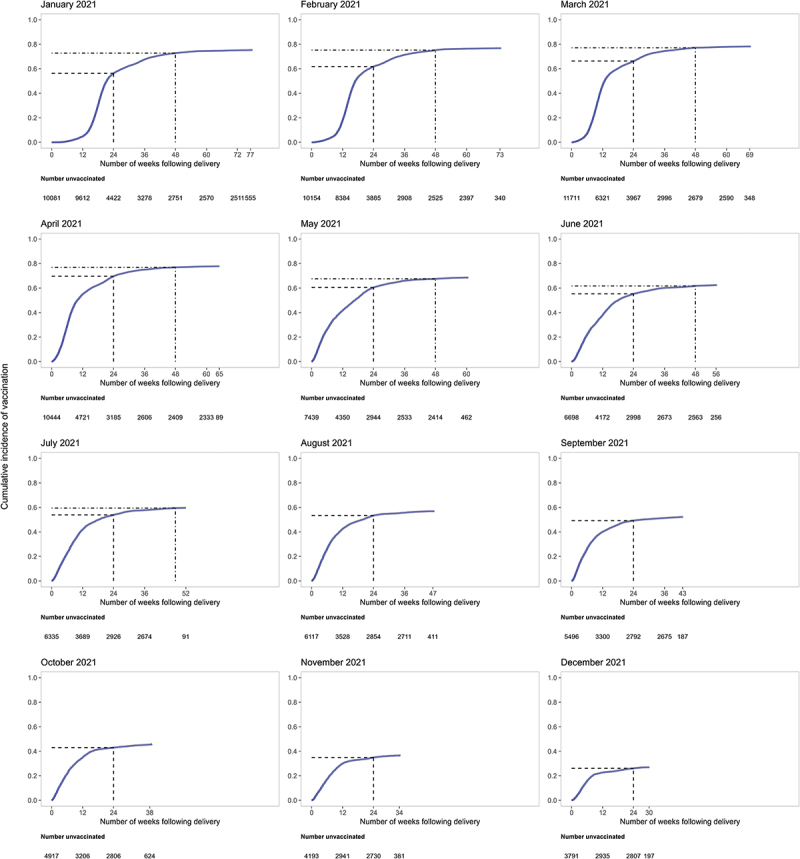
Cumulative incidence plots of time-to-COVID-19 vaccine series initiation after pregnancy, by calendar month of deliverya,b.
a Dashed line displays COVID-19 vaccine series initiation by 6 months (24 weeks).
b Dash-dotted line displays COVID-19 vaccine series initiation by 12 months (48 weeks).
Overall COVID-19 vaccine series initiation by 6 months after pregnancy was higher among those 30 y of age and older at delivery (60.2%) compared with those under 30 y of age (49.5%), and while this was a consistent pattern in each calendar month of delivery, the absolute differences between age groups decreased over time (Figure S2 and Table S5). The 6-month cumulative incidence of series initiation did not differ appreciably according to newborn feeding status at the time of discharge from hospital/birth center (55.1% among those who were exclusively breastfeeding; 57.2% among those who were using other methods for newborn feeding; Figure S3 and Table S5) and did not demonstrate any clear temporal patterns.
Determinants of COVID-19 vaccine series initiation after pregnancy
Among 44,986 individuals who gave birth between May 1 and December 31, 2021, the crude incidence rates of COVID-19 vaccine series initiation after pregnancy were lower in the lowest (<25 y of age: 2.2 per 1,000 person-days) and highest (≥40 y: 2.6 per 1,000 person-days) maternal age groups, compared with those 30–34 y of age (3.4 per 1,000 person-days). Even after adjustment for neighborhood income quintile, rural residence, and region of residence, COVID-19 vaccine series initiation was significantly lower in these age groups relative to those 30–34 y of age (<25 vs. 30–34 y aHR: 0.73, 95% CI: 0.70–0.77; ≥40 vs. 30–34 y aHR: 0.79, 95% CI: 0.74–0.84) (Table 1). Vaccine series initiation was also significantly lower in each month of delivery, relative to the month of May 2021, and decreased monotonically from June until December. Compared with nonsmokers, those who smoked during pregnancy had lower likelihood of initiating vaccination (smoking vs. nonsmoking aHR: 0.68, 95% CI: 0.65–0.72); a similar pattern was observed for substance use during pregnancy (vs. nonuse aHR: 0.67, 95% CI: 0.63–0.71). Other factors associated with a lower likelihood of COVID-19 vaccine series initiation after delivery included rural residence (vs. urban aHR: 0.72, 95% CI: 0.69–0.74), lower neighborhood income (lowest quintile vs. highest aHR: 0.79, 95% CI: 0.76–0.83), higher neighborhood material deprivation (highest quintile vs. lowest aHR: 0.74, 95% CI: 0.70–0.79), higher neighborhood social dependency (highest quintile vs. lowest aHR: 0.89, 95% CI: 0.85–0.93), and exclusive breastfeeding at time of hospital discharge (vs. other type of newborn feeding aHR: 0.81, 95% CI: 0.79–0.84). Having a preexisting chronic medical condition was associated with a higher likelihood of vaccine series initiation after pregnancy (vs. no condition aHR: 1.09, 95% CI: 1.05–1.14), as was having pregnancy complications (vs. no complication aHR: 1.18, 95% CI: 1.14–1.22) and residing in a neighborhood with the highest ethnic concentration (highest quintile vs. lowest aHR: 1.30, 95% CI: 1.24–1.37).
Table 1.
COVID-19 vaccine series initiation after pregnancy among individuals who gave birth between May 1 and December 31, 2021, and had not yet been vaccinated by the end of pregnancya.
| Characteristic | All |
Received ≥1 dose of COVID-19 vaccine after pregnancya |
||||
|---|---|---|---|---|---|---|
| N n = 44,986 |
%b | Number vaccinated by June 30, 2022 n = 24,141 |
Incidence rate of vaccination per 1000 person-days | Unadjusted hazard ratio (95% CI) |
Adjusted hazard ratioc (95% CI) |
|
| Maternal age (years) | ||||||
| <25 | 5,419 | 12.0 | 2,436 | 2.2 | 0.69 (0.66 to 0.72) | 0.73 (0.70 to 0.77) |
| 25–29 | 12,498 | 27.8 | 6,651 | 3.0 | 0.89 (0.87 to 0.92) | 0.92 (0.89 to 0.95) |
| 30–34 | 16,229 | 36.1 | 9,173 | 3.4 | 1.00 | 1.00 |
| 35–39 | 8,696 | 19.3 | 4,835 | 3.3 | 0.97 (0.93 to 1.00) | 0.96 (0.92 to 0.99) |
| ≥40 | 2,144 | 4.8 | 1,046 | 2.6 | 0.79 (0.75 to 0.85) | 0.79 (0.74 to 0.84) |
| Month of delivery | ||||||
| May 2021 | 7,439 | 16.5 | 5,095 | 3.7 | 1.00 | 1.00 |
| June 2021 | 6,698 | 14.9 | 4,171 | 3.2 | 0.86 (0.83 to 0.90) | 0.87 (0.83 to 0.90) |
| July 2021 | 6,335 | 14.1 | 3,771 | 3.2 | 0.84 (0.80 to 0.88) | 0.84 (0.81 to 0.88) |
| August 2021 | 6,117 | 13.6 | 3,475 | 3.3 | 0.82 (0.78 to 0.85) | 0.82 (0.79 to 0.86) |
| September 2021 | 5,496 | 12.2 | 2,858 | 3.1 | 0.74 (0.71 to 0.78) | 0.75 (0.72 to 0.78) |
| October 2021 | 4,917 | 10.9 | 2,227 | 2.7 | 0.62 (0.59 to 0.65) | 0.62 (0.59 to 0.65) |
| November 2021 | 4,193 | 9.3 | 1,528 | 2.2 | 0.47 (0.45 to 0.50) | 0.48 (0.45 to 0.50) |
| December 2021 | 3,791 | 8.4 | 1,016 | 1.7 | 0.34 (0.32 to 0.36) | 0.34 (0.32 to 0.36) |
| Parity | ||||||
| 0 (nulliparous) | 18,598 | 41.3 | 10,630 | 3.4 | 1.03 (1.01 to 1.07) | 1.07 (1.04 to 1.10) |
| 1 (primiparous) | 14,780 | 32.9 | 8,273 | 3.3 | 1.00 | 1.00 |
| ≥2 (multiparous) | 11,608 | 25.8 | 5,238 | 2.3 | 0.72 (0.69 to 0.74) | 0.71 (0.69 to 0.74) |
| Multiple birth | ||||||
| No | 44,307 | 98.5 | 23,773 | 3.0 | 1.00 | 1.00 |
| Yes | 679 | 1.5 | 368 | 3.1 | 1.02 (0.92 to 1.13) | 1.01 (0.91 to 1.12) |
| Any preexisting maternal medical conditiond | ||||||
| No | 40,463 | 89.9 | 21,560 | 3.0 | 1.00 | 1.00 |
| Yes | 4,523 | 10.1 | 2,581 | 3.4 | 1.11 (1.07 to 1.16) | 1.09 (1.05 to 1.14) |
| Exclusive breastfeeding from birth to discharge from hospital or birth centere | ||||||
| No | 22,291 | 49.6 | 12,944 | 3.5 | 1.00 | 1.00 |
| Yes | 22,695 | 50.4 | 11,197 | 2.6 | 0.80 (0.78 to 0.83) | 0.81 (0.79 to 0.84) |
| Healthcare provider who caught/delivered baby | ||||||
| Midwife | 5,728 | 12.7 | 2,344 | 2.0 | 0.65 (0.62 to 0.68) | 0.67 (0.64 to 0.70) |
| Registered Nurse or CNS/NP | 392 | 0.9 | 197 | 2.7 | 0.85 (0.74 to 0.98) | 0.88 (0.77 to 1.02) |
| Family Physician | 2,579 | 5.7 | 1,271 | 2.6 | 0.83 (0.78 to 0.87) | 0.87 (0.82 to 0.92) |
| Obstetrician | 32,482 | 72.2 | 18,298 | 3.3 | 1.00 | 1.00 |
| Other | 3,618 | 8.0 | 1,968 | 3.1 | 0.95 (0.91 to 1.00) | 0.99 (0.94 to 1.04) |
| Unattended (None) | 187 | 0.4 | 63 | 1.4 | 0.49 (0.38 to 0.63) | 0.52 (0.40 to 0.66) |
| Smoked during pregnancy | ||||||
| No | 40,894 | 90.9 | 22,454 | 3.2 | 1.00 | 1.00 |
| Yes | 4,092 | 9.1 | 1,687 | 1.9 | 0.65 (0.62 to 0.68) | 0.68 (0.65 to 0.72) |
| Substance use during pregnancyf | ||||||
| No | 41,844 | 93.0 | 22,886 | 3.1 | 1.00 | 1.00 |
| Yes | 3,142 | 7.0 | 1,255 | 1.9 | 0.64 (0.60 to 0.67) | 0.67 (0.63 to 0.71) |
| Pregnancy complicationsg | ||||||
| No | 38,626 | 85.9 | 20,347 | 2.9 | 1.00 | 1.00 |
| Yes | 6,360 | 14.1 | 3,794 | 3.7 | 1.20 (1.16 to 1.24) | 1.18 (1.14 to 1.22) |
| Preterm birth | ||||||
| No (≥37 weeks) | 41,675 | 92.6 | 22,441 | 3.1 | 1.00 | 1.00 |
| Yes (<37 Weeks) | 3,311 | 7.4 | 1,700 | 2.8 | 0.93 (0.89 to 0.98) | 0.93 (0.88 to 0.98) |
| Maternal BMI (kg/m2) | ||||||
| ≥30.0 | 9,654 | 21.5 | 5,258 | 3.1 | 1.02 (0.99 to 1.05) | 1.04 (1.00 to 1.07) |
| <30.0 | 35,332 | 78.5 | 18,883 | 3.0 | 1.00 | 1.00 |
| Neighborhood median family income quintiles | ||||||
| 1 (Lowest) | 10,885 | 24.2 | 5,441 | 2.7 | 0.81 (0.78 to 0.85) | 0.79 (0.76 to 0.83) |
| 2 | 9,656 | 21.5 | 5,109 | 2.9 | 0.89 (0.86 to 0.93) | 0.88 (0.85 to 0.92) |
| 3 | 9,606 | 21.4 | 5,276 | 3.2 | 0.96 (0.92 to 1.00) | 0.94 (0.90 to 0.98) |
| 4 | 8,637 | 19.2 | 4,858 | 3.3 | 1.00 (0.96 to 1.05) | 0.99 (0.95 to 1.04) |
| 5 (Highest) | 6,202 | 13.8 | 3,457 | 3.3 | 1.00 | 1.00 |
| Rural residence | ||||||
| No | 37,663 | 83.7 | 20,917 | 3.2 | 1.00 | 1.00 |
| Yes | 7,323 | 16.3 | 3,224 | 2.2 | 0.73 (0.70 to 0.76) | 0.72 (0.69 to 0.74) |
| PHU region of residence | ||||||
| South West | 6,503 | 14.5 | 3,169 | 2.6 | 0.90 (0.86 to 0.94) | 0.97 (0.92 to 1.02) |
| Central West | 9,827 | 21.8 | 5,251 | 3.0 | 1.02 (0.98 to 1.07) | 1.03 (0.99 to 1.07) |
| Central East | 13,126 | 29.2 | 7,534 | 3.4 | 1.13 (1.08 to 1.17) | 1.09 (1.05 to 1.13) |
| Greater Toronto Area | 7,736 | 17.2 | 4,110 | 3.0 | 1.00 | 1.00 |
| Eastern | 5,210 | 11.6 | 2,733 | 2.9 | 1.00 (0.95 to 1.05) | 1.04 (0.99 to 1.09) |
| North West | 810 | 1.8 | 462 | 3.3 | 1.12 (1.01 to 1.23) | 1.25 (1.14 to 1.38) |
| North East | 1,774 | 3.9 | 882 | 2.7 | 0.91 (0.85 to 0.98) | 1.03 (0.96 to 1.12) |
| Marginalization indicesh | ||||||
| Residential instability quintile | ||||||
| 1 (least unstable) | 8,907 | 19.8 | 4,968 | 3.3 | 1.00 | 1.00 |
| 2 | 8,387 | 18.6 | 4,652 | 3.2 | 0.99 (0.95 to 1.03) | 1.03 (0.99 to 1.07) |
| 3 | 8,640 | 19.2 | 4,611 | 3.0 | 0.93 (0.90 to 0.97) | 0.98 (0.94 to 1.02) |
| 4 | 8,516 | 18.9 | 4,399 | 2.9 | 0.89 (0.85 to 0.93) | 0.96 (0.92 to 1.01) |
| 5 (most unstable) | 10,536 | 23.4 | 5,511 | 2.9 | 0.90 (0.86 to 0.93) | 1.01 (0.96 to 1.06) |
| Material deprivation quintile | ||||||
| 1 (least deprived) | 8,086 | 18.0 | 4,746 | 3.6 | 1.00 | 1.00 |
| 2 | 8,348 | 18.6 | 4,652 | 3.3 | 0.91 (0.87 to 0.95) | 0.91 (0.87 to 0.95) |
| 3 | 8,434 | 18.7 | 4,583 | 3.1 | 0.87 (0.84 to 0.91) | 0.88 (0.84 to 0.92) |
| 4 | 8,882 | 19.7 | 4,654 | 2.9 | 0.82 (0.78 to 0.85) | 0.82 (0.78 to 0.86) |
| 5 (most deprived) | 11,236 | 25.0 | 5,506 | 2.6 | 0.73 (0.70 to 0.76) | 0.74 (0.70 to 0.79) |
| Dependency quintile | ||||||
| 1 (least dependent) | 13,974 | 31.1 | 8,082 | 3.5 | 1.00 | 1.00 |
| 2 | 9,364 | 20.8 | 5,010 | 3.0 | 0.89 (0.86 to 0.92) | 0.94 (0.90 to 0.97) |
| 3 | 7,717 | 17.2 | 3,969 | 2.8 | 0.84 (0.81 to 0.87) | 0.90 (0.86 to 0.93) |
| 4 | 7,247 | 16.1 | 3,795 | 2.9 | 0.86 (0.83 to 0.90) | 0.93 (0.89 to 0.97) |
| 5 (most dependent) | 6,684 | 14.9 | 3,285 | 2.7 | 0.80 (0.77 to 0.84) | 0.89 (0.85 to 0.93) |
| Ethnic concentration quintile | ||||||
| 1 (lowest concentration) | 7,003 | 15.6 | 3,286 | 2.5 | 1.00 | 1.00 |
| 2 | 7,329 | 16.3 | 3,674 | 2.7 | 1.10 (1.05 to 1.16) | 1.04 (0.99 to 1.09) |
| 3 | 7,469 | 16.6 | 3,917 | 2.9 | 1.18 (1.12 to 1.24) | 1.08 (1.02 to 1.13) |
| 4 | 9,249 | 20.6 | 5,038 | 3.1 | 1.23 (1.18 to 1.29) | 1.11 (1.06 to 1.17) |
| 5 (highest concentration) | 13,936 | 31.0 | 8,226 | 3.5 | 1.35 (1.30 to 1.41) | 1.30 (1.24 to 1.37) |
Abbreviations: BMI, body mass index; PHU, public health unit.
aFollow-up period extended from the day of delivery until June 30, 2022.
bColumn percentages.
cModels were adjusted for maternal age at delivery, neighborhood income quintile, rural residence, and public health unit region.
dComposite of asthma, chronic hypertension, diabetes, heart disease, thyroid disease.
eExclusive breastfeeding vs. other methods of newborn feeding from birth to discharge from hospital or birth center. Other methods of feeding comprised: breast milk substitute (formula only), breast milk substitute (other), combination of breastmilk and breastmilk substitute, and unknown feeding practices.
fSelf-reported cannabis, opioid, or alcohol use during pregnancy.
gComposite of gestational hypertension, eclampsia, pre-eclampsia, gestational diabetes, and pulmonary embolism.
hOntario Marginalization Index included neighborhood-level quintiles for residential instability (family or housing instability), material deprivation (inability to attain basic material needs), dependency (receipt of income support), and ethnic concentration (geographic areas with high concentrations of recent immigrants and/or ‘visible minorities;’ see Table S1 for details.
Subgroup and sensitivity analyses
Following stratification by time period of delivery, we generally observed the same patterns of associations with COVID-19 vaccine series initiation, with respect to both direction and magnitude of the hazards ratios (Table S6). However, the likelihood of COVID-19 vaccine series initiation among those ≥40 y of age compared with those 30–34 y of age was qualitatively lower between September and December 2021 (aHR: 0.69, 95% CI: 0.62–0.78) than between May and August 2021 (aHR: 0.84, 95% CI: 0.78–0.91). Conversely, COVID-19 vaccine series initiation was higher among those living in neighborhoods with the highest ethnic concentration compared with the lowest ethnic concentration between September and December 2021 (aHR: 1.47, 95% CI: 1.34–1.62) than between May and August 2021 (aHR: 1.22, 95% CI: 1.14–1.29). When we restricted the follow-up period to only the first 6 months after pregnancy, there were no changes in direction or magnitude of the results (Table S7). As can be seen in the Lexis diagram (Figure S4), there were no clear temporal patterns in series initiation in the first 6 months after pregnancy with respect to time since the delivery; the most intense calendar period of COVID-19 vaccine series initiation occurred from May to June 2021, corresponding to when COVID-19 vaccines became widely available to the general adult population in the province.
Discussion
In this population-wide study of 87,376 individuals who gave birth in 2021 and were not yet vaccinated by the end of their pregnancy, the cumulative incidence of COVID-19 vaccine series initiation after delivery reached 65.0% by the end of June 2022. Series initiation after pregnancy by June 30, 2022, was highest among those who gave birth in the first 4 months of 2021; nevertheless, it was still considerably lower among these individuals than in the general population of women of reproductive age in Ontario (92% coverage by early July 202233), despite having had 15–18 months to initiate vaccination by the end of the study period. Factors associated with lower likelihood of COVID-19 vaccine series initiation after pregnancy included younger and older maternal age, smoking and substance use during pregnancy, rural residence, lower neighborhood income, higher material deprivation and social dependency, and exclusive breastfeeding at the time of discharge from hospital/birth center. Individuals with a family doctor-attended or midwife-attended delivery (vs. obstetrician) also had lower likelihood of initiating their COVID-19 vaccine series after giving birth. In contrast, having a preexisting chronic medical condition, having complications during pregnancy, and residing in a neighborhood with the highest ethnic concentration were associated with a higher likelihood of vaccine series initiation after pregnancy.
There are few studies that have specifically assessed COVID-19 vaccine uptake among postpartum individuals – consistent with our findings, a few small studies from the U.S. have demonstrated that lower COVID-19 vaccine uptake among pregnant and postpartum individuals was associated with a number of sociodemographic characteristics, including younger age, lower education, reported substance use, and higher parity.34,35 Similar patterns of association have also been observed in studies assessing COVID-19 vaccine coverage during pregnancy.20,22,25,36,37 Some of the sociodemographic subgroups in which vaccine initiation after delivery was lowest in our study (e.g., rural residence, lower neighborhood income, and higher neighborhood material deprivation) may reflect individuals who have less access to health services during pregnancy, even within the context of a publicly funded healthcare system.38 Healthcare providers play a critical role in enhancing vaccination rates in the obstetric population both by providing access to vaccination, as well as by providing information and education to their patients about safety and effectiveness of vaccination during pregnancy.39,40 A U.S. survey of pregnant and postpartum individuals related to vaccination status 3–6 months after an initial baseline assessment of intention to receive COVID-19 vaccination showed hesitancy at baseline was associated with persistent hesitancy at follow-up; only a few individuals who reported hesitancy at baseline were later vaccinated.41 In addition, surveys, conducted in more than 15 countries, of pregnant and postpartum individuals about perceptions and attitudes toward COVID-19 vaccination found that barriers to accessing healthcare services, negative attitudes toward other vaccinations routinely recommended during pregnancy, and lack of information and concerns about side effects of the vaccine for themselves or their baby – especially in this context of a newly developed vaccine – were associated with a lower likelihood of getting vaccinated against COVID-19.23–34,41–43 These factors, combined with the prominence of misinformation about COVID-19 vaccination and adverse effects on fertility, pregnancy outcomes, and breastfeeding44,45 as well as concerns about transitory changes in the menstrual cycle following COVID-19 vaccination46, may have contributed to the lower vaccination rates in this population. An additional important consideration in the postpartum population that may impact willingness to become vaccinated is breastfeeding. The World Health Organization and other pediatric associations recommend exclusive breastfeeding for the first 6 months of life.47,48 Although breastfeeding people might be more willing to get vaccinated against COVID-19 than pregnant people,49 there could be some reluctance to initiate vaccination while still breastfeeding if there are perceived safety concerns,24,45,50 despite recommendations for lactating individuals to get vaccinated.8
It has been suggested that immunization should become a part of routine obstetrical care to increase vaccine confidence and uptake and decrease misinformation.39,40 One small study conducted at a single hospital in mid-2021 explored willingness of obstetric patients admitted for delivery to initiate their COVID-19 vaccine series during their hospitalization; among 221 obstetric patients who had not yet received any doses of COVID-19 vaccine, 19.5% successfully received their first dose in that clinical setting.51 Inpatient vaccination has also been found to improve postpartum vaccination rates for other vaccines; for example, offering human papillomavirus and pertussis vaccination prior to hospital discharge substantially increased vaccination rates among postpartum patients not yet vaccinated.52,53 Addressing concerns about COVID-19 vaccines by obstetrical care providers and administering vaccines in hospital could be an efficient way to increase COVID-19 vaccine coverage among individuals who are not yet vaccinated by the time of delivery, particularly in settings where the majority of births take place in a hospital. Nevertheless, efforts should be made to ensure COVID-19 vaccination before or during pregnancy in order to protect individuals during their pregnancy, when they are at higher risk of serious COVID-19 disease,1–4 and also to provide passive protection to newborns.11–13
Strengths and limitations
This study was population-based and included all individuals in Ontario, who gave birth in 2021 and had not yet received any doses of COVID-19 vaccine by the time of delivery. We followed these individuals until June 30, 2022, to assess COVID-19 vaccine series initiation after pregnancy, with a minimum of 6 months of follow-up for everyone in the study population (maximum follow-up was 18 months). We were able to explore associations between COVID-19 vaccine series initiation after pregnancy with a wide range of sociodemographic, pregnancy-related, and health-related factors. Nevertheless, we were unable to measure some factors that may have been important – for example, we had no information about individual or contextual factors that could have influenced individuals’ decisions about whether, or when, to initiate their primary COVID-19 vaccine series (i.e., knowledge, beliefs, attitudes, intentions, past experience, confidence or trust in the healthcare system or healthcare providers, social or cultural norms and values). Although we explored COVID-19 vaccine series initiation by 6 months after pregnancy, which is a time when recommendations for newborn feeding change,47 we did not have information on the actual method of newborn feeding after discharge from hospital/birth center.
Conclusion
This study found that among individuals not yet vaccinated against COVID-19 by the end of their pregnancy in 2021, the initiation of COVID-19 vaccination after delivery – even many months after giving birth – was lower than among women of reproductive age in the general population. This suggests that there may be persistent vaccine hesitancy among individuals experiencing a recent pregnancy or possibly reduced access to health care. Barriers to access, vaccine hesitancy, and vaccine misinformation could be addressed by targeted interventions to improve both pregnant and postpartum individuals’ awareness of the risks of COVID-19 and the benefits of COVID-19 vaccines. Given ongoing recommendations for vaccination of pregnant and lactating individuals, additional research is needed to better understand barriers to COVID-19 vaccination during pregnancy and after giving birth, and to monitor trends in COVID-19 vaccine coverage in these populations over time.
Supplementary Material
Acknowledgments
The authors thank the Ontario Ministry of Health for granting access to the COVaxON database and the Public Health Case and Contact Management Solution (CCM). The authors also thank maternal-newborn hospitals and midwifery practice groups in Ontario for providing maternal-newborn data to BORN Ontario. Finally, the authors thank BORN Ontario staff for their assistance with data extraction, linkage, code review, and results review.
Funding Statement
This study was supported by funding from the Public Health Agency of Canada, through the Vaccine Surveillance Reference Group and the COVID-19 Immunity Task Force; S. Håberg was partly supported by the Research Council of Norway (project number, 324312) and through its Centers of Excellence funding scheme (project number, 262700) and by NordForsk (project number, 105545).
Disclosure statement
K. Wilson is CEO of CANImmunize Inc, which hosts a national digital immunization record, and is a member of the independent data safety board for the Medicago COVID-19 vaccine trial; no other relationships or activities that could appear to have influenced the submitted work.
Data sharing
The dataset from this study is held securely by BORN Ontario. Although the dataset cannot be made publicly available, the analytic code may be available on request.
Disclaimer
The study sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Parts of this material are based on data and information compiled and provided by BORN Ontario and the Ontario Ministry of Health; however, the analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2215150.
References
- 1.McClymont E, Albert AY, Alton GD, Boucoiran I, Castillo E, Fell DB, Kuret V, Poliquin V, Reeve T, Scott H, et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA. 2022;327(20):1983–11. doi: 10.1001/jama.2022.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Precautions for people with certain medical conditions. [accessed 2023 May 1]. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- 3.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Zhou D, Coomar D, Sheikh J, Lawson H, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, Roggero P, Prefumo F, Do Vale MS, Cardona-Perez JA, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan AK, Arah OA, Fell DB, Sullivan SG.. SARS-CoV-2 infection during pregnancy and associated perinatal health outcomes: a national US cohort study. J Infect Dis. 2022;225(5):759–67. doi: 10.1093/infdis/jiab626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karasek D, Baer RJ, McLemore MR, Bell AJ, Blebu BE, Casey JA, Coleman-Phox K, JM Costello, JN Felder, Flowers E, JD Fuchs. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Heal Am. 2021;2:100027. doi: 10.1016/j.lana.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, Ellington SR. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization — United States, March 2020–September 2021. Morb Mortal Wkly Rep. 2021;70(47):1640–5. 10.15585/mmwr.mm7047e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman Institute of Bioethics & Center for Immunization Research JHU . COVID-19 Maternal Immunization Tracker (COMIT): COVID-19 Vaccine policies for pregnant and lactating people worldwide. 2021. [accessed 2023 May 1]. https://www.comitglobal.org.
- 9.Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, Hernán MA, Lipsitch M, Hernandez-Diaz S, Reis BY, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–5. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 10.Magnus MC, Håberg SE, Carlsen EØ, Kwong JC, Buchan SA, Fell DB. Pregnancy status at the time of COVID-19 vaccination and incidence of SARS-CoV-2 infection. Clin Infect Dis. 2022:ciac739. doi: 10.1093/cid/ciac739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsen EØ, Magnus MC, Oakley L, Fell DB, Greve-Isdahl M, Kinge JM, Håberg SE. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med. 2022;182(8):825–31. doi: 10.1001/jamainternmed.2022.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Pannaraj PS, Boom JA, Sahni LC, Chiotos K, Cameron MA, et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med. 2022;387(2):109–19. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen SC, Hernandez A, Fell DB, Austin PC, D’Souza R, Guttmann A, Brown KA, Buchan SA, Gubbay JB, Nasreen S, et al. Effectiveness of maternal mRNA COVID-19 vaccination during pregnancy against Delta and Omicron SARS-CoV-2 infection and hospitalization in infants: a test-negative design study. BMJ. 2023;380:e074035. doi: 10.1136/bmj-2022-074035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharbanda EO, Haapala J, DeSilva M, Vazquez-Benitez G, Vesco KK, Naleway AL, Lipkind HS. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326(16):1629–31. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008–10. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, Boyce TG, Daley MF, Fuller CC, Getahun D, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth — eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. Morb Mortal Wkly Rep. 2022;71(1):26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnus MC, Örtqvist AK, Dahlqwist E, Ljung R, Skår F, Oakley L, Macsali F, Pasternak B, Gjessing HK, Håberg SE, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469–77. doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, Sprague AE, Buchan SA, Kwong JC, Wilson SE, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478–87. doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fell DB, Dimanlig-Cruz S, Regan AK, Håberg SE, Gravel CA, Oakley L, Alton GD, Török E, Dhinsa T, Shah PS, et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after COVID-19 vaccination during pregnancy: population based retrospective cohort study. BMJ. 2022;378:e071416. doi: 10.1136/bmj-2022-071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, Kenigsberg TA, Lamias MJ, Irving SA, Kauffman TL, et al. COVID-19 vaccination coverage among pregnant women during pregnancy — eight integrated health care organizations, United States, December 14, 2020–May 8, 2021. Morb Mortal Wkly Rep. 2021;70(24):895–9. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stock SJ, Carruthers J, Calvert C, Denny C, Donaghy J, Goulding A, Hopcroft LEM, Hopkins L, McLaughlin T, Pan J, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28(3):504–12. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fell DB, Török E, Sprague AE, Regan AK, Dhinsa T, Alton GD, Dimanlig-Cruz S, MacDonald SE, Buchan SA, Kwong JC, et al. Temporal trends and determinants of COVID-19 vaccine coverage and series initiation during pregnancy in Ontario, Canada, December 2020 to December 2021: a population-based retrospective cohort study. Vaccine. 2023;41(10):1716–25. doi: 10.1016/j.vaccine.2023.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski DF, Wu JW. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skirrow H, Barnett S, Bell S, Riaposova L, Mounier-Jack S, Kampmann B, Holder B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022;22(1):33. doi: 10.1186/s12884-021-04321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharya O, Siddiquea BN, Shetty A, Afroz A, Billah B. COVID-19 vaccine hesitancy among pregnant women: a systematic review and meta-analysis. BMJ Open. 2022;12(8):e061477. doi: 10.1136/bmjopen-2022-061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid P, Rauber D, Betsch C, Lidolt G, Denker M-L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005 - 2016. PLos One. 2017;12(1):e0170550. doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statistics Canada . Live births and fetal deaths (stillbirths), by type of birth (single or multiple). 10.25318/1310042801-eng. [DOI]
- 28.National Advisory Committee on Immunization . Recommendations on the use of COVID-19 vaccines. 2021 Apr 23 [accessed 2023 May 1]. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/april-23-2021.html.
- 29.Ontario Ministry of Health and Long-Term Care . COVID-19 vaccine booking expanding to Ontarians 18+ ahead of schedule. 2021. [accessed 2023 May 1]. https://news.ontario.ca/en/release/1000143/covid-19-vaccine-booking-expanding-to-ontarians-18-ahead-of-schedule.
- 30.Murphy MSQ, Fell DB, Sprague AE, Corsi DJ, Dougan S, Dunn SI, Holmberg V, Huang T, Johnson M, Kotuba M, et al. Data resource profile: better outcomes registry & network (BORN) Ontario. Int J Epidemiol. 2021;50(5):1416–25. doi: 10.1093/ije/dyab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn S, Lanes A, Sprague AE, Fell DB, Weiss D, Reszel J, Taljaard M, Darling EK, Graham ID, Grimshaw JM, et al. Data accuracy in the Ontario birth registry: a chart re-abstraction study. BMC Health Serv Res. 2019;19(1):1001. doi: 10.1186/s12913-019-4825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Public Health Ontario . Ontario marginalization index (ON-Marg). [accessed 2023 May 1]. https://www.publichealthontario.ca/en/data-and-analysis/health-equity/ontario-marginalization-index.
- 33.Ontario Agency for Health Protection and Promotion (Public Health Ontario) . COVID-19 vaccine uptake in Ontario: December 14, 2020 to July 4, 2022. Toronto, ON: Queen’s Printer for Ontario; 2022. [Google Scholar]
- 34.Kiefer MK, Mehl R, Costantine MM, Johnson A, Cohen J, Summerfield TL, Landon MB, Rood KM, Venkatesh KK. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: a cross-sectional study. BJOG. 2022;129(8):1342–51. doi: 10.1111/1471-0528.17110. [DOI] [PubMed] [Google Scholar]
- 35.Siegel MR, Lumbreras-Marquez MI, James K, McBay BR, Gray KJ, Schantz-Dunn J, Diouf K, IT Goldfarb. Perceptions and attitudes toward COVID-19 vaccination among pregnant and postpartum individuals. Am J Perinatol. 2022. doi: 10.1055/a-1877-5880. [DOI] [PubMed] [Google Scholar]
- 36.Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, Magee LA, O’Brien P, Rezvani A, von Dadelszen P, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1-236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Örtqvist AK, Dahlqwist E, Magnus MC, Ljung R, Jonsson J, Aronsson B, Pasternak B, Håberg SE, Stephansson O. COVID-19 vaccination in pregnant women in Sweden and Norway. Vaccine. 2022;40(33):4686–92. doi: 10.1016/j.vaccine.2022.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Origlia P, Jevitt C, Sayn-Wittgenstein FZ, Cignacco E. Experiences of antenatal care among women who are socioeconomically deprived in high-income industrialized countries: an integrative review. J Midwifery Women's Health. 2017;62(5):589–98. doi: 10.1111/jmwh.12627. [DOI] [PubMed] [Google Scholar]
- 39.Alhendyani F, Jolly K, Jones LL. Views and experiences of maternal healthcare providers regarding influenza vaccine during pregnancy globally: a systematic review and qualitative evidence synthesis. PLos One. 2022;17(2):e0263234. doi: 10.1371/journal.pone.0263234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacDougall DM, Halperin SA. Improving rates of maternal immunization: challenges and opportunities. Hum Vaccines Immunother. 2016;12(4):857–65. doi: 10.1080/21645515.2015.1101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Germann K, Kiefer MK, Rood KM, Mehl R, Wu J, Pandit R, Lynch CD, Landon MB, Grobman WA, Costantine MM, et al. Association of initial COVID-19 vaccine hesitancy with subsequent vaccination among pregnant and postpartum individuals. BJOG. 2022;129(8):1352–60. doi: 10.1111/1471-0528.17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L, Riggan KA, Ashby GB, Rivera-Chiauzzi EY, Allyse MA. Pregnant and postpartum patients’ views of COVID-19 vaccination. J Community Health. 2022;47(5):871–8. doi: 10.1007/s10900-022-01118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oluklu D, Goncu Ayhan S, Menekse Beser D, Uyan Hendem D, Ozden Tokalioglu E, Turgut E, Sahin D. Factors affecting the acceptability of COVID-19 vaccine in the postpartum period. Hum Vaccin Immunother. 2021;17(11):4043–7. doi: 10.1080/21645515.2021.1972710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327(11):1013–15. doi: 10.1001/jama.2022.2404. [DOI] [PubMed] [Google Scholar]
- 45.Oliver J, Gibney Z, Danchin C, Bradfield KB, Homer M, Gibney KB, Danchin M. Drivers of COVID-19 vaccine hesitancy among women of childbearing age in Victoria, Australia: a descriptive qualitative study. Vaccine: X. 2022;12:100240. doi: 10.1016/j.jvacx.2022.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dellino M, Vimercati A, D’Amato A, Damiani GR, Laganà AS, Cicinelli E, Pinto V, Malvasi A, Scacco S, Ballini A, et al. “GONE with the WIND”: the transitory effects of COVID-19 on the gynecological system. J Pers Med. 2023;13(2):312. doi: 10.3390/jpm13020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization . Breastfeeding. 2022. [accessed 2023 May 1]. https://www.who.int/health-topics/breastfeeding#tab=tab_2.
- 48.Critch JN, Canadian Paediatric Society N and GC . Nutrition for healthy term infants, birth to six months: an overview. Paediatr Child Health. 2013;18(4):206–7. doi: 10.1093/pch/18.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaal NK, Zöllkau J, Hepp P, Fehm T, Hagenbeck C. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch Gynecol Obstet. 2022;306(2):365–72. doi: 10.1007/s00404-021-06297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton D, D’Alton M, Zhang Y, Kahe K, Cepin A, Goffman D, Staniczenko A, Yates H, Burgansky A, Coletta J, et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. 2021;3(5):100403. doi: 10.1016/j.ajogmf.2021.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez MJ, Paul R, Hirshberg JS, Aibangbee E, Ford T, Cooke E, Carter EB, Odibo AO, Raghuraman N, Kelly JC. Administration of the coronavirus disease 2019 (COVID-19) vaccine to hospitalized postpartum patients. Obstet Gynecol. 2021;138(6):885–7. doi: 10.1097/AOG.0000000000004590. [DOI] [PubMed] [Google Scholar]
- 52.Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A human papillomavirus vaccination program for low-income postpartum women. Am J Obstet Gynecol. 2016;215(3):318.e1–e9. doi: 10.1016/j.ajog.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernstein HH, Monty M, Yang P, Cohen A. Increasing Tdap coverage among postpartum women: a quality improvement intervention. Pediatrics. 2017;139(3):e20160607. doi: 10.1542/peds.2016-0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


