Summary
Background
Familial hypercholesterolemia (FH) is an autosomal inherited disorder characterised by elevated low-density lipoprotein cholesterol and premature cardiovascular events. Despite being declared as a public health priority, FH remains highly underdiagnosed, generally due to the lack of awareness and shortcomings in the available infrastructure, particularly in lower income countries.
Methods
To map the existing infrastructure for the management of FH, a survey was conducted among 128 physicians (cardiologists, paediatricians, endocrinologists, and internal medicine specialists) from different regions of Pakistan.
Findings
The respondents encountered a limited number of adults or children with diagnosed FH. A very small proportion of the population had access to free cholesterol and genetic testing even when indicated by a physician. In general, cascade screening of the relatives was not performed. Uniform diagnostic criteria for FH had not been established even within the same institution or province. The use of statins and ezetimibe in addition to lifestyle changes were the most common recommended treatment option for FH patients. The respondents considered lack of financial resources as a major barrier for the management of FH and stressed on taking relevant measures for a uniform FH screening programs around the country.
Interpretation
National FH screening programmes are not in place worldwide hence FH is commonly undiagnosed, and many individuals are at a high risk for cardiovascular diseases. Timely screening of population for FH requires knowledge about FH among the clinicians and the availability of fundamental infrastructure coupled with sufficient financial resources.
Funding
The authors confirm independence from the sponsor. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. FS received funding from Higher Education Commission, Pakistan (Grant 20-15760) and UG received grants from Slovenian Research Agency (J3-2536, P3-0343).
Keywords: Cardiovascular disease, Familial hypercholesterolemia, Mapping survey, Statins, FH screening, Opportunistic testing
Research in context.
Evidence before this study
Articles were searched on PubMed and Google Scholar using the terms related to “familial hypercholesterolemia and dyslipidaemia” in any language (keywords used for screening relevant studies listed in supplementary information). The reference list of the articles was further screened for more relevant articles. After screening of titles and abstracts, articles that included surveys and questionnaire methods from the physicians and clinicians were reviewed. A questionnaire was designed to address the topics of available familial hypercholesterolemia (FH) screening strategies, accessibility and cost of screening and treatment, potential barriers and strategies that could be implemented in the country for management of FH. Studies conducted across the world suggested that significant gaps existed among the knowledge and consensus of clinicians regarding the screening and management of FH, which leads to the underdiagnosis and undertreatment of FH.
Added value of this study
To our knowledge, this study is the first effort towards identifying and mapping the infrastructure for FH and its management, that includes clinicians from many regions of Pakistan. It not only provides an insight into the practices employed within the country but also helps assessing the potential barriers in managing FH/dyslipidaemia patients. We found that majority of the clinicians had rarely encountered FH affected individuals. Moreover, the inaccessibility of genetic testing, consolidated screening guidelines, and the non-availability of free of cost cholesterol testing and medicines remain a challenge for the participants of the study. Evidence from studies conducted across the region also validate similar issues faced by the clinicians. This points towards implementation of uniform screening and treatment guidelines at least at the regional level, which could potentially address underdiagnosis and undertreatment.
Implications of all the available evidence
The findings of this study identify several shortcomings in the existing FH screening and management practices and suggest the development of framework for systematic FH awareness and screening programmes within the country. This would also set a stage for implementing a consolidated programme involving all the stakeholders of the society for effective and robust strategy for FH screening, diagnosis, and treatment within the country, which could also be implemented within the region in future.
Introduction
Pakistan is a low-middle income country from South Asia with a population of around 220 million people where cardiovascular diseases (CVD) accounted for almost 15% of the total deaths in 2020.1 Annual indirect cost of CVD to Pakistan's economy as a consequence of productivity loss was estimated to be 0.21 billion USD in 2007, furthermore, the direct costs to a single patient with CVD is estimated at 563 USD per annum.2 High cumulative burden of low-density lipoproteins (LDL) due to the reduced hepatic clearance of these particles from the circulation is characteristic of familial hypercholesterolemia (FH), an inherited autosomal dominant disorder resulting from pathogenic mutations in the genes encoding LDL receptor function.3 Individuals with FH have a 10-fold higher risk for coronary artery disease (CAD) compared to healthy peers.4 Early diagnosis and treatment of FH can lead to the reduction of LDL-C burden which in turns reduces the progression of atherosclerosis and improves coronary outcomes.5 Therefore, screening programmes based on cascade testing or universal screening for early identification of FH are recommended to prevent a proportion of population from substantial CVD risk.5
Globally, it is estimated that more than 25 million people are affected by FH, however <10% of these individuals are diagnosed and treated.6 This could be due to the lack of awareness among the general public and medical community and use of different criteria for the diagnosis of FH patients.6,7
Although the WHO recognised FH as a public health priority and outlined the recommendations to prevent premature atherosclerosis almost two decades ago, very limited progress has been made.8 Therefore, revised public policy recommendations under the ambit of the Global Call to Action on FH include diagnosis, management, and awareness among healthcare professionals and the public.4,7 Slovenia has been a pioneer for FH universal screening among children since 1995 as the implemented nation-wide programme covers 91% of eligible individuals.4,9 Other countries such as the Netherlands and Norway have also implemented robust FH diagnosis strategies, in response to the global call, and now they have 86% and 37% diagnosis rates, respectively.10 In Asia, excluding Japan, <1% of individuals with FH are diagnosed.10
Statins and ezetimibe remain the drugs of choice for treating FH patients. Newer and advanced therapies that include proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, inclisiran, bempedoic acids are not easily available in the developing and undeveloped countries mostly due to their cost which poses additional burden on patients with FH.11,12 The monthly median treatment price of some essential statins range from 5.00 to 7.50 US $, while the average monthly wage in Pakistan is 119 US $ (24,028 PKR) suggesting statins cost patients 6–7% (1500–1700 PKR) of their monthly salary.1,13
To date, Pakistan has not implemented systematic screening and management of patients with FH and data are scarce on the existing barriers and country's infrastructure to manage these patients. Hence, in this study we aimed to map specific activities to identify FH patients and to assess the national situation regarding available infrastructure that could be applied to systematically identify FH patients in the future.
Methods
We conducted a survey in Pakistan among physicians predominantly from four specialties: cardiology, paediatrics, general medicine, and endocrinology during November 2020 and February 2022. The questionnaire was adapted from the Country Mapping analysis survey from the World Heart Foundation and modified according to the situation in the country.14 The questionnaire was distributed in a paper form and was also available on the online platform Survey Monkey®. The questionnaire (n = 340) was sent to 27 institutions across all provinces, Islamabad Capital Territory, Gilgit Baltistan and Azad Kashmir of Pakistan. The institutions were chosen based on our nation-wide network for lipid research throughout the country. Questionnaires were sent out to a contact person in each institute and the numbers of the questionnaires was decided based on the availability of physicians in each of the healthcare facility.
Data acquisition and analyses were completed by May 2022. The questionnaire was composed of 44 questions on the topics of i) available FH identification strategies, ii) accessibility of diagnostic and treatment options for FH patients, iii) costs of care, iv) barriers for performing a systematic FH screening program, and v) potential to expand the existing FH programs. Details of the design of the questionnaire, the general rules for unification of questionnaire responses, and the definition of valid and not applicable group have been provided in the Supplementary Materials.
A total of 128 clinicians responded and of these, 15 were excluded due to inadequate answers (not applicable group) to more than 30% of the questions in the whole survey (Supplementary Materials Fig. S1). Hence the response rate was 33.2%. All respondents were invited for group co-authorship of the paper and those who consented were included.
The questionnaire was administered to the clinicians across all provinces (Punjab, Sindh, Balochistan and Khyber Pakhtunkhwa), Islamabad Capital Territory, Gilgit Baltistan and Azad Kashmir of Pakistan. The number of respondents from each province was approximately proportional to the population of provinces. The largest number of respondents (n = 61; 54%) were from Punjab which constitute 52.8% of the population of Pakistan, followed by Sindh (n = 20; 17.7%) that accounts for 23% of Pakistani population, Khyber Pakhtunkhwa (n = 14; 12.4%), Azad Kashmir (n = 10; 8.8%), Islamabad Capital Territory (n = 6; 5.3%) and Balochistan (n = 2; 1.8%) accounting for 23%, 14.7%, 1.9%, 1% and 5.9% of Pakistani population, respectively.1,15 There were no respondents from Gilgit-Baltistan; that accounts for less than 1% of the Pakistani population.16 Around 70% of the participants were working in the public sector while the remaining were from the private sector, and only one was from a semi-private sector. Respondents were from 24 institutions across 12 cities of Pakistan as shown in Fig. 1.
Fig. 1.
Pakistani provinces/administrative units and distribution of respondents throughout the country. Name of provinces and regions are capitalized, and the name of cities are non-capitalised.
Included and excluded questions
During the analyses, we excluded questions that (i) had several non-consistent or contradictory answers from the same respondent to other related questions; (ii) clearly opposing answers from different respondents; and (iii) questions assessed as inadequately understood or inappropriately formulated by the authors of the questionnaire. Details of the included and excluded questions have been presented in the Supplementary Materials.
Calculations
Some questions were composed of a series of four or more Likert-type answers. To calculate the relevance of measures needed to implement FH screening we used the average score of responses. Each response was given its numerical analogue, for example “irrelevant” was “1”, “somewhat important” was “2”, “important” was “3”. The average score was calculated as an average of these numerical analogues for each statement. We used the exchange rate of 1 US $ = 200 Pakistan Rupees (PKR) from June 2022.
Statistical analysis
Data was collected, analysed, and visualized in Microsoft Office 365 Excel (Microsoft Corporation, USA) and IBM SPSS Statistics, version 26.0 (IBM, USA). Data was presented as absolute frequencies, proportion in percentages and as 95% CI. Mann–Whitney U test was used to compare ordinal data between different groups (in example sectors) and Wilcoxon signed-rank test for comparison of data from the same respondent. Categorical variables were compared with Pearson's chi squared test. All statistical tests were two-tailed and p value of ≤0.05 was considered statistically significant.
Role of funding source
The funding agencies had no role in study design, data collection, data analysis, interpretation, or writing of the report. The corresponding authors had full responsibility for data handling and manuscript submission.
Results
Characterisation of the respondents
Most of the respondents were cardiologists (30%), followed by paediatricians (26%), general medicine physicians (18%) and internal medicine specialists (16%). The duration of practice within their specialty was an average of 9.2 years (95% CI: 7.7–10.7, minimum half a year, and maximum 45 years). Around 8.3% (95% CI: 2.8%–13.9%) of the respondents had rarely managed patients with FH, while others had treated patients with FH for an average of 7.6 (95% CI: 7.4–7.7) years. Furthermore, 75.0% (95% CI: 66.7%–83.3%) of the physicians had seen <50 adult patients, while 86.2% (95% CI: 79.8%–92.7%) had seen <10 paediatric patients.
Access to diagnostic testing
For both the adult and paediatric population, a large majority of clinicians (71.4% for adults [95% CI: 62.8%–80.1%] and 66.7% for children [95% CI: 57.4%–76.0%]) estimated that less than 10% of individuals have access to free of cost cholesterol measurement. Moreover the respondents (40.0% for adults [95% CI: 30.6%–49.4%] and 45.5% for children [95% CI: 35.6%–55.3%]) most frequently estimated that <1% of the population has access to free cholesterol measurement in their region, while the estimation that more than 75% of adults and children have access to it was expressed by only 1.9% of the clinicians for adults (95% CI: 0%–4.5%) and 5.0% for children (95% CI: 0.7%–9.4%). The estimated accessibility to free cholesterol measurement did not differ between adults and children (p = 0.531).
In their region of practice, 75.8% (95% CI: 67.3%–84.2%) and 74.7% (95% CI: 66.2%–83.3%) of clinicians estimated that less than 10% of adults and children have access to FH genetic testing if indicated by a physician. No differences between estimations for adults and children were observed (p = 0.690). Most physicians (52.7% [95% CI: 43.4%–61.9%]) indicated that FH genetic testing is very rarely performed or not performed at all.
No significant differences in accessibility to either free of cost cholesterol measurement for adults and children (p = 0.053 and p = 0.497, respectively) and accessibility to genetic testing (p = 0.153 and p = 0.389) were observed between the private and public sector.
Use of guidelines
For the management of FH patients in general, American Heart Association (AHA) guidelines are most used (in around 3/4 of clinicians or 73.4% [95% CI: 65.1%–81.7%]), followed by The National Institute for Health and Care Excellence (NICE) guidelines (1/3 of clinicians or 33.0 [95% CI: 24.2%–41.9%]). According to the physicians included in the survey, 70.3% (95% CI: 60.9%–79.7%) of the adults and 77.6% (95% CI: 68.8%–86.5%) of children are being diagnosed based on clinical criteria only. Genetic testing is seldom used as the only diagnostic method (8.8% [95% CI: 3.0%–14.6%] of adults and 16.5% [95% CI: 8.6%–24.4%] of children) and is more often combined with clinical criteria in adults (20.9% [95% CI: 12.5%–29.2%]) than in the paediatric population (5.9% [95% CI: 0.9%–10.9%]; p = 0.004). When using clinical criteria to diagnose FH, the most used clinical guidelines for children were from the AHA and the European Atherosclerosis Society (EAS) (68.2 [95% CI: 56.9%–79.4%] and 16.7% [95% CI: 7.7%–25.7%] respectively). In adults MedPed and Simon Broome criteria (39.1% [95% CI: 27.1%–51.0%] and 32.4% [95% CI: 22.7%–46%], respectively) were most commonly used. However, significant variation existed when responses from the same province and institution were compared.
Available structure for a systematic FH screening program
Only 48.2% (95% CI: 38.8%–57.5%) of the physicians screened patients for hypercholesterolemia in their respective institution. Knowledge about registry for FH patients in their region was only reported by 9.9% (95% CI: 4.4%–15.5%) of the respondents. Responses about FH registry from within the same institution were however inconsistent.
Management of patients with premature cardiovascular disease
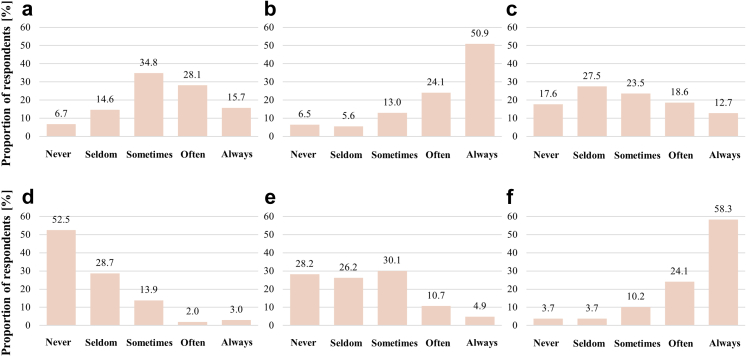
Patients with premature cardiovascular disease had their cholesterol measured often or always by 75.0% (95% CI: 66.8%–83.2%) of the physicians and in 82.4% (95% CI: 75.2%–89.6%) of cases were often or always prescribed lipid lowering therapy when cholesterol levels were above the treatment threshold, as presented in Fig. 2. On the other hand, 6.5% (95% CI: 1.8%–11.1%) of the respondents expressed that they never measured cholesterol in these patients and when cholesterol levels were already available, 3.7% (95% CI: 0.1%–7.3%) never initiated lipid lowering therapy. Actively considering FH was seldom or never done by 21.3% (95% CI: 12.8%–29.9%) of the physicians. Applying clinical criteria to diagnose FH was seldom or never by 45.1% (95% CI: 35.4%–54.8%) of the physicians. Genetic testing was never done in 52.5% (95% CI: 42.7%–62.2%) of the patients with premature CVD, while cascade screening of relatives was performed only sometimes by 30.1% (95% CI: 21.2%–39.0%) of the physicians.
Fig. 2.
Measures taken at a patient with a premature cardiovascular disease. a) actively considering familial hypercholesterolemia [FH]; b) Cholesterol measurement; c) Evaluation of FH with clinical criteria; d) Genetic testing for FH; e) Cascade testing of relatives; f) Prescription of lipid lowering therapy.
Treatment of adults with FH
Adults with FH are most frequently treated with lifestyle changes, statins and ezetimibe (46.6% [95% CI: 37.0%–56.2%]), followed by a combination of statins and lifestyle changes (40.8% [95% CI: 31.3%–50.3%]). PCSK9 inhibitors are used only by one physician (1.0% [95% CI: 0%–2.9%]). Statins alone or in combination with ezetimibe (with/without lifestyle changes) are prescribed to adult FH patients by 92.2% (95% CI: 87.1%–97.4%) of the physicians (Fig. 3).
Fig. 3.
Treatment of adult and paediatric individuals with familial hypercholesterolemia.
Treatment of children with FH
Statins accompanied by lifestyle changes is the most common (40.9% [95% CI: 30.9%–50.9%]) practice of treatment in paediatric patients with FH, followed by statins in combination with ezetimibe and lifestyle changes (28.0% [95% CI: 18.8%–37.1%]). Lifestyle changes as the only method of treatment is used in 21.5% (95% CI: 13.2%–29.9%) of the cases. Statins alone or in combination with ezetimibe (with/without lifestyle changes) are accessible to 78.5% (95% CI: 70.1%–86.8%) of children with FH. Only 2.2% (95% CI: 0%–5.1%) of the physicians prescribe PCSK9 inhibitors (Fig. 3). Treatment is started at an average age of 12.4 (95% CI: 12.1–12.7) years, however the interval for the age at treatment initiation spans from 2 months to 30 years (Fig. 3).
Economic aspects of FH care
The average cost of FH genetic testing, according to 20.4% of the respondents, was approximately 7000 PKR (95% CI: 6700–7200) which amounts to 35 US $ (95% CI: 33.5–36). Physicians estimated that the mean monthly dose of statins cost was approximately 1700 PKR (95% CI: 1660–1730) or 8.5 USD (95% CI: 8.3–8.6) for adult patients and 1500 PKR (95% CI: 1440–1520) or 7.5 US $ (95% CI: 7.2–7.6) for paediatric patients. According to the Pakistan Bureau of Statistics, the average monthly wage in 2020–21 was 24,028 PKR.1 The cost of genetic testing, monthly statin dose of statins for an adult, and monthly statin dose for a child represent 29.1% (95% CI: 27.9%–30.0%), 7.1% (95% CI: 6.9%–7.2%) and 6.2% (95% CI: 6.0%–6.3%) of the average monthly wage in Pakistan, respectively.
Out of pocket expenditure was the only option of financing FH treatment for patients of 63.1% (95% CI: 54.1%–72%) of the physicians. Other sources of finances were federal government (3.6% [95% CI: 0.1%–7.1%]), provincial government (7.2% [95% CI: 2.4%–12.0%]), hospital funds (12.6% [95% CI: 6.4%–18.8%]), charity (6.3% [95% CI: 1.8%–10.8%]), and in some cases a combination of these options (7.2% [95% CI: 2.4%–12.0%]).
Barriers
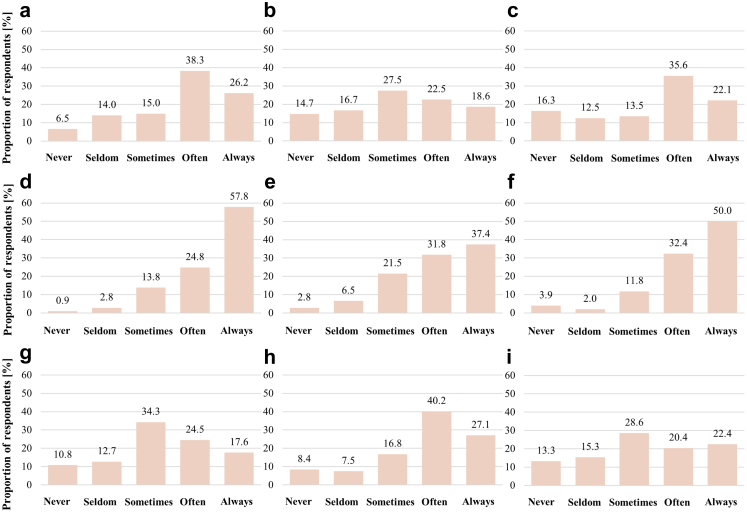
The predominant barriers identified were the lack of financial resources (Fig. 4d) followed by the lack of government support (Fig. 4f) and that FH was not considered a healthcare priority (Fig. 4h). A considerable proportion of respondents suggested that the lack of national consensus (Fig. 4a) and the lack of clear guidance or recommendations (Fig. 4c) was often the case for not implementing a systematic FH screening programme. There was a mixed response towards lack of professional consensus on an international level (Fig. 4b).
Fig. 4.
Main barriers to performing a systematic familial hypercholesterolemia screening program. a) lack of professional consensus on national level; b) lack of professional consensus on international level; c) lack of clear guidance/recommendations; d) lack of financial resources; e) lack of institutional support; f) lack of support by government; g) too difficult to be implemented in practice; h) not a healthcare priority in our country; i) possibility of genetic or other discrimination of patients.
Requirements for the implementation of a systematic FH screening programme
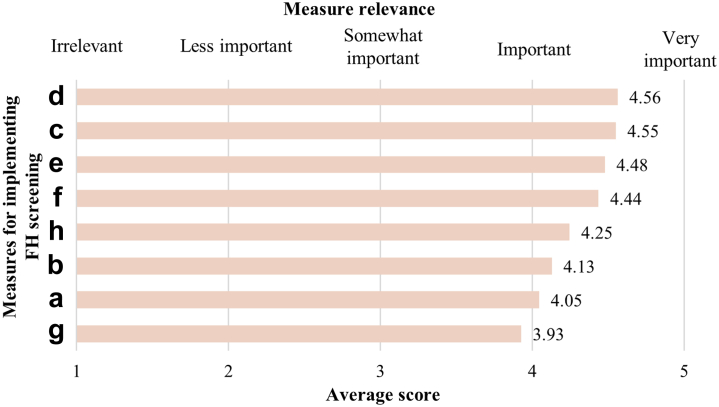
Physicians expressed that the most important and the most relevant measure needed to implement the systematic FH program was resources and support at the institutional level (average score 4.56 [95% CI: 4.55–4.57]; Fig. 5), followed by clear professional and national guidelines/recommendations (average score 4.55 [95% CI: 4.54–4.56]), and resources and support from the government (average score 4.48 [95% CI: 4.46–4.49]). The least important or relevant were measures to combat discrimination of FH patients (average score: 3.93 [95% CI: 3.91–3.95]).
Fig. 5.
Relevancy of measures required for the implementation of a systematic familial hypercholesterolemia screening program. a) clear professional consensus on national level; b) clear professional consensus on international level; c) clear professional guidance/recommendations; d) resources and support by institution; e) resources and support by government; f) assistance with practical implementation; g) measures against genetic or other discrimination of patients; h) support from patient advocacy organizations.
Discussion
This study is the first investigation to map the currently available infrastructure and the capability of the healthcare system for the management of FH patients in Pakistan. Similar studies have also been conducted across the region including India, Malaysia, and a few countries from Asia Pacific.17, 18, 19, 20
The WHO recommends a physician-population ratio of at least 1:1000, however 44% of the countries fail to meet this goal. On the other hand, Pakistan has a ratio of 1.12 physicians per 1000 people, but the question is if these physicians are equipped with the awareness and knowledge about FH.21,22
The results of the present study suggest that most of the respondents encountered <50 adult patients and <10 paediatric patients with FH, displaying the existing situation of FH in Pakistan. According to the majority (75%) of the participants of the present study, patients with the incidence of premature CVD had their cholesterol levels measured and were prescribed lipid lowering therapy, however genetic testing was rarely performed. Our results suggest that the median age for diagnosis and treatment initiation is 10 years. However, this is mostly a result of opportunistic screening as universal screening programs are not implemented in these institutions. A majority (73%) of the physicians and specialists who took part in the present study used AHA guidelines, 33% followed NICE guidelines for the diagnosis and management of FH. Moreover, differences exist among the responses within the same institution suggesting a potential gap in awareness and knowledge regarding FH. Majority of the physicians included in this study considered statins and ezetimibe combined with lifestyle changes as a recommended treatment option for adult FH patients (46.6%) and statins in combination with lifestyle changes for children with FH (40.9%). The participants of the study considered lack of financial resources as the major barrier in implementing a systematic FH screening program and estimated. The participants of the study estimated the mean monthly cost of statins to be 8.5 US $ (1700 PKR) for adults and 7.5 US $ (1500 PKR) for children.
The results of the current study are no exception as several previous studies from around the world have also identified similar gaps. A survey conducted in France suggests that FH is generally underdiagnosed and undertreated mostly due to the lack of knowledge among physicians and difficulties in implementing consolidated guidelines.23 Similarly, only 34% of the physicians from ten countries in the Asia–Pacific region (Table 1) considered themselves fully aware of FH and less than half were aware of the national and international guidelines.20 A mapping survey conducted among 95 respondents across 78 countries showed that only a few countries were able to achieve a diagnosis rate above 10% and most of these were in the Europe.14 In a study conducted by American College of Cardiology (ACC), approximately 10% of the cardiologists were fully confident about their knowledge and awareness of FH and stressed on the need to develop extensive FH awareness programs.24 Around 30% of the physicians from India had above average knowledge and awareness about FH.17,18 More than 50% of the physicians surveyed in Saudi Arabia, had no familiarity with FH.25 In contrast, a study conducted in Australia showed that 76% of the clinicians had knowledge regarding FH and 43% were aware of the available lipid guidelines.26
Table 1.
Comparison of familial hypercholesterolemia (FH) care in South Asia and Southeast Asia.
| India17 | India18 | Malaysia19 | Malaysia20 | Taiwan20 | Vietnam20 | Philippines20 | Pakistan -current study | |
|---|---|---|---|---|---|---|---|---|
| Awareness about FH guidelines (%) | 40.6 | 31.0 | 39.0 | 35.0 | 53.0 | 28.0 | N/Aa | N/A |
| Cascade screening of relatives (%) | 41.4 | 52.0 | 53.0 | 82.0 | 77.0 | 83.0 | 53.0 | 71.8 |
| Prescription of statins to FH patients (%) | 91.7 | 50.0 | 96.2 | 96.0 | 95.0 | 75.0 | 95.0 | 92.2 (for adults); 78.7 (for children) |
| Prescription of statins & ezetimibe to FH patients (%) | 19.5 | 33.0 | 58.3 | 56.0 | 63. | 31.0 | 48.0 | 49.5 (for adults); 35.5 (for children) |
Proportion percentage of the clinicians surveyed from the respective countries are mentioned.
N/A indicates not applicable or not available.
There are no definite FH diagnosis guidelines in Pakistan, hence physicians employ different guidelines for the diagnosis and management of FH. Whilst international guidelines agree that high cholesterol, positive family history, and physical features of FH suggest a diagnosis, there is variation among guidelines with regards to the level of cholesterol and the importance of secondary features. Cascade screening combined with genetic testing of the family members of the known index case is generally recommended as an effective approach for FH screening.27 Previous FH mapping studies have shown that cascade screening of FH patients is performed by physicians in Saudi Arabia and a few countries in Asia Pacific region.20,25 However, a study conducted among physicians in India, reported that only 41.4% of the participants screened close relatives of FH patients.17 The screening of FH patients and their family members is widely performed in high income countries, however only a fraction of population in low-income and middle income countries has access to genetic testing.10,12
Generally, lifestyle changes are considered the first and fundamental strategy for the management of FH in both children and adolescents followed by statin therapy for children aged 8–10 years, whose LDL-C levels are persistently ≥160 mg/dL even after lifestyle modification and adults with baseline LDL-C levels >190 mg/dL.11 According to the EAS guidelines, primary prevention of individuals with heterozygous FH is aimed at reaching an LDL-C reduction of ≥50% from baseline and an LDL-C goal of <55 mg/dL for very high-risk patients.27 Physicians from India and Asia pacific countries, recommended statins combined with lifestyle changes and ezetimibe as a primary treatment option for FH (Table 1), consistent with the results from the present study.17, 18, 19, 20 Apart from statins other lipid lowering therapies, such as PCSK9 inhibitors (evolucumab and alirocumab), mipomersen, lomitapide and LDL apheresis have been developed over the years and used in several countries, typically for those with homozygous FH or severe LDL-C elevation.11 Despite their promising activities, these drugs are not widely available in Pakistan and come at a high cost. Only three respondents in the present study prescribed PCSK9 inhibitors to their patients.
Early lipid screening of a child with family history of premature onset of CAD, at 2 years and general screening between 9 and 11 years is recommended.5 FH mapping studies in different countries suggest that screening age might differ from country to country. General practitioners in Australia screen children aged 13–18 years whereas in India, almost fifty percent of the physicians screen FH patients aged between 13 and 18 years.17,26 Different clinical practices and lack of uniform guidelines on the age for FH screening lead to a delayed diagnosis of FH i.e., in adults, mean age for diagnosis of FH among males and females is 43 and 46 years, respectively and only 2% are diagnosed before the age of 18 years.6 The Slovenian model for FH universal screening involves mandatory screening of children at the age of 5 years, followed by genetic and cascade screening of children referred to the tertiary care level.28
Pakistan spent 1.2% of the total GDP in 2020–2021 on health expenses compared to 5% recommended by the WHO.13 Thus, lack of financial resources and low budget allocation in healthcare sector can be a major barrier in implementing FH screening, diagnosis, and treatment, which was also stressed out by the present study respondents. Consequently, low financial resources can shift the burden on the patients to rely on out-of-pocket payments for healthcare needs which would lead to catastrophic impacts on the quality of life of the patients and push the households into extreme poverty. In Pakistan, about 32% of health expenditure is borne by the Government, while the remaining 64% relies on out-of-pocket treatment payments.13 A same trend can also be seen in the present study where 63% of the physicians consider out-of-pocket treatment as the only financing option for FH treatment. The higher price of monthly dose of statins with respect to average monthly income also pose serious financial burdens on the patients and their family. Compared to Pakistan, in other countries such as Australia, the monthly cost of statins is estimated at 7 USD representing 0.4% of the average monthly wage.29,30 In Slovenia, the average monthly dose of high intensity statins (rosuvastatin 40 mg or atorvastatin 80 mg) is around 4.30 USD (0.3% of the net monthly salary) and the cost is covered by the insurance. Extended public healthcare expenditure could lead to the provision of better healthcare facilities, which in turn could lead to positive outcomes in terms of FH screening and treatment in Pakistan.
One of the major limitations of this study is the lack of consistent responses from respondents even from within the same institutions. We assume that respondents did not comprehend some of the questions. Non-coherent responses to simple questions about the programs and strategies in their host institutions were excluded from the analysis. In addition, low response rate and a relatively small number of included clinicians from the country impedes the generalization of the study results. This implies that FH care is a low priority area in Pakistan, which is already facing a significant disease burden that includes, diabetes, cardiovascular, gastrointestinal, and respiratory conditions. Hence only a small fraction of dedicated professionals working on FH. Extended studies need to be conducted to analyse the availability of FH screening programmess in the country. The participants of the study stressed the importance of provision of resources and support at the institutional level for FH screening. They also emphasised the provision of clear and consolidated guidelines for FH screening.
Despite being considered a public health priority, FH remains underdiagnosed and undertreated in various regions of the world. Being a low-middle income country, the Pakistani population has a substantially high risk of CVD and FH can be a contributing factor. The study provides a first comprehensive mapping of the capacity of the existing infrastructure for the diagnosis and treatment of FH and other dyslipidemias. The study highlighted the shortcomings in the healthcare system of Pakistan regarding FH; hence it is crucial to develop a network involving stakeholders from healthcare sector to implement a consolidated FH screening, diagnosis and treatment program in the country.
Contributors
FS, UG, and SS contributed to the conception and design of the study. SS and FS contributed to contacting the participants for data acquisition. JS analysed the data and all authors contributed to the interpretation of data. FS, MK, and JS drafted the manuscript and directly accessed and verified the data. All authors critically reviewed the manuscript and approved the final draft.
Data sharing statement
The dataset from the current study is not publicly available.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
None.
Acknowledgments
The contribution of facilitators in each healthcare organisation across Pakistan is highly appreciated.
PAKISTAN Familial Hypercholesterolemia Collaborators:
Jamshed Alami, Muhammad Alij, Jamal Anwerk, Waheed Akhtar Awanl, Shireen Qassim Bhamj, Nousheen Fatimam, Farah Guln, Syeda Sughra Hameedl, Maryam Haroono, Mujtaba Hasanp, Arzu Jadoonq, Shazia Jamilr, Asaad Akbar Khann, Sabeen Abid Khann,s, Saera Suhail Kidwaij, Atif Munirt, Muhammad Talha Bin Naziru, Gul Zaman Khan Niaziu, Sumera Nawaz Qabulioq, Muhammad Asim Ranav, Adeel ur Rehmanu, Saba Safdarq, Saeedullah Shahn, Tanzeel Ur Rehman Ahmed Sheikhw, Abeer Yousufx, Kaneez Zehraq, Tehzeeb Zehras
iPeshawar Institute of Cardiology, Peshawar, Pakistan
jFazaia Ruth Pfau Medical College (FRPMC), Karachi, Pakistan
kSheikh Zayed Hospital, Rahimyar Khan, Pakistan
lAbbass Institute of Medical Sciences, Muzaffarabad, Azad Kashmir, Pakistan
mQuaid e Azam Medical College, Bahawal Victoria Hospital Bahawalpur, Pakistan
nShifa International Hospital, Islamabad, Pakistan
oServices Hospital Lahore, Pakistan
pAkhtar Saeed Medical and Dental College, Lahore, Pakistan
qZiauddin Hospital Karachi, Pakistan
rM Islam Medical College, Gujranwala, Pakistan
sShifa College of Medicine, Shifa Tameer e Millat University, Islamabad, Pakistan
tFatima Memorial Hospital, Lahore, Pakistan
uRawalpindi Institute of Cardiology, Rawalpindi, Pakistan
vBahria International Hospital, Lahore, Pakistan
wBenazir Bhutto Hospital, Rawalpindi, Pakistan
xRehman Medical Institute, Peshawar, Pakistan
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100163.
Contributor Information
Fouzia Sadiq, Email: director.research@stmu.edu.pk.
Urh Groselj, Email: urh.groselj@kclj.si.
PAKISTAN Familial Hypercholesterolemia Collaborators:
Jamshed Alam, Muhammad Ali, Jamal Anwer, Waheed Akhtar Awan, Shireen Qassim Bham, Nousheen Fatima, Farah Gul, Syeda Sughra Hameed, Maryam Haroon, Mujtaba Hasan, Arzu Jadoon, Shazia Jamil, Asaad Akbar Khan, Sabeen Abid Khan, Saera Suhail Kidwai, Atif Munir, Muhammad Talha Bin Nazir, Gul Zaman Khan Niazi, Sumera Nawaz Qabulio, Muhammad Asim Rana, Adeel ur Rehman, Saba Safdar, Saeedullah Shah, Tanzeel Ur Rehman Ahmed Sheikh, Abeer Yousuf, Kaneez Zehra, and Tehzeeb Zehra
Appendix A. Supplementary data
References
- 1.Pakistan Bureau of Statistics [Internet]. Available from: https://www.pbs.gov.pk/; 2021. Accessed May 11, 2022.
- 2.Walker I.F., Garbe F., Wright J., et al. The economic costs of cardiovascular disease, diabetes mellitus, and associated complications in South Asia: a systematic review. Value Heal Reg Issues. 2018;15:12–26. doi: 10.1016/j.vhri.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Wiegman A., Panel for the EASC, Gidding S.S., et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425–2437. doi: 10.1093/eurheartj/ehv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groselj U., Wiegman A., Gidding S.S. Screening in children for familial hypercholesterolaemia: start now. Eur Heart J. 2022;43:3209. doi: 10.1093/eurheartj/ehac224. [DOI] [PubMed] [Google Scholar]
- 5.Gidding S.S., Wiegman A., Groselj U., et al. Paediatric familial hypercholesterolaemia screening in Europe: public policy background and recommendations. Eur J Prev Cardiol. 2022;16:2301. doi: 10.1093/eurjpc/zwac200. [DOI] [PubMed] [Google Scholar]
- 6.Vallejo-Vaz A.J., Stevens C.A.T., Lyons A.R.M., et al. Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) Lancet. 2021;398(10312):1713–1725. doi: 10.1016/S0140-6736(21)01122-3. [DOI] [PubMed] [Google Scholar]
- 7.Wilemon K.A., Patel J., Aguilar-Salinas C., et al. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol. 2020;5(2):217–229. doi: 10.1001/jamacardio.2019.5173. [DOI] [PubMed] [Google Scholar]
- 8.Familial hypercholesterolaemia (FH): report of a second WHO consultation, Geneva, 4 September 1998. World Health Organization; Geneva: 1999. [Google Scholar]
- 9.Sustar U., Kordonouri O., Mlinaric M., et al. Universal screening for familial hypercholesterolemia in 2 populations. Genet Med. 2022;24(10):2103–2111. doi: 10.1016/j.gim.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard B.G., Benn M. Genetic testing for familial hypercholesterolaemia is essential in individuals with high LDL cholesterol: who does it in the world? Eur Heart J. 2017;38(20):1580–1583. doi: 10.1093/eurheartj/ehx136. [DOI] [PubMed] [Google Scholar]
- 11.McGowan M.P., Hosseini Dehkordi S.H., Moriarty P.M., Duell P.B. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2019;8(24):e13225. doi: 10.1161/JAHA.119.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tromp T.R., Hartgers M.L., Hovingh G.K., et al. Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. Lancet. 2022;399(10326):719–728. doi: 10.1016/S0140-6736(21)02001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeed A., Saeed F., Saeed H., et al. Access to essential cardiovascular medicines in Pakistan: a national survey on the availability, price, and affordability, using WHO/HAI methodology. Front Pharmacol. 2021;11:2260. doi: 10.3389/fphar.2020.595008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Familial hypercholesterolemia: country mapping survey - preliminary analysis - World Heart Federation [Internet] 2022. https://world-heart-federation.org/resource/familial-hypercholesterolemia-country-mapping-survey-preliminary-analysis/ Available from:
- 15.Ajk at a glance-azad Jammu & Kashmir: official portal [Internet] 2019. https://www.ajk.gov.pk/ajk-at-a-glance Available from:
- 16.Planning and development department, Gilgit-baltistan [Internet] 2020. https://www.pnd.gog.pk/Reports Available from:
- 17.Rangarajan N., Balasubramanian S., Pang J., Watts G.F. Knowledge and awareness of familial hypercholesterolaemia among registered medical practitioners in Tamilnadu: are they suboptimal? J Clin Diagn Res. 2016;10(5):OC52. doi: 10.7860/JCDR/2016/18798.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavanya Reddy L., Ashavaid T.F. Familial hypercholesterolemia (FH) awareness amongst physicians in Mumbai, India. J Assoc Physicians India. 2018;66:66. [PubMed] [Google Scholar]
- 19.Azraii A.B., Ramli A.S., Ismail Z., et al. Knowledge, awareness and practice regarding familial hypercholesterolaemia among primary care physicians in Malaysia: the importance of professional training. Atherosclerosis. 2018;277(August):508–516. doi: 10.1016/j.atherosclerosis.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Pang J., Hu M., Lin J., et al. An enquiry based on a standardised questionnaire into knowledge, awareness and preferences concerning the care of familial hypercholesterolaemia among primary care physicians in the Asia-Pacific region: the “Ten Countries Study”. BMJ Open. 2017;7(10) doi: 10.1136/bmjopen-2017-017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank Open Data-Physicians (per 1,000 people) - Pakistan [Internet]. Available from: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS?locations=PK; 2019. Accessed April 22, 2022.
- 22.Kumar R., Pal R. India achieves WHO recommended doctor population ratio: a call for paradigm shift in public health discourse! J Fam Med Prim Care. 2018;7(5):841. doi: 10.4103/jfmpc.jfmpc_218_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Béliard S., Rabès J.P., Cariou B., et al. Hypercholestérolémie familiale peu diagnostiquée, insuffisamment traitée. Enquête auprès de 495 médecins. Presse Med. 2018;47(9):e159–e167. doi: 10.1016/j.lpm.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Foody J.M. Familial hypercholesterolemia: an under-recognized but significant concern in cardiology practice. Clin Cardiol. 2014;37(2):119–125. doi: 10.1002/clc.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alaqeel A., Alrashidi A. Gaps in knowledge and practice for familial hypercholesterolemia among physicians caring for children in Saudi Arabia. Eur Rev Med Pharmacol Sci. 2022;26:2727–2739. doi: 10.26355/eurrev_202204_28603. [DOI] [PubMed] [Google Scholar]
- 26.Mirzaee S., Rashid H.N., Tumur O., et al. Awareness of familial hypercholesterolemia among healthcare providers involved in the management of acute coronary syndrome in victoria, Australia. CJC Open. 2019;1(4):168–172. doi: 10.1016/j.cjco.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 28.Groselj U., Kovac J., Sustar U., et al. Universal screening for familial hypercholesterolemia in children: the Slovenian model and literature review. Atherosclerosis. 2018;277:383–391. doi: 10.1016/j.atherosclerosis.2018.06.858. [DOI] [PubMed] [Google Scholar]
- 29.Pharmaceutical Benefits Scheme (PBS) [Internet]. Available from: https://www.pbs.gov.au/medicine/item/2594M-2636R; 2022. Accessed June 8 2022.
- 30.Australian Bureau of Statistics [Internet]. Available from: https://www.abs.gov.au/; 2022. Accessed June 8, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.