Abstract
Baicalin, the main active component of Scutellaria baicalensis, has antioxidant and anti-apoptotic effects and is used to treat acute pancreatitis; however, its specific mechanism is unclear. This study aims to determine the protective effect and underlying mechanism of baicalin on AR42J pancreatic acinar cell injury. AR42J acinar cells (caerulein, 10 nmol/L) were induced in vitro to establish a cell model for acute pancreatitis. Cell relative survival was measured by thiazolyl blue tetrazolium bromide, and cell apoptosis and death were examined by flow cytometry. The expression levels of superoxide dismutase1 (SOD1), Bax, survivin, Bcl-2, caspase-3, and caspase-7 proteins were analyzed by Western blot, and those of SOD1 mRNA and miR-136-5p were determined by RT-PCR. The activities of GSH, SOD1, ROS, and MDA were also investigated. Compared with those of the caerulein group, the relative survival rate and activity of AR42J pancreatic acinar cells with different baicalin concentrations were significantly increased (p < 0.05), and the supernatant amylase level was markedly decreased (p < 0.05). In addition, the ROS and MDA activities and mir-136-5p expression were significantly decreased, and the GSH activities and SOD1 gene and protein expression levels were markedly increased (p < 0.05). These results suggest that baicalin reduced the caerulein-induced death of AR42J acinar cells and alleviated the caerulein-induced injury in pancreatic acinar cells by inhibiting oxidative stress. The mechanism may be related to the decreased expression of Mir-136-5p and the increased expression of SOD1 gene and protein.
Keywords: Baicalin, oxidative stress, AR42J acinar cells, acute pancreatitis, miR-136-5p, superoxide dismutase 1
Introduction
Characterized by intracellular trypsin activation and subsequent self-digestion in the pancreas, acute pancreatitis (AP) leads to pancreatic acini damage, systemic inflammatory response syndrome, and persistent multi-organ failure. 1 Although no specific treatment has been developed for this disease, the common remedies mainly include the inhibition of pancreatic juice and trypsin release, rehydration, nutritional support, correction of electrolyte disturbance, and acid−base balance. Severe acute pancreatitis (SAP) has high treatment cost and unsatisfactory therapeutic effect, and mild pancreatitis heals on its own. 2 Approximately 20%−30% mild pancreatitis can progress into severe cases with fatality rate of 36%−50%. 2 The diagnosis and treatment of this disease have been globally investigated, but the pathogenesis of AP has not been fully elucidated. Owing to the improvement of living standards, pancreatitis incidence and SAP proportion in China have increased. Developing effective therapeutic drugs to improve the survival rate of SAP and reduce the treatment cost has become a hot spot in clinical research.
Oxidative stress plays an important role in pancreatic tissue damage during SAP. 3 As the first line of defense for the body’s antioxidant system, 4 SOD is a biological antioxidant enzyme that removes superoxide anions and protects tissues from superoxide anion damage. MDA is the degradation product of lipid peroxidation, which in turn is the product of the interaction between free radicals and lipids and can indirectly reflect the level of free radicals. 3 ROS products react with NO to produce strong oxidant peroxynitrite, which further reacts with tyrosine residues in tissue proteins to produce 3-nitrotyrosine residues. This process leads to protein degeneration and tissue dysfunction. Nitrotyrosine is a marker of endogenous peroxynitrite formation. In addition, ROS products can cause DNA single-strand breakage and activate ribose PARP, leading to NAD+ consumption, glycolysis rate decline, rapid intracellular ATP depletion, and eventually cell death. 5
Oxidative stress can easily lead to cell apoptosis and necrosis. 6 Apoptosis plays an important role in AP development, 6 and apoptosis and necrosis are the two major forms of pancreatic acinar cell death in AP. Their main difference is that apoptotic cells are rapidly devoured by macrophages and rarely or not at all cause inflammation. Pancreatic acinar cell necrosis damage pancreatic enzymes and inflammatory mediators and thus aggravate pancreatic and organ damage. In mild pancreatitis, a large number of pancreatic acinus cells undergo apoptosis.7,8 In SAP, only a small number of pancreatic acinar cells undergo apoptosis, and most undergo substantial necrosis. 9 AP severity is positively correlated with the degree of necrosis but negatively correlated with the degree of apoptosis. 9 Therefore, regulating the oxidative stress in the pancreas and inducing the early apoptosis of acinar cells are the key steps in AP treatment.
MicroRNAs are a class of endogenous, highly conserved, single-stranded non-coding small RNAs of nucleotide size that negatively regulate the gene expression at the post-transcriptional level by complementing and pairing with the non-transcriptional 3′-non-transcriptional region (3′UTR) of the target gene, resulting in degradation or translation inhibition. 10 MiRNA genes account for 1%−2% of eukaryotic genomes. A single miRNA can regulate multiple target genes and can also be regulated by multiple miRNAs, thus constituting a complex and fine regulatory system. 11 Hence, miRNAs become an important regulatory molecule in various physiological and pathological processes, cell development, differentiation, apoptosis, and proliferation. These molecules also affect the occurrence and development of some pathological processes, such as tumors, autoimmune diseases, and inflammatory reactions. MiR-136 plays an anti-tumor role in various tumor types and is closely related to their occurrence and development.12,13 Yang et al.14,15 found that miR-136 promotes apoptosis and plays an anti-tumor role in glioma cells by inhibiting astrocyte-elevated gene1 (aeg-1) and bcl-2 genes.
Baicalin, an ancient Chinese medicine, is still widely used in clinical treatment. Radix Scutellariae element mainly comes from high scutellaria root extract, and its chemical name is 5,6,7-3 hydroxy flavone. Scutellaria baicalensis flavone compounds can inhibit inflammation, antioxidant, apoptosis, oxidative stress, and neuron degeneration and therefore are often used for the treatment of traumatic brain injury, Parkinson’s disease, lung disease, and liver disease.16–19 Baicalin also exhibits a neuroprotective effect on liver ischemia reperfusion models and liver injury diseases, and this action may be related to its regulation of oxidative stress of cells.18,19 This medicine can also activate NF-E2 related factor-2 factor to produce the antioxidant enzyme manganese superoxide dismutase, thereby scavenging free radicals and repairing mitochondrial dysfunction during the antioxidant stress response.20,21
Baicalin inhibits the metaplasia of pancreatic acinar cells AR42J in the ducts by improving the inflammatory microenvironment and preventing the hollow formation of pancreatic acinar cells, which is induced by sodium taurine cholic and the autophagy of pancreatic acinar cells. 22 This compound also reduces serum IL-6 and TNF-concentrations, pancreatic histopathological scores in SAP rats, and ascite production; prevents pancreatic injury; and improves survival rate. 23 However, the mechanism by which baicalin improves acute pancreatitis remains unclear. This study aims to determine whether baicalin ameliorates caerulein-induced oxidative stress, improves cell apoptosis, reduces cell death, and reverses caerulein-induced pancreatic acinar cell damage. TargetScan was used to verify whether SOD1 is the regulation target of miR-136-5p. This work provides new targets for the treatment of acute pancreatitis and serves as a theoretical basis for the application of radix scutellariae element.
Materials and methods
AR42J drum acinar cells in 1640 liquid containing 10% fetal bovine serum were cultured to 70% at a density of 1 × 104/mL per well at 37°C in a 5% CO2 incubator with 0.25% trypsin for 7 days at 1:2 passage. When the cells were inoculated in 25 cm2 culture flask at a density of 1 × 105/mL and reached approximately 60% fusion, they were synchronized to grow for 24 h and then grouped. Different concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) were gathered for 24 h to observe the effect of caerulein on the activity and survival of AR42J testacinar cells.24,25 The cells were cultured with baicalin at different concentrations (5−75 μM) for 2 h to observe the effect of baicalin on the caerulein-induced injury of AR42J testacinar cells and on the caerulein (10 nmol/L) solution for 24 h to measure the targeted indexes.
CCK-8 test for AR42J testacinar cell activity
CCK-8 kit (CCK-8, Septicum) was used to measure the proliferative activity of AR42J testacinar cells in various treatment groups. AR42J pacing inar cells were inoculated on 96-well plates at 70% and cultured in serum-free medium with either caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) alone for 24 h or baicalin first (25–75 μM) for 2 h and then caerulein (10 nmol/L) solution after 2 h. After 24 h of culture, 10 L of CCK-8 solution was added to each well and incubated in dark for 2−4 h. Absorbance (A450 nm) was measured with a marker at the wavelength of 450 nm.
Proliferation inhibition rate of AR42J pacing acinar cells
AR42J pacing inar cells were inoculated on 96-well plates at 70% and cultured in serum-free medium with either caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) alone for 24 h or baicalin (25−75 μM) for 2 h and then caerulein (10 nmol/L) solution after 2 h. After 24 h of culture, the liquid in each well of the 96-well plate was absorbed, added into 270 L of DMEM medium, mixed with 5 g/L MTT working solution 30 L, and incubated at 37°C for 4 h. The inhole medium was discarded, and 270 L of dimethyl sulfoxide (DMSO) was added to each well. The solution was fully dissolved after 10 min of oscillation. The OD value of each well was measured at the wavelength of 490 nm. The formula for measuring the inhibition rate of cell proliferation (%) was as follows: (1-A570 nm administration group/A570 nm blank group) × 100%. 5
Cell transfection
Appropriate quantities of AR42J dyacinar cells at logarithmic growth stage were inoculated into six-well plates overnight until the cells were adherent to 60%. According to the instructions for transfection reagent 2000, miR-136-5p mimic, miR-136-5p inhibitor, miR-136-5p mimic control, and miR-136-5p inhibitor control were transfected into AR42J tadacinar cells at 50 nmol/L. After transfection for 6 h, the old medium was replaced with a fresh one, and the cells were cultured for 24 h and then collected for PT-PCR and Western blot detection.
Flow cytometry for cell apoptosis detection
Developed cells were digested, heavily suspended, vaccinated in six orifices, and added with 2 mL of mixed suspension culture cells in each hole for 24 h. For one sample group, different concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) were added to the culture for 24 h. For another, different concentrations of baicalin (25−75 μM) were added to the culture for 2 h, followed by the incorporation of caerulein (10 nmol/L) solution and culturing for 24 h. The cells were collected, and the suspended cells were directly transferred to the centrifugal tube. The adherent cells were digested with trypsin and transferred to a centrifuge tube. The cells were then washed with PBS for three times and centrifuged at 5000 r/min for 6 min to collect the cells. Cell apoptosis was determined by flow cytometry after resuspension by using Annexin V 5 L and PI dark staining.
AO/EB assay to detect cell morphology and apoptosis
AR42J pacing acinar cells at logarithmic growth stage were inoculated in six-well plates at a density of 5 × 107/L at 3 mL per well. The cells were cultured in a constant temperature incubator until they adhered to the wall. For one treatment, caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) at different concentrations was added for 24 h. For another, baicalin (25–75 μM) with different concentrations was added for 2 h, and caerulein (10 nmol/L) solution was added after 2 h of culture. After 24 h of culture, the solution was washed twice with PBS solution, and 1 mL of PBS was added for each well. AO solution: EB solution = 1:1 working solution was prepared. Each well was added with 20 L of working solution and stored at room temperature for 5 min. Finally, the samples were observed under an inverted fluorescence microscope and photographed.
Detection of MDA and NO contents and SOD activity
AR42J testacinar cells at logarithmic growth stage were collected, washed, digested, and resuspended, and inoculated in six-well plates with 5 × 105/well. Each group was prepared with six replicates and was placed in an incubator at 37°C and 5% CO2 for overnight cultivation. The cells were cultured either with different concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h or with different concentrations of baicalin (25–75 μM) for 2 h and then with caerulein (10 nmol/L) solution for 2 h. The final volume of the cells containing the drug was 1 mL/well. After 24 h of drug action, the cells were collected and lysed into homogenate. MDA and NO content and SOD activity were determined following the reagent instructions.
Detection of intracellular reactive oxygen species
AR42J pacing inar cells at logarithmic growth stage were inoculated on six-well plates at 2 × 105/mL and cultured with either different concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h or with baicalin at different concentrations (25–75 μM) for 2 h and then with caerulein (10 nmol/L) solution for 24 h. The collected cells were suspended in serum-free medium with final concentration of 10 mol/L H2DCFDA and incubated at 37°C for 20 min. The samples were mixed upside-down every 3–5 min to allow the H2DCFDA light probe to be in full contact with the cells. The cells were washed once with serum-free cell culture medium and then twice with PBS to fully remove the H2DCFD that did not enter the cells. The cells were resuspended with 0.5−1 mL of PBS solution, and their fluorescence intensity (OD value) was determined by fluorescence spectrophotometer at the excitation wavelength of 488 nm and emission wavelength of 525 nm. Each treatment was repeated at least three times.
Target gene prediction
Target gene prediction software miR-Base (http://www.mirbase.org/), TargetScan (http://www.targetscan.org/), and PicTar (http://pictar.mdc-Berlin.DE/) were used to predict miR-136-5p target genes for SOD1. Wild-type (WT)-SOD1 and mutation-type (MUT)-SOD1 luciferase gene vectors were constructed by conventional methods, and these two plasmids were mixed and co-transfected into AR42J with miR-136-5p mimics, miR-136-5p inhibitor, mimics control and inhibitor control to produce Wt+ miR-136-5p mimics, Mut+ miR-136-5p, Wt+ negative control, and Mut+ negative control, respectively. After a 48 h culture in an incubator at 37°C and 5%CO2, dual-luciferase activity was detected by the dual-luciferase reporter gene kit. RT-PCR and Western blot were used to detect SOD1 level in AR42J overexpressing miR-136-5p.
Real-time quantitative PCR
AR42J pacing cells at logarithmic growth stage were washed, digested, and resuspended, and inoculated in six-well plates with 5 × 105/well. Each group was assigned to six compound wells and cultured overnight at 37°C in a 5% CO2 incubator. After the AR42J gathers acinar cells were collected, one group was cultured for 24 h with different concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L), and the other was cultured with baicalin at different concentrations (25–75 μM) for 2 h and then with caerulein (10 nmol/L) solution for 24 h. RNA was extracted by total RNA extraction kit and reverse-transcribed to cDNA by PrimeScript TM RT reagent kit. The expression levels of SOD1mRNA and Mir-136-5P were detected by real-time quantitative PCR with the following reaction system: SYBR Premix Ex TaqTM II 10 μL, PCR primers2 μL, DNA template 2 uL, DEPC water 6 uL, and total reaction system 20 uL. Three parallel multiple holes were prepared for each sample. The reaction system was placed on a PCR instrument for completion. TaqMan MicroRNA assay kit (Applied Biosystems, Foster City, CA, USA) was used to measure the expression level of Mir-136-5p using the 2−ããCt method.26,27 The reaction conditions were as follows: 40 cycles of pre-denaturation at 95°C for 30 s, reaction at 95°C for 5 s, annealing at 60°C for 30 s. The primer series are shown in Table 1. U6 was used as the internal reference of miR-136-5p, and β-actin was used as the internal reference of SOD1 gene expression. After the reaction, the circulating threshold (Ct) of each gene was calculated and compared with that of the internal reference gene U6 to determine the expression level.
Table 1.
Shows the primer sequences for RT-PCR analysis.
| Gene | Primer | Product (bp) |
|---|---|---|
| miR-136-5p | F-5′-AGCTGGTGTTGTGAATCAGGCCG-3′ | 198 |
| R-5′-TGGTGTCGTGGAGTCG-3′ | ||
| U6 | F-5′-CTCGCTTCGGCAGCACA-3′ | 144 |
| R-5′-AACGCTTCACGAATTTGCGT-3′ | ||
| SOD1mRNA | F-5′-AGTGATTGGCAGCAGGTC-3′ | 171 |
| R-5′-GGTGTCTGGGATGTTTAGG-3′ | ||
| β-Actin | F-5′-ATGGTGAAGGTCGGTGTG-3′ | 154 |
| R-5′-AACTTGCCGTGGGTAGAG-3′ |
Western blot analysis of the protein expression levels in cells
AR42J pacing cells at logarithmic growth stage were washed, digested, and resuspended, and inoculated in six-well plates with 5 × 105/well. Each group was prepared with six replicates an incubated at 37°C for overnight at 5% CO2. One group was cultured for 24 h with different concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L), and the other was cultured for 2 h with different concentrations of baicalin (25–75 M) and then with caerulein (10 nmol/L) solution for 2 h. The cells were collected, and lysed, and their protein concentrations were determined after 24 h of drug action. Sample buffer was added to the cell lysis buffer, heated at 100°C, and boiled for 10 min. At the time of electrophoresis, the amount of protein in each well was 40 g. Electrophoresis separation was conducted in 12% SDS-PAGE gel. The voltage of concentrated gel electrophoresis was set at 70 V, and that of gel electrophoresis separation was set at 120 V. After the separation, the membrane was transferred at 275 mA for 60 min. The NC film was sealed with 5% skim milk powder at room temperature for 1 h. SOD1, Bax, survivin, Bcl-2, caspase-3, and caspase-7 primary antibodies (1:1000) were added overnight at 4°C. After three times of TBST washing on the next day, hrP-labeled goat anti-rabbit IgG (volume dilution ratio: 1:3000) was added and reacted for 1 h at room temperature. TBST was used to wash the film for three times at 10 min each. ECL chemiluminescence solution was added for color development. Image Lab software was employed to analyze the gray value of the strip, and β-actin was used as internal reference.
Determination of amylase activity
The iodine-starch colorimetry kit of Nanjing Jiancheng Biological Engineering Research Institute was used and operated following the instructions. The calculation formula was as follows: Culture medium supernatant amylase (U/dL) = (blank tube absorbance − measuring tube absorbance)/blank tube absorbance × 800.
Statistical analysis
All data were expressed as mean ± standard deviation, and SPSS 17.0 statistical software package was used for statistical analysis. LSD-T test was used for pairwise comparison, and one-way ANOVA was used for data measurement after the non-normal distribution data were converted into normal distribution data. Statistical data were tested by two test, and p < 0.05 was considered statistically significant.
Results
Effect of caerulein on the viability and proliferation inhibition rate of AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h to observe the effect of caerulein on AR42J pancreatic acinar cells viability and proliferation inhibition rate. AR42J pancreatic acinar cell viability was measured using CCK-8, and proliferation inhibition rate was determined using MTT assay. As shown in Figure 1, caerulein concentration-dependently decreased the viability and proliferation inhibition rate of AR42J pancreatic acinar cells. Caerulein concentrations as low as 10 nmol/L effectively decreased the viability and proliferation inhibition rate of AR42J pancreatic acinar cells.
Figure 1.
Effect of caerulein on AR42J pancreatic acinar cells viability, proliferation inhibition rate, apoptosis, and cells death rate. AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h. AR42J pancreatic acinar cells viability was measured using CCK-8, proliferation inhibition rate was determined using MTT assay, and cells apoptosis and cells death rate was determined using flow cytometry. Data from three independent experiments are presented as mean ± SEM.
*p < 0.05 versus the control group. #p < 0.05 versus the caerulein (7.5 nmol/L) group. p > 0.05 the caerulein (10 nmol/L) group versus the caerulein (15 nmol/L) group.
Effect of caerulein on the apoptosis and death rate of AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h to analyze the effect of caerulein on cell apoptosis. Apoptosis and cell death rate were determined using flow cytometry. As shown in Figure 1, caerulein concentration-dependently increased the apoptosis and decreased the death rate of AR42J pancreatic acinar cells. Caerulein concentrations as low as 10 nmol/L effectively the apoptosis and decreased the death of AR42J pancreatic acinar cells.
Effect of caerulein on oxidative stress in AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h to explain the effect of caerulein on their oxidative stress. The activities of ROS, MDA, GSH, and SOD1 in AR42J pancreatic acinar cells were determined. As shown in Figure 2, caerulein concentration-dependently decreased the activities of GSH and SOD1 and increased the activities of ROS and MDA in AR42J pancreatic acinar cells. Caerulein concentrations as low as 10 nmol/L effectively decreased the activities of GSH and SOD1 and increased the activities of ROS and MDA in AR42J pancreatic acinar cells.
Figure 2.
Effect of caerulein on oxidative stress in AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h. The activities of ROS, MDA, GSH, and SOD1 in AR42J pancreatic acinar cells were determined. Data from three independent experiments are presented as mean ± SEM.
*p < 0.05 versus the control group. #p < 0.05 versus the caerulein (7.5 nmol/L) group. p > 0.05 the caerulein (10 nmol/L) group versus the caerulein (15 nmol/L) group.
Effect of caerulein on Bax, survivin, Bcl-2, caspase-3, and caspase-7 protein expression in AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h to investigate the effect of caerulein on Bax, survivin, Bcl-2, caspase-3, and caspase-7 protein expression in these cells. The protein expression levels of Bax, survivin, Bcl-2, caspase-3, and caspase-7 in AR42J pancreatic acinar cells were determined by Western blot analysis. As shown in Figure 3, caerulein concentration-dependently increased the expression of survivin and Bcl-2 protein and decreased the expression of Bax, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells. Caerulein concentrations as low as 10 nmol/L effectively decreased the activities of survivin and Bcl-2 protein and increased the expression of Bax, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells.
Figure 3.
Effect of caerulein on Bax, survivin, Bcl-2, caspase-3, caspase-7 protein expression in AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h. The activities of Bax, survivin, Bcl-2, caspase-3, caspase-7 protein expression in AR42J pancreatic acinar cells were determined by Western blot analysis. Data from three independent experiments are presented as mean ± SEM.
*p < 0.05 versus the control group. #p < 0.05 versus the caerulein (7.5 nmol/L) group. p > 0.05 the caerulein (10 nmol/L) group versus the caerulein (15 nmol/L) group.
miR-136-5p inhibitor increased the apoptosis and decreased the death rate of AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h. Cell apoptosis and death rate were determined using flow cytometry. As shown in Figure 4, miR-136-5p inhibitor increased the apoptosis and decreased the death rate of AR42J pancreatic acinar cells. By contrast, miR-136-5p mimic decreased the apoptosis and increased the death rate of AR42J pancreatic acinar cells.
Figure 4.
Effect of miR-136-5p inhibitor on AR42J pancreatic acinar cells apoptosis, decreased cells death rate, viability, and proliferation rate. The AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h: (a and b) AR42J pancreatic acinar cells apoptosis and cells death rate was determined using flow cytometry, (c) AR42J pancreatic acinar cells viability was measured by CCK-8, and (d) AR42J pancreatic acinar cells proliferation rate was measured by MTT. The data from three independent experiments are presented as the mean ± SD.
*p < 0.05 versus the miR-136-5p mimic control group. #p < 0.05 versus the miR-136-5p inhibitor control group.
miR-136-5p inhibitor increased the viability and proliferation rate of AR42J pancreatic acinar cells.
AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h. Cell viability was measured by CCK-8, and proliferation rate was measured by MTT. As shown in Figure 4, miR-136-5p inhibitor increased the viability and proliferation rate of AR42J pancreatic acinar cells. By contrast, miR-136-5p mimic decreased their viability and proliferation rate.
miR-136-5p inhibitor attenuated oxidative stress in AR42J pancreatic acinar cells.
AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h. The activities of ROS, MDA, GSH, and SOD1 in AR42J pancreatic acinar cells were determined. As shown in Figure 5, miR-136-5p inhibitor increased the viability and proliferation rate of AR42J pancreatic acinar cells. By contrast, miR-136-5p mimic decreased their viability and proliferation rate.
Figure 5.
Effect of miR-136-5p inhibitor on oxidative stress in AR42J pancreatic acinar cells. The AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h. The activities of ROS, MDA, GSH, and SOD1 in AR42J pancreatic acinar cells were determined. Data from three independent experiments are presented as mean ± SEM. AR42J pancreatic acinar cells proliferation rate was measured by MTT. The data from three independent experiments are presented as the mean ± SD.
*p < 0.05 versus the miR-136-5p mimic control group. #p < 0.05 versus the miR-136-5p inhibitor control group.
miR-136-5p inhibitor decreased miR-136-5p expression and increased SOD1 expression in caerulein-induced AR42J pancreatic acinar cells.
AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h. MiR-136-5p expression in AR42J pancreatic acinar cells was determined by RT-PCR, and SOD expression was determined by Western blot. As shown in Figure 6, miR-136-5p inhibitor decreased miR-136-5p expression and increased SOD expression in AR42J pancreatic acinar cells.
Figure 6.
Effect of miR-136-5p inhibitor on miR-136-5p and SOD1 expression in AR42J pancreatic acinar cells. The AR42J pancreatic acinar cells were transfected with miR-136-5p mimic and miR-136-5p inhibitor in the presence of 10 nmol/L caerulein for 24 h. The levels of miR-136-5p expression in AR42J pancreatic acinar cells were determined by RT-PCR. The levels of SOD expression in AR42J pancreatic acinar cells were determined by Western blot. Data from three independent experiments are presented as mean ± SD.
*p < 0.05 versus the control group. #p < 0.05 versus the model group. *p < 0.05 versus the miR-136-5p mimic control group. #p < 0.05 versus the miR-136-5p inhibitor control group.
Effect of baicalin on the oxidative stress, viability, apoptosis, and death rate of AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of baicalin (10, 25, 50, and 75 μM) for 24 h to investigate the effect of baicalin on the oxidative stress, viability, apoptosis, and death rate of these cells. Apoptosis and cell death rate were determined using flow cytometry. Cell viability was measured by CCK-8. ROS, MDA, and SOD1 activities in AR42J pancreatic acinar cells were also determined. As shown in Figure 7, baicalin treatment did not substantially change the ROS, MDA, and SOD1 activities; viability; apoptosis; and death rate of AR42J pancreatic acinar cells.
Figure 7.
Effect of baicalin on AR42J pancreatic acinar cells oxidative stress, viability, apoptosis, and death rate. AR42J pancreatic acinar cells were incubated with various concentrations of baicalin (10, 25, 50, and 75 μM) for 24 h. AR42J pancreatic acinar cells apoptosis and cells death rate was determined using flow cytometry. AR42J pancreatic acinar cells viability was measured by CCK-8. The activities of ROS, MDA, and SOD1 in AR42J pancreatic acinar cells were determined. Data from three independent experiments are presented as mean ± SD.
p > 0.05 versus the control group.
Effect of baicalin on the mRNA expression of miR-136-5p and SOD1 and the protein expression of Bax, Bcl-2, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of baicalin (10, 25, 50, and 75 μM) for 24 h to observe effect of baicalin on the mRNA expression of miR-136-5p and SOD1 and the protein expression of Bax, Bcl-2, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells. The mRNA expression of miR-136-5p and SOD1 in AR42J pancreatic acinar cells was measured by RT-PCR, and the protein expression of Bax, Bcl-2, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells was measured by Western blot. As shown in Figure 8, baicalin treatment did not substantially change the expression of miR-136-5p and SOD1 mRNA and the protein expression of Bax, Bcl-2, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells.
Figure 8.
Effect of baicalin on the mRNA expression of miR-136-5p and SOD1 and the protein expression Bax, Bcl-2, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with various concentrations of baicalin (10, 25, 50, and 75 μM) for 24 h. The mRNA expression of miR-136-5p and SOD1 in AR42J pancreatic acinar cells was measured by RT-PCR, the protein expression Bax, Bcl-2, caspase-3, and caspase-7 protein in AR42J pancreatic acinar cells were measured by Western blotting. Data from three independent experiments are presented as mean ± SD.
p > 0.05 versus the control group.
Effect of baicalin on the mRNA and protein expression of miR-136-5p and SOD1 in caerulein-induced AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h to analyze the effect of caerulein on miR-136-5p and SOD1 mRNA and protein expression. miR-136-5p and SOD1 mRNA expression was measured by RT-PCR, and SOD1 protein expression was detected by Western blot analysis. As shown in Figure 9, caerulein concentration-dependently increased the miR-136-5p expression and reduced the SOD1 mRNA and protein expression in AR42J pancreatic acinar cells. Caerulein concentrations as low as 10 nmol/L effectively increased the miR-136-5p expression and reduced the SOD1 mRNA and protein expression in AR42J pancreatic acinar cells. For further analysis, AR42J pancreatic acinar cells were incubated with 10 nmol/L caerulein and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h. MiR-136-5p and SOD1 mRNA expression was measured using RT-PCR, and SOD1 protein expression was detected using Western blot. Baicalin concentration-dependently decreased the miR-136-5p expression and increased the SOD1 mRNA and protein expression in caerulein-induced AR42J pancreatic acinar cells. Baicalin concentrations as low as 50 μM effectively decreased the miR-136-5p expression and increased the SOD1mRNA and protein expression in caerulein-induced AR42J pancreatic acinar cells.
Figure 9.
Effect of baicalein on miR-136-5p and SOD1 mRNA and protein expression in caerulein induced AR42J pancreatic acinar cells: (a) effect of caerulein on miR-136-5p and SOD1 mRNA and protein expression in caerulein induced AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with various concentrations of caerulein (2.5, 5.0, 7.5, 10, and 15 nmol/L) for 24 h. miR-136-5p and SOD1 mRNA expression were measured by RT-PCR. SOD1 protein expression by Western blot analysis. Data from three independent experiments are presented as mean ± SEM. *p < 0.05 versus the control group. #p < 0.05 versus the caerulein (7.5 nmol/L) group. p > 0.05 the caerulein (10 nmol/L) group versus the caerulein (15 nmol/L) group. (b) Effect of Baicalein on miR-136-5p and SOD1 mRNA and protein expression in caerulein induced AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h. miR-136-5p and SOD1 mRNA expression were measured by RT-PCR. SOD1 protein expression by Western blot analysis. Data from three independent experiments are presented as mean ± SEM.
(a) *p < 0.05; **p < 0.01 versus the control group. (b) *p < 0.05; **p < 0.01 versus the control group; #p < 0.05; ##p < 0.01versus the only caerulein group.
Effect of baicalin on oxidative stress in caerulein-induced AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h to evaluate the effect of baicalin on the caerulein-induced oxidative stress in AR42J pancreatic acinar cells. ROS, MDA, GSH, and SOD1 activities in AR42J pancreatic acinar cells apoptosis were determined. As shown in Figure 10, baicalin concentration-dependently decreased the caerulein-induced apoptosis in AR42J pancreatic acinar cells (Figure 10(a)–(d)). Approximately 50 μM baicalin effectively decreased the caerulein-induced apoptosis of AR42J pancreatic acinar cells (Figure 10(a)–(d)).
Figure 10.
Effect of baicalein on oxidative stress in caerulein induced AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h. ROS, MDA, GSH, and SOD1 in AR42J pancreatic acinar cells apoptosis was determined. Data from three independent experiments are presented as mean ± SD.
*p < 0.05 versus the control group. #p < 0.05 versus the baicalin (25 μM) group. p > 0.05 the baicalin (50 μM) group versus the baicalin (75 μM) group.
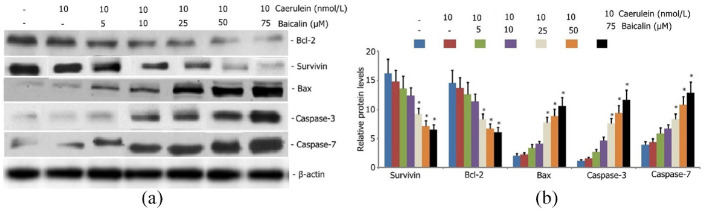
Effect of baicalin on survivin, Bax, Bcl-2, caspase-3, and caspase-7 protein expression in caerulein-induced AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h to investigate the effect of baicalin on survivin, Bax, Bcl-2, caspase-3, and caspase-7 protein expression in caerulein-induced AR42J pancreatic acinar cells. Survivin, Bax, Bcl-2, caspase-3, and caspase-7 protein expression levels were measured by Western blot analysis. As shown in Figure 11, baicalin increased the Bax, caspase-3, and caspase-7 protein expression levels and reduced the survivin and Bcl-2 protein expression levels in caerulein-induced AR42J pancreatic acinar cells. Baicalin concentrations as low as 50 μM effectively increased the Bax, caspase-3, and caspase-7 protein expression levels and decreased the survivin and Bcl-2 protein expression levels in caerulein-induced AR42J pancreatic acinar cells
Figure 11.
Effect of baicalein on survivin, Bax, Bcl-2, caspase-3, caspase-7 protein expression in caerulein induced AR42J pancreatic acinar cells. AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h. Survivin, Bax, Bcl-2, caspase-3, caspase-7 protein expression levels were measured by Western blot analysis. Data from three independent experiments are presented as mean ± SEM.
*p < 0.05. **p < 0.01 versus the only caerulein group.
Baicalin decreased the supernatant amylase content in caerulein-induced AR42J pancreatic acinar cells
Amylase activity is an important indicator that reflects pancreatic damage. The effect of baicalin on supernatant amylase in caerulein-induced AR42J pancreatic acinar cells was investigated. AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h. Supernatant amylase AMS in culture medium was measured. As shown in Figure 12, baicalin concentration-dependently decreased the supernatant amylase in caerulein-induced AR42J pancreatic acinar cells.
Figure 12.
Effect of baicalin on AR42J pancreatic acinar cells supernatant amylase, viability, proliferation inhibition rate, apoptosis, and death rate. AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h: (a) culture medium supernatant amylase AMS was measured, (b) AR42J pancreatic acinar cells viability was measured using CCK-8, (c) AR42J pancreatic acinar cells proliferation inhibition rate was determined using MTT, (d and e) AR42J pancreatic acinar cells apoptosis and death rate was determined using flow cytometry. Data from three independent experiments are presented as mean ± SEM.
*p < 0.05; **p < 0.01 versus the control group. #p < 0.05; ##p < 0.01 versus the only caerulein group.
Effect of baicalin on the viability and proliferation inhibition rate of caerulein-induced AR42J pancreatic acinar cells
Caerulein-induced AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h to observe the effect of baicalin on the viability and growth inhibition rate of these cells. Cell viability was measured using CCK-8, and proliferation inhibition rate was determined using MTT. As shown in Figure 12, baicalin concentration-dependently increased the cell viability and proliferation inhibition rate in caerulein-induced AR42J pancreatic acinar cells (Figure 12(a) and (b)). Approximately 50 μM baicalin effectively increased the cell viability and proliferation inhibition rate in caerulein-induced AR42J pancreatic acinar cells (Figure 12(a) and (b)).
Effect of baicalin on the apoptosis and death rate of caerulein-induced AR42J pancreatic acinar cells
AR42J pancreatic acinar cells were incubated with caerulein (10 nmol/L) and various concentrations of baicalin (5, 10, 25, 50, and 75 μM) for 24 h to evaluate effect of baicalin on caerulein-induced apoptosis of AR42J pancreatic acinar cells. Cell apoptosis was determined using flow cytometry. As shown in Figure 12, baicalin concentration-dependently increased the apoptosis and decreased the death rate of caerulein-induced AR42J pancreatic acinar cells (Figure 12(c) and (d)). Approximately 50 μM baicalin effectively increased the caerulein-induced apoptosis of AR42J pancreatic acinar cells (Figure 12(c) and (d)).
SOD1 is a regulatory target of miR-136-5p
Targetscan was used to predict the possible targets of miR-136-5p and determine how miR-136-5p mediates caerulein-induced oxidative stress in AR42J pancreatic acinar cells. SOD1 was found to be an important regulator of caerulein-induced oxidative stress in these cells. Analysis revealed that SOD1 mRNA and protein levels were dramatically decreased after miR-136-5p overexpression (Figure 13(a)–(c)). Wild-type and mutant SOD1 were constructed for dual luciferase reporter assay to validate the interaction between miR-136-5p and SOD1. MiR-136-5p bound to the wild-type SOD1, rather than to the mutants (Figure 13(d) and (e)).
Figure 13.
SOD1 is a downstream target of miR-136-5p: (a) decreased mRNA levels of SOD1 in caerulein-induced AR42J pancreatic acinar cells after miR-136-5p overexpression (p < 0.05). Levels of SOD1 mRNA were enhanced after miR-136-5p downregulation (p < 0.05), (b and c) levels of SOD1 protein was decreased in caerulein-induced AR42J pancreatic acinar cells with miR-136-5p overexpression relative to the empty vector control (p < 0.05). Levels of SOD1 protein was significantly increased in cells with miR-136-5p downregulation. (d) miR-136-5p was bound to the 3′-UTR regions of SOD1. Binding was interrupted in mutant SOD1. (e) Dual luciferase reporter assay indicated that miR-136-5p mimic was bound to the 3′-UTR region of the wild-type SOD1, rather than to SOD1 mutants (p < 0.05).
Correlation between SOD1 and miR-136-5p in AR42J pancreatic acinar cells
A decreased SOD1 protein was found in the caerulein-induced AR42J pancreatic acinar cells with miR-136-5p overexpression relative to that in the empty vector control. SOD1 protein was significantly increased in the cells with miR-136-5p downregulation. Further analysis revealed a negative correlation between SOD1 protein and miR-136-5p expression in caerulein-induced AR42J pancreatic acinar cells (r = −0.397, p < 0.05).
Correlation between AR42J pancreatic acinar cells apoptosis and death rate
Substantially increased apoptosis and decreased death were noted in caerulein-induced AR42J pancreatic acinar cells. Further analysis revealed a negative correlation between apoptosis and death rate in caerulein-induced AR42J pancreatic acinar cells (r = −0.508, p < 0.05).
Discussion
As a polypeptide that has similar chemical structure and pharmacological action to cholecystokinin, frogs’ peptide has strong stimulating effect on gallbladder contraction and trypsin secretion. Hyla peptide can cause abnormalities in pancreatic enzyme activation, apoptosis gene expression, and calcium channel, thus inducing pancreatic acinar cell apoptosis and causing tissue damage. 28 Many models for pancreatitis acinar cell AP have been established. Hylapeptide or hyla peptides combined with lipopolysaccharide (LPS) processing cell AP model are easy to operate. 29 AR42J cells are rat pancreatic exocrine cells, which act as the cellular receptor expression and signal transduction similar to normal pancreatic acinar cells. In addition, its culture is free from other interference, its research system is simple and beneficial because of its high transfection efficiency and high sensitivity to stimulation. 30 Therefore, this study used frog’s peptide to treat pancreatic AR42J to establish an AP model.
Acute pancreatitis is a local and systemic inflammatory response triggered by the death of pancreatic acinar cells. Apoptosis and necrosis are the two main forms of acinar cell death. Compared with necrosis, apoptosis is an autonomous and orderly cell death mode regulated by internal genes. Pancreatic acinar cell apoptosis occurs without pancreatic enzyme leakage; hence, inducing acinar cell apoptosis can reduce the disease progression of SAP (or AP).31,32 In this experiment, a small dose of rainfrog peptide increased the apoptosis of pancreatic acinar cells. As a result, many pancreatic acinar cells died, and the number of apoptotic cells gradually decreased. After baicalin treatment, supernatant amylase content and pancreatic acinar cell death were reduced, and the number of apoptotic cells gradually increased. This finding suggested that baicalin has a protective effect on pancreatic acinar cells induced by frog’s peptide; however, the mechanism remains unclear. Further exploration was therefore conducted. Under the action of various stimulators, death receptors or intracellular death signals are activated to form apoptotic complexes, in which caspase-9 is released and downstream caspase-3 and 7 substrates are activated to induce apoptosis.33,34 Caspase 3 can interact with the BCL-2 family. When a signal induces apoptosis to stimulate the cell, Bax is moved from cytoplasm to the mitochondria. Hence, the mitochondrial membrane activity 34 is decreased, and cytochrome C is released. Cytochrome C, apoptosis promoting factor 1 (Apaf-1), and caspase-9 combine into complex, in ATP, caspase protease activation cascade cause apoptosis. 35 Bcl-2 protein can be cleaved by caspase-3, and the fragment formed after the clipping further promotes the activation of downstream caspase-3 and the amplification of the caspase cascade reaction. 33 Hence, apoptosis is promoted. Survivin is a member of the inhibitor of apoptosis protein (IAP) family 36 and can inhibit the activity of caspase-3 and caspase-7. 36 In this experiment, a small dose of hyla peptide increased the protein expression of Bax, caspase-3, and caspase-7 but reduced that of the anti-apoptosis proteins survivin and Bcl-2 in pancreatic acinar cells. With the increase in hyla peptide dose, Bax, caspase 3, caspase-7 expression levels were reduced, and whereas those of anti-apoptosis proteins survivin and Bcl-2 were increased. In addition, pancreatic acinar cell apoptosis was reduced, and pancreatic acinar cell death was increased. After baicalin treatment, the expression levels of Bax, caspase-3, and caspase-7 were increased, whereas those of survivin and Bcl-2 decreased. In addition, cell apoptosis was increased, and cell death was decreased. This finding suggested that baicalin could increase the expression of pro-apoptotic proteins and inhibit the expression of anti-apoptotic proteins. This compound could also promote the apoptosis of pancreatic acinus cells, reduce the death of pancreatic acinus cells, and exhibit a protective effect on pancreatic acinus cells in acute pancreatitis.
Oxidative stress is the imbalance of cells and tissues between oxidation and anti-oxidation system, exposure to excessive oxidative stress, leading to increased intracellular ROS. ROS may be reduced by increasing the oxidative stress cell mitochondrial membrane potential, lead to mitochondrial dysfunction, impact energy supply in the cell, mitochondria mediated apoptosis induction, and even necrosis. 37 SOD is an important antioxidant enzyme that can remove excessive ROS in cells and protect cells from oxidative damage caused by free radicals. Glutathione (GSH) is an important substance to reduce intracellular oxidative stress. When GSH-PX action is reduced, the production of GSH is also reduced, leading to intracellular oxidative stress response. As a product of lipid peroxidation, MDA level can reflect the degree of lipid peroxidation in oxidative damage to a certain extent. 38 In this experiment, rainfrog peptide significantly reduced the activity of SOD and GSH, increased the production of ROS, thus increasing the death of pancreatic acinar cells and decreasing the cell apoptosis. After baicalin treatment, the activities of SOD and GSH were increased, the production of ROS was decreased. Consequently, the death of pancreatic acini cells was decreased, and the apoptosis was increased. This finding suggested that baicalin can significantly increase the activity of SOD and reduce the production of ROS. It can also promote the apoptosis of pancreatic acini cells, reduce the death of pancreatic acini cells, and exhibit a protective effect on pancreatic acini cells in acute pancreatitis.
MiR-136 promotes LPS-induced ATDC5 cell damage and inflammatory cytokine expression by targeting myeloid leukemia 1. 38 Overexpressed Mir-136-5p inhibited the expression of A20 protein and promoted the production of inflammatory factors, chemokines, and P-NF-kB in rats with acute spinal cord injury. It also increased the infiltration and injury of inflammatory cells in the spinal cord. 39 Silencing Mir-136-5p significantly reduced the protein expression outcomes after Mir-136-5p overexpression and improved the inflammatory cell infiltration and spinal cord injury. 40 In this experiment, rainfrog peptide increased the expression of Mir-136-5p, reduced the expression of SOD1 protein, and promoted the production of ROS, thus leading to increased death rate and decreased apoptosis of pancreatic acinar cells. Baicalin treatment can reduce the expression of Mir-136-5p, increase the expression of SOD1 protein, and reduce the production of ROS, thus decreasing pancreatic acinus cell death and increasing the apoptosis. Therefore, baicalin could reduce the expression of mir-136-5p, increase the expression of SOD1 protein, and exhibit a protective effect on pancreatic acinus cells during acute pancreatitis. Mir-136-5p and SOD1 regulate apoptosis. After transfection with AR42J cells, Mir-136-5p inhibitor decreased the expression of Mir-136-5p and increased the expression of SOD1. After transfection with AR42J cells, Mir-136-5p mimic increased the expression of Mir-136-5p and decreased the expression of SOD1. Therefore, Mir-136-5p might regulate the apoptosis and death of pancreatic acinus cells by targeting the regulation of SOD1 and the oxidative stress response induced by frog’s peptide. To prove the correlation between Mir-136-5p and SOD1 expression, we predicted that SOD1 was the regulatory target of Mir-136-5p through Targetscan software. Wild-type and mutant SOD1 were constructed, and dual luciferase reporter gene detection was conducted. The result showed that Mir-136-5p bound to wild-type SOD1, but not to mutants, confirming that SOD1 is the regulatory target of Mir-136-5P.
In conclusion, miR-136-5p may regulate the expression of SOD1 and the hyla peptide-induced apoptosis and death of pancreatic acinar cells. Baikal skullcap root element can reduce the expression of miR-136-5p and increase the expression of SOD1. It can also promote the hyla-peptide-induced apoptosis and reduce the death rate of pancreatic acinar cells, thus improving pancreatic acinar cell injury after acute pancreatitis. This work has limitations. For example, the regulation of baicalin on pancreatitis may involve inflammation, autophagy, and oxidative stress. The mechanism is still unclear and needs further study. In addition, this study did not prove that baicalin regulates the ED50 and MIC of AR42J cells. Further exploration on this effect is needed.
Acknowledgments
The authors are grateful for the excellent technical assistance provided by Prof. Li Hao.
Author biographies
Zhu-fen Zhao is an Attending physician in Institute of the First Affiliated Hospital of Kunming Medical University. and has long been engaged in Intensive Care Unit. His research focuses on immunomodulatory treatment of sepsis.
Ye Zhang is an Attending physician in Institute of the Third People’s Hospital of Yunnan Province, and has long been engaged in Traditional Chinese Medicine. His research focuses on lung injury.
Yang Sun is an Associate Professor in Institute of the Sixth Affiliated Hospital of Kunming Medical University. and has long been engaged in Nephrology. His research focuses on immunomodulatory treatment of kidney injury.
Chun-hai Zhang is an Attending physician in Institute of the First Affiliated Hospital of Kunming Medical University, and has long been engaged in Emergency medicine. His research focuses on immunomodulatory treatment of sepsis.
Ming-wei Liu is an Associate Professor in Institute of the First Affiliated Hospital of Kunming Medical University, and has long been engaged in Emergency medicine. His research interest lies in the immunomodulatory treatment of sepsis, the regulation of immune disorders in acute lung injury and pulmonary fibrosis.
Footnotes
Author contributions: Conceived and designed the experiments: YZ, YH, and WX; Performed the experiments: YZ and YH; Analyzed the data: ND, YC, and JCH. Contributed reagents/materials/analysis tools: YC and JCH. Wrote the paper: YZ. All authors have contributed to, seen, and approved the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the National Natural Science Foundation of China (Grant No. 81960350).
ORCID iD: Ming-wei Liu  https://orcid.org/0000-0002-3728-2350
https://orcid.org/0000-0002-3728-2350
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
References
- 1.Amas Gómez L, Zubia Olaskoaga F. Results of the modification of an acute pancreatitis management protocol in Intensive Care medicine. Med Intensiva 2019; 43(9): 546–555. [DOI] [PubMed] [Google Scholar]
- 2.Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ 2019; 367: l6227. [DOI] [PubMed] [Google Scholar]
- 3.Ma Z, Song G, Zhao D, et al. Bone marrow-derived mesenchymal stromal cells ameliorate severe acute pancreatitis in rats via hemeoxygenase-1-mediated anti-oxidant and anti-inflammatory effects. Cytotherapy 2019; 21(2): 162–174. [DOI] [PubMed] [Google Scholar]
- 4.Lv C, Jin Q. Maresin-1 inhibits oxidative stress and inflammation and promotes apoptosis in a mouse model of caerulein-induced acute pancreatitis. Med Sci Monit 2019; 25: 8181–8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Máté G, Gazdag Z, Mike N, et al. Regulation of oxidative stress-induced cytotoxic processes of citrinin in the fission yeast Schizosaccharomyces pombe. Toxicon 2014; 90: 155–166. [DOI] [PubMed] [Google Scholar]
- 6.Wang CM, Huo X, Chen J, et al. An acute lytic cell death induced by xanthohumol obstructed ROS detecting in HL-60 cells. Toxicol In Vitro 2020; 62: 104667. [DOI] [PubMed] [Google Scholar]
- 7.Ren YF, Wang MZ, Bi JB, et al. Irisin attenuates intestinal injury, oxidative and endoplasmic reticulum stress in mice with L-arginine-induced acute pancreatitis. World J Gastroenterol 2019; 25(45): 6653–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong K, Chen X, Xie L, et al. Spautin-A41 attenuates cerulein-induced acute pancreatitis through inhibition of dysregulated autophagy. Biol Pharm Bull 2019; 42(11): 1789–1798. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong JA, Cash NJ, Ouyang Y, et al. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem 2018; 293(21): 8032–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo CJ, Hou WH, Jiang DG, et al. Circular RNAs in early brain development and their influence and clinical significance in neuropsychiatric disorders. Neural Regen Res 2020; 15(5): 817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez B, Peplow PV. MicroRNAs in blood and cerebrospinal fluid as diagnostic biomarkers of multiple sclerosis and to monitor disease progression. Neural Regen Res 2020; 15(4): 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao RZ, Que Q, Lin P, et al. Clinical roles of miR-136-5p and its target metadherin in thyroid carcinoma. Am J Transl Res 2019; 11(11): 6754–6774. [PMC free article] [PubMed] [Google Scholar]
- 13.Jin C, Wang A, Liu L, et al. Hsa_circ_0136666 promotes the proliferation and invasion of colorectal cancer through miR-136/SH2B1 axis. J Cell Physiol 2019; 234(5): 7247–7256. [DOI] [PubMed] [Google Scholar]
- 14.Li DX, Fei XR, Dong YF, et al. The long non-coding RNA CRNDE acts as a ceRNA and promotes glioma malignancy by preventing miR-136-5p-mediated downregulation of Bcl-2 and Wnt2. Oncotarget 2017; 8(50): 88163–88178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Wu J, Guan H, et al. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Lett 2012; 586(20): 3608–3612. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Yang Y, Du L, et al. Baicalin exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int Immunopharmacol 2017; 50: 38–47. [DOI] [PubMed] [Google Scholar]
- 17.Zhao WZ, Wang HT, Huang HJ, et al. Neuroprotective effects of baicalin on acrolein-induced neurotoxicity in the nigrostriatal dopaminergic system of rat brain. Mol Neurobiol 2018; 55(1): 130–137. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Zhang Y, Zhang W, et al. Inhibition of myeloid differentiation factor 2 by baicalin protects against acute lung injury. Hytomedicine 2019; 63: 152997. [DOI] [PubMed] [Google Scholar]
- 19.Wu R, Murali R, Kabe Y, et al. Baicalin targets GTPase-mediated autophagy to eliminate liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition. Hepatology 2018; 68(5): 1726–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai C, Tang S, Wang Y, et al. Baicalin acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J Antimicrob Chemother 2017; 72(9): 2562–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Zhang T, Wang Y, et al. Baicalin ameliorates neuropathology in repeated cerebral ischemia–reperfusion injury model mice by remodeling the gut microbiota. Aging (Albany NY) 2020; 12(4): 3791–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu WL, Luo YY, Bai RY, et al. Baicalin inhibits acinar-to-ductal metaplasia of pancreatic acinal cell AR42J via improving the inflammatory microenvironment. J Cell Physiol 2018; 233(8): 5747–5755. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wu Y, Zhang S, et al. Baicalin protect pancreatic injury in rats with severe acute pancreatitis by inhibiting pro-inflammatory cytokines expression. Biochem Biophys Res Commun 2015; 466(4): 664–649. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Tian J, Li J. MiR-92b-3p ameliorates inflammation and autophagy by targeting TRAF3 and suppressing MKK3-p38 pathway in caerulein-induced AR42J cells. Int Immunopharmacol 2020; 88: 106691. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Chen B, Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1alpha/BNIP3 pathway. Artif Cells Nanomed Biotechnol 2019; 47(1): 3657–3663. [DOI] [PubMed] [Google Scholar]
- 26.Rao X, Huang X, Zhou Z, et al. An improvement of the 2(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 2013; 3(3): 71–85. [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Cheng X, MingYu Q, et al. The effects of microRNA-1224-5p on hepatocellular carcinoma tumor endothelial cells. J Cancer Res Ther 2019; 15(2): 329–335. [DOI] [PubMed] [Google Scholar]
- 28.Pan X, Fang X, Wang F, et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol 2019; 176(23): 4446–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang HL, Tang GD, Liang ZH, et al. Role of Wnt/β-catenin pathway agonist SKL2001 in caerulein-induced acute pancreatitis. Can J Physiol Pharmacol 2019; 97(1): 15–22. [DOI] [PubMed] [Google Scholar]
- 30.Jian J, Li S, Fang N, et al. Pim-3 alleviates lipopolysaccharide-stimulated AR42J pancreatic acinar cell injury via improving the inflammatory microenvironment. Exp Ther Med 2019; 18(6): 4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munir F, Jamshed MB, Shahid N, et al. Advances in immunomodulatory therapy for severe acute pancreatitis. Immunol Lett 2019; 217: 72–76. [DOI] [PubMed] [Google Scholar]
- 32.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol 2019; 16(8): 479–496. [DOI] [PubMed] [Google Scholar]
- 33.Lyu D, Tang N, Womack AW, et al. Neonatal ketamine exposure-induced hippocampal neuroapoptosis in the developing brain impairs adult spatial learning ability. Neural Regen Res 2020; 15(5): 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao C, Chen W, Tan X, et al. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J Ethnopharmacol 2020; 247: 112256. [DOI] [PubMed] [Google Scholar]
- 35.Fang X, Miao XL, Liu JL, et al. Berberine induces cell apoptosis through cytochrome C/apoptotic protease-activating factor 1/caspase-3 and apoptosis inducing factor pathway in mouse insulinoma cells. Chin J Integr Med 2019; 25(11): 853–860. [DOI] [PubMed] [Google Scholar]
- 36.Abbaszadeh H, Valizadeh A, Mahdavinia M, et al. 3-Bromopyruvate potentiates TRAIL-induced apoptosis in human colon cancer cells through a reactive oxygen species- and caspase-dependent mitochondrial pathway. Can J Physiol Pharmacol 2019; 97(12): 1176–1184. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Wang Y, Li C, et al. The effect of benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) treatment on regulation of reactive oxygen species metabolism involved in wound healing of potato tubers during postharvest. Food Chem 2020; 309: 125608. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Xiong Y, Zhou L, et al. Soluble E-cadherin is associated with oxidative stress in patients with chronic HBV infection. J Med Virol 2020; 92(1): 34–44. [DOI] [PubMed] [Google Scholar]
- 39.Peng X, Shi X, Zhao J, et al. The effects of miR-136-5p-mediated rgulation of A20 in astrocytes from cultured spinal cord cultured cells in vitro. Cell Physiol Biochem 2017; 41(4): 1596–1604. [DOI] [PubMed] [Google Scholar]
- 40.Deng G, Gao Y, Cen Z, et al. miR-136-5p regulates the inflammatory response by targeting the IKKβ/NF-κB/A20 pathway after spinal cord injury. Cell Physiol Biochem 2018; 50(2): 512–524. [DOI] [PubMed] [Google Scholar]