Summary
Background
Rare cancers (RCs) are challenging to manage and are “forgotten cancers” though they collectively constitute a significant proportion of all cancers (∼20%). As a first step towards streamlining care, there is an unmet need to map the epidemiology of RCs in South Asian Association for Regional Collaboration (SAARC) countries.
Methods
The authors collected data from 30 Population-Based Cancer Registries (PBCR) of India and the published national registries of Nepal, Bhutan and Sri Lanka (SL) and compared them with the standard RARECAREnet RC list.
Findings
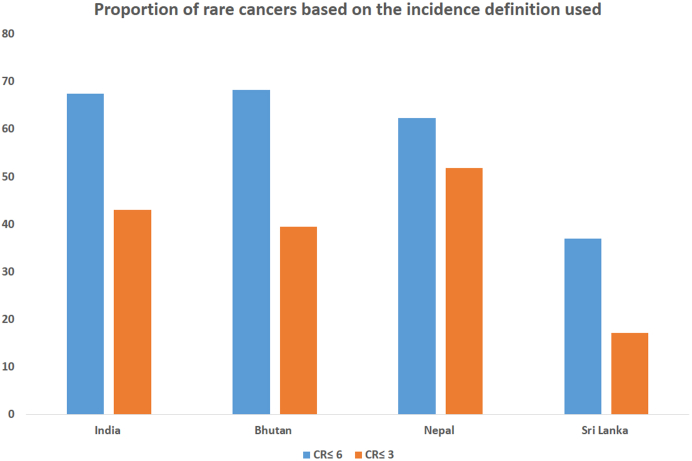
With the standard definition of crude incidence rates (CR) ≤6/100,0000 per population, 67.5%, 68.3%, 62.3% and 37% of all incident cancers qualify as RCs in India, Bhutan, Nepal and SL, respectively. An arbitrary cut-off CR ≤3 appears more appropriate with 43%, 39.5%, 51.8% and 17.2% of cancers identified as RCs, respectively, due to the lower cancer incidence.
There are similarities and notable differences between the RC lists of the SAARC region with that of the European RC list. Oral cavity cancers are rare in Europe, while pancreas, rectum, urinary bladder and melanomas are common. In addition, uterine, colon and prostatic cancers are rare in India, Nepal and Bhutan. In SL, thyroid cancer is common. There are gender-related and regional differences in RC trends in the SAARC countries.
Interpretation
There is an unmet need in SAARC nations to capture epidemiological nuances in rare cancers. Understanding the unique issues in the developing world may guide policymakers to adopt appropriate measures to improve RC care and tailor public health interventions.
Funding
None.
Keywords: Rare cancers, Incidence, Rare cancer list, Epidemiology, India, Nepal, Bhutan, Sri Lanka, South Asian Association for Regional Cooperation (SAARC) countries
Research in context.
Evidence before this study
Rare cancers (RCs) have been identified as a separate subset of “forgotten cancers” requiring special care as the treatment outcomes are worse due to persistent lack of attention. When considered together as a single group, RCs form a significant proportion of the cancer burden (∼20%) though the individual entities are rare. There are barriers like diagnostic issues, lack of randomized data on treatment and uncertainty in treatment protocols among RCs. Identifying an RC list helps in appropriate research prioritization, collaboration and development of strategies for improving outcomes of treatment of rare cancers. The European group RARECARE net identified a three-tier list of 198 cancers based on the incidence of cancers ≤6/100,000 populations. Later there were concentrated efforts like the RARECAREnet Asia project from Japan and East Asia. They found similar cancer burden and RC characteristics, except among head and neck and gastrointestinal cancers. However, there is not much data on the RC burden and epidemiology in South Asian Association for Regional Cooperation (SAARC) countries, where the cancer incidence is relatively lower.
Added value of this study
Our study explored the RC epidemiology in SAARC countries from the population-based cancer registries. Due to lower incidence, RCs with incidence ≤6/100,000 population formed 67.5% of total incident cancers and included many common cancers in India. An arbitrary cut-off of incidence of ≤3/100,000 populations may delineate better the RC epidemiology in this unique population. The study identified the arbitrary list at that potential cut-off. In addition, it identified an ultra-rare subset of cancers, with incidence ≤0.1/100,000 population.
Further, this study described the differences in the RC lists based on gender and regions of India. The study compared the Indian list with the RARECAREnet European list; there were disparities among the head and neck and gastrointestinal cancers.
The study also compiled RC lists for other SAARC nations, including Bhutan, Nepal and Sri Lanka wherein the authors noted similar RC epidemiology, with many common cancers having incidences ≤6/100,000.
Implications of all the available evidence
Rare cancers deserve special attention, as they form a significant proportion of cancers and are associated with inferior outcomes. Organizational support for collaborative research, multicenter trials and strategies like resource-adapted management protocols are vital to improving outcomes. Data on RCs will also help in national cancer control policies. The interpretations from this study are widely applicable to countries with similar cancer epidemiology patterns like other SAARC and other Low- and Middle- Income countries and hence merit wider recognition.
Introduction
Rare diseases and tumors are variably defined in the literature as per the prevalence or incidence in the defined geographical territory. Rare diseases are those diseases with a prevalence of <50–86/100,0000 population, while rare cancers (RCs) are commonly defined as those with an incidence of ≤6/100,000 population, although others defined an incidence of <15/100,000 persons as well.1, 2, 3 Individually, each RC represents a small fraction of cases. Nevertheless, as a group, RCs constitute ∼20% of all cancers in Europe, comparable to the burden of many common cancers.1,4,5 RCs form a conundrum in oncological practice, with inherent uncertainties in all treatment stages, ultimately leading to inferior outcomes.1,5, 6, 7 Diagnostic delays, difficulties conducting clinical trials with the resultant shortage of randomized evidence and prevalent non-uniform practices, inadequate expert centers and lack of awareness are a few pressing issues in RC treatment.1,4,8, 9, 10 Clinicians and researchers often focus on common cancers; conferences and continuing education programs are also usually conducted on common topics rather than rare cancers. Pharmaceutical companies are also inclined to sponsor research on molecules intended for more patients. When experts discuss the cancer epidemiology of any region, they focus on the most common cancers. Hence rare cancers are described as forgotten.
International organizations like the European Society for Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO) recognize the importance of research and collaboration in the RC field.11 The project for surveillance of RCs in Europe, RARECAREnet published a list of 198 RCs belonging to 12 cancer families, thus demarcating cancers with an incidence of ≤6/100,000 persons across Europe.9,12,13 However, the RC incidence cut-off is arbitrary, as acknowledged by the RARECARE group, due to variable incidences of cancers in different populations.1,4,12
There is a paucity of data on RCs from Asia. To mitigate challenges and enhance education and research, ESMO launched the Rare Cancers Asia initiative in 2018.11 RARECAREnet Asia group reported the epidemiology of RCs in East Asia. It concluded that the overall European RC list applies to East Asia also, with a few differences.5
With a highly heterogeneous population of 1.3 billion, comprising nearly 18% of the world population and almost 1.8 times Europe, India has distinct cancer epidemiology.14, 15, 16 Though the cancer incidence is lower in India; cancer is a significant public health problem due to the vast population and the resultant absolute numbers of patients involved. Prioritization of available resources requires the establishment of the epidemiological trend of all cancers, including the RCs. South Asian Association for Regional Cooperation (SAARC) countries share similarities in population characteristics, disease epidemiology and healthcare system challenges. However, there is a significant deficiency of information on RC epidemiology from SAARC countries. The population-based cancer registration system was long-laid in India under National Cancer Registry Program, with the network of registries covering about 9% of India's population. Though there may be some issues with coverage and lack of the exact histopathology for some sites of cancers, these population-based cancer registries (PBCRs) are stable and cover the urban and rural populations across the length and breadth of India.17 It is paramount to identify a correct RC list from South Asian countries that will enable focus on research collaborations, awareness and networking activities for these “forgotten cancers” globally.12 Correct identification of RCs will also help resource allocation and national cancer control policies.
To the best of our knowledge, the current study is the first study aimed to identify the RC epidemiology in SAARC countries from the published data of PBCR and compare the differences and similarities between the RC lists of India (RCI) and select SAARC countries (Nepal, Bhutan and Sri Lanka) with that of Europe (RCE). The analysis included only the SAARC countries with published epidemiological data on cancer incidence.
Methods
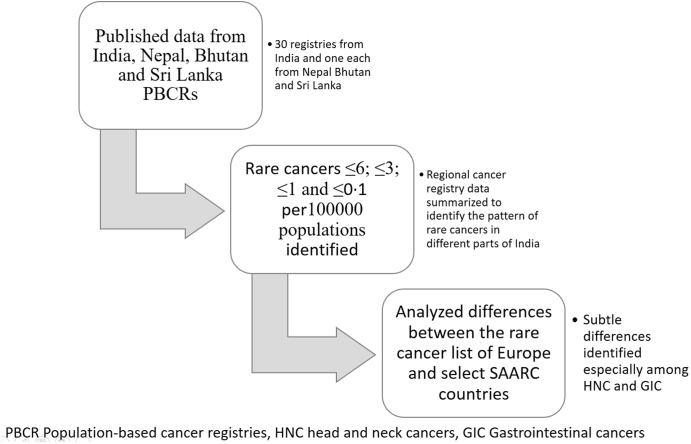
This study is based on the published reports of 30 Indian PBCR and published national reports of Nepal, Bhutan and Sri Lanka (SL) (Fig. 1). For the Indian data, the authors used the published consolidated report of the national cancer registry programme (NCRP) as well as Tata Memorial Centre (TMC), Mumbai-an autonomous unit under the Department of Atomic Energy, Government of India. For other SAARC countries, the authors used Columbo (year 2019), Bhutan (the year 2014–2018) and for Kathmandu Valley, Nepal (year 2018) data. These registry reports are available online in the public domain.
Fig. 1.
Schematic representation of the study methodology in identification of rare cancers in SAARC countries. CR Crude rate, SAARC South Asian Association for Regional Cooperation.
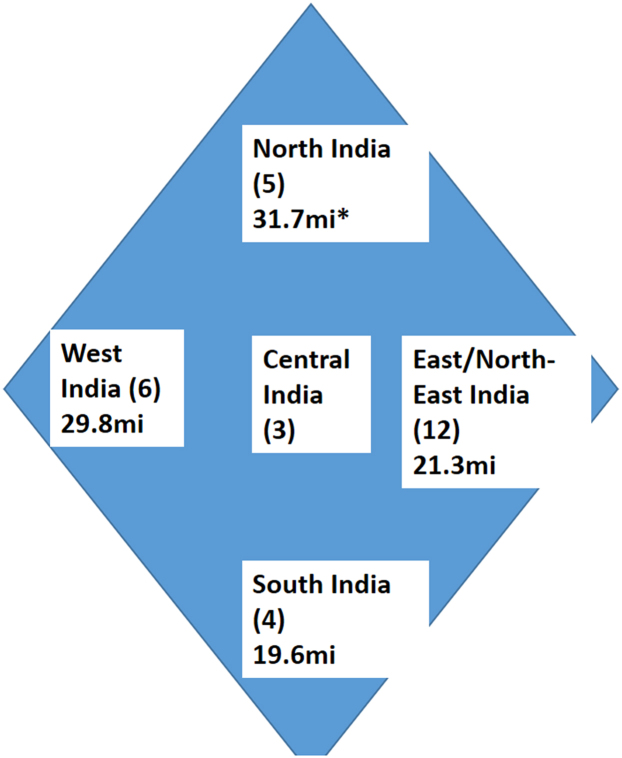
The government scientifically estimates the population every 10 years with the census. Each PBCR covers a well-defined region with an assigned population, a standard number mentioned in the PBCR reports. For the analysis, we calculated the sum of the PBCR population and obtained the proportion of coverage from the population of the country. The PBCR included patients diagnosed from 2012 to 2014 in 27 registries [Indian Council of Medical Research (ICMR) recognized registries and part of the National Cancer Registry Programme] and 2015–2017 in three other PBCR (associated with tertiary cancer care centers).18, 19, 20, 21 The authors included data from the following PBCR; Patiala, New Delhi, Varanasi, Sangrur, Chandigarh, Wardha, Nagpur and Bhopal in North and Central India (NC); Mumbai, Ahmedabad, Barshi (rural), Barshi (expanded), Aurangabad and Pune from West India; Kolkata, Nagaland, Mizoram, Pasighat, Manipur, Tripura, Naharlagun, Sikkim, Dibrugarh, Kamrup, Cachar and Meghalaya from East and North-East India (NE); Chennai, Thiruvananthapuram, Kollam and Bengaluru from South India; national cancer registries of Nepal (Kathmandu Valley PBCR), Bhutan and SL (Columbo PBCR), among the SAARC countries22, 23, 24 (Fig. 2). The other SAARC countries, like Pakistan and Afghanistan, do not have published PBCR data at present, hence were not included in the analysis. Maldives and Bangladesh do not have PBCRs, while other countries with PBCRs have limited data.
Fig. 2.
Schematic representation of the population based cancer registries in different regions of India. The number in parenthesis represents the number of population based registries in each region. The population in millions (mi) is also denoted. ∗ includes the population of central Indian registries too.
The authors collected details of the number of cancer cases, crude rates (CR) and age-adjusted rates (AAR) of cancers based on the topographic classification of the ICD-10 nomenclature to calculate the consolidated incidence rates for India.25 The PBCR data is separate for men and women. The crude incidence is the rate (CR) at which new cases occur in a population during a specific period. It is calculated from the number of new cancer cases observed in the 0–75+ year age group population in the specified period divided by the estimated population of the registry area of the same year, multiplied by a factor of 100,000. To calculate the CR for India, the authors divided the sum of the number of cases of each cancer type from the 30 PBCRs for a given duration and divided it by the total population at risk (sum of the individual populations of the PBCRs) for the same duration. Age adjusted incidence rate (AAR) is a statistical method that corrects for the changing age distribution of the population and allows comparisons to be made in the adjusted rates between different population sub-groups over time. Age-specific rate is multiplied by the world standard population. The AAR for each PBCR was calculated using the same standard population, as published in the consolidated report of the National Cancer Registry Program.26 Using the standard formula, the pooled data from the PBCRs was used to calculate AARs for entire India.
By arranging the cancers in decreasing order of CRs, the study identified four groups of RCs; with incidence rates of ≤6, ≤3, ≤1 and ≤0.1.12 The incidence of cancers is six times higher in Europe compared to India. So the first identified RC group (incidence ≤6/100,000) includes the majority of cancers, some of which are among the most common cancers in the countries.12,15 Additionally, the authors set an arbitrary cut-off of ≤3/100,000 incidence to enlist the more real RCs in this region as per the unique epidemiology of India (Rare Cancer list India, RCI) based on a consensus opinion of the authors. The study also identified separate subsets of cancers with an arbitrary incidence cut-off of ≤1 and ≤0.1/100,000 to capture data on the ‘ultra-rare’ cancers. The RCI is derived from primarily topographic diagnosis for most of the sites. For comparison with the European RC list, the total incidence of oral cavity and lip malignancies was calculated by consolidating the subsets of lip, tongue, gum, floor of mouth and palate (C00-C06) for India, Nepal, Bhutan and SL.
Characterization of the RCI
To understand the RC epidemiology further, the mean CR and AAR for each RC for the pre-defined regions of India from the constituent registry data, separately for men and women, were calculated, allowing the authors to study the RC epidemiology in terms of gender and regions of India. Exploring the RC epidemiology further, we compared the RCI and SAARC countries with that of Europe.
Results
Details of PBCR included
The data from 30 Indian, Kathmandu Valley, Bhutan and Columbo PBCRs covering nearly 8.5%, 10.5%, 100% and 11.4% of India, Nepal, Bhutan and SL, respectively, were analysed.
Quality indicators of the PBCR
There are stringent International Association of Cancer Registries (IARC)-based quality checks on the PBCR registry data at entry time and subsequent time points.27 For their publication, the National Cancer Registry Programme (NCRP) under the Indian Council of Medical Research (ICMR) consolidated the data from all the available PBCRs and included them in the published report. Before reporting the data, the NCRP, ICMR and Tata Memorial Centre (TMC) conduct and ensure quality control. NCRP conducts quality control exercises in individual PBCRs for internal consistency and assures data quality. TMC follows IARC guidelines and performs quality control through the IARC check program. Similarly, for Nepal, Bhutan and Columbo cancer registries, quality control exercise is conducted in collaboration with IARC regional hub Mumbai, India. Senior-level workforce officials cross-check the data for all these cancer registries and ensure continuous quality control. We have included only those registries whose results are published by ICMR in their consolidated report or IARC assured. In India, 27 of the chosen PBCRs are ICMR-recognized registries under the NCRP under the rigorous ICMR regulations (IARC norms); many of the PBCR data were also included in the formal IARC publication, Cancer Incidence in Five Continents (CI5 Vol-XI). The other three PBCRs (Sangrur, Chandigarh and Varanasi), under Tata Memorial Centre, are under stringent quality control procedures with available published reports.28, 29, 30 Table 1 shows the quality parameters of the included registries, with the percentage of microscopically verified (MV) cases, death certificate only (DCO) rate, and other/unspecified cancer percentages. The other and unspecified cancers are the topographically unspecified set of cancers.12 As per comparability and quality control in cancer registration by IARC, MV is an indicator of data quality and is used as a measure of diagnostic validity, because, by definition, any cancer should be confirmed by a pathological assessment. The percentage of MV-confirmed cases varies across countries. A value close to 100% for MV indicates that the registry is missing radiologically/clinically diagnosed cases. Patients with advanced malignancy and poor general conditions may sometimes not have a histological diagnosis. The MV%, shown in the table, indicates that the PBCR captures data on patients without an exact histopathological diagnosis. A lower DCO rate suggests that the registry has captured data before the patients’ death.25,27
Table 1.
Select quality indices of the included population-based cancer registries.
| Registry | Microscopically verified (%) | Death certificate only (%) | Others and unspecified seta (%) |
|---|---|---|---|
| Indiab | 74–98 | 0–24c | 6.3 |
| Nepal | 90.5 | 1.5 | 4.1 |
| Bhutan | 98.6 | 1.4 | 3.7 |
| Columbo | 56.7 (M) 63.4 (F) |
4.7 | 12.2 |
F Female, M Male.
Other and unspecified refers to the topographically unspecified set.
Includes 30 PBCRs.
Percentage of registries with Death certificate only % >5 are only 7.
Derivation of RC list India
Of the 167,083 cancers reported from all the included cancer registries, the rates (CR and AAR) of each cancer site and the mean value for the entire India were compiled. With a standard definition of CR ≤6/100,000 populations, 67.5% of the total cancers, including some common cancers, were included in the RC list (Table 2, Fig. 3).
Table 2.
Rare cancers of India with incidence ≤6/1,00,000 populations.
| ICD code | Cancer site | Total number | Percentage | AAR | CR | 95% CI |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| C56 | Ovary | 4972 | 3.0 | 5.9 | 5.7 | 5.50 | 5.81 |
| C03-06 | Mouth | 9439 | 5.6 | 5.6 | 5.2 | 5.10 | 5.31 |
| C15 | Oesophagus | 8887 | 5.3 | 5.6 | 4.9 | 4.80 | 5.00 |
| C 61 | Prostate | 4287 | 2.6 | 5.8 | 4.6 | 4.73 | 4.45 |
| C16 | Stomach | 6899 | 4.1 | 4.3 | 3.8 | 3.71 | 3.89 |
| C01-02 | Tongue | 6460 | 3.9 | 3.9 | 3.6 | 3.47 | 3.65 |
| Cancers with incidence ≤3/1,00,000 population | |||||||
| C54-55 | Corpus uteri and uterus uns | 3010 | 1.8 | 3.8 | 2.8 | 2.65 | 2.87 |
| C23-24 | Gallbladder etc. | 4973 | 3.0 | 3.1 | 2.7 | 2.67 | 2.82 |
| C22 | Liver | 4452 | 2.7 | 2.8 | 2.5 | 2.38 | 2.53 |
| C82-85 | Non-Hodgkin lymphoma | 4411 | 2.6 | 2.7 | 2.4 | 2.36 | 2.50 |
| C32 | Larynx | 4295 | 2.6 | 2.7 | 2.4 | 2.30 | 2.44 |
| C19-20 | Rectum | 4039 | 2.4 | 2.5 | 2.2 | 2.16 | 2.30 |
| C18 | Colon | 4024 | 2.4 | 2.5 | 2.2 | 2.15 | 2.29 |
| C73 | Thyroid | 4023 | 2.4 | 2.1 | 2.2 | 2.15 | 2.29 |
| C70-72 | Brain, nervous system | 3632 | 2.2 | 2.1 | 2.0 | 1.94 | 2.07 |
| C12-13 | Hypopharynx | 3308 | 2.0 | 2.1 | 1.8 | 1.76 | 1.89 |
| C67-68 | Ureter, bladder, uns urinary organs | 3059 | 1.8 | 2.0 | 1.7 | 1.61 | 1.76 |
| C92-C94 | Myeloid Leu. | 2999 | 1.8 | 1.7 | 1.7 | 1.59 | 1.71 |
| C09-10 | Tonsil and oth oropharynx | 2292 | 1.4 | 1.4 | 1.3 | 1.19 | 1.34 |
| C91 | Lymphoid Leu. | 2222 | 1.3 | 1.4 | 1.2 | 1.17 | 1.28 |
| C44 | Skin | 2149 | 1.3 | 1.3 | 1.2 | 1.13 | 1.23 |
| C25 | Pancreas | 2125 | 1.3 | 1.3 | 1.2 | 1.12 | 1.22 |
| C90 | Multiple Myeloma | 1983 | 1.2 | 1.3 | 1.1 | 1.05 | 1.14 |
| C64 | Kidney | 1942 | 1.2 | 1.2 | 1.1 | 1.02 | 1.12 |
| Cancers with incidence ≤1/1,00,000 population | |||||||
| C40-41 | Bone | 1709 | 1 | 1 | 0.9 | 0.90 | 0.99 |
| C47,C49 | Connective and soft tissue | 1473 | 0.9 | 0.9 | 0.8 | 0.77 | 0.85 |
| C 60 | Penis | 753 | 0.5 | 0.5 | 0.8 | 0.86 | 0.75 |
| C81 | Hodgkin disease | 1163 | 0.7 | 0.6 | 0.6 | 0.60 | 0.68 |
| C11 | Nasopharynx | 1091 | 0.7 | 0.6 | 0.6 | 0.57 | 0.64 |
| C62-63 | Testis and other MGO | 583 | 0.3 | 0.6 | 0.6 | 0.69 | 0.56 |
| C52, C57 | Vagina and other FGO | 406 | 0.2 | 0.5 | 0.5 | 0.52 | 0.40 |
| C07-08 | Salivary glands | 798 | 0.5 | 0.5 | 0.4 | 0.41 | 0.47 |
| C14 | Pharynx uns. | 645 | 0.4 | 0.4 | 0.4 | 0.33 | 0.38 |
| C95 | Leu uns. | 610 | 0.4 | 0.4 | 0.3 | 0.31 | 0.36 |
| C51 | Vulva | 291 | 0.2 | 0.4 | 0.3 | 0.37 | 0.29 |
| C21 | Anus and Anal Canal | 547 | 0.3 | 0.3 | 0.3 | 0.28 | 0.33 |
| C30-31 | Nose, sinuses etc. | 545 | 0.3 | 0.3 | 0.3 | 0.28 | 0.33 |
| C00 | Lip | 506 | 0.3 | 0.3 | 0.3 | 0.25 | 0.30 |
| C43 | Melanoma of skin | 504 | 0.3 | 0.3 | 0.3 | 0.25 | 0.30 |
| C17 | Small intestine | 389 | 0.2 | 0.2 | 0.2 | 0.19 | 0.24 |
| C37-38 | Oth. thoracic organs | 296 | 0.2 | 0.2 | 0.2 | 0.14 | 0.18 |
| Cancers with incidence ≤0.1/1,00,000 population | |||||||
| C69 | Eye | 269 | 0.2 | 0.2 | 0.1 | 0.13 | 0.17 |
| C74 | Adrenal gland | 140 | 0.1 | 0.1 | 0.1 | 0.06 | 0.09 |
| C58 | Placenta | 67 | 0.0 | 0.1 | 0.1 | 0.09 | 0.06 |
| C96 | Oth malignancies of lymphoid, hematopoietic and related tissues | 98 | 0.1 | 0.1 | 0.1 | 0.04 | 0.06 |
| C65 | Renal pelvis | 17 | 0.0 | 0.0 | 0.0 | 0.00 | 0.01 |
| C88 | Malig IPD | 16 | 0.0 | 0.0 | 0.0 | 0.00 | 0.01 |
| C45 | Mesothelioma | 15 | 0.0 | 0.0 | 0.0 | 0.00 | 0.01 |
| C46 | KS | 9 | 0.0 | 0.0 | 0.0 | 0.00 | 0.01 |
Cancers listed in italics represent the ultra-rare cancers with incidence <0.1/1,000,000 population.51
AAR Age-adjusted rate, CI confidence interval, CR Crude rate, FGO Female genital organs, ICD International Classification of Diseases, KS Kaposi sarcoma, Leu Leukemia, Malig IPD Malignant immunoproliferative disorder, MGO male genital organs, Oth Other, uns unspecified.
Fig. 3.
Graphical representation of the proportion of rare cancers in India and selected SAARC countries based on the incidence definitions.
Hence we proposed an arbitrary CR of ≤3/100,000 for RCI corresponding to 42 cancer sites (Table 2). The proposed incidence cut-off included all the relevant RCs, which are known to present diagnostic and treatment difficulties like acute myeloid leukemia and soft tissue/bone sarcomas while excluding the known common cancers like prostate, lung and ovarian cancers.12,13 Table 2 shows the CR and AAR for RCI. The total number of RCs was 68,868, constituting 43% of all incident cancers (Fig. 3).
Cancers with incidence ≤1/100,000 constitute 9.5% of the incident cancers and are genuinely uncommon even among the RCs. These include bone and soft tissue, penile, nasopharyngeal and testicular cancers.
The ‘ultra-rare’ cancers, defined as an incidence of ≤0.1/100,000, constituted only 0.5% of total incident cancers (Table 2). These included cancers of the adrenal gland, placenta, ureter, renal pelvis, mesotheliomas and Kaposi sarcoma.
Characteristics of the Indian RC list
Sites of RCs
Head and neck cancers
Sites like nasopharynx, nasal cavity and sinuses, salivary glands, lip, larynx and hypopharynx and tonsillar malignancies are rare. Oral cavity cancers are common.
Gastrointestinal cancers
Pancreas, colon, rectum, small intestine, liver, gall bladder and anal canal cancers are rare in India.
Gender differences
Among RCs, there is a disparity (CR difference > 1) between male and female incidences of thyroid, oral cavity, hypopharynx and larynx, esophagus, stomach and gall bladder malignancies. There are similar trends in males and females in renal pelvic and ureter, mesotheliomas and Kaposi sarcoma. Table S1 summarizes the gender differences in the RC list.
Regional differences
We listed the AARs of the RCs in four regions of India. As seen in Table S2, there is some variation in the distribution of RCs (Fig. 4). While gall bladder cancers seem truly rare in West and South India, esophageal and stomach cancers seem very common in NE India. The profile of cancers in South India is similar to the national list. However, incidence rates are generally higher than the national average. The very rare cancers are essentially comparable between the different regions of India.
Fig. 4.
Regional variation in rare cancer distribution in India.
Comparison of the Indian rare cancer list with the RARECAREnet list
There are similarities and differences between the published RARECAREnet list of RCs and the RCI (Table 3). Notably, differences are seen among head and neck cancers and gastrointestinal cancers. Cancer of the oral cavity is common in India and rare in Europe. In contrast, the RCI included cancers of the colo-rectum, pancreas, melanomas and body uterus, which are common in Europe.
Table 3.
Comparison of rare cancer lists of Europe and select SAARC countries.
| Cancer sites | Europe | India | Nepal | Bhutan | Sri Lanka | |
|---|---|---|---|---|---|---|
| Head and neck cancers | ET larynx | R | R∗ | R∗ | R∗ | R∗ |
| ET hypopharynx | R | R∗ | R∗ | R∗ | R∗ | |
| ET nasal cavity and sinuses | R | R∗ | R∗ | R∗ | R∗ | |
| ET nasopharynx | R | R∗ | R∗ | R∗ | R∗ | |
| ET major salivary glands and salivary gland type tumors | R | R∗ | R∗ | R∗ | R∗ | |
| ET oropharynx | R | R∗ | R∗ | R∗ | R∗ | |
| ET oral cavity and lipa | R | C | R∗ | R∗ | C | |
| ET eye and adnexa | R | R∗ | R∗ | R∗ | R∗ | |
| ET middle ear | R | NA | NA | NA | NA | |
| Gastrointestinal tumors | ET stomach | C | R | R | C | R |
| ET esophagus | C | R | R∗ | R | R | |
| ET small intestine | R | R∗ | R∗ | R∗ | R∗ | |
| ET pancreas | C | R∗ | R∗ | R∗ | R∗ | |
| ET colon | C | R∗ | R∗ | R∗ | C | |
| ET rectum | C | R∗ | R∗ | R∗ | R | |
| Liver | C | R∗ | R∗ | R | C | |
| ET gall bladder and extrahepatic biliary tract | R | R∗ | R | R∗ | R∗ | |
| ET anal canal | R | R∗ | R∗ | R∗ | R∗ | |
| Thoracic tumors | Lung | C | C | C | R | C |
| ET trachea | R | R∗ | ||||
| Thymomas and thymic carcinomas | R | NA | NA | NA | NA | |
| Malignant mesothelioma | R | R∗ | R∗ | R∗ | R∗ | |
| Breast | Breast | C | C | C | R | C |
| Female genital cancers | Ovary | C | R | R | R | C |
| Non ET ovary | R | NA | NA | NA | NA | |
| ET vulva and vagina | R | R∗ | R∗ | R∗ | R∗ | |
| Trophoblastic tumors of placenta | R | R∗ | R∗ | R∗ | R∗ | |
| Uterus | C | R | R∗ | R∗ | C | |
| Male genital and urogenital cancers | Testis and paratestis | R | R∗ | R∗ | R∗ | R∗ |
| ET penis | R | R∗ | R∗ | R∗ | R∗ | |
| Extragonadal germ cell tumors | R | NA | NA | NA | NA | |
| ET renal pelvis, ureter and urethra | R | R∗ | R∗ | R∗ | R∗ | |
| Kidney | C | R∗ | R∗ | R∗ | R | |
| Bladder | C | R∗ | R∗ | R∗ | R | |
| Prostate | C | R | R∗ | R∗ | C | |
| Skin cancers and non cutaneous melanomas | Skin | C | R∗ | R∗ | R∗ | R∗ |
| Cutaneous melanoma | C | R∗ | R∗ | R∗ | R∗ | |
| Mucosal and uveal melanoma | R | NA | NA | NA | NA | |
| Kaposi sarcoma | R | R∗ | R∗ | R∗ | R∗ | |
| Adnexal skin carcinomas | R | NA | NA | NA | NA | |
| Sarcomas | Soft tissue sarcoma | R | R∗ | R∗ | R∗ | R∗ |
| Bone sarcoma | R | R∗ | R∗ | R∗ | R∗ | |
| Gastrointestinal stromal tumors | R | NA | NA | NA | NA | |
| Neuroendocrine tumor (NET) | NET gastrointestinal pancreatic | R | NA | NA | NA | NA |
| NET lung | R | NA | NA | NA | NA | |
| NET other sites | R | NA | NA | NA | NA | |
| Endocrine cancers | Thyroid cancers | R | R∗ | R∗ | R | C |
| Parathyroid cancer | R | NA | NA | NA | NA | |
| Adrenal cortex cancer | R | R∗ | R∗ | R∗ | R∗ | |
| Pituitary gland cancer | R | NA | NA | NA | NA | |
| Central nervous system | Glial tumors and others | R | R∗ | R∗ | R∗ | R |
| Malignant meningioma | R | |||||
| Embryonal tumors of CNS | R | |||||
| Pediatric | Pediatric tumor subsets | R | NA | NA | NA | NA |
| Hematological | Lymphoid malignancies | R | R∗ | R∗ | R∗ | R |
| MDS | R | NA | NA | NA | NA | |
| MPN | R | NA | NA | NA | NA | |
| Acute myeloid leukemia and other related neoplasms | R | R∗ | R∗ | R∗ | R∗ | |
| Myeloid or lymphoid neoplasms with eosinophilia and abnormalities of growth factors | R | NA | NA | NA | NA | |
| Myelodysplastic/myeloproliferative neoplasms | R | NA | NA | NA | NA |
C common, CR Crude rate, ET Epithelial tumors, HL Hodgkin lymphoma, MDS/MPD Myelodysplastic syndromes/Myeloproliferative disorders, NHL Non Hodgkin lymphoma, R rare.
NA separate data not available.
R ( ) indicates rare cancers (CR ≤ 6/100,000), R∗ (
) indicates rare cancers (CR ≤ 6/100,000), R∗ ( ) group includes entities with CR ≤ 3. C (
) group includes entities with CR ≤ 3. C ( ) indicates the common cancers.
) indicates the common cancers.
For the comparison with the European rare cancer list, the entities C00-C06 (sites of lip, mouth, tongue) were combined to form a single entity of ‘oral cavity and lip’ from the individual site data. CR C00-C06: for India 9, Nepal 2.3, Bhutan 2.4, Sri Lanka 9.
Rare cancers in Bhutan, Nepal and Sri Lanka
Table 4 shows the RC list of Bhutan, Nepal and SL. Similar to RCI, cancers with CR ≤6 formed 68.3%, 62.3% and 37% and the cut-off of ≤3 included 39.5%, 51.8% and 17.2% of the total incident cancers in Bhutan, Nepal and SL respectively. In Bhutan, myeloid leukemias, prostate, bone, mouth, hypopharynx and laryngeal cancers are rare. In Nepal, thyroid, colon, NHL, bladder, rectal and prostate cancers are rare. A few of the RCs of SL are stomach, larynx, bladder, brain and kidney cancers. Compared to RARECAREnet list, colon and prostate cancers are rare in Bhutan and Nepal, while bladder cancers are rare in SL. Kidney cancers are rare in all three countries, similar to India, unlike the European list. In SL, cancers of the esophagus, cervix and tongue are common, similar to India and liver cancer is also excluded from the RC list. Table 3 demonstrates the similarities and differences between the RARECAREnet list and the RC lists of Nepal, Bhutan and SL.
Table 4.
Rare cancers in Bhutan, Nepal and Sri Lanka.
| Bhutan |
Nepal |
Sri Lanka |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sites | CR | 95% CI |
Sites | CR | 95% CI |
Sites | CR | 95% CI |
|||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||
| CR < 6.0 | CR < 6.0 | CR ≤ 6 | |||||||||
| Ovary | 5.4 | 4.32 | 6.53 | Stomach | 4.6 | 3.83 | 5.35 | Rectum | 6.0 | 5.07 | 7.02 |
| Lung | 4.4 | 3.68 | 5.05 | Gallbladder etc. | 4.6 | 3.80 | 5.31 | Mouth | 5.7 | 4.77 | 6.67 |
| Thyroid | 4.3 | 3.58 | 4.93 | Ovary | 4.3 | 3.26 | 5.37 | Oesophagus | 5.1 | 4.25 | 6.05 |
| Oesophagus | 4.1 | 3.48 | 4.81 | NHL | 3.7 | 2.95 | 4.48 | ||||
| Liver | 3.4 | 2.77 | 3.97 | Kidney | 3.2 | 2.52 | 3.94 | ||||
| Breast | 3.2 | 2.61 | 3.78 | Stomach | 3.2 | 2.48 | 3.89 | ||||
| Ureter, bl & oth UO | 3.2 | 2.36 | 4.09 | ||||||||
| Brain, NS | 3.1 | 2.44 | 3.85 | ||||||||
| CR < 3.0 | CR < 3.0 | CR ≤ 3 | |||||||||
| Rectum | 2.6 | 2.06 | 3.11 | Thyroid | 2.7 | 2.15 | 3.32 | Tongue | 3.0 | 2.30 | 3.67 |
| Gallbladder etc. | 2.5 | 1.99 | 3.02 | Corpus uteri, Ut uns | 2.6 | 1.55 | 3.71 | Myeloid Leu. | 2.5 | 1.90 | 3.16 |
| Myeloid Leukaemia | 2.3 | 1.79 | 2.77 | Colon | 2.5 | 1.98 | 3.10 | Pancreas | 2.5 | 1.87 | 3.12 |
| Brain, NS | 2.1 | 1.64 | 2.59 | NHL | 2.5 | 1.95 | 3.07 | Larynx | 2.3 | 1.72 | 2.93 |
| Prostate | 1.8 | 1.20 | 2.41 | Ureter, bl & oth UO | 2.1 | 1.49 | 2.81 | Multiple Myeloma | 2.2 | 1.65 | 2.84 |
| Bone | 1.7 | 1.25 | 2.09 | Rectum | 1.8 | 1.32 | 2.26 | Gallbladder etc. | 2.2 | 1.58 | 2.75 |
| Mouth | 1.5 | 1.08 | 1.87 | Prostate | 1.6 | 1.01 | 2.27 | Connective and ST | 1.3 | 0.82 | 1.71 |
| Colon | 1.3 | 0.96 | 1.71 | Skin | 1.5 | 1.06 | 1.93 | Lymphoid Leu. | 1.3 | 0.82 | 1.71 |
| NHL | 1.1 | 0.74 | 1.43 | Pancreas | 1.5 | 1.04 | 1.89 | Vagina and other FGO | 1.2 | 0.35 | 2.05 |
| Hypopharynx | 1.1 | 0.72 | 1.39 | Oesophagus | 1.4 | 0.98 | 1.82 | ||||
| Myeloid leu. | 1.4 | 0.98 | 1.82 | ||||||||
| Brain, NS | 1.4 | 0.95 | 1.78 | ||||||||
| Larynx | 1.2 | 0.84 | 1.63 | ||||||||
| Mouth | 1.2 | 0.82 | 1.59 | ||||||||
| CR < 1.0 | CR < 1.0 | CR ≤ 1 | |||||||||
| Larynx | 1.0 | 0.65 | 1.30 | Kidney | 1.0 | 0.68 | 1.40 | Testis and oth MGO | 1.0 | 0.29 | 1.70 |
| Vulva | 0.9 | 0.43 | 1.32 | Tongue | 1.0 | 0.63 | 1.33 | Eye | 0.9 | 0.56 | 1.32 |
| Corpus uteri, Ut uns | 1.4 | 0.61 | 2.19 | Hypopharynx | 1.0 | 0.63 | 1.33 | Other Skin | 0.9 | 0.52 | 1.27 |
| Placenta | 0.9 | 0.43 | 1.32 | Liver | 0.9 | 0.60 | 1.29 | Leukaemia Uns | 0.9 | 0.52 | 1.27 |
| Nasopharynx | 0.9 | 0.56 | 1.17 | Multiple myeloma | 0.8 | 0.52 | 1.17 | Pharynx Uns | 0.9 | 0.49 | 1.22 |
| Ureter, bl & oth UO | 0.9 | 0.53 | 1.25 | Connective and ST | 0.8 | 0.47 | 1.09 | Hodgkins Disease | 0.8 | 0.46 | 1.18 |
| Melanoma of skin | 0.8 | 0.49 | 1.07 | Lymphoid leuk. | 0.6 | 0.34 | 0.90 | Tonsil and oth oropharynx | 0.8 | 0.33 | 1.31 |
| Pancreas | 0.7 | 0.45 | 1.00 | Vulva | 0.6 | 0.21 | 1.00 | Bone | 0.7 | 0.36 | 1.02 |
| Tongue | 0.7 | 0.42 | 0.97 | Small intestine | 0.5 | 0.22 | 0.69 | Hypopharynx | 0.7 | 0.33 | 0.97 |
| Other skin | 0.6 | 0.38 | 0.90 | Leu. uns | 0.5 | 0.08 | 0.44 | Vulva | 0.6 | 0.15 | 0.98 |
| Connective and ST | 0.6 | 0.31 | 0.80 | Tonsil and oth oropharynx | 0.5 | 0.15 | 0.83 | Adrenal & oth end. organs | 0.5 | 0.13 | 0.93 |
| Kidney | 0.6 | 0.31 | 0.80 | Testis and oth MGO | 0.4 | −0.02 | 0.78 | Oth Thoracic Organs | 0.4 | 0.18 | 0.72 |
| Multiple Myeloma | 0.6 | 0.31 | 0.80 | Melanoma of skin | 0.4 | 0.19 | 0.65 | Penis | 0.4 | 0.05 | 0.78 |
| Testis and other MGO | 0.5 | 0.20 | 0.86 | Salivary glands | 0.4 | 0.17 | 0.61 | Nose, Sinuses etc. | 0.4 | 0.16 | 0.66 |
| Lymphoid Leuk. | 0.5 | 0.29 | 0.77 | Nasopharynx | 0.4 | 0.15 | 0.57 | Anus & Anal Canal | 0.4 | 0.18 | 0.72 |
| Eye | 0.4 | 0.21 | 0.63 | Bone | 0.4 | 0.15 | 0.57 | Salivary Gland | 0.3 | 0.10 | 0.55 |
| Salivary glands | 0.4 | 0.19 | 0.59 | Hodgkin disease | 0.4 | 0.15 | 0.57 | Nasopharynx | 0.3 | 0.10 | 0.55 |
| Hodgkin disease | 0.4 | 0.19 | 0.59 | Penis | 0.3 | 0.01 | 0.50 | Lip | 0.3 | 0.07 | 0.50 |
| Leu unspecified | 0.4 | 0.19 | 0.59 | Eye | 0.3 | 0.08 | 0.44 | Small Intestine | 0.2 | 0.05 | 0.44 |
| Vagina and oth FGO | 0.4 | 0.07 | 0.63 | Anus | 0.2 | 0.02 | 0.31 | ||||
| Tonsil and oth oropharynx | 0.4 | 0.12 | 0.71 | ||||||||
| Nose, sinuses etc. | 0.3 | 0.13 | 0.49 | ||||||||
| Penis | 0.3 | 0.03 | 0.50 | ||||||||
| Lip | 0.2 | 0.07 | 0.38 | ||||||||
| Adrenal & oth end. organs | 0.2 | 0.03 | 0.30 | ||||||||
| O&U | 0.2 | 0.03 | 0.30 | ||||||||
| CR < 0.1 | CR < 0.1 | CR ≤ 0.1 | |||||||||
| Pharynx unspecified | 0.1 | 0.00 | 0.22 | Lip | 0.1 | 0.00 | 0.26 | Melanoma of Skin | 0.1 | −0.02 | 0.26 |
| Small intestine | 0.1 | 0.00 | 0.22 | Nose, sinuses etc. | 0.1 | −0.01 | 0.21 | Mesothelioma | 0.1 | −0.03 | 0.19 |
| Other thoracic organs | 0.1 | 0.00 | 0.22 | Oth thoracic organ | 0.1 | −0.01 | 0.21 | Placenta | 0.1 | −0.04 | 0.12 |
| Anus and Anal Canal | 0.1 | −0.02 | 0.13 | Vagina and other FGO | 0.1 | −0.06 | 0.20 | KS | 0.0 | −0.04 | 0.12 |
| Mesothelioma | 0.1 | −0.02 | 0.13 | Placenta | 0.1 | −0.05 | 0.32 | Renal pelvis | 0.0 | 0.00 | 0.00 |
| Renal pelvis | 0.1 | −0.02 | 0.13 | Adrenal & oth end. organs | 0.1 | −0.01 | 0.21 | Malig IPD | 0.0 | 0.00 | 0.00 |
| KS | 0.0 | 0.00 | 0.00 | Malig IPD | 0.0 | −0.03 | 0.10 | ||||
| Malig lmn. Pro D | 0.0 | 0.00 | 0.00 | Pharynx unspecified | 0.0 | 0.00 | 0.00 | ||||
| MPD | 0.0 | 0.00 | 0.00 | KS | 0.0 | 0.00 | 0.00 | ||||
| Mesothelioma | 0.0 | −0.03 | 0.10 | ||||||||
| Renal pelvis | 0.0 | −0.03 | 0.10 | ||||||||
Cancers listed in italics represent the ultra-rare cancers with incidence <0.1/1,000,000 population.
Bl bladder, Conn and ST Connective and soft tissue, CR Crude rate, End endocrine, KS Kaposi Sarcoma, Leu Leukemia, Malig IPD malignant immunoproliferative disorder, NHL Non Hodgkins lymphoma, NS nervous system, O & U other and unspecified, Oth Other, Oth FGO Other female genital organ, Oth MGO Other Male genital organ, Uns unspecified, Ut UterusUO urinary organs.
aMany common cancers have incidence <6/1,00,000 population, due to lower cancer incidence.
Discussion
There is very little data on the epidemiology of RCs from India and SAARC countries. This study represents the first formal attempt to identify the RCs in this region and organize the available information on RCs from all the national reports. The population in this region has distinct age distribution and lifestyle patterns compared to many developed countries, hence unique cancer epidemiology.
Using the most widely accepted RC definition ≤6/100,000 population, most cancers (68%, including some most common cancers in this region like ovary, mouth and prostate) in India come under RCs due to lower incidence. The above factors emphasize the relatively low cancer burden in India. The findings have a significant potential impact on the national cancer control program to allocate resources for public health interventions. The study additionally set an arbitrary threshold of CR ≤3/100,000 population for identification of RCs. Forty-three percent of cancers, a high proportion, fall under RCs with this cut-off, underscoring the relative rarity of cancers in India.
The cancer burden is different in LMICs and HICs, due to differences in exposure and risk factors such as age, screening uptake and treatment availability. The structure of the Indian population pyramid is different from the European structure, with a predominance of a younger population.25 Also, the life expectancy in India is lower (68.6–73.2 years) compared to many HICs.31 According to the most recent NFHS-5 survey, less than 2% of individuals (ages 30–49) participate in cervix, breast, and oral cancer screening. Compared to the European region, where screening coverage for cervical cancer (age group: 30–59) and breast cancer (age group: 45–49) is 40.2% and 68.9%, respectively, the screening coverage in India is alarmingly low.32,33 As per the Global Adult Tobacco Survey (GATS)- India, though there has been a reduction of tobacco consumption by 17% still, every 5th adult (199 Million) uses smokeless tobacco and every 10th adult (100 Million) smokes tobacco.34 However, the exact contribution of each risk factor cannot be determined as the distribution of the risk factors across the included countries is not known correctly. Also, for many rare cancers, there is limited data on the putative factors.
Though the RCI was similar to the published RARECAREnet list in some aspects, there were differences among the head and neck cancers and GI cancers.13 This is an expected finding given the distinct regional study population and cancer epidemiology.12 India has an absolute higher burden of head and neck cancers. The exact reason for the higher incidence is not apparent; however, risk factors for head and neck cancers like smokeless tobacco use, human papillomavirus (HPV) infection and periodontal disease are common in India.35,36 The pattern of tobacco use is different in India, compared to HIC, with increased use of chewing tobacco.37 Though tobacco is a significant cause for oral cancer, the HPV prevalence in oral cavity, oropharynx, hypopharynx, and larynx tumor tissues are 28.43%, 41.67%, 38.89%, and 15.79%, respectively, as per an Indian study.38,39
The possible explanations for the higher rates of gastro-esophageal cancers could be genetic, dietary and infective causes.40 There is a high incidence of infection-related cancers, such as liver, stomach and cervical cancers, in Eastern Asia.41,42 Studies report linkage of lifestyle factors with stomach and esophageal cancers in India.40,43 Arecanut chewing, common in South Asia, is linked with esophageal cancer.44 The incidence of gallbladder cancer varies noticeably, with the highest risk in the Northeast and the lowest in South India.45
The RARECAREnet ASIA group also reported a disparity in gastrointestinal and head and neck cancers between Asia and Europe.5 Unlike in developed countries, cervical cancer is not rare in India due to various predispositions.46 Due to genetic, environmental/lifestyle differences, colorectal, pancreatic and urinary bladder, cutaneous and endometrial cancers are rare in India as compared to Europe.47,48
RCs with incidence ≤1 fit the actual prototypes of RCs, including bone and soft tissue sarcomas and testicular and penile cancers. Soft tissue sarcomas (STS), including the subset of ‘ultra-rare’ sarcomas, have a high rate (≈40%) of misdiagnosis at regional centers and mandate expert pathologist review of histology with additional sophisticated molecular tests for proper diagnosis.49 The evidence for adjuvant chemotherapy in STS is derived from a meta-analysis, which did not have a sufficient number of each subset of STS.50 The above factors epitomize the logistic challenges in treating RCs across all subsets, more prominent among some. The cut-off incidence of ≤0.1/100,000 population, established for the definition of ultra-rare sarcomas, was used to identify an arbitrary subgroup of ultra-rare cancers in our populations.51
There are definite gender-based differences in cancer epidemiology and treatment due to genetic and environmental factors.52 From our data, cancers of the hypopharynx, liver, larynx and urinary bladder are more common among males; thyroid and gallbladder cancers are common among women. This distribution has implications for planning public health programs and health research.
Indian population is diverse, sometimes described as having a ‘nations within nations’ pattern in regional cancer epidemiology.14 This was proven true in our study of RC lists also. Some parts of India like Kerala, have higher literacy rates, lower rural populations, and better health indicators, leading to more common lifestyle-related cancers in South India than in the rest of India.53
We compared the cancer incidences in the neighboring countries of India, from where published data was available; Bhutan, SL and Nepal.22, 23, 24 Table 4 demonstrates the RC incidence rates in Bhutan, SL and Nepal. Cancer incidence rates are low in Bhutan and Nepal, and the RCs with CR ≤6/100,000 population include many common cancers. Available data indicates that cervical, gastro-esophageal, laryngeal, oral cavity and hepatobiliary cancers are more common in Pakistan, Afghanistan and Bangladesh, too, as in India.54, 55, 56, 57 The population of these countries is small compared to India. However, there may be similarities in the environmental/lifestyle patterns and genetic constitutions. The data is from only single tumor registries in some countries. The GLOBOCAN data represents only estimates of cancer rates. GLOBOCAN data may also be estimated in smaller countries based on neighboring PBCR data. Hence, the epidemiological data in this study is derived from the actual tumor registries rather than from the GLOBOCAN estimates.
Despite being the first study to characterize the epidemiological trends in RCs and redefine them for relevant interpretations and comparisons from SAARC countries, this study had several limitations. The PBCR distribution is not uniform across India; they have an urban predominance with better coverage in some states. Though coverage of PBCRs is 40–100% in HICs, it is known to be lower in LMICs. The lack of full coverage of PBCRs and the heterogeneity of the regions in terms of age, sex, social class, occupational and healthcare access issues in the populations is a major limitation of this study. Many existing PBCRs were established as early as the 1970s and are now stable registries providing good data. Though there are pitfalls in the PBCR system, most health documentation in India is not digital. Hence data on cancer incidence is challenging to trace. The PBCR personnel actively identify patients with cancers (as identified from hospital/clinical records, radiology/pathology, or death certificates) and then work to collect and enter the desired information. But there are undocumented cancer treatments by traditional healthcare providers or unqualified personnel, which the PBCRs may miss. The PBCR data represent the best available cross-sectional data covering most regions of India.15,17,30 With further organizational support and the national digital health mission initiation, there may be better future data on the epidemiology of rare cancers.
The Indian population is heterogeneous in many aspects, including cancer patterns. But this study represents the initiation of RC epidemiology studies in India and other SAARC nations. Also, the RC list from this study is based on the ICD-based classification of cancer sites. There is an unmet need for a correct RC histology list, like the tier-wise classification in the RARECAREnet list. However, the RC experts now recommend using the broader 12-family RC list (tier 1 entities), rather than the rare cancer histologies, to facilitate a convenient referral pattern. The real-world clinical oncology practice is site-based, not histology-based, which aligns with the tier 1 RC list. The current study compares the RC list of India and the SAARC countries to the RC families (Table 3).12 Estimates of RCs based on single countries may not be relevant to other parts of the world. Nevertheless, LMIC/SAARC countries share several similarities in age distribution pyramids and lifestyle (predominantly rural/traditional), making the SAARC RC list currently pertinent until more data is available on regional differences in incidences.
Though establishing an arbitrary cut-off and identifying the RC list may seem a low priority given the other more considerable challenges in cancer care in India, the RC list has to be considered in the context of the enormous proportion of cancers in clinical practice. RCs pose specific and different challenges in clinical practice than common cancers and hence require organizational and other support to improve outcomes. The first step towards the organization for better care for RCs is correctly identifying the RCs.
A multi-pronged approach is essential in tackling the complex issue of RCs. Developing a locally relevant RC list is the first step in improving the outcomes of RCs. The list will help to prioritize the public health programs and identify the critical areas for focus. Resource-appropriate and feasible solutions to improving RC treatment outcomes are necessary for India, where many patients have difficulty accessing primary cancer care. More information from collaborative research, increased awareness on some RCs, like pregnancy-associated cancers, and organizational support from international and national organizations like ESMO and the Indian Society of Medical and Pediatric Oncology (ISMPO) to identify regional issues in RCs are essential to mitigate the massive challenges in their management. Further, countries with similar cancer distribution and epidemiology and logistic difficulties, like the SAARC countries, should join forces for collaborative research and data sharing for better treatment of rare cancers.
The cancer epidemiology is distinct in India and SAARC countries, underlining the need for separate recognition of RCs in this region based on the standard definition and arbitrary lower cut-offs, which appear more realistic. RCs deserve more attention from the professional oncology community and funding organizations. There is a need for data on rare cancer histologies and patterns of RC care and survival, to enable better comparison with the international RC patterns. The current RC list is based on the data on rare sites of cancer. However, the unique observation of the current study is that RCs constitute 40–50% of the total incident cancers in India and the SAARC region, as the incidence is low in these regions. This percentage has implications for policymakers’ decisions to streamline cancer control policies based on regional cancer burden. The findings are widely applicable in other LMICs with similar epidemiological trends and merit wider recognition.
Contributors
J.B.: conception, design, analysis, forming first draft and final manuscript. S.M.: design, data collection, analysis, forming first draft and final manuscript. J.B. and S.M. contributed equally to the paper. A.B.: data collection, data analysis, final manuscript. R.S.: data analysis, final manuscript. R.D.: data analysis, final manuscript. M.D.: data analysis, final manuscript. S.P.: data analysis, final manuscript. U.T.: data analysis, final manuscript. S.D.B.: data analysis, final manuscript. M.B.: data analysis, final manuscript. P.C.: data analysis, final manuscript. S.B.: data analysis, final manuscript. S.G.: data analysis, final manuscript. R.A.B.: data analysis, final manuscript. A.T.: data analysis, final manuscript.
Data sharing statement
The data that support the findings of this study are openly available at the following websites (links appended as supplementary material) (cited in the paper as references18, 19, 20, 21, 22, 23, 24). The derived data supporting the findings of the study are available from the first and corresponding authors upon reasonable request [sharadajayaram27@gmail.com and dr_jyotibajpai@yahoo.co.in].
Declaration of interests
None of the authors have competing interests.
Acknowledgments
We are grateful to the leaders of Rare cancer Europe and Asia mission for their relentless efforts in this space and the Indian society of Medical and Paediatric Oncology for taking it forward in India. We also thank all the patients and families of rare cancers for their continued trust in us despite their unique challenges.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100168.
Contributor Information
Jyoti Bajpai, Email: drjyotibajpai25@gmail.com, dr_jyotibajpai@yahoo.co.in.
Annalisa Trama, Email: Annalisa.Trama@istitutotumori.mi.it.
Appendix A. Supplementary data
References
- 1.Gatta G., Trama A., Capocaccia R. Epidemiology of rare cancers and inequalities in oncologic outcomes. Eur J Surg Oncol. 2019;45(1):3–11. doi: 10.1016/j.ejso.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee R.T., Goodman M.T., Lynch C.F., Platz C.E., Havener L.A., Howe H.L. The occurrence of rare cancers in U.S. adults, 1995–2004. Public Health Rep. 2010;125(1):28–43. doi: 10.1177/003335491012500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguengang Wakap S., Lambert D.M., Olry A., et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28(2):165–173. doi: 10.1038/s41431-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSantis C.E., Kramer J.L., Jemal A. The burden of rare cancers in the United States. CA Cancer J Clin. 2017;67(4):261–272. doi: 10.3322/caac.21400. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda T., Won Y.-J., Chun-ju Chiang R., et al. Rare cancers are not rare in Asia as well: the rare cancer burden in East Asia. Cancer Epidemiol. 2020;67:101702. doi: 10.1016/j.canep.2020.101702. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki T., Dong Y., Ohno Y., Sobue T., Nishimoto H., Shibata A. The burden of rare cancer in Japan: application of the RARECARE definition. Cancer Epidemiol. 2014;38(5):490–495. doi: 10.1016/j.canep.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Gatta G., Capocaccia R., Botta L., et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18(8):1022–1039. doi: 10.1016/S1470-2045(17)30445-X. [DOI] [PubMed] [Google Scholar]
- 8.Gatta G., Ciccolallo L., Kunkler I., et al. Survival from rare cancer in adults: a population-based study. Lancet Oncol. 2006;7(2):132–140. doi: 10.1016/S1470-2045(05)70471-X. [DOI] [PubMed] [Google Scholar]
- 9.Botta L., Gatta G., Trama A., et al. Incidence and survival of rare cancers in the US and Europe. Cancer Med. 2020;9(15):5632–5642. doi: 10.1002/cam4.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandrucci S., Naredi P., Bonvalot S. Centers of excellence or excellence networks: the surgical challenge and quality issues in rare cancers. Eur J Surg Oncol. 2019;45(1):19–21. doi: 10.1016/j.ejso.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 11.ESMO | Press Release | rare cancers, Asia, patient care [internet] https://www.esmo.org/newsroom/press-office/rare-cancers-asia-improve-patient-care Available from:
- 12.Casali P.G., Trama A. Rationale of the rare cancer list: a consensus paper from the Joint Action on Rare Cancers (JARC) of the European Union (EU) ESMO Open. 2020;5(2) doi: 10.1136/esmoopen-2019-000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatta G., van der Zwan J.M., Casali P.G., et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390(10111):2437–2460. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Oncol. 2018;19(10):1289–1306. doi: 10.1016/S1470-2045(18)30447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World population prospects - Population Division - United Nations [internet] https://population.un.org/wpp/default.aspx?aspxerrorpath=/wpp/Publications/Files/WPP2017_KeyFindings.pdf Available from:
- 17.Behera P., Patro B.K. Population based cancer registry of India – the challenges and opportunities. Asian Pac J Cancer Prev. 2018;19(10):2885–2889. doi: 10.22034/APJCP.2018.19.10.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Registry Programme – Indian Council of Medical Research: three year report of PBCR 2012-2014, Bengaluru 2016. [internet] 2016. https://ncdirindia.org/ncrp/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/index.htm Available from:
- 19.Varanasi Population-Based Cancer Registry Report: 2017 – Tata Memorial Centre, Mumbai, a Homi Bhabha National Institute (HBNI) Homi Bhabha Cancer Hospital (HBCH) & Mahamana Pandit Madan Mohan Malaviya Cancer Centre (MPMMCC), Varanasi, Uttar Pradesh, India I [internet] https://tmc.gov.in/tmh/pdf/Reports/PBCRVaranasiReport-Summary.pdf Available from:
- 20.Sangrur Population-Based Cancer Registry Report: 2015-2016 – Tata Memorial Centre, Mumbai, and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. [Internet] https://pgimer.edu.in/PGIMER_PORTAL/AbstractFilePath?FileType=E&FileName=SangrurReport2015-2016.pdf&PathKey=EDITORREPOSITORY_PATH Available from:
- 21.Chandigarh Population-Based Cancer Registry Report: 2015-2016 – Tata Memorial Centre, Mumbai and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh [internet] https://pgimer.edu.in/PGIMER_PORTAL/AbstractFilePath?FileType=E&FileName=ChandigarhReport2015-2016.pdf&PathKey=EDITORREPOSITORY_PATH Available from:
- 22.Dhimal M., Subedi R., Kafle U., et al. Cancer incidence and Mortality in Kathmandu Valley in 2018. Nepal Health Research Council, Government of Nepal. 2020 [internet] http://nhrc.gov.np/wp-content/uploads/2020/08/KTM-Valley-Report-final.pdf Available from:
- 23.Cancer incidence and mortality in Bhutan 2014-2018. Bhutan. 2020. [Google Scholar]
- 24.Cancer incidence and mortality data Colombo District, Sri Lanka (Population Based Cancer Registry) 2013-2019: National Cancer Control Programme, Ministry of Health, Sri Lanka [internet] https://www.nccp.health.gov.lk/storage/post/pdfs/PCBRBooks.pdf Available from:
- 25.Bray F., Ferlay J. In: No. 166. Bray F., Colombet M., Mery L., et al., editors. IARC Scientific Publications; Lyon, France: 2017. Cancer incidence in five continents, Volume XI; pp. 127–130. [Google Scholar]
- 26.Report of National Cancer Registry Programme (ICMR-NCDIR), Bengaluru, India 2020. [Internet] https://www.ncdirindia.org/All_Reports/Report_2020/default.aspx Available from:
- 27.Parkin D.M., Chen V.W., Ferlay J., Galceran J., Storm H.H., Whalen S.L. International Agency for Research on Cancer (IARC), World Health Organisation,International Association of Cancer Registries; Lyon, France: 1994. Comparability and quality control in cancer registration. IARC technical report no. 19. [Google Scholar]
- 28.Bashar M.A., Thakur J.S., Budukh A. Evaluation of data quality of four new population based cancer registries (PBCRs) in Chandigarh and Punjab, North India- a quality control study. Asian Pac J Cancer Prev. 2021;22(5):1421–1433. doi: 10.31557/apjcp.2021.22.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budukh A., Dikshit R., Thakur J., et al. Linkage of cancer registration with cancer treatment in predominantly rural district: a model form Sangrur district, Punjab state, India. Int J Noncommunicable Dis. 2018;3(2):56–59. [Google Scholar]
- 30.Bhatia A., Victora C.G., Beckfield J., Budukh A., Krieger N. “Registries are not only a tool for data collection, they are for action”: cancer registration and gaps in data for health equity in six population-based registries in India. Int J Cancer. 2021;148(9):2171–2183. doi: 10.1002/ijc.33391. [DOI] [PubMed] [Google Scholar]
- 31.44377 @ censusindia.gov.in [internet] https://censusindia.gov.in/nada/index.php/catalog/44377.?page=1&sort_by=popularity&sort_order=desc&ps=15 Available from:
- 32.Factsheet_NFHS-5 @ rchiips.org [internet] http://rchiips.org/nfhs/factsheet_NFHS-5.shtml Available from:
- 33.Smith R.A. Cancer screening in the USA. J Med Screen. 2006;13(SUPPL.1):23–26. [PubMed] [Google Scholar]
- 34.TISS-Ministry of Health & Family Welfare-Government of India . vol. 1. International Institute for Population Sciences; 2017. pp. 1–314.https://mohfw.gov.in/sites/default/files/GlobaltobacoJune2018.pdf (Global adult tobacco surey 2016-2017 [internet]). Available from: [Google Scholar]
- 35.Laprise C., Shahul H.P., Madathil S.A., et al. Periodontal diseases and risk of oral cancer in Southern India: results from the HeNCe Life study. Int J Cancer. 2016;139(7):1512–1519. doi: 10.1002/ijc.30201. [DOI] [PubMed] [Google Scholar]
- 36.Zeng X.-T., Deng A.-P., Li C., Xia L.-Y., Niu Y.-M., Leng W.-D. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0079017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European tobacco. 2019. https://apps.who.int/iris/bitstream/handle/10665/346817/WHO-EURO-2019-3711-43470-61063-eng.pdf?sequence=1&isAllowed=y Available from:
- 38.Gholap D., Mhatre S., Chaturvedi P., et al. Prevalence of human papillomavirus types in head and neck cancer sub-sites in the Indian population. Ecancermedicalscience. 2022;16:1358. doi: 10.3332/ecancer.2022.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nandi S., Mandal A., Chhebbi M. The prevalence and clinicopathological correlation of human papillomavirus in head and neck squamous cell carcinoma in India: a systematic review article. Cancer Treat Res Commun. 2021;26:100301. doi: 10.1016/j.ctarc.2020.100301. [DOI] [PubMed] [Google Scholar]
- 40.Dikshit R.P., Mathur G., Mhatre S., Yeole B.B. Epidemiological review of gastric cancer in India. Indian J Med Paediatr Oncol. 2011;32(1):3–11. doi: 10.4103/0971-5851.81883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pourhoseingholi M.A., Vahedi M., Baghestani A.R. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol Hepatol Bed Bench. 2015;8(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 42.Ng C.J., Teo C.H., Abdullah N., Tan W.P., Tan H.M. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer. 2015;15:613. doi: 10.1186/s12885-015-1615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gajalakshmi C.K., Shanta V. Lifestyle and risk of stomach cancer: a hospital-based case-control study. Int J Epidemiol. 1996;25(6):1146–1153. doi: 10.1093/ije/25.6.1146. [DOI] [PubMed] [Google Scholar]
- 44.Akhtar S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies. Cancer Causes Control. 2013;24(2):257–265. doi: 10.1007/s10552-012-0113-9. [DOI] [PubMed] [Google Scholar]
- 45.Mhatre S.S., Nagrani R.T., Budukh A., et al. Place of birth and risk of gallbladder cancer in India. Indian J Cancer. 2016;53(2):304–308. doi: 10.4103/0019-509X.197723. [DOI] [PubMed] [Google Scholar]
- 46.Sreedevi A., Javed R., Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405–414. doi: 10.2147/IJWH.S50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karimkhani C., Green A.C., Nijsten T., et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lortet-Tieulent J., Ferlay J., Bray F., Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110(4):354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 49.Ray-Coquard I., Montesco M.C., Coindre J.M., et al. Sarcoma: concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann Oncol. 2012 Sep;23(9):2442–2449. doi: 10.1093/annonc/mdr610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pervaiz N., Colterjohn N., Farrokhyar F., Tozer R., Figueredo A., Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 51.Stacchiotti S., Frezza A.M., Blay J.-Y., et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934–2942. doi: 10.1002/cncr.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haupt S., Caramia F., Klein S.L., Rubin J.B., Haupt Y. Sex disparities matter in cancer development and therapy. Nat Rev Cancer. 2021;21(6):393–407. doi: 10.1038/s41568-021-00348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathew A., Sara George P., Kalavathy M.C., Padmakumari G., Jagathnath Krishna K.M., Sebastian P. Cancer incidence and mortality: district cancer registry, Trivandrum, South India. Asian Pac J Cancer Prev. 2017;18(6):1485–1491. doi: 10.22034/APJCP.2017.18.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhurgri Y. Karachi Cancer Registry Data--implications for the National Cancer Control Program of Pakistan. Asian Pac J Cancer Prev. 2004;5(1):77–82. [PubMed] [Google Scholar]
- 55.Joya M., Stanikzai Z., Akbarzadeh I., Babaloui S., Bradley D.A., Jafari S.M. Prevalence of cancers diagnosed in Jamhuriyat Hospital, Kabul, Afghanistan. Heliyon. 2020;6(3) doi: 10.1016/j.heliyon.2020.e03603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abul M., Sarker B., Hirosawa T. Trends and distributions of common types of cancer in Bangladesh : results from the Cancer Registry Data of 2008-10. 2012;20(1):32–38. [Google Scholar]
- 57.Hussain S.A., Sullivan R. Cancer control in Bangladesh. Jpn J Clin Oncol. 2013;43(12):1159–1169. doi: 10.1093/jjco/hyt140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.