Summary
Background
Congenital hypothyroidism (CH) is the leading cause of preventable mental retardation, which is currently not universally screened in India. Knowledge of the country-specific prevalence of the disease can guide in establishing a universal screening program.
Methods
We performed a systematic review and meta-analysis to assess the prevalence, screen positivity rates, compliance to recall and etiology of CH in India. The databases of PubMed, Embase, Google scholar and IMSEAR were searched on 1st October 2021. All observational studies reporting at least one of the outcomes of interest were included. Two reviewers independently extracted the data and appraised the quality of studies using the Joanna Briggs tool for prevalence studies. Estimates were pooled using a random-effects model with double arcsine transformation (MetaXL software). PROSPERO database registration number was CRD42021277523.
Findings
Of the 2 073 unique articles retrieved, 70 studies were eligible for inclusion. The prevalence of CH (per 1 000 neonates screened) was 0·97 (95% confidence intervals/CI: 0·9, 1·04) in non-endemic areas (54 studies and 819 559 neonates), 79 (95% CI: 72, 86) in endemic areas (3 studies, 5 060 neonates), 50 (95% CI: 31, 72) in neonates born to mothers with thyroid disorders, and 14 (95% CI: 8, 22) in preterm neonates. At thyroid stimulation hormone cut-off of 20 mIU/L, the screen positivity rates were 5·6% (95% CI: 5·4%, 5·9%) for cord blood samples and 0·19% (95% CI: 0·18%, 0·2%) for postnatal sample. About 70% (95% CI: 70, 71) of screen positive neonates were retested with diagnostic tests. Among neonates with permanent hypothyroidism, thyroid dysgenesis 56·6% (95% CI: 50·9%, 62·2%) was more common than dyshormonogenesis 38·7% (95% CI: 33·2%, 44·3%).
Interpretation
The prevalence of congenital hypothyroidism in India is higher than global estimates. Screen positivity rate was higher for cord blood screening when compared to postnatal screening. Compliance with confirmatory testing was higher for cord blood screening.
Funding
The study was not funded by any source.
Keywords: Congenital hypothyroidism, India, Prevalence, Screen positivity rate, Meta-analysis
Research in context.
Evidence before this study
The databases of PubMed, Embase, Google Scholar and IMSEAR (Index Medicus for South East Asian Region) were searched from their inception till 1st October 2021. We also searched citations and went through references of the eligible articles to identify further studies. For grey literature and unpublished data, google was searched and researchers who are working on these topics were contacted. The search terms used were (newborn OR neonatal OR birth OR childhood OR “heel prick” OR “cord blood”) AND (screening OR diagnosis OR detection OR incidence OR prevalence) AND (hypothyroidism OR TSH OR "thyroid stimulating hormone" OR thyroxine) AND India. We included all observational studies and cross-sectional prevalence studies from India which assessed one or more of the three outcomes of interest i.e., prevalence of CH, or screen-positivity rates, or etiology of CH. Studies done on a consecutive population of a selected subgroup of neonates were also included. Conference abstracts without formal publication, systematic reviews, meta-analysis, letters or correspondences without original data, guidelines, and multi-national studies where separate data of Indian neonates could not be obtained were excluded. None of the included studies had concerns related to the sample frame used, recruitment of study participants, data analysis, and statistical methods. The pooled estimates of prevalence of CH (per 1 000 neonates screened) from the meta-analysis were: (a) 0·97 (95% confidence intervals/CI: 0·9–1·04) in non-endemic areas, (b) 79 (95% CI: 72–86) in endemic areas, (c) 49·7 (95% CI: 31·3 to 71·9) in neonates born to mothers with thyroid disorders, and (d) 14·1 (95% CI: 8·1 to 21·6) in preterm neonates. The pooled estimates of recall/ screen positivity rates from the meta-analysis were: (a) 5·6% (95% CI: 5·4% to 5·9%) for cord TSH of 20 mIU/L, (b) 23·5% (95% CI: 22% to 25%) for cord TSH of 10 mIU/L, (c) 3·3% (95% CI: 3·2% to 3·4%) for cord TSH cut-offs more than 20 mIU/L, (d) 0·19% (95% CI: 0·18% to 0·2%) for postnatal TSH of 20 mIU/L, (e) 2% (95% CI: 1·9% to 2%) for postnatal TSH of 10 mIU/L, and (f) 17% for postnatal T4 cut-off of 80 ng/ml. The pooled estimates for compliance to recall from the meta-analysis were: (a) 70% (95% CI: 70%, 71%) overall, (b) 79% (95% CI: 78%, 80%) for cord blood TSH screening, and (c) 58% (95% CI: 56%, 59%) for postnatal TSH screening. The pooled estimates for etiology were: (a) Among all neonates with confirmed diagnosis, transient hypothyroidism in 14% (95% CI: 10·8%, 17·8%) and permanent hypothyroidism in 86% (95% CI: 82·2%, 89·2%), (b) Among those with permanent hypothyroidism, dysgenesis in 56·6% (95% CI: 50·9%, 62·2%), dyshormonogenesis in 38·7% (95% CI: 33·2%, 44·3%), unascertained etiology in 4·4% (95% CI: 2·3%, 7·1%), and (c) Among those with dysgenesis, agenesis in 74·2% (95% CI: 63·3%, 83·8%), ectopy in 23·3% (95% CI: 14·1%, 33·9%) and hypoplasia in 3·3% (95% CI: 1·8%, 9·1%).
Added value of this study
From this meta-analysis, we noted that the practices on newborn screening for congenital hypothyroidism (type of sample, analytical method, screen positivity cut-offs, criteria for confirmation of diagnosis, etc) were variable in India. The prevalence of congenital hypothyroidism in non-endemic regions of India is about 1 in 1 031 neonates, which is higher than several countries. The recall rates for postnatal TSH screening were about 0·19% for a cut-off of 20 mIU/L, which increased to 2% when cut-off is lowered to 10 mIU/L, and 5·6% when cord blood TSH is used for newborn screening. Only 70% of neonates who screened positive could be tested for confirmation of diagnosis.
Implications of all the available evidence
The findings of this meta-analysis indicate that postnatal TSH is preferred over cord blood TSH for a newborn screening for congenital hypothyroidism. A screen positivity cut-off for TSH of 20 mIU/L can be used initially. More emphasis has to be placed on adherence to guidelines provided by national organizations, and to improve the compliance for diagnostic testing in screen positive neonates.
Alt-text: Unlabelled box
Introduction
Congenital hypothyroidism (CH) is regarded as the commonest cause of preventable mental retardation.1 With the introduction of newborn screening, early diagnosis and timely initiation of thyroxine replacement therapy have improved the outcomes of affected children.2 Congenital hypothyroidism fulfils all the criteria provided by Wilson and Jungner for a condition requiring a screening test. It is easy to screen, has a lag time before symptoms manifest, has a definitive diagnostic test, is inexpensive to treat, and the affected children have excellent outcomes when timely treatment is initiated. Over the last few decades, most developed countries have established newborn screening programmes to facilitate the early diagnosis of CH and other disorders. India, like several other developing countries, does not have a universal newborn screening program for early diagnosis of CH. In an attempt to operationalize the screening, several pilot studies were conducted in the last 3 decades. Despite these efforts, only 3 states/union territories have been able to initiate and sustain a newborn screening program, namely Chandigarh, Goa, and Kerala. The expert estimate on the number of neonates being screened in India is about 3%.3 The barriers identified include poor access to healthcare, finances, availability of diagnostic services, logistics involved, and commitment of the policymakers.3
Various operational factors must be considered while establishing a newborn screening program for CH.4 They include the sampling strategy for the initial screening test (i.e., cord blood or postnatal sample), the biochemical test for the postnatal screen (i.e., thyroid-stimulating hormone/TSH or thyroxine/T4 levels, or a combination of both the tests), the cut-offs of TSH/T4 for defining screen positivity, the analytical method for estimating TSH/T4 levels,5 and the method of blood sample collection (venous or filter paper sample). Apart from these factors, it is also important to know the population characteristics- i.e., the prevalence, screen positivity rates for cord blood and postnatal sampling strategies, and the aetiology of CH as they have implications for a national program on newborn screening.
The reported prevalence of congenital hypothyroidism in developed countries increased after the introduction of newborn screening programs from about 1 in 7 000-10 000 to about 1 in 3 000-4 000 neonates.6 The studies published in India reported varying prevalence ranging from 1 in 727 to 1 in 2 640.7 The screen positivity rates for cord blood and postnatal sampling strategies, as well as etiology, are not well studied. With this background, we did a systematic review and meta-analysis of the literature published in India to assess the prevalence, screen positivity rates, and the etiology of CH.
Methods
Search strategy and selection criteria
The protocol of this systematic review and meta-analysis was prospectively registered in the PROSPERO database with a registration number of CRD42021277523 and can be accessed from the website https://www.crd.york.ac.uk/PROSPERO/. We have followed the PRISMA (preferred reporting items for systematic reviews and meta-analyses) reporting guidelines. The differences between the protocol and final review are provided in the supplementary material.
The databases of PubMed, Embase, Google Scholar and IMSEAR (Index Medicus for South-East Asian Region) were searched from their inception till 1st October 2021. As the data on the prevalence of CH is obtained from newborn screening, we designed our search strategy to identify all studies on newborn screening from India. For PubMed and Embase search, we combined medical subject heading and generic terms from literature search using “OR” and separated them into 4 search strands. These search strands were: 1) newborn OR neonatal OR birth OR childhood OR “heel prick” OR “cord blood”, 2) screening OR diagnosis OR detection OR incidence OR prevalence, 3) hypothyroidism OR TSH OR ‘thyroid-stimulating hormone’ OR thyroxine, and 4) India. We also searched citations and went through references of the eligible articles to identify further studies. For grey literature and unpublished data, we searched google and contacted researchers who are working on these topics. The search strategy is provided in Table S1.
We included all observational studies from India which assessed one or more of the three outcomes of interest i.e., the prevalence of CH, screen-positivity rates, or etiology of CH. We considered the study to be eligible for inclusion when it is a cross-sectional prevalence study (i.e., all the neonates born or admitted to the unit or from a particular locality were screened for congenital hypothyroidism), cord blood or postnatal blood samples were used for screening, and the diagnosis of congenital hypothyroidism was confirmed by estimating venous thyroid-stimulating hormone (TSH) or tetraiodothyronine (T4) or free T4 levels. We also included studies done on a consecutive population of a selected subgroup of neonates (e.g., preterm neonates, neonates born to mothers with thyroid disease, neonates born in endemic areas, etc). We excluded conference abstracts without formal publication, systematic reviews, meta-analysis, letters or correspondences without original data, guidelines, and multi-national studies when separate data of Indian neonates could not be obtained.
The primary outcomes of interest were the prevalence of CH and the screen positivity rate. The diagnostic criteria used were those suggested by the Indian Society of Pediatric and Adolescent Endocrinology (ISPAE) in 2018, i.e., TSH >20 mIU/L for screen positivity; venous TSH >20 mIU/L before 2 weeks age or >10 mIU/L after 2 weeks age or T4 <10 ug/dL or free T4 <1·17 ng/dL for confirmation.8 The secondary outcomes studied were compliance with confirmatory testing among screen positive neonates and the etiology of CH. Prevalence was calculated per 1000 populations screened. Screen positivity rate was estimated as the percentage of neonates who screened positive to the number of newborns who underwent the screening test. Compliance with confirmatory testing was defined as the percentage of screen positive neonates who returned for confirmatory testing to the total number of screen positive neonates. Low compliance rates to confirmatory testing can lead to an underestimation of the actual prevalence of CH, as a proportion of neonates who did not undergo confirmatory tests may be having CH. Hence, we corrected the estimated prevalence of CH, to account for the low compliance to follow-up for confirmatory testing. This was analyzed by using the data on screen positivity rate from each study. The adjusted prevalence was calculated as reported prevalence x number of neonates who tested screen positive/the number of neonates who retested.
With regards to the etiology, the percentage of cases attributable to thyroid dysgenesis and dyshormonogenesis was calculated. Among those with dysgenesis, we calculated the contribution from the three common reasons for dysgenesis i.e., ectopic gland, aplasia, and hypoplasia.
Data analysis
The articles retrieved on database search were exported to Rayyan software. After removing the duplicates, the titles and abstracts were assessed for potentially eligible articles by the 2 reviewers (RPA and EAR) in a blinded manner, and any discrepancy was resolved by mutual discussion. Full texts of eligible articles were retrieved and the following data were extracted- study identification details; study and population characteristics including study setting (hospital-based, community-based, or laboratory-based), place of study, duration, study design (prospective or retrospective), inclusion and exclusion criteria; details of screening test including the sampling method (cord blood or postnatal sampling), age at sample collection for postnatal samples, other disorders tested, screening strategy (TSH or T4 based or combined), analytical methods used, and the cut-off for screen positivity; details of diagnostic testing- sampling strategy for screen positive neonates, and criteria for confirming the diagnosis of congenital hypothyroidism; and the data on outcomes. When the full texts could not be retrieved, we contacted the authors. If the authors have not responded in 1 month, we have excluded those studies. We have not contacted the authors for missing/ additional information in the full texts. Duplication of the dataset was assumed when the study duration and hospital/place of study were similar or overlapping, and such studies were not included in the meta-analysis.
The quality of included studies was assessed using the Joanna Briggs tool for assessing the quality of prevalence studies independently by the 2 reviewers (RPA and EAR).9 The assessment included 9 questions related to the appropriateness of the sample frame used, recruitment of study participants, sample size, description of study subjects and study setting, data analysis, diagnostic testing, statistical analysis, and response rate. The quality was rated down when the neonates were excluded inappropriately, consecutive neonates were not included, the sample size was inadequate (<15 350 based on a prevalence estimate of 0·001 and precision of 0·0005), neonatal characteristics were not described in detail, analytical methods used were inappropriate, the confirmation of the diagnosis of CH was not by ISPAE (Indian Society of Pediatric and Adolescent Endocrinology) guidelines,8 or when the follow-up rates were inadequate (<80%). We included all eligible studies in the meta-analysis irrespective of the quality.
As the prevalence of congenital hypothyroidism is below 0·5 (about 0·001 to 0·002 in previous studies), the double arcsine transformation method was used to stabilize the variance.10 The prevalence estimates were calculated separately for term neonates in endemic and non-endemic regions (as defined by the study authors), preterm neonates (born before 37 weeks gestational age), and neonates born to mothers with thyroid disease (either hypothyroidism or hyperthyroidism). We used the random-effects model for meta-analysis using the software MetaXL version 5·3 for windows (EpiGear International, Sunrise Beach, Australia). The heterogeneity was assessed using the I2 statistic, the variability of estimates across the studies in the forest plots, and the variance of the pooled estimates. We performed a subgroup analysis of studies as per the important covariates- the type of the study (hospital-based versus community-based), area/region of the study (coastal, hills/mountains, plateau, and plains), type of sample used for screening (cord blood versus postnatal sample), screening strategy used (TSH based versus T4 based versus a combination of the tests), screen positivity threshold (20 mIU/L of TSH versus 10 mIU/L), and the sample size (>15 000 versus <15 000). We also performed a sensitivity analysis to estimate the effect of the year of study (excluding studies done before 2000 AD), adherence to the ISPAE guidelines for the diagnosis of CH (excluding studies which did not adhere to/ did not state the guidelines used), and the risk of bias (low risk versus the high risk of bias) on the prevalence estimates. Publication bias was assessed using the funnel plot when the number of publications was more than 10.
Role of the funding source
The study was not funded by any source.
Results
On database and register search, we identified 2 423 articles. After removing duplicates, and screening titles and abstracts, full texts of 227 articles were retrieved. Seventy-nine studies were included in the systematic review, and 70 studies in the meta-analysis.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80 The flow of the studies is shown in the PRISMA flow diagram (Figure 1). The details of studies excluded after retrieving the full text are provided in Table S2. The study and participant characteristics are described in Table 1 and Table S3. The details of data extraction are shown in Table S4.
Figure 1.
PRISMA flow diagram for the study.
Table 1.
Study setting, population characteristics, and strategies for screening and diagnosis of congenital hypothyroidism in the included studies.
| S No | Author | Sampling technique | Screening strategy | Cut-off for screen positivity (mIU/L) | Cut-off for confirmation of CH | Sample size |
|---|---|---|---|---|---|---|
| 1 | Palanisamy 202111 | Postnatal venous at 48 h-7 days | TSH-based | 10 and 11.3 | Unclear | 14 738 |
| 2 | Patel S 202112 | Postnatal filter paper at 48 h-8 weeks | TSH-based | 10 | Unclear | 1 282 |
| 3 | Choudhary SS 202013 | Cord blood | TSH-based | 20 | Unclear | 1 200 |
| 4 | Prakash Sharma 201914 | Postnatal venous, timing unclear | TSH + T4 | cord blood-25; postnatal-10 | Unclear | 161 |
| 5 | Preeti Sharma 201815 | After 24 h | TSH-based | Unclear | Unclear | 70 590 |
| 6 | Kapil 201416 | Cord blood | TSH-based | 20 | Venous TSH >10 mIU/L | 613 |
| 7 | Ilamaran 201417 | Cord blood | TSH-based | 20 | Elevated TSH and low FT4 | 785 |
| 8 | Lodh 201318 | Unclear | TSH-based | Unclear | Unclear | 600 |
| 9 | John J 201319 | Cord blood | TSH-based | 20 and 40 | Unclear | 79 |
| 10 | Ramadevi 200420 | Postnatal filter paper at 6 +/- 2 days age | TSH-based | 20 | Unclear | 10 300 |
| 11 | Kochupillai 198642 | Cord blood | TSH + T4 | Unclear | Unclear | 6 265 |
| 12 | Venugopalan 202022 | Postnatal venous at 72-120 h | TSH-based | 20 | Venous TSH > 10 μIU/mL | 8 064 |
| 13 | Venugopalan 202123 | Postnatal filter paper on day 3 | TSH-based | 6 | Venous TSH≥6 mIU/L (in>3 weeks), ≥ 20 mIU/L (≤3weeks) or Free T4<0.9 ng/dL | 249 |
| 14 | Verma J 202024 | Postnatal filter paper. Timing: 24-48 h; NICU admission- beyond 7 days age; premature- close to 7 days age; immediately before any transfusion for sick neonates. | TSH-based | 18 | Unclear | 13 376 |
| 15 | Kommalur 201925 | Postnatal filter paper between 48 h - discharge | TSH-based | 20 | Unclear | 41 027 |
| 16 | Verma P 201926 | Postnatal filter paper after 24 h of birth or at discharge, whichever was later. Preterm- not later than 36 completed weeks of gestation or 14 days of life, whichever was later. | TSH-based | 20 | Venous TSH >10 mU/L and fT4 <12 pmol/L | 174 000 |
| 17 | Nasheeda C M 201827 | Cord blood | TSH-based | 20 | TSH>20; FT4<0.76; TSH 10-20 with FT4<0.76 | 1 200 |
| 18 | Chaudhary M 201828 | Cord blood | TSH-based | 20 | 20 | 9 558 |
| 19 | Bhatia R 201829 | Cord blood | TSH-based | 20 | Unclear | 1 824 |
| 20 | ICMR taskforce 201830 | Postnatal filter paper between 24 h- 7 days | TSH-based | 20 | Unclear | 104 066 |
| 21 | Lakshminarayana SG 201631 | Cord blood | TSH-based | <1 and >16.1 | TSH >10 μIU/mL with T4 <6.5 μg/dl and FT4 <0.8 ng/dl. | 979 |
| 22 | Anand 201533 | Cord blood | TSH-based | 10 | TSH on day 5 >40 mU/L with a low FT4 or at 2 weeks: TSH >10 mU/L with low FT4 | 1 950 |
| 23 | Verma IC 201534 | Unclear | NA | NA | NA | 16 832 |
| 24 | Gopalakrishnan 201435 | Postnatal filter paper after 24 h | TSH-based | Initially 20, later 20 for >48 h, 34 for 24-48 h | Unclear | 13 426 |
| 25 | Raj S 201436 | Cord blood | TSH-based | 13.2 | NA | 430 |
| 26 | Gupta A 201437 | Cord blood | TSH-based | 20 | Unclear | 952 |
| 27 | Kaur 201038 | Postnatal filter paper at 24-48 h after birth | TSH-based | 18 | Unclear | 6 813 |
| 28 | Kaur 201632 | Postnatal filter paper at 24-48 h after birth | TSH-based | 9 | Unclear | 25 395 |
| 29 | Sanghvi 200839 | Postnatal filter paper at 72-120 h | TSH-based | 10 | serum TSH ≥20 mU/L and T4 <7 μg/dL | 2 964 |
| 30 | Manglik 200540 | Cord blood | TSH-based | 20 | Unclear | 1 200 |
| 31 | Desai MP 198741 | Cord blood | TSH-based | 30 | Unclear | 12 407 |
| 32 | Desai MP 199421 | Postnatal filter paper at 24-96 h | T4-based | T4 values of 51- 80 ng/ml as borderline and < 50 ng/ml as high risk | NA | 25 244 |
| 33 | Shankar M 201944 | Cord blood | TSH-based | 20 | Unclear | 200 |
| 34 | Singh RA 201345 | Cord blood | TSH-based | 20 | Unclear | 1 000 |
| 35 | Sangeeta 201380 | Cord blood | TSH-based | 20 | Unclear | 500 |
| 36 | Ramya 201446 | Postnatal sample | TSH + T4 | 20 | >50- permanent CH, 20-50 with repeat of 20-50 –> transient CH | 2 376 |
| 37 | Kumari N 202147 | Cord blood | TSH-based | 20 | Repeat serum TSH was above the age appropriate cut off | 1 512 |
| 38 | Bhatia R 201948 | Cord blood | TSH-based | 20 | 20 | 2 916 |
| 39 | Tiwari 201949 | Postnatal filter paper on 3rd day | TSH-based | Not mentioned | Not mentioned | 1 373 |
| 40 | Jacob AS 201950 | Postnatal venous after 48 h | TSH-based | 10 | venous TSH levels ≥10 mIU/L after 3 weeks of age irrespective of T4, low free T4 levels (<1.1 ng/dl) irrespective of TSH, free T4 <1.17 ng/dl with TSH >20 mIU/L (TSH >10 mIU/L for age >2 weeks), | 2 407 |
| 41 | Patil 202152 | Postnatal filter paper on day 1 | TSH-based | 10 | Unclear | 3 420 |
| 42 | Bhandar 201753 | Postnatal venous at 48-96 h | TSH + backup T4 | 20 | TSH >20 mIU/L or T4 is low. | 3 150 |
| 43 | Prasad H 202154 | Postnatal filter paper at 72-120 h | TSH-based | 6 | venous TSH ≥20 or free T4 <0.9ng/dl at GA <37 weeks or venous TSH ≥ 10 or Free T4 < 0.9 ng/dl in a term infant | 1 147 |
| 44 | Sahoo 202055 | Postnatal venous sample at 72-96 h of birth in two health institutions and 96-120 h in one, and neonates attending the private clinic at 72-120 h of life | TSH-based | 6 | TSH > 20 mIU/L irrespective of FT4 levels. Low serum FT4 regardless of TSH |
1 530 |
| 45 | Seeralar 201656 | Postnatal filter paper at 48 h-7 days | TSH-based | 20 | 20 | 1 695 |
| 46 | Gupta V 202157 | Cord blood | TSH-based | 20 | Unclear | 4 057 |
| 47 | Rai R 202158 | Postnatal venous at 48-72 h of life for all babies admitted within 24 h of life and on the day of admission for babies admitted after 48 h of life; repeat at 14-21 days | TSH + T4 | TSH: > 20 mIU/L in the first week and > 10 mIU/L after the first week; T4: < 16.7 pmol/L in babies with a gestational age of 31-36 weeks and < 6.4 pmol/L for gestation of 25-30 weeks | Diagnosed as per cut-offs for TSH | 64 |
| 48 | Kulshrestha 202059 | Cord blood | TSH-based | 20 | Unclear | 750 |
| 49 | Ranjan 201960 | Unclear | Unclear | Unclear | Unclear | 120 |
| 50 | Gunasundari 202061 | Postnatal filter paper at 2-5 days, preferable >72 h | TSH + T4 | Unclear | Age normal reference ranges | 80 |
| 51 | Kadam 201662 | Postnatal filter paper at 48-120 h | TSH-based | 20 | Unclear | 57 |
| 52 | Gulhane 202163 | Cord blood | TSH-based | 10 and 20 | Unclear | 122 |
| 53 | Paul 202164 | Cord blood | TSH-based | 25 | Unclear | 164 163 |
| 54 | Kalouni 201565 | Postnatal filter paper at 48-72 h | TSH-based | Unclear | Unclear | 715 |
| 55 | Poyekar 201966 | Cord blood | TSH-based | 20 | Unclear | 726 |
| 56 | Garg MD 201867 | Cord blood | TSH-based | 20 | TSH >10microIU/ml and FT4 <0.7ng/ml after 72 h of life | 1 465 |
| 57 | Katyayani 201968 | Cord blood | TSH-based | 20 | Unclear | 2 012 |
| 58 | Patel L M 201969 | Unclear | Unclear | Not mentioned | Not mentioned | 18 573 |
| 59 | Prasad R 201770 | Postnatal filter paper at 3-7 days | TSH-based | 20 | Not mentioned | 150 |
| 60 | Devi ARR 201871 | Unclear | Unclear | Unclear | Unclear | 47 900 |
| 61 | George 202072 | Cord blood | TSH-based | 10 and 20 | Persistently elevated TSH and low FT4 | 272 |
| 62 | Vidhydhara 202073 | Postnatal at 3-5 days, type of sample unclear | TSH + backup T4 | 20 | Persistently elevated TSH >20 | 2 212 |
| 63 | Kishore RK 201474 | Postnatal filter paper at 36-48 h | TSH-based | 12 | Unclear | 19 800 |
| 64 | Kishore RK 201751 | Postnatal filter paper at 36-48 h | TSH-based | 12 | Unclear | 48 600 |
| 65 | Singh BP 202175 | Cord blood | TSH-based | 20 | 20 | 1 470 |
| 66 | Raguvaran 201676 | Cord blood | TSH-based | 13 | Unclear | 110 |
| 67 | Yadav 201677 | Cord blood | TSH + T4 | 20 | Unclear | 1 000 |
| 68 | Gurnani 202178 | Cord blood | TSH-based | 20 | Unclear | 856 |
| 69 | Goa programme 201179 | Postnatal filter paper | TSH-based | Unclear | Unclear | 27 578 |
| 70 | Kochupillai 198443 | Unclear | TSH + T4 | Unclear | Unclear | 1 647 |
CH- congenital hypothyroidism, TSH- thyroid stimulating hormone, T4- tetraiodothyronine, FT4- free tetraiodothyronine, FT3- free triiodothyronine, NICU- neonatal intensive care unit, FAODs- fatty acid oxidation defects, CNS- central nervous system, VLBW- very low birth weight.
A detailed assessment of the quality of studies using the Joanna Briggs tool is provided in Table S5. None of the included studies had concerns related to the sample frame used, recruitment of study participants, data analysis, and statistical methods. However, 58 (83%) studies used inadequate sample size while two studies did not describe the study setting in sufficient detail. Only 13 (19%) studies used the diagnostic criteria as suggested by the ISPAE guidelines,8 5 studies used other definitions, and 52 studies did not describe the criteria used. Fourteen studies (20%) did not describe the analytical technique.
The prevalence of CH was estimated separately for neonates in endemic and non-endemic regions, neonates born to mothers with thyroid disease and preterm neonates. Meta-analysis of 54 studies that included term neonates from non-endemic regions (55 datasets, and 819 559 neonates)11, 12, 13, 14,17,19, 20, 21, 22,24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53,55, 56, 57,62, 63, 64, 65, 67, 68, 69,71, 72, 73, 74, 75, 76,80 gave a pooled prevalence of 0·97 per 1 000 neonates (95% confidence interval/CI: 0·9 to 1·04), which is about 1 in 1 031 neonates. The I2 statistic was 92%, while the variance in pooled estimates was narrow. The forest plot demonstrates a wide and non-overlapping confidence interval in studies with smaller weights (Figure 2a). The funnel plot was asymmetric (Figure S1) indicating possible publication bias.
Figure 2.
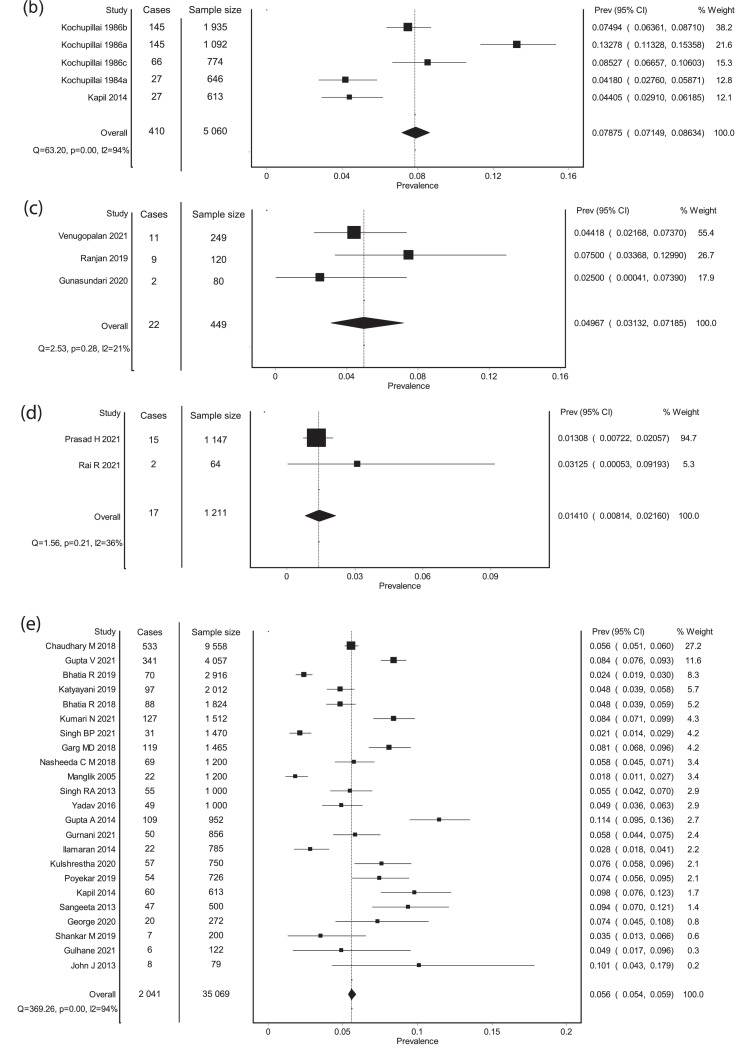
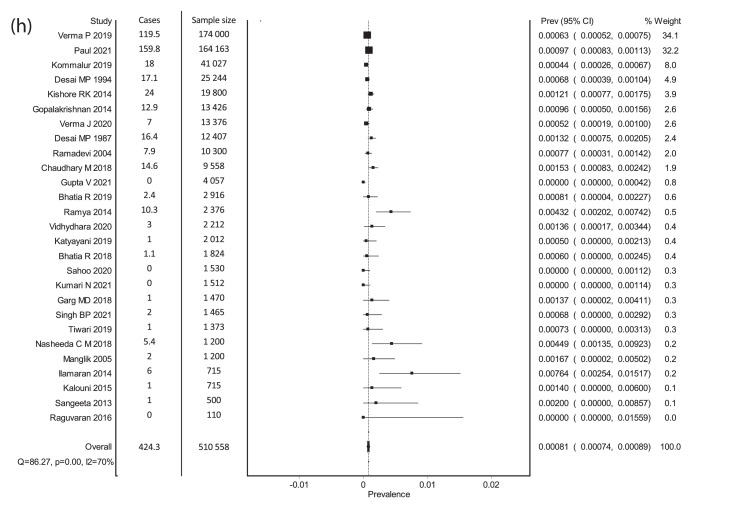
Forest plots. (a) Meta-analysis of Prevalence (non-endemic regions). (b) Meta-analysis of prevalence- Endemic regions. (c) Meta-analysis of prevalence- Neonates born to mothers with thyroid disorders. (d) Meta-analysis of prevalence- Preterm neonates. (e) Meta-analysis of screen positivity rate- Cord TSH at a cut-off of 20 mIU/L. (f) Meta-analysis of screen positivity rate- postnatal TSH at a cut-off of 20 mIU/L. (g) Meta-analysis of compliance to recall. (h) Meta-analysis of corrected Prevalence (non-endemic). X-axis shows the event rate, Y-axis represents the data from each study, each square represents the estimate from that study, size of the square is proportional to the sample size.
On a meta-analysis of 3 studies (5 data sets, and 5 060 neonates) in16,42,43 that included newborns from endemic regions, the pooled estimate of prevalence was 78·8 per 1 000 neonates (95% CI: 71·5 to 86·3), which is about 1 in every 13 neonates screened. Substantial heterogeneity was noted (I2 of 94%). The forest plot is shown in Figure 2b.
In neonates born to mothers with thyroid disorders (3 studies, 449 neonates),23,60,61 the pooled prevalence was 49·7 per 1 000 neonates screened (95% CI: 31·3 to 71·9), approximating about 1 in every 20 neonates screened (forest plot shown in Figure 2c). Heterogeneity was not significant (I2 = 21%).
In preterm neonates (2 studies, 1 211 neonates),54,58 the pooled prevalence was 14·1 per 1000 neonates screened (95% CI: 8·1 to 21·6), which is about 1 in every 71 preterm neonates (forest plot shown in Figure 2d). Mild heterogeneity was noted (I2 = 36%). None of the studies from endemic regions studied the prevalence of CH in neonates born to mothers with thyroid disease, or preterm neonates.
The pooled prevalence estimates from studies using cord blood versus postnatal samples were 0·9 (95% CI: 0·7 to 1) and 0·9 (95% CI: 0·9 to 1), respectively. The pooled estimates from hospital-based studies were 0·96 per 1 000 neonates (95% CI: 0·99 to 1·03), from studies with a low risk of bias was 0·94 per 1 000 neonates (95% CI: 0·86 to 1·03), and from studies with large sample size was 0·87 per 1 000 neonates (95% CI: 0·80 to 0·94). The subgroup analysis of prevalence with the sampling strategy (cord blood versus postnatal sample; TSH based versus combined TSH and T4 strategies), and different screen positivity thresholds used (TSH of 10 mIU/L versus 20 mIU/L) is shown in Table 2.
Table 2.
Subgroup analysis of prevalence in studies from non-endemic regions.
| Co-variate | No of studies and sample size | Prevalence (confirmed cases per 1000 neonates screened) | 95% CI | I2 |
|---|---|---|---|---|
| Cord blood | 25 studies, 26 datasets, 396 354 neonates | 0·9 | 0·7 to 1 | 70% |
| Postnatal sample | 25 studies, 524 342 neonates | 0·9 | 0·9 to 1 | 92% |
| TSH-based screening | 44 studies, 699 646 | 0·9 | 0·9 to 1 | 88% |
| TSH + T4 based | 6 studies, 7 datasets, 13 828 neonates | 2·1 | 1·4 to 3·1 | 66% |
| Screen positivity cut-off of TSH- 20 mIU/L | 26 studies, 203 119 neonates | 1·3 | 1·1 to 1·4 | 89% |
| Screen positivity cut-off of TSH- 10 mIU/L | 7 studies, 200 761 neonates | 0·79 | 0·71 to 0·76 | 92% |
| Hospital based studies | 52 studies, 816 934 | 0.96 | 0.89 to 1.03 | 92% |
| Community based studies | 2 studies, 3 datasets, 2 625 neonates | 3.91 | 1.84 to 6.72 | 82% |
| Studies with a sample size > 15 000 | 11 studies, 685 600 neonates | 0.87 | 0.80 to 0.94 | 97% |
| Studies with sample size < 15 000 | 43 studies, 45 datasets, 133 959 neonates | 1.54 | 1.34 to 1.76 | 84% |
Prevalence from various regions
|
23 studies, 134 160 neonates 15 studies, 240 484 neonates 9 studies, 303 100 neonates 5 studies, 34 372 neonates |
1.73 0.67 0.81 0.84 |
1.52 to 1.96 0.57 to 0.77 0.71 to 0.91 0.56 to 1.18 |
95% 68% 74% 85% |
TSH- thyroid stimulating hormone, T4- tetraiodothyronine, CI- confidence interval.
The pooled estimates after removing studies that did not adhere to ISPAE guidelines, studies conducted before 2000 AD, studies with high/unclear risk of bias, and studies with low sample size (<15 000) were similar to the overall estimate (Table S6).
Studies included in this meta-analysis reported varying screen positivity cut-offs for cord TSH (10–40 mIU/l) and postnatal TSH (6–20 mIU/L). On meta-analysis of 23 studies (35 069 neonates)16,17,19,27, 28, 29, 37,40,44,45,47,48,57,59,63,66, 67, 68,72,75,77,78,80 that used a cord TSH cut-off of 20 mIU/L for screen positivity, the pooled estimate of screen positivity rate was 5·6% (95% CI: 5·4% to 5·9%). The forest plot is shown in Figure 2e. The funnel plot was asymmetric (Figure S2) indicating the presence of publication bias. Two studies (2 950 neonates) provided data on screen positivity rate at a cord TSH cut-off of 10 mIU/L.33,45 The pooled estimate of screen positivity rate was 23·5% (95% CI: 22% to 25%). When cord blood TSH cut-off exceeding 20 mIU/L was used (3 studies, 176 649 neonates),19,41,64 the pooled estimate of screen positivity rates was 3·3% (95% CI: 3·2% to 3·4%). The cord blood TSH cut-offs used in these 3 studies were 25,64 3041 and 40 mIU/L,19 respectively.
Meta-analysis of 14 studies (364 728 neonates) that used postnatal TSH cut-off of 20 mIU/L,20,22,25,26,30,35, 46,50,52,53,56,62,70,73 gave a pooled estimate for screen positivity rates of 0·19% (95% CI: 0·18% to 0·2%). The forest plot is shown in Figure 2f. The funnel plot (Figure S3) showed presence of publication bias. On analysis of 9 studies that used a postnatal TSH cut-off of 10 mIU/L (202 355 neonates)11,12,26,39,50,52,53,63,72 the estimated pooled screen positivity rate was 2% (95% CI: 1·9% to 2%). No study used a postnatal TSH cut-off exceeding 20 mIU/L.
Only one study used T4-based screening.21 A high screen positivity rate of 17% (4 775 of 25 244 neonates screened) was noted at a T4 cut-off of 80 ng/ml.
Analysis for hospital-based versus community-based and region on screen positivity rate on screening with postnatal TSH at cut off 20mIU/L and at cord TSH cut off 20 mIU/L was not performed due to a lack of an adequate number of studies. The pooled estimate for screen positivity rate on using postnatal TSH at cut-off 20mIU/L after removing studies that did not adhere to ISPAE guidelines was 0·82% (95% CI: 0·67% to 0·99%), which was higher than the overall prevalence (Table S7). The pooled estimate on removing studies with a low sample size (< 15 000), as well as a high/unclear risk of bias, was 0·14% (95% CI: 0·13% to 0·16%), which was lower than the overall screen positivity rate (Table S7). The pooled estimate for screen positivity rate on using cord TSH at cut-off 20mIU/L after removing studies that did not adhere to ISPAE guidelines was 4·7% (95% CI: 4·4% to 5·0%), which was lower than the overall screen positivity rate (Table S8).
Ten studies provided data on the etiology of CH. In the 9 studies that included 355 657 neonates17,22,25,30,33,35,46,64,74 born to mothers without thyroid disorders, 419 had confirmed CH, and 376 of them were evaluated for the specific etiology. The pooled estimates of transient hypothyroidism were 14% (95% CI: 10·8%, 17·8%) and permanent hypothyroidism were 86% (95% CI: 82·2%, 89·2%). Among those with permanent hypothyroidism, dysgenesis was seen in 56·6% (95% CI: 50·9%, 62·2%), dyshormonogenesis in 38·7% (95% CI: 33·2%, 44·3%), and etiology could not be ascertained in 4·4% (95% CI: 2·3%, 7·1%). Five studies provided further details on 195 neonates with dysgenesis.25,30,33,46,74 Agenesis was reported as the commonest etiology (74·2%, 95% CI: 63·3%, 83·8%), followed by ectopy (23·3%, 95% CI: 14·1%, 33·9%) and hypoplasia (3·3%, 95% CI: 1·8%, 9·1%).
One study (249 neonates) assessed the etiology of CH in neonates born to mothers with thyroid disorders.23 Of 11 neonates with a confirmed diagnosis, 6 were evaluated further- 2 each had transient hypothyroidism due to maternal antibodies and thyroid dysgenesis, while 1 each had dyshormonogenesis and an ectopic gland.
Twenty-eight studies (515 975 neonates)16,17,19, 20, 21, 24, 25, 26, 27, 28, 29,35,38,40,41,46, 47, 48,55,57,64,67,68,73, 74, 75, 76,80 provided data on compliance with confirmatory testing for screen positive neonates (Figure 2g). Except one study from endemic region,16 the rest were from non-endemic regions. An estimated 70 neonates (95% CI: 70, 71) per 100 neonates who screened positive came for a follow-up visit for confirmatory testing. Funnel plot showed evidence of publication bias (Figure S4). Heterogeneity was significant.
Among these 28 studies, 17 studies used cord TSH16,17,19,27, 28, 29,40,41,47,48,57,64,67,68,75,76,80 and 11 studies used postnatal TSH20,21,24, 25, 26,35,38,46,55,73,74 for screening. The compliance for confirmatory testing for studies using cord TSH and postnatal TSH were 79% (95% CI: 78%, 80%) and 58% (95% CI: 56%, 59%), respectively (Table S9). The pooled estimate for compliance with confirmatory testing on removing studies before 2000 AD, studies with high/unclear risk of bias, and studies not adhering to ISPAE guidelines was higher at 81·3% (95% CI: 80·4% to 82·2%), 78% (95% CI: 76·9% to 79%) and 89·2% (95% CI: 87·3% to 90·9%), respectively (Table S10). On removing studies with a low sample size (< 15 000), the pooled estimate for compliance with confirmatory testing was 67·1% (95% CI: 66·2% to 68%), which was similar to the overall compliance (Table S10).
Prevalence, corrected for the loss to follow-up for confirmatory testing was derived from the data available in 27 studies (510 558 neonates) from non-endemic regions.17,20,21,24, 25, 26, 27, 28, 29,35,40,41,46, 47, 48, 49,55,57,64,65,67,68,73, 74, 75, 76,80 The pooled estimate of corrected prevalence was 0·81 per 1 000 neonates screened (95% CI: 0·74, 0·89), which approximates to 1 in 1 234 neonates screened. The forest plot is shown in Figure 2h. The funnel plot indicates the presence of publication bias (Figure S5). There was significant heterogeneity (I2 = 70%). When the reported prevalence estimates were pooled from these same 27 studies, a pooled prevalence of 0·68 per 1 000 neonates was noted (95% CI: 0·61, 0·75), which is about 1 in 1 471 neonates screened.
Discussion
The findings of our meta-analysis show that congenital hypothyroidism affects about 1 in 1 031 term neonates from non-endemic regions, 1 in 13 term neonates from endemic regions, 1 in 20 neonates born to mothers with thyroid disorders, and 1 in 71 preterm neonates. When corrected for the loss to follow up for confirmatory testing, the estimated prevalence was noted to be about 20% higher than the prevalence reported from the same set of studies (1 in 1 234 versus 1 in 1 471 neonates). Hence, the true prevalence of congenital hypothyroidism may be higher than the estimate of 1 in 1 031 neonates derived from this meta-analysis.
On evaluating the screen positivity rates, we found that the screen positivity rates were 0·19% for postnatal TSH and 5·6% for cord TSH, at a cut-off of 20 mIU/L. Only 7 of 10 neonates who screened positive have reported for confirmatory testing. Most neonates (86%) identified on screening had permanent congenital hypothyroidism, with dysgenesis being more common than dyshormonogenesis. Among those neonates with thyroid dysgenesis, agenesis was the most common cause followed by the ectopic gland.
The possible reasons for the higher burden of CH in India include more consanguineous marriages,81,82 higher prevalence of maternal hypothyroidism, and the presence of endemic iodine deficiency.83 India is currently in the transition phase of iodine deficiency to sufficiency, by universal iodization of salt. Studies have observed that although it was decided to fortify all consumed salt by 1992, only 71% of households had access to adequately iodized salt by 2009.83 It was also observed that the prevalence of hypothyroidism in older age groups is also higher in India (11%) when compared to other countries (2-4·6%).84 In pregnant women, the pooled prevalence of hypothyroidism in India was 11·07% when compared to 2-4%, reported in other countries.85 Similarly, the results of this meta-analysis indicate that the prevalence of hypothyroidism in newborns in India is higher than that reported in developed countries like Japan (prevalence of 1 in 2 500 – 3 500 children),86 Germany (1 in 3 330 children),87 and United Kingdom (1 in 1 887 children)88 and developing countries like Egypt (1 in 3 587 children)89 and Serbia (1 in 1 872 children),90 where the prevalence ranged from 1 in 1 800 to 1 in 3 500. However, it is lower than that observed in a few other regions like Iran (1 in 500 children),91 and Sicily of Italy (1 in 469 children),92 where a prevalence of about 1 in 500 was reported. Hence, it seems that the burden of thyroid disorders is higher in India, across all age groups and especially in newborns. Although the exact reasons for the same are not clear, the postulated attributes are iodine deficiency, exposure to industrial and agricultural contaminants, and a higher prevalence of auto-immune antibodies.
In meta-analyses of prevalence, we observed that funnel plots were asymmetric, with the absence of studies in the lower-left region of the plot. Meta-analysis of smaller studies (<15 000 sample size) indicated the pooled estimate of prevalence to be 1·54 per 1 000 neonates (95% CI: 1·34, 1·76). These factors indicate that the asymmetry in the funnel plot is probably due to the non-reporting of studies with a small sample size where the prevalence was lower. Migliavaca et al observed that the I2 values are often high when a larger number of studies (>10) are included in the meta-analysis of prevalence. As I2 statistic measures the degree of overlapping of the confidence interval of prevalence estimates, elevated values do not necessarily indicate high heterogeneity.93 Hence, we used additional methods of sensitivity and subgroup analyses and assessment of variability on forest plots and the confidence intervals of the pooled estimates. The prevalence estimates on the meta-analysis of high-quality studies (i.e., studies with low risk of bias) were very close to the prevalence estimates obtained from the meta-analysis of all studies (0·94 and 0·97 per 1 000 neonates, respectively), and this substantiates that the prevalence estimates obtained are reliable.
In our meta-analysis, we observed that the screen positivity rate varied from 0·01 to 25%. This observation is similar to another analysis that assessed the worldwide screen positivity rates for congenital hypothyroidism in which they noted that screen positivity rates varied from 0·01% to 13·3% in different programs.94 We observed that the screen positivity rate increases by almost 10-fold on lowering the postnatal TSH cut-off from 20 mIU/L to 10 mIU/L (0·19% and 2%, respectively). Similarly, the screen positivity rate increases by 30-fold when cord TSH is used in place of postnatal TSH (5·6%). These findings should be taken into consideration while implementing a newborn screening program. However, the findings need to be interpreted cautiously as the comparisons were indirect. The results were inconsistent in subgroups. For example, at the postnatal TSH cut-off of 20 mIU/L, the screen positivity rates were noted to be lower in studies with large sample sizes and higher in studies adhering to ISPAE guidelines. Further studies with a large sample size are required, focusing on a direct comparison of various screening strategies for CH.
Compliance with confirmatory testing following a positive screen is a problem faced while implementing a newborn screening program. While some studies from Italy95 and China96 have reported excellent (>95%) compliance rates, the same may not be the case in settings with low socio-economic status and poor parental awareness. In this meta-analysis, we noted that a sizable proportion of screen-positive neonates could not be retested. Importantly, the loss to follow up was higher for postnatal TSH screening compared to cord blood screening. This observation has a bearing on the decision-making of which strategy to be adopted while establishing a screening program. It has been observed that an active tracking system using telephones and the availability of centralized tracking systems can improve the compliance for confirmatory testing from 44% to 99%, in the setting of newborn screening for cystic fibrosis.97 A similar strategy may be required in our country as well to ensure re-testing, confirmation, and follow-up. It is also noted that compliance with confirmatory testing is better in studies done after 2000 AD (81%).
The etiology of CH has been traditionally classified into thyroid dysgenesis or dyshormonogenesis. Previous studies have attributed 75-85% to TD and 15-25% to dyshormonogenesis. However, recent studies have reported a higher prevalence of CH, along with an increased incidence of dyshormonogenesis.98 Currently, dyshormonogenesis accounts for 30-40% of CH, compared with around 15% in the previous century, during the early years of newborn screening.99 In this meta-analysis, although dysgenesis is more common than dyshormonogenesis, the latter accounted for 40% of CH, mirroring the recent evidence, worldwide. The ectopic gland is the most common cause of dysgenesis, followed by aplasia and hypoplasia.6,100 Agenesis accounted for three-fourths of neonates with dysgenesis and was more common than ectopic gland. One of the possible reasons for the increased contribution of dyshormonogenesis, an autosomal recessively inherited spectrum of CH, is the higher burden of consanguineous marriages in various communities/parts of the country.81,82 However, this observation requires to be validated by multi-centric studies.
This systematic review and meta-analysis had several limitations. The overall quality of included studies was low. The prominent reasons included non-adherence to ISPAE guidelines for screen positivity and diagnosis of CH, inadequate description of analytical techniques used, and small sample size for a disorder with low prevalence. We also could observe reasonable heterogeneity, the effect of which we have assessed using subgroup and sensitivity analyses. We were unable to obtain data from a few studies published before the year 2000 despite the attempts to contact the authors. Although we had access to some data from screening programs of the smaller states/union territories of Goa and Chandigarh, we could not access data on newborn screening from Kerala state, which is the largest public-sector initiative on newborn screening in India. We observed limited data on the prevalence of CH in preterm neonates and in those born to mothers with thyroid disorders. Also, we could not find any published literature on the prevalence of central hypothyroidism in India.
To conclude, the pooled estimate of the prevalence of congenital hypothyroidism in India is about 1 in 1,031 neonates and is higher than in several other countries. This indicates that newborn screening must be initiated on a priority basis in India to avoid the morbidity related to missed/delayed diagnosis of congenital hypothyroidism. Postnatal TSH-based screening is preferred over cord blood TSH screening as the indirect comparison of prevalence found by the cord blood versus postnatal approaches were similar, for a much lower screen positivity rate with the postnatal approach. Postnatal TSH allows for the testing of other disorders simultaneously. As only 7 in 10 neonates who were screened positive could be subjected to diagnostic tests, more efforts are required to ensure follow-up of the neonates being screened. In setups where early discharge and postnatal follow-up are a problem, cord blood TSH may be used for initiation of newborn screening for CH.
Further research should focus on the bottlenecks and potential solutions for the operationalization of universal newborn screening in large countries like India in particular and developing countries in general. Strategies to improve coverage of newborn screening and increase compliance with confirmatory testing in screen-positive neonates should be evaluated in future studies. More studies evaluating the etiology of congenital hypothyroidism, and the prevalence of central hypothyroidism are needed in India.
Contributors
Conceptualization- RPA; Literature search- RPA, EAR; Study design- RPA, EAR; Data collection- RPA, EAR; Data analysis- RPA; Data interpretation- RPA, EAR; Manuscript writing- RPA, EAR; Review of article and editing- RPA, EAR.
Data sharing statement
The details of the data search and data extracted from the included studies are provided in the supplementary material. The study protocol, including the statistical analysis plan, can be accessed freely from the PROSPERO database, using the trial number mentioned. We do not possess any additional data to share.
Declaration of interests
We declare no competing interests.
Acknowledgements
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lansea.2022.100040.
Appendix. Supplementary materials
References
- 1.Brady J, Cannupp A, Myers J, Jnah AJ. Congenital hypothyroidism. Neonatal Netw. 2021;40:377–385. doi: 10.1891/11-T-699. [DOI] [PubMed] [Google Scholar]
- 2.Ehsani R, Alijanpour M, Salehiomran M, Kheirkhah F, Moslemi L, Aghajanpour F. Evaluation of the developmental outcome in children with congenital hypothyroidism. Casp J Intern Med. 2021;12:315–322. doi: 10.22088/cjim.12.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mookken T. Universal implementation of newborn screening in India. Int J Neonatal Screen. 2020;6:24. doi: 10.3390/ijns6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Büyükgebiz A. Newborn screening for congenital hypothyroidism. J Clin Res Pediatr Endocrinol. 2013;5:8–12. doi: 10.4274/Jcrpe.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matyjaszek-Matuszek B, Pyzik A, Nowakowski A, Jarosz MJ. Diagnostic methods of TSH in thyroid screening tests. Ann Agric Environ Med AAEM. 2013;20:731–735. [PubMed] [Google Scholar]
- 6.Dayal D, Prasad R. Congenital hypothyroidism: current perspectives. Res Rep Endocr Disord. 2015;5:91–102. [Google Scholar]
- 7.Prabhu SR, Mahadevan S, Jagadeesh S, Suresh S. Congenital hypothyroidism: recent Indian data. Indian J Endocrinol Metab. 2015;19:436–437. doi: 10.4103/2230-8210.152800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai MP, Sharma R, Riaz I, Sudhanshu S, Parikh R, Bhatia V. Newborn screening guidelines for congenital hypothyroidism in India: recommendations of the Indian Society for Pediatric and Adolescent Endocrinology (ISPAE) - part I: screening and confirmation of diagnosis. Indian J Pediatr. 2018;85:440–447. doi: 10.1007/s12098-017-2575-y. [DOI] [PubMed] [Google Scholar]
- 9.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 10.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 11.Palanisamy S, Srinivasan R, Kannappan S, Ganesan J. Serum thyroid stimulating hormone screening and influence of perinatal factors. Indian J Pediatr. 2021;88:516. doi: 10.1007/s12098-021-03674-z. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Priya R, Padhi P, et al. Prevalence of congenital hypothyroidism and G6PD deficiency in newborns in a tertiary care hospital of central India. J Neonatol. 2021;35:5–9. [Google Scholar]
- 13.Choudhary SS, Langade RA, Kshirsagar VY. Normal values and variation of Thyroid Stimulating Hormone (Tsh) in cord blood of babies bornover a period of 1 year. Int J Res Pharm Sci. 2020;11:3177–3182. [Google Scholar]
- 14.Sharma CP, Dubey W, Nagtilak S. Prevalence of Congenital Hypothyroidism; in rural area of district Gautam Budha Nagar (U.P.) Medico-Leg Update. 2019;19:49. [Google Scholar]
- 15.Sharma P, Kumar P, Tyagi MS, Sharma R, Ps D. Prevalence of inborn errors of metabolism in neonates. J Clin Diagn Res. 2018:7–13. [Google Scholar]
- 16.Kapil U, Kabra M, Sareen N, Khenduja P, Pande S. Iodine nutrition status amongst neonates in Kangra district, Himachal Pradesh. J Trace Elem Med Biol. 2014;28:351–353. doi: 10.1016/j.jtemb.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Ilamaran V, Rathisharmila R, Uvaraj P, Saraswathi N. Neonatal screening for congenital hypothyroidism using cord blood thyroid stimulating hormone. Curr Pediatr Res. 2014;18:76–78. [Google Scholar]
- 18.Lodh M, Kerketta A. Inborn errors of metabolism in a tertiary care hospital of eastern India. Indian Pediatr. 2013;50:1155–1156. doi: 10.1007/s13312-013-0303-x. [DOI] [PubMed] [Google Scholar]
- 19.John J, Abraham A, Sahu S. Umbilical cord blood TSH: a predictor of congenital hypothyroidism. Indian J Physiol Pharmacol. 2013;57:452–453. [PubMed] [Google Scholar]
- 20.Rama Devi AR, Naushad SM. Newborn screening in India. Indian J Pediatr. 2004;71:157–160. doi: 10.1007/BF02723099. [DOI] [PubMed] [Google Scholar]
- 21.Desai MP, Upadhye P, Colaco MP, et al. Neonatal screening for congenital hypothyroidism using the filter paper thyroxine technique. Indian J Med Res. 1994;100:36–42. [PubMed] [Google Scholar]
- 22.Venugopalan L, Paranjyothi S, Sankaran A, Prasad HK, Murugesan G, Shanmughasundaram R. Utility of the new Indian Society of Paediatric and Adolescent Endocrinology (ISPAE) guidelines for congenital hypothyroidism screening in a high risk unit. Indian J Pediatr. 2020;88:1075–1079. doi: 10.1007/s12098-020-03555-x. [DOI] [PubMed] [Google Scholar]
- 23.Venugopalan L, Rajan A, Prasad HK, Sankaran A, Murugesan G, Ramanathan S. Impact of maternal thyroid disease on neonatal thyroid status. J Pediatr Endocrinol Metab. 2021;34:237–241. doi: 10.1515/jpem-2020-0349. [DOI] [PubMed] [Google Scholar]
- 24.Verma J, Roy P, Thomas DC, et al. Newborn screening for congenital hypothyroidism, congenital adrenal hyperplasia, and glucose-6-phosphate dehydrogenase deficiency for improving health care in India. J Pediatr Intensive Care. 2020;09:040–044. doi: 10.1055/s-0039-1698424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kommalur A, Devadas S, Kariyappa M, et al. Newborn screening for five conditions in a tertiary care government hospital in Bengaluru, South India—three years experience. J Trop Pediatr. 2020;66:284–289. doi: 10.1093/tropej/fmz067. [DOI] [PubMed] [Google Scholar]
- 26.Verma P, Kapoor S, Kalaivani M, et al. An optimal capillary screen cut-off of thyroid stimulating hormone for diagnosing congenital hypothyroidism: data from a Pilot Newborn Screening Program in Delhi. Indian Pediatr. 2019;56:281–286. [PubMed] [Google Scholar]
- 27.Nasheeda CM, Philip P, Shenoy RD, Shetty S. Diagnostic utility of cord blood thyroid stimulating hormone in congenital hypothyroidism in the era of expanded newborn screening. Indian J Clin Biochem. 2018;33:461–466. doi: 10.1007/s12291-017-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhary M, Soni J, Goyal V, Sharma P, Makwana M, Lora S. Incidence of congenital hypothyroidism in Western Rajasthan using cord blood thyroid-stimulating hormone levels as a screening tool: a cross-sectional hospital-based study. Indian J Endocrinol Metab. 2018;22:417. doi: 10.4103/ijem.IJEM_354_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia R, Rajwaniya D. Congenital hypothyroidism screening in term neonates using umbilical cord blood TSH values. Indian J Endocrinol Metab. 2018;22:277. doi: 10.4103/ijem.IJEM_640_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ICMR Task Force on Inherited Metabolic Disorders Newborn screening for congenital hypothyroidism and congenital adrenal hyperplasia. Indian J Pediatr. 2018;85:935–940. doi: 10.1007/s12098-018-2645-9. [DOI] [PubMed] [Google Scholar]
- 31.Lakshminarayana S, Sadanandan N, Mehaboob A, Gopaliah L. Effect of maternal and neonatal factors on cord blood thyroid stimulating hormone. Indian J Endocrinol Metab. 2016;20:317. doi: 10.4103/2230-8210.179998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur G, Thakur K, Kataria S, et al. Current and future perspective of newborn screening: an Indian scenario. J Pediatr Endocrinol Metab. 2016;29:5–13. doi: 10.1515/jpem-2015-0009. [DOI] [PubMed] [Google Scholar]
- 33.Anand MR, Ramesh P, Nath D. Congenital hypothyroidism screening with umbilical cord blood: retrospective analysis. Indian Pediatr. 2015;52:435–436. doi: 10.1007/s13312-015-0652-8. [DOI] [PubMed] [Google Scholar]
- 34.Verma IC, Bijarnia-Mahay S, Jhingan G, Verma J. Newborn screening: need of the hour in India. Indian J Pediatr. 2015;82:61–70. doi: 10.1007/s12098-014-1615-0. [DOI] [PubMed] [Google Scholar]
- 35.Gopalakrishnan V, Joshi K, Phadke S, et al. Newborn screening for congenital hypothyroidism, galactosemia and biotinidase deficiency in Uttar Pradesh, India. Indian Pediatr. 2014;51:701–705. doi: 10.1007/s13312-014-0485-x. [DOI] [PubMed] [Google Scholar]
- 36.Raj S, Baburaj S, George J, Abraham B, Singh S. Cord blood TSH level variations in newborn—experience from a rural centre in Southern India. J Clin Diagn Res. 2014;8:PC18–PC20. doi: 10.7860/JCDR/2014/9058.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Srivastava S, Bhatnagar A. Cord blood thyroid stimulating hormone level—interpretation in light of perinatal factors. Indian Pediatr. 2014;51:32–36. doi: 10.1007/s13312-014-0330-2. [DOI] [PubMed] [Google Scholar]
- 38.Kaur G, Srivastav J, Jain S, et al. Preliminary report on neonatal screening for congenital hypothyroidism, congenital adrenal hyperplasia and glucose-6-phosphate dehydrogenase deficiency: a Chandigarh experience. Indian J Pediatr. 2010;77:969–973. doi: 10.1007/s12098-010-0150-x. [DOI] [PubMed] [Google Scholar]
- 39.Sanghvi U, Diwakar KK. Universal newborn screening for congenital hypothyroidism. Indian Pediatr. 2008;45:331–332. [PubMed] [Google Scholar]
- 40.Manglik AK, Chatterjee N, Ghosh G. Umbilical cord blood TSH levels in term neonates: a screening tool for congenital hypothyroidism. Indian Pediatr. 2005;42:1029–1032. [PubMed] [Google Scholar]
- 41.Desai MP, Colaco MP, Ajgaonkar AR, et al. Neonatal screening for congenital hypothyroidism in a developing country: problems and strategies. Indian J Pediatr. 1987;54:571–581. doi: 10.1007/BF02749056. [DOI] [PubMed] [Google Scholar]
- 42.Kochupillai N, Pandav CS, Godbole MM, Mehta M, Ahuja MM. Iodine deficiency and neonatal hypothyroidism. Bull World Health Organ. 1986;64:547–551. [PMC free article] [PubMed] [Google Scholar]
- 43.Kochupillai N, Godbole MM, Pandav CS, Karmarkar MG, Ahuja MM. Neonatal thyroid status in iodine deficient environments of the sub-Himalayan region. Indian J Med Res. 1984;80:293–299. [PubMed] [Google Scholar]
- 44.Shankar M, Chaudhary R, Chaudhary AK. Use of umbilical cord blood TSH as a marker for screening of congenital hypothyroidism in a tertiary care centre in Jharkhand. IOSR J Dent Med Sci. 2019;18:31–36. [Google Scholar]
- 45.Singh R, Banik U, Devi K, Devi K. New-born screening for congenital hypothyroidism in Manipur by measurement of umbilical cord thyroid stimulating hormone: a hospital based study. J Med Soc. 2013;27:127. [Google Scholar]
- 46.Ramya HS, Srinivasa S, Sharanya R. Incidence of congenital hypothyroidism in a 2 year study period, identified by newborn screening at a tertiary level hospital, Bangalore. J Evol Med Dent Sci. 2014;3:95–99. [Google Scholar]
- 47.Kumari N, Kumar R, Ansari A, Singh BK. Incidence of congenital hypothyroidism and G6pd deficiency in new born. Int J Health Clin Res. 2021;4:169–172. [Google Scholar]
- 48.Bhatia R, Rajwaniya D. Cord blood thyroid-stimulating hormone as a screening tool for congenital hypothyroidism: a single-center 5-year experience. Thyroid Res Pract. 2019;16:76. [Google Scholar]
- 49.Tiwari AK, Bandyopadhyay D, Saha B. A prelimiary report on newborn screening of inborn metabolic disorders. Indian J Child Health. 2019;6:39–41. [Google Scholar]
- 50.Jacob AS, Gopinath R, Divakaran B. Screening for congenital hypothyroidism and the association of congenital hypothyroidism with screening thyroid stimulating hormone levels of 10-20 mIU/L among inborn neonates in a government tertiary care hospital of north Kerala. Indian J Child Health. 2019;06:431–434. [Google Scholar]
- 51.Kumar RK, Chandrakumar N, Savinay KS, Tajamul S, Kini P. Neborn screening: saves lives—why India is lagging behind in implementing newborn screening? Indian Pract. 2017;70:28–31. [Google Scholar]
- 52.Patil P, Nikam P, Hosamani N, Patil S, Nikam S. Neonatal screening for congenital hypothyroidism at BIMS hospital, Belagavi: original research article. World J Pharamaceutical Res. 2021;10:1294–1302. [Google Scholar]
- 53.Bhandar R, Kumar KS, Patil S. Newborn screening for congenital hypothyroidism in India is still overdue. Pediatr Educ Res. 2017;5:13–17. [Google Scholar]
- 54.Krishna Prasad H, Pulluru P, Venugopalan L, Murugesan G, Ramanathan S. Congenital hypothyroidism in Indian preterm babies—screening, prevalence, and aetiology. Pediatr Endocrinol Diabetes Metab. 2021;27:82–86. doi: 10.5114/pedm.2021.105295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahoo PK, Swain A, Sahoo G, Mohanty S, Barik K. Newborn screening for congenital hypothyroidism in institutional set up in an urban area in Odisha. Int J Curr Res Rev. 2020;12:179–182. [Google Scholar]
- 56.Seeralar A, Ganesh T, Padmanaban S. Newborn screening for congenital hypothyroidism in a tertiary care centre. Int J Contemp Pediatr. 2016;3:456–460. [Google Scholar]
- 57.Gupta V, Sood A, Sood M. Newborn screening for congenital hypothyroidism: a local perspective from Himachal Pradesh, India. Thyroid Res Pract. 2021;18:11. [Google Scholar]
- 58.Rai R, Singh DK, Bhakhri BK. Transient hypothyroxinemia of prematurity and its risk factors in an extramural neonatal intensive care unit. Arch Endocrinol Metab. 2021:723–729. doi: 10.20945/2359-3997000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulshrestha MR, Kulshrestha R, Kalra RK. The study of relationship between season of delivery and cord blood TSH. Int J Clin Obstet Gynaecol. 2020;4:1–3. [Google Scholar]
- 60.Ranjan P, Singh N. Assessment of prevalence of thyroid dysfunction in newborns born to mothers with thyroid dysfunction: an observational study. J Adv Med Dent Sci Res. 2019;7:213–216. [Google Scholar]
- 61.Gunasundari P, Satyamoorthy M, Paul CM, et al. A cross sectional study on the thyroid profile of neonates born to hypothyroid mothers in a tertiary care centre in Chennai. Indian J Sci Res. 2020;10:81–83. [Google Scholar]
- 62.Kadam N, Dhar R, Gill RK. Neonatal screening for G6PD, 17-OHP and TSH levels. Int J Sci Res. 2016;5:1771–1777. [Google Scholar]
- 63.Gulhane R, Gulhane A, Muley S, Khandelwal A, Maldhuri S. Cord blood versus peripheral venous blood sample of thyroid stimulating hormone for neonatal thyroid screening. Ann RSCB. 2021;25:7223–7229. [Google Scholar]
- 64.Paul PG, Rebekah G, Korula S, et al. Optimizing cord blood thyroid stimulating hormone cutoff for screening of congenital hypothyroidism—experience from screening 164,000 newborns in a tertiary hospital in India. Indian J Endocrinol Metab. 2021;25:348–353. doi: 10.4103/ijem.ijem_220_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalouni MR, Badade ZG, Mohanty N, Bhatta BD, Pandey BR, Bhatt RD. Screening of newborn babies for thyroid stimulating hormone, glucose-6-phosphate dehydrogenase, 17-hydroxyprogesterone and sickle cell disease. ACCLM. 2015;1:27–30. [Google Scholar]
- 66.Poyekar S, Pratinidhi S, Prasad SS, Sardar ZS, Kankariya B, Bhole O. Cord blood thyroid stimulating hormone level - and the influence of perinatal and other factors on it. Pediatr Oncall. 2019;16:79–82. [Google Scholar]
- 67.Garg MD, Kumar P, Abirami S, Mani M, Krishnan L. Perinatal variables influencing cord blood thyroid stimulating hormone. Int J Contemp Pediatr. 2018;5:1537. [Google Scholar]
- 68.Katyayani M, Kumari K, Laxmi V, Sravanthi L. Unfolding the perinatal factors that affect cord blood thyroid stimulating hormone levels—an experience from a rural centre in Southern India. Int J Paediatr Geriatr. 2020;3:107–112. [Google Scholar]
- 69.Patel LM, Reotutar LP, Reotutar MJ, Reotutar AJP. Proceeding Surabaya International Health Conference. Vol.1. 2019. Newborn screening in the Philippines and India: its status and problems encountered in the program; pp. 419–426. [Google Scholar]
- 70.Prasad R, Verma A, Verma A. Neonatal screening for hypothyroidism by time resolved fluoroimmunoassay in Jharkhand. Indian J Child Health. 2017;04:542–544. [Google Scholar]
- 71.Devi RRA, Ananthalakshmi Y, Rao SK. Challenges and opportunities in establishing and maintaining newborn screening in a rural area of Andhra Pradesh—task force study by Indian Council of Medical Research. Indian J Pediatr. 2018;85:948–951. doi: 10.1007/s12098-017-2497-8. [DOI] [PubMed] [Google Scholar]
- 72.George RT, Stephen ST, Ramachandran R, Vazhayil PP. Comparison of cord blood thyroid stimulating hormone with thyroid stimulating hormone levels from venous samples on 3rd day of life in detecting congenital hypothyroidism in newborn—a retrospective study. J Evol Med Dent Sci. 2020;9:353–356. [Google Scholar]
- 73.Vidhyadhara NTL, Jena SK, Mishra NR. Incidence of congenital hypothyroidism and associated congenital anomalies at VSS Medical College & Hospital, Burla. Int J Paediatr Geriatr. 2020;3:157–161. [Google Scholar]
- 74.Kumar RK, Ranieri E, Fletcher J. Newborn screening for congenital hypothyroidism in India—is overdue. J Neonatal Biol. 2014;03:129. [Google Scholar]
- 75.Singh PB, Motwani NP, Sudhakar C, Chaturvedi U. Congenital hypothyroidism screening with umbilical cord blood thyroid-stimulating hormone at birth and peripheral venous blood thyroid-stimulating hormone after 72 h at a hospital in suburban area of Chhattisgarh. Indian J Child Health. 2021;8:38–41. [Google Scholar]
- 76.Raghuvaran R. A study on mean TSH levels and various perinatal factors affecting TSH level in cord blood of newborn. Doctoral Dissertation, Kilpauk Medical College; Chennai: 2016. [Google Scholar]
- 77.Yadav AK, Gupta MM, Singh VK. A study to determine the normative data for cord blood free thyroxine and thyroid stimulating hormone in Indian babies. Int J Res Rev. 2016;3:11–17. [Google Scholar]
- 78.Gurnani R, Engade MB, Mohammed H, Sindhu SM, Goiporia MH, Choudhary AS. Effect of mode of delivery on cord blood thyroid stimulating hormone levels—a cross-sectional study. Indian J Neonatal Med Res. 2021;9:33–35. [Google Scholar]
- 79.The Goa newborn screening programme- 3 year review: 2008-2011, www.dhsgoa.gov.in/documents/new_born.pdf, Accessed 1 December 2021.
- 80.Sangeeta N, Kamala L, Karki P, et al. Assessment of umbilical cord TSH in term neonates in Manipur. IOSR J Dent Med Sci. 2013;9:14–17. [Google Scholar]
- 81.Kalam MA, Sharma SK, Ghosh S, Roy S. Change in the prevalence and determinants of consanguineous marriages in India between National Family and Health Surveys of 1992-1993 and 2015–2016. Hum Biol. 2021;92:93–113. doi: 10.13110/humanbiology.92.2.02. [DOI] [PubMed] [Google Scholar]
- 82.Sharma SK, Kalam MA, Ghosh S, Roy S. Prevalence and determinants of consanguineous marriage and its types in India: evidence from the National Family Health Survey, 2015-2016. J Biosoc Sci. 2021;53:566–576. doi: 10.1017/S0021932020000383. [DOI] [PubMed] [Google Scholar]
- 83.Pandav CS, Yadav K, Srivastava R, Pandav R, Karmarkar MG. Iodine Deficiency Disorders (IDD) control in India. Indian J Med Res. 2013;138:418–433. [PMC free article] [PubMed] [Google Scholar]
- 84.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults: an epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–652. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Negro R, Mestman JH. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:927–943. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Nagasaki K, Sato H, Sasaki S, et al. Re-evaluation of the prevalence of permanent congenital hypothyroidism in Niigata, Japan: a retrospective study. Int J Neonatal Screen. 2021;7:27. doi: 10.3390/ijns7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lüders A, Blankenstein O, Brockow I, et al. Neonatal screening for congenital metabolic and endocrine disorders—results from Germany for the years 2006–2018. Dtsch Arzteblatt Int. 2021;118:101–108. doi: 10.3238/arztebl.m2021.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knowles RL, Oerton J, Cheetham T, et al. Newborn screening for primary congenital hypothyroidism: estimating test performance at different TSH thresholds. J Clin Endocrinol Metab. 2018;103:3720–3728. doi: 10.1210/jc.2018-00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bekhit OEM, Yousef RM. Permanent and transient congenital hypothyroidism in Fayoum, Egypt: a descriptive retrospective study. PLoS One. 2013;8:e68048. doi: 10.1371/journal.pone.0068048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitrovic K, Vukovic R, Milenkovic T, Todorovic S, Radivojcevic J, Zdravkovic D. Changes in the incidence and etiology of congenital hypothyroidism detected during 30 years of a screening program in central Serbia. Eur J Pediatr. 2016;175:253–259. doi: 10.1007/s00431-015-2630-5. [DOI] [PubMed] [Google Scholar]
- 91.Veisani Y, Sayehmiri K, Rezaeian S, Delpisheh A. Congenital hypothyroidism screening program in Iran; a systematic review and metaanalysis. Iran J Pediatr. 2014;24:665–672. [PMC free article] [PubMed] [Google Scholar]
- 92.Maggio MC, Ragusa SS, Aronica TS, Granata OM, Gucciardino E, Corsello G. Neonatal screening for congenital hypothyroidism in an Italian Centre: a 5-years real-life retrospective study. Ital J Pediatr. 2021;47:108. doi: 10.1186/s13052-021-01053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Migliavaca CB, Stein C, Colpani V, et al. Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. 2022;13:363–367. doi: 10.1002/jrsm.1547. [DOI] [PubMed] [Google Scholar]
- 94.Mehran L, Khalili D, Yarahmadi S, et al. Worldwide recall rate in newborn screening programs for congenital hypothyroidism. Int J Endocrinol Metab. 2017;15:e55451. doi: 10.5812/ijem.55451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Dalmazi G, Carlucci MA, Semeraro D, et al. A detailed analysis of the factors influencing neonatal TSH: results from a 6-year congenital hypothyroidism screening program. Front Endocrinol. 2020;11:456. doi: 10.3389/fendo.2020.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang C, Tan M, Xie T, et al. Screening for neonatal inherited metabolic disorders by tandem mass spectrometry in Guangzhou. Zhejiang Xue Xue Bao Yi Xue Ban J Zhejiang Univ Med Sci. 2021;50:463–471. doi: 10.3724/zdxbyxb-2021-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gramer G, Brockow I, Labitzke C, et al. Implementing a tracking system for confirmatory diagnostic results after positive newborn screening for cystic fibrosis-implications for process quality and patient care. Eur J Pediatr. 2021;180:1145–1155. doi: 10.1007/s00431-020-03849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corbetta C, Weber G, Cortinovis F, et al. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH) Clin Endocrinol. 2009;71:739–745. doi: 10.1111/j.1365-2265.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 99.Olivieri A, Fazzini C, Medda E. Italian Study Group for congenital hypothyroidism. Multiple factors influencing the incidence of congenital hypothyroidism detected by neonatal screening. Horm Res Paediatr. 2015;83:86–93. doi: 10.1159/000369394. [DOI] [PubMed] [Google Scholar]
- 100.Al-Qahtani M. Congenital hypothyroidism. J Matern Fetal Neonatal Med. 2020:1–9. doi: 10.1080/14767058.2020.1838480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.