Abstract
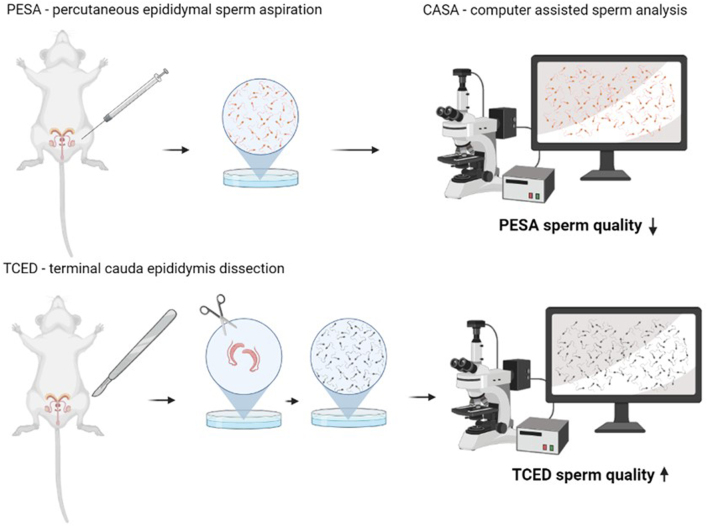
Graphical abstract
Abstract
In laboratory mice, sperm quality is usually assessed in spermatozoa collected from the cauda epididymidis of freshly sacrificed males. Percutaneous epididymal sperm aspiration (PESA) is a non-terminal alternative that would allow repeated sperm collection for sperm quality assessment in living males. To test whether PESA is a suitable method to assess sperm quality, we compared sperm traits between samples collected by PESA vs the commonly applied terminal cauda epididymidis dissection. The collected sperm samples were analyzed using computer-assisted sperm analysis and various parameters, including sperm motility, swimming velocity and morphology, were determined. We were able to retrieve motile sperm from all mice using PESA and the terminal cauda epididymidis dissection. Based on computer-assisted sperm analysis, however, sperm motility and swimming velocity were significantly lower after PESA compared to samples obtained by cauda epididymidis dissection. In addition, we found significantly more morphological abnormalities in PESA samples, probably induced as a side effect of the sampling technique. Although sperm samples collected by PESA are successfully used for in vitro fertilization, we cannot recommend PESA as a suitable method to assess sperm quality in mice, since the procedure seems to impair various sperm traits.
Lay summary
In mice, sperm quality is usually assessed in sperm collected from the epididymis (organ where ripe sperm is stored) of euthanized males. However, there is one non-terminal and minimal invasive alternative to collect sperm, called percutaneous epididymal sperm aspiration (PESA), which allows repeated sample collections from the same individual. Given that individual sperm quality is variable and can change according to various factors, PESA could allow to track sperm quality over time and would be highly appreciated in different research fields. Here, we tested the suitability of PESA to determine sperm quality by comparing sperm samples collected by PESA vs the commonly applied terminal epididymis dissection. We used computer-assisted sperm analysis to determine various sperm quality traits. Surprisingly, we found that sperm collected by PESA showed significantly reduced motility, swimming velocity and more morphological abnormalities compared to sperm samples collected by epididymis dissection. Thus, we cannot recommend PESA as a suitable method to determine sperm quality traits as the procedure itself seems to affect collected sperm cells.
Keywords: sperm quality assessment, sperm collection, non-terminal, sperm traits, CASA, mouse, epididymal sperm
Introduction
Sperm collection in mice is frequently applied in laboratory rodent facilities, and it is especially important in assisted reproduction. Spermatozoa are collected to cryopreserve valuable mouse strains (Nakagata 2000, Wilson & Sheardown 2011) or for the distribution of mouse models across laboratories. Archiving mouse spermatozoa is a fast and cost-effective way to store and save the continuously growing number of genetically engineered mouse lines. In addition, collected spermatozoa are used for breeding mouse lines via in vitro fertilization (IVF). IVF with fresh or frozen-thawed sperm is furthermore an important routine practice to overcome fertility problems, for the rederivation of contaminated strains and to accelerate the expansion of a line (Behringer et al. 2014, Taft 2017). In addition, sperm collection is performed to investigate sperm quality traits like sperm motility or swimming velocity. These traits can work as a proxy to estimate male fertility in inbred (Kawai et al. 2006) and mutant mice (Danshina et al. 2010, Khatun et al. 2018) or to investigate the effect of specific environmental (Ozkosem et al. 2015) and social factors (Koyama & Kamimura 1999, Ramm & Stockley 2009) on sperm production.
The standard procedure for sperm collection in mice is the terminal caudaepididymidis dissection (TCED). TCED is conducted post mortem with freshly sacrificed males and results in a high number of mature spermatozoa (Nakagata 2000, Behringer et al. 2014). Alternatively, a unilateral epididymectomy under anesthesia can be performed, although this procedure can only be conducted twice in each male. Yet, in a number of experiments, it is required to repeatedly collect sperm from a single male, that is, due to a specific study design or to preserve a valuable individual. Besides practical issues, there are also ethical concerns. It is important to refine sperm collection techniques and pursue different, non-terminal sperm retrieval procedures, which can save animal lives and preserve their fertility. In establishing a non-terminal in vivo sperm collection procedure that allows to subsequently assess sperm quality, we can contribute to the reduction and refinement aspect of the 3Rs as postulated by Russell and Burch (Russell & Burch 1992).
To date, there are a number of different non-terminal sperm retrieval procedures in mice that would potentially allow subsequent sperm quality assessment, namely electroejaculation (Tecirlioglu et al. 2002), injection of drugs (Loewe 1937, 1938) and flushing of uteri from previously mated females (King et al. 1994). However, none of these procedures has arrived in routine practice, since they are risky and/or stressful for the animals, they are unsuitable as they affect sperm quality or the outcome is not proportional to the effort. There have been multiple studies about electroejaculation in mice confirming its feasibility but reporting several drawbacks, such as lower fertilization rates, lower motility and sperm number in comparison with epididymal sperm, a relatively high mortality rate and the need for specialized equipment (Scott & Dziuk 1959, Snyder 1966, Anderson et al. 1983, Tecirlioglu et al. 2002). Similarly, there are also some concerns regarding drug injection since success rates are inconsistent and negative side effects can occur (Loewe 1937, 1938). To collect sperm from female uterus horns post ejaculation has also proven unsatisfactory, as this collection method adds additional variation to the sample and requires euthanizing the female for harvesting the ejaculated sample. In addition, this method requires a functional sex drive in males, causing a problem in some strains. Furthermore, some studies have demonstrated lower developmental rates in embryos, presumably due to increased nuclease activity in capacitated sperm (Yamauchi & Ward 2007).
A silver lining in the area of non-terminal sperm collection techniques in mice is the microsurgical epididymal sperm aspiration (MESA) (Moreno del Val & Muñoz-Robledano 2013) and the percutaneous epididymal sperm aspiration (PESA) (Boersma et al. 2015). These minimal invasive procedures were originally developed in human medicine to overcome infertility problems in cases of obstructive and non-obstructive azoospermia (Craft et al. 1995, Collins et al. 1996, Meniru et al. 1998, Glina et al. 2003, Esteves et al. 2011, Jensen et al. 2016, Hao et al. 2017, Bromage et al. 2008). MESA allows in vivo serial sampling of epididymal sperm by making a small scrotal incision to puncture the caudaepididymidis under eyesight, whereas in PESA, the caudaepididymidis is punctured through the skin of the scrotum. Thus, PESA is a refined procedure of MESA. In addition, PESA have been shown to retrieve enough sperm of sufficient quality to successfully perform IVF (Boersma et al. 2015). Besides the numerous studies that have been conducted in humans, there is also a literature confirming the efficiency of PESA as an acceptable sperm retrieval technique in other mammals like dogs (Varesi et al. 2013).
It has been shown that in vivo serial sampling of mice via PESA is possible and that the procedure does not impair male fecundity (Moreno del Val & Muñoz-Robledano 2013, Boersma et al. 2015). However, there are no studies to our knowledge, which have investigated the suitability of PESA to collect sperm for sperm quality assessment. In rats, repeated PESA can cause significant epididymal inflammatory changes as well as reduced sperm concentration and motility (Saade et al. 2008, Zhang et al. 2014). Although the number of performed PESAs did not correlate with the severity of inflammation or the reduced sperm motility, these studies show that the procedure can be associated with negative side effects. In mice, no inflammatory reactions were observed after a single PESA (Boersma et al. 2015), though the long-term effects of repeated sperm collection by PESA are unknown.
Here, we aimed to test whether PESA can be applied as a suitable method to collect sperm for quality assessment in mice. We collected sperm samples by PESA and by the standard sperm retrieval method, which is the dissection of the caudaepididymidis, and compared sperm motility, swimming velocity and morphology traits between samples. Given that PESA samples have been successfully used for IVFs, we assume that PESA will provide sperm samples that are representative of the overall sperm population of a male. If PESA proves suitable as a sperm collection method to assess sperm quality, this procedure would find broad applicability in various research fields from reproductive biology to behavioral ecology.
Materials and methods
Animals and husbandry
We used ten sexually mature, male RjOrl:SWISS mice. We decided to use an outbred stain since outbred mice have good fertility and are expected to have greater variation in their sperm quality parameters. Animals originated from the institute’s own SPF breeding colony and stock founders were purchased from Janvier Labs, France. Experimental mice were surplus males from the breeding colony and were 15 weeks old with a body mass ranging from 38 to 49 g when used for the experiment.
Prior to the experiment, mice were kept individually in open-top Makrolon type II cages (252 × 167 mm, Tecniplast, Buguggiate, Italy) equipped with soiled bedding (Lignocel®, heat treated, Rettenmaier KG, Vienna, Austria) and enriched with cellulose swabs (Pur-Zellin 4 × 5 cm; Paul Hartmann GmbH, Wiener Neudorf, Austria) as nesting material. Males were kept under optimal hygienic conditions with a light/darkness photoperiod of 12 h (lights on at 03:00 am). The room temperature was maintained at 20°C ± 1°C and relative humidity was constantly held at 55% ± 10%. Water and food (ssniff® M-Z Extrudate, Spezialdiäten GmbH, Soest, Germany) were provided ad libitum. Previous studies in mice have shown that males can differ in their sperm quality depending on their social status (Koyama & Kamimura 1999). Single housing avoided the establishment of dominance relationships between males, and thus any a priori differences in sperm quality between males.
This study has been performed in accordance with the Good Scientific Practice guidelines (01.07.2019) of the University of Veterinary Medicine, Vienna, Austria and national legislation. No ethical board decision was required for this study since all experimental procedures were conducted post mortem, and no animals were bred or purchased for the purpose of this study.
Study design
To test whether PESA is a suitable method to collect sperm for quality assessment, we compared sperm traits of samples obtained by PESA to those obtained by TCED. We applied a within-subject design and collected four samples from each of the ten males, two per epididymis side. First, we collected sperm by PESA from the right and left caudaepididymidis. Immediately afterward, we dissected both epididymides and collected separate sperm samples from the right and left caudaepididymidis. To rule out any side bias, the side of the first collection was randomized for both PESA and TCED. We collected PESA and TCED samples sequentially to be able to detect side differences in sperm traits.
PESA is usually performed in anesthetized males. However, we performed both PESA and TCED sample collections post mortem, since no in vivo sampling is required to test the suitability of PESA for sperm quality assessment in mice, and we thereby avoided unnecessary handling and stress related to the administration of the anesthesia for the animals.
Percutaneous epididymal sperm aspiration (PESA)
For sample collection, each mouse was placed in dorsal recumbency after cervical dislocation and the abdomen was gently massaged downward to ensure that the testes were located in the scrotum. The abdomen and scrotum were then rinsed with distilled water to improve visualization of the caudaepididymidis and to avoid contamination with loose hair.
The caudaeepididymidis were then visually located at the base of the scrotum. The caudaepididymidis normally appears to be right next to the scrotal raphe on the medial apical side of the scrotum. It presents itself as a pinhead-sized circular light yellow-greyish tissue with a visible curly structure, the ductusepididymidis, which has to be distinguished from the similar-looking circumjacent fatty tissue. Once located, the caudaepididymidis was fixed using a curved forceps. Therefore, the scrotal skin between testis and epididymis was carefully squeezed by forceps to separate the epididymal tissue from the testis and to restrain the tissue (Fig. 1). We then used a 30-gauge (0.3 mm × 8 mm) needle on an insulin syringe (Omnican 20, B Braun, Melsungen, Germany), containing 0.05 mL of incubator pre-warmed TYH medium (for medium composition see Guan et al. 2014). A gentle resistance was perceived once the epididymal tissue was punctured with the needle. Negative pressure was applied before the needle was retrieved. The content of the syringe was then transferred onto a culture dish and examined for the presence of sperm under a stereomicroscope. On average, 2.35 punctures were performed to successfully locate sperm (a maximum of four punctures was once required).
Figure 1.
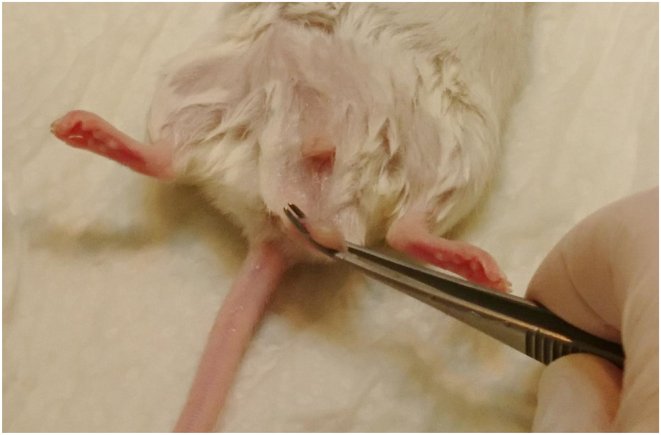
Picture of a fixated cauda epididymidis in a male RjOrl:SWISS mouse. For PESA, the testis was moved to the scrotum. The cauda epididymidis was then visually identified and fixated with curved forceps by applying gentle pressure. Sperm aspiration was subsequently performed by puncturing the cauda epididymidis with a 30G needle on an insulin syringe.
The successfully collected sperm samples were then transferred into a 35 mm culture dish containing a 100 µL drop of TYH covered with oil before incubation at 37°C under 5% CO2 for 30 min until the first sperm analysis was performed. The contralateral caudaepididymidis was subsequently sampled in exactly the same manner.
Dissection of the cauda epididymidis (TCED)
After performing a bilateral PESA, we opened the abdominal cavity and dissected both caudaeepididymidis. We made a horizontal cut on the lower third of the abdomen with surgical scissors. The testes were then pulled out of the scrotum in a cranial direction. The dissection was performed carefully to prevent damaging any tissue or to accidentally squeezing the epididymis. The caudaepididymidis was extracted using scissors and forceps and surrounding fat tissue was removed.
The isolated caudaepididymidis was then transferred into a pre-warmed 200 µL drop of TYH in a 35 mm culture dish covered with mineral oil and the second epididymis was prepared. The isolated epididymal tails were then cut four to six times with a Vannas-Tübingen spring scissors (Fine Science Tools, Heidelberg, Germany) before the dish was incubated at 37°C under 5% CO2 for 10 min to allow the sperm to swim out. Subsequently, the epididymal tissue was removed, and 10 µL of the sperm suspension were transferred into a 190 µL drop of TYH covered with mineral oil (1:20 dilution). The diluted sperm sample was then incubated for another 20 min at 37°C and 5% CO2 before sperm analyses were performed. The dilution was required to gain an appropriate sperm density for the analyses.
Sperm analysis
Sperm motility and velocity
We used computer-assisted sperm analysis (CASA) to assess various sperm motility parameters. The CASA system (SCA®, Sperm Class Analyzer, Microptics, Spain) consists of a high-resolution camera (Basler acA1300-200uc, Basler, Germany) connected to a phase-contrast microscope (Nikon Eclipse E200, Nikon) using a 10× objective (Nikon 10×/0.25 Ph1 BM, Nikon) under negative phase contrast to track and record individual sperm. After gently shaking the incubated sperm samples on a heating plate (37°C) for 1 min for homogenization, we transferred 3 µL on an imaging slide (Leja Products B.V., Nieuw-Vennep, Netherlands). Ten videos were then recorded (with a frame rate of 50 frames per second, 25 images) at different sites evenly distributed throughout the slide to ensure an objective sample estimate.
We used the SCA® software (Version 6.5.0.15) to determine the following sperm parameters: sperm motility (percentage of sperm moving faster than 10 µm/s), curvilinear velocity (VCL in µm/s, velocity along the actual sperm path), straight-line velocity (VSL in µm/s, velocity along a straight line between the first and last position of a sperm track) and average path velocity (VAP in µm/s, time-averaged velocity of a sperm head along its average path), path linearity (LIN in %, linearity of the actual sperm track), path wobble (WOB in %, departure of actual sperm track from average path), path straightness (STR in %, linearity of the average path), average lateral head displacement (ALH in µm) and beat-cross frequency (BCF in Hz). We visually inspected all recorded videos from each sample and performed manual corrections when incorrect classifications were observed. Corrections were performed blind to sample origin.
All samples were evaluated twice; the first analysis was performed after sperm collection (T0) to assess fresh sperm quality traits and the second analysis was performed after 2 h of incubation (T1) to assess the decline in sperm quality traits as an estimate for sperm longevity.
Sperm morphology
We further assessed sperm morphology to determine whether and how the sperm collection method affects sperm morphology traits. Therefore, air-dried smears of a 15 µL aliquot of each sperm sample were fixed on slides and stained with SpermBlue (Microptic S.L., Barcelona, Spain) according to instructions of the manufacturer. Stained samples were then analyzed using a 60× objective (Nikon Eclipse E200 microscope). Observers blinded for the sperm collection method inspected 50 spermatozoa per sample and determined the frequency and type of morphological abnormalities. Multiple abnormalities within one spermatozoon were counted individually. An overview of all observed morphological abnormalities is provided in Fig. 2.
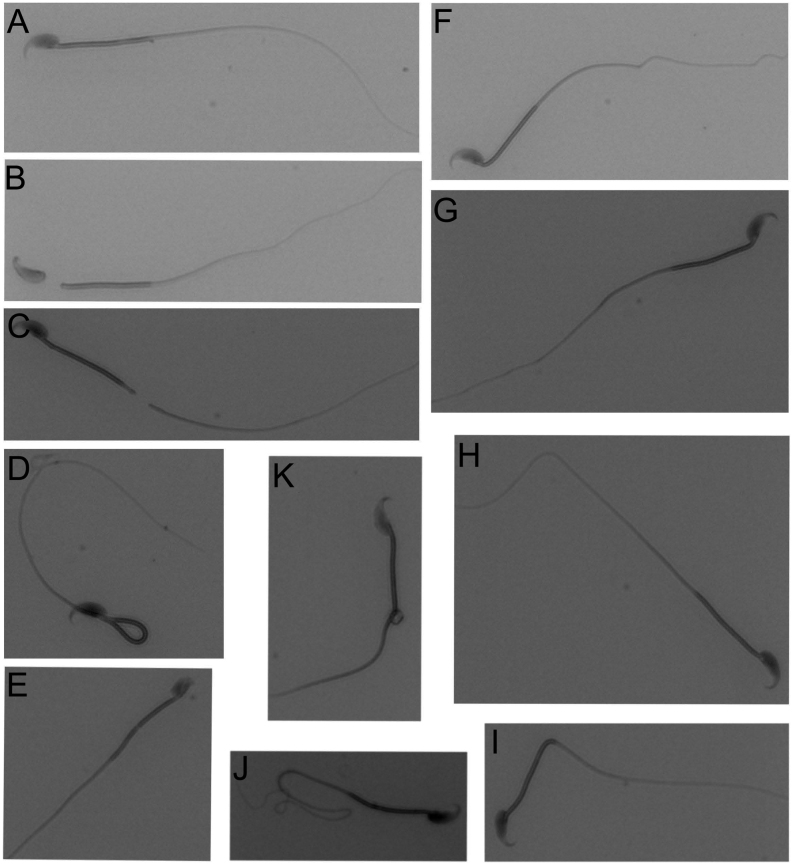
Figure 2.
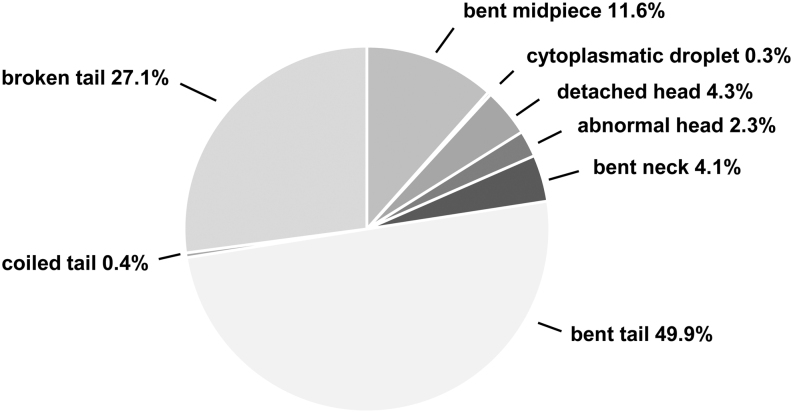
Pictures of morphological abnormalities observed in spermatozoa from RjOrl:SWISS mice collected by PESA and TCED. (A) Normal shaped sperm for comparison, (B) sperm with detached head, (C) sperm with broken tail, (D) sperm with bent midpiece, (E) sperm with abnormal head shape, (F) sperm with bent neck and tail, (G) sperm with bent neck, (H) sperm with bent tail, (I) sperm with bent midpiece, (J) sperm with coiled tail and (K) sperm with cytoplasmatic droplet.
Statistical analysis
We used SPSS for Windows (IBM SPSS, Version 28.0 Inc.) for statistical analyses. To test for differences in sperm collected by PESA vs TCED, we performed repeated measures ANOVAs on the following sperm parameters: sperm motility, VCL, VSL, VAP, LIN, STR, WOB, ALH, BCF, all measured at T0 and T1, and the sum of observed morphological abnormalities. We always included collection side as fixed factor to test for sperm differences between collection sides. The probability value used to identify significance was set at P < 0.05.
Results
Sperm motility and velocity
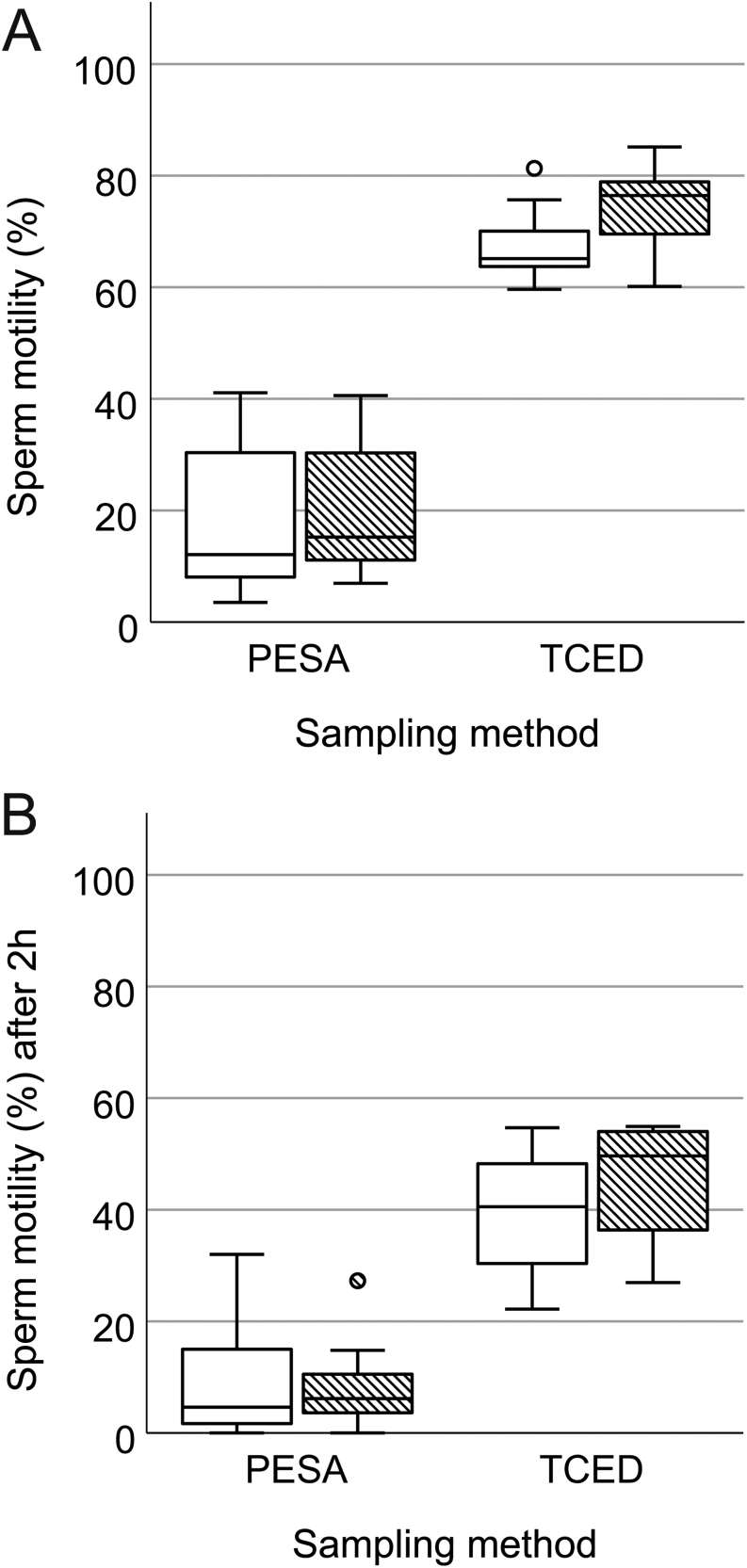
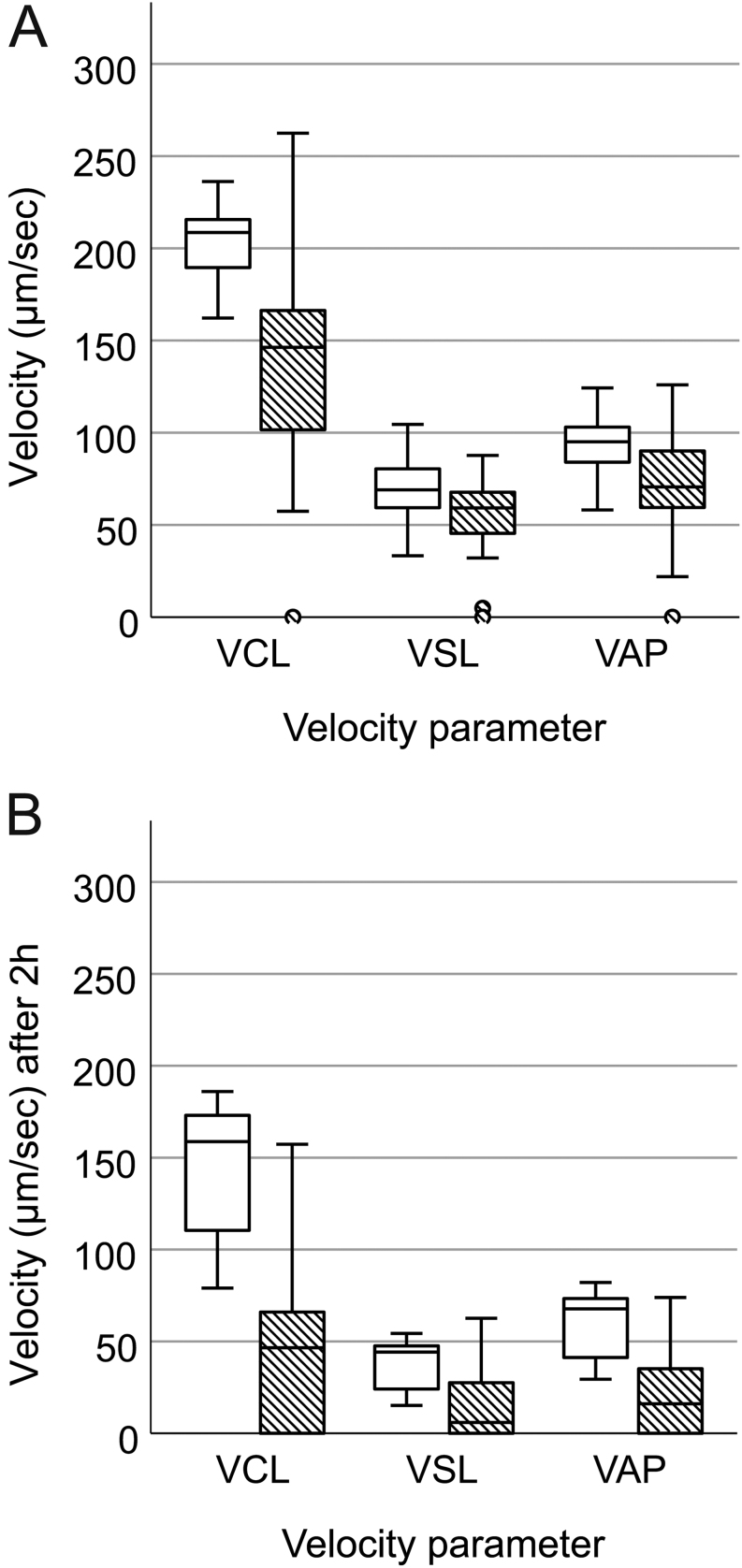
We were able to retrieve motile sperm from all ten mice by applying PESA and TCED. However, PESA samples showed a significantly lower percentage of motile sperm (F = 227.46, P < 0.001, Fig. 3A) and reduced VCL (F = 14.32, P = 0.001, Fig. 4A), VSL (F = 7.99, P = 0.011, Fig. 4A) and VAP (F = 7.92, P = 0.011, Fig. 4A) compared to TCED samples (Table 1). In addition, sperm ALH (F = 9.23, P = 0.007) was significantly lower in PESA compared to TCED samples. Other measured sperm kinematic parameters like STR (F = 0.80, P = 0.383), LIN (F = 1.57, P = 0.226), WOB (F = 1.50, P = 0.237) and BCF (F = 3.33, P = 0.085) did not differ between PESA and TCED samples (Table 1). In addition, we did not find a difference in any of the recorded sperm parameters between collection sides: Sperm motility (F = 2.52, P = 0.130), VCL (F = 0.13, P = 0.723), VSL (F = 0.30, P = 0.591), VAP (F = 0.040, P = 0.844), STR (F = 0.04, P = 0.838), LIN (F = 0.49, P = 0.494), WOB (F = 0.09, P = 0.770), ALH (F = 0.46, P = 0.505) and BCF (F = 0.52, P = 0.481).
Figure 3.
Boxplot of sperm motility (%) in PESA and TCED samples collected from RjOrl:SWISS mice and assessed (A) after sperm collection (T0) and (B) after 2 h of incubation (T1). White bars show data from left epididymal samples and patterned bars from right epididymal samples. Circles (○) refer to mild outliers (Q3 + 1.5 × IQR).
Figure 4.
Sperm swimming velocities in PESA (patterned bars) and TCED (white bars) samples collected from RjOrl:SWISS mice. Sperm curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s) and average path velocity (VAP, µm/s) assessed (A) after sperm collection (T0) and (B) after 2 h of incubation (T1). Circles (○) refer to mild outliers (Q1 + 1.5 × IQR).
Table 1.
Comparison of mouse sperm traits obtained by left- and right-sided PESA and TCED in RjOrl:SWISS mice. Sperm traits were assessed after sample collection (T0) and after 2 h of incubation (T1). Data are presented as mean ± s.d.
| Mouse sperm traits | PESA | TCED | ||
|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |
| Sperm motility, % | 18.9 ± 12.4 | 8.9 ± 9.2 | 71.0 ± 7.8 | 42.6 ± 10.6 |
| Sperm curvilinear velocity, µm/s | 143.4 ± 64.1 | 45.1 ± 51.0 | 203.7 ± 18.9 | 144.6 ± 34.5 |
| Sperm average path velocity, µm/s | 72.7 ± 30.0 | 20.8 ± 24.3 | 94.2 ± 14.7 | 58.8 ± 18.7 |
| Sperm straight line velocity, µm/s | 54.7 ± 23.3 | 13.8 ± 17.9 | 69.9 ± 15.8 | 37.6 ± 13.8 |
| Path straightness, % | 65.1 ± 21.2 | 32.4 ± 34.0 | 69.0 ± 6.1 | 57.6 ± 5.0 |
| Path linearity, % | 37.7 ± 16.9 | 16.1 ± 19.9 | 33.7 ± 5.4 | 25.4 ± 4.6 |
| Path wobble, % | 50.7 ± 16.7 | 25.0 ± 25.6 | 46.3 ± 4.0 | 41.0 ± 4.7 |
| Average lateral head displacement, µm | 4.7 ± 2.2 | 1.7 ± 1.8 | 6.4 ± 0.6 | 4.9 ± 1.2 |
| Beat cross frequency, Hz | 15.4 ± 4.1 | 7.9 ± 7.8 | 17.1 ± 1.1 | 16.9 ± 1.3 |
After 2 h of incubation, sperm motility (F = 89.01, P < 0.001, Fig. 3B), VCL (F = 52.47, P < 0.001, Fig. 4B), VSL (F = 26.80, P < 0.001, Fig. 4B) and VAP (F = 32.38, P < 0.001, Fig. 4B) were significantly lower in PESA compared to TCED samples. In addition, all assessed sperm kinematic parameters were significantly reduced in PESA compared to TCED samples: STR (F = 11.73, P = 0.003), LIN (F = 4.51, P = 0.048), WOB (F = 7.39, P = 0.014), ALH (F = 42.78, P < 0.001) and BCF (F = 25.96, P < 0.001). Again, no differences in the recorded sperm parameters were detected after 2 h of incubation between collection sides: sperm motility (F = 0.44, P = 0.516), VCL (F = 0.03, P = 0.873), VSL (F = 0.19, P = 0.667), VAP (F = 0.04, P = 0.851), STR (F = 0.34, P = 0.566), LIN (F = 0.27, P = 0.611), WOB (F = 0.30, P = 0.592), ALH (F = 0.19, P = 0.665) and BCF (F = 0.15, P = 0.708).
Finally, we did not find any relationship between sperm traits measured after TCED and PESA, neither after sample collection nor after 2 h of incubation (Table 2).
Table 2.
Relationship between mouse sperm traits in samples collected by PESA vs TCED in RjOrl:SWISS mice. Sperm traits were assessed after sample collection (T0) and after 2 h of incubation (T1). Depending on the data distribution, Pearson or Spearman correlation coefficients and respective P values are provided.
| Mouse sperm traits | T0 | T1 | |||
|---|---|---|---|---|---|
| ρ | r | P | ρ | P | |
| Sperm motility, % | −0.111 | 0.640 | −0.139 | 0.558 | |
| Sperm curvilinear velocity, µm/s | −0.189 | 0.424 | −0.013 | 0.958 | |
| Sperm average path velocity, µm/s | 0.009 | 0.971 | 0.054 | 0.822 | |
| Sperm straight line velocity, µm/s | 0.325 | 0.163 | 0.158 | 0.507 | |
| Path straightness, % | 0.808 | <0.001 | 0.393 | 0.087 | |
| Path linearity, % | −0.582 | 0.007 | 0.221 | 0.350 | |
| Path wobble, % | −0.111 | 0.640 | 0.006 | 0.979 | |
| Average lateral head displacement, µm | 0.368 | 0.111 | 0.079 | 0.741 | |
| Beat cross frequency, Hz | −0.257 | 0.274 | 0.244 | 0.299 | |
Sperm morphology
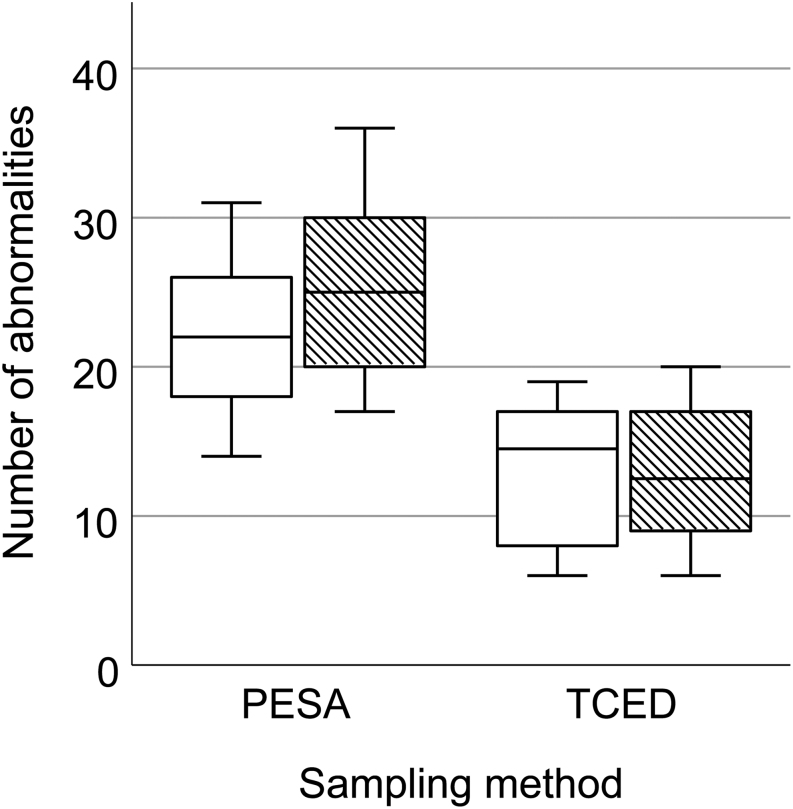
A total of 2000 spermatozoa (1000 per sampling method) were screened for morphological abnormalities, and we found that the number of morphological abnormalities was significantly higher in PESA compared to TCED samples (F = 31.30, P < 0.001, Fig. 5). On average, 45.9% of spermatozoa in PESA samples and 24.5% of spermatozoa in TCED samples showed morphological abnormalities. The total number of observed morphological abnormalities was 471 and 256, respectively. Interestingly, the sampling procedure did not seem to affect the prevalence of specific morphological abnormalities, as we did not find major differences in the relative occurrence of specific abnormalities (Table 3). Across all samples, morphological abnormalities occurred mainly at the tail, followed by the midpiece and the head (Fig. 6). Overall, 3.2% of abnormal spermatozoa showed multiple abnormalities. No significant differences in morphological abnormalities were found between the sampling sides (F = 1.55, P = 0.229).
Figure 5.
Boxplot of the observed abnormalities in RjOrl:SWISS sperm samples collected by PESA and TCED. White bars show results from left epididymal samples and patterned bars from right epididymal samples.
Table 3.
Relative occurrence (%) of specific morphological abnormalities in mouse spermatozoa collected by PESA and TCED in RjOrl:SWISS mice.
| Abnormalities | PESA | TCED |
|---|---|---|
| Abnormal head | 2.8 | 1.6 |
| Detached head | 5.1 | 2.7 |
| Bent neck | 3.6 | 5.1 |
| Bent midpiece | 12.3 | 10.2 |
| Bent tail | 47.1 | 55.1 |
| Coiled tail | 0.6 | 0.0 |
| Broken tail | 28.2 | 25.0 |
| Cytoplasmatic droplet | 0.2 | 0.4 |
Figure 6.
Classification and frequency distribution of observed morphological sperm abnormalities in RjOrl:SWISS mice. Type and prevalence (%) of observed morphological abnormalities in all sperm samples collected by PESA and TCED (n = 2000).
Discussion
The aim of this study was to evaluate the suitability of PESA to collect sperm for sperm quality assessments. Therefore, we compared the quality of sperm samples retrieved by PESA vs the standard collection procedure TCED. We applied a within-subject design to control for individual differences in sperm quality. In the majority of mice, the caudaepididymidis was located on the medial apical side of the scrotum, with one side being more prominent in size compared to the contralateral organ. The epididymal tail can be described as a pinhead-sized circular light yellow-greyish organ with a visible curly structure embedded in fat tissue. In order to yield sufficient sperm by PESA, training to locate the correct spot to puncture is required, since the caudaepididymidis is not always easy to locate through the scrotal skin, especially in strains with pigmented skin, like C57BL/6. Inaccurate location and aspiration at adjacent tissues lead to samples without sperm or to sperm samples of decreased quality, that is, samples contaminated with blood, fat tissue, immature spermatozoa and other cells. We required on average 2.35 trials per epididymis to retrieve an adequate sperm sample for quality assessment. We pooled two samples to average potentially appearing quality differences in samples related to the site of collection and to ensure that sperm density was high enough for quality assessment. Similar to our study, also others needed multiple scrotal punctures to collect sufficient sperm and failed to retrieve sperm from all animals when the number of scrotal punctures was limited to a maximum of three trials (Boersma et al. 2015).
In rats, repetitive PESA caused significant epididymal inflammatory changes (Saade et al. 2008, Zhang et al. 2014), suggesting that multiple scrotal punctures might be problematic. In human reproductive medicine, PESA has established a reputation for being a safe and fast technique with minimal trauma (Ron-El et al. 1998, Rosenlund et al. 1998). In mice, no histopathologic alterations were detected and in vivo fertility was confirmed after performing PESA once with a maximum number of three epididymal punctures (Boersma et al. 2015). Similarly, in vitro and in vivo fertility was unimpaired after MESA when the procedure was performed twice (Moreno del Val & Muñoz-Robledano 2013). However, further research is necessary to determine the risk of inflammation and traumata after repetitive PESA in mice.
As anticipated, we observed a clear difference in the mean total number of spermatozoa between PESA and TCED, due to the different volumes in sperm samples obtained with both procedures. Furthermore, we found a major drop in sperm motility: The overall mean sperm motility in PESA samples was 19% compared to 71% in TCED samples. Similarly, all measured sperm velocity parameters were significantly reduced in PESA compared to TCED samples, indicating that we were not able to retrieve a qualitatively comparable sample with PESA, or that the PESA procedure itself had a damaging effect on sperm traits. In line with this, none of the recorded sperm parameters were correlated between PESA and TCED samples, once more indicating that our measurements arise from qualitatively distinct sperm populations. The initial difference in sperm motility and swimming velocity between PESA and TCED samples did not disappear after 2 h of incubation, suggesting that sperm traits do not recover from the potentially harmful effects of PESA. In addition, all sperm direction parameters declined more severely in PESA compared to TCED samples during the 2 h of incubation, implying that PESA might even have delayed negative effects on sperm motility parameters.
One possibility of how PESA could affect sperm quality is related to the negative suction pressure during the aspiration. It has been shown that repeated pipetting with a 1 mL pipette significantly affects sperm motility parameters measured by CASA in mice and rats, though this effect was not observed in bulls, boars and rams (Varisli et al. 2009). Rodent sperm is much more sensitive to physical stress factors, like shear and centrifugal forces, likely due to the considerably longer tail of their spermatozoa. To reduce shear forces, several protocols for IVF in mice recommend the use of wide-bore pipette tips for the handling of murine spermatozoa (Marschall et al. 2009, Bath 2010). The comparatively strong suction pressure caused by injection needles with 30G (0.3 mm) in diameter, as used for PESA in our study, could have had a damaging effect on spermatozoa and might explain the overall poor sperm motility and swimming velocity in our PESA samples. We always applied minimum suction pressure to gain epididymal content in our PESA trials. However, to examine how differences in suction pressure affect sperm quality, we performed an additional test (see supplemental material, see section on supplementary materials given at the end of this article). We could confirm that independent of the suction pressure PESA significantly reduced sperm quality compared to TCED, though higher suction pressure was associated with the strongest declines in sperm quality.
We also found that the percentage of morphological abnormalities in PESA samples was about twice as large as in TCED samples (45.9 vs 24.5%). Likewise, and as explained above for motility, the negative suction pressure during aspiration could induce a higher incidence of bent and/or broken necks, midpieces and tails in sperm. Indeed, we found higher incidences of all these abnormalities in PESA compared to TCED samples; however, the relative proportion of specific abnormalities did not differ. While there are many studies showing the influence of genetic and toxicological factors on spermatogenesis and spermiogenesis, respectively, and thereby on sperm morphology, the relationship between the method of sperm collection and sperm morphology has not been well-studied. A mutational disorder of spermatogenesis is most often the cause of severe defects in the sperm head, midpiece or tail morphology (primary abnormalities, for review, see Boer et al. 2015, Beurois et al. 2020). In contrast, suboptimal handling or unfavorable storage conditions are more likely to result in, for example, a bent tail of an otherwise normal sperm and can be considered a secondary abnormality. Severe morphological abnormalities usually lead to fertility disorders, but only few and partially inconclusive data exist for the relationship between secondary abnormalities and fertility. One study found a positive relationship between a proximal bent tail and a light-type cytoplasmic droplet with IVF outcome, while a distal bent tail and heavy-type cytoplasmic droplet were associated with poor IVF rates (Kawai et al. 2006). It is unclear how these morphological saliences affected fertility in vitro. We found that PESA samples – which showed more morphological abnormalities – had reduced motility, providing a potential mechanistic explanation for how secondary abnormalities could impair fertility.
An alternative explanation for the higher number of morphological abnormalities in PESA compared to TCED samples could be a sampling error within the epididymal duct, or by accidentally puncturing wrong sampling sites, for example, the corpus epididymidis or the testis. We can exclude the latter, as a testis puncture would lead to immature and immotile spermatozoa and progenitor cells, which we did not observe in our samples.
Interestingly, no reduction in sperm traits was observed in samples obtained by PESA in dogs (Varesi et al. 2013). This discrepancy in results could on one hand be explained by the significantly longer average tail length in rodent spermatozoa and the larger sensibility of murine compared to the canine spermatozoa (Cummis & Woodall 1985). On the other hand, in the canine study, a 26G cannula for percutaneous puncture was used, which has a larger lumen in contrast to the 30G needle we used for our experiment. The use of larger needles than 30G for PESA in mice would lead to disproportional damage of the epididymal tissue and is not reconcilable with later fertility (Moreno del Val & Muñoz-Robledano 2013). Regardless of the collection method, sperm quality traits and the prevalence of morphological deformities did not differ between the right- and left-sided caudaepididymidis within the same animal. These findings are in line with the study in dogs (Varesi et al. 2013) and confirm that both methods yield reproducible results and that the puncture or dissection of one epididymis is representative of the animal. In mice, PESA has been developed as a non-terminal method to collect spermatozoa for IVF. A mean fertilization rate of 84.4% and no difference in litter size after IVF have been reported with this sperm-collected method (Boersma et al. 2015). Thus, even though PESA seems to negatively affect sperm traits, the sperm quality within a sample proves to be sufficient for successful IVF, most likely as only a small number of vital and progressively motile spermatozoa are required. In addition, recent IVF protocols often include a pre-incubation and selection of progressively motile spermatozoa (Guan et al. 2014). Further research is needed to determine whether PESA samples are suitable for cryopreservation. In most mouse strains, the cryopreservation process in combination with cryo-injuries reduces the percentage of progressively motile sperm to a range of 30–50%, for example, for C57BL/6J from 43% pre-freeze to 25% post-freeze (Sztein et al. 2000). It remains to be tested, whether the reduced level of progressively motile sperm harvested by PESA is sufficient for a successful fertilization after cryopreservation.
In conclusion, we cannot recommend PESA as a suitable method to collect sperm for sperm quality assessment in mice, as the procedure itself seems to affect the collected sperm cells, resulting in diminished sperm quality. Changes to the PESA protocol, which would potentially reduce its negative impact, like the use of needles with a larger diameter than 30G needles, can be considered. Alternatively, MESA might be anticipated, since a high number of sperm cells for quality analysis can be collected without applying a damaging suction pressure (Moreno del Val & Muñoz-Robledano 2023). Even though we cannot recommend the use of PESA to assess sperm quality in mice, the possibility to sample spermatozoa from living animals has great potential for other purposes in research fields investigating reproductive biology, pharmacokinetics, toxicology and mutagenesis in mice. Being the most refined sperm sampling method available today – compared to other methods that imply higher burdens for the animals, like electroejaculation, or require a higher number of animals, like flushing uteri of mated females – PESA can make a valuable contribution to reduce animal numbers in biomedical research.
Supplementary Materials
Declaration of interest
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
KEA and TR designed the study; AB and KEA established the methodology; KEA, AB and LW performed the study; KEA analyzed and visualized the data; KEA, LW and AB wrote the original draft and KEA, AB, MD and TR reviewed and edited the final manuscript.
Acknowledgements
We gratefully acknowledge Jasmin Primus, Lin Hui and Elisabeth Kavan for their support in conducting the study. The graphical abstract was created with BioRender.com.
References
- Anderson RA Oswald C Willis BR & Zaneveld LJD. 1983Relationship between semen characteristics and fertility in electroejaculated mice. Journal of Reproduction and Fertility 681–7. ( 10.1530/jrf.0.0680001) [DOI] [PubMed] [Google Scholar]
- Bath ML.2010Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione. PLoS One 5e9387. ( 10.1371/journal.pone.0009387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R Gertsenstein M Nagy KV & Nagy A. 2014Manipulating the Mouse Embryo: a Laboratory Manual. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Beurois J Cazin C Kherraf Z-E Martinez G Celse T Touré A Arnoult C Ray PF & Coutton C. 2020Genetics of teratozoospermia: back to the head. Best Practice and Research. Clinical Endocrinology and Metabolism 34 101473. ( 10.1016/j.beem.2020.101473) [DOI] [PubMed] [Google Scholar]
- Boer P Vries M & de Ramos L. 2015A mutation study of sperm head shape and motility in the mouse: lessons for the clinic. Andrology 3174–202. ( 10.1111/andr.300) [DOI] [PubMed] [Google Scholar]
- Boersma A Olszanska O Walter I & Ruelicke T. 2015Microsurgical and percutaneous epididymal sperm aspiration for sperm collection from live mice. Journal of the American Association for Laboratory Animal Science 54471–477. [PMC free article] [PubMed] [Google Scholar]
- Bromage SJ Falconer DA Lieberman BA Shafar B & Payne SR. 2008Percutaneous sperm retrieval in secondary azoospermia. Urologia Internationalis 81252–255. ( 10.1159/000151399) [DOI] [PubMed] [Google Scholar]
- Collins GN Critchlow JD Lau MWM & Payne SR. 1996Open versus closed epididymal sperm retrieval in men with secondarily obstructed vasal systems—a preliminary report. British Journal of Urology 78437–439. ( 10.1046/j.1464-410x.1996.00133.x) [DOI] [PubMed] [Google Scholar]
- Craft I Tsirigotis M Bennett V Taranissi M Khalifa Y Hogewind G & Nicholson N. 1995Percutaneous epididymal sperm aspiration and intrazytoplasmic sperm injection in the management of infertility due to obstructive azoospermia. Fertility and Sterility 631038–1042. ( 10.1016/s0015-0282(1657544-x) [DOI] [PubMed] [Google Scholar]
- Cummis JM & Woodall PF. 1985On mammalian sperm dimensions. Journal of Reproduction and Fertility 75153–175. ( 10.1530/jrf.0.0750153) [DOI] [PubMed] [Google Scholar]
- Danshina PV Geyer CB Dai Q Goulding EH Willis WD & Kitto G. 2010Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice. Biology of Reproduction 82136–145. ( 10.1095/biolreprod.109.079699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves SC Miyaoka R & Agarwal A. 2011Sperm retrieval techniques for assisted reproduction. International Braz J Urol 37570–583. ( 10.1590/s1677-55382011000500002) [DOI] [PubMed] [Google Scholar]
- Glina S Fragoso JB Martins FG Soares JB Galuppo AG & Wonchockier R. 2003Percutaneous epididymal sperm aspiration (PESA) in men with obstructive azoospermia. International Braz J Urol 29141–145. ( 10.1590/s1677-55382003000200008) [DOI] [PubMed] [Google Scholar]
- Guan M Bogani D Marschall S Raspa M Takeo T Nakagata N Taft R & Fray M. 2014Contemporary techniques for freezing mouse spermatozoa. Current Protocols in Mouse Biology 485–104. ( 10.1002/9780470942390.mo140065) [DOI] [PubMed] [Google Scholar]
- Hao L Li ZG He HG Zhang ZG Zhang JJ Dong Y Li ZB & Han CH. 2017Application of percutaneous epididymal sperm aspiration in azoospermia. European Review for Medical and Pharmacological Sciences 211032–1035. [PubMed] [Google Scholar]
- Jensen CFS Ohl DA Hiner MR Fode M Shah T Smith GD & Sonksen J. 2016Multiple needle‐pass percutaneous testicular sperm aspiration as first‐line treatment in azoospermic men. Andrology 4257–262. ( 10.1111/andr.12143) [DOI] [PubMed] [Google Scholar]
- Kawai Y Hata T Suzuki O & Matsuda J. 2006The relationship between sperm morphology and in vitro fertilization ability in mice. Journal of Reproduction and Development 52561–568. ( 10.1262/jrd.18023) [DOI] [PubMed] [Google Scholar]
- Khatun A Kang K-H Ryu D-Y & Rahman Md. 2018Effect of Aminopeptidase N on functions and fertility of mouse spermatozoa in vitro. Theriogenology 118182–189. ( 10.1016/j.theriogenology.2018.06.005) [DOI] [PubMed] [Google Scholar]
- King WW Stamant LG & Lee WR. 1994A technique for serial spermatozoa collection in mice. Laboratory Animal Science 44295–296. [PubMed] [Google Scholar]
- Koyama S & Kamimura S. 1999Lowered sperm motility in subordinate social status of mice. Physiology and Behavior 65665–669. ( 10.1016/s0031-9384(9800205-4) [DOI] [PubMed] [Google Scholar]
- Loewe S.1937A pharmacological ejaculation test for bio-assay of male sex hormone. Experimental Biology and Medicine 37483–486. ( 10.3181/00379727-37-9616P) [DOI] [Google Scholar]
- Loewe S.1938Influence of autonomic drugs on ejaculation. Journal of Pharmacology and Experimental Therapeutics 6370–75. [Google Scholar]
- Marschall S Boersma A & Angelis MH. 2009Sperm Cryopreservation and In Vitro Fertilization. Gene Knockout Protocols 2nd ed., pp. 407–420. ( 10.1007/978-1-59745-471-1_22) [DOI] [PubMed] [Google Scholar]
- Meniru GI Gorgy A Batha S Clarke RJ Podsiadly BT & Craft IL. 1998Studies of percutaneous epididymal sperm aspiration (PESA) and intracytoplasmic sperm injection. Human Reproduction Update 457–71. ( 10.1093/humupd/4.1.57) [DOI] [PubMed] [Google Scholar]
- Moreno del Val GM & Robledano PM. 2013In vivo serial sampling of epididymal sperm in mice. Laboratory Animals 47168–174. ( 10.1177/0023677213478411) [DOI] [PubMed] [Google Scholar]
- Moreno del Val G Muñoz-Robledano P Caler AJ & Morante J. 2023A method for multiple sampling mouse sperm in vivo†. Biology of Reproduction 108197–203. ( 10.1093/biolre/ioac194) [DOI] [PubMed] [Google Scholar]
- Nakagata N.2000Cryopreservation of mouse spermatozoa. Mammalian Genome 11572–576. ( 10.1007/s003350010109) [DOI] [PubMed] [Google Scholar]
- Ozkosem B Feinstein SI Fisher AB & O’Flaherty C. 2015Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biology 515–23. ( 10.1016/j.redox.2015.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm SA & Stockley P. 2009Adaptive plasticity of mammalian sperm production in response to social experience. Proceedings. Biological Sciences 276745–751. ( 10.1098/rspb.2008.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron-El R Strauss S Friedler S Strassburger D Komarovsky D & Raziel A. 1998Serial sonography and colour flow Doppler imaging following testicular and epididymal sperm extraction. Human Reproduction 133390–3393. ( 10.1093/humrep/13.12.3390) [DOI] [PubMed] [Google Scholar]
- Rosenlund B Westlander G Wood M Lundin K Reismer E & Hillensjö T. 1998Sperm retrieval and fertilization in repeated percutaneous epididymal sperm aspiration. Human Reproduction 132805–2807. ( 10.1093/humrep/13.10.2805) [DOI] [PubMed] [Google Scholar]
- Russell WMS & Burch RL. 1992The Principles of Humane Experimental Technique. Wheathampstead,UK: Universities Federation for Animal Welfare. [Google Scholar]
- Saade RD Neves PA Glina S D’Ancona CAL Dambros M & Lúcio MA. 2008Quantitative (stereological) and qualitative study of rat epididymis after vasectomy and percutaneous epididymal sperm aspiration. Journal of Urology 179381–384. ( 10.1016/j.juro.2007.08.095) [DOI] [PubMed] [Google Scholar]
- Scott JV & Dziuk PJ. 1959Evaluation of the electroejaculation technique and the spermatozoa thus obtained from rats, mice and guinea pigs. Anatomical Record 133655–664. ( 10.1002/ar.1091330406) [DOI] [Google Scholar]
- Snyder RL.1966Collection of mouse semen by electroejaculation. Anatomical Record 15511–14. ( 10.1002/ar.1091550103) [DOI] [PubMed] [Google Scholar]
- Sztein JM Farley JS & Mobraaten LE. 2000In vitro fertilization with cryopreserved inbred mouse sperm. Biology of Reproduction 631774–1780. ( 10.1095/biolreprod63.6.1774) [DOI] [PubMed] [Google Scholar]
- Taft R.2017In vitro fertilization in mice. Cold Spring Harbor Protocols 2017pdb.prot094508. ( 10.1101/pdb.prot094508) [DOI] [PubMed] [Google Scholar]
- Tecirlioglu RT Hayes ES & Trounson AO. 2002Semen collection from mice: electroejaculation. Reproduction, Fertility, and Development 14363–371. ( 10.1071/rd02015) [DOI] [PubMed] [Google Scholar]
- Varesi S Vernocchi V Faustini M & Luvoni GC. 2013Quality of canine spermatozoa retrieved by percutaneous epididymal sperm aspiration. Journal of Small Animal Practice 5487–91. ( 10.1111/jsap.12020) [DOI] [PubMed] [Google Scholar]
- Varisli O Uguz C Agca C & Agca Y. 2009Various physical stress factors on rat sperm motility, integrity of acrosome, and plasma membrane. Journal of Andrology 3075–86. ( 10.2164/jandrol.107.004333) [DOI] [PubMed] [Google Scholar]
- Wilson S & Sheardown SA. 2011Identification of Germline Competent Chimaeras by Copulatory Plug Genotyping. Transgenic Research 20429–433. ( 10.1007/s11248-010-9413-6) [DOI] [PubMed] [Google Scholar]
- Yamauchi Y & Ward MA. 2007Preservation of ejaculated mouse spermatozoa from fertile C57BL/6 and infertile Hook1/Hook1 mice collected from the uteri of mated females. Biology of Reproduction 761002–1008. ( 10.1095/biolreprod.106.059881) [DOI] [PubMed] [Google Scholar]
- Zhang Y Chohan KR Landas SK Reeder JE & Trussell JC. 2014Repetitive percutaneous epididymal sperm aspirations (PESA's) resulted in asthenospermia and significant inflammation. Canadian Journal of Urology 217475–7478. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a