Summary
Background
Although key populations (KPs), such as men-who-have-sex-with-men (MSM) are disproportionately affected by HIV, many prevention and treatment services are not easily accessible for KP members. To address the needs of KPs, Thailand established pre-exposure prophylaxis (PrEP) service delivery together with and led by KP members. This study determines the epidemiological impact and cost-effectiveness of key population-led (KP-led) PrEP.
Methods
We calibrated a compartmental deterministic HIV transmission model to the HIV epidemic among Thai MSM. Besides KP-led PrEP, we included other Thai service delivery models of PrEP (fee-based PrEP, the government PrEP program).Data on consistent PrEP use (5 years daily use, 95% effectiveness for preventing HIV) came from Thai PrEP delivery models. For the period 2015–2032, we ranged the number of PrEP starters (40,000–120,000), effectiveness of PrEP (45%–95%), and proportion of consistent users (10%–50%). The analysis started in 2015 when PrEP was introduced. A cost-effectiveness ratio of <160,000 Baht per quality-adjusted life year (QALY) over 40 years was cost-effective.
Findings
Without PrEP, 53,800 (interquartile range 48,700–59,700) new HIV infections are expected in 2015–2032. KP-led PrEP was found to have the strongest epidemiological impact of all delivery models averting 58% of infections compared to without PrEP. The epidemiological impact depends on the number of PrEP starters and proportion of consistent use. Although all PrEP service delivery models are cost-effective, KP-led PrEP is most cost-effective with incremental cost-effectiveness ratios of 28,000–37,300 Thai Baht per QALY.
Interpretation
Our model projects KP-led PrEP having the greatest epidemiological impact and being the most cost-effective service delivery model of PrEP in Thailand.
Funding
This study was supported by the US Agency for International Development and U.S. President’s Emergency Plan for AIDS Relief through the Linkages Across the Continuum of HIV Services for Key Populations cooperative agreement (AID-OAA-A-14- 0045) managed by FHI 360.
Keywords: Key population, Preexposure prophylaxis, Thailand, Men-who-have-sex-with-men
Research in context.
Evidence before this study
In many countries the HIV epidemic is concentrated among key populations, including men who have sex with men (MSM) and transgender women. Key population members are often marginalised in traditional HIV service delivery due to enduring stigma and criminalisation of same-sex relationships, drug use and sex work. Traditional prevention and treatment services (hospital-based) receive most funding, while focussing on the general population. Only 10% of global HIV prevention funds were available for key populations in the period 2016–2018. The WHO expects that the cost-effectiveness of the response to HIV could be improved by greater investment in any country's key populations. This underscores the need to investigate alternative methods of service delivery tailored to key populations. Key population-led pre-exposure prophylaxis (PrEP) is a unique and innovative model of PrEP delivery designed in consultation with and led by key population members.
This service delivery model therefore fits the specific needs of those individuals disproportionally affected by HIV and empowers key populations to provide non-judgemental HIV services. To showcase the important role that key population-led PrEP programs can play in HIV service delivery, demonstrating the impact of this delivery model on the HIV epidemic is crucial. However, to our knowledge, the epidemiological impact and cost-effectiveness of key population-led PrEP programs have not yet been determined.
We searched PubMed for studies up to August 15th, 2022, with the terms “HIV” and “model” or “modeling” or “modelling”, and “PrEP” or “pre-exposure prophylaxis” or “preexposure prophylaxis”, and “cost-effectiveness”, and “key population-led” or “key population led” or “lay provider” or “lay providers” or “differentiated care” or “differentiated service delivery” or “task shifting” or “task-shifting,” with no language or start date restrictions. We identified no other mathematical modelling studies that estimated the epidemiological impact or cost-effectiveness of key population-led PrEP delivery. Widening our search to “HIV” and “model” or “modelling” or “modelling”, and “PrEP” or “pre-exposure prophylaxis” or “preexposure prophylaxis”, and “cost-effectiveness”, with no language or start date restrictions, we identified no other studies comparing multiple implementedreal-world PrEP service delivery programs in terms of number of infections averted or health economic impact.
Added value of this study
We used a compartmental deterministic model to estimate the epidemiological impact and cost-effectiveness of three different service delivery models of PrEP in Thailand, directly informed by real-world program data. Our study shows the added value of key population-led PrEP delivery in terms of number of infections averted and cost-effectiveness, compared to both no PrEP delivery and other delivery models of PrEP. Furthermore, our study provides a unique overview of the epidemiological and health economic impact of the implementation of different PrEP service delivery models.
Implications of all the available evidence
Our study shows that key population-led PrEP is the largest and most cost-effective provider of PrEP in Thailand with the greatest epidemiological impact. This builds on the growing body of evidence that community-based (or non-hospital based) and key population-led approaches to HIV prevention could benefit PrEP programs worldwide, providing equitable access to healthcare for marginalized communities. For all delivery models in Thailand, increasing consistent PrEP use in individuals most-at-risk of acquiring HIV is imperative to realize the full benefit PrEP has to offer. Future data collection should therefore prioritize clarifying reasons for non-consistent PrEP use while at increased risk and the impact of episodes of risk among those at high risk of acquiring HIV. Gaining insight in episodes of risk could guide provision of service delivery to be more effective.
Introduction
Key populations (KPs), including men who have sex with men (MSM) and transgender women, are disproportionally affected by HIV with KPs and their sexual partners accounting for 70% of HIV infections globally 2021.1 Nonetheless, these KPs are often marginalised in public health HIV service delivery approaches due to enduring stigma and insufficient availability of appropriate care.2 Although the World Health Organization (WHO) expects that cost-effectiveness of the HIV response can be improved by targeting KPs, traditional HIV treatment and prevention services are not always easily accessible to KP.1 This leads to an urgent need for tailored pre-exposure prophylaxis (PrEP) service delivery approaches that recognize the experiences and needs of KP members.3 Approaches can be tailored to fit the lifestyle of the individual at risk, aiming to make PrEP more convenient to use and to minimise participant burden. Offering KP members the option to be served by or even become KP-lay providers, recognizes KPs real-life experience with HIV and empowers them to receive or provide non-judgemental PrEP services.3 Different aspects of PrEP service delivery such as delivery settings or delivery channels can be tailored to bring HIV and related health services closer to the most vulnerable KPs. Delivery settings in which PrEP is provided can range from highly centralised specialist clinics to community health centres,3 to offering PrEP at the client's home.4 Delivery models can range from traditional in-person visits3 to self-management options.4 Even though PrEP is a very effective HIV prevention method, consistent use in real-world settings in which individuals adhere to PrEP is unfortunately challenging.4
Thailand has implemented KP-led health services (KPLHS), an innovative model of service delivery targeted at and led by KP members. Services delivered as part of KPLHS are designed in consultation with KP members and are therefore needs-based, demand-driven and client-centred, while also targeting resources effectively and ensuring that the highest-risk clients are targeted for the greatest impact.5 KP members have been trained to provide high-quality and non-judgemental HIV counselling and testing.3 In Thailand, KP-led PrEP, integrated in KPLHS, involves the same day initiation of PrEP for MSM and transgender women, and is implemented in 9 community-based clinics in 6 high burden provinces (Table S2).3 KP-led PrEP accounts for 57% of all PrEP users (10,576) in the country in 2020, and as a result of this success, PrEP has been included in the Universal Health Coverage Scheme in Thailand since 2019.6 Other service delivery models of PrEP in Thailand are fee-based PrEP (or PrEP-15), where PrEP is available for 15 Thai baht a day in an HIV-testing clinic, and PrEP service delivery at ART clinics in public hospitals funded through the government. These programs account for 19% (3525) and 24% (4453) of individuals that have started using PrEP, respectively.
The aim of this study is to assess the epidemiological impact and cost-effectiveness of PrEP provided through KPLHS and other service delivery models in Thailand among MSM at risk of HIV infection.
Methods
Study design and population
We adapted an existing compartmental deterministic HIV transmission model originally used for assessing the cost-effectiveness of PrEP for HIV prevention among MSM in Germany,7 to the Thai HIV epidemic among MSM aged 15 years and older.
Model assumptions and calibration
The model was adapted using parameters that represent the Thai HIV epidemic among MSM from 2015 to 2019 (Table 1, Fig. S1, Table S1). Due to limited data being available on the HIV epidemic among transgender women in Thailand, our model focusses solely on MSM. Our model distinguishes disease progression into the acute stage, three chronic stages (CD4+ T-cell count ≥500 cells/μL, 350–499 cells/μL, and 200–349 cells/μL), and an AIDS stage. A schematic representation of the model and the equations used can be found in supplement Fig. S1. The duration and infectiousness of each stage varies (Table S1).15 In our calibration, we assume that individuals are tested at a rate which matches the CD4+ T-cell count distribution at diagnosis as reported in the Thai Ministry of Public Health Department of Disease Control National Database.10 Individuals living with HIV start antiretroviral drug treatment immediately after diagnosis In our model, it is assumed that individuals who are virally suppressed cannot transmit HIV to others.16
Table 1.
Variables used to calibrate and accept simulations of the historic HIV epidemic among men-who-have-sex-with-men (MSM) in Thailand, and variables used to calibrate the different pre-exposure prophylaxis (PrEP) service delivery models into the model.
| Parameters used for calibration | Data in real world | Values accepted in calibration Median (min–max) |
Reference |
|---|---|---|---|
| MSM population (15+) | |||
| 2017 | 790,476 | 802,470 (491,584–1,098,150) | 8,9 |
| 2018 | 790,476 | 808,930 (491,584–1,098,150) | |
| 2019 | 790,476 | 815,700 (491,584–1,098,150) | |
| Proportion diagnosed at a CD4 of | |||
| ≥500 cells/μl | 10 | ||
| 350–499 cells/μl | |||
| 200–349 cells/μl | 19.60% | 20.25% (10%–30%) | |
| <200 cells/μl | 53.40% | 55.34% (40%–70%) | |
| Number of MSM living with HIV | |||
| 2019 | 46,314 | 41,711 (16,884–103,154) | 8,9 |
| Number of new infections among MSM in Thailand | |||
| 2015 | 3418 | 3830 (3000–4000) | 11 |
| 2016 | 3405 | 3570 (3000–4000) | |
| 2017 | 3394 | 3350 (3000–4000) | |
| Proportion of individuals diagnosed with HIV receiving treatment | 69% | 69% (25%–80%) | 12,13 |
| Proportion of individuals treated that are virally supressed | 93% | ||
| Total | 69% · 93% = 64.2% | ||
| Key population-led PrEP | |||
| Cumulative new users | 14 | ||
| 2015 | 150 | 189.7 (119.7–317.5) | |
| 2016 | 600 | 605.5 (381.0–1010.2) | |
| 2017 | 1450 | 1471.3 (928.3–2451.9) | |
| 2018 | 2900 | 2942 (1860–4892) | |
| 2019 | 5820 | 5807 (3,6595–9671) | |
| 2020 | 10,576 | 10,550 (6719–17,601) | |
| 12-month program retention rate | 27% | 27% | |
| PrEP-15 | |||
| Cumulative new users | 14 | ||
| 2015 | 200 | 189.4 (119.7–317.6) | |
| 2016 | 500 | 492.6 (309.9–823.1) | |
| 2017 | 950 | 937.2 (591.0–1562.6) | |
| 2018 | 1500 | 1506.8 (952.3–2508.0) | |
| 2019 | 2500 | 2517 (1598–4189) | |
| 2020 | 3525 | 3545 (2255–5889) | |
| 12-month program retention rate | 17% | 17% | |
| Government PrEP | |||
| Cumulative new users | 14 | ||
| 2016 | 150 | 154.57 (97.59–256.63) | |
| 2017 | 400 | 422.0 (268.2–681.4) | |
| 2018 | 850 | 855.8 (541.0–1396.4) | |
| 2019 | 1864 | 1857 (1186–3077) | |
| 2020 | 4453 | 4360 (2776–7273) | |
| 12-month program retention rate | 37% | 37% | |
We calibrated our model to the Thai HIV epidemic based on national and subnational estimates of the number of MSM,8,9 the percentage of MSM diagnosed at a CD4+ T-cell count between 200 and 349 cells/μl and less than 200 cells/μl, the number of MSM living with HIV,8,9 the number of new infections among MSM,11 the estimated proportion of individuals diagnosed with HIV receiving antiretroviral drug treatment and the proportion of treated individuals that are virally suppressed,12 the historic cumulative number of people starting PrEP, and yearly PrEP program retention rates (Table 1,14). Three existing Thai PrEP delivery models were considered in the model, including KP-led PrEP, fee-based PrEP (PrEP-15), and the government PrEP program. In total, nearly 19,000 individuals have started PrEP through one of the three service delivery models since 2015 until the end of 2020.
The model included four different risk groups with different levels of sexual activity based on the annual number of new sexual partners (Table S1). PrEP was targeted to the three most sexually active groups in the model. We utilize the term consistent use to describe both high adherence to PrEP, characterized by 95% effectiveness, and high retention in the PrEP programs, characterized by an average duration of use of five years. Consistent use of PrEP is suboptimal in the current delivery programs, with approximately 10% of individuals consistently using PrEP in the KP-led PrEP program. Individuals using PrEP were assumed to receive an HIV-test between 1 and 4 times per year. As the number of individuals starting PrEP through the programs is currently still expanding, we assumed a growing number of individuals starting PrEP over the next years, ranging from 40,000 to 120,000 individuals in 2032. Furthermore, we varied the effectiveness of PrEP between 45% and 95% for non-consistent users,7 whose duration of use was estimated based on current program data. Lastly, we varied the proportion of consistent PrEPusers between 10% and 50%. Monte Carlo filtering techniques using wide ranges of sexual activity allowed us to identify which sexual risk group combinations resulted in the appropriately calibrated HIV epidemic model. We accepted 154 out of 290,000 simulations that matched the HIV epidemic among MSM in Thailand. We assessed the epidemiological impact of PrEP on the short-term, between 2015 and 2032, reported as median number of infections averted in comparison to a scenario with no PrEP in the same period.
Cost-effectiveness
Each compartment in our model was assigned a cost and a quality-adjusted life year (QALY, supplementary Tables S3–S5). The cost-effectiveness was calculated from the third-party payer perspective without end-user costs. Costing data were only available for the KP-led PrEP program and the government PrEP program.17 Therefore, the average cost of the other programs was used for the fee-based PrEP program.
The costs of PrEP included the price of tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), the costs of clinical visits for PrEP care, the costs of monitoring side effects of tenofovir (e.g., creatinine) and monitoring sexually transmitted infections (including HIV, syphilis, and other bacterial sexually transmitted infections). The cost components that we included for HIV-infected individuals were direct healthcare costs, including outpatient visits to an HIV specialist and costs of testing for sexually transmitted diseases. We also included the costs of antiretroviral drugs based on the cost of the most frequently prescribed antiretroviral drug regimen, namely TDF/FTC and efavirenz.
We calculated average and incremental cost-effectiveness ratios (ICERs) over a 40 year period (2015–2055), with costs and QALYs discounted at 3% a year. Average cost-effectiveness ratios were calculated as the difference in costs divided by the QALY difference comparing PrEP to no PrEP. Incremental cost-effectiveness ratios were calculated similarly, only comparing PrEP to the previous cost-effective scenario. The cost-effectiveness threshold is a willingness to pay threshold by the Thai government currently set at 160,000 Baht or 5104 United States Dollars (USD) per QALY.18 This study uses the current exchange rate of 1 Thai Baht equalling 0.032 USD.19 Additionally, we calculated the short-term budget impact over 2021–2025 using undiscounted costs, representing the short-term financial impact. Findings >2500 were rounded off to the nearest 100s. Cost-effectiveness analysis was conducted using R.
Sensitivity analysis
We performed a one-way sensitivity analysis of the change in costs of HIV care and prevention after introduction of PrEP in comparison to a scenario where PrEP is not used. For the analysis, we assumed a baseline scenario of all three service delivery models with 120,000 combined PrEP starters in 2032, 85% PrEP effectiveness20 and 10% consistent PrEP use. We considered several key variables to assess the sensitivity of our model. We varied the 12- month retention of the three service delivery models between 7% and 67%, based on actual program retention rates (Table S2) and to account for future developments. The annual cost of providing PrEP care via each service delivery model and the annual cost of providing care for HIV-infected individuals including ART were varied between a 50% decrease or increase in cost. For PrEP-15, the minimum and maximum values in costs of KP-led PrEP and the government PrEP program were used. Finally, the yearly cost and QALY discounting was varied between 0% and 4.5%.
Role of the funding source
The study sponsor had no role in study design, analysis and interpretation of data. FHI360 approved the study before submission.
Results
Impact of PrEP on the HIV epidemic
Without PrEP, 53,800 (interquartile range (IQR) 48,700–59,700) new HIV infections are expected to occur among Thai MSM between 2015 and 2032. KP-led PrEP has the strongest impact on infections averted, accounting for 58% of infections prevented on average in scenarios with a varying amount of PrEP starters. PrEP service delivery in ART clinics that are part of public hospitals, is expected to account for 31% of infections averted on average, and PrEP-15 for 11% of infections averted. The epidemiological impact depends on the number of individuals that have started using PrEP up until 2032 (Table 2). At 40,000 cumulative PrEP starters, with 85% effectiveness and 10% consistent use yearly, PrEP is expected to avert 370 (0.7%) new infections. For 120,000 PrEP starters, the number of infections averted increases to 995 (1.9%). In comparison, varying the effectiveness of PrEP between 45% and 95% has a relatively modest impact on the number of infections averted. The range between the lowest and highest effectiveness when 120,000 individuals have started PrEP is only 215 additional infections averted. Moreover, the epidemiological impact depends on increasing the proportion of consistent users (Table 3). Increasing the proportion from 10% to 50% with 120,000 PrEP starters results in an increase of 1410 infections averted through PrEP. Even if only 40,000 individuals have started PrEP, an increase in the proportion of consistent users to 50% averts 340 additional infections in comparison to 10% consistent use.
Table 2.
Number of infections averted via pre-exposure prophylaxis (PrEP) use (2015–2032) in comparison to no PrEP.
| Cumulative starters | PrEP effectiveness (%) |
|||||
|---|---|---|---|---|---|---|
| 45% | 55% | 65% | 75% | 85% | 95% | |
| 120,000 | 810 (1.5%) | 850 (1.6%) | 900 (1.7%) | 945 (1.8%) | 995 (1.9%) | 1025 (1.9%) |
| 100,000 | 660 (1.2%) | 700 (1.3%) | 745 (1.4%) | 780 (1.5%) | 810 (1.5%) | 850 (1.6%) |
| 80,000 | 550 (1.0%) | 580 (1.1%) | 625 (1.2%) | 645 (1.2%) | 675 (1.3%) | 685 (1.3%) |
| 60,000 | 435 (0.8%) | 460 (0.9%) | 485 (0.9%) | 495 (0.9%) | 525 (1.0%) | 550 (1.0%) |
| 40,000 | 290 (0.5%) | 310 (0.6%) | 345 (0.6%) | 355 (0.7%) | 370 (0.7%) | 385 (0.7%) |
The analysis is stratified by effectiveness of PrEP in preventing HIV infections and number of cumulative starters, with 10% using PrEP consistently defined as daily PrEP use with with high retention (average duration of five years) and a 95% effectiveness.
Table 3.
Number of infections averted via pre-exposure prophylaxis (PrEP) use (2015–2032) in comparison to no PrEP.
| Cumulative starters | Consistent use (%) |
||||
|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | |
| 120,000 | 995 (1.9%) | 1355 (2.5%) | 1680 (3.1%) | 2075 (3.9%) | 2405 (4.5%) |
| 100,000 | 810 (1.5%) | 1120 (2.1%) | 1365 (2.5%) | 1675 (3.1%) | 1955 (3.6%) |
| 80,000 | 675 (1.3%) | 895 (1.7%) | 1100 (2.0%) | 1330 (2.5%) | 1540 (2.9%) |
| 60,000 | 525 (1.0%) | 695 (1.3%) | 840 (1.6%) | 1025 (1.9%) | 1160 (2.2%) |
| 40,000 | 370 (0.7%) | 455 (0.8%) | 530 (1.0%) | 635 (1.2%) | 710 (1.3%) |
The analysis are stratified by percentage of users using PrEP consistently from 2021 onwards. We defined consistent use as daily PrEP use with 95% effectiveness of PrEP, with high retention (average duration of five years) in the PrEP programs. Non-consistent PrEP use has an effectiveness of 85%.
Cost-effectiveness
Treatment and monitoring of HIV for all MSM seeking healthcare will result in a discounted cumulative cost of 6.7 billion Thai Baht (IQR: 6.4 billion – 6.9 billion) over 2015–2055. All service delivery models of PrEP are cost-effective (Table 4 and Fig. 1), with all cost-effectiveness ratios below the 160,000 THB/QALY willingness to pay threshold. PrEP delivery via KP-led PrEP is the most cost-effective service delivery model of PrEP in Thailand. Average cost-effectiveness ratios for KP-led PrEP range from 62,400THB/QALY to 67,600 THB/QALY and incremental cost-effectiveness ratios range from 28,000 THB/QALY to 37,300/QALY when varying the number of starters in 2032 and the percentage of consistent use. Implementing all PrEP programs is most cost-effective (64,500 THB/QALY) when only 40,000 individuals start using PrEP and 10% uses PrEP consistently.
Table 4.
Cost-effectiveness of pre-exposure prophylaxis (PrEP) delivery via service delivery models individually or combined.
| 40,000 starters in 2032, 85% effectiveness, 10% consistent use with 95% effectiveness | Total costs (Billions THB) | QALYs Gained compared to no PrEP (Median) |
Average cost- effectiveness ratio compared to no PrEP | Incremental cost- effectiveness ratio compared to last cost-effective intervention | Conclusion | Budget impact 2021–2025 (Millions THB) | |
|---|---|---|---|---|---|---|---|
| no PrEP (baseline) | 6.7 B | ||||||
| 1 | PrEP via government (24% of starters) | 6.8 B | 1000 | 90,600 | Cost- effective | 26 M | |
| 2 | PrEP via KPLHS (57% of starters) | 6.8 B | 2000 | 64,000 | 37,300 | Cost- effective | 30 M |
| 3 | PrEP via KPLHS + government (81% of starters) | 6.9 B | 3000 | 72,500 | 90,000 | Cost- effective | 60 M |
| 4 | PrEP via KPLHS + PrEP-15 + government (100% of starters) | 7.0 B | 4000 | 64,500 | 40,500 | Cost- effective | 68 M |
| 120,000 starters, 85% effectiveness, 10% cons use with 95% effectivenesses |
Total costs (Billions THB) |
QALYs Gained compared to no PrEP (Median) |
Average cost- effectiveness ratio |
Incremental cost- effectiveness ratio |
Conclusion |
Budget impact 2021–2025 (Millions THB) |
|
| no PrEP (baseline) | 6.7 B | ||||||
| 1 | PrEP via government (24% of starters) | 7.1 B | 4000 | 88,900 | Cost- effective | 90 M | |
| 2 | PrEP via KPLHS (57% of starters) | 7.2 B | 7000 | 62,400 | 28,000 | Cost-effective | 98 M |
| 3 | PrEP via KPLHS + government (81% of starters) | 7.5 B | 10,000 | 79,200 | 87,800 | Cost- effective | 183 M |
| 4 | PrEP via KPLHS + PrEP-15 + government (100% of starters) | 7.6 B | 12,000 | 77,000 | 65,900 | Cost- effective | 204 M |
|
120,000 starters in 2032, 85% effectiveness, 50% cons use with 95% effectiveness |
Total costs (Billions THB) |
QALYs Gained compared to no PrEP (Median) |
Average cost- effectiveness ratio | Incremental cost- effectiveness ratio | Conclusion | Budget impact 2021–2025 (Millions THB) | |
| no PrEP (baseline) | 6.7 B | ||||||
| 1 | PrEP via government (24% of starters) | 7.7 B | 10,000 | 93,400 | Cost- effective | 198 M | |
| 2 | PrEP via KPLHS (57% of starters) | 8.0 B | 19,000 | 67,600 | 37,000 | Cost- effective | 233 M |
| 3 | PrEP via KPLHS + government (81% of starters) | 8.9 B | 28,000 | 78,200 | 100,700 | Cost- effective | 424 M |
| 4 | PrEP via KPLHS + PrEP-15 + government (100% of starters) | 9.4 B | 33,500 | 78,500 | 88,200 | Cost- effective | 482 M |
In each scenario, average and incremental cost-effectiveness ratios are calculated from the total additional cost and total quality adjusted life years (QALYs) gained. The budget impact represents the undiscounted short-term costs of each intervention over 2021–2025. For non-consistent PrEP starters 85% effectiveness is assumed, while for individuals using PrEP consistently 95% effectiveness is assumed. We defined consistent use as daily PrEP use with high retention (average duration of five years) in the PrEP programs.
Fig. 1.
Costs and quality adjusted life years (QALYs) of pre-exposure prophylaxis (PrEP) delivery programs over 2015–2055. The costs and QALYS of the different programs using different scenarios including 40,000 (Fig. 1a–c), 80,000 (1d-f) or 120,000 (1g-i) individuals starting PrEP. The figure also includes different proportions of consistent use (defined as using daily PrEP for 5 years with an effectiveness for preventing new infections of 95%). The proportions of consistent use are 10% (Fig. 1a, d and g), 30% (1b, e, h) and 50% (c, f, i). The blue line represents the cost-effectiveness threshold of 160,000 THB/QALY in Thailand. The different PrEP delivery systems are represented by the coloured squares with black representing via government PrEP, blue key population-led PrEP, green a combination of government and key population-led PrEP delivery, and red a combination of all service delivery models combined
The budget impact of different scenarios varies, ranging from an undiscounted 26 million Baht for only the government PrEP program that serves 24% of 40,000 starters, to 482 million Baht for all programs combined with 120,000 starters and 50% consistent use (Table 4). Costs of KP- led PrEP range between 30 million Baht (40,000 starters, 10% consistent use) and 233 million Baht (120,000 starters, 50% consistent use). If the number of individuals that has started PrEP increases to 120,000, the budget impact of each service delivery model triples.
Sensitivity analysis
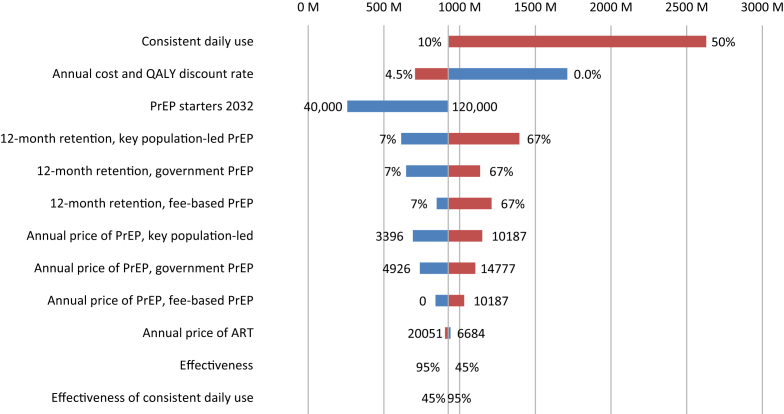
The sensitivity analysis indicates that the key driver of the total costs is the proportion of individuals using PrEP consistently, with costs ranging from 923 million Baht for 10% consistent use to 2629 million Baht for 50% consistent use (Fig. 2). Furthermore, the total PrEP costs also vary strongly with the discount rate, with a range of 1006 million Baht between no discounting and a 4.5% discounting rate. Additionally, the total costs range 781 million Baht between a 7% and 67% 12-month retention rate in the KP-led PrEP program. Lastly, the costs range is 665 million Baht between a scenario with 40,000 individuals starting PrEP and a scenario with 120,000 PrEP starters. Other variables, including variation in the 12-month retention of the other service delivery models and the annual pricing of PrEP care, have a relatively modest impact on total costs. The total ranges in cost vary from 363 to 488 million Baht for the 12-month retention rate in the fee-based PrEP program and the government PrEP program, respectively. The effectiveness of PrEP and annual price of ART has no impact on the total PrEP costs.
Fig. 2.
One-way sensitivity analysis. One-way sensitivity analysis of the change in the total costs of preexposure prophylaxis (PrEP) in comparison to a baseline scenario with 120,000 starters in 2032 and 10% consistent use over a 40 year period (2015–2055) with twelve key input variables. We defined consistent use as daily PrEP use with 95% effectiveness of PrEP, with high retention (average duration of five years) in the PrEP programs. The vertical grey line represents the costs of this baseline scenario. The red bars represent an increase in the key input variable, while the blue bars represent a decrease as compared to the baseline scenario.
Discussion
HIV prevention among Thai MSM via each service delivery model of PrEP is a cost-effective intervention. Although all service delivery models of PrEP in Thailand are cost-effective, KP- led PrEP is most cost-effective with the greatest epidemiological impact. This can be explained due to KP-led PrEP being the largest provider of PrEP in Thailand, while simultaneously having the lowest PrEP service delivery costs. Nonetheless, an increase in individuals that start using PrEP and the proportion of consistent users is essential for current programs in Thailand to reach their full potential, which could lead to averting up to 5% of new HIV infections until 2032.
Our findings substantiate the message by Vannakit et al.21 that bold investments in KP-led organisations and support for their involvement in service delivery in the face of declining external investments are urgently needed. Our cost-effectiveness analysis highlights that although health gains can be attained, implementation of PrEP also leads to an increase in costs. Domestic investments are crucial to sustain gains made and continue to defy the HIV epidemic, since short-term treatment and prevention costs could be up to 481 million Thai Baht. Although UNAIDS recognizes the importance of HIV investments, more efficient spending of funds is warranted.1,22 This could be achieved by investing in HIV services for KPs, which globally account for 70% of new infections, but for whom only 11% of global HIV funds were available in the period of 2016–2018.22 Therefore, recognition of KP-led programs and regulation of PrEP provision via KP-members is essential to achieve HIV epidemic control in a cost-effective manner. KP-led PrEP in Thailand has provided PrEP for nearly 60% of individuals that have started using PrEP,14 the highest number of all service delivery models of PrEP in Thailand. To attain maximum individual and societal benefit through PrEP, it is essential to reach more individuals that are most-at-risk of acquiring HIV. Investing in KP-led outreach activities and recruitment for testing, are important tools to reach individuals interested in PrEP. Since reaching individuals eligible for PrEP is not by itself enough to ensure actual PrEP uptake, the importance of gain framing and demand generation outreach activities has been recognized in KP-led PrEP.23
Our sensitivity analysis showed that consistent use of PrEP is crucial to reach the full potential of current programs. A strategy currently under development that could play an important role in increasing consistent use are long-acting formulations of PrEP. Different long-acting formulations are expected to become available. Currently, only cabotegravir given once every eight weeks has been investigated as PrEP.24 Formulations that can be administered less frequently are in development. Long-acting formulations reduce the need to take PrEP daily or on-demand, which has shown to be an important barrier for PrEP uptake.25 Another approach to increase consistent use of PrEP could include offering PrEP service delivery at the individual's home, possibly reducing the four annual in-person PrEP visits to one per year. More than one third of participants who tried this form of PrEP delivery via a pilot system reported a greater likelihood of persisting in care.4 Furthermore, other important facilitators of PrEP use are found to be partner and peer support and the ability to have control over the prevention option,25 in which further rollout of KP-led PrEP could play an important role. The number of individuals using PrEP consistently is a key driver for the total costs which is explained by fewer secondary infections that can be prevented as more sexual partners of PrEP are likely to also use PrEP.
A previous study by Suraratdecha et al.17 showed that incorporating PrEP delivery into an existing ARV program in Thailand was a cost-effective HIV prevention intervention, if offered to either MSM at high-risk of infection or all MSM. Ten Brink et al.26 modelled that if PrEP were scaled-up from 2022 with a 15% extra coverage by the end of 2026, 1200 additional new HIV infections among MSM could be averted in Thailand. This would result in 12,700 USD/DALY averted for daily generic PrEP, or 6100 USD/DALY averted for event-driven generic PrEP by 2051, both considered not cost-effective. Our study however, the first to model the effect of KP-led PrEP and now able to use data of existing PrEP programs, shows that PrEP delivery among Thai MSM is a cost-effective HIV prevention strategy. Other modelling studies in the Asia–Pacific region have also found similar results, with Choi and colleagues27 finding that targeting PrEP at the 20% of the highest risk MSM in South-Korea is a cost-effective strategy. Kazemian et al.28 found that providing PrEP at a lifetime horizon with HIV testing every six months is a cost-effective strategy for HIV prevention among MSM in India. However, PrEP among Indian MSM was not cost-effective when testing for HIV every 3 months.
Our mathematical modelling analysis has several strengths. First, to our knowledge this is the first study assessing the epidemiological impact and cost-effectiveness of PrEP delivery through KPLHS. As such, our study adds to the growing body of work providing the evidence base for KP-led programming, enabling other countries and policy makers to learn from the benefit of KPLHS.21 Another strength of our study is that our analyses are based on real-world program data which allowed us to include the impact of non-continuous use of PrEP, the actual uptake of PrEP in each of the service delivery models and realistic data regarding adherence and coverage. Furthermore, we considered the population benefit of PrEP by using a HIV transmission model, considering the population benefit of PrEP, rather than only the individual benefit of infection prevention.
Our study has several limitations. First, a compartmental deterministic model is restricted in accurately representing the reality of an epidemic. Necessarily, a model is a simplification of reality, since the actual epidemic depends on more intangible variables than a compartmental deterministic model can consider. Nonetheless, mathematical modelling is an essential, yet simplified, tool for gaining insights in the development of epidemics and determining what course of action to take. Furthermore, costing data were not available for the PrEP-15 program, which led to assessing cost-effectiveness based on data of the KP-led PrEP and the government PrEP program. However, these programs are the two largest service delivery models of PrEP in Thailand, and we believe that we have therefore estimated the cost-effectiveness of fee-based PrEP based on representable costing data. Additionally, we did not take all direct and indirect costs of providing ART care into account (such as hospitalization costs and costs of sick leave), because these data were not available. Nevertheless, if we included these data, PrEP would be considered even more cost-effective. Also, we have not been able to consider non-continuous use of PrEP in relation to episodes of risk, since data regarding intentionally stopping PrEP because of low HIV risk is scarce. However, if considered, PrEP would likely be more cost-effective, considering individuals using PrEP inconsistently in our model could actually be stopping and restarting at a later moment according to their HIV risk. Consistent use as we have defined in our study is mainly beneficial in those at high risk of acquiring HIV, while non-continuous use in relation to episodes of risk could be an effective solution for others. Lastly, our data collection ended at the end of 2019, therefore the effect of the COVID-19 pandemic is not reflected in our model.
We found that limited data are available on the HIV epidemic among transgender women in Thailand. Even though PrEP program data on transgender women are available, sufficient data to calibrate our model were lacking. Future research should focus on this data gap, as transgender women are an important population disproportionally affected by HIV.29 Furthermore, determining the needs, impact, and effectiveness of PrEP among transgender women is essential for policy makers to make informed decisions regarding PrEP service delivery. Additionally, we found that little data on seasons of risk or changes in risk status among MSM have been published. Seasons of risk as have been determined by Namey et al.30 among women at higher risk of HIV, could be a valuable contribution to accurately modelling PrEP use among Thai MSM.
All service delivery models of PrEP in Thailand are cost-effective in comparison to a scenario without PrEP, however KP-led PrEP integrated in KPLHS is most cost-effective with the highest epidemiological impact. This stresses the imperative role that KPLHS plays in realizing the full potential of PrEP for KPs, an approach to HIV prevention that could benefit PrEP programs worldwide. KPLHS and consistent use of PrEP are key components of HIV prevention in the Thai epidemic among MSM, in which long-acting formulations could eventually also have an important role to play. Therefore, we recommend the investment in and scale-up of PrEP service delivery in Thailand, with a focus on KPLHS.
Contributors
NP, RR, DC and DvdV conceptualized the study. LV, NP, RR, SM and DvdV designed the adapted compartmental deterministic model. LV and DvdV performed the mathematical modelling analysis. LV, RR, SM, SP and DvdV were involved in data analysis. SA, TC, NT, RT, AP, MA, SM, NP and RR collected cost and epidemiological data. All authors read and approved the final version of the paper.
Data sharing statement
The data that has been used for calibration and validation of the model is available in Table 1 and in the supplement.
Declaration of interests
Shreoshee Mukherjee and David van de Vijver report research grants from Gilead Sciences and ViiV outside of the submitted work. David van de Vijver reports funding from the National Institutes of Health, outside of the submitted work.
Acknowledgements
This study was supported by the US Agency for International Development and US President's Emergency Plan for AIDS Relief through the Linkages Across the Continuum of HIV Services for Key Populations cooperative agreement (AID-OAA-A-14- 0045) managed by FHI 360. The contents are the responsibility of the authors and do not necessarily reflect the views of US Agency for International Development and US President's Emergency Plan for AIDS Relief, or the United States Government.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2022.100097.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organization . WHO; Geneva: 2016. WHO Guidelines approved by the Guidelines Review Committee. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations – 2016 Update. [Google Scholar]
- 2.Yang F., Janamnuaysook R., Boyd M.A., Phanuphak N., Tucker J.D. Key populations and power: people-centred social innovation in Asian HIV services. Lancet HIV. 2020;7(1):e69–e74. doi: 10.1016/S2352-3018(19)30347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phanuphak N., Sungsing T., Jantarapakde J., et al. Princess PrEP program: the first key population-led model to deliver pre-exposure prophylaxis to key populations by key populations in Thailand. Sex Health. 2018;15(6):542–555. doi: 10.1071/SH18065. [DOI] [PubMed] [Google Scholar]
- 4.Siegler A.J., Mayer K.H., Liu A.Y., et al. Developing and assessing the feasibility of a home-based preexposure prophylaxis monitoring and support program. Clin Infect Dis. 2019;68(3):501–504. doi: 10.1093/cid/ciy529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Thai Red Cross AIDS Research Centre/FHI 360 . 2018. Differentiated HIV-service delivery along the cascade for men who have sex with men and transgender women in Thailand: lessons learned from the LINKAGES project. [Google Scholar]
- 6.National Health Security Office NHSO board adds PrEP for high risk populations to fiscal year 2020 budget (Thai language) 2019. https://www.nhso.go.th/frontend/NewsInformationDetail.aspx?newsid=MjUzMg
- 7.van de Vijver D., Richter A.K., Boucher C.A.B., et al. Cost-effectiveness and budget effect of pre-exposure prophylaxis for HIV-1 prevention in Germany from 2018 to 2058. Euro Surveill. 2019;24(7) doi: 10.2807/1560-7917.ES.2019.24.7.1800398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Disease Control . Ministry of Public Health; Thailand: July 2019. Estimation of PrEP Targets for Key and High-Risk Populations in Thailand, 2020-2022. [Google Scholar]
- 9.National Statistical Office Thailand . Demography Population and Housing Branch; 2019. Number of Population from Registration by Age, Sex and Province. [Google Scholar]
- 10.Department of Disease Control Thailand . 2018. CD4 at diagnosis derived from Minisitry of Public Health National Database. [Google Scholar]
- 11.Thailand Working Group on HIV/AIDS Projection . Nonthaburi; Thailand: 2010. AIDS Epidemic Model Projection for HIV/AIDS in Thailand 2010 -2030 Summary Report. [Google Scholar]
- 12.Thai Red Cross AIDS Research Centre . 2020. Virological failure data from Thai same day ART (SDART) program. unpublished. [Google Scholar]
- 13.UNAIDS . 2019. 90-90-90 achievements among MSM in Thailand, unofficial data. unpublished. [Google Scholar]
- 14.Phanuphak N. 23rd International AIDS Conference; Virtual2020; 6-10 July 2020. 2020. Elements of success in Thailand. [Google Scholar]
- 15.Lodi S., Phillips A., Touloumi G., et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 Cells/mm³: assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53(8):817–825. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 16.Rodger A.J., Cambiano V., Bruun T., et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi: 10.1016/S0140-6736(19)30418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suraratdecha C., Stuart R.M., Manopaiboon C., et al. Cost and cost-effectiveness analysis of pre-exposure prophylaxis among men who have sex with men in two hospitals in Thailand. J Int AIDS Soc. 2018;21(Suppl 5) doi: 10.1002/jia2.25129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National List of Essential Medicines Committee . 2013. Sub-committee of Thai Working Group on Health Technology Assessment, Meeting report of 2nd annual meeting. [Google Scholar]
- 19.Board of Governors of the Federal Reserve System Foreign exhange rates, historical rates for the Thai baht. 2021. https://www.federalreserve.gov/releases/h10/hist/dat00_th.htm
- 20.McCormack S., Dunn D.T., Desai M., et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vannakit R., Andreeva V., Mills S., et al. Fast-tracking the end of HIV in the Asia Pacific region: domestic funding of key population-led and civil society organisations. Lancet HIV. 2020;7(5):e366–e372. doi: 10.1016/S2352-3018(20)30077-1. [DOI] [PubMed] [Google Scholar]
- 22.Aidsfonds . 2019. FAST-TRACK or OFF TRACK? How insufficient funding for key populations jeopardises ending AIDS by 2030. Amsterdam. [Google Scholar]
- 23.Rivet Amico K., Bekker L.G. Global PrEP roll-out: recommendations for programmatic success. Lancet HIV. 2019;6(2):e137–e140. doi: 10.1016/S2352-3018(19)30002-5. [DOI] [PubMed] [Google Scholar]
- 24.Landovitz R.J., Donnell D., Clement M.E., et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koechlin F.M., Fonner V.A., Dalglish S.L., et al. Values and preferences on the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention among multiple populations: a systematic review of the literature. AIDS Behav. 2017;21(5):1325–1335. doi: 10.1007/s10461-016-1627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ten Brink D.C., Martin-Hughes R., Minnery M.E., et al. Cost-effectiveness and impact of pre-exposure prophylaxis to prevent HIV among men who have sex with men in Asia: a modelling study. PLoS One. 2022;17(5) doi: 10.1371/journal.pone.0268240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi H., Suh J., Lee W., et al. Cost-effectiveness analysis of pre-exposure prophylaxis for the prevention of HIV in men who have sex with men in South Korea: a mathematical modelling study. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-71565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazemian P., Costantini S., Kumarasamy N., et al. The cost-effectiveness of human immunodeficiency virus (HIV) preexposure prophylaxis and HIV testing strategies in high-risk groups in India. Clin Infect Dis. 2020;70(4):633–642. doi: 10.1093/cid/ciz249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baral S.D., Poteat T., Strömdahl S., Wirtz A.L., Guadamuz T.E., Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 30.Namey E., Agot K., Ahmed K., et al. When and why women might suspend PrEP use according to perceived seasons of risk: implications for PrEP-specific risk-reduction counselling. Cult Health Sex. 2016;18(9):1081–1091. doi: 10.1080/13691058.2016.1164899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.