Summary
Background
Diphtheria, Tetanus, and whole-cell Pertussis (DTwP) vaccination-associated seizures form the commonest type of serious adverse event following immunization in India and are an important reason for vaccine hesitancy. Our study explored the genetic explanation of DTwP vaccination-associated seizures or subsequent epilepsies.
Methods
Between March 2017 and March 2019, we screened 67 children with DTwP vaccination-associated seizures or subsequent epilepsies, and of those, we studied 54 without prior seizures or neurodevelopmental deficits. Our study design was cross-sectional with a 1-year follow-up having both retrospective and prospective cases. We performed clinical exome sequencing focused on 157 epilepsy-associated genes and multiplex ligation-dependent probe amplification of the SCN1A gene at enrolment. We applied the Vineland Social Maturity Scale for neurodevelopmental assessment at follow-up.
Findings
Of 54 children enrolled and underwent genetic testing (median age 37.5 months, interquartile range 7.7–67.2; diagnosis at enrolment: epilepsy 29, febrile seizure 21, and febrile seizure-plus 4), we found 33 pathogenic variants of 12 genes. Of 33 variants, 13 (39%) were novel. Most pathogenic variants were found in SCN1A gene (n = 21/33; 64%), SCN8A in 2 children, and 10 children had one variant in CDKL5, DEPDC5, GNAO1, KCNA2, KCNT1, KCNQ2, NPRL3, PCDH19, RHOBTB2, and SLC2A1. Five or more seizures (odds ratio [OR] = 5.3, confidence interval [CI]: 1.6–18.4, p = 0.006), drug-resistant epilepsy (OR = 9.8, 95% CI: 2.6–30.7, p = 0.001) and neurodevelopmental impairment (social quotient < 70) (OR = 5.6, 95% CI: 1.65–17.6, p = 0.006) were significant predictors of genetic diagnosis.
Interpretation
Our study provides proof-of-concept for genetic aetiology in children with DTwP vaccination-associated seizures or subsequent epilepsies and has important implications for vaccination policies in developing countries.
Funding
International Pediatric Association Foundation, Inc. (IPAF): İhsan Doğramaci research award 2016/2017; Indian Council of Medical Research (ICMR), New Delhi, India: No.3/1/3/JRF-2016/HRD/LS/71/10940.
Keywords: Vaccine, Seizure, Neurodevelopment, Diphtheria-tetanus-pertussis, Next-generation sequencing, Vaccine hesitancy
Research in context.
Evidence before this study
Since 1933, whole-cell pertussis (wP) vaccination-associated seizures and subsequent epilepsy constitute a significant cause of vaccine hesitancy globally. We searched PubMed and Embase for papers published in English up to March 2022, reviewing the causal association of pertussis vaccine-associated seizures and subsequent epilepsy using the terms “pertussis”, “vaccine”, and “seizures”. A landmark cohort study by the Centers for Disease Control and Prevention in 2001 demonstrated a significantly elevated risk of febrile seizures after receiving wP vaccination (adjusted relative risk 5.7; 95 per cent confidence interval, 1.9–16.4). Subsequently, multiple small case series (Berkovic, 2006; Reyes, 2011; Verbeek, 2014) reported SCN1A genetic variation as a plausible explanation in children with epileptic encephalopathy with onset related to DTwP vaccination. In 2020, Damiano et al performed SCN1A genetic testing in prospectively enrolled 69 children with acellular pertussis vaccine-proximate seizures and diagnosed two children with SCN1A variants. Small sample size in retrospective studies, limited genetic testing via Sanger sequencing, and lack of objective psychometric assessment were important limitations of these studies.
Added value of this study
Our paper, the most extensive study on whole-cell pertussis vaccine-associated seizures using next-generation sequencing as a primary diagnostic technique, had a high yield of pathogenic variants related to 12 genes in 33/54 (61%) probands. We highlighted the genetic heterogeneity with 13 novel implicated genetic variants. SCN1A genetic variation was the most common explanation for vaccine-associated seizures. Sodium channel gene SCN8A and potassium channel genes KCNA2, KCNT1, and KCNQ2 were also affected, comprising 26 out of 33 variants from ion channels, suggesting vaccine-associated seizures are predominantly due to channelopathies. Five or more subsequent seizures (odds ratio [OR] = 5.3, 95% confidence interval [CI]: 1.6–18.4, p = 0.006), drug-resistant epilepsy (OR = 9.8, 95% CI: 2.6–30.7, p = 0.001) and neurodevelopmental impairment (social quotient < 70) (OR = 5.6, 95% CI: 1.65–17.6, p = 0.006) were significant predictors of genetic diagnosis.
Implications of all the available evidence
Our data suggest that genetic testing, preferably next-generation sequencing, should be offered routinely in children with vaccine-proximate children due to its diagnostic, therapeutic, and prognostic implications. Clinicians should offer SCN1A Sanger sequencing at least to all such children. In countries with the resource-limited setting, children with vaccine-proximate seizures may be followed-up clinically with developmental surveillance and seizure recurrences. Subsequently, clinicians may prioritize genetic testing for children who evolved into epilepsy or show developmental impairment on follow-up. Vaccine-proximate seizures should not be a barrier to immunization and a reason to adopt policy for acellular-pertussis vaccination.
Introduction
Globally, diphtheria and tetanus toxoids and pertussis (DTP) immunization in infants is an essential component of national policies and preventive public health interventions. Since 1933, neurological complications following whole-cell pertussis (wP) vaccination have been reported, there are concerns regarding its safety.1 A landmark cohort study by the Centers for Disease Control and Prevention demonstrated a significantly elevated risk of febrile seizures after receipt of the wP vaccine, with an incidence of 6–9 per 100,000 children.2 Subsequently, a population-based 10-year cohort study by Verbeek et al3 demonstrated that a small proportion (4.2%) of children with vaccine-proximate seizures evolve into epilepsies, including Dravet syndrome (1.2%). Dravet syndrome is a developmental and epileptic encephalopathy with seizure onset in infancy and is often triggered by febrile illness or vaccination in a previously healthy child. There is an underlying genetic explanation in most children with Dravet syndrome, and a preponderance has sodium channel alpha-1 subunit gene (SCN1A) pathogenic variants.4 SCN1A pathogenic variants have phenotypic pleiotropy with Dravet syndrome in a severe phenotype and febrile seizures in a mild phenotype.5
With the advent of genetics, the causal relationship between vaccination and seizures is under further exploration. In 2006, Berkovic et al6 demonstrated that among 14 cases of epileptic encephalopathy after DTP vaccination, 11 had SCN1A gene pathogenic variations implicated as a direct cause. Similarly, retrospective case series studies by Reyes et al7 (n = 5) and Verbeek et al (n = 23) identified SCN1A genetic variations as a plausible explanation. In a prospective epidemiological study in 2020, Damiano et al8 performed SCN1A genetic testing in 69 children with acellular pertussis (aP) vaccine-proximate seizures and found two with SCN1A variants while one in 75 controls. The limitations of these studies were the small sample size (in retrospective studies), limited genetic testing via Sanger sequencing, and lack of objective psychometric assessments.
Incidents of vaccine-proximate seizures escalate vaccine hesitancy among parents and pediatricians. These often trigger unnecessary litigations that threaten the success of the universal vaccination program and endanger pertussis outbreaks.9 Epidemiological studies and animal studies suggest a higher risk of vaccine-proximate seizures with whole-cell pertussis vaccination than acellular pertussis.10,11 Therefore, acellular pertussis vaccination is preferred to whole-cell pertussis in developed countries despite less immunogenicity.12 WHO reported that most developing countries use whole-cell pertussis vaccines due to their low cost.13 Hence, assessing the etiological relation between wP and vaccine-proximate seizures is crucial by performing extensive genetic testing in those cases. We hypothesized that molecular testing through clinical exome sequencing with a specific reference to 157 known epilepsy-associated genes and correlating genetic diagnoses with clinical phenotype would be able to explore the genetic landscape of DTwP-associated seizures or subsequent epilepsy.
Methods
Study design
Between March 2017 and March 2019, we conducted this ambispective (prospective and retrospective case recruitment), cross-sectional study with a one-year follow-up at Advanced Pediatric Centre, Postgraduate Institute of Medical Education and Research, Chandigarh, India. We obtained informed consent from the parents of eligible children.
Participants
We screened children with a history of first seizure within 72 h of the DTwP vaccine, attending the outpatient department or emergency services for eligibility for the study. We also included follow-up children with epilepsy and a history of first seizure associated with the DTwP vaccine. Exclusion criteria were known explanation of underlying aetiology—structural, metabolic, or known genetic syndrome, e.g., tuberous sclerosis complex or Down syndrome; or a history of prior seizure or pre-existing developmental delay as reported by the clinician. To avoid recall bias in retrospectively recruited cases, we restricted enrollment to patients with precise documentary records of the temporal relationship of vaccine administration with the occurrence of seizures.
Phenotypic ascertainment
At recruitment, a pediatric neurologist ascertained phenotypic details of participants, including age at occurrence of first seizure, seizure characteristics, examination, and investigation findings. We classified seizures and epilepsy diagnoses according to the International League Against Epilepsy Criteria 2017, based on available seizure semiology (history and video), neuroimaging, and electroencephalogram details.14,15 We followed up with the recruited children for a year since the initial presentation and noted evolution of clinical phenotype, details on antiseizure medications, control of seizures and neurodevelopmental status. We defined drug-resistant epilepsy as a lack of seizure freedom for six months despite an optimum trial of two appropriately selected and dosed antiseizure medications either as monotherapies or in combination.16 Children were assessed for their neurodevelopmental status by a child psychologist using the Vineland Social Maturity Scale (VSMS)- Indian adaptation, which correlates 0.85–0.96 with Stanford-Binet Intelligence Scale.17 Social Quotients (SQ) were estimated to classify children's intellectual ability as profound (<20), severe (20–35), moderate (35–50), mild (50–70), borderline (70–80), below average (80–90), average (90–110) and above-average (>110).
Genetic study
We extracted DNA from the whole blood using the QIAamp DNA blood mini kit (Qiagen N.V.) and tested the quality of DNA with a Qubit fluorometer (Thermo). Readings above 50 ng/μl were considered suitable for performing genetic testing.
Next-generation sequencing (NGS)
Clinical exome sequencing, a panel of 8332 genes targeted for gene capture and sequencing of the protein-coding regions, was used in the study. Furthermore, 157 epilepsy-associated genes (Supplementary Table S1) relevant to this study were short-listed by SN, JKS, PB, and AK after an extensive literature search and review. Mean coverage of 99.7% with a depth of >25× using the HiSeq platform (Illumina Inc., San Diego, CA, USA) was ensured for these 157 genes. Sequences were aligned to the human reference genome (GRCh37/hg19) using the Burrows-Wheeler Aligner (BWA)18 program. Picard19 and Genome Analysis Toolkit20 tools (v3.6) were applied to recognize variants of clinical significance. Ensembl variant effect predictor was used for gene annotation of clinically relevant pathogenic variants, based on published variants in literature and disease databases ClinVar, OMIM, GWAS, HGMD, and SwissVar. Common variants were filtered based on allele frequency in population databases like 1000Genome Phase 3, Exome Aggregation Consortium, gnomAD, and Indian population databases SAGE (South Asian Genomes & Exomes) and INDEX-db (INDian EXome database).21,22 The non-synonymous and splice site variants were assessed for pathogenicity using multiple tools PolyPhen-2, SIFT, PROVEAN, Mutation Taster2, LRT, Mutation Assessor, FATHMM, Human Splicing Finder 3.1, and various algorithms using the tool and engine CADD23 and VarSome (Educational version 9.2.2).24 Those variants that predicted deleterious were used for clinical interpretation. Sanger sequencing was used for the validation of each identified potentially pathogenic variant. A multidisciplinary team of a pediatric neurologist, clinical geneticist, and molecular lab pathologist (J.K.S., A.P., P.B.) reviewed all variants with regard to clinical phenotype and reported variants using the American College of Medical Genetics and Genomics (ACMG) guideline.25 Furthermore, we also considered sequencing of variant(s) in other affected or non-affected family members to confirm the significance wherever needed and feasible.
Multiplex ligation-dependent probe amplification (MLPA) for SCN1A gene
We also tested for copy number variation of the SCN1A gene using MLPA among cases with no pathogenic variants detected by NGS as SCN1A gene mutation is widely known to cause febrile seizures. We used SALSA MLPA P137 SCN1A probemix (MRC Holland lot no. B2 and B3), SALSA MLPA EK1 reagent kit (MRC Holland) having FAM dye, and size marker LIZ GeneScane 500–250 (ThermoFisher Scientific), and analyzed results on Coffalyser.net.
Statistical analysis
We calculated a sample size of 49, based on an empirical 15% genetic yield, with a total 20% width of the confidence interval and 95% confidence level. We collected data on a predesigned proforma and entered it into a Microsoft Excel spreadsheet. We analyzed data using GraphPad Prism (9.1.0). We applied Kolmogorov Smirnov and Shapiro–Wilk tests on variables with continuous data to assess normality distribution. Continuous variables were presented as median with interquartile range and compared by the Mann–Whitney U test. We classified the first seizure into two categories: generalized and focal-onset for analysis. The first seizure with a duration of more than or equal to 15 min was considered a prolonged seizure. Children were also grouped based on the number of seizures in infancy into two categories <5 and ≥5 seizures. Clustering of seizures was considered for children with two or more seizures within 24 h of onset. Categorical variables were presented as frequencies and compared by Fisher's exact test. We performed multivariate logistic regression to calculate the odds ratio (OR) for potential predictors of genetic diagnosis. A p-value of less than 0.05 was considered statistically significant.
Ethics approval: The study was approved by the Institute Ethics Committee.
Consent to participate: Informed consent was obtained from the parents.
Role of the funding source
Indian Council of Medical Research (ICMR), New Delhi, India [No.3/1/3/JRF-2016/HRD/LS/71/10940]; and International Pediatric Association Foundation, Inc. (IPAF) [İhsan Doğramaci research award 2016/2017]; had no role in design, patient recruitment, data collection, analysis, interpretation, writing of the manuscript, the decision to submit it for publication, or any other aspect pertinent to the study. The authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
Results
Cohort recruitment
We screened 67 consecutive children with a history of DTwP vaccination-associated seizures or subsequent epilepsies. Of 67, ten were excluded: history of prior seizures (n = 4), hypoxic-ischemic brain injury (n = 2), hypoglycemic brain injury (n = 2), hypocalcemia (n = 1), and unexplained pre-existing delay (n = 1). Of 57 eligible cases, three were further excluded due to the low quality of DNA for next-generation sequencing. Fifty-four children (median age at enrolment 37.5 months, interquartile range [IQR] 7.7–67.2; 33 [61%] boys) formed the study cohort.
The median age at onset of vaccination-proximate seizures was four months (IQR 3–5.6). Children received wP vaccine as following combinations: [DTwP alone (n = 42), DTwP-Hepatitis B (HepB)-Haemophilus influenzae type b (HiB) (n = 10), DTwP-HepB-HiB and MMR (n = 1) and DTwP-HiB (n = 1)]. The relation of the timing of seizure with a dose of vaccine was as follows: a first dose of DTwP (n = 9), the second dose of DTwP (n = 15), the third dose of DTwP (n = 21), first booster of DTwP (n = 8), and second booster of DTwP (n = 1). Forty-eight children had seizure episodes within 24 h of vaccination, five during 24–48 h, and one during 48–72 h post-vaccination. None of the children had other triggers except one had co-existing upper respiratory tract infection.
Characteristics of vaccine-proximate seizures and phenotypes
All children, except four, had febrile vaccine seizures. The semiology characteristics of vaccine-proximate seizure type were generalized clonic in 25 children, generalized tonic in seven, generalized tonic-clonic in five, focal impaired awareness motor onset in nine, focal impaired awareness nonmotor onset in five, and epileptic spasms in three with unknown-onset. The clustering of seizures (more than one) within 24 h was present in 22 children. Twenty-one children had a prolonged seizure of ≥15 min (Table 1).
Table 1.
Clinical characteristics of probands at recruitment along with the genes having pathogenic variants
| Code no. | Sex | DTP dose | Vaccine type | Age at first seizure in months | Age at enrolment in months | Seizure semiology | Seizure duration range in minutes | Clustering | Number of seizures in infancy | Diagnosis | Gene with pathogenic variant |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 3rd | DTwP | 5.5 | 40 | GC | >30 | Yes | >10 | Dravet Syndrome | ·· |
| 2 | M | 3rd | DTwP | 4.5 | 96 | GC | 5–14 | No | >10 | Dravet Syndrome | SCN1A |
| 3 | M | 3rd | DTwP | 6 | 59 | GC | 15–30 | No | >10 | Dravet Syndrome | SCN1A |
| 4 | M | 3rd | DTwP-HepB-HiB | 4 | 14 | GC | <5 | Yes | 0–4 | Complex febrile seizure | ·· |
| 5 | M | 1st | DTwP | 1.5 | 71 | GC | <5 | No | >10 | Dravet Syndrome | SCN1A |
| 6 | M | 3rd | DTwP | 4 | 5 | ES | <5 | Yes | >10 | West syndrome | ·· |
| 7 | M | 3rd | DTwP | 3.5 | 41 | GC | <5 | Yes | 0–4 | Simple febrile seizure | ·· |
| 8 | F | 3rd | DTwP | 4 | 21 | FIAM | >30 | No | >10 | Dravet Syndrome | SCN1A |
| 9 | M | 2nd | DTwP-HepB-HiB | 3 | 56 | GC | <5 | Yes | >10 | Self-limited myoclonic epilepsy in infancy | ·· |
| 10 | M | 2nd | DTwP-HepB-HiB | 3.5 | 11 | FIANM | <5 | Yes | >10 | Febrile seizure plus | ·· |
| 11 | M | 2nd | DTwP | 3 | 159 | FIAM | <5 | No | >10 | Dravet Syndrome | SCN1A |
| 12 | M | 2nd | DTwP-HepB-HiB | 4 | 12 | GT | <5 | Yes | 0–4 | Complex febrile seizure | ·· |
| 13 | F | 1st | DTwP | 2 | 36 | ES | <5 | Yes | >10 | West syndrome | CDKL5 |
| 14 | F | 1st booster | DTwP | 26 | 49 | FIANM | <5 | Yes | 0–4 | Doose syndrome | ·· |
| 15 | F | 2nd | DTwP | 3 | 94 | FIAM | 5–14 | Yes | 5–10 | Dravet Syndrome | SCN1A |
| 16 | M | 2nd | DTwP | 3 | 47 | GC | 5–14 | Yes | >10 | Dravet Syndrome | SCN1A |
| 17 | M | 2nd | DTwP | 4 | 48 | FIAM | >30 | No | >10 | Dravet Syndrome | SCN1A |
| 18 | M | 2nd | DTwP | 4 | 90 | GC | >30 | Yes | >10 | Dravet Syndrome | SCN1A |
| 19 | M | 2nd | DTwP | 4.5 | 62 | GC | 5–14 | No | >10 | Dravet Syndrome | SCN1A |
| 20 | F | 3rd | DTwP | 5 | 148 | GC | <5 | No | >10 | Dravet Syndrome | SCN1A |
| 21 | F | 2nd | DTwP | 3 | 126 | FIAM | 15–30 | No | 5–10 | Dravet Syndrome | SCN1A |
| 22 | M | 1st | DTwP | 2 | 15 | FIAM | 5–14 | No | >10 | Dravet Syndrome | SCN1A |
| 23 | M | 3rd | DTwP | 5 | 5 | GC | 15–30 | Yes | 5–10 | Complex febrile seizure | ·· |
| 24 | F | 2nd | DTwP | 3 | 39 | GTC | <5 | Yes | >10 | Dravet Syndrome | SCN1A |
| 25 | F | 1st | DTwP | 1.5 | 1.5 | FIANM | <5 | No | 0–4 | Complex febrile seizure | ·· |
| 26 | M | 3rd | DTwP | 6 | 71 | GC | 5–14 | No | >10 | Dravet Syndrome | SCN1A |
| 27 | F | 3rd | DTwP-HepB-HiB | 4 | 4 | GC | <5 | Yes | 0–4 | Complex febrile seizure | KCNQ2 |
| 28 | M | 3rd | DTwP | 5 | 123 | GC | 15–30 | No | 5–10 | Dravet Syndrome | SCN8A |
| 29 | M | 1st booster | DTwP | 16 | 16 | GC | >30 | No | 0–4 | Complex febrile seizure | ·· |
| 30 | M | 1st | DTwP-HepB-HiB | 1.5 | 1.5 | FIANM | <5 | No | 0–4 | Simple febrile seizure | ·· |
| 31 | M | 1st booster | DTwP | 18 | 66 | GC | >30 | Yes | 0–4 | Complex febrile seizure | ·· |
| 32 | M | 3rd | DTwP | 4.5 | 207 | GC | <5 | No | 0–4 | Dravet Syndrome | SCN1A |
| 33 | F | 3rd | DTwP-HepB-HiB | 3.5 | 8 | GC | <5 | No | >10 | Intractable Childhood epilepsy with GTCs | ·· |
| 34 | F | 1st booster | DTwP | 18 | 29 | GTC | 5–14 | No | 0–4 | Simple febrile seizure | DEPDC5 |
| 35 | M | 1st booster | DTwP | 18 | 71 | GT | 5–14 | No | 0–4 | Complex febrile seizure | ·· |
| 36 | F | 2nd | DTwP-HepB-HiB | 3.5 | 59 | GT | <5 | No | 0–4 | Febrile seizure plus | NPRL3 |
| 37 | M | 1st booster | DTwP | 20 | 26 | GC | 5–14 | No | 0–4 | Complex febrile seizure | ·· |
| 38 | F | 1st booster | DTwP-HepB-HiB, MMR | 15 | 17 | GC | 5–14 | No | 0–4 | Simple febrile seizure | PCDH19 |
| 39 | M | 3rd | DTwP | 7 | 90 | GC | 15–30 | No | >10 | Complex febrile seizure | SCN1A |
| 40 | M | 3rd | DTwP | 6 | 7 | GC | <5 | No | 0–4 | Febrile seizure plus | ·· |
| 41 | M | 1st | DTwP-HepB-HiB | 1.5 | 2 | GT | <5 | No | 0–4 | Simple febrile seizure | RHOBTB2 |
| 42 | M | 2nd booster | DTwP | 54 | 59 | GTC | <5 | No | 0–4 | Simple febrile seizure | ·· |
| 43 | F | 3rd | DTwP | 4 | 4 | GTC | 5–14 | No | 5–10 | Complex febrile seizure | SCN1A |
| 44 | F | 2nd | DTwP-HepB-HiB | 3 | 3 | GT | 5–14 | Yes | 0–4 | Complex febrile seizure | ·· |
| 45 | F | 2nd | DTwP | 2.5 | 3 | GT | <5 | Yes | 0–4 | Complex febrile seizure | KCNA2 |
| 46 | F | 1st | DTwP | 1.5 | 7 | ES | <5 | Yes | >10 | West syndrome | GNAO1 |
| 47 | M | 3rd | DTwP | 4 | 36 | FIAM | >30 | No | >10 | Dravet Syndrome | SCN1A |
| 48 | M | 2nd | DTwP | 2.5 | 5 | GT | 5–14 | Yes | >10 | Dravet Syndrome | SCN8A |
| 49 | F | 1st | DTwP | 1.5 | 20 | FIAM | 15–30 | Yes | 5–10 | Dravet Syndrome | ·· |
| 50 | M | 3rd | DTwP | 3 | 9 | FIAM | 15–30 | No | >10 | Dravet Syndrome | SCN1A |
| 51 | M | 3rd | DTwP | 3.5 | 154 | GC | 15–30 | No | 0–4 | Febrile seizure plus | SLC2A1 |
| 52 | M | 1st booster | DTwP | 18 | 43 | GTC | 5–14 | No | 0–4 | Simple febrile seizure | ·· |
| 53 | F | 3rd | DTwP-HepB | 3 | 48 | GC | 5–14 | Yes | 5–10 | Dravet Syndrome | SCN1A |
| 54 | F | 1st | DTwP | 1.5 | 1.5 | FIANM | >30 | No | >10 | Complex febrile seizure | KCNT1 |
ES = Epileptic spasms, F = Female, FIANM = Focal impaired awareness non motor, FIAM = Focal impaired awareness motor GC = Generalized clonic, GT = Generalized tonic, GTC = Generalized tonic-clonic, M = Male.
At recruitment, the diagnoses of children were as following: 21 children with febrile seizures, [complex febrile seizure (n = 14; 26%) and simple febrile seizure (n = 7; 13%)], 4 with febrile seizure plus (n = 4; 7%), 29 children with epilepsy [Dravet syndrome (n = 23; 43%), West syndrome (n = 3; 5.5%), Doose syndrome (n = 1; 2%), self-limited myoclonic epilepsy in infancy (n = 1; 2%) and intractable childhood epilepsy with generalized tonic-clonic (n = 1; 2%) (Fig. 1A). Of 21 children with febrile seizures, 14 were recruited soon after the first vaccination seizure, while seven were enrolled after 2–5 episodes of febrile seizures (Supplementary Fig. S1). All children with epilepsy were drug-resistant, except one child, each with Doose syndrome and self-limited myoclonic epilepsy in infancy.
Fig. 1.
Genetic outcome with syndromic diagnoses at the time of recruitment. (a) Yield in 54 children according to syndromic diagnoses of epilepsy or seizures at recruitment. FS = Febrile seizure, MEI = Myoclonic epilepsy in infancy, ICEGTC = Intractable childhood epilepsy with generalized tonic-clonic seizures. (b) Percentage wise distribution of 33 pathogenic variants according to epilepsy or seizures diagnoses.
Forty-nine children underwent magnetic resonance imaging (MRI) brain. Imaging was unremarkable except in eight children [mild cerebral atrophy (n = 4), white matter signal changes (n = 2), mild hemispheric atrophy (n = 1), and mesial temporal lobe signal change (n = 1). The primary inheritance pattern was sporadic, except autosomal recessive in one family, based on the family history.
Neurodevelopmental profile
Before the vaccine-associated seizure, all recruited children had a history of normal development, as reported by the clinicians. However, the neurodevelopmental status of 54 children during follow-up, as assessed by VSMS, revealed a profound delay in 5 (9.2%), severe delay in 8 (14.8%), moderate delay in 9 (16.7%), mild delay in 4 (7.4%), borderline delay in 3 (5.5%), below average in 3 (5.5%), average in 15 (27.8%) and above-average in 7 (13.0%) children. The median social quotient was 72 (IQR 35.5–99.3).
Genetic landscape
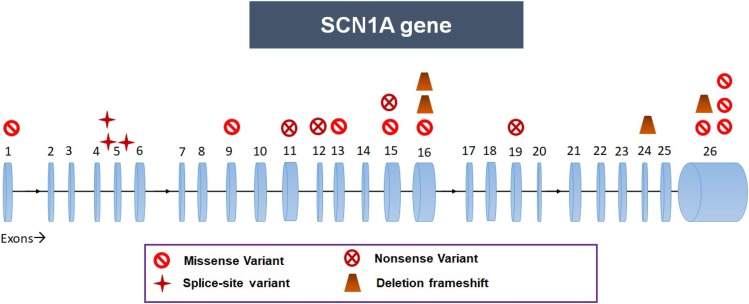
Fifty-four children underwent genetic testing (52-NGS with Sanger validation, 2-SCN1A Sanger sequencing). Thirty-three children (61%) had pathogenic or likely pathogenic variants in 12 different genes; of these, 13 children had novel variants (Table 2). These variations included 26 single nucleotide variants (20 missense, four nonsense, and two splice site) and six indel variants (four deletions, one deletion splice site, and one insertion splice site), with one already reported deletion variant found in two children. Thirty-one children had heterozygous variants with autosomal dominance, while two had X-linked inheritance. As shown in Fig. 1B, there is a broad spectrum of implicated genes. We found most pathogenic variants in the SCN1A gene (n = 21/34; 62%). One variant was recurrent in two children, while the other variants found were non-recurrent and spread across the SCN1A gene (Fig. 2).
Table 2.
Characterization of causative genetic variants identified in the study (n = 33)
| Code No. | Gene | Exon/ Intron | Chromosomal location | Variant using Grch37 | Variant amino acid change | Variant Type | CADD Score | Inheritance | RS ID | ACMG Criteria for Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | SCN1A | Ex19 | chr2-166868646-C-T | NM_006920.6:c.3819G>A | p.Trp1273Ter | SNV Nonsense |
40 | Autosomal Dominant | rs1553531178 | PVS1, PM2, PP3, & PP5 |
| 3 | SCN1A | Ex16 | chr2-166893015-A-T | NM_006920.6:c.2939T>A | p.Leu980His | SNV Missense |
27.4 | Autosomal Dominant | Novel | PM1, PM2, PP2, & PP3 |
| 5 | SCN1A | Ex16 | chr2-166892660-G- | NM_006920.6:c.3294delC | p.Ser1099ValfsTer4 | Deletion Frameshift | 25.9 | Autosomal Dominant | Novel | PVS1, PM2, & PP3 |
| 8 | SCN1A | In5 | chr2-166908499-C-G | NM_006920.6:c.695-1G>C | 3′Splice site | SNV Splice site |
33 | Autosomal Dominant | Novel | PVS1, PM2, & PP3 |
| 11 | SCN1A | Ex26 | chr2-166848362-A-G | NM_006920.6:c.5390T>C | p.Phe1797Ser | SNV Missense |
29.6 | Autosomal Dominant | Novel | PM1, PM2, PM5, PP2, & PP3 |
| 13 | CDKL5 | In9 | chrX-18602478-G-A | NM_003159.2:c.554+5G>A | 5′ splice site | SNV Splice site |
26.2 | X-Linked Dominant | Reported (without RS ID) | PM2, PM4, &PP3 |
| 15 | SCN1A | Ex11 | chr2-166900385-G-A | NM_006920.6:c.1837C>T | p.Arg613Ter | SNV Nonsense |
35 | Autosomal Dominant | rs398123585 | PVS1, PP5, PM2, & PP3 |
| 16 | SCN1A | Ex26 | chr2-166848614-G-T | NM_006920.6:c.5138C>A | p.Ala1713Asp | SNV Missense |
27.4 | Autosomal Dominant | Novel | PM1, PM2, PM5, PP2, & PP3 |
| 17 | SCN1A | Ex13 | chr2-166897827-A-G | NM_006920.6:c.2296T>C | p.Cys766Arg | SNV Missense |
27.6 | Autosomal Dominant | Novel | PM1, PM2, PP2, & PP3 |
| 18 | SCN1A | In4 | chr2-166911147-C- | NM_006920.6:c.602+1del | 5′ splice site | Deletion Splice site | 33 | Autosomal Dominant | Novel | PVS1, PM2, & PP3 |
| 19 | SCN1A | Ex26 | chr2-166848246-GTTT- | NM_006920.6:c.5503_5506del | p.Lys1835SerfsTer11 | Deletion Frameshift |
34 | Autosomal Dominant | rs794726726 | PVS1, PP5, PM2, & PP3 |
| 20 | SCN1A | Ex16 | chr2-166892963-TTCATTA- | NM_006920.6:c.2985_2991del | p.Asp995GlufsTer2 | Deletion Frameshift |
33 | Autosomal Dominant | Novel | PVS1, PM2, & PP3 |
| 21 | SCN1A | Ex12 | chr2-166898844-G-A | NM_006920.6:c.2101C>T | p.Arg701Ter | SNV Nonsense |
36 | Autosomal Dominant | rs794726730 | PVS1, PP5, PM2, & PP3 |
| 22 | SCN1A | Ex26 | chr2-166848246-GTTT- | NM_006920.6:c.5503_5506del | p.Lys1835SerfsTer11 | Deletion Frameshift |
34 | Autosomal Dominant | rs794726726 | PVS1, PP5, PM2, & PP3 |
| 24 | SCN1A | Ex15 | chr2-166894450-G-A | NM_006920.6:c.2749C>T | p.Gln917Ter | SNV Nonsense |
29.8 | Autosomal Dominant | rs796052985 | PVS1, PM2, & PP5 |
| 26 | SCN1A | Ex15 | chr2-166894438-A-G | NM_006920.6:c.2761T>C | p.Trp921Arg | SNV Missense |
28.4 | Autosomal Dominant | Reported (without RS ID) | PM1, PM2, PM5, PP2 & PP3 |
| 27 | KCNQ2 | Ex7 | chr20-62070003-C-T | NM_172107.4:c.998G>A | p.Arg333Gln | SNV Missense |
28 | Autosomal Dominant | rs118192216 | PP5, PM1, PM2, PM5, PP2, & PP3 |
| 28 | SCN8A | Ex27 | chr12-52201011-G-C | NM_014191.4:c.5741G>C | p.Gly1914Ala | SNV Missense |
22.4 | Autosomal Dominant | Novel | PS2, & PM2 |
| 32 | SCN1A | Ex1 | chr2-166929882-A-G | NM_006920.6:c.250T>C | p.Tyr84His | SNV Missense |
27.7 | Autosomal Dominant | Novel | PM1, PM2, PM5, PP2 & PP3 |
| 34 | DEPDC5 | Ex5 | chr22-32162622-C-T | NM_001242896.3:c.331C>T | p.Arg111Cys | SNV Missense |
29.4 | Autosomal Dominant | rs866638369 | PS2, PM2, & PP3 |
| 36 | NPRL3 | Ex10 | chr16-142715-G-A | NM_001077350.3:c.1040C>T | p.Pro347Leu | SNV Missense |
22.7 | Autosomal Dominant | rs755232774 | PS2, PM2, & PP3 |
| 38 | PCDH19 | Ex1 | chrX-99661953-G-A | NM_001184880.2:c.1643C>T | p.Thr548Met | SNV Missense |
26.6 | X-linked | Novel | PM1, PM2, PP2, & PP3 |
| 39 | SCN1A | Ex26 | chr2-166848119-A-C | NM_006920.6:c.5633T>G | p.Met1878Arg | SNV Missense |
27.3 | Autosomal Dominant | Reported (without RS ID) | PM2, PM6, PP2, & PP3 |
| 41 | RHOBTB2 | Ex7 | chr8-22864480-C-G | ENST00000519685.1:c.788C>G | p.Pro263Arg | SNV Missense |
25.9 | Autosomal Dominant | Novel | PM2, PM6, & PP3 |
| 43 | SCN1A | Ex9 | chr2-166903479-C-T | NM_006920.6:c.1178G>A | p.Arg393His | SNV Missense |
31 | Autosomal Dominant | rs121917927 | PP5, PM1, PM2, PP2, & PP3 |
| 45 | KCNA2 | Ex3 | chr1-111147170-C-T | NM_004974.4:c.235G>A | p.Asp79Asn | SNV Missense |
29.1 | Autosomal Dominant | rs747844549 | PM2, PM6, & PP3 |
| 46 | GNAO1 | Ex8 | chr16-56377732-A-G | NM_138736.3:c.935A>G | p.Asn312Ser | SNV Missense |
25.4 | Autosomal Dominant | rs758503575 | PM1, PM6, PP2, PP3, & PP4 (VUS with pathogenic evidence) |
| 47 | SCN1A | Ex26 | chr2-166848437-G-A | NM_006920.6:c.5315C>T | p.Ala1772Val | SNV Missense |
27.8 | Autosomal Dominant | rs121917921 | PP5, PM1, PM2, PM5, PP2, & PP3 |
| 48 | SCN8A | Ex25 | chr12-52184185-G-A | NM_014191.4:c.4423G>A | p.Gly1475Arg | SNV Missense |
26.3 | Autosomal Dominant | rs796053216 | PP5, PS1, PM1, PM2, PP2, & PP3 |
| 50 | SCN1A | Ex24 | chr2-166852550-TT- | NM_006920.6:c.4520_4521del | p.Lys1507ThrfsTer18 | Deletion Frameshift | 33 | Autosomal Dominant | Novel | PVS1, PM2, & PP3 |
| 51 | SLC2A1 | Ex4 | chr1-43396356-G-A | NM_006516.4:c.457C>T | p.Arg153Cys | SNV Missense |
28.4 | Autosomal Dominant | Reported (without RS ID) | PM1, PM2, PM5, PP5, PP2, & PP3 |
| 53 | SCN1A | In4 | chr2-166911146--A | NM_001353960.2:c.602+2dup | 5′ splice site | Insertion Splice site |
24.5 | Autosomal Dominant | rs796053054 | PM2, PM6, & PP3 |
| 54 | KCNT1 | Ex3 | chr9-138641945-G-A | NM_020822.3:c.256G>A | p.Val86Ile | SNV Missense |
26.3 | Autosomal Dominant | rs1172819191 | PM2, PM6, & PP3 |
CADD = Combined Annotation Dependent Depletion, Ex = exon, In = intron, SNV = Single Nucleotide Variant.
Note: Population ethnicity was preferred to be South Asian for allele frequency calculations; Pathogenicity of variant might evolve with new evidence such as change in allele frequency.
Fig. 2.
Location of SCN1A gene variants in our study. Pathogenic variants are missense, nonsense, splice-site, and frameshift deletion, which are spread across the gene.
A variant of uncertain significance was found in one child (code 34) in the CPA6 gene. Another child (code 43) seemingly had a pathogenic variant in the SCN9A gene by multiple computational evidence; however, the neuropathic pain phenotype was absent in the child, so we considered it a variant of uncertain significance. A child (code 15) with Doose syndrome had a pathogenic variant in ADGRV1. However, the gene-disease phenotype did not match the ClinGen criteria and was considered unrelated to the syndrome. We found single nonsense pathogenic variants in three genes, TBC1D24 (code 10), NDUFV2 (code 13), and PNPO (code 45), with autosomal recessive inheritance, lacking a second variant.
We performed MLPA for the SCN1A gene in 21 children with no pathogenic variant yield by NGS testing. However, it did not yield any copy number variation of the SCN1A gene.
Genotype-phenotype association
Of 14 children recruited after the first febrile vaccination seizure, 4 (29%) had pathogenic variants. In contrast, four children (57%) had pathogenic variants among seven children enrolled after 2-5 febrile seizures. The genetic yield was 79% (23/29) with epilepsy diagnoses, whereas it was 40% (10/25) of children with the diagnosis of either simple or complex febrile seizures or febrile seizure plus. Of 27 children with drug-resistant epilepsy, 23 (85%) had pathogenic variants (Supplementary Fig. S1).
The genetic yield as per diagnoses was 91% (21/23) in Dravet syndrome, 36% (5/14) in complex febrile seizure, 43% (3/7) in simple febrile seizure, 50% (2/4) in febrile seizure plus, and 67% (2/3) in West syndrome. None of the children with self-limiting myoclonic epilepsy in infancy (n = 1), Doose syndrome (n = 1), and intractable childhood epilepsy with generalized tonic-clonic (n = 1) had a pathogenic variant (Fig. 1A).
Of 23 children with Dravet syndrome, 19 (79%) had pathogenic variants of the SCN1A gene, while two (8%) had SCN8A variants (Fig. 1B and Table 2). Of 14 children with complex febrile seizures, six had pathogenic variants, and of these, SCN1A was pathogenic in two (14%), while other variants were related to KCNT1, KCNQ2, and KCNA2 genes (Fig. 1B and Table 2).
Predictors of genetic diagnosis
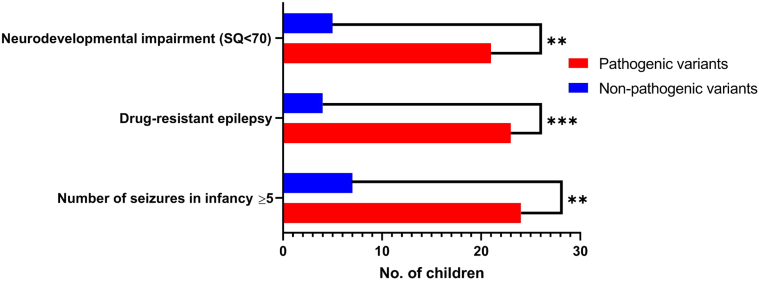
Pathogenic variants were more frequent in children with five or more seizures in infancy (24/31, 77%) than those with four or fewer seizures (9/23, 39%), (OR = 5.3, 95% CI: 1.6–18.4, p = 0.006). Furthermore, the genetic yield was higher (23/27, 85%) in children with drug-resistant epilepsy than in pharmaco-responsive (10/27, 37%), and the difference was statistically significant (OR = 9.8, 95% CI: 2.6–30.7, p = 0.001) (Fig. 3). However, the age at vaccination-associated seizure and characteristics of vaccination-proximate seizures were not associated with the genetic diagnosis (Table 3).
Fig. 3.
Association of genetic diagnosis and phenotypic characteristics. Neurodevelopmental impairment (OR = 5.6, 95% CI: 1.65–17.6, p = 0.006); Drug-resistant epilepsy (OR = 9.8, 95% CI: 2.6–30.7, p = 0.001); No. of seizures in infancy ≥5 (OR = 5.3, CI: 1.6–18.4, p = 0.006 are seen significantly associated with genetic pathogenicity, based on Fisher's exact test.
Table 3.
Association of phenotypic characteristics with genetic diagnosis
| Characteristics | Children with genetic diagnosis (n = 33) | Children without genetic diagnosis (n = 21) | P-value | Odds ratio, 95% CI |
|---|---|---|---|---|
| Median age at vaccine-seizure in months (Q1,Q3) | 3.5 (2.75, 4.5) | 4 (3.25, 18) | 0.07 | ·· |
| Characteristics of Vaccine-proximate seizures | ||||
| • Focal onseta | 9 | 5 | >0.99 | 1.2, 95% CI: 0.4–4.1 |
| • Clustering | 10 | 12 | 0.09 | 0.3, 95% CI: 0.1–1.0 |
| • Seizure duration ≥15 min | 11 | 5 | 0.55 | 1.6, 95% CI: 0.4–5.1 |
| ≥5 seizures in infancyb | 24 | 7 | 0.006 | 5.3, 95% CI: 1.6–18.4 |
| Drug-resistance epilepsy | 23 | 4 | 0.001 | 9.8, 95% CI: 2.6–30.7 |
| Neurodevelopment impairment (SQ < 70) | 21 | 5 | 0.006 | 5.6, 95% CI: 1.65–17.6 |
Three children with unknown onset of epileptic spasms (two with genetic diagnosis and one without genetic diagnosis) were excluded from the analysis.
Based on total number of seizures during one year of follow-up for children with febrile seizures.
Follow-up
Children were routinely followed-up, and we updated the phenotypic data at one-year follow-up. During follow-up, one child with KCNQ2-associated complex febrile seizure and another with PCDH19-associated simple febrile seizure evolved into the phenotype of febrile seizure plus. Additionally, one child with KCNT1-associated complex febrile seizure evolved into the phenotype of early infantile epileptic encephalopathy, and another with SCN1A variant evolved into Dravet syndrome. The child with febrile seizure plus with underlying SLC2A1 variant was reclassified as Glut1DS. The final phenotype was as following: complex febrile seizure (n = 11; 20%), simple febrile seizure (n = 6; 11%), FS plus (n = 5; 9%), Dravet syndrome (n = 24; 44%), West syndrome (n = 3; 5.5%), self-limited myoclonic epilepsy in infancy (n = 1; 2%) and Doose syndrome (n = 1; 2%), intractable childhood epilepsy with generalized tonic-clonic (n = 1; 2%), KCNT1 associated early infantile epileptic encephalopathy (n = 1; 2%) and Glut1DS (n = 1; 2%).
We categorized the children according to SQ score into two groups ─ SQ <70 (neurodevelopmental impairment) and SQ ≥70 (normal intellect). Twenty-six children had neurodevelopmental impairment in the follow-up period of one year. Genetic diagnosis was higher in children with neurodevelopmental impairment (SQ < 70) than children without neurodevelopmental impairment (81% vs. 43%, OR = 5.6, 95% CI: 1.65–17.6, p = 0.006) (Fig. 3). The median SQ in children with pathogenic variants was 45 (IQR 32–97), while it was 94 (IQR 70–109) in children without pathogenic variants. Children with focal-onset, vaccine-proximate seizures were significantly associated with neurodevelopmental impairment than those with generalized-onset seizures (71% vs. 35%, OR = 4.6, CI 95% 1.3–15.0, p = 0.0286).
During follow-up, two children died. One child with KCNT1-associated early infantile epileptic encephalopathy died due to status epilepticus, and another child with CDLK5-associated West syndrome due to sudden unexplained death in epilepsy.
Discussion
In our study, 54 children tested with next-generation sequencing had a high yield of pathogenic genetic variants. We highlighted the genetic heterogeneity with 12 genes and 13 novel implicated genetic variants. SCN1A genetic variation was the most common explanation for vaccine-associated seizures. Another sodium channel gene, SCN8A, and potassium channel genes, KCNA2, KCNT1, and KCNQ2, were also affected, resulting in 26 out of 33 variants from ion channels, suggesting vaccine-associated seizures are predominantly due to channelopathies. Moreover, the study points out that nearly one-third (29%, 4/14) of cases with the first vaccine-proximate febrile seizure can have genetic diagnoses and further escalated yield up to 57% (4/7) in children with two to five febrile seizures.
In the study by Damiano et al, the genetic yield after the first vaccine-proximate seizure was low (2.8%, 2/69) compared with the 29% yield in our study cohort of first vaccine-proximate febrile seizure (n = 14). It could be due to the isolated use of Sanger sequencing of the SCN1A gene in their study. It suggests that next-generation sequencing for epilepsy-associated genes should be a better testing modality than Sanger sequencing for the SCN1A gene alone in such cases.
Among 29 children with epilepsy at recruitment, 27 had drug-resistant epilepsy with pathogenic variants in 85% (23/27) of children. In concurrence with published studies, SCNIA-associated Dravet syndrome was recognized as the most common epilepsy syndrome.6,26 In a cohort of 14 cases of epileptic encephalopathy after DTP vaccination, Berkovic et al6 demonstrated that 11 (79%) had SCN1A gene pathogenic variations. Similarly, in the study by Verbeek et al26, of nine children with drug-resistant epilepsy after wP, eight (89%) had genetic diagnoses (SCN1A-7, PCDH19-1). Our study is peculiar to demonstrate genetic heterogeneity (SCN1A-19, SCN8A-2, GNAO1-1, CDKL5-1) (Fig. 1B). A mice animal model study (SCN1A+/−) has shown that elevated body temperature spontaneously induces seizures.27 McIntosh et al28 studied patients with SCN1A positive Dravet syndrome and observed that vaccination might trigger the earlier onset of symptoms in such children who were destined to develop seizures. In our study, two children with Dravet syndrome phenotype also had SCN8A pathogenic variants.
Interestingly, our cohort included three children with West syndrome, characterized by clustered epileptic spasms and hypsarrhythmia in electroencephalogram, with the onset of epileptic spasms coinciding temporarily with DTwP vaccination. Two of them had genetic diagnoses. One girl child with West syndrome had CDKL5 splice site pathogenic variant (code 13), which explained the severe phenotype. Eventually, the child expired on follow-up at 11 months of age due to sudden unexpected death in epilepsy. Another girl child with West syndrome had GNAO1 variant of uncertain significance having pathogenic evidence, which affects guanine nucleotide-binding proteins (G proteins) coupled signal transduction. The association of wP vaccination and subsequent West syndrome has been a concern since 1983.29,30 These findings might explain the significant role of genetics in such cases.
We identified a unique case of Glut1DS presented as a ‘febrile seizure plus,’ with the first seizure triggered by the wP vaccine at 3.5 months of age. The diagnosis was made after the genetic test with the detection of the SLC2A1 pathogenic variant. Early diagnosis is crucial because of therapeutic implications, as the ketogenic diet is a preferred choice.
Focal-onset characteristic of the first vaccine-proximate seizure was associated with neurodevelopmental impairment in follow-up. It highlights the importance of semiological classification for distinguishing febrile seizures into simple versus complex for prognostic significance. However, none of the characteristics of vaccine-proximate seizures (focal-onset, clustering, or seizure duration) was significant in predicting genetic diagnosis. It could be due to the small sample size and inadequately powered for these comparisons.
A lower incidence of vaccination-associated seizures with acellular pertussis than wP (3.6 per million vs. 13.4 per million, respectively) is a fundamental reason to adopt acellular pertussis in developed countries.10 Ours and Verbeek's study assessed the relation of wP with vaccine-proximate seizures or subsequent epilepsy, while Reyes's and Damiano's studies were on acellular-pertussis-associated seizures. Data from these studies and ours suggest that vaccination was just a chance trigger for seizures in children with underlying genetic variations. The cross-talk between genetics and immunological response is possibly independent of the nature of the pertussis vaccine. These findings are essential for policymakers, and wP vaccine-associated seizures should not be considered the reason for adopting acellular pertussis vaccination, especially being less immunogenic and costly than wP.
Our findings suggest that the clinical follow-up of children with vaccine-associated seizures is necessary, with emphasis on developmental surveillance and the occurrence of epilepsy. These three phenotypic markers strongly enhance the odds of genetic diagnosis by NGS. Our study demonstrated that only testing the SCN1A gene may also give a high yield of 39% (21/54), and Sanger sequencing of SCN1A might be cost-effective. However, MLPA for the SCN1A gene was non-yielding and could be avoided. These findings should be helpful to triage resources in low-resource settings. However, the NGS panel run for epilepsy genes should be preferred routinely after all vaccination-associated seizures to resolve diagnostic uncertainty and find genetic etiology. It is also crucial in the therapeutic decision of some monogenetic conditions by precision treatment approaches, which might impact future prognosis.31 Potential precision therapies are possible for various monogenetic etiologies diagnosed in our studies, such as the use of stiripentol with cannabidiol in SCN1A, ketogenic diet in SLC2A1, sodium channel blockers in SCN8A, and KCNQ2, everolimus in DEPDC5, and NPRL3, quinidine in KCNT1, and 4-Aminopyridine for KCNA2.31
Our study had a few inadvertent limitations. First, the number of enrolled children with first febrile seizure was limited during the study period due to the condition's rarity. Second, due to cost considerations, we have not enrolled a control group of vaccinated children without seizures. To overcome this limitation, we used Indian population databases SAGE (South Asian Genomes & Exomes)21 and INDEX-db (INDian EXome database)22 to assess minor allele frequency in a healthy population. Lastly, we have not examined the health economics of genetic testing in children with DTP seizures.
To conclude, our study proves that the genetic variations in the epilepsy genes are the fundamental cause of vaccine-associated seizures and subsequent epilepsy. Our data suggest that genetic testing, preferably next-generation sequencing, should be offered routinely in children with vaccine-proximate seizures due to its diagnostic, therapeutic, and prognostic implications. Clinicians should offer SCN1A Sanger sequencing at least to all such children. In countries with resource-limited settings, children with vaccine-proximate seizures may be followed up clinically with developmental surveillance and seizure recurrences. Subsequently, clinicians may prioritize genetic testing for children who evolved into epilepsy or show developmental impairment on follow-up. Vaccine-proximate seizures should not be a barrier to immunization and a reason to adopt policy for acellular-pertussis vaccination. Future studies should examine the health economics of early genetic testing in children with vaccine-associated seizures.
Contributors
S.N.: study design, drafting the work, data collection, data analysis, data interpretation, manuscript writing and approval of the manuscript; P.B. & A.K.: study design, data analysis, data interpretation, drafting the work and approval of the manuscript; P.M.: study design, data collection, data interpretation, revising work critically and approval of the manuscript; G.S.: data collection, data interpretation, revising work critically and approval of the manuscript; A.A., N.S. & P.S.K.: study design, data interpretation, revising work critically and approval of manuscript, J.K.S.: conceptualization of study and design, drafting the work, data collection, data interpretation, manuscript writing and approval of the manuscript.
Data sharing statement
All important data generated or analyzed during this study are included in this published article or uploaded as supplementary information.
Declaration of interests
Nothing to report.
Acknowledgment
We sincerely acknowledge the Indian Council of Medical Research (ICMR), New Delhi, India and the International Pediatric Association Foundation, Inc. (IPAF) for funding the study. We also acknowledge the families and children who participated.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2022.100094.
Appendix A. Supplementary data
References
- 1.Madsen T. Vaccination against whooping cough. J Am Med Assoc. 1933;101:187–188. [Google Scholar]
- 2.Barlow W.E., Davis R.L., Glasser J.W., et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345:656–661. doi: 10.1056/NEJMoa003077. [DOI] [PubMed] [Google Scholar]
- 3.Verbeek N.E., van der Maas N.A.T.T., Jansen F.E., van Kempen M.J.A.A., Lindhout D., Brilstra E.H. Prevalence of SCN1A-related Dravet syndrome among children reported with seizures following vaccination: a population-based ten-year cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marini C., Scheffer I.E., Nabbout R., et al. The genetics of Dravet syndrome. Epilepsia. 2011;52:24–29. doi: 10.1111/j.1528-1167.2011.02997.x. [DOI] [PubMed] [Google Scholar]
- 5.Ceulemans B.P.G., Claes L.R., Lagae L.G. Clinical correlations of mutations in the SCN1A gene: from febrile seizures to severe myoclonic epilepsy in infancy. Pediatr Neurol. 2004;30:236–243. doi: 10.1016/j.pediatrneurol.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Berkovic S.F., Harkin L., McMahon J.M., et al. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5:488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- 7.Reyes I.S., Hsieh D.T., Laux L.C., Wilfong A.A. Alleged cases of vaccine encephalopathy rediagnosed years later as Dravet syndrome. Pediatrics. 2011;128:e699–e702. doi: 10.1542/peds.2010-0887. [DOI] [PubMed] [Google Scholar]
- 8.Damiano J.A., Deng L., Li W., et al. SCN1A Variants in vaccine-related febrile seizures: a prospective study. Ann Neurol. 2020;87:281–288. doi: 10.1002/ana.25650. [DOI] [PubMed] [Google Scholar]
- 9.Phadke V.K., Bednarczyk R.A., Salmon D.A., Omer S.B. Association between vaccine refusal and vaccine-preventable diseases in the United States a review of measles and pertussis. JAMA. 2016;315:1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geier D.A., Geier M.R. An evaluation of serious neurological disorders following immunization: a comparison of whole-cell pertussis and acellular pertussis vaccines. Brain Dev. 2004;26:296–300. doi: 10.1016/S0387-7604(03)00169-4. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly S., Loscher C.E., Lynch M.A., Mills K.H.G. Whole-cell but not acellular pertussis vaccines induce convulsive activity in mice: evidence of a role for toxin-induced interleukin-1β in a new murine model for analysis of neuronal side effects of vaccination. Infect Immun. 2001;69:4217–4223. doi: 10.1128/IAI.69.7.4217-4223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheridan S.L., Ware R.S., Grimwood K., Lambert S.B. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308(5):454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 13.WHO Pertussis vaccines: WHO position paper, August 2015-recommendations. Vaccine. 2016;34:1423–1425. doi: 10.1016/j.vaccine.2015.10.136. [DOI] [PubMed] [Google Scholar]
- 14.Fisher R.S., Cross J.H., French J.A., et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 15.Scheffer I.E., Berkovic S., Capovilla G., et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan P., Arzimanoglou A., Berg A.T., et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 17.Malin A.J., Raj J.B. Swayamsiddha Prakashana; Mysore, India: 1992. Vineland social maturity scale and manual, Indian adaptation. [Google Scholar]
- 18.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard Tools - By Broad Institute. http://broadinstitute.github.io/picard/
- 20.McKenna A., Hanna M., Banks E., et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hariprakash J.M., Vellarikkal S.K., Verma A., et al. SAGE: a comprehensive resource of genetic variants integrating South Asian whole genomes and exomes. Database (Oxford) 2018;2018:1–10. doi: 10.1093/database/bay080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husayn Ahmed P., Vidhya V., More R.P., et al. INDEX-db: the Indian exome reference database (phase I) J Comput Biol. 2019;26:225–234. doi: 10.1089/cmb.2018.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kircher M., Witten D.M., Jain P., O'Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopanos C., Tsiolkas V., Kouris A., et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeek N.E., Jansen F.E., Vermeer-de Bondt P.E., et al. Etiologies for seizures around the time of vaccination. Pediatrics. 2014;134:658–666. doi: 10.1542/peds.2014-0690. [DOI] [PubMed] [Google Scholar]
- 27.Oakley J.C., Kalume F., Yu F.H., Scheuer T., Catterall W.A. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntosh A.M., McMahon J., Dibbens L.M., et al. Effects of vaccination on onset and outcome of Dravet syndrome: a retrospective study. Lancet Neurol. 2010;9:592–598. doi: 10.1016/S1474-4422(10)70107-1. [DOI] [PubMed] [Google Scholar]
- 29.Bellman M.H., Ross E.M., Miller D.L., Meade T.W. Pertussis vaccine and infantile spasms. Lancet. 1983;322:278–279. [Google Scholar]
- 30.Lapatsanis P., Lapatsanis D. Pertussis vaccine and infantile spasms. Vaccine. 2007;25:404–405. doi: 10.1016/j.vaccine.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Kearney H., Byrne S., Cavalleri G.L., Delanty N. Tackling epilepsy with high-definition precision medicine: a review. JAMA Neurol. 2019;76:1109–1116. doi: 10.1001/jamaneurol.2019.2384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.