Abstract
Context
Prior studies of the relationship between physical activity and incident type 2 diabetes mellitus (T2DM) relied primarily on questionnaires at a single time point.
Objective
We sought to investigate the relationship between physical activity and incident T2DM with an innovative approach using data from commercial wearable devices linked to electronic health records in a real-world population.
Methods
Using All of Us participants’ accelerometer data from their personal Fitbit devices, we used a time-varying Cox proportional hazards models with repeated measures of physical activity for the outcome of incident T2DM. We evaluated for effect modification with age, sex, body mass index (BMI), and sedentary time using multiplicative interaction terms.
Results
From 5677 participants in the All of Us Research Program (median age 51 years; 74% female; 89% White), there were 97 (2%) cases of incident T2DM over a median follow-up period of 3.8 years between 2010 to 2021. In models adjusted for age, sex, and race, the hazard of incident diabetes was reduced by 44% (95% CI, 15%-63%; P = 0.01) when comparing those with an average daily step count of 10 700 to those with 6000. Similar benefits were seen comparing groups based on average duration of various intensities of activity (eg, lightly active, fairly active, very active). There was no evidence for effect modification by age, sex, BMI, or sedentary time.
Conclusion
Greater time in any type of physical activity intensity was associated with lower risk of T2DM irrespective of age, sex, BMI, or sedentary time.
Keywords: activity monitoring, diabetes, physical activity, risk factors
Physical activity (PA) offers a variety of acute and sustained benefits on cardiometabolic health. A wealth of observational epidemiology has supported an association of PA (including intensity and frequency) with increased metabolic resilience, including protection from obesity, cardiovascular disease (1), and diabetes (2–4), and enhanced recovery from illness (5). Moreover, increased PA is also associated with greater cardiorespiratory fitness (2), itself a powerful risk factor for mortality (6). While these observations have codified PA in contemporary prevention guidelines across diabetes (7) and cardiovascular disease (8), the observational design of most investigations supporting PA recommendations have left several important questions unanswered. In most studies, PA has been assessed either via questionnaire (9, 10) or via single-point, time-limited measures of accelerometry (11, 12), missing opportunities to examine repeated, longitudinal measures continuously in a given individual with long-term outcome disease risk. This study design also introduces biases in determining effect of different forms of PA within an individual, given the insensitivity of a single measure of PA and its propensity to change over time in a given individual (13). Integrating quantitative, continuous measures of PA in large, contemporary observational studies may directly address these limitations.
In 2018, the National Institutes of Health inaugurated the All of Us Research Program, a national effort to collect, integrate, and report phenotypic, clinical, and genomic data from >1 million Americans underrepresented in biomedical research with a goal of advancing precision health (14). The All of Us program leverages the electronic health record to capture an individual's contact with the medical system, in order to expand the array of clinical characterization for phenotypic study. Recently, the All of Us program has integrated wearable data on PA to characterize the personalized, longitudinal effects of amount, type, and sustainability of PA on medical health. In this study, we examine continuous PA data from 5677 participants free from diabetes in the All of Us effort collected between 2010 and 2021 to address the impact of longitudinal PA and pattern over approximately 4 years on diabetes risk and whether associations of PA with diabetes risk are different by age, sex, or obesity status. We investigated the range of physical activity from sedentary time (15) through various intensities of activity (lightly active, fairly active, very active). Our ultimate goal was to leverage this highly unique longitudinal dataset, containing a large number of repeated measures of PA, to examine the association of repeated measures of PA with diabetes risk in contemporary practice.
Methods
All of Us Study Participants
The study population comes from the All of Us Research Program, a program funded by the National Institutes of Health, from >340 participating sites across the United States (as previously described (14)). All of Us strives to collect detailed phenotypic data (including questionnaires, electronic health record data, physical measurements, and biospecimens) from a diverse, representative population across the United States. For this analysis, we included participants who voluntarily provided accelerometry data from their personal Fitbit device. Of note, accelerometer use was optional in All of Us; currently only participants who own a Fitbit device could share their data under the “Bring Your Own Device Program.” For this study, we used registered tier data (R2022Q2R2), which was available on the on the All of Us Researcher Workbench.
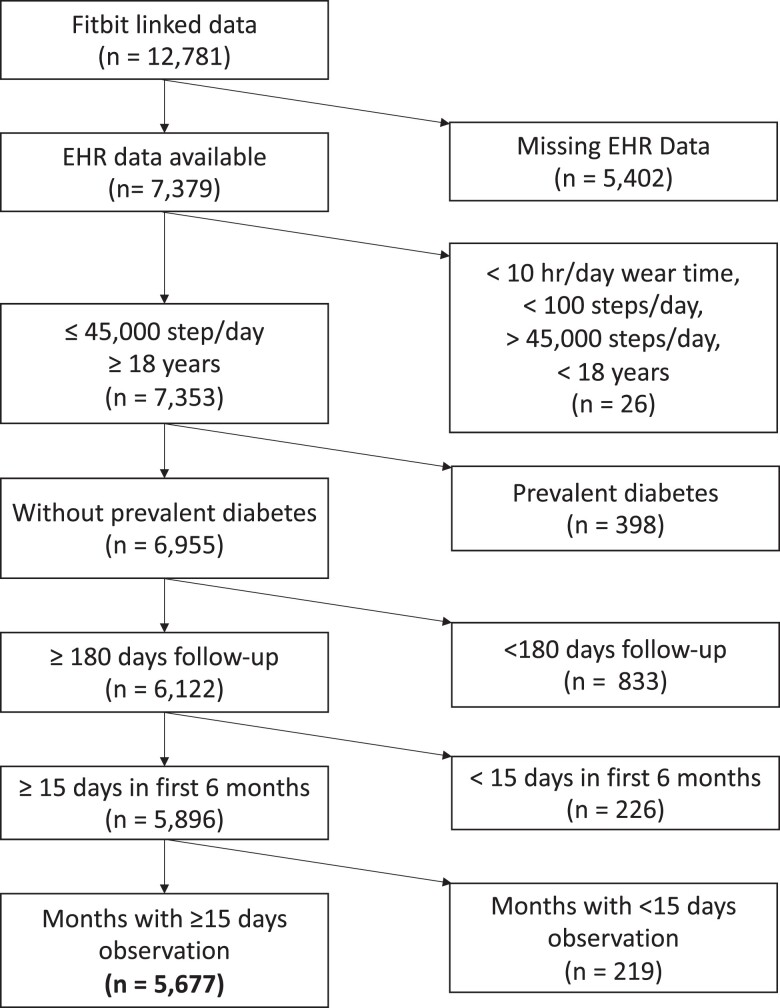
Of the 362 674 participants’ data available on the All of Us Researcher Workbench, 12 781 participants provided Fitbit data linked with electronic health records. Using the registered tier dataset, we excluded participants (1) younger than 18 years, (2) those who did not have linked electronic health record data, and (3) those with prevalent diabetes (definition below). In addition, to account for changes in PA during the initial monitoring period, we also excluded those participants with < 15 days of activity monitoring in the initial 180 days of monitoring and with ≤ 180 days of follow-up (Fig. 1). Participants were also excluded if they were diagnosed with diabetes within the initial 180-day monitoring period. Daily step counts were estimated using data from days during which a participant wore the device for ≥ 10 hours and had between 100 and 45 000 steps. An hour of wear time was defined as any hour in which step data was recorded. Months with fewer than 15 days of monitoring were excluded.
Figure 1.
Inclusion flow diagram. Exclusion factors were a combination of patient level and Fitbit level factors. Exclusions of Fitbit data often resulted in exclusion of participants, such as removing days with less than 10 hours of wear time resulted in the exclusion of 24 individuals. Participants need to have one month of Fitbit data available in the first 6 months of observation and another month afterwards to be included. Abbreviation: EHR, electronic health record.
Age, sex, and race were obtained from participant surveys. Body mass index (BMI) was both provided by linked health records and calculated from measured height and weight if BMI was not reported. Baseline BMI was defined as the BMI measurement reported closest to when activity monitoring began so long as this occurred within 180 days. Notably, there was a high degree of missingness of baseline BMI (34%), with minimal missingness in sex (4%) and race (7%). Given our desire to include all data (and the inclusion of BMI adjustment as a sensitivity analysis), we imputed data for sex, race, and BMI with multiple imputation using bootstrap and predictive mean matching with 5 imputations using “aregImpute” from the Hmisc package in R (16). History of smoking was ascertained through participant questionnaire and defined as smoking ≥ 100 cigarettes in their lifetime.
Assessment of Type 2 Diabetes Status
Type 2 diabetes was defined according to a Northwestern University algorithm for extracting status from electronic health records using diagnosis codes, medication prescriptions, serum glucose, and hemoglobin A1c values (17). Prevalent diabetes was defined as being present within 180 days of initial activity monitoring. Incident diabetes was assessed throughout the study period (2010-2021).
Measures of Physical Activity by Fitbit
PA was quantified with the use of participants’ personal Fitbit device. This device counts “steps” and uses a proprietary algorithm to quantify “sedentary minutes,” “lightly active minutes,” “fairly active minutes,” and “very active minutes.” Fitbit data agree well with “research-grade” accelerometry measures (18–20). Comparing Fitbit defined activity level to metabolic equivalent task (MET) indicated “lightly active” minutes correspond to 1.5 to 3 METs, “fairly active” minutes correspond to 3 to 6 METs, and “very active” minutes correspond to ≥ 6 METs (21). A strength of our study was the availability and use of longitudinal PA data over time in a real-world setting: PA measures were summarized on a daily basis and averaged on a monthly basis. Therefore, each participant had an average value for daily steps, sedentary minutes, lightly, fairly and very active minutes for each month with valid Fitbit data (with valid months defined as those with > 15 days of monitoring data available). These measures were summarized by age, sex, race, and BMI status.
Statistical Analysis
Our first aim was to quantify how longitudinal measures of PA were associated with incident diabetes, including potential modification by age, sex, or BMI. In order to reduce the risk of reverse causation between physical activity and incident diabetes, participants with events that occurred within 180 days of starting activity monitoring were excluded from analysis. We estimated time-varying Cox proportional hazards models for the association of incident diabetes with measures of PA (mean steps, sedentary time, and light, fairly active, and very active minutes), adjusted for age, sex, and race. We additionally adjusted for BMI and smoking in separate sensitivity analyses. Of note, we included adjustment for the mean of each PA measure during the initial 180 days of monitoring to account for initial potential increases in PA with each wearable (eg, the model evaluating daily steps includes adjustment for the mean daily steps in the first 180 days of monitoring) (22, 23). A key aspect of our model is that measures of PA were treated as a time-varying covariates (updated for each month of observation), thereby accounting for individual-level variability in PA exposure over time. We tested for effect modification with age, sex, and BMI using multiplicative interaction terms. Of note, given the significant imbalance by race in our All of Us subsample, we do not report effect modification by race. All continuous variables were initially modeled as restricted cubic spline with 3 knots. Chunk tests for nonlinearity were performed and terms not meeting significance at the 0.05 level were removed. We found evidence for nonlinearity for time-updated lightly active minutes and fairly active minutes (all model variants). For BMI interaction models, we found evidence for nonlinearity in fairly active minutes. For age and sex interaction models, nonlinearity was found in lightly and fairly active minutes. Finally, to address the question of whether effects of PA would be different based on the degree of sedentary time, we additionally generated Cox models for each measure of PA including a multiplicative interaction between the PA measure and mean sedentary time (treating both PA and sedentary time as time-varying covariates). Hazard ratios were calculated for an increase of interquartile range using the 25th percentile of the PA measure as the reference. All analyses were performed in R (version 4.2.0, R Project https://www.r-project.org). The Institutional Review Board at Vanderbilt University Medical Center approved this study.
Results
Characteristics of All of Us Study Participants
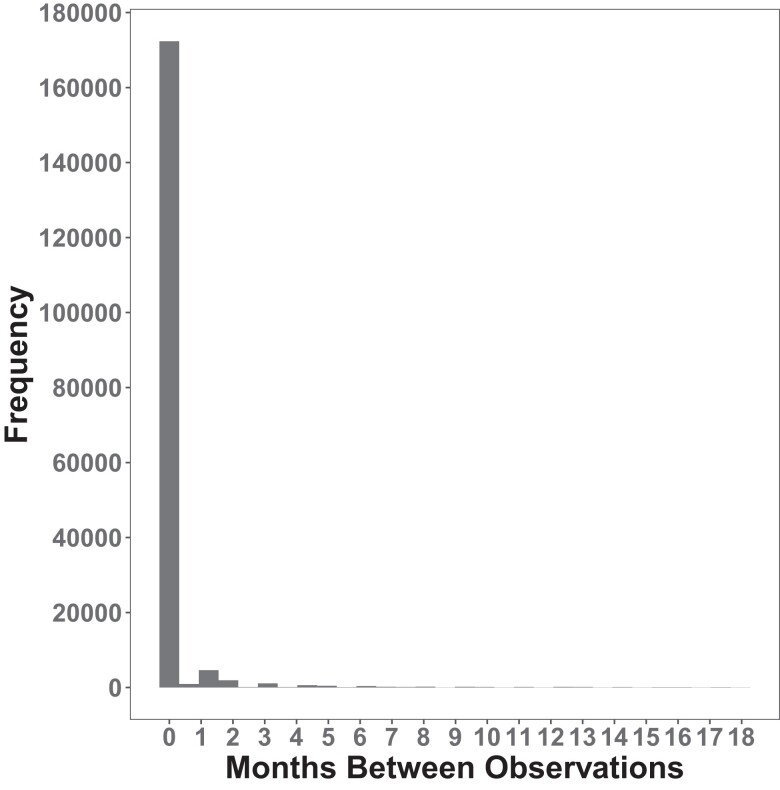
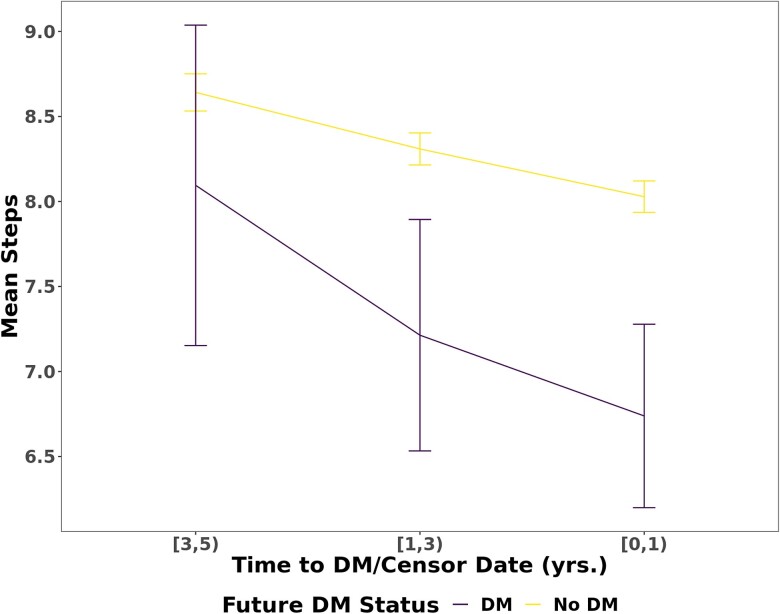
Of 12 781 participants with linked Fitbit data, 5677 individuals were included in our sample (Fig. 1). The median age was 50.9 years. The cohort was predominantly female (74%), White (89%), college graduates (74%), and overweight (median BMI 27.8 kg/m2, Table 1). Of 2457 participants who had a fasting glucose or hemoglobin A1c measured within 180 days of initial activity monitoring, we identified 454 (18%) participants who had prediabetes (fasting glucose 100-125 mg/dL or hemoglobin A1c 5.7%-6.4%). Unique to this study is the high number of repeated assessments of PA longitudinally. While we excluded months with fewer than 15 days of monitoring (15.1% of total months), participants in our study had a median 27 months of Fitbit observation (25th-75th percentile, 12-49 months), which is a substantially greater length of monitoring compared to other studies (11, 24). While our study design allowed for breaks in monitoring, it was rare to have a gap in monitoring (Fig. 2). The median daily step count, across all monthly averages of monitoring, was 7924 (25th-75th percentile, 5997-10 019) with trends for decreasing steps throughout the study period (Fig. 3). We observed a median of 15.96 minutes of fairly active minutes per day (25th-75th percentile, 9.09-26.26) and a median of 16.06 minutes of very active minutes per day (25th-75th percentile, 7.56-28.88).
Table 1.
Baseline characteristics of study population
| Variable | N | Ntotal = 5677 |
|---|---|---|
| Age (years) | 5677 | 50.9 (36.0-61.9) |
| Female | 5470 | 0.74 4028⁄5470 |
| Race | 5266 | |
| ȃBlack | 0.05 269⁄5266 | |
| ȃOther | 0.06 292⁄5266 | |
| ȃWhite | 0.89 4705⁄5266 | |
| Highest education achieved | 5471 | |
| ȃCollege graduate or advanced degree | 0.74 4033⁄5471 | |
| ȃ1-3 years of college | 0.21 1150⁄5471 | |
| ȃHigh school diploma, GED, or less | 0.05 288⁄5471 | |
| Baseline body mass index (kg/m2) | 3726 | 27.8 (24.3-32.1) |
| History of smoking | 5584 | 0.32 1782⁄5584 |
| Prediabetes | 2457 | 0.18 454⁄2457 |
| Monitoring duration (months) | 5677 | 27 (12-49) |
| Mean baseline daily steps (thousands) | 5677 | 8.17 (6.25-10.33) |
| Mean baseline daily sedentary minutes | 5677 | 801 (708-1010) |
| Mean baseline daily lightly active minutes | 5677 | 217.6 (172.9-265.0) |
| Mean baseline daily fairly active minutes | 5677 | 17.72 (9.15-42.04) |
| Mean baseline daily very active minutes | 5677 | 15.8 (7.1-29.1) |
| Mean daily steps (thousands) | 5677 | 7.92 (6.00-10.02) |
| Mean daily sedentary minutes | 5677 | 788 (699-951) |
| Mean daily lightly active minutes | 5677 | 223.3 (184.9-266.2) |
| Mean daily fairly active minutes | 5677 | 15.96 (9.09-26.26) |
| Mean daily very active minutes | 5677 | 16.06 (7.56-28.88) |
Continuous variables are reported with median and 25th-75th percentile. Categorial variables are reported as frequency and the corresponding ratio. Prediabetes is defined as a fasting glucose of 100 to 125 mg/dL or hemoglobin A1c 5.7% to 6.4%.
Figure 2.
Discontinuous months of monitoring were rare. Histogram of the number of months without Fitbit monitoring between months with Fitbit monitoring. Most months of monitoring were continuous.
Figure 3.
Mean step count decreased over study period. Trend of mean step count as a function of time to event (incident diabetes or censoring). The x-axis is grouped into ranges of time (ie, [3,5) = a range of 3-5 years prior to event/censoring, which is inclusive of 3.00 and noninclusive of 5.00). Error bars indicate interquartile range. Mean steps is reported in thousands.
Longitudinal Measures of PA Are Associated With Diabetes Risk Without Effect Modification by Age, Sex, or Obesity Status
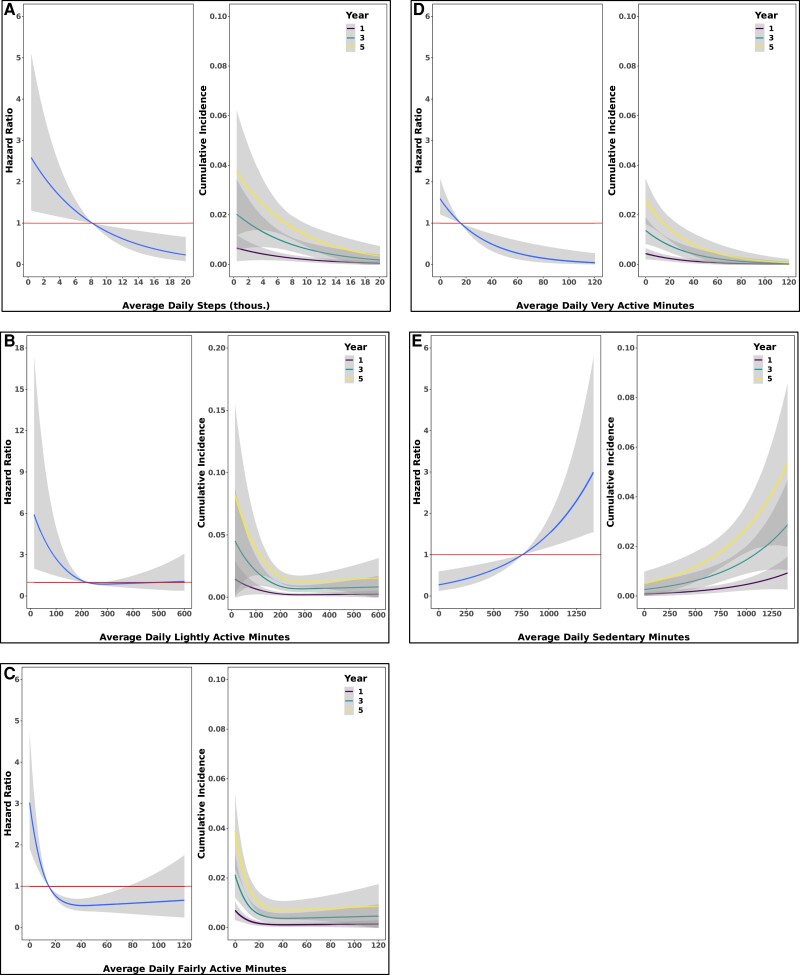
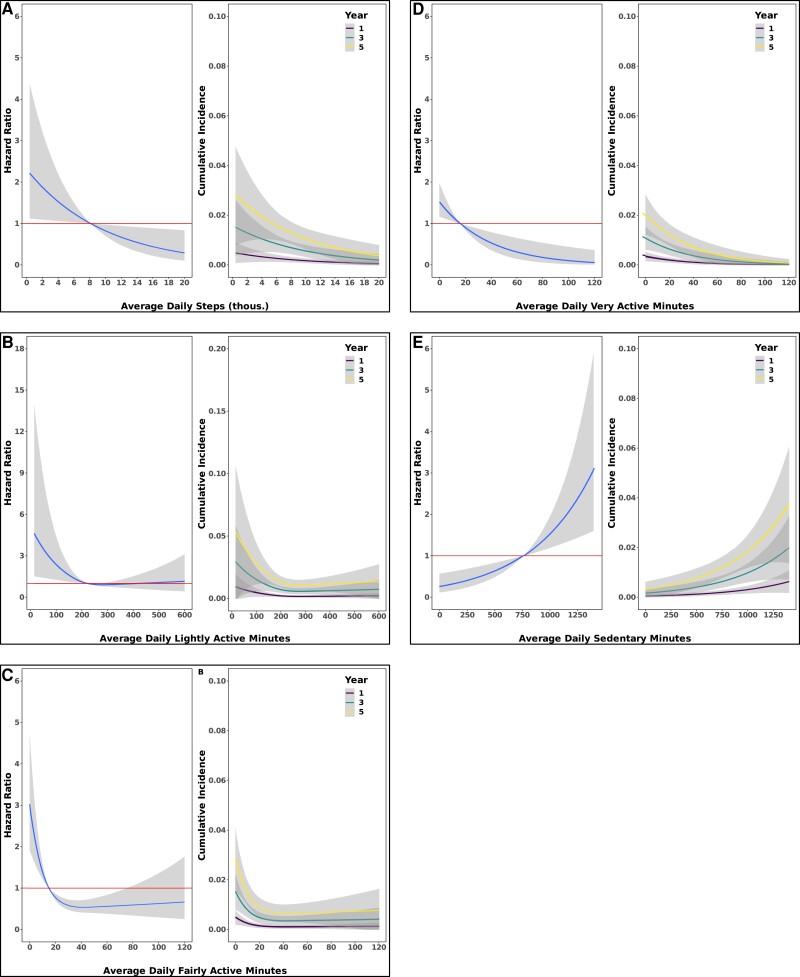
Over a median follow-up period of 3.8 years (25th-75th percentile, 2.1-5.6 years), 97 (2%) individuals developed diabetes. In models adjusted for age, sex, and race, the hazard of incident diabetes was reduced by 44% (95% CI, 15%-63%) when comparing those with an average daily step count of 10 700 to those with 6000. This relation between daily step count and incident diabetes persisted after additional adjustment for BMI (hazard ratio, 0.62; 95% CI, 0.41-0.93). The predicted cumulative incidence of diabetes for the 90th percentile of daily steps (13 245) was 0.8% (95% CI, 0.3%-1.3%) at 5 years, whereas for the 10th percentile of daily steps (4301 steps) the incidence was 2.3% (95% CI, 1.4%-3.3%), a nearly 3-fold increased risk of diabetes. We found similar results when stratifying the type of PA into light, fairly, or very active minutes; consistently, greater time of PA was associated with lower diabetes risk and greater sedentary time with increased hazard (Table 2; Fig. 4). Similar results were found when including adjustment for BMI and smoking (see Table 2 and Fig. 5). We did not observe evidence of interaction by age, sex, or BMI with any measures of PA on incident diabetes (P > 0.05 for all).
Table 2.
Cox proportional hazards models for outcome of incident type 2 diabetes mellitus by measures of physical activity
| 25th percentile | 75th percentile | Hazard ratio (75th vs 25th percentile) | P | |
|---|---|---|---|---|
| Adjusted for age, sex, race | ||||
| ȃMean steps per day (thousands) | 5.97 | 10.69 | 0.56 (0.37-0.85) | 0.01 |
| ȃMean sedentary minutes per day | 673.33 | 965.65 | 1.65 (1.22-2.22) | 0.001 |
| ȃMean lightly active minutes per day | 181.32 | 271.57 | 0.67 (0.52-0.87) | 0.003 |
| ȃMean fairly active minutes per day | 7.63 | 27.84 | 0.37 (0.25-0.55) | <0.001 |
| ȃMean very active minutes per day | 6.03 | 32.61 | 0.46 (0.30-0.72) | <0.001 |
| Adjusted for age, sex, race, BMI | ||||
| ȃMean steps per day (thousands) | 5.97 | 10.69 | 0.62 (0.41-0.93) | 0.02 |
| ȃMean sedentary minutes per day | 673.33 | 965.65 | 1.68 (1.24-2.26) | <0.001 |
| ȃMean lightly active minutes per day | 181.32 | 271.57 | 0.72 (0.55-0.94) | 0.01 |
| ȃMean fairly active minutes per day | 7.63 | 27.84 | 0.43 (0.28-0.64) | <0.001 |
| ȃMean very active minutes per day | 6.03 | 32.61 | 0.50 (0.32-0.77) | 0.001 |
| Adjusted for age, sex, race, history of smoking | ||||
| ȃMean steps per day (thousands) | 5.97 | 10.69 | 0.57 (0.38-0.86) | 0.008 |
| ȃMean sedentary minutes per day | 673.33 | 965.65 | 1.65 (1.23-2.23) | 0.001 |
| ȃMean lightly active minutes per day | 181.32 | 271.57 | 0.67 (0.52-0.87) | 0.003 |
| ȃMean fairly active minutes per day | 7.63 | 27.84 | 0.37 (0.25-0.55) | <0.001 |
| ȃMean very active minutes per day | 6.03 | 32.61 | 0.47 (0.30-0.73) | 0.001 |
Cox proportional hazards models for the outcome of incident type 2 diabetes mellitus were created for various measures of daily physical activity. Reference values for hazard ratios are the 25th percentile with comparison against the 75th percentile. Daily measures of physical activity were averaged over a calendar month of observation and treated as time-varying variables in models.
Figure 4.
Greater amounts of physical activity are associated with lower risk for incident type 2 diabetes mellitus. Spline curves demonstrating the hazard ratio and 95% CI for incident type 2 diabetes mellitus as a function of measures of physical activity (left figures). Spline curves demonstrated the cumulative incidence and 95% CI for type 2 diabetes mellitus as a function of measures of physical activity (right figures). (A) daily steps (B) lightly active minutes (C) fairly active minutes (D) very active minutes (E) sedentary minutes. Data come from Cox regression models adjusted for age, sex, and race. Avg. = average.
Figure 5.
Greater amounts of physical activity are associated with lower risk for incident type 2 diabetes mellitus. Spline curves demonstrating the hazard ratio and 95% CI for incident type 2 diabetes mellitus as a function of measures of physical activity (left figures). Spline curves demonstrated the cumulative incidence and 95% CI for type 2 diabetes mellitus as a function of measures of physical activity (right figures). (A) daily steps (B) lightly active minutes (C) fairly active minutes (D) very active minutes (E) sedentary minutes. Data come from Cox regression models adjusted for age, sex, race, and body mass index.
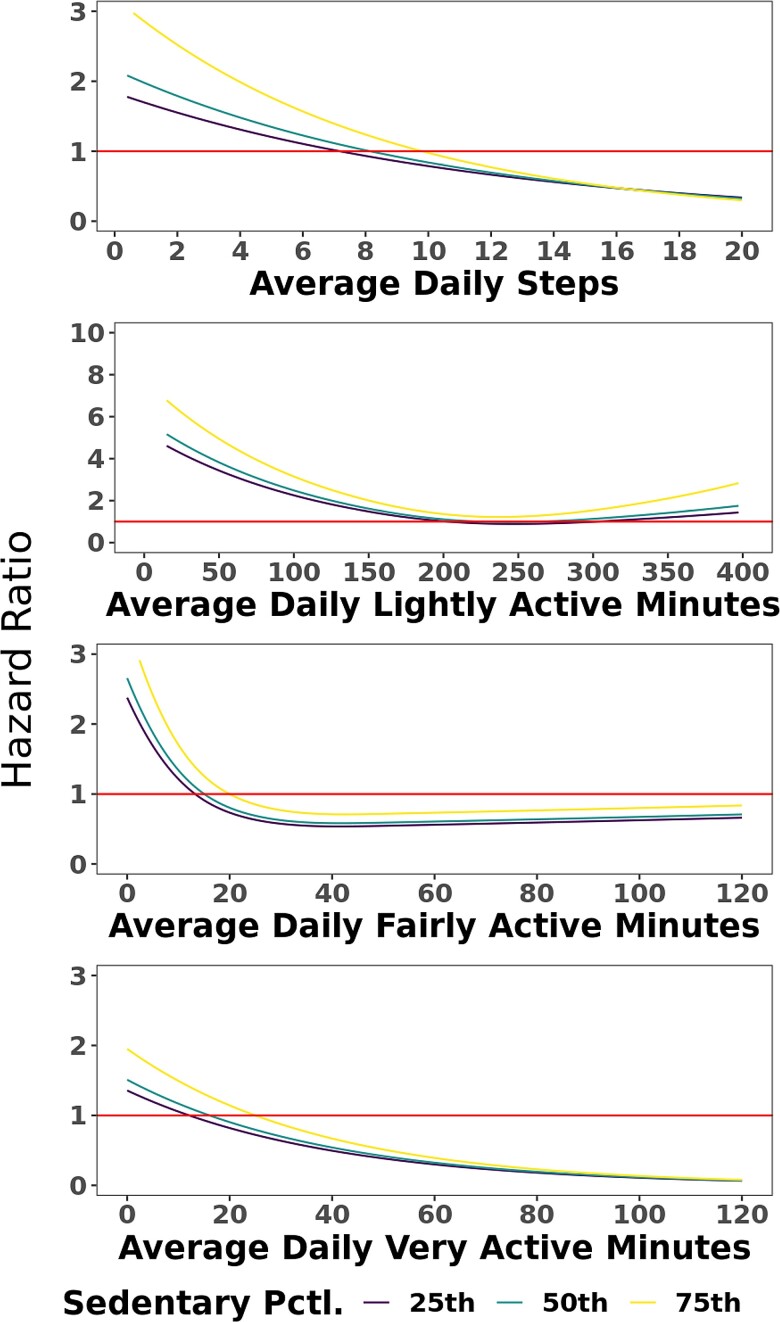
Considering the deleterious effect of sedentary time on overall cardiometabolic health (15, 25), we next evaluated whether the benefits of increased PA would be mitigated in those individuals with higher average sedentary time. We created separate Cox models for diabetes as a function of each measure of PA and its interaction with sedentary time. None of these models suggested significant interaction (P > 0.05 for all), indicating that the association of PA with reduced risk of diabetes persisted regardless of the amount of time an individual was sedentary (Fig. 6).
Figure 6.
Spline curves demonstrating the hazard ratio for incident type 2 diabetes mellitus as a function of measures of physical activity with separate curves for the 25th, 50th, and 75th percentile of sedentary function. These plots indicate no significant interaction between sedentary time and measures of physical activity on the outcome of incident type 2 diabetes mellitus, which was corroborated in Cox model analyses.
Conclusions
In these analyses using data from the All of Us Research Program, we characterized the relationship between longitudinal measures of PA via a commercial wearable device (Fitbit) and incident diabetes over 4 years. Beyond the use of accelerometry in quantifying PA, our study is highly unique in including a longitudinal assessment of many repeated measures of PA within an individual subject (median 27 monthly assessments per participant) with concurrent follow-up using validated methods within an electronic health record to ascertain diabetes. In summary, we describe a consistent association between higher amounts of PA and lower risk of incident diabetes, regardless of an individual's age, sex, or BMI. This “protective” association of PA with diabetes risk remained, regardless of the time an individual spent in sedentary behavior. These results provide real-world evidence for the benefits of maintaining PA over time as a key step to reducing risk.
While the notion that higher amounts of PA are related to lower incident diabetes risk is not new (2–4, 6, 8, 26, 27), most studies remain limited by non-quantitative measures of PA made at either a single time point or during a short observation period. In a recent meta-analysis of 3 studies with > 260 000 individuals with > 19 000 diabetes events over a mean 7.5-year follow-up period, increases in PA from inactive to guideline-recommended targets (> 150 minutes moderate intensity activity per week) were associated with a 26% reduction in incident type 2 diabetes (an effect size comparable to our findings), sustained after adjustment for weight (28). Each of these 3 studies used questionnaires or interviews to quantify an individual's PA, and none used repeated assessments of PA in their models. While these results have been replicated in other settings (29), the reliance on self-reported, questionnaire-based instruments of PA (with attendant risk of instrument and recall bias (30)) or limited periods of wearable PA monitoring (generally < 1 month) has limited our understanding of how average trends in PA over a longer time period may impact diabetes risk, after “early,” observer biases from wearing the device diminish (22, 23). In addition, approximately half of participants in a large study demonstrated changes in amount of PA over time, highlighting the importance of using a time-updated model (1). The approach taken here allowed us to “time-update” the PA exposure in our models, thereby reducing the impact any given time epoch of monitoring has on an outcome (a limitation of questionnaire and limited [1- to 2-week] collection of PA data) (13). In comparison with the general United States population, our cohort is more active and modest increases in activity may be of benefit in more sedentary populations (31). Whereas some have observed a “physical activity paradox” (wherein leisure time activity is associated with lower risk of cardiovascular disease, but occupational time activity is not) (32) our study was not designed to examine for a similar phenomenon in relation to diabetes. While we do not report race effect modification here (due to limited diversity of our analytic sample), we did not observe interactions of PA by age, sex, or BMI on diabetes, consistent with the universal benefits of PA across demographics. Importantly, our data did not include concurrent dietary assessment, so we were unable to test for confounding by diet.
Notably the All of Us cohort studied differs from other large studies, and one of the main strengths is the use of data from real-world observation. The cohort studied here has relatively few risk factors and accordingly there was a low incidence of diabetes (2%). This cohort was also quite active with a median of 16 very active minutes daily, which corresponds to 112 very active minutes weekly (guidelines recommend 150 minutes) (7, 8). Yet, despite being low risk, we still detected a signal of benefit from increased PA. These results indicate that greater amounts of physical activity are correlated with lower risk of diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend.
Despite these key innovations, there are several limitations relevant to our conclusions. Our population—predominantly White women—was likely a reflection that the data provided was voluntary and biased by factors that influence accelerometer/wearable use. Further studies within All of Us are attempting to address this imbalance. While the Fitbit has demonstrated reliability in measuring active time, there may be discrepancies with the estimate of sedentary time (19). Additionally, Fitbit does not discriminate between type of physical activity (ie, aerobic activity, strength training etc.). Our analysis did not account for differences in leisure time activity or occupational activity, which may be an important distinction provided the “physical activity paradox” (32). Our data set also does not include concurrent dietary assessments, so it is possible that our findings are confounded by dietary habits. Although use of the electronic health record is a strength in some respects, data missingness (eg, BMI) and ICD code–defined diabetes definitions may lead to ascertainment bias (with misclassification); nevertheless, these biases would have likely limited our power to detect an effect (null bias). The relatively low incidence of diabetes in this cohort may result in imprecise estimates of the association between PA and diabetes. We excluded events within 180 days of monitoring initiation, which protects against reverse causation but may increase the risk of immortal time bias. Provided this is an observational study, we are unable to infer conclusions related to causation and there may be unaccounted confounders such as environmental factors, diet, socioeconomic factors, or differences in Fitbit use.
In conclusion, this analysis of 5677 participants from the All of Us study leveraged numerous, serial quantifications of PA from a wearable monitor, demonstrating increased risk of diabetes with decreased activity type and frequency over approximately 4 years of follow-up, with consistent effects across age, sex, BMI, or sedentary time. As a corollary, increased PA time, regardless of PA intensity, was associated with decreased risk. Future studies should aim to extend these findings to more racially diverse populations to confirm the generalizability of these findings.
Acknowledgments
The All of Us Research Program would not be possible without the partnership of its participants.
Abbreviations
- BMI
body mass index
- PA
physical activity
- T2DM
type 2 diabetes mellitus
Contributor Information
Andrew S Perry, Vanderbilt Translational and Clinical Cardiovascular Research Center, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Jeffrey S Annis, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Hiral Master, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Matthew Nayor, Sections of Cardiovascular Medicine and Preventive Medicine and Epidemiology, Department of Medicine, Boston University School of Medicine, Boston, MA 02118, USA.
Andrew Hughes, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN 37203, USA.
Aymone Kouame, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Karthik Natarajan, Department of Biomedical Informatics, Columbia University, New York, NY 10032, USA.
Kayla Marginean, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Venkatesh Murthy, Department of Medicine and Radiology, University of Michigan, Ann Arbor, MI 48109, USA.
Dan M Roden, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN 37203, USA; Department of Pharmacology, Vanderbilt University Medical Center, Nashville, TN 37203, USA; Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, TN 37203, USA.
Paul A Harris, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University School of Medicine, Nashville, TN 37203, USA; Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, TN 37203, USA; Department of Biomedical Engineering, Vanderbilt University Medical Center, Nashville, TN 37203, USA.
Ravi Shah, Vanderbilt Translational and Clinical Cardiovascular Research Center, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Evan L Brittain, Vanderbilt Translational and Clinical Cardiovascular Research Center, Vanderbilt University School of Medicine, Nashville, TN 37203, USA.
Funding
NHLBI R61 HL158941
NHLBI R01 HL146588
NIDDK R01 DK124845
The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
Role of the Funder/Sponsor
The sponsor, All of Us Research Program, as well as Fitbit, were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
V.M.: grant support from Siemens Healthineers, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Aging (NIA), National Heart, Lung, and Blood Institute (NHLBI), and American Heart Association (AHA). He has received other research support from NIVA Medical Imaging Solutions. He owns stock in Eli Lilly, Johnson & Johnson, Merck, Bristol-Myers Squibb, and Pfizer and stock options in Ionetix. He has received research grants and speaking honoraria from Quart Medical.
R.S.: grant support from the NHLBI, NIA, and NIDDK and has served as a consultant for Amgen, Cytokinetics, Myokardia, and Best Doctors. He is a co-inventor on a patent for ex-RNAs signatures of cardiac remodeling.
M.N.: received speaking honoraria from Cytokinetics.
A.S.P., J.A., H.M., A.H., A.K., K.N., K.M., D.M.R., P.A.H., and E.B.: none.
Author Contributions
V.M., J.A., R.S., and E.B. were involved in the conception, design, and conduct of the study and were joined by A.S.P. for the analysis and interpretation of the results. A.S.P. wrote the first draft of the manuscript, and all authors edited, reviewed, and approved the final version of the manuscript. E.B. had full access to the data and is responsible for the integrity of the data and the accuracy of the data analysis.
Data Availability
To ensure privacy of participants, data used for this study are available to approved researchers following registration, completion of ethics training, and attestation of a data use agreement through the All of Us Research Workbench platform, which can be accessed via https://workbench.researchallofus.org/login.
References
- 1. Lee DH, Rezende LFM, Joh HK, et al. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: a prospective cohort of US adults. Circulation. 2022;146(7):523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799‐815. [DOI] [PubMed] [Google Scholar]
- 3. Carbone S, Del Buono MG, Ozemek C, Lavie CJ. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 2019;62(4):327‐333. [DOI] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8(3):201‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kokkinos P, Faselis C, Samuel IBH, et al. Cardiorespiratory fitness and mortality risk across the spectra of age, race, and sex. J Am Coll Cardiol. 2022;80(6):598‐609. [DOI] [PubMed] [Google Scholar]
- 7. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Sbcommittee on Physical Activity). Circulation. 2003;107(24):3109‐3116. [DOI] [PubMed] [Google Scholar]
- 9. Guo W, Key TJ, Reeves GK. Accelerometer compared with questionnaire measures of physical activity in relation to body size and composition: a large cross-sectional analysis of UK biobank. BMJ Open. 2019;9(1):e024206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skender S, Ose J, Chang-Claude J, et al. Accelerometry and physical activity questionnaires - a systematic review. BMC Public Health. 2016;16(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garduno AC, LaCroix AZ, LaMonte MJ, et al. Associations of daily steps and step intensity with incident diabetes in a prospective cohort study of older women: the OPACH study. Diabetes Care. 2022;45(2):339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1S):S68‐S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee DH, Rezende LFM, Ferrari G, et al. Physical activity and all-cause and cause-specific mortality: assessing the impact of reverse causation and measurement error in two large prospective cohorts. Eur J Epidemiol. 2021;36(3):275‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. All of Us Research Program Investigators, Denny JC, Rutter JL, et al. The “All of Us” research program. N Engl J Med 2019;381(7):668‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND. Sedentary behavior and cardiovascular disease risk: mediating mechanisms. Exerc Sport Sci Rev. 2017;45(2):80‐86. [DOI] [PubMed] [Google Scholar]
- 16. Harrell F. Hmisc: Harrell Miscellaneous_. R package version 4.7-0.2022. Accessed April 19, 2022. https://CRAN.R-project.org/package=Hmisc
- 17. Jennifer Pacheco WT. Northwestern University Type 2 diabetes mellitus algorithm.2012. Accessed June 1, 2022. https://phekb.org/phenotype/18
- 18. Brewer W, Swanson BT, Ortiz A. Validity of Fitbit's Active minutes as compared with a research-grade accelerometer and self-reported measures. BMJ Open Sport Exerc Med. 2017;3(1):e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carpenter C, Yang CH, West D. A comparison of sedentary behavior as measured by the fitbit and ActivPAL in college students. Int J Environ Res Public Health. 2021;18(8):3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evenson KR, Wen F, Furberg RD. Assessing validity of the fitbit indicators for U. S. Public Health Surveillance. Am J Prev Med. 2017;53(6):931‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Semanik P, Lee J, Pellegrini CA, Song J, Dunlop DD, Chang RW. Comparison of physical activity measures derived from the Fitbit Flex and the ActiGraph GT3X+ in an employee population with chronic knee symptoms. ACR Open Rheumatol. 2020;2(1):48‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaudhry UAR, Wahlich C, Fortescue R, Cook DG, Knightly R, Harris T. The effects of step-count monitoring interventions on physical activity: systematic review and meta-analysis of community-based randomised controlled trials in adults. Int J Behav Nutr Phys Act. 2020;17(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferguson T, Olds T, Curtis R, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4(8):e615‐e626. [DOI] [PubMed] [Google Scholar]
- 24. Jefferis BJ, Whincup PH, Lennon L, Wannamethee SG. Longitudinal associations between changes in physical activity and onset of type 2 diabetes in older British men: the influence of adiposity. Diabetes Care. 2012;35(9):1876‐1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young DR, Hivert MF, Alhassan S, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134(13):e262‐e279. [DOI] [PubMed] [Google Scholar]
- 26. Ballin M, Nordstrom P, Niklasson J, et al. Daily step count and incident diabetes in community-dwelling 70-year-olds: a prospective cohort study. BMC Public Health. 2020;20(1):1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hall KS, Hyde ET, Bassett DR, et al. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int J Behav Nutr Phys Act. 2020;17(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wahid A, Manek N, Nichols M, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(9):e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol. 2016;31(10):967‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46(1):99‐106. [DOI] [PubMed] [Google Scholar]
- 31. Blackwell DL, Clarke TC. State variation in meeting the 2008 federal guidelines for both aerobic and muscle-strengthening activities through leisure-time physical activity among adults aged 18-64: United States, 2010-2015. Natl Health Stat Report. 2018;112:1‐22. [PubMed] [Google Scholar]
- 32. Holtermann A, Schnohr P, Nordestgaard BG, Marott JL. The physical activity paradox in cardiovascular disease and all-cause mortality: the contemporary Copenhagen general population study with 104 046 adults. Eur Heart J. 2021;42(15):1499‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To ensure privacy of participants, data used for this study are available to approved researchers following registration, completion of ethics training, and attestation of a data use agreement through the All of Us Research Workbench platform, which can be accessed via https://workbench.researchallofus.org/login.