Abstract
BACKGROUND & AIMS:
Policy changes in the United States have lengthened overall waiting times for patients with hepatocellular carcinoma (HCC). We investigated temporal trends in utilization of locoregional therapy (LRT) and associated waitlist outcomes among liver transplant (LT) candidates in the United States.
METHODS:
Data for primary adult LT candidates listed from 2003 to 2018 who received HCC exception were extracted from the Organ Procurement and Transplantation Network database. Explant histology was examined, and multivariable competing risk analysis was used to evaluate the association between LRT type and waitlist dropout.
RESULTS:
There were 31,609 eligible patients with at least 1 approved HCC exception, and 34,610 treatments among 24,145 LT candidates. The proportion with at least 1 LRT recorded increased from 42.3% in 2003 to 92.4% in 2018. Chemoembolization remains the most frequent type, followed by thermal ablation, with a notable increase in radioembolization from 3% in 2013 to 19% in 2018. An increased incidence of LRT was observed among patients with tumor burden beyond Milan criteria, higher α-fetoprotein level, and more compensated liver disease. Receipt of any type of LRT was associated with a lower risk of waitlist dropout; there was no significant difference by number of LRTs. In inverse probability of treatment weighting–adjusted analysis, radioembolization or ablation as the first LRT was associated with a reduced risk of waitlist dropout compared with chemoembolization.
CONCLUSIONS:
In a large nationwide cohort of LT candidates with HCC, LRT, and in particular radioembolization, increasingly was used to bridge to LT. Patients with greater tumor burden and those with more compensated liver disease received more treatments while awaiting LT. Bridging LRT was associated with a lower risk of waitlist dropout.
Keywords: Ablation, Chemoembolization, Liver Cancer, Liver Transplantation, Radioembolization, Waitlist Dropout
Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality in the United States.1 Liver transplantation (LT) is the curative option for patients with unresectable HCC meeting prespecified size criteria.2–4 A mandated waiting time of 6 months before gaining exception points has been implemented in the United States to allow for consideration of tumor biology and reduce the disparities in waitlist dropout between HCC and non-HCC patients.5 This policy change intentionally has lengthened waiting times for patients with HCC.
Advances in locoregional therapy (LRT) have expanded treatment options for patients with unresectable HCC. Several forms of LRT now are available, including chemoembolization, radioembolization, radiofrequency or microwave ablation, and external beam radiation. Not all patients are eligible for all LRT modalities, and the type of LRT chosen can be influenced by the size, location, number, and characteristics of the tumor, liver function, provider preference, or center expertise.6 For example, percutaneous ablation may be preferred for a single lesion less than 3 cm, while intra-arterial modalities such as chemoembolization may be selected for multifocal disease or lesions near major vascular and biliary structures, hollow viscera, or diaphragm.7 These therapies also can be combined or used in sequence. Overall, efficacies of different LRT modalities are considered to be similar; recent data have suggested that radioembolization may prolong time to progression compared with chemoembolization.8
In the setting of LT, LRT allows for treatment and control of intrahepatic disease while patients await LT, termed bridging therapy. An international consensus statement has recommended that bridging strategies using LRT should be used in LT candidates who are likely to wait 6 months or longer.9 In addition, response to LRT increasingly is recognized as an indicator of favorable tumor biology.10,11 In the United States, patients whose tumors initially exceed Milan criteria may undergo LRT to reduce tumor burden to become eligible for LT.12
As a result of longer waiting times and wider availability of LRT, LT candidates with HCC often receive LRT as bridging therapy while awaiting LT, although the quality of evidence for bridging therapy is low.13 The aim of this study was to investigate temporal trends and waitlist outcomes of LRT among LT candidates in the United States.
Materials and Methods
Relevant candidate, donor, and procurement data were extracted from the Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) database, which include data regarding candidate demographics, comorbidities, and laboratory values; applications for HCC exception including AFP, tumor burden, and LRT; and donor characteristics and explant histology if transplanted. All adults listed for primary liver transplantation who received at least 1 approved HCC exception between 2003 to 2018 were included.
The date and type of LRT, if any, was reported with each submitted HCC exception application. Combination therapy (ie, ablation + chemoembolization; ablation + radioembolization) was defined as receipt of these therapies within 30 days. In the OPTN database, LRT was coded as follows: chemical ablation, chemoembolization, cryoablation, external beam radiation, radiation micro-spheres, radiofrequency ablation, and thermal ablation. Radiofrequency ablation was not valid after 2013, and therefore radiofrequency ablation and thermal ablation were combined. The number of LRTs was capped at 4 because few patients received greater than this number of treatments (n = 127). UNOS T2 criteria were defined using conventional Milan criteria, with 1 lesion larger than 2 cm and smaller than 5 cm or 2 to 3 lesions each smaller than 3 cm. Patients qualifying for exception points via downstaging protocols, identified by initial recorded tumor burden beyond Milan criteria, also were included.
Multiple listings were merged so that each patient had a single entry, and the earliest date of listing was considered. Patients removed from the list for reasons other than transplant, death, being too sick, or condition improved were excluded. Outcomes were categorized by removal code as follows: (1) waitlist dropout (death or being too sick), (2) liver transplant, deceased or living donor, and (3) still waiting or removed for condition improved. The UNOS region was assigned based on the center at the time of the first approved exception application. Region wait times were categorized based on previously used definitions, with regions 3, 10, and 11 considered as short; regions 2, 4, 6, 7, and 8 as medium; and 1, 5, and 9 as long wait time regions.14
We characterized trends in types of locoregional therapy received by year of initial listing. P values for trend across eras were assessed using the Mann–Kendall test for continuous variables and the Cochran–Armitage test for categoric variables. Incidence rates of LRT per 100 waitlist days were calculated.
Multivariable competing risk analysis was used to evaluate the association between type of first LRT and time to waitlist dropout, defined by removal from the list for death or being too sick. Removal from the list for being too sick in these cases likely would represent progression of disease beyond transplantable criteria, and beyond which patients were unlikely to benefit from or be eligible for further LRT. Liver transplant was considered as a competing risk, and patients who did not experience either event were censored at the time of removal from the list (for condition improved) or last follow-up evaluation (for those still waiting). To account for potential bias with regard to type of treatment, an inverse probability of treatment weighting (IPTW)-adjusted competing risk analysis was performed among patients who received chemoembolization, radio-embolization, or ablation as the first recorded LRT. The propensity of receiving each LRT type was modeled by a multinomial logistic regression by the other covariates in the competing risk model. IPTW-adjusted cumulative incidence curves were generated using the methods described by Neumann and Billionnet.15
Secondary analyses were performed to consider model for end-stage liver disease (MELD) instead of Child-Pugh class and to evaluate the impact of LRT type on the time from the first to a second treatment, with liver transplantation and death considered as competing risks. Need for a second LRT was considered in light of evidence suggesting that radioembolization may prolong time to progression compared with chemoembolization.8 Explant histology for the transplant recipients (available after April 2012) was examined, and incomplete or no necrosis was defined as any viable tumor on explant.
For all analyses, a P value less than .05 was considered significant. Variables were compared among groups using t tests, chi-square tests, 1-way analysis of variance, and Wilcoxon rank-sum tests, as appropriate. Statistical analyses were performed using R version 3.6.1 (Vienna, Austria) and SAS 9.4 (Cary, NC).
Results
During the study time period, there were 31,609 eligible liver transplant candidates with at least 1 approved HCC exception application. Baseline demographics of these patients, tumor characteristics, and outcome, stratified by era of listing, are shown in Table 1. The median age was 59 years (interquartile range [IQR], 54–64 y), 76.8% were male, and the predominant etiology of liver disease was hepatitis C (53.1%). The median initial α-fetoprotein (AFP) level was 10 (IQR, 5–38), 4.2% were beyond Milan criteria, and the median follow-up time on the waitlist was 214 days (IQR, 87–416). The majority ultimately underwent deceased or living donor liver transplantation (79.0%), while 18.6% were removed for death or being too sick for transplant. The median waitlist time increased in more recent years (257 days for patients listed in 2015–2018, compared with 123 days for patients listed in 2003–2006); there was no similar trend for severity of liver disease (MELD) or initial total tumor diameter (Table 1).
Table 1.
Baseline Demographics, Tumor Characteristics, and Outcomes (n = 31,609) Over 4 Different Time Periods
| 2003–2006 | 2007–2010 | 2011–2014 | 2015–2018 | P | |
|---|---|---|---|---|---|
| N | 5258 | 7477 | 9327 | 9547 | |
| Median age at listing, y (IQR) | 55 (51–61) | 57 (53–62) | 60 (55–64) | 62 (58–66) | <.01 |
| Sex, male, n (%) | 4074 (77.5) | 5763 (77.1) | 7122 (76.4) | 7329 (76.8) | .32 |
| Race, n (%) | <.01 | ||||
| White | 3429 (65.2) | 4857 (65.0) | 6071 (65.1) | 6103 (63.9) | |
| Black | 441 (8.4) | 685 (9.2) | 943 (10.1) | 883 (9.2) | |
| Hispanic | 804 (15.3) | 1118 (15.0) | 1518 (16.3) | 1722 (18.0) | |
| Asian | 521 (9.9) | 704 (9.4) | 683 (7.3) | 680 (7.1) | |
| Other | 63 (1.2) | 113 (1.5) | 112 (1.2) | 159 (1.7) | |
| Etiology of liver disease, n (%) | <.01 | ||||
| Alcohol | 911 (17.3) | 1072 (14.3) | 1263 (13.5) | 1669 (17.5) | |
| Hepatitis B | 429 (8.2) | 486 (6.5) | 525 (5.6) | 497 (5.2) | |
| Hepatitis C | 2842 (54.1) | 4100 (54.8) | 5331 (57.2) | 4524 (47.4) | |
| Metabolic | 68 (1.3) | 77 (1.0) | 74 (0.8) | 92 (1.0) | |
| NASH | 151 (2.9) | 409 (5.5) | 750 (8.0) | 1424 (14.9) | |
| Other or not specified | 857 (16.3) | 1333 (17.8) | 1383 (14.8) | 1341 (14.0) | |
| Median initial biochemical MELD (IQR) | 11 (9–14) | 11 (8–14) | 11 (8–14) | 10 (8–14) | <.01 |
| Initial Child–Pugh class, n (%) | <.01 | ||||
| A | 1668 (31.8) | 2758 (36.9) | 3859 (41.4) | 4796 (50.2) | |
| B | 2620 (49.9) | 3531 (47.3) | 3995 (42.8) | 3640 (38.1) | |
| C | 962 (18.3) | 1184 (15.8) | 1472 (15.8) | 1110 (11.6) | |
| Median initial AFP (IQR) | 14 (6–71) | 12 (5–51) | 11 (5–38) | 7 (4–20) | <.01 |
| Median total tumor diameter, cm (initial) (IQR)a | 3.1 (2.4–4.1) | 3.1 (2.4–4.2) | 3.0 (2.3–4.1) | 3.0 (2.2–4.2) | <.01 |
| Median largest tumor, cm (initial) (IQR)a | 2.6 (2.1–3.4) | 2.6 (2.1–3.4) | 2.5 (2.1–3.3) | 2.6 (2.1–3.4) | .11 |
| Number of lesions (initial), n (%) | <.01 | ||||
| 0 | 14 (0.3) | 29 (0.4) | 52 (0.6) | 242 (2.5) | |
| 1 | 3632 (69.1) | 5272 (70.5) | 7037 (75.5) | 7834 (82.1) | |
| 2 | 1119 (21.3) | 1577 (21.1) | 1690 (18.1) | 1144 (12.0) | |
| 3 | 486 (9.2) | 578 (7.7) | 530 (5.7) | 303 (3.2) | |
| ≥4 | 7 (0.1) | 21 (0.3) | 19 (0.2) | 24 (0.3) | |
| Initial tumor outside Milan, n (%)b | 176 (3.3) | 368 (4.9) | 356 (3.8) | 418 (4.4) | .26 |
| Median waitlist time, d (IQR) | 123 (35–365) | 160 (57–378) | 226 (103–444) | 257 (174–419) | <.01 |
| Wait time region of initial listing | <.01 | ||||
| Short | 1229 (23.4) | 1776 (23.8) | 2464 (26.4) | 2653 (27.8) | |
| Medium | 1959 (37.2) | 2572 (34.4) | 3028 (32.5) | 2825 (29.6) | |
| Long | 2070 (39.4) | 3129 (41.8) | 3835 (41.1) | 4069 (42.6) | |
| Number of LRT per patient, n (%) | <.01 | ||||
| 0 | 2594 (49.3) | 2364 (31.6) | 1634 (17.5) | 872 (9.0) | |
| 1 | 2255 (42.9) | 3897 (52.1) | 5030 (53.9) | 5196 (54.4) | |
| 2 | 356 (6.8) | 978 (13.1) | 1948 (20.9) | 2463 (25.9) | |
| 3 | 47 (0.9) | 188 (2.5) | 515 (5.5) | 750 (7.9) | |
| ≥4 | 6 (0.1) | 50 (0.7) | 200 (2.1) | 266 (2.8) | |
| Outcome, n (%) | <.01 | ||||
| Liver transplant | 4547 (86.5) | 6190 (82.8) | 7239 (77.6) | 6995 (73.3) | |
| Waitlist dropout | 674 (12.8) | 1231 (16.5) | 1951 (20.9) | 2014 (21.1) | |
| Still waiting/condition improved | 37 (0.7) | 56 (0.7) | 137 (1.5) | 538 (5.6) |
AFP, α-fetoprotein; IQR, interquartile ratio; LRT, locoregional therapy; MELD, model for end-stage liver disease; NASH, nonalcoholic steatohepatitis.
Largest of either listing or original tumor size.
Reported either at listing or the original tumor size.
Overall, there was an increase in the number of treatments per patient by year of listing, and population-level differences in treatment type based on the year of treatment. In total, there were 34,610 LRT among 24,145 LT candidates. The proportion of patients with at least 1 LRT recorded increased from 42.3% in 2003 to 92.4% in 2018 (Figure 1A). The timing of the first LRT to date of waitlisting was a median of 37 days (IQR, 115 days before listing to 14 days after listing). The majority (67.8%) of patients receiving liver-directed therapy recorded a single LRT; 23.8% had 2 LRTs, 6.2% had 3 LRTs, and 2.2% had 4 or more LRTs. Chemoembolization was the most frequent type of LRT, followed by thermal ablation (radiofrequency or microwave). There was a notable increase in radioembolization over the study period, comprising 19% of LRT in 2018, compared with less than 5% as recently as 2013 (Figure 1B). External beam radiation also increased during this time, from 1% to 3%. In 2018, chemoembolization accounted for 50% of LRTs, and thermal ablation accounted for 22%.
Figure 1.
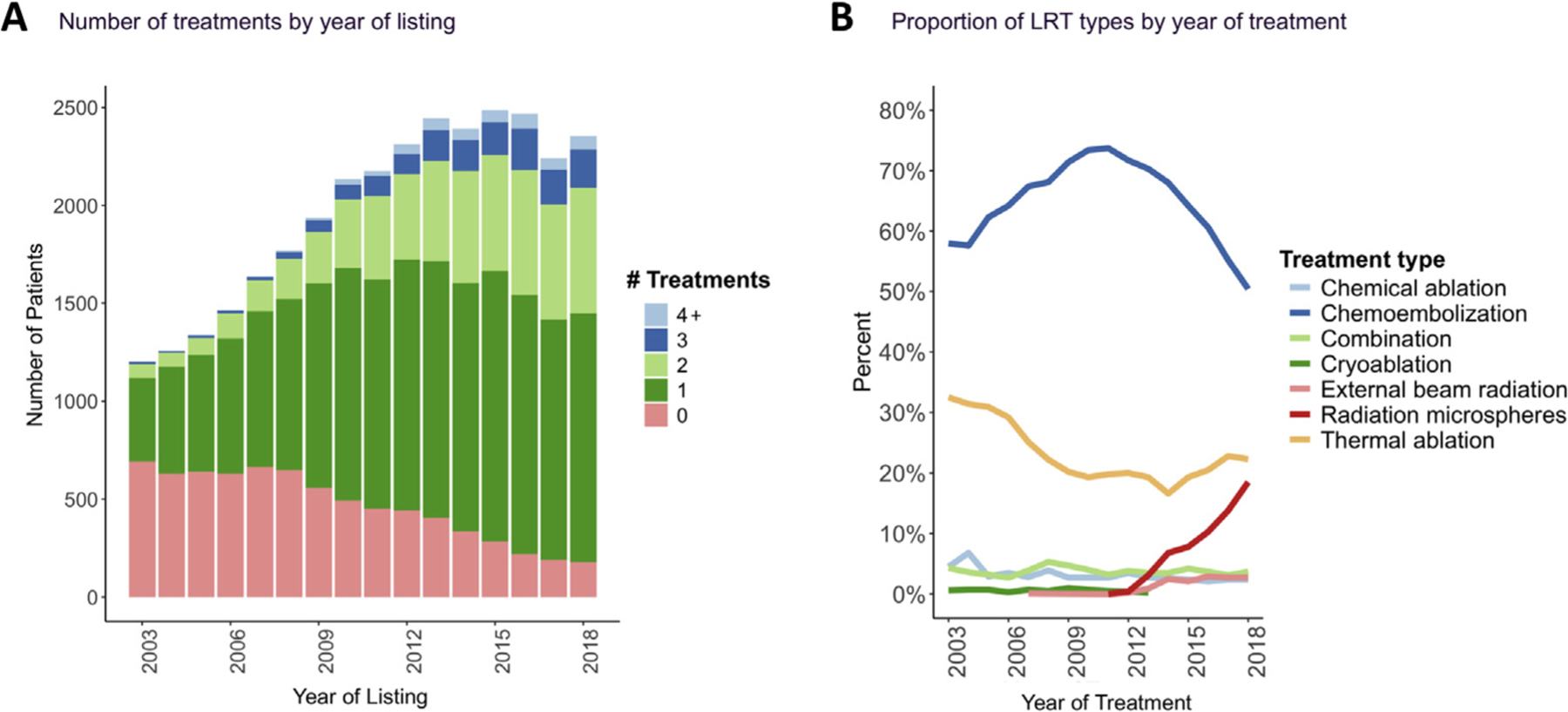
(A) Number of treatments by year of listing. (B) Proportion of locoregional therapy (LRT) types by year of treatment.
Figure 2 illustrates the distribution of LRT by individual centers, stratified by era of listing. Use of radioembolization ranged from 0% to 84% among centers, even during the most recent period (median, 7.8%; IQR, 2.7%–17.0%). In 2015 to 2018, radioembolization accounted for more than 20% of recorded LRTs at 21 of 114 (18.4%) centers and was not performed at all in 14 (12.3%) centers. During this period, chemoembolization remained the most common type of LRT, making up more than 50% of LRTs at 76 (66.7%) centers.
Figure 2.
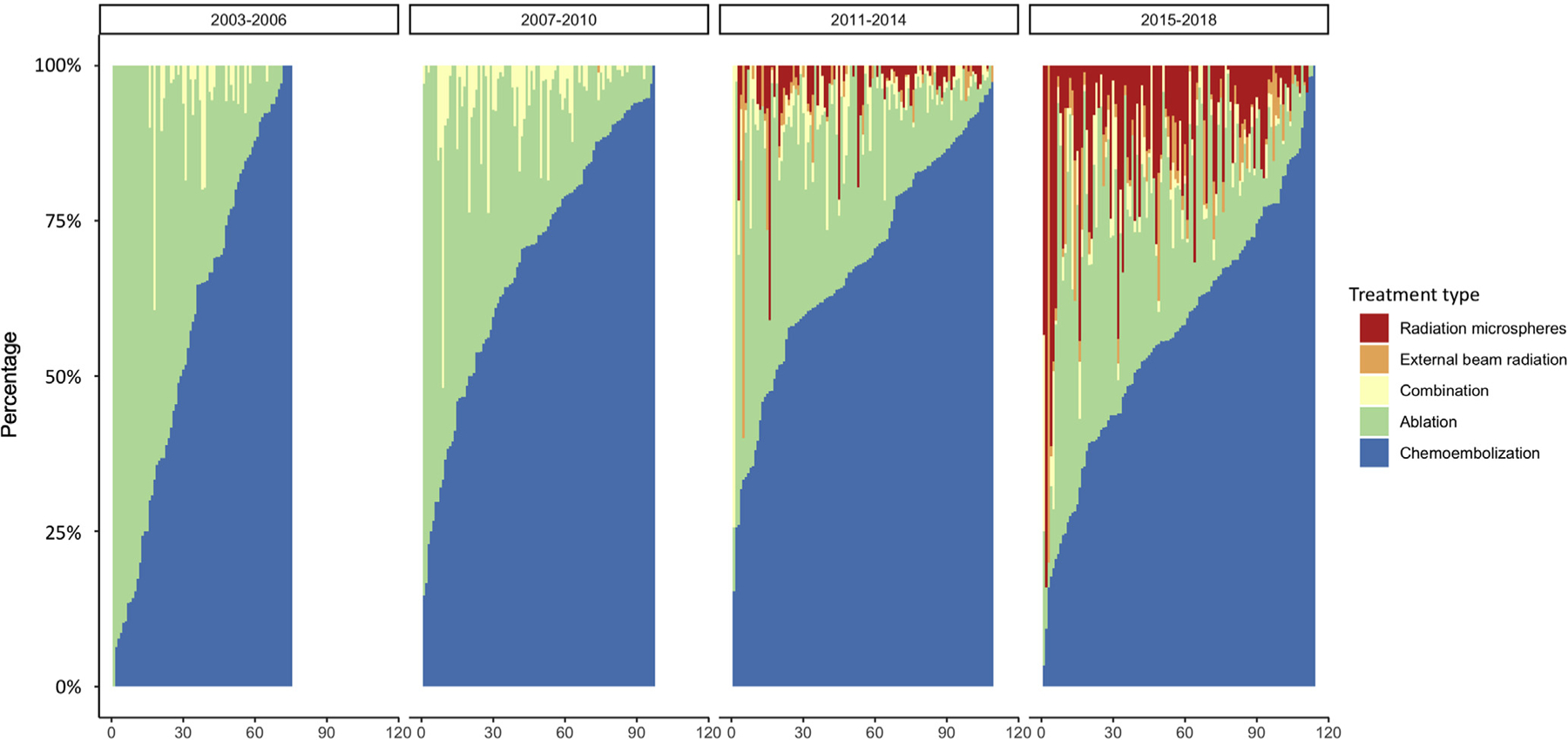
Distribution of different types of locoregional therapy (LRT) by individual centers over 4 different time periods. The x-axis represents individual centers, ordered by proportion of LRT at that center being chemoembolization. A center must have 10 or more LRTs recorded within the era to be included. Ablation indicates thermal, chemical, or cryoablation.
The incidence rate of LRT per 100 waitlist days was 0.143, and above average among those with an initial tumor burden beyond Milan criteria (0.188), AFP level of 21 to 40 or 41 to 500 ng/mL (0.171 and 0.179, respectively), Child–Pugh class A (0.160), those in short (0.151) and medium (0.154) wait time regions, and those listed after implementation of cap-and-delay on October 8, 2015 (0.192) (Supplementary Table 1).
In the multivariable competing-risk analysis for waitlist dropout, adjusting for initial tumor burden and AFP, Child–Pugh class, wait region, and listing era, receipt of no locoregional therapy was associated with an increased risk of waitlist dropout compared with chemoembolization as the first LRT (subhazard ratio [sHR], 1.37; 95% CI, 1.28–1.47) (Table 2). Larger initial tumor diameter, AFP level greater than 20 ng/mL, and more decompensated liver disease, as well as listing in longer wait time regions and in more recent years, also predicted a higher risk of waitlist dropout. In IPTW-adjusted analysis, radioembolization compared with chemoembolization was associated with a reduced risk of waitlist dropout (sHR, 0.85; 95% CI, 0.81–0.89), as was thermal ablation (sHR, 0.95; 95% CI, 0.91–0.99) (Supplementary Figure 1). Findings for both the multivariable competing-risks analysis and IPTW-adjusted analysis were similar when MELD was considered instead of Child–Pugh score (Supplementary Table 2).
Table 2.
Univariable and Multivariable Competing-Risk Analysis for Time From Listing to Waitlist Dropout, With Transplant Considered as a Competing Risk; Considering Type of First LRT
| Univariable sHR (95% CI) | Multivariable sHR (95% CI) | |
|---|---|---|
| Initial total tumor diameter, cm | 1.10 (1.09–1.12) | 1.13 (1.11–1.15) |
| Initial tumor number (ref: 0–1) | ||
| 2 | 1.01 (0.94–1.08) | 0.96 (0.89–1.03) |
| 3 | 1.16 (1.04–1.28) | 0.96 (0.86–1.07) |
| ≥4 | 1.51 (0.97–2.36) | 1.06 (0.68–1.63) |
| Initial AFP level (ref: <20) | ||
| 21–40 | 1.19 (1.09–1.30) | 1.25 (1.14–1.36) |
| 41–500 | 1.53 (1.44–1.63) | 1.65 (1.55–1.75) |
| 501–1000 | 1.76 (1.52–2.05) | 2.25 (1.94–2.61) |
| ≥1000 | 2.64 (2.32–2.99) | 3.20 (2.81–3.64) |
| Child–Pugh class (ref: A) | ||
| B | 1.34 (1.26–1.42) | 1.53 (1.44–1.62) |
| C | 1.93 (1.80–2.08) | 2.25 (2.08–2.42) |
| Region of initial listing (ref: short) | ||
| Medium | 1.91 (1.76–2.07) | 2.02 (1.86–2.20) |
| Long | 2.70 (2.49–2.92) | 3.17 (2.92–3.44) |
| Type of first LRT (ref: chemoembolization) | ||
| Radioembolization | 1.26 (1.12–1.42) | 1.03 (0.91–1.17) |
| Thermal ablation | 0.89 (0.82–0.96) | 0.97 (0.90–1.05) |
| Combination | 1.02 (0.88–1.18) | 1.05 (0.91–1.21) |
| External beam radiation | 1.04 (0.74–1.45) | 0.88 (0.63–1.23) |
| Other | 0.92 (0.76–1.12) | 0.92 (0.76–1.12) |
| None | 1.07 (1.01–1.15) | 1.37 (1.28–1.47) |
| Listing era (ref: 2003–2006) | ||
| 2007–2010 | 1.31 (1.19–1.44) | 1.50 (1.36–1.65) |
| 2011–2014 | 1.73 (1.58–1.89) | 2.30 (2.10–2.52) |
| 2015–2018 | 1.78 (1.63–1.95) | 2.69 (2.44–2.96) |
AFP, α-fetoprotein; LRT, locoregional therapy; sHR, subhazard ratio.
Bold indicates statistical significance.
A secondary analysis considering the number of LRTs instead of the type of first treatment in the multivariable competing-risk analysis showed a lower risk of waitlist dropout among those who received 1 or more LRTs, compared with zero, although there was no appreciable difference with more LRTs (Supplementary Table 3).
Among the 24,145 patients who had LRTs recorded, the first type of LRT received was chemoembolization in 66.2%, thermal ablation in 20.2%, and radioembolization in 5.7%. Patients who received chemoembolization as the first treatment compared with other types of LRT were more likely to have a greater initial tumor burden in terms of tumor number, total tumor diameter, and size of largest tumor (Supplementary Table 4). In the most recent era (2015–2018), chemoembolization was the first type of LRT in 59.2% of cases, thermal ablation in 19.0%, and radioembolization in 13.6%. Radioembolization, thermal ablation, combination therapy, and external beam radiation were associated with a lower risk of second LRT, compared with chemoembolization (Supplementary Table 5). Conversely, other types of LRT, which included chemical ablation and cryoablation, were associated with a higher risk of subsequent LRT.
There were 11,488 LT recipients with available explant data. Patients with complete necrosis on explant were more likely to have lower MELD or Child–Pugh class, lower AFP level, smaller totaltumor diameter, and fewer tumors at baseline, as well as longer waitlist time (Table 3) (P < .01). Two or more LRTs was associated with a higher risk of incomplete or no necrosis on explant, as was chemoembolization as the first or last treatment compared with other types of LRT (P < .01).
Table 3.
Baseline, Tumor, and Treatment Characteristics for Incomplete or No Tumor Necrosis on Explant Histology (n = 11,488)
| Complete necrosis (N = 3542) | Incomplete or no necrosis (N = 8029) | P | |
|---|---|---|---|
| Initial total tumor diameter, cm (IQR) | 2.7 (2.2–3.7) | 3.0 (2.3–4.1) | <.01 |
| Initial tumor number, n (%) | <.01 | ||
| 0–1 | 3066 (86.6) | 6349 (79.1) | |
| 2 | 384 (10.8) | 1294 (16.1) | |
| 3 | 88 (2.5) | 369 (4.6) | |
| ≥4 | 4 (0.1) | 17 (0.2) | |
| Initial AFP level, ng/mL, n (%) | <.01 | ||
| <20 | 2735 (77.6) | 5714 (71.7) | |
| 21–40 | 25 (0.7) | 60 (0.8) | |
| 41–500 | 289 (8.2) | 775 (9.7) | |
| 501–1000 | 451 (12.8) | 1327 (16.7) | |
| ≥1000 | 23 (0.7) | 91 (1.1) | |
| Child–Pugh class, n (%) | <.01 | ||
| A | 1992 (56.3) | 3954 (49.3) | |
| B | 1302 (36.8) | 3314 (41.3) | |
| C | 247 (7.0) | 760 (9.5) | |
| Region of initial listing, n (%) | .09 | ||
| Short | 1034 (29.2) | 2451 (30.5) | |
| Medium | 1012 (28.6) | 2142 (26.7) | |
| Long | 1496 (42.2) | 3436 (42.8) | |
| Type of first LRT, n (%) | <.01 | ||
| Chemoembolization | 1887 (53.3) | 4981 (62.0) | |
| Radioembolization | 293 (8.3) | 497 (6.2) | |
| Thermal ablation | 840 (23.7) | 1180 (14.7) | |
| Combination | 160 (4.5) | 312 (3.9) | |
| Other | 110 (3.1) | 225 (2.8) | |
| None | 252 (7.1) | 834 (10.4) | |
| Type of last LRT, n (%) | <.01 | ||
| Chemoembolization | 1693 (47.8) | 4661 (58.1) | |
| Radioembolization | 301 (8.5) | 512 (6.4) | |
| Thermal ablation | 979 (27.6) | 1423 (17.7) | |
| Combination | 159 (4.5) | 264 (3.3) | |
| Other | 158 (4.5) | 335 (4.2) | |
| None | 252 (7.1) | 834 (10.4) | |
| Waiting time, d (per 100 days) | 284 (191–460) | 259 (156–436) | <.01 |
| Number of LRTs, n (%) | <.01 | ||
| 0 | 252 (7.1) | 834 (10.4) | |
| 1 | 2152 (60.8) | 4190 (52.2) | |
| 2 | 873 (24.6) | 2084 (26.0) | |
| 3 | 196 (5.5) | 667 (8.3) | |
| ≥4 | 69 (1.9) | 254 (3.2) |
AFP, α-fetoprotein; IQR, interquartile range; LRT, locoregional therapy.
Conclusions
In this large nationwide cohort of LT candidates with HCC, LRT was used increasingly to bridge to LT. Only 42.3% of LT candidates waitlisted in 2003 received LRT; this increased to 92.4% for candidates waitlisted in 2018. The use of radioembolization as bridging LRT has increased notably in the past 5 years, now making up 19% of LRTs in 2018, compared with less than 5% as recently as 2013. Adjusting for initial tumor characteristics and underlying severity of liver disease, LRT was associated with a lower risk of waitlist dropout. In the IPTW-adjusted competing-risks analysis, waitlist dropout was lower among those who received radioembolization or thermal ablation as the first treatment compared with chemoembolization. As expected, larger initial tumor diameter, higher initial AFP level, greater severity of liver disease, and listing in longer wait time regions and in more recent years were associated with a higher risk of waitlist dropout.
We observed a higher incidence of LRT among patients with a greater tumor burden and higher AFP level, and a lower incidence among those with more severe liver disease. These observations are consistent with contemporary clinical realities—larger tumors outside Milan criteria must be downstaged by LRT to qualify for LT, and increased severity of the underlying liver disease can preclude the ability to administer LRT due to concern for worsening hepatic decompensation.16
Few head-to-head comparisons of different types of LRT exist, which contributes to the various practice patterns seen worldwide. Specific types of LRT may be better suited for certain types of tumors, such as ablation for solitary tumors smaller than 3 cm, but ablation is relatively contraindicated for tumors in close proximity to major vascular or biliary structures, hollow viscera, and the diaphragm, owing to the risks of collateral damage or incomplete treatment.7 The occurrence of tract seeding from percutaneous ablation, although uncommon, also may compromise LT candidacy. Comparisons of efficacy between specific modalities of LRT thus are limited because eligibility for these treatments is variable and rarely reported. In our IPTW-adjusted analysis, either radioembolization and thermal ablation as the first LRT was associated with a lower risk of waitlist dropout compared with chemoembolization. Similarly, in 1 randomized controlled trial of 45 patients, increased time to progression was observed in those receiving radioembolization compared with chemoembolization.8 Based on this experience, this center adopted radioembolization as the first-line LRT for HCC.17 This is not yet a common practice, however; our study shows that use of radioembolization by center still varies widely, ranging from 0% to 84%, even during the most recent period (2015–2018). No specific guidelines exist yet on the preferred type of LRT for specific characteristics of HCC. Future prospective studies or randomized trials, although often more difficult to execute, are needed to directly compare treatment modalities for early to intermediate stage HCC. For now, center variation in experience and expertise with types of LRT makes it challenging to project future trends in LRT and analyze LRT-related outcomes.
Expanded criteria for HCC beyond Milan, as well as longer wait times, necessitate the use of bridging LRT to maintain control of tumor burden, increasing health care utilization in the pretransplant setting, exposing patients to greater risk of complications from LRT, and potentially increasing costs within the health care system. Based on Medicare reimbursement rates, the cost of each chemoembolization (including overnight hospitalization) has been estimated to be approximately $17,000 vs $31,000 to $48,000 for unilobar or bilobar radioembolization.18 We observed a lower risk of receiving subsequent treatment when the first treatment was radioembolization, thermal ablation, external beam radiation, or combination therapy, compared with chemoembolization. In addition, LT recipients who received radioembolization, thermal ablation, or combination therapy as the first LRT were more likely to have complete necrosis (no viable tumor) on explant, compared with those who received chemoembolization. Techniques such as radioembolization, although more costly and resource-intensive initially, may be more cost effective if fewer treatments are needed in total. Still, the majority of patients received only 1 LRT, the most common being chemoembolization. Additional studies considering not only waitlist outcome and explant pathology, but also post-transplant survival and cost effectiveness, may help to identify patients who might benefit upfront from 1 type of LRT vs another. There also is growing interest in the use of systemic therapy or even immunotherapy among LT candidates, either alone or in combination with LRT.19 Use of these therapies remains relatively rare and is not yet collected or reported in the OPTN database but deserves further study.
This study leverages the national OPTN database, a comprehensive record of all waitlist and transplant events in the United States spanning decades. Transplant programs are mandated to enter clinical information for each waitlist registration, with auditing for accuracy, and the HCC exception pathway in particular requires updates every 3 months. Nonetheless, potential data inconsistencies remain a limitation of this study. Specifically, the accuracy of information regarding dates and types of locoregional therapy may vary depending on the center and data entry personnel. In addition, reasons for removal from the waitlist beyond death or being too sick are not provided, so waitlist dropout as a result of HCC progression vs liver failure are not distinguished. Additional considerations are that selection of LRT is dependent on tumor characteristics, which are not completely captured in the OPTN database; and that the analyses involving explant data are susceptible to selection bias because they represent only patients who were able to receive a transplant.
Changes in exception points and US liver allocation over the years also may have influenced results. Starting in 2005, patients with HCC not yet meeting T2 criteria no longer received standardized exception points, which would tend to delay LRT until patients met the size criteria for listing. In 2015, the implementation of cap-and-delay limited accrued exception points to a maximum of 34 and instituted a mandatory 6-month waiting period before granting exception points.20 We did observe that the incidence of LRT increased after cap-and-delay on October 8, 2015. Overall, access to LT for patients with HCC has decreased, lengthening waiting times and increasing the need for bridging therapies while awaiting LT. In addition, downstaging protocols implemented in the past several years have increased the acceptable initial tumor burden for LT, and how much locoregional therapy LT candidates must undergo to qualify and remain eligible for LT. Still, patients who qualified for LT through downstaging, as defined by initial tumor burden beyond Milan criteria, made up a minority of cases in this cohort. How recent changes in liver allocation for exception points based on regional median MELD at transplant, implemented after the timeframe of this study, will influence these patterns remains to be seen. Geographic differences were evident in this study, with patients in longer wait time regions at greater odds of receiving more LRT and at higher risk of waitlist dropout. Changing demographics and management of the underlying liver disease during the study period also may have contributed to the observed results, the major event being the availability of effective direct-acting antiviral therapy for hepatitis C starting in 2013, which could slow progression of underlying liver disease, leading to reduced risk of waitlist dropout or transplant and thus longer waiting times, and allow patients with hepatitis C to tolerate additional LRT while awaiting LT.21
In summary, we show that the use of bridging LRT before LT has increased over the past 15 years, with a notable increase in radioembolization, although its use is still not as prevalent as chemoembolization. Our findings corroborate those from previous studies suggesting that use of bridging LRT is associated with improved waitlist and post-LT outcomes.18 Longer waitlist times for patients with HCC, although helpful to clarify tumor biology, are associated with greater use of bridging LRT, incurring increased costs and risk of complications during the waiting period. Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context. Further study is needed to clarify the impact of the various LRT types on waitlist and post-LT outcomes.
Supplementary Material
What You Need to Know.
Background
Waiting times for patients with hepatocellular carcinoma have lengthened, leading to changes in utilization of locoregional therapy among liver transplant candidates in the United States.
Findings
Use of locoregional therapy while awaiting liver transplantation has increased over the past 15 years, up to 92.4% in 2018. Chemoembolization remains the most frequent type, followed by ablation and radioembolization. Receipt of locoregional therapy is associated with improved waitlist outcomes.
Implications for patient care
Longer waitlist times for patients with hepatocellular carcinoma help to clarify tumor biology but necessitate increased use of bridging locoregional therapy. Locoregional therapy, although associated with a lower risk of waitlist dropout, may increase costs and the risk of complications during the waiting period.
Funding
Supported in part by National Institute of Allergy and Infectious Diseases grant R25 AI-147369 and an American Association for the Study of Liver Diseases Foundation Clinical, Translational, and Outcomes Research Award (A.J.K.). The funding organizations played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations used in this paper:
- AFP
α-fetoprotein
- HCC
hepatocellular carcinoma
- IPTW
inverse probability of treatment weighting
- IQR
inter-quartile range
- LRT
locoregional therapy
- LT
liver transplantation
- MELD
model for end-stage liver disease
- OPTN
Organ Procurement and Transplantation Network
- sHR
subhazard ratio
- UNOS
United Network for Organ Sharing database
Footnotes
CRediT Authorship Contributions
Allison J. Kwong, MD (Data curation: Lead; Formal analysis: Lead; Investigation: Equal; Methodology: Lead; Writing – original draft: Lead)
T. Tara Ghaziani (Writing – review & editing: Equal)
Francis Yao (Writing – review & editing: Equal)
Daniel Sze (Writing – review & editing: Equal)
Ajitha Mannalithara (Formal analysis: Supporting)
Neil Mehta (Conceptualization: Lead; Investigation: Equal; Methodology: Supporting; Supervision: Lead; Writing – review & editing: Equal)
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.07.048.
References
- 1.Ferrante ND, Pillai A, Singal AG. Update on the diagnosis and treatment of hepatocellular carcinoma. Gastroenterol Hepatol 2020;16:506–516. [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 3.Mehta N, Bhangui P, Yao FY, et al. Liver transplantation for hepatocellular carcinoma. Working group report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020;104:1136–1142. [DOI] [PubMed] [Google Scholar]
- 4.Verna EC, Patel YA, Aggarwal A, et al. Liver transplantation for hepatocellular carcinoma: management after the transplant. Am J Transplant 2020;20:333–347. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology 2015;61:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67:358–380. [DOI] [PubMed] [Google Scholar]
- 7.Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89–97. [DOI] [PubMed] [Google Scholar]
- 8.Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016;151:1155–1163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavien P-A, Lesurtel M, Bossuyt PMM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012; 13:e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006; 12:1260–1267. [DOI] [PubMed] [Google Scholar]
- 11.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation: alpha-fetoprotein increase and m RECIST progression. Liver Transpl 2013;19:1108–1118. [DOI] [PubMed] [Google Scholar]
- 12.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology 2018;67:381–400. [DOI] [PubMed] [Google Scholar]
- 14.Mehta N, Dodge JL, Hirose R, et al. Increasing liver transplantation wait-list dropout for hepatocellular carcinoma with widening geographical disparities: implications for organ allocation. Liver Transpl 2018;24:1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann A, Billionnet C. Covariate adjustment of cumulative incidence functions for competing risks data using inverse probability of treatment weighting. Comput Methods Programs Biomed 2016;129:63–70. [DOI] [PubMed] [Google Scholar]
- 16.Mehta N, Guy J, Frenette CT, et al. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: a multicenter study. Clin Gastroenterol Hepatol 2018;16:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018; 68:1429–1440. [DOI] [PubMed] [Google Scholar]
- 18.Rostambeigi N, Dekarske AS, Austin EE, et al. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:1075–1084. [DOI] [PubMed] [Google Scholar]
- 19.Vitale A, Volk ML, Pastorelli D, et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology 2010;51:165–173. [DOI] [PubMed] [Google Scholar]
- 20.Yohanathan L, Heimbach JK. The impact of allocation changes on patients with hepatocellular carcinoma. Clin Liver Dis 2020; 24:657–663. [DOI] [PubMed] [Google Scholar]
- 21.Kwong A, Kim WR, Mannalithara A, et al. Decreasing mortality and disease severity in hepatitis C patients awaiting liver transplantation in the United States. Liver Transpl 2018; 24:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


