Abstract
Background
Child temperament styles characterized by increased emotionality or pleasure seeking may increase risk for less healthful eating patterns, while strong executive control (EC) may be protective. The interaction of these characteristics with longitudinal outcomes has not yet been examined.
Purpose
The aim of this study was to examine the association of preschool temperament and EC, as well as their interaction with adolescent eating.
Methods
Preschoolers (N = 313) were recruited into a longitudinal study, with behavioral measurement of EC at age 5.25 years, temperament assessed multiple times across preschool, and eating outcomes assessed in adolescence (mean age = 15.34 years).
Results
Separate latent moderated structural equation models demonstrated that weaker EC was associated with eating less healthful foods, including high sugar foods, sugar-sweetened beverages (SSBs), and convenience foods (p < .05). In the moderation models, negative affectivity temperament was correlated with eating less healthful foods, high sugar foods, and SSBs (p < .05). Children lower in surgency/extraversion temperament were more likely to drink SSBs. There was an interaction between temperament and EC, such that children high in negative affectivity with weaker EC were particularly more likely to consume less healthful foods, high sugar foods, and SSBs (p < .05). There was no interaction of surgency with EC and food consumption.
Conclusions
Child characteristics measured early in development were associated with later adolescent eating behaviors. Adequate EC could be necessary to counteract the drive toward eating associated with temperaments high in negative affectivity.
Keywords: Temperament, Executive control, Preschool, Adolescence, Diet
Temperament styles and executive function in preschoolers predicted eating patterns a decade later in adolescence.
Establishing healthful eating behaviors is critically important during childhood and adolescence, as less healthful eating imbues risk for cardiometabolic health conditions, such as obesity and type 2 diabetes, and certain types of cancer [1, 2]. Pediatric obesity has reached epidemic proportions (18.5% of ages 2–19 in the USA) [3], and rates of obesity-related comorbidities are also on the rise [4]. Multiple factors shape eating behaviors, with most of the available research on factors external to the child, such as parent feeding, the home environment, and neighborhood food access [5–7]. Individual child factors, such as executive control (EC, also commonly referred to as executive function) and temperament, have also received some attention, but previous research has not yet explored possible interactions between these factors; nor has the extant research examined longitudinal data into adolescence. Adolescence is a critically important developmental period to study eating behaviors, as it is a time of emerging autonomy in eating with implications for long-term trajectories of health behaviors and outcomes. Identifying interactions between EC and temperament that may be associated with less healthful eating patterns (e.g., consumption of energy-dense, nutrient-poor food and beverages) into adolescence has the potential to inform tailored health promotion interventions.
Executive control is a set of higher order neurocognitive abilities including working memory, inhibitory control, and flexible shifting that allow individuals to direct attention and plan behavior. Snapshots at different points across the lifespan demonstrate links between EC and eating behaviors [8–11]. For example, cross-sectional and short-term longitudinal studies reveal that youth with weak EC were more likely to consume less healthy foods such as high sugar snacks and beverages than children with strong EC [11–15]. Youth with weak EC also exhibited potentially obesogenic eating patterns such as emotional eating [16] and binge eating [9, 17]. An innovative cross-sectional, randomized study using theta-burst stimulation (i.e., brain stimulation that mimics natural brain patterns) in young adults demonstrated that weakened EC caused an increase in sugary and salty high-fat snack foods (i.e., milk chocolate and chips) and food cravings [18]. Although warranted given existing cross-sectional evidence, extended longitudinal studies have not yet tracked how early EC may be associated with later dietary patterns.
Nelson and colleagues [19] described a conceptual framework for how EC may bolster or undermine health trajectories. Specifically, EC may influence dietary choices through attentional, behavioral, and emotional pathways. Youth with weak EC may have difficulties shifting their attention from prominent external and internal cues urging consumption of energy-dense, nutrient-poor foods (e.g., high sugar foods/drinks, convenience foods). With weak EC, they may not have strong top-down behavioral control when making dietary decisions. Executive control is also critical for managing emotional responses. Individuals with weak EC may have difficulty implementing healthy coping strategies in response to strong emotions, potentially resulting in a drive to eat to soothe negative emotions. Thus, weak EC may undermine healthy dietary choices, while strong EC may be protective.
In addition to EC, children’s temperament styles (i.e., biologically based tendencies toward behavioral and emotional patterns) may increase the risk for less healthful eating. Rothbart et al. [20] conceptualized temperament into two main styles of reactivity: negative affectivity and surgency/extraversion. Children high in negative affectivity frequently experience strong negative emotions including anger, sadness, and anxiety, and are difficult to soothe. Children high in surgency/extraversion tend to impulsively seek pleasurable activities and have a high activity level. Both negative affectivity and surgency are characterized by traits that could increase the consumption of energy-dense, nutrient-poor foods. Studies have mostly focused on the role of negative affectivity rather than surgency/extraversion (for a review, see [21]), with findings indicating that negative affectivity predicted children’s emotional eating [22] and food approach behaviors cross-sectionally [23]. Temperament styles have even been linked to whether preschoolers eat breakfast or not [24]. By adolescence, negative affectivity has been identified as a risk factor for disordered eating behaviors and attitudes [25–27]. In the limited research on surgency, preschool surgency predicted eating more candy than fruit during a lab-based task, while there was no difference in preference for preschoolers high in negative affectivity [28]. In another study, preschoolers high in surgency were more likely to derive pleasure from food, to have a frequent desire to eat, and to eat in the absence of hunger [29]. Types of eating styles (e.g., food approach/avoidance or emotional eating) have typically been the outcomes of interest [22, 29], rather than studying dietary intake (e.g., sugary foods, fruits/vegetables consumed). Further, because temperament is often assessed in early childhood, much of the research has focused on how parents feed their children [30, 31], with less research on how temperament correlates with independent eating choices.
Existing literature suggests that both child EC and temperament may be important individual correlates of dietary behaviors. However, studying these factors in isolation may miss critical interactions between these constructs. Executive control and temperament are distinct constructs, but possibly correlated (mixed evidence for correlations; e.g., for significant correlations, see [32]; null correlations, see [33]). Executive control and temperament share some overlapping features (e.g., positive anticipation in surgency with inhibition in EC), with other aspects that are entirely unique. In addition to the two temperament reactivity styles (negative affectivity and surgency), temperament is comprised of effortful control, which facilitates self-regulation through modulating behavioral and emotional impulses. Although similar-sounding and related in that they both include attentional processes, EC (aka executive function) differs from effortful control in its inclusion of cognitive processes such as working memory, while effortful control includes regulatory/approach processes. Executive control and effortful control have emerged from separate research lines and are considered different constructs despite some overlapping features [34].
The interaction of EC and temperament reactivity styles could be particularly important in understanding risk for less healthful eating and have implications for how to promote healthier eating habits. Theoretically, negative affect and surgency may lead to seeking out highly rewarding, but less healthful foods, such as highly palatable foods/beverages that are high in sugar and fat. For negative affect, in particular, emotional eating may be a way of soothing emotionality. In the case of surgency, youth with weak EC may have limited inhibition to modulate the drive toward immediately reinforcing, highly palatable foods. These youth may have a greater need for strong EC to regulate the tendency for pleasure seeking and positive anticipation of reward toward healthy alternatives other than food. Strong EC is particularly needed for refraining from the consumption of these tempting (and often easily available, inexpensive foods) and directing attention toward more healthful foods (e.g., fruits and vegetables). Examining the interaction of “top-down” EC with “bottom-up” temperament styles is needed to understand how these individual differences may convey risk or protection for later eating behaviors.
This conceptual framework applies particularly during adolescence. Executive control strengthens from childhood into adolescence [35] and differentiates into specific components through adolescence [19]. Adolescence is a time of increasing autonomy in eating [36], and behaviors formed in adolescence may lay the foundation for lifelong patterns [37]. Adolescents generally consume high levels of unhealthful foods (especially sugary foods) and fall short on fruits and vegetables [38]. Adolescence is also a time of developmental mismatch between still-developing EC abilities and adult-like demands for healthy decision-making, particularly within an obesogenic society. Thus, individuals who have poor EC and/or temperaments that put them at risk would be expected to struggle with healthy decision-making and impulsivity in an obesogenic environment. Further, considering the potential interaction of EC and temperament, adolescents with poor EC may have a low chance of enacting healthy behaviors in the context of environmental and temperamental risk.
The Need for the Current Study
While previous studies of EC and temperament hint at their potential role in eating behaviors, important weaknesses limit our understanding of the complex interplay between these constructs. The research base thus far has been primarily cross-sectional and relied on parent report of EC (or related constructs) rather than objective, performance-based EC tasks. When objective behavioral EC tasks have been included, they are often limited to measuring inhibition, only one aspect of EC. Moreover, the few longitudinal studies on either preschool EC or temperament have not extended into examining adolescent behavior, although adolescents have more access to foods of their choosing as they gain more autonomy. Long-term longitudinal studies have demonstrated that early EC and temperament independently correlate with other important adolescent and young adult outcomes, such as academic performance and achievement [39, 40], but such studies are much rarer in exploring EC, temperament, and health behaviors. Therefore, a long-term longitudinal study to examine the role of early childhood EC and temperament as early correlates of eating behaviors during the critical period of adolescence is needed.
The current study seeks to address these gaps by leveraging a long-term longitudinal study with rigorous measurement of preschool EC and temperament correlated with adolescent eating behaviors, testing three hypotheses. First, weaker EC was expected to be associated with greater consumption of energy-dense, nutrient-poor foods and fewer healthful foods. Second, both negative affectivity and surgency temperament styles were expected to be associated with greater consumption of energy-dense, nutrient-poor foods and less consumption of healthful foods. Third, an interactive effect of temperament styles and EC was expected, such that individuals high in either negative affectivity or surgency with weak EC would be particularly likely to consume energy-dense, nutrient-poor foods and be less likely to consume healthful foods. To supplement these hypotheses focused on self-report of food consumption, a secondary analysis was conducted with BMI percentile as an objective outcome measure. It was hypothesized that EC, negative affectivity, and surgency would be associated with higher BMI percentiles.
Method
Participants
The study method and results are reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. The participants were 313 children and their caregivers who were recruited through flyer distribution in a small Midwestern city from 2006 to 2012 when the target child was preschool aged. The larger longitudinal study employed a lagged cohort sequential design with participants entering the study at age 3, 3.75, 4.5, or 5.25 years. Eligibility criteria included English as the primary language spoken at home. Exclusion criteria included having a diagnosed developmental, behavioral, or language disorder at the time of initial recruitment. Recruitment was stratified by sex (51.1% female), and lower income families were oversampled to increase their representation in the study. The median household income at study entry for the sample was $42,000. Reflective of the population demographics of the area, 63.6% were European American, 18.8% were multiracial, 13.4% were Hispanic, 3.8% were African American, and 0.3% were Asian. The mean BMI percentile was 69.72 ± 25.90, with 38.4% with BMI percentiles at or above the 85th percentile (overweight/obese).
Procedures
During the preschool phase of the longitudinal study, parents and children attended study visits at the laboratory every 9 months. Preschool data for the current paper included parent report of temperament when children were 3.0, 3.75, 4.5, and 5.25 years, and performance-based measurement of EC at 5.25 years. Participating families were also followed through elementary school and then invited to participate in an adolescent follow-up study focusing on health behaviors starting in 2017. Participants were first eligible to participate in the adolescent phase at age 14, and invitations to participate were extended around the time of the adolescent’s birthday each year up to age 18. The age at which participants first completed the adolescent data collection protocol varied for two reasons. First, because the broader study employed a lagged cohort sequential design, some of the participants were already older than age 14 at the time funding for the adolescent phase began, so their first opportunity to participate was at an older age. Second, a small number of adolescents did not participate in the first year they were invited but did participate in a subsequent year. Overall, the majority participated in the adolescent study between 14 and 16 years of age (85%), with most participants completing their adolescent assessment at age 15. The mean age at participation in the adolescent data collection was 15.34 years (SD = .99, range = 14–18). Of the 313 preschoolers who participated, 217 (67.8%) returned to the lab for a follow-up adolescent study assessment. Parents provided consent, and adolescents provided assent for participation. Adolescents completed measures in a quiet room at the laboratory. Parents and youth were compensated for their time at each study visit. The university’s Institutional Review Board approved all procedures.
Measures
Preschool executive control
At age 5.25 years, all participating children completed a developmentally appropriate, performance-based battery of nine EC tasks in the laboratory, covering different aspects of EC [41]. Working memory tasks included Nine Boxes, Delayed Alternation, and Nebraska Barnyard. Inhibitory control was assessed using Big-Little Stroop, Go/No-Go, Shape School—Inhibit Condition, and a modified Snack Delay. Flexible shifting tasks included Shape School—Switching Condition and Trails—Switching Condition. Outlier scores were trimmed to three standard deviations from the mean to reduce skewness and kurtosis. These EC behavioral tasks have high inter-rater reliability (95%–100%) in preschool children [42]. Confirmatory factor analyses with this battery have found that a unitary EC construct, with all nine tasks loading on a latent EC factor, provides the preferred structure [43]; therefore, this representation is retained in the current study.
Temperament
Parents completed the Children’s Behavior Questionnaire (CBQ) [20] to assess temperament across the preschool developmental period. Parents answered 36 questions about how true the characteristic is of their child on a seven-point Likert scale ranging from extremely untrue to extremely true. The CBQ provides subscales for negative affectivity (high in anger, discomfort, sadness, and fear; difficult to soothe) and surgency/extraversion (high-intensity pleasure seeking, high activity level). The 36-item version of the CBQ has good psychometric properties [44]. Because temperament is considered a relatively stable characteristic, the four available measurements from across the preschool timepoints were included when creating the latent temperament factors to increase construct validity and reliability. Internal consistency in the current study was acceptable to good across timepoints (surgency: α = 0.74–0.81; negative affectivity α = 0.70 at each timepoint).
Dietary intake
Developed by the National Cancer Institute, the Family Life, Activity, Sun, Health, and Eating (FLASHE) Dietary Screener includes 27 items assessing the frequency of beverages, foods, and food groups commonly consumed by adolescents in the USA [45]. Items are summed to create subscales representing dietary intake across the past 7 days for convenience foods (e.g., pizza), high sugar foods (e.g., cookies, cake), sugar-sweetened beverages (SSBs; e.g., sweetened fruit drinks, soda), and fruits/vegetables (e.g., green salad). In addition to examining each subscale on its own, analyses examined two total composite scores separately. A total score for energy-dense, nutrient-poor foods was calculated by summing convenience foods, high sugar foods, SSBs, and other less healthful foods (e.g., processed meat). A total score of more healthful foods was calculated by summing fruits, vegetables, beans, whole grains, and water. In terms of validity, the FLASHE Dietary Screener closely corresponds with 24-hr dietary recalls [46]. The research comparing food frequency questionnaires to doubly labeled water measures of energy intake has primarily been conducted in adults or children, with little research on adolescents to provide valid information. Pearson’s correlation between the food frequency questionnaire administered in the same way as the current study and doubly labeled water was 0.56 in children (large correlation) [47]. However, caloric intake from food frequency measures can be markedly lower than objectively measured caloric intake. It is important to note that correlation coefficients assess differences in rank order rather than the level of the variables.
BMI Percentile
Trained researchers measured adolescents’ height and weight on a stadiometer and scale. Height and weight were used to calculate BMI for age percentiles using CDC guidelines.
Analysis Plan
Separate latent moderated structural equation (LMS) models were used to examine the conditional main effects of EC and temperament (negative affectivity or surgency/extraversion), as well as their interaction, in correlating with adolescent eating behaviors. All predictor and control variables were standardized into Z-scores, so that the relative magnitude of the path coefficients could be compared [48]. A single-factor latent EC variable was created from the nine EC task scores. Separate latent variables for negative affectivity and surgency/extraversion were created using the temperament subscale scores measured at 3.0, 3.75, 4.5, and 5.25 years. The fit of the factor structures for EC, negative affectivity, and surgency/extraversion were confirmed with the current sample. By default in Mplus, latent EC and latent temperament were allowed to correlate in all models (Note: preliminary analyses indicated significant bivariate Pearson’s correlations between latent EC and latent negative affectivity [r = −.15, p = .048, small correlation] and latent surgency (r = −.33, p < .001, medium correlation]). FLASHE dietary behavior subscale scores and the two total composite scores were included as observed outcomes in separate models. A secondary analysis included BMI percentile as the outcome variable using the same analysis structure. All models controlled for maternal education during preschool, child sex, and age at the adolescent assessment. Race and ethnicity were examined as potential control variables; however, they were not significantly correlated with eating outcomes and were not included in models.
The two-step procedure for latent moderator analyses [49] was implemented in Mplus version 8.4 using maximum likelihood estimation. First, the measurement models that estimated the relationships between the latent predictors and observed dietary outcomes were evaluated to ensure acceptable fit statistics. Second, the latent interaction (EC X negative affectivity or EC X surgency) was added to the structural equation models. Because LMS models do not provide fit statistics, likelihood ratio tests were used to compare the LMS models with the measurement models [49]. To probe each significant interaction, the Johnson–Neyman technique examining regions of significance was applied in Mplus v8.4 using Model Constraints, LOOP, and PLOT commands.
Results
Participant Retention
Attrition analyses were conducted to examine if preschool demographics (race, ethnicity, age, gender, maternal education) differed between participants who did/did not continue to participate into adolescence. There were no significant differences in demographic factors.
Structural Equation Model
The first measurement model that estimated the associations among the latent predictors (i.e., EC and negative affectivity) and composite energy-dense, nutrient-poor foods demonstrated adequate fit [44–46], RMSEA = .05, 90% CI RMSEA = .04–.06, CFI = .83, SRMR = .08. All item loadings contributing to latent EC and negative affectivity were significant, with standardized values ranging from .29 to .65 for EC and .69 to .84 for negative affectivity. The likelihood ratio test comparing the latent moderator model to the measurement model supported including the latent moderator when predicting the composite energy-dense, nutrient-poor foods scale, χ2 (1) = 10, p < .001. Supplemental Table 1 provides the likelihood ratio test results for models with negative affectivity and each food subscale.
The second measurement model that estimated the associations among the latent predictors (i.e., EC and surgency/extraversion) and composite energy-dense, nutrient-poor foods demonstrated adequate fit, RMSEA = .06, 90% CI RMSEA = .05–.07, CFI = .85, SRMR = .08. All item loadings contributing to latent EC and surgency were significant, with standardized values ranging from .29 to .63 for EC and .78 to .92 for surgency. The likelihood ratio test comparing the latent moderator model to the measurement model indicated that the EC × surgency interaction did not significantly contribute to the model when the outcome was composite energy-dense, nutrient-poor foods scale, χ2 (1) = 0, p = .50. See Supplemental Table 1 for likelihood ratio test results for surgency and each food subscale.
Control variables
In the final models, there were no significant age, sex, or SES differences (i.e., maternal education) in most dietary variables. However, there was a sex difference in convenience foods only, with boys eating more convenience foods than girls (negative affectivity model: b = .22, p = .022; surgency model: b = .22, p = .036).
Negative Affectivity Models
EC conditional main effects
See Table 1 for all model results. In the latent moderator model including control variables, the EC conditional effect represents the effect of EC when negative affectivity equals zero. Weaker EC significantly correlated with eating more energy-dense, nutrient-poor foods (b = −1.60, SE = .50, p = .001), convenience foods (b = −.43, SE = .21, p = .036), high sugar foods (b = −.47, SE =.19, p = .012), and SSBs (b = −.59, SE = .21, p = .005). Weaker EC was also associated with eating more healthful foods (b = −1.11, SE = .48, p = .02) and more fruits/vegetables (b = −.76, SE = .31, p = .013).
Table 1.
Results from Each Latent Moderated Model Predicting Dietary Outcomes
| Outcome: energy-dense, nutrient-poor total composite score | b | p | 95% CI | Outcome: energy- dense, nutrient-poor total composite score | b | p | 95% CI |
|---|---|---|---|---|---|---|---|
| EC | −1.60* | .001 | −2.59 to −.62 | EC | −2.26* | <.001 | −3.42 to −1.11 |
| Negative affectivity | 1.33* | .001 | .52 to 2.13 | Surgency | −.73 | .155 | −1.74 to.28 |
| EC X negative affectivity | −1.98* | .004 | −3.32 to −.64 | EC X surgency | .28 | .754 | −1.48 to 2.02 |
| Outcome: convenience foods | b | p | 95% CI | Outcome: convenience foods | b | p | 95% CI |
| EC | −.43* | .036 | −.84 to −.03 | EC | −.57* | .016 | −1.04 to −.11 |
| Negative affectivity | .30 | .111 | −.07 to .66 | Surgency | .09 | .725 | −.40 to .57 |
| EC X negative affectivity | −.15 | .627 | −.75 to .45 | EC X surgency | −.47 | .359 | −1.46 to.53 |
| Outcome: high sugar foods | b | p | 95% CI | Outcome:high sugar foods | b | p | 95% CI |
| EC | −.47* | .012 | −.83 to −.10 | EC | −.65* | .003 | −1.08 to −.22 |
| Negative affectivity | .30* | .032 | .03 to .58 | Surgency | −.15 | .466 | −.56 –.26 |
| EC X negative affectivity | −.72* | .004 | −1.19 to −.23 | EC X Surgency | −.18 | .682 | −1.01 to .66 |
| Outcome: sugar−sweetened beverages | b | p | 95% CI | Outcome: sugar−sweetened beverages | b | p | 95% CI |
| EC | −.59* | .005 | −1.00 to −.18 | EC | −.92* | <.001 | −1.40 to −.44 |
| Negative affectivity | .51* | .002 | .19 to .84 | Surgency | −.41* | .028 | −.77 to −.04 |
| EC X negative affectivity | −1.26* | .001 | −2.02 to −.50 | EC X surgency | .62 | .067 | −.04 to 1.29 |
| Outcome: healthful foods total composite score | b | p | 95% CI | Outcome: healthful foods total composite score | b | p | 95% CI |
| EC | −1.11* | .020 | −2.05 to −.17 | EC | −.76 | .133 | −1.76 to.23 |
| Negative affectivity | −.48 | .203 | −1.23 to.26 | Surgency | .72 | .060 | −.03 to 1.47 |
| EC X negative affectivity | −.29 | .642 | −1.52 to .93 | EC X Surgency | −.81 | .170 | −1.96 to.34 |
| Outcome: fruits and vegetables | b | p | 95% CI | Outcome: fruits and vegetables | b | p | 95% CI |
| EC | −.76* | .013 | −1.36 to −.16 | EC | −.61 | .057 | −1.25 to .02 |
| Negative affectivity | −.14 | .552 | −.61 to .33 | Surgency | .45 | .065 | −.03 to .92 |
| EC X negative affectivity | −.42 | .316 | −1.23 to .40 | EC X surgency | −.29 | .428 | −1.02 to .43 |
| Secondary outcome: BMI percentile | b | p | 95% CI | Secondary outcome: BMI percentile | b | p | 95% CI |
| EC | .64 | .865 | −6.70 to 7.98 | EC | 2.01 | .610 | −5.72 to 9.73 |
| Negative affectivity | .65 | .829 | −5.23 to 6.53 | Surgency | 2.83 | .363 | −3.27 to 8.93 |
| EC X negative affectivity | 2.96 | .570 | −7.26 to 13.18 | EC X surgency | 3.83 | .525 | −10.31 to 13.81 |
Notes: Separate models were conducted for each temperament factor and each outcome. Each model controlled for maternal education, child sex, and adolescent age (not depicted in table). Negative affectivity models on the left and surgency models on the right.
Negative affectivity conditional main effects
In the latent moderator model including control variables, the negative affectivity conditional effect represents the effect of negative affectivity when EC equals zero. Greater negative affectivity significantly correlated with eating more energy-dense, nutrient-poor foods (b = 1.33, SE = .41, p = .001), high sugar foods (b = .30, SE = .14, p = .032), and SSBs (b = .51, SE = .17, p = .002). Negative affectivity was not associated with convenience foods, more healthful foods, or fruits/vegetables.
EC X negative affectivity interaction
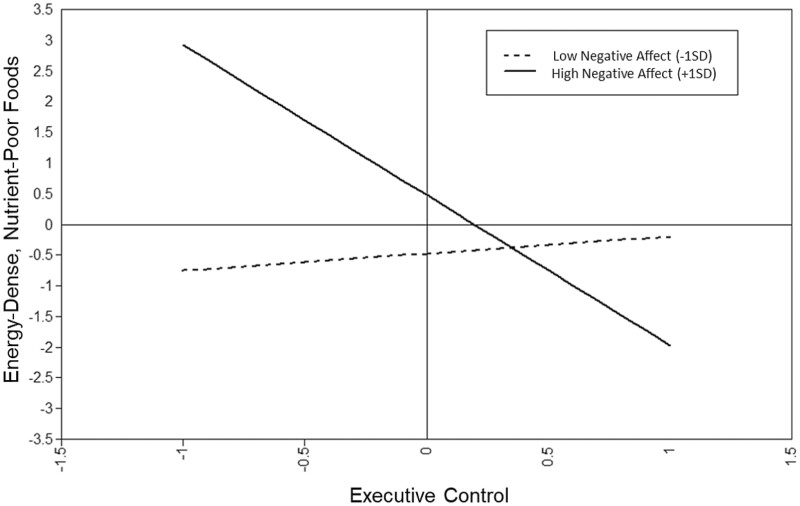
The EC X negative affectivity interaction significantly correlated with eating more energy-dense, nutrient-poor foods (b = −1.98, SE = .41, p = .004), high sugar foods (b = −.72, SE = .25, p = .004), and SSBs (b = −1.26, SE = .39, p = .001). The latent interaction did not significantly correlate with convenience foods, more healthful foods, or fruits/vegetables. See Figure 1.
Fig. 1.
Graph of the interactive effect of the negative affectivity temperament style with preschool executive control correlated with adolescent consumption of energy-dense, nutrient-poor foods (total composite score from the FLASHE).
Probing the significant interactions revealed that negative affectivity mattered most for individuals with weaker EC. While negative affectivity correlated with energy-dense food intake when EC was lower, for individuals, .40 SD above the average level of EC, negative affectivity did not correlate with the energy-dense, nutrient-poor total composite score. Similarly, negative affectivity was associated with individuals with weaker EC for high sugar foods (less than .05 SD above the average level of EC) and SSBs (less than .25 SD above the average level of EC).
Surgency Models
EC conditional main effects
In the latent models including EC, surgency, their interaction, and control variables, EC significantly correlated with more energy-dense, nutrient-poor foods (b = −2.26, SE = .59, p < .001), convenience foods (b = −.57, SE = .24, p = .016), high sugar foods (b = −.65, SE = .22, p = .003), and SSBs (b = −.92, SE = .25, p < .001). Unlike the models including negative affectivity, EC was not associated with more healthful foods nor fruits/vegetables.
Surgency conditional main effects and interaction
Lower surgency was associated with more SSBs (b = −.41, SE = 0.18, p = 0.028). Otherwise, surgency was not associated with eating behaviors. Surgency did not significantly interact with EC for any eating or drinking behaviors.
Secondary BMI Percentile Models
In the latent models including control variables, neither EC, negative affectivity or surgency, nor their interaction were associated with BMI percentile. See Table 1 for results.
Discussion
The current study leveraged and extended a longitudinal design to model how preschool EC and temperament styles were associated with adolescent food choices, with an emphasis on how the interaction of EC and particular temperament styles may convey health risk or protection. Using rigorous measurement of EC, EC assessed in preschool was a significant correlate of adolescent eating behaviors when accounting for temperament and their interaction. Another individual factor assessed in preschool, negative affectivity temperament, was also a key correlate of adolescent eating. In contrast, surgency temperament was generally not associated with eating patterns (with the exception of SSBs). Finally, EC was particularly important for modulating negative affectivity, but not surgency, in relationship to eating behaviors.
The finding of preschool EC correlating with adolescent eating behaviors expands on previous cross-sectional and short-term longitudinal studies. Specifically, the current study adds further support to the idea that EC development in preschool is important for later health behaviors and outcomes [50], with performance at age 5 associated with key eating behaviors approximately a decade later.
The findings of temperament styles on eating patterns are also consistent with and expand upon the existing literature. Negative affectivity was associated with eating more energy-dense, nutrient-poor foods in general and more high sugar foods and SSBs. Concerningly, youth high in negative affectivity were consuming sugar from two pathways—food and drinks—at a higher frequency than youth lower in negative affectivity, potentially contributing to long-term risk for obesity and type 2 diabetes [51]. Theoretically, this finding may reflect more engagement in emotional eating for youth who experience stronger and more persistently negative emotions [52].
Surgency/extraversion largely was not associated with eating patterns. The one exception was for SSBs, in that lower levels of surgency correlated with greater SSBs. Perhaps, having a lower energy temperament promotes seeking perceived quick energy from SSBs which often contain both sugar and caffeine. This finding was unexpected, as it was predicted that greater surgency would be correlated with less healthful eating/drinking patterns. This unsupported hypothesis was based partially on research findings by Zhou et al. [28]. in which high levels of surgency predicted greater sugary food intake in young children [28]. Conceivably, the relationship between child surgency and child intake of sugary foods [28] may disappear by adolescence, because EC contributes to stronger regulatory processes as it continues to develop in adolescence. This may be the case for surgency more so than negative affectivity, because children lower in surgency may tend to have stronger EC (based on medium-sized correlations of EC with surgency vs. marginally significant small correlation with negative affectivity in the current study). Cross-sectional and longitudinal replication studies are needed for both children and adolescents.
Interestingly, EC and negative affectivity interacted, such that weaker EC and greater negative affectivity were associated with eating less healthful foods. This reflects an interaction of a “top-down” EC process with a “bottom-up” temperament process, demonstrating the importance of these two individual-level factors in influencing eating patterns. Although the findings for EC and temperament are important contributions to the literature on their own, the interaction finding is a novel addition to this field. Having adequate EC allows for an integration of prefrontal cognitive abilities with subcortical affective areas to enable healthier eating choices despite strong affective states [9]. The finding that strong EC can be protective in the context of negative affectivity is consistent with adult research on EC and personality styles. One study with adults examining EC and personality factors [53], a succedent of childhood temperament [54], provided an initial indication that the interaction of EC and personality may be important for predicting eating behaviors [53]. However, this relationship has not received as much attention in childhood and adolescence.
The finding that stronger EC was associated with greater consumption of not only energy-dense, nutrient-poor foods, but also more healthful foods (like fruits and vegetables) was at first surprising. However, it is worth noting that the total composite scales for energy-dense, nutrient-poor foods and more healthy foods were positively correlated (r = .23, p < .001), suggesting that youth with weak EC were eating more in general than youth with strong EC. The pattern of findings may suggest that EC may correlate with the amount of food eaten, while temperament may correlate with specific intake choices.
Finally, EC and temperament did not significantly correlate with BMI percentile. Prior research with young adults demonstrated that low inhibitory control predicted weight gain over a year period [55]. Food intake and BMI are distinct constructs that unfold on different timelines. It is possible that any effects on BMI could lag behind eating behaviors and might not become apparent until later into young adulthood. Questions remain regarding the causal relationships between executive function and weight status. Future research can further explore these issues by examining BMI trajectories over time and into later adolescence and young adulthood.
Clinical Implications
Because findings indicate preschool traits may be associated with health behaviors into adolescence, early prevention and intervention strategies may be needed. Cognitive remediation training with adults with obesity has demonstrated success in promoting stronger EC and weight loss [56, 57], and these strategies may complement existing pediatric nutrition education or weight management programs. Given the strong links between SSB intake and obesity, identifying temperament styles (high negative affectivity; low surgency) that are correlated with SSB intake may provide insights into novel interventions. Finally, the EC X negative affectivity interaction may point to novel ways to specifically target interventions toward those who might benefit the most. Behavioral medicine clinicians could screen for both EC deficits and temperament styles early, and then target healthful eating interventions toward particularly high-risk groups. Finally, behavioral medicine clinicians are encouraged to teach healthy alternatives to emotional eating as part of their clinical work with youth high in negative affectivity.
Limitations
The findings must be considered in light of limitations. First, although the self-report food frequency questionnaire used in this study correlates with 24-hr dietary recalls [46], self-report of dietary intake via food frequency questionnaires has important limitations. Adolescents tend to systematically under-report dietary intake relative to objective measures. For example, in a systematic review [58], intake from food frequency questionnaires in children compared to doubly labeled water was under-reported in three studies (7%–23%), over-reported in one study (+13%), and accurately reported across four scenarios in one study (i.e., 47). In a study of 21 undergraduates comparing food frequency measures to doubly labeled water, there was a range of reporting accuracy, with less than half of the participants accurately reporting caloric intake (7 of 17 were identified as acceptable reporters, 8 of 17 as under-reporters, and 2 of 17 were identified as over-reporters) [59]. The current study relied exclusively on adolescent-reported eating behaviors, which have rarely been validated against objective measures such as doubly labeled water. Future researchers are encouraged to validate adolescent food frequency questionnaires against objective measures and to use objective measures of eating when feasible. Second, as with any longitudinal study, there was attrition from preschool to adolescence. Attrition analyses did not reveal demographic differences based on race, ethnicity, child sex, or maternal education, and maximum likelihood estimation was used to account for missing data. However, there could be differences in other factors that were not measured between individuals who continued to participate and those who did not. Third, the majority of the sample was non-Hispanic White, potentially limiting generalizability to more diverse samples. Although the current sample is representative of the local area in terms of race and ethnicity, and oversampled for low family income, future research with more racially and ethnically diverse samples is needed. Fourth, models were estimated separately for negative affect and surgency and for different eating subscales, resulting in a series of models. This approach was implemented to facilitate a clearer interpretation of EC and temperament conditional and interaction effects (as compared to one integrated model). Finally, this study employs a rigorous longitudinal design, but the results are correlational, precluding the ability to make causal claims.
Conclusions
Individual differences detected as early as preschool were associated with later adolescent eating behaviors. EC and temperament in preschool both significantly correlated with consumption of energy-dense, nutrient-poor foods in adolescence. Notably, of the two temperament factors, negative affectivity but not surgency consistently correlated with eating behaviors. There was also a significant interaction between these constructs, with poorer EC being particularly problematic in the context of high negative affect. These results point to potentially novel obesity prevention strategies, including early intervention to promote strong EC and screening for temperament styles.
Supplementary Material
Acknowledgments
We are grateful to the participating families, as well as the research technicians, undergraduate and graduate students, and lab coordinators who made this research possible. This work was supported by the National Institute of Mental Health [Award Number R01MH065668], the National Institute of Diabetes and Digestive and Kidney Diseases [R01DK116693], and the National Institute on Drug Abuse of the National Institutes of Health [R01DA041738]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Katherine M Kidwell, Department of Psychology, Syracuse University, Syracuse, NY, USA.
Tiffany D James, Office of Research and Economic Development, University of Nebraska-Lincoln, Lincoln, NE, USA.
Rebecca L Brock, Department of Psychology, University of Nebraska-Lincoln, Lincoln, NE, USA.
Amy Lazarus Yaroch, Gretchen Swanson Center for Nutrition, Omaha, NE, USA.
Jennie L Hill, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA.
Jennifer Mize Nelson, Office of Research and Economic Development, University of Nebraska-Lincoln, Lincoln, NE, USA.
W Alex Mason, Department of Preventive Medicine, Tennessee Health Science Center, University of Tennessee, Memphis, TN, USA.
Kimberly Andrews Espy, Department of Psychology, University of Texas at San Antonio, San Antonio, TX, USA; Department of Psychiatry and Behavioral Science, UT Health San Antonio, Long School of Medicine, San Antonio, TX, USA.
Timothy D Nelson, Department of Psychology, University of Nebraska-Lincoln, Lincoln, NE, USA.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest The authors do not have conflicts of interest.
Authors’ Contributions Dr. Kidwell drafted the initial manuscript, conducted data analyses, led data interpretation, and oversaw subsequent revisions. Ms. James served as data manager, consulted on statistical analyses, and provided critical review, commentary, and revision of the manuscript. Dr. Brock consulted on statistical analyses, assisted with data interpretation, and provided critical review, commentary, and revision of the manuscript. Drs. Lazarus Yaroch and Hill provided critical review, commentary, and revision of the manuscript. Dr. Mize Nelson oversaw and managed data collection, coordinated research activity planning and execution, and reviewed the final manuscript. Dr. Mason acquired financial support for the project leading to this publication and provided critical review, commentary, and revision of the manuscript. Dr. Espy acquired financial support for the project leading to this publication, developed/designed methodology, coordinated research activity planning and execution, and reviewed the final manuscript. Dr. Nelson formulated the overarching research goals and aims, acquired financial support for the project leading to this publication, coordinated research activity planning and execution, and provided critical review, commentary, and revision of the manuscript.
Data Availability
This study is not a clinical trial and was not formally registered. The analysis plan was not formally registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the senior author. Analytic code used to conduct the analyses presented are not available in a public archive. They may be available by emailing the corresponding author. Materials used to conduct this study are not publicly available.
References
- 1. Mozaffarian D. Foods, obesity, and diabetes—are all calories created equal? Nutr Rev. 2017; 75:19–31. [DOI] [PubMed] [Google Scholar]
- 2. Zhang FF, Cudhea F, Shan Z, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. 2019; 3:pkz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018; 319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caprio S, Santoro N, Weiss R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab. 2020; 2:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell KJ, Crawford DA, Salmon J, et al. Associations between the home food environment and obesity-promoting eating behaviors in adolescence. Obesity. 2007; 15:719–730. [DOI] [PubMed] [Google Scholar]
- 6. Farrow CV, Haycraft E, Blissett JM. Teaching our children when to eat: how parental feeding practices inform the development of emotional eating—a longitudinal experimental design. Am J Clin Nutr. 2015; 101:908–913. [DOI] [PubMed] [Google Scholar]
- 7. Larson N, Miller JM, Eisenberg ME, et al. Multicontextual correlates of energy-dense, nutrient-poor snack food consumption by adolescents. Appetite. 2017; 112:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allom V, Mullan B. Individual differences in executive function predict distinct eating behaviours. Appetite. 2014; 80:123–130. [DOI] [PubMed] [Google Scholar]
- 9. Ames SL, Kisbu-Sakarya Y, Reynolds KD, et al. Inhibitory control effects in adolescent binge eating and consumption of sugar-sweetened beverages and snacks. Appetite. 2014; 81:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall PA. Executive control resources and frequency of fatty food consumption: findings from an age-stratified community sample. Health Psychol. 2012; 31:235–241. [DOI] [PubMed] [Google Scholar]
- 11. Riggs NR, Spruijt-Metz D, Chou CP, Pentz MA. Relationships between executive cognitive function and lifetime substance use and obesity-related behaviors in fourth grade youth. Child Neuropsychol. 2012; 18:1–11. [DOI] [PubMed] [Google Scholar]
- 12. Egbert AH, Bohnert AM, Bates CR, et al. The heat is on: a mixed-method examination of eating behavior and executive functions among low income minority girls during summertime. J Early Adolesc. 2018; 39:625–641. [Google Scholar]
- 13. Pieper JR, Laugero KD. Preschool children with lower executive function may be more vulnerable to emotional-based eating in the absence of hunger. Appetite. 2013; 62:103–109. [DOI] [PubMed] [Google Scholar]
- 14. Riggs NR, Huh J, Chou CP, Spruijt-Metz D, Pentz MA. Executive function and latent classes of childhood obesity risk. J Behav Med. 2012; 35:642–650. [DOI] [PubMed] [Google Scholar]
- 15. Riggs NR, Spruijt-Metz D, Sakuma KL, Chou CP, Pentz MA. Executive cognitive function and food intake in children. J Nutr Educ Behav. 2010; 42:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson TD, James TD, Nelson JM, et al. Associations between specific components of executive control and eating behaviors in adolescence: a study using objective and subjective measures. Appetite. 2020; 154:104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gowey MA, Lim CS, Dutton GR, et al. Executive function and dysregulated eating behaviors in pediatric obesity. J Pediatr Psychol. 2018; 43:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowe CJ, Hall PA, Staines WR. The effects of continuous theta burst stimulation to the left dorsolateral prefrontal cortex on executive function, food cravings, and snack food consumption. Psychosom Med. 2014; 76:503–511. [DOI] [PubMed] [Google Scholar]
- 19. Nelson TD, Mize Nelson J, Alex Mason W, et al. Executive control and adolescent health: toward a conceptual framework. Adolesc Res Rev. 2019; 4:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: the Children’s Behavior Questionnaire. Child Dev. 2001; 72:1394–1408. [DOI] [PubMed] [Google Scholar]
- 21. Anzman-Frasca S, Stifter CA, Birch LL. Temperament and childhood obesity risk: a review of the literature. J Dev Behav Pediatr. 2012; 33:732–745. [DOI] [PubMed] [Google Scholar]
- 22. Haycraft E, Farrow C, Meyer C, Powell F, Blissett J. Relationships between temperament and eating behaviours in young children. Appetite. 2011; 56:689–692. [DOI] [PubMed] [Google Scholar]
- 23. Liew J, Zhou Z, Perez M, Yoon M, Kim M. Parental child-feeding in the context of child temperament and appetitive traits: evidence for a biopsychosocial process model of appetite self-regulation and weight status. Nutrients. 2020; 12:3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skogheim TS, Vollrath ME. Associations of child temperament with child overweight and breakfast habits: a population study in five-year-olds. Nutrients. 2015; 7:10116–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin GC, Wertheim EH, Prior M, et al. A longitudinal study of the role of childhood temperament in the later development of eating concerns. Int J Eat Disord. 2000; 27:150–162. [DOI] [PubMed] [Google Scholar]
- 26. Sutin AR, Kerr JA, Terracciano A. Temperament and body weight from ages 4 to 15 years. Int J Obes. 2017; 41:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Eeden AE, Hoek HW, van Hoeken D, Deen M, Oldehinkel AJ. Temperament in preadolescence is associated with weight and eating pathology in young adulthood. Int J Eat Disord. 2020; 53:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Z, SooHoo M, Zhou Q, Perez M, Liew J. Temperament as risk and protective factors in obesogenic eating: relations among parent temperament, child temperament, and child food preference and eating. J Genet Psychol. 2019; 180:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leung CY, Lumeng JC, Kaciroti NA, et al. Surgency and negative affectivity, but not effortful control, are uniquely associated with obesogenic eating behaviors among low-income preschoolers. Appetite. 2014; 78:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horn MG, Galloway AT, Webb RM, Gagnon SG. The role of child temperament in parental child feeding practices and attitudes using a sibling design. Appetite. 2011; 57:510–516. [DOI] [PubMed] [Google Scholar]
- 31. Kidwell KM, Kozikowski C, Roth T, Lundahl A, Nelson TD. Concurrent and longitudinal associations among temperament, parental feeding styles, and selective eating in a preschool sample. J Ped Psychol. 2018; 43:572–583. [DOI] [PubMed] [Google Scholar]
- 32. Liu R, Blankenship TL, Broomell AP, Garcia-Meza T, Calkins SD, Bell MA. Executive function mediates the association between toddler negative affectivity and early academic achievement. Early Educ Dev. 2018; 29(5):641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krieger V, Amador-Campos JA, Gallardo-Pujol D. Temperament, executive function, and attention-deficit/hyperactivity disorder (ADHD) in adolescents: the mediating role of effortful control. J Clin Exp Neuropsychol. 2019; 41(6):615–633. [DOI] [PubMed] [Google Scholar]
- 34. Putnam SP, Ellis LK, Rothbart MK. The structure of temperament from infancy through adolescence. In: Eliasz A, Angleitner A, eds. Advances/Proceedings in Research on Temperament. Germany: Pabst Scientific; 2001:163–180. [Google Scholar]
- 35. Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010; 81(6):1641–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bassett R, Chapman GE, Beagan BL. Autonomy and control: the co-construction of adolescent food choice. Appetite. 2008; 50:325–332. [DOI] [PubMed] [Google Scholar]
- 37. Mikkilä V, Räsänen L, Raitakari O, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in Young Finns Study. Br J Nutr. 2005; 93:923–931. [DOI] [PubMed] [Google Scholar]
- 38. Story M, Neumark-Sztainer D, French S. Individual and environmental influences on adolescent eating behaviors. J Am Diet Assoc. 2002; 102:S40–S51. [DOI] [PubMed] [Google Scholar]
- 39. Ahmed SF, Tang S, Waters NE, Davis-Kean P. Executive function and academic achievement: longitudinal relations from early childhood to adolescence. J Educ Psychol. 2019; 111:446–458. [Google Scholar]
- 40. DiLalla LF, Marcus JL, Wright-Phillips MV. Longitudinal effects of preschool behavioral styles on early adolescent school performance. J Sch Psychol. 2004; 42:385–401. [Google Scholar]
- 41. Espy KA. The changing nature of executive control in preschool [monograph]. Monogr Soc Res Child Dev. 2016; 81:1–179. [DOI] [PubMed] [Google Scholar]
- 42. James T, Choi HJ, Wiebe S, Espy KA. The changing nature of executive control in preschool: II. The preschool problem solving study: sample, data, and statistical methods. Monogr Soc Res Child Dev. 2016. [DOI] [PubMed] [Google Scholar]
- 43. Nelson JM, James TD, Chevalier N, Clark CA, Espy KA. Structure, measurement, and development of preschool executive function. In Griffin JA, McCardle P, Freund LS, eds. Executive Function in Preschool-Age Children: Integrating Measurement, Neurodevelopment, and Translational Research. American Psychological Associati; on; 2016:65–89. [Google Scholar]
- 44. Putnam SP, Rothbart MK. Development of short and very short forms of the Children’s Behavior Questionnaire. J Pers Assess. 2006; 87:102–112. [DOI] [PubMed] [Google Scholar]
- 45. Nebeling LC, Hennessy E, Oh AY, et al. The FLASHE study: survey development, dyadic perspectives, and participant characteristics. Am J Prev Med. 2017; 52:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith TM, Calloway EE, Pinard CA, et al. Using secondary 24-hour dietary recall data to estimate daily dietary factor intake from the FLASHE study dietary screener. Am J Prev Med. 2017; 52:856–862. [DOI] [PubMed] [Google Scholar]
- 47. Dutman AE, Stafleu A, Kruizinga A, et al. Validation of an FFQ and options for data processing using the doubly labelled water method in children. Public Health Nutr. 2011; 14(3):410–417. [DOI] [PubMed] [Google Scholar]
- 48. Maslowsky J, Jager J, Hemken D. Estimating and interpreting latent variable interactions: a tutorial for applying the latent moderated structural equations method. Int J Behav Dev. 2015; 39:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Methods Res. 1992; 21:230–258. [Google Scholar]
- 50. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999; 6:1–55. [Google Scholar]
- 51. Whitley Jr BE, Kite ME. Principles of Research in Behavioral Science. Routledge; 2012. [Google Scholar]
- 52. Nelson TD, Kidwell KM, Hankey M, Nelson JM, Espy KA. Preschool executive control and sleep problems in early adolescence. Behav Sleep Med. 2018; 16:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Astrup A. Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr. 2001; 4:499–515. [DOI] [PubMed] [Google Scholar]
- 54. Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010; 21:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010; 29(4):389–393. [DOI] [PubMed] [Google Scholar]
- 56. Hall PA, Fong GT, Epp LJ. Cognitive and personality factors in the prediction of health behaviors: an examination of total, direct and indirect effects. J Behav Med. 2014; 37:1057–1068. [DOI] [PubMed] [Google Scholar]
- 57. Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: longitudinal evidence from a birth cohort. Child Dev. 1995; 66:486–498. [DOI] [PubMed] [Google Scholar]
- 58. Burrows T, Goldman S, Rollo M. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labelled water. Eur J Clin Nutr. 2020; 74(5):669–681. [DOI] [PubMed] [Google Scholar]
- 59. Andersen LF, Tomten H, Haggarty P, Løvø A, Hustvedt BE. Validation of energy intake estimated from a food frequency questionnaire: a doubly labelled water study. Eur J Clin Nutr. 2003; 57(2):279–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is not a clinical trial and was not formally registered. The analysis plan was not formally registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the senior author. Analytic code used to conduct the analyses presented are not available in a public archive. They may be available by emailing the corresponding author. Materials used to conduct this study are not publicly available.