Abstract
Objective:
Existing ultra-high dose rate (UHDR) electron sources lack dose rate independent dosimeters and a calibrated dose control system for accurate delivery. In this study, we aim to develop a custom single-pulse dose monitoring and a real-time dose-based control system for a FLASH enabled clinical linear accelerator (Linac).
Approach:
A commercially available point scintillator detector was coupled to a gated integrating amplifier and a real-time controller for dose monitoring and feedback control loop. The controller was programmed to integrate dose for each radiation pulse and stop the radiation beam when the prescribed dose was delivered. Additionally, the scintillator was mounted in a solid water phantom and placed underneath mice skin for in vivo dose monitoring. The scintillator was characterized in terms of its radiation stability, mean dose-rate (Ḋm), and dose per pulse (Dp) dependence.
Main Results:
The Dp exhibited a consistent ramp-up period across ~4–5 pulse. The plastic scintillator was shown to be linear with Ḋm (40–380 Gy/s) and Dp (0.3–1.3 Gy/Pulse) to within +/− 3%. However, the plastic scintillator was subject to significant radiation damage (16%/kGy) for the initial 1 kGy and would need to be calibrated frequently. Pulse-counting control was accurately implemented with one-to-one correspondence between the intended and the actual delivered pulses. The dose-based control was sufficient to gate on any pulse of the Linac. In-vivo dosimetry monitoring with a 1 cm circular cut-out revealed that during the ramp-up period, the average Dp was ~0.045 ± 0.004 Gy/Pulse, whereas after the ramp-up it stabilized at 0.65 ±0.01 Gy/Pulse.
Significance:
The tools presented in this study can be used to determine the beam parameter space pertinent to the FLASH effect. Additionally, this study is the first instance of real-time dose-based control for a modified Linac at ultra-high dose rates, which provides insight into the tool required for future clinical translation of FLASH-RT.
Keywords: Scintillator, Cherenkov, FLASH, Pulse Resolved Dosimetry, In-vivo Dosimetry
1. Introduction:
FLASH is a novel ultra-high dose-rate (UDHR) radiation therapy technique, which has gained significant interest due to its ability to spare some normal tissues as compared to conventional forms of radiotherapy1 while still providing effective tumor control. The FLASH effect has now been observed in multiple animal and organ2–4 models. Moreover, the first human patient was treated using FLASH5 and a clinical human trial is currently in progress6. One of the factors hindering widespread pre-clinical and clinical adaption of FLASH is the issue of accurate real-time beam monitoring and beam control systems for user-modified clinical linear accelerator (linac) which can deliver dose at UHDR7–12. It is critical that the pre-clinical studies being performed have accurate dosimetry, because the radiobiological underpinnings of FLASH are not currently well understood and accurate dosimetry will be needed to build this understanding.
There is an emergence of user-modified clinical accelerators which enable delivery of beam in the UHDR regime (i.e. > 40 Gy/s)13–15. At our institution, a clinical linac was modified by disabling feedback mechanisms and retracting the target to deliver a 10 MeV UHDR beam, with a dose per pulse of 1 Gy at iso-center15. Conventional linear accelerators rely on ionization chambers for the purpose of real-time beam monitoring and feedback based on beam flatness, symmetry, dose-rate and total delivered dose. However, ionization chambers are known to be dependent on dose-rate due to ion-recombination issues at high instantaneous dose-rates, although ion recombination models and appropriately designed ionization (electrode spacing ~0.25mm) have been suggested which can result in negligible ion recombination at 5 Gy/ pulse.16–19 Due to this dose rate dependence, most of the aforementioned feedback systems were disabled to realize the FLASH beam. Even if the internal linac feedback systems were to be used, they lack the ability to offer single pulse temporal resolution, which is critical for accurate FLASH studies.
Moreover, the FLASH effect has been said to be highly dependent on the temporal structure of the beam. Quantities such as the repetition rate and dose per pulse play a crucial role in successful elicitation of the FLASH effect and therefore by measuring these quantities for each beam delivery, the FLASH effect can be understood in terms of its relation to the temporal structure of the beam. Unfortunately, most FLASH studies use passive dosimeters such as radiochromic film, alanine rods and thermoluminscent dosimeters (TLD)10,11 which can only report integrated dose.
For beam control purpose, the popular method has been to use external pulse counting circuitry as a beam-stop for the linac. The current beam control system at our institution relies on a remote trigger unit (RTU) interfaced with an Arduino Controller and a relay which sends a beam gating signal to Varian’s real-time position management (RPM) gating box. The RTU consists of two Bismuth Germanium Oxide (BGO) scintillators coupled with silicon photomultiplier (SiPM) detectors which are used to detect stray radiation inside the room and produce a TTL output only where there is a radiation beam present20. The two-scintillator configuration is used for coincidence detection to minimize detection of spurious pulses. Spurious RTU pulses here refer to pulses that are not representative of the electron pulse produced from the linac but are still registered as valid pulses by the pulse counting circuity. These spurious pulses are primarily due to scintillator afterglow, and/or the neutron activated products that decay at their own rate.
With external pulse counting circuitry, there is significant uncertainty in dose delivery because a ramp-up period is observed for our accelerator where the first 4–5 pulses underdose significantly. The ramp-up period can itself be variable in terms of how many pulses are required to achieve beam stability. The ramp-up is perhaps attributable to the fact the automatic frequency control (AFC) is delayed by ~ 36 ms after beam-on for Varian’s C-series series linacs. It should be noted this is simply a byproduct of Varian’s implementation of the AFC, and the presence and extent of the ramp-up might differ for other linacs. Nonetheless, with a dose per pulse near 1 Gy, any variability in the ramp-up period results in significant uncertainty in the dose delivery for low doses.
Real-time monitoring of beam dose rate is required for accurate control of radiotherapy beamlines, including ultra-high dose rate (UHDR) electron linacs. In high energy physics, beam current transformers21, wakefield monitors22 and beam induced fluorescence monitors23 are typically used to measure ultra-high dose rate charged particle beams in real time. However, these techniques are mostly limited to research beamlines where the user has access to the accelerating waveguide, and other internal structures where the current monitoring device can be placed easily. Field size requirements also preclude the use of beam current monitors24 for large field sizes, especially if the beam monitor is meant to be placed at the exit window. For UHDR-converted clinical linacs used for pre-clinical and translational clinical studies, an ideal dosimeter should be small enough to be used in-vivo, dose-rate independent, offer per pulse resolution, and be readily available to the medical physics community. Ionization chambers and diodes with zero applied bias are commonly used in radiation dosimetry, but suffer from non-linear response at high doses per pulse10,11,25,26. Other commonly available dose-rate independent detectors, such as radiochromic film, optically stimulated luminescence dosimeters (OSLD), TLD and alanine rods do not offer any temporal resolution. Luminescence based dosimeters can potentially be a viable choice because of their high spatio-temporal resolution (~ ns and ~1 mm, respectively), dose linearity (above ~ 100 keV) and dose-rate linearity27. Therefore, for this study a commercially available plastic scintillator detector (PSD) was used and characterized at FLASH dose-rates. The commercially available PSD, Exradin W1 (Standard Imaging, Middleton, WI) has a diameter of 1.0 mm and is 3 mm long with a physical density of 1.05 g/cm3. The detector itself is packaged in a housing which has a 2.8 mm diameter and is 42 mm long. The scintillator system is compared against radiochromic film; a passive detector which has also been established to be dose-rate independent by multiple investigators8,25. This study is motivated by the need to enable accurate dose delivery and monitoring in UHDR operation.
2. Methods and Materials:
2.1. Real-Time Beam Monitoring Hardware
To enable deterministic control of the linac based on dose accumulation and reporting of temporal structure of the beam, a field programmable gate array (FPGA) based embedded controller was used. FPGAs are advantageous for highly deterministic tasks because each independent processing task can be assigned to a dedicated section on the chip and can function independently of other tasks with minimal jitter. Figure 1 depicts the hardware, detectors, and signal routing for pulse-resolved beam monitoring. There are three major components of the hardware solution: 1) A Compact Real-Time Input/Output (CompactRIO NI 9045) controller, 2) External Detectors, and 3) Trigger signals to and from the linac. These components and their interaction are explained next. A real-time FPGA based controller, (CompactRIO, NI-9045) manufactured by National Instruments (Austin, TX) was used for online beam monitoring. The controller consisted of an Kintex-7 70T FPGA board which interfaces directly with input/output (I/O) modules connected to external detectors. The FPGA was programmed through the LabView graphical programming language using the LabView FPGA module. Additionally, included with the controller is a dedicated real-time operating system (RTOS) which was useful for floating point operations and streaming of data, i.e., processes which would otherwise take up significant resources on the FPGA circuit. The RTOS was also programmed via the LabView environment. The RTOS displayed real-time data communicated from the FPGA, mainly information about the dose per pulse and the pulse width. The FPGA portion of the hardware was programmed to accomplish four tasks which were implemented physically on different parts of the FPGA chip, thus ensuring true parallel control. The different input/output modules used to achieve these tasks are given in the supplementary section.
Figure 1.
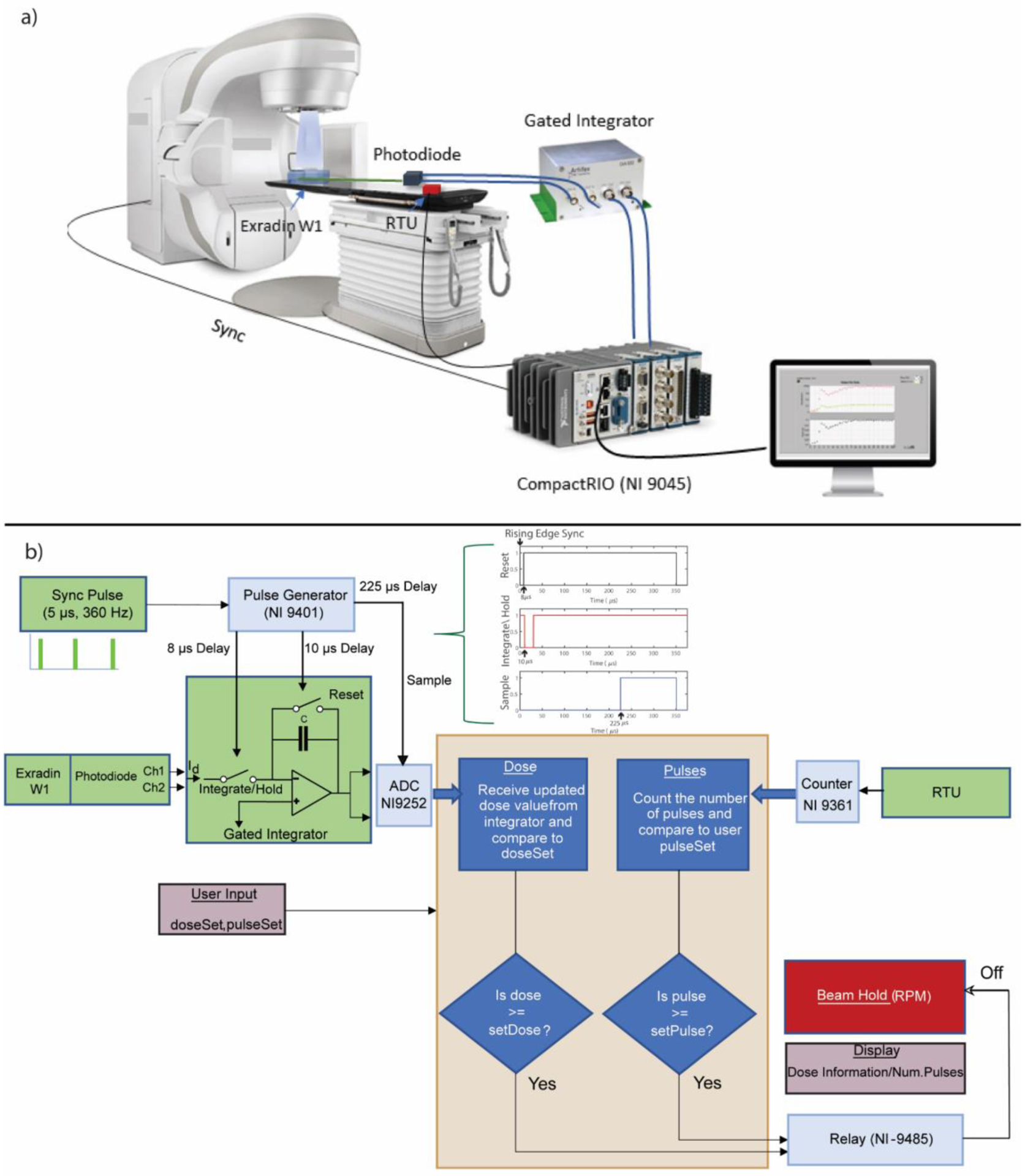
a) Schematic of the hardware and signal routing for real-time beam monitoring. The beam monitoring hardware primarily consisted of a real-time controller, a gated integrator, a plastic scintillator, and a remote trigger unit. b) Shows the control algorithm consisting of redundant dose feedback based on pulse counting and dose accumulation. The signal routing is also depicted here along with the different I/O modules and radiation detectors. The pulse sequence realized via the hardware to integrate dose per pulse is also shown in b). Green colored boxes represent external signals from the linac and detectors, whereas the light blue boxes represent the I/O modules used in this study. The yellow box in the middle denotes the control algorithm used in this study. Boxes with light shade of red denote the information required from the user and displayed after dose delivery.
2.1.1. Synchronization & signal sampling.
The first task implemented on the hardware was to provide signals to the gated integrator which initiated with input from the ‘Sync’ signal from the linac and generated signals for a gated integrator, which is described briefly. The ‘Sync’ signal consisted of a 5 μs pulse with a repetition rate of 360 Hz. The radiation pulse occurred 12 μs after the rising edge of the Sync pulse. Taking these timing considerations into account, the FPGA was programmed to generate reset, integrate/hold, and sample signals for the gated integrator with delays of 8 μs, 10 μs and 225 μs, respectively from the leading edge of the Sync signal, as shown in Figure 1. These timing signals resulted in a dose integration period of ~ 20 μs. The time delays were optimized by generating artificial signals mimicking the linac pulse structure from a function generator and taking into consideration the settling time of the integrator.
2.1.2. Dose integration.
The second task was to perform dose integration per pulse and sample the dose value, as triggered by a signal delayed by 225 μs from the rising edge of the sync pulse and send a beam gating signal to the linac via the relay if the desired dose value is reached. The integration of dose within each pulse is realized via a dual channel gated integrator, GIA100 (Artifex, Emden, Germany). The gated integrator acts as simple integrator (i.e., a capacitor in the feedback loop of an operational amplifier) with the additional ability to gate the integrating process if provided with the appropriate timing signals. A schematic of a generic gated integrator is shown in Figure 1b), where it is connected to the output from the W1’s photodiode. If the reset switch was open and the integrate/hold switch was closed, a current source would charge the capacitor C. Since the inverting input acts as a virtual ground, the output is given by:
After integration, the voltage is held constant for a specified duration and sampled by the ADC until the reset switch is closed, after which the capacitor discharges and is ready to integrate the next pulse. The GIA100 employs a 100 pF capacitor and the nominal switching time is ~ 1 μs. Charge leakage through the feedback capacitor can occur in such a circuit which would lead to an offset voltage. The offset voltages were seen to be small (~ 0.2–0.5%) when compared to the radiation induced signal. Nonetheless, before every experiment, an offset voltage was subtracted by reading the output of the ADC without any beam. The gated integrator essentially replaces the role of the electrometer that would otherwise be typically used with point detectors for signal accumulation in conventional radiotherapy dosimetry. A dual channel gated integrator is required to acquire data in different wavelength bands for removal of the stem effect in the Exradin W1. The Exradin W1 is equipped with a beam splitter and two photodiodes, which acquire signals in two different wavelengths bands that can be used to suppress the Cherenkov stem effect using a chromatic removal technique28,29.
2.1.3. LINAC control.
The third task was to provide control to the linac primarily in terms of dose accumulation, but also pulse counting was to be used as a secondary form of control. The dose accumulation process is described above. Pulse counting was achieved using the RTU as the external detector. The pulse counting and dose counting routines send a beam gating signal to the linac via Varian’s Real-time Position Management (RPM) gating box, if the required number of pulses or dose have been reached. The different I/O modules used in this study to accomplish the aforementioned tasks are described in the supplementary section.
2.1.4. Pulse Counting.
Finally, the fourth task was to detect the radiation pulses from the RTU and report the total number of pulses and pulse width of each pulse to the user.
2.2. Dose Calibration
As mentioned earlier, Cherenkov radiation and/or fluorescence produced in the light guide can be a source of large uncertainty for any fiber optic-based dosimeter. The stem signal is typically proportional to the amount of light guide/fiber optic in the radiation beam. The stem removal in the Exradin W1 is implemented according to technique presented by Fontbonne et al28 and Frelin et al29 and entails measurement of two factors; 1) gain and 2) Cherenkov light ratio (CLR). To determine the calibration coefficients 5 repeated measurements were performed with the W1 placed at iso-center (100 cm SAD) for both the minimum and maximum fiber configuration. A total of 35 linac pulses were delivered to minimize the effect of ramp-up on the final accumulated dose, and the measurements were repeated three times, unless otherwise stated. A 40 × 40 cm2 jaw size was used. Additionally, a 1 cm build up solid water slab was placed on top of the W1. In each case, the total dose delivered was verified using GafChromic film. FilmQA™ (Ashland, Wilmington, DE) software was used, which employs the triple channel dosimetry formalism with non-uniformity correction30, for calibrating and reading-out the films.
2.3. Exradin W1 Characterization
Once the calibration coefficients were obtained, a series of experiments were performed to characterize the W1 under ultra-high dose-rate conditions. Dose verification was performed for each beam delivery using GafChromic film.
2.3.1). Dose-Rate Dependence
The dose-rate dependence of the detector was evaluated by either changing the repetition rate of the linac or changing the source to surface distance (SSD). The former case tests the mean dose-rate dependence, and the latter case tests the instantaneous dose-rate dependence (i.e., dose-rate within the pulse) of the detector. For Varian based linacs, the radiation beam is typically delivered at a repetition rate of 360 Hz (600 MU/min). To achieve lower average dose-rates, pulses are dropped by delaying the gun pulse relative to the accelerating klystron pulse. In this study, repetition rates of ~360 Hz, 240 Hz, 120 Hz and 60 Hz were used to test the mean-dose rate dependence. For the instantaneous dose-rate study, four different SSDs were used: 77 cm, 90 cm, 110 cm, and 130 cm. To note, no beam collimation was used for this study.
2.3.2). Field Size Dependence
For most pre-clinical FLASH studies, small field sizes are typically used for irradiating mice and other small animals. The Exradin W1 has been shown to be an ideal small field dosimeter, requiring minimal correction factors. The purpose of this test was to primarily assess if the Cherenkov discrimination was implemented accurately, since changing field size from the calibration conditions (40 × 40 cm2) to smaller field sizes results in a vastly different amount stem irradiation. Therefore, 4 cm ⌀, 3 cm ⌀, 1.5 cm ⌀ and a 1 cm ⌀ circular beams were delivered to a solid water slab with the W1 at a SAD of 100 cm.
2.3.3). Radiation Damage
It is also critical that radiation damage sustained by the W1 and its effect on the sensitivity of the detector is quantified. The radiation damage response of the W1 was quantified by keeping track of the sensitivity over an extended period and periodically recalibrating the CLR and gain values to quantify the change in sensitivity.
2.3.4). In-Vivo Dosimetry
As an example of the new beam monitoring solution, the hardware presented in this study was used for in-vivo pulse-resolved dosimetry of murine experiments and verified against GafChromic film. All animal procedures were approved by the Dartmouth Institutional Animal Care and Use Committee (IACUC) and the work in this paper followed these approved procedures throughout the study. A small 1 cm ⌀ circular field was used to irradiate 4 different mice around the leg region. Mice were placed on top of the calibration solid water slab provided by Standard Imaging (Figure 2). 40 pulses were delivered such that the final cumulative dose was ~ 20 Gy to the surface of the leg. The W1 was used to measure the exit dose. Additionally, film was placed underneath the mice and on top of the W1 to serve as a reference.
Figure 2.
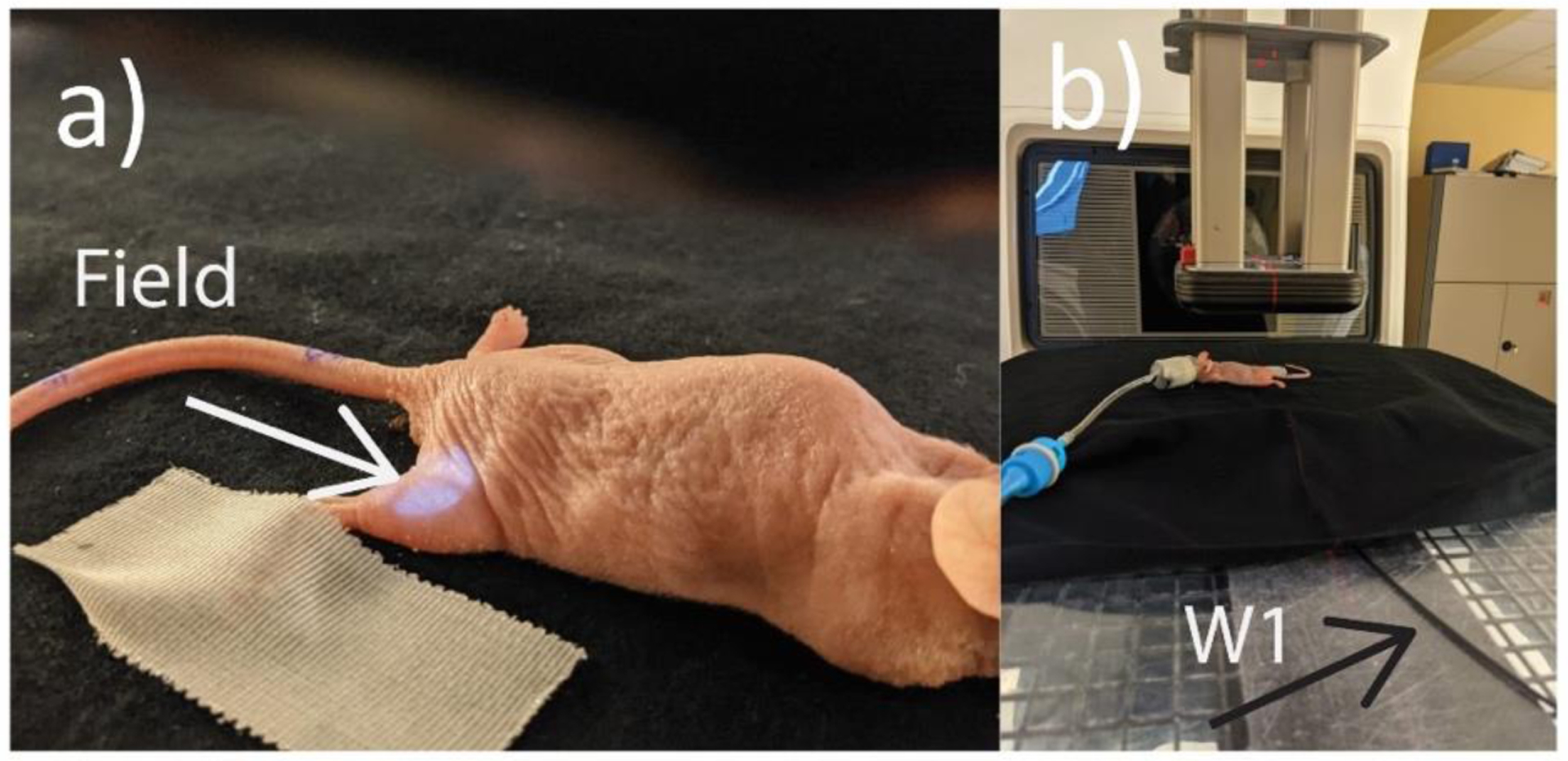
a) and b) In-Vivo Dosimetry Setup for the mice experiments, with the mouse lined up in the beam (as shown by the field light) underneath the applicator.
Only type A uncertainty is considered in this text and therefore standard deviation values based on repeated measures are presented, unless otherwise noted. A rigorous uncertainty analysis would entail accounting for Type B uncertainty to include any systematic errors and any limitations of the individual components used in the control system. However, the purpose of this study is to demonstrate the potential of the system, therefore we only consider type A uncertainty.
3. Results:
3.1. Pulse Counting Feedback
The pulse counting implementation was tested by placing the RTU at different locations from the iso-center and using different field sizes or beam collimation. The issue of spurious pulses is depicted in Figure A1 a-c) in the supplementary section. As beam collimation is increased and the distance of the RTU from the isocenter is decreased the issue of spurious pulses after the main radiation becomes pronounced. Note that moving the RTU closer to the projected field and/or adding beam collimation also results in a larger pulse width than what is expected of a typical linac pulse. This is because of the abundance of stray radiation closer to the source and the decay kinetics of the BGO (~ 300ns decay time + afterglow) scintillator. The pulse counting routine implemented in the FPGA accounts for these spurious pulses by detecting the rising edge of only those peaks which have a minimum pulse width of 1 μs, thereby rejecting the high frequency noise. The one-to-one correspondence between the number of pulses counted by this technique versus an offline comparison with a digital oscilloscope is shown Figure A1d-f) for two different beam collimations and RTU distances in the supplementary section.
The pulse counting feedback loop was tested by disabling the transmission chamber control and the linac was controlled using the pulse counting routine implemented on the hardware. The RTU was placed at 0.5 m from iso-center and a circular cutout of 1 cm was used to maximize the issue of spurious pulses. Two different pulse repetition rates were used (360 Hz and 60 Hz) to verify that the pulse counting feedback was valid over the complete range of repetition rates. The results for 360 Hz and 60 Hz are displayed in Table 1. The experiment was repeated 5 times and number of pulses delivered in each instance is show in the table. The delivered pulses were exactly equal to the intended pulses for a repetition rate of 60 Hz. However, for 360 Hz, one measurement each for the 5 and the 30-pulse delivery, delivered one less pulse (4 and 29). All other measurements had exact one-to-one correspondence. One reason for this discrepancy might be due to uncertainty related to exact time at which the relay switch is closed, and the beam gating signal is sent to the linac. The beam gating latency is also a function of the repetition rate31. This would entail that for each repetition rate, the exact timing of when to close the switch will need to be optimized.
Table 1.
Accuracy assessment of the pulse counting feedback at different repetition rates. Results from 5 repeated measurements are shown below.
| Intended Pulses | Delivered Pulses (360 Hz) |
Delivered Pulses (60 Hz) |
|---|---|---|
| 5 | 4,5,5,5,5 | 5,5,5,5,5 |
| 10 | 10,10,10,10,10 | 10,10,10,10,10 |
| 20 | 20,20,20,20,20 | 20,20,20,20,20 |
| 30 | 30,30,29,30,30 | 30,30,30,30,30 |
3.2. Dose Integration and Calibration
Figure 3 depicts the different waveforms generated and captured during the dose delivery process. In particular, Figure 3 a and b) show the dose accumulation process for a typical FLASH irradiation. Dose integration takes place for the two outputs of the photodiode synchronously with the radiation pulse. The integrated dose is then reset before the onset of the next Sync pulse. Channel 1 (Ch 1) here denotes the scintillation signal (green wavelength band), whereas channel 2 (Ch 2) denotes the signal which is dominated by stem (blue wavelength band). These voltage values are then sampled after a specified duration (225 us) and used for the dose calibration procedure.
Figure 3.
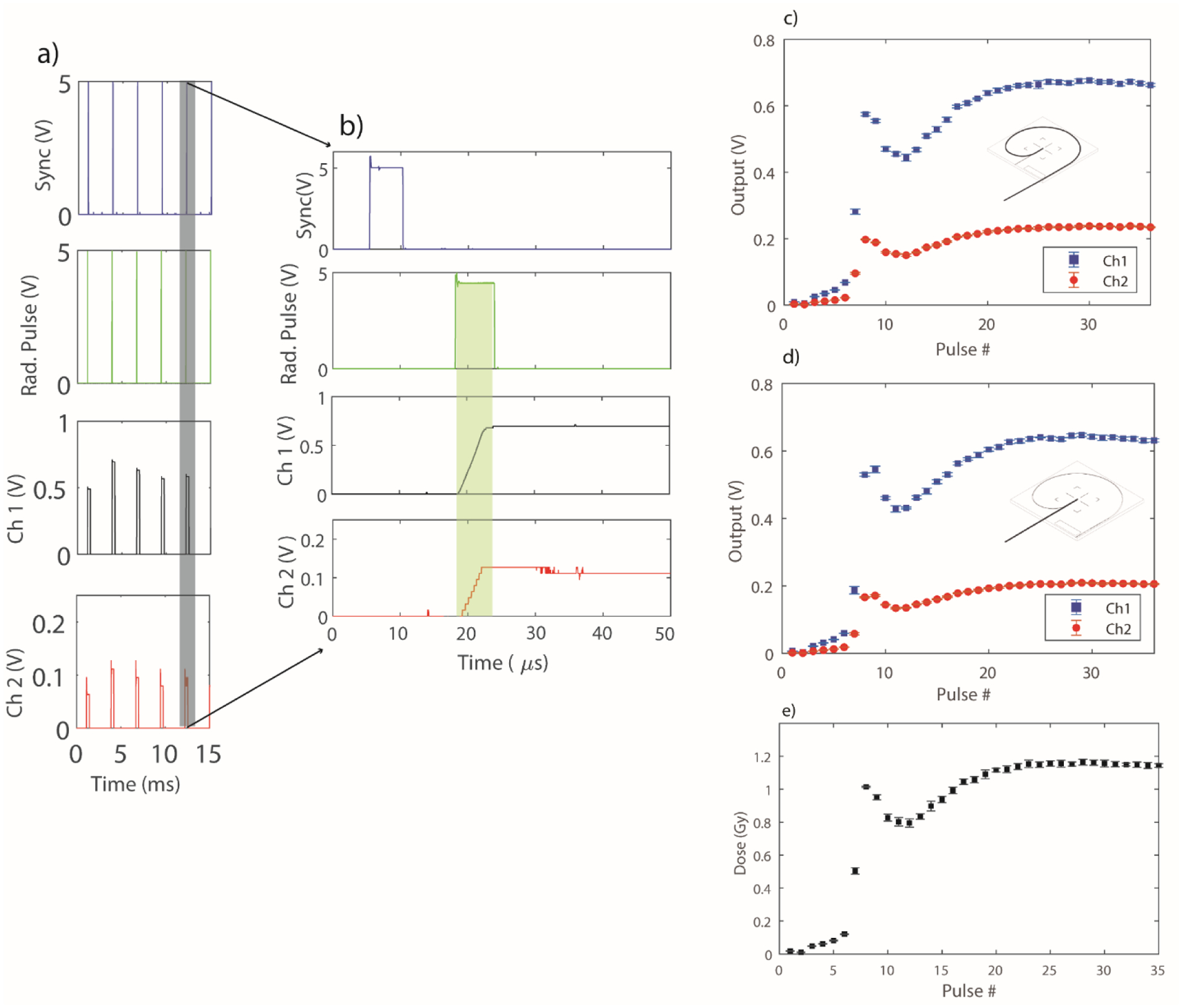
a) Shows a waveform capture of the sync signal, radiation pulse and the integrated two channel output of the photodiode for a sample irradiation. b) Shows a zoomed-in portion of Figure 3a, depicting the dose integration for an individual linac pulse (shaded in green). c, and d) Output from the Exradin-W1 photodiode for the maximum and minimum configuration, respectively. e) Depicts a sample dose delivery of 35 pulses. The error bars for each graph represent the standard deviation obtained from 5 repeated measurements.
Figure 3 c) and d) show the output for the maximum and minimum fiber configuration, respectively. Considering a nominal dose per pulse of 1 Gy for the FLASH beam and the 100pF feedback capacitor in the gated integrator, the output voltage can be calculated to be approximately ~0.6 V for each integrated linac pulse. This agrees with the values seen in Figure 3 c) and d). For determination of the CLR, the output per pulse values of Ch 1 and Ch 2 were accumulated. The CLR value for this calibration was found to be 1.16 ± 0.02. Similarly, the gain value was found to be 3.09 ± 0.13. The uncertainty in the measurement of the CLR and gain values were obtained by adding in quadrature the standard deviations of the Ch1, Ch2 and film values from 5 repeated measurements. Figure 3 e) shows the 5 repeated measures of a 35-pulse dose delivery sequence. The ramp-up period, which includes the first 5–6 pulses, is clearly visible. On average, the first 5 pulses delivered ~ 0.040 ± 0.002 Gy/Pulse. Once the calibration was performed, the next step was to characterize the W1 under FLASH dose-rates, since for any dose-based feedback mechanism to work, a dosimeter which is linear at high dose-rates and does not suffer from saturation issues is needed.
3.3. Exradin W1 Characterization
The results of Exradin W1 characterization are presented in Figure 4. The W1 was found to be independent (within 3%) of any mean dose-rate effects when compared against film (Figure 4a). Since the Sync signal continuously pulses at 360 Hz, independent of the radiation pulses, a threshold value was used to only sample after a valid radiation pulse. The dose per pulse linearity, as shown in Figure 4 b), yielded a close agreement between the two detectors with a R2 value of 0.9994. The results for the field size dependency are shown in Figure 4 c). An average difference of 2.1%, with film as the reference, was seen between the two detectors indicating that the Cherenkov removal technique was accurately implemented. The radiation damage study, shown in Figure 4 d), indicates significant reduction in sensitivity of the dosimeter with increasing cumulative dose. A 16.2% loss in sensitivity per 1 kGy of dose was seen when the dosimeter was initially calibrated and characterized. However, with subsequent irradiations, a decrease in the rate of degradation was seen. For example, for experiment # 2 performed the next day, a decrease of ~7% was observed. The same decrease was observed again for experiment # 3, which was performed after a gap of 5 days. A slight recovery of the output was seen between experiment # 2 and # 3, which had a gap of 5 days between them. The spread of the values around the linear fit can be attributed to the fact that the reference dataset is film which has inherent 2–4% accuracy, which adds more noise to the data since the W1 response is normalized with film values. Therefore, for any experiments where accurate dosimetry is required, the W1 would have to be recalibrated just prior to the experiment, without any additional irradiations. Barring the radiation stability, the W1 was proven to be good candidate for online dose-based feedback. Therefore, the next step was to characterize the accuracy of the feedback system based on dose accumulation.
Figure 4.
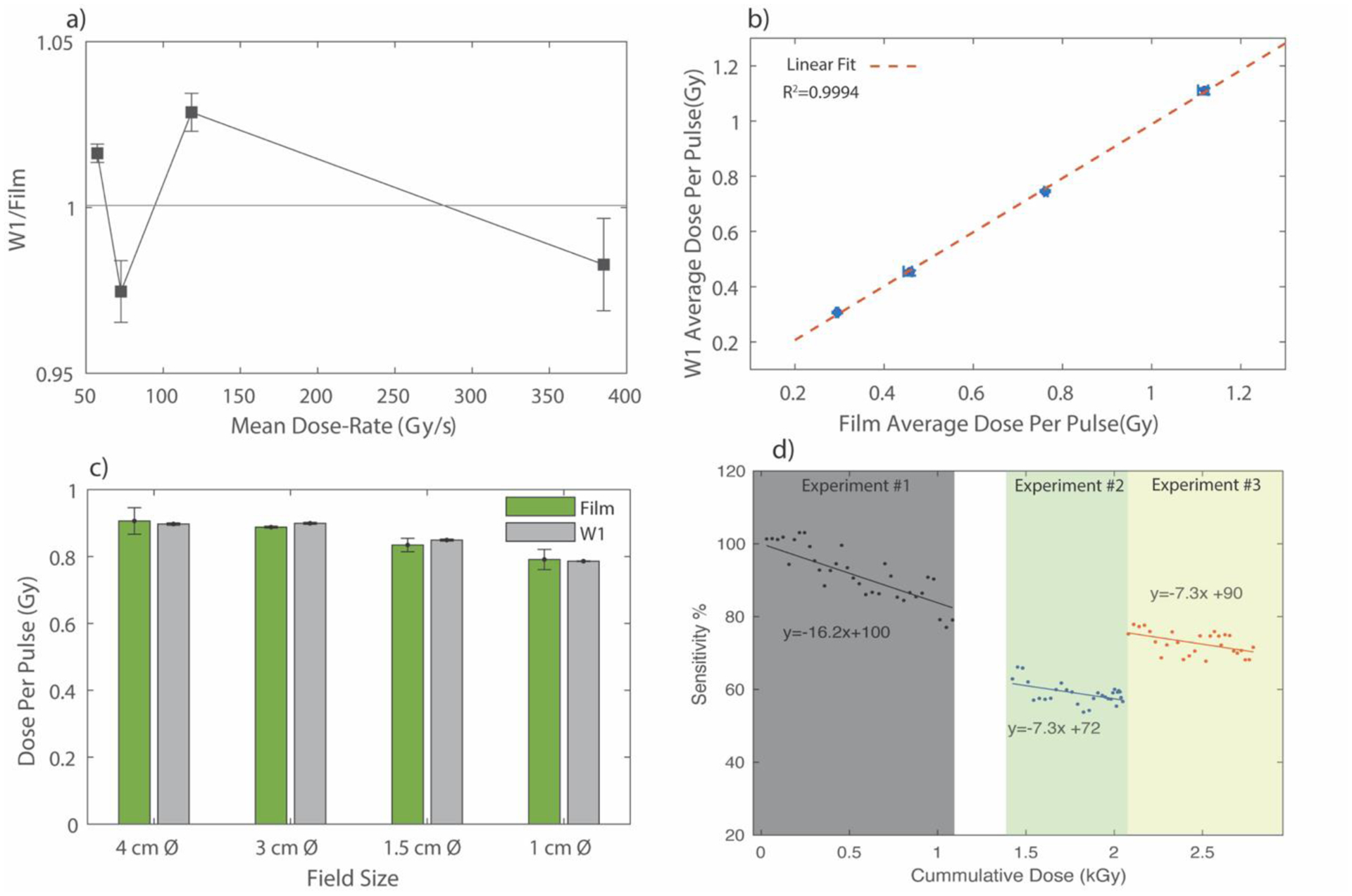
a) Mean dose-rate dependence of the W1 plastic scintillator. b) Dose-per pulse linearity of the W1. The red dotted line is a linear fit. c) Field size characterization and d) shows the radiation damage study. Experiment 1 and 2 were conducted on consecutive days, whereas experiment 3 was conducted after a gap of 5 days. The white space on the graph between experiment 1 and experiment 2 denotes points for which output of W1 was not recorded, but the dose delivered to the W1 was confirmed with film readings. The uncertainty bars in a), b) and c) represent standard deviation from 5 repeated measurements.
3.4. Dose-Based Feedback
For the dose-based feedback, the W1 was placed at iso-center in the calibration slab. To offset the radiation damage the W1 had accumulated prior to the study, a new calibration was obtained for this study. For the calibration, the built-in transmission chamber was used for beam control. Once calibrated, the internal feedback was turned off and the controller (via the dose-feedback implementation) was used as a beam gating signal. The accuracy of the feedback system was tested by delivering 5 Gy, 10 Gy, 20 Gy and 30 Gy with 5 repeated measures and verified against film data. The results of this study are shown in Table 2, where the difference was calculated based on the final averaged dose value reported by the controller and the film. Of note, there is a difference between the 1) intended dose and the controller dose and 2) between the controller dose and film dose which were read out 24 hours after the study. The first difference between the controller and intended dose can be attributed to the fact that the controller does not yet have the capability of modulating either the pulse width or dose delivered in the last pulse of the dose sequence. For the 5 and 10 Gy delivery, the average dose delivered in the last pulse was 0.38 Gy and 0.68 Gy, respectively. For the 20 Gy and the 30 Gy delivery, the average dose per pulse in the last pulse were 0.75 Gy and 0.79 Gy, respectively. The difference between the dose reported by the controller and film is because once the delivered dose is greater than the dose set by the user, the dose monitoring loops exits and stops accumulation. At this point, a relay signal is sent to the RPM system. The relay and the gating system31,32 have their own latency which may sometimes result in an additional pulse. Interestingly, the dose difference was lower for smaller dose deliveries, which can be explained by the fact that the nominal dose per pulse is lower. Other sources of uncertainty are film accuracy (2–4%) and accrual of radiation damage during the study. More importantly, it is worth noting that the number of pulses for similar doses vary significantly; a fact pointing towards the importance of dose-based control instead of simple pulse counting circuitry. The graphical user interface, which depicts relevant temporal metrics of the radiation beam is shown in the supplementary data in Figure A2.
Table 2.
Accuracy assessment of the dose-based feedback. Standard deviation from 5 repeated measurements is presented in the table.
| Intended Dose (Gy) | 5 | 10 | 20 | 30 |
|---|---|---|---|---|
| Controller Dose (Gy) | 5.2 ± 0.1 | 10.4 ± 0.2 | 20.5 ± 0.5 | 30.4 ± 0.2 |
| Film Dose (Gy) | 5.7 ± 0.1 | 11.1 ± 0.1 | 21.8 ± 0.7 | 31.7 ± 0.3 |
| Difference (Gy) | 0.5 ± 0.1 | 0.6 ± 0.2 | 1.3 ± 0.9 | 1.4 ± 0.4 |
| Num.Pulses | {15,14,14,16,15} | {28,24,28,29,25} | {42,38,38,43,42} | {56,53,53,54,58,58} |
3.5. In-vivo Dosimetry
As another example of the usefulness of the pulse-resolved beam monitoring system, in-vivo dosimetry results from 4 mice are presented in Figure 5. Note, only pulse counting feedback was used for this experiment. The ramp-up period was again clearly visible. With the 1 cm cut-out, the average dose delivered for the first 4 pulses across the 4 mice was measured to be ~ 0.045 ± 0.004 Gy/Pulse. The dose/pulse eventually stabilized to a nominal value of around 0.65 ±0.01 Gy. When comparing the cumulative dose with GafChromic film, the W1 exhibited an under-response of ~3%. Since it was seen earlier that the sensitivity of the detector deteriorates with dose, the dosimeter was calibrated prior to the in-vivo measurements with no additional doses delivered between the calibration setup and the actual experiment.
Figure 5.
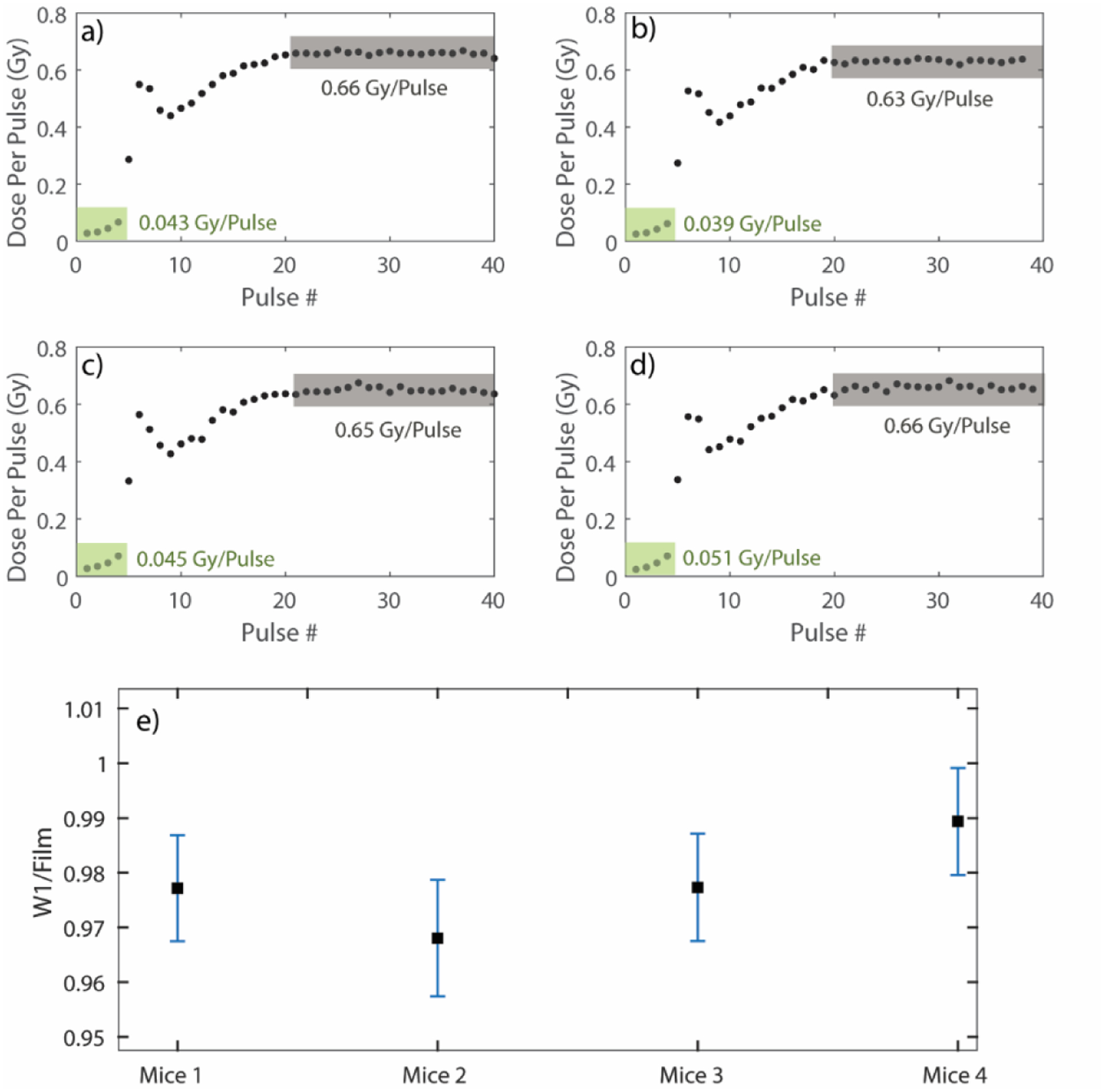
a), b), c) and d) In-vivo pulse-resolved dosimetry for the 4 mice. The green shaded areas signify the ramp-up period along with the average dose per pulse delivered in that domain. The grey shaded areas denote the stable output of the LINAC and the average dose per pulse delivered in that domain. e) Comparison of the cumulative dose against radiochromic film for the 4 mice. The uncertainty bars here indicate the uncertainty in dose measured with W1 propagated from the uncertainity in the calibration coefficients (CLR and Gain).
4. Discussions:
In this study, a hardware solution for real-time and pulse-resolved beam monitoring and control of a FLASH enabled linac were introduced. A gated integrator was used to accumulate dose in real-time. It should be noted that pulse-resolved dosimetry techniques have been proposed before33–39, but none of the solutions employed an FPGA-based system and were not used in the context of beam control of a FLASH beamline. To the best of our knowledge, this is first instance of both dose-based feedback for FLASH-RT and characterization of the W1 at FLASH dose-rates. In particular, pulse counting, and dose-based control were implemented and characterized. The dose-accumulation based control was designed to take precedence, while the pulse-counting was only used as a secondary form of control. The dose-based control was found to be accurate within 1.5 Gy of the intended dose, and more accurate results were seen at lower doses. A solution is currently being worked on to modulate the dose delivered within the last pulse, thereby giving an even better beam delivery system. It is expected that with these changes, the dose accuracy can be comparable to normal clinical tolerance. The control system can be used to measure pulse width and repetition rate as well. In this study, the pulse width was measured using the RTU. However, due to the decay time of BGO, and the position dependent response of the RTU, the current implementation is not ideal.
The issue of ramp-up needs to be addressed to obtain accuracy at smaller doses. Ideally, the ramp-up would need to be eliminated to ensure that all pulses are delivered at UDHR. One potential way of overcoming the ramp-up for C-series linacs would be to disable the AFC and build external circuitry to manually control the frequency control by changing the reflected power waveform, such that the system is in resonance even prior to the delivery of the first pulse.
It should be noted that the solution presented in this study is specific to Varian C-series linacs. However, by using an easily accessible industrial-grade controller with widely used LabVIEW programming environment, the tools presented in this study can be readily implemented to other linac models. Most linacs use trigger signals to monitor waveforms. Those triggers signal can be used to trigger the dose per pulse accumulation as well. In this manuscript, the ‘Sync’ signal was used as trigger source to enable accumulation of dose within each pulse. As mentioned previously, the radiation pulse occurs 12 μs after the rising edge of the Sync signal. This time delay might be different for other linacs but can be easily tuned by the user by sampling adjusting the delay value in the code. Additionally, the gun current pulse can also be used as a gating signal for the gated integrator; this would be the most universal solution. The code is available upon request. Importantly, the tools presented in this study are not limited to luminescence-based dosimeters and can be used for other potentially attractive dosimeters for FLASH, making this hardware solution a flexible choice for online beam monitoring.
The W1 PSD has been extensively characterized at conventional dose-rates40,41, but for FLASH monitoring it was prudent to also assess its efficacy at ultra-high dose-rates. The W1 was found to be independent of any dose-rate effects in the FLASH regime (50– 350 Gy/s). The dose per pulse dependence, which is directly related to the instantaneous dose-rate, was also minimal at values ranging from 0.3–1.1 Gy/Pulse. Additionally, no field size dependency was seen, indicating that the Cherenkov removal technique was sufficiently implemented. However, at FLASH dose-rates, the W1 accrued radiation damage at a much faster rate (16%/kGy) when compared to literature and vendor provided specification sheet (2%/kGy), which were conducted at conventional dose-rates40,41. Essentially, the dosimeter would need to be re-calibrated before irradiation to ensure accurate dose monitoring. However, it was seen the rate of radiation damage did decrease with increasing cumulative dose (~ 7% for the next two iterations). Some recovery was seen after a gap of 5 days, but more studies are required to elucidate the temporal evolution of the radiation damage process at ultra-high dose rates. Of note, there have been reports of dose-rate dependence of radiation damage of plastic scintillators42,43. To rule out the possibility of radiation damage to the gated integrator, which was also placed inside the radiation bunker, a constant voltage source coupled to a resistor was provided to the gated integrator during radiation delivery to test constancy of a constant current signal readout. No degradation of the integrator was seen after delivery of ~ 1000 pulses. Furthermore, within a single dose delivery, the presence of radiation did not interfere with the integrator. This is presented in Figure A3 in the supporting document where, the peak-peak voltage value of the integrated signal in the pre delivery, beam-on and post-delivery period was the same. In this regard, liquid based scintillators might be a viable solution. Further research is required to identify radiation hardened plastic scintillators. Certain base materials, such as polyvinyl toluene (PVT), have shown to be less susceptible to dose-rate dependent radiation damage Moreover, it has been observed that for plastic scintillators, the change in transmission with increasing radiation damage occurs around 400–600 nm and remains relatively constant in the 600–800 nm range. Red shifting dopants might therefore also improve the detector’s radiation hardness. It should also be noted that the light guide can lead to radiation induced attenuation and fading. In general, high OH- content, pure silica light guides should be used, since they offer superior radiation resistance47.
Nonetheless, if calibrated prior to irradiation, the W1 can be considered an important dosimetric tool for FLASH-RT, because of its linearity at high dose-rates, tissue equivalency and its ability to offer real-time, pulse resolved monitoring. The ability to verify dose for each pulse is crucial to understanding in-vivo FLASH studies. For example, the first 4 pulses delivered on average 0.04 Gy/Pulse for the 1 cm cut-out used for the murine study and this type of information needs to be recorded to fully compare outcomes from different investigators. These values are comparable with conventional forms of radiotherapy and therefore, cannot be regarded as dose delivery under FLASH dose-rate regimes. Moreover, the quantities measured with this new control system will lead to more accurate reporting of FLASH effect and its relation to the temporal aspects of dose delivery.
5. Conclusions:
A real-time embedded industrial controller was presented for online beam monitoring and dose feedback control, which was an improvement over the pulse counting based method. Dose delivered within each pulse was measured and displayed in real-time. A commercially available scintillating detector was characterized under ultra-high dose-rate regime and was found to be suitable for dose verification if routine recalibrations are performed to account for the loss in sensitivity with radiation damage. For more accurate dose control and measurement of pulse width, the current system needs to be optimized further. Future iterations would focus on identifying radiation resistant beam monitors suitable at FLASH dose-rates and implement the ability of modulate the dose per pulse in real-time. Moreover, a failure modes and effects analysis approach will be applied to analyze the underlying programming routines to enable safe clinical translation.
Supplementary Material
References:
- 1.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra93. doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 2.Levy K, Natarajan S, Wang J, et al. FLASH irradiation enhances the therapeutic index of abdominal radiotherapy in mice. bioRxiv. Published online December 12, 2019:2019.12.12.873414. doi: 10.1101/2019.12.12.873414 [DOI] [Google Scholar]
- 3.Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol. 2017;124(3):365–369. doi: 10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 4.Vozenin MC, De Fornel P, Petersson K, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(1):35–42. doi: 10.1158/1078-0432.CCR-17-3375 [DOI] [PubMed] [Google Scholar]
- 5.Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 6.Medical Varian. Feasibility Study of FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases. clinicaltrials.gov; 2021. Accessed March 23, 2021. https://clinicaltrials.gov/ct2/show/NCT04592887 [Google Scholar]
- 7.Favaudon V, Lentz JM, Heinrich S, et al. Time-resolved dosimetry of pulsed electron beams in very high dose-rate, FLASH irradiation for radiotherapy preclinical studies. Nucl Instrum Methods Phys Res Sect Accel Spectrometers Detect Assoc Equip. 2019;944:162537. doi: 10.1016/j.nima.2019.162537 [DOI] [Google Scholar]
- 8.Jaccard M, Petersson K, Buchillier T, et al. High dose‐per‐pulse electron beam dosimetry: Usability and dose‐rate independence of EBT3 Gafchromic films. Med Phys. 2017;44(2):725–735. doi: 10.1002/mp.12066 [DOI] [PubMed] [Google Scholar]
- 9.Jaccard M, Durán MT, Petersson K, et al. High dose-per-pulse electron beam dosimetry: Commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys. 2018;45(2):863–874. doi: 10.1002/mp.12713 [DOI] [PubMed] [Google Scholar]
- 10.Esplen NM, Mendonca MS, Bazalova-Carter M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: a topical review. Phys Med Biol. Published online 2020. doi: 10.1088/1361-6560/abaa28 [DOI] [PubMed] [Google Scholar]
- 11.Ashraf MR, Rahman M, Zhang R, et al. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front Phys. 2020;8. doi: 10.3389/fphy.2020.00328 [DOI] [Google Scholar]
- 12.Di Martino F, Barca P, Barone S, et al. FLASH Radiotherapy With Electrons: Issues Related to the Production, Monitoring, and Dosimetric Characterization of the Beam. Front Phys. 2020;8. doi: 10.3389/fphy.2020.570697 [DOI] [Google Scholar]
- 13.Lempart M, Blad B, Adrian G, et al. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother Oncol. 2019;139:40–45. doi: 10.1016/j.radonc.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 14.Schüler E, Trovati S, King G, et al. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int J Radiat Oncol Biol Phys. 2017;97(1):195–203. doi: 10.1016/j.ijrobp.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 15.Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH Delivery at Treatment Room Isocenter for Efficient Reversible Conversion of a Clinical LINAC. Int J Radiat Oncol Biol Phys. 2021;0(0). doi: 10.1016/j.ijrobp.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersson K, Jaccard M, Germond JF, et al. High dose-per-pulse electron beam dosimetry - A model to correct for the ion recombination in the Advanced Markus ionization chamber. Med Phys. 2017;44(3):1157–1167. doi: 10.1002/mp.12111 [DOI] [PubMed] [Google Scholar]
- 17.Konradsson E, Ceberg C, Lempart M, et al. Correction for Ion Recombination in a Built-in Monitor Chamber of a Clinical Linear Accelerator at Ultra-High Dose Rates. Radiat Res. 2020;194(6):580–586. doi: 10.1667/RADE-19-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kranzer R, Poppinga D, Weidner J, et al. Ion collection efficiency of ionization chambers in ultra-high dose-per-pulse electron beams. Med Phys. n/a(n/a). doi: 10.1002/mp.14620 [DOI] [PubMed] [Google Scholar]
- 19.UHDpulse. UHDpulse. Accessed November 19, 2021. http://uhdpulse-empir.eu/
- 20.Ashraf MR, Bruza P, Krishnaswamy V, Gladstone DJ, Pogue BW. Technical Note: Time-gating to medical linear accelerator pulses: Stray radiation detector. Med Phys. 2019;46(2):1044–1048. doi: 10.1002/mp.13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unser K A Toroidal DC Beam Current Transformer with High Resolution. IEEE Trans Nucl Sci. 1981;28(3):2344–2346. doi: 10.1109/TNS.1981.4331686 [DOI] [Google Scholar]
- 22.Palumbo L, Vaccaro VG. Wake fields measurements. In: Month M, Turner S, eds. Frontiers of Particle Beams; Observation, Diagnosis and Correction. Lecture Notes in Physics Springer; 1989:312–354. doi: 10.1007/BFb0018294 [DOI] [Google Scholar]
- 23.Becker F Beam Induced Fluorescence Monitors. Published online 2011:5.
- 24.Oesterle R, Gonçalves Jorge P, Grilj V, et al. Implementation and validation of a beam-current transformer on a medical pulsed electron beam LINAC for FLASH-RT beam monitoring. J Appl Clin Med Phys. 2021;22(11):165–171. doi: 10.1002/acm2.13433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsch L, Beyreuther E, Burris‐Mog T, et al. Dose rate dependence for different dosimeters and detectors: TLD, OSL, EBT films, and diamond detectors. Med Phys. 2012;39(5):2447–2455. doi: 10.1118/1.3700400 [DOI] [PubMed] [Google Scholar]
- 26.Jursinic PA. Dependence of diode sensitivity on the pulse rate of delivered radiation. Med Phys. 2013;40(2):021720. doi: 10.1118/1.4788763 [DOI] [PubMed] [Google Scholar]
- 27.Beddar AS, Mackie TR, Attix FH. Water-equivalent plastic scintillation detectors for high-energy beam dosimetry: II. Properties and measurements. Phys Med Biol. 1992;37(10):1901–1913. doi: 10.1088/0031-9155/37/10/007 [DOI] [PubMed] [Google Scholar]
- 28.Fontbonne JM, Iltis G, Ban G, et al. Scintillating fiber dosimeter for radiation therapy accelerator. IEEE Trans Nucl Sci. 2002;49(5):2223–2227. doi: 10.1109/TNS.2002.803680 [DOI] [Google Scholar]
- 29.Frelin AM, Fontbonne JM, Ban G, et al. Spectral discrimination of Čerenkov radiation in scintillating dosimeters. Med Phys. 2005;32(9):3000–3006. doi: 10.1118/1.2008487 [DOI] [PubMed] [Google Scholar]
- 30.Micke A, Lewis DF, Yu X. Multichannel film dosimetry with nonuniformity correction. Med Phys. 2011;38(5):2523–2534. doi: 10.1118/1.3576105 [DOI] [PubMed] [Google Scholar]
- 31.Shepard AJ, Matrosic CK, Radtke JL, Jupitz SA, Culberson WS, Bednarz BP. Technical Note: Characterization of clinical linear accelerator triggering latency for motion management system development. Med Phys. 2018;45(11):4816–4821. doi: 10.1002/mp.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M, Sano N, Ueda K, et al. Technical Note: Evaluation of the latency and the beam characteristics of a respiratory gating system using an Elekta linear accelerator and a respiratory indicator device, Abches. Med Phys. 2018;45(1):74–80. doi: 10.1002/mp.12664 [DOI] [PubMed] [Google Scholar]
- 33.Beierholm AR, Ottosson RO, Lindvold LR, Behrens CF, Andersen CE. Characterizing a pulse-resolved dosimetry system for complex radiotherapy beams using organic scintillators. Phys Med Biol. 2011;56(10):3033–3045. doi: 10.1088/0031-9155/56/10/009 [DOI] [PubMed] [Google Scholar]
- 34.Velthuis JJ, Page RF, Purves TM, Beck L, Hanifa MAM, Hugtenburg RP. Toward Pulse by Pulse Dosimetry Using an SC CVD Diamond Detector. IEEE Trans Radiat Plasma Med Sci. 2017;1(6):527–533. doi: 10.1109/TRPMS.2017.2750799 [DOI] [Google Scholar]
- 35.Pettinato S, Orsini A, Girolami M, Trucchi DM, Rossi MC, Salvatori S. A High-Precision Gated Integrator for Repetitive Pulsed Signals Acquisition. Electronics. 2019;8(11):1231. doi: 10.3390/electronics8111231 [DOI] [Google Scholar]
- 36.Pettinato S, Girolami M, Olivieri R, Stravato A, Caruso C, Salvatori S. A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy. Materials. 2021;14(18):5203. doi: 10.3390/ma14185203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettinato S, Girolami M, Olivieri R, Stravato A, Caruso C, Salvatori S. Diamond-based Detection Systems for Accurate Pulsed X-rays Diagnostics in Radiotherapy. ArXiv210914547 Phys. Published online September 28, 2021. Accessed December 1, 2021. http://arxiv.org/abs/2109.14547 [Google Scholar]
- 38.Ashraf MR, Rahman M, Zhang R, et al. Technical Note: Single Pulse Beam Characterization for FLASH-RT Using Optical Imaging in a Water Tank. Med Phys. n/a(n/a). doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman M, Ashraf MR, Zhang R, et al. Spatial and temporal dosimetry of individual electron FLASH beam pulses using radioluminescence imaging. Phys Med Biol. Published online 2021. doi: 10.1088/1361-6560/ac0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrasco P, Jornet N, Jordi O, et al. Characterization of the Exradin W1 scintillator for use in radiotherapy: Characterization of Exradin W1 for radiotherapy. Med Phys. 2014;42(1):297–304. doi: 10.1118/1.4903757 [DOI] [PubMed] [Google Scholar]
- 41.Dimitriadis A, Patallo IS, Billas I, Duane S, Nisbet A, Clark CH. Characterisation of a plastic scintillation detector to be used in a multicentre stereotactic radiosurgery dosimetry audit. Radiat Phys Chem. 2017;140:373–378. doi: 10.1016/j.radphyschem.2017.02.023 [DOI] [Google Scholar]
- 42.Birks JB. The Theory and Practice of Scintillation Counting. :671.
- 43.collaboration CH. Dose rate effects in the radiation damage of the plastic scintillators of the CMS Hadron Endcap Calorimeter. J Instrum. 2016;11(10):T10004–T10004. doi: 10.1088/1748-0221/11/10/T10004 [DOI] [Google Scholar]
- 44.Belloni A Radiation Effects on Plastic Scintillators for Current and Future HEP Experiments. :47.
- 45.Zorn C A pedestrian’s guide to radiation damage in plastic scintillators. Radiat Phys Chem. 1993;41(1):37–43. doi: 10.1016/0969-806X(93)90040-2 [DOI] [Google Scholar]
- 46.Bross AD, Pla-Dalmau A. Radiation damage of plastic scintillators. IEEE Trans Nucl Sci. 1992;39(5):1199–1204. doi: 10.1109/23.173178 [DOI] [Google Scholar]
- 47.Ito C, Naito H, Nishimura A, et al. Development of radiation-resistant optical fiber for application to observation and laser spectroscopy under high radiation dose. J Nucl Sci Technol. 2014;51(7–8):944–950. doi: 10.1080/00223131.2014.924883 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


