Abstract
The clinical benefit associated with anti-amyloid immunotherapies, a new class of drugs for the treatment of Alzheimer’s disease, is predicated on their ability to modify disease course by lowering brain amyloid levels. At the time of writing, two amyloid-lowering antibodies, aducanumab and lecanemab, have obtained United States Food and Drug Administration accelerated approval, with further agents of this class in the Alzheimer’s disease treatment pipeline. Based on limited published clinical trial data to date, regulators, payors and physicians will need to assess their efficacy, clinical effectiveness and safety, as well as cost and accessibility. We propose that attention to three important questions related to treatment efficacy, clinical effectiveness and safety should guide evidence-based consideration of this important class of drugs. These are: (1) Were trial statistical analyses appropriate and did they convincingly support claims of efficacy? (2) Do reported treatment effects outweigh safety concerns and are they generalizable to a representative clinical population of people with Alzheimer’s disease? and (3) Do the data convincingly demonstrate disease course modification, suggesting that increasing clinical benefits beyond the duration of the trials are likely? We suggest specific approaches to interpreting trial results for these drugs and highlight important areas of uncertainty where additional data and a cautious interpretation of existing results is warranted. Safe, effective and accessible treatments for Alzheimer’s disease are eagerly awaited by millions of patients and their caregivers worldwide. While amyloid-targeting immunotherapies may be promising disease-modifying Alzheimer’s disease treatments, rigorous and unbiased assessment of clinical trial data is critical to regulatory decision-making and subsequently determining their provision and utility in routine clinical practice. Our recommendations provide a framework for evidence-based appraisal of these drugs by regulators, payors, physicians and patients.
Keywords: amyloid immunotherapy, Alzheimer, dementia, MCI, disease-modifying
Amyloid-targeting immunotherapies may be promising disease-modifying treatments for Alzheimer’s disease. Rigorous and unbiased assessment of clinical trial data is critical for regulatory decision-making and evidence-based clinical guidelines. Liu et al. present a framework for regulators, payors, physicians, and patients to evaluate the potential clinical benefits and safety of amyloid-targeting immunotherapies.
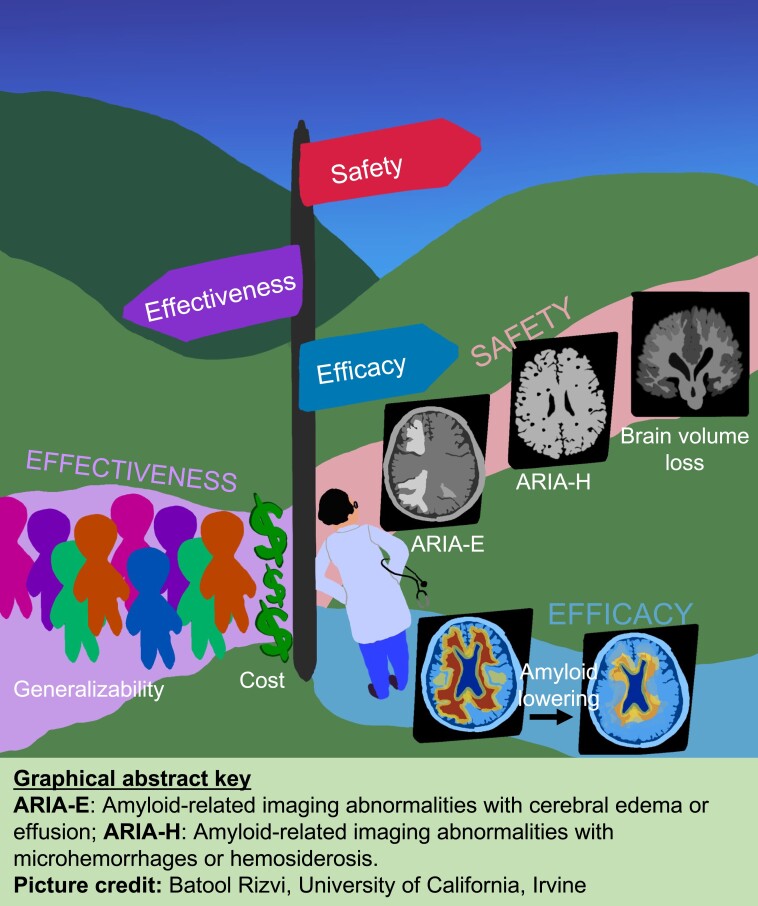
Graphical Abstract
Graphical abstract.
The US Food and Drug Administration (FDA)’s accelerated approval of aducanumab and lecanemab, based on an assumption that brain amyloid-beta (Aβ) lowering is likely to predict clinical benefit in Alzheimer’s disease, has set a precedent for this drug class.1 Two additional anti-amyloid monoclonal antibodies have also been granted ‘Breakthrough Therapy’ status by the FDA, signalling that their respective Biologics License Applications (BLAs) are eligible for accelerated review and approval (Tables 1 and 2). However, of these two passive immunotherapies, the FDA declined to grant accelerated approval for donanemab based on only limited phase 2 trial data,20 and the gantenerumab phase 3 trials did not meet their primary endpoints. Furthermore, the US Centers for Medicare and Medicaid Services’ (CMS) decision to limit coverage of anti-amyloid immunotherapies approved by the FDA shows that the pathway to Market may not be straightforward.21 Deliberations by regulators and payors over access to drugs in this class have already spurred passionate arguments over whether treatment efficacy, clinical meaningfulness, and safety have been demonstrated, as well as regarding treatment costs and implications for provision and access.
Table 1.
Efficacy and safety of amyloid lowering antibodies granted ‘breakthrough therapy’ designation by FDA for the treatment of sporadic/late-onset Alzheimer’s disease
| Antibody | Efficacy | Safety |
|---|---|---|
| Aducanumab | One phase 3 trial, EMERGE, showed a 0.39-CDR-SB point (23%) better outcome for the high-dose treatment group at 18 months, but the identical ENGAGE trial high-dose group showed a 0.03 CDR-SB point (2%) worsening.2 Both trials were prematurely discontinued following futility analyses. | 425 of 1029 patients (41.3%) in the high-dose group experienced ARIA (ARIA-E; 362/1029—35.2%, ARIA-H; 291/1029—28.3%) with serious cases in 14 patients (1.4%).3 Four reported deaths in patients potentially exposed to the drug.4 A significant increase in lateral ventricular volume occurred in all drug groups.2 |
| Lecanemab | Phase-2b: did not meet primary endpoint at 12 months.5 Additional pre-specified Bayesian analyses indicated a > 95% probability of being superior to placebo by any magnitude at 12 and 18 months. Phase 3: met primary endpoint, a between-group treatment difference of a −0.45 CDR-SB point at 18 months, presented as a 27% reduction in clinical decline.6 | Phase 2b: 20 of 161 patients (12.4%) in the 10 mg/kg arm experienced ARIA (ARIA-E; 16/161—9.9%, ARIA-H; 11/161—6.8%) with serious cases in 3 patients (1.9%).5 Greater reduction in whole brain volume observed at all doses, and greater expansion in total ventricular volume with 5 mg/kg and 10 mg/kg doses, relative to placebo. Phase 3: 10 mg/kg versus placebo was associated with: ARIA-E 12.6% versus 1.7%; ARIA-H 17.3% versus 9.0%; ARIA-E or ARIA-H 21.5%versus 9.5%; concurrent ARIA-E and ARIA-H 8.2%, 1.0%,6 respectively. Serious cases in 10 patients (1.1%).6 Three reported patient deaths due to brain swelling or macrohaemorrhages in open-label extensions.7,8 |
| Donanemab | Phase-2 trial met primary endpoint—a 3.2 iADRS point benefit (designated minimally clinically significant effect size = 6 points). No significant effects on secondary outcomes.9 | 51 of 131 patients (38.9%) experienced ARIA (ARIA-E; 35/131–26.7%, ARIA-H; 40/131–30.5%) with serious cases occurring in 2 patients (1.5%).9 Greater decrease in whole-brain volume and greater increase in ventricular volume observed versus placebo.9 |
| Gantenerumab | Two phase-3 trials in early Alzheimer’s disease (Scarlet RoAD and Marguerite) terminated early for futility.10 A trial in asymptomatic patients with dominantly inherited Alzheimer’s disease did not show evidence of slowing cognitive decline.11 Two phase-3 trials (GRADUATE-1 and 2, testing higher doses in early Alzheimer’s disease than Marguerite and Scarlet RoAD) did not meet primary endpoints in early Alzheimer’s diseasea.12 |
For the 225 mg arm versus placebo in Scarlet RoAD trial, incidence of ARIA-E was 13.5% versus 0.8%, ARIA-H was 16.2% versus 13.2%.10 No difference between groups for whole brain, hippocampal and ventricular volumes. For the pooled treatment arms of Scarlet RoAD, incidence of ARIA-E was 25% with most being asymptomatic. ARIA-H incidence not reported but stated to be balanced across groupsa.13 |
Data were primarily compiled from published peer-reviewed literature. Early Alzheimer’s disease is here defined as MCI and mild dementia due to Alzheimer’s disease.
Non-peer-reviewed data that were obtained from news reports and/or online sources.
Table 2.
Regulatory status, generalizability and cost of amyloid lowering antibodies granted ‘breakthrough therapy’ designation by FDA for the treatment of sporadic/late-onset Alzheimer’s disease
| Antibody | Regulatory Status | Generalizability of Findings | COST and CMS Assessment |
|---|---|---|---|
| Aducanumab | Accelerated FDA approval in June 20211 based on phase 3 trial data in early Alzheimer’s disease. | 19 of 3285 enrolled participants were Black/African American, only 6 were randomized to high dose arm.2 < 1% of memory clinic patients would be eligible to receive treatment based on phase 3 trial inclusion/exclusion criteria.14 | Initial annual drug cost of $56 000 revised to $28 200.15 ICER estimated cost-effective annual pricing between $3000–8400.16 CMS issued a NCD providing coverage with evidence development requiring demonstration of clinical efficacy in clinical trial(s).17 |
| Lecanemab | Accelerated FDA approval in January 2023 based on phase 2b trial data in early Alzheimer’s disease.5 Supplemental BLA for traditional approval under consideration by FDA (includes results from phase-3 CLARITY-AD trial in early Alzheimer’s disease).6 | Phase-2b: No race/ethnicity data published. Phase 3: 27% of US enrolment included Hispanic and African American participants.6 | Cost $26 500 annually per patient.18 ICER estimated cost-effective annual pricing between $8900–21 500.19 Broader CMS coverage may be provided if lecanemab receives full FDA approval. |
| Donanemab | BLA for accelerated approval based on phase-2 TRAILBLAZER-ALZ (early Alzheimer’s disease) rejected by the FDA.20 Ongoing phase-3 TRAILBLAZER-ALZ 2 (early Alzheimer’s disease) results expected in 2023. | 2.9% Black and 1.1% Asian compared to 94.9% White patients enrolled.9 | N/A |
| Gantenerumab | FDA Breakthrough Therapy designation in October 2021. | No race/ethnicity data reported from published phase-2 Scarlet RoAD trial. | N/A |
Data were primarily compiled from published peer-reviewed literature. Early Alzheimer’s disease is here defined as MCI and mild dementia due to Alzheimer’s disease.
NCD, national coverage determination.
Here, we highlight three key questions that should be asked in the evaluation of the potential clinical benefit and safety of BLAs for amyloid-targeting antibodies in Alzheimer’s disease:
Were trial statistical analyses appropriate and did they support claims of efficacy?
Do reported treatment effects outweigh safety concerns, and are they generalizable to a representative clinical population of people with Alzheimer’s disease?
Do the data convincingly demonstrate disease course modification, suggesting that increasing clinical benefits beyond the duration of the trials are likely?
We have summarized proposed approaches to address these key questions and associated challenges in text boxes at the end of each section. Since this review primarily concerns evaluation of clinical benefit/safety of amyloid-targeting drugs following accelerated approval in the USA, we have not included a discussion on the appropriateness of biomarker selection, whether accelerated approval based on biomarker changes alone is appropriate, or on additional considerations related to cost-effectiveness analyses. These related, important issues lie outside the intended scope of this article and warrant further discussion in separate focused analyses.
Were statistical analyses appropriate and did they support efficacy?
While independent evaluation and peer-reviewed publication of clinical trial data analyses are not requirements for FDA approval, it is important to ensure that adequate and appropriate statistical analyses are conducted to support any claims of treatment efficacy. Phase-3 trial primary outcomes of the approved agents, aducanumab and lecanemab, are not inconsistent with prior negative trials from this drug class, where uncertainty estimates have included potential for small clinical benefit,22 and scores on cognitive and functional outcome scales lie within the range of values attributed to heterogeneous rates of decline.23 Key considerations include whether any small, statistically significant differences are likely to mainly reflect the contribution of biases from loss to follow-up or functional unblinding, as the detection of any such effects will be amplified with an increase in sample size.
Amyloid-related imaging abnormalities (ARIAs) represent an adverse event associated with exposure to amyloid-lowering immunotherapies, and analyses should assess the extent to which this could have resulted in both functional unblinding to treatment allocation24 and group differences in loss to follow-up. Potential unblinding of patients, their caregivers and clinicians arises from infusion reactions, dose suspensions or alterations and additional unscheduled MRI surveillance, as well as, for a minority of cases, ARIA symptoms such as headache, confusion, altered consciousness, seizures, unsteadiness and vomiting. Effects of unblinding on patient and caregiver responses to questions assessing subjective impression of changes in functional abilities may be especially important. These subjective reports contribute to a significant part of key primary and secondary trial outcome scores, including the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) and Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory-Mild Cognitive Impairment.
Disappointingly, publications from phase 2/3 trials of amyloid targeting antibodies have included few details on statistical analyses conducted to address potential confounding due to unblinding. In two phase 3 aducanumab trials, 425 of 1029 patients (41.3%) in the 10-mg/kg group experienced ARIA [ARIA with cerebral oedema or effusion (ARIA-E) and/or ARIA with microhaemorrhages or haemosiderosis (ARIA-H)].3 While trial protocols may attempt to limit functional unblinding via the blinding of independent raters to ARIA diagnoses and other medical information, they fail to address potential unblinding of patients and their caregivers who were the primary sources of information for assessing clinical outcomes. Analyses that the FDA considered in its evaluation of Biogen’s BLA for aducanumab25,26 included a comparison of the primary analysis using the complete intention-to-treat dataset, versus a reduced intention-to-treat dataset, where all assessments after occurrences of ARIA were excluded. These analyses reportedly found no evidence of a systematic bias due to functional unblinding. However, the FDA’s statistical reviewer noted that such analyses of censored data have significant limitations, as they break the randomization and/or imbalance the treatment and placebo groups’ distributions of follow-up. This is especially true of sensitivity analyses in ‘post-randomization event-defined subgroups’, where the adverse event (i.e. ARIA) is strongly associated with drug exposure.26
In the phase 3 lecanemab (CLARITY-AD) trial for early AD, reported incidences of ARIA-E in the lecanemab and placebo groups were 12.6% and 1.7%, and 17.3% and 9.0%, respectively, for ARIA-H. In addition, 26.4% of the lecanemab group experienced infusion-related reactions compared with 7.4% of the placebo group. The investigators reported a pre-specified mixed-model repeated measure, censoring assessment after occurrence of ARIA-E, to conclude that the results were generally consistent with the primary analysis.6 However, no sensitivity analyses were performed to account for potential unblinding due to infusion-related reactions or ARIA-H.
The phase 2 donanemab (TRAILBLAZER-ALZ) trial in patients with early symptomatic Alzheimer’s disease reported incidences of ARIA-E and ARIA-H of 27.5% and 30.5%, respectively,9 which required additional MRI surveillance every 4–6 weeks until ‘resolution’ and for all new cases of ARIA-H until they had ‘stabilized’. The published report of the trial does not detail sensitivity analyses performed to assess the effects of the risk of unblinding in patients with ARIA. The authors merely state that they visually inspected the curves representing change in the integrated Alzheimer’s Disease Rating Scale (iADRS) between those with and without ARIA-E and concluded that they were similar. Furthermore, no sensitivity analyses were reported to address potential unblinding due to ARIA-H.
Finally, given the potential effect of unblinding on patients’ and caregivers’ subjective impressions of change on functional outcome scales, it would be important to systematically assess whether an efficacy signal on these functional scales is supported by converging results on neuropsychological outcomes such as the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog).27 For instance, in the CLARITY-AD trial, a statistically significant effect favouring lecanemab over placebo on the primary outcome (CDR-SB) was also observed on secondary outcomes including the cognitive subscale of the Alzheimer’s Disease Assessment Scale (14-item ADAS-Cog).6 The main points in this section are summarized in Box 1.
Box 1. Were statistical analyses appropriate and did they support efficacy?
Potential challenges
Testing a hypothesis multiple times using trials’ data increases the risk that a statistically significant finding will occur by chance.
ARIA, an adverse and frequently symptomatic effect of treatment and associated with treatment modification and/or MRI surveillance and infusion-related reactions increase the risk of functional unblinding and biased outcome scoring.
Proposed approaches
A full tabulation of all analyses performed including exploratory and post-hoc assessments should be presented.
Details of statistical analyses to adjust for multiple hypotheses testing should be provided.
Sensitivity analyses can investigate whether outcome results from scales assessing functional abilities are convergent with those from cognitive assessments.
Future trials should consider including dose suspensions and unscheduled dummy MRI scans in placebo groups to balance sources of unblinding.
Sponsors should implement a data-sharing plan to make individual patient-level clinical trial data publicly available in a timely manner to qualified investigators, allowing external evaluation of study design and analyses.
Do reported treatment effects outweigh safety concerns?
Treatment effects
A statistically significant (P < 0.05) drug-placebo difference in a well-designed and appropriately analysed trial indicates that, assuming no drug effect, a difference between groups at least as extreme as observed was unlikely to have occurred due to chance. It is thus important to ascertain the clinical relevance of any such statistically significant result, as even very small, clinically trivial differences can achieve statistical significance if the sample size is sufficiently large.28
Although there are no consensus-based gold-standard methods to establish minimal clinically important difference (MCID) in Alzheimer’s disease clinical trials, anchor-based methods [i.e. clinical outcome assessment (COA) score changes linked to clinical opinion] are prioritized over distribution-based approaches (i.e. statistical calibration of MCID based on variation in COA score changes). For individuals with mild cognitive impairment (MCI) due to Alzheimer’s disease, an MCID estimate of around 1 point on the CDR-SB scale has been reported from separate studies using different Alzheimer’s disease databases and methodological approaches.29,30 Moreover, the MCID is likely to increase with progression along the Alzheimer’s disease severity spectrum.29
It has been proposed that MCIDs applied to group means do not reflect potentially heterogeneous treatment effects at the level of the individual.31 In other words, there may be a distinction between overall between-group treatment effects and the MCID defined as a within-individual meaningful change in a proportion of participants. Although this is a reasonable assumption, parallel-arm trials are not designed to assess within-individual treatment responses and doing so has implications for the presentation of trial findings. For example, rather than applying an MCID threshold to between-group differences, applying the same threshold to individual participants’ score changes can define subgroups who reached the MCID threshold (‘responders’) and those who did not (‘non-responders’). ‘Responder analyses’ of these groups present (potentially more favourable) differences in response rates between treatment arms, which can then be presented as probability/risk ratios or number needed to treat (NNT) to be a ‘responder’, but this approach is associated with serious statistical limitations.32,33 A post-hoc ‘responder analysis’ does not actually reflect individual responses to treatment in a parallel arm trial, since we do not know how a given individual’s data would have changed if they had been left untreated. Hence, this approach is less effective at disentangling the treatment outcome from any true treatment effect compared to a between-group analysis of randomized controlled trial (RCT) data.32–34 Also, in addition to loss of statistical power, the post-hoc dichotomization of a continuous COA variable risks mainly identifying ‘borderline’ cases for whom even a marginal response to the drug pushes them past the cut-off to be labelled as a ‘responder’, rather than being a distinguishable group who experience notable benefit from treatment. This concept is also relevant for risk calculations that depend on (potentially arbitrarily) dichotomized or time-to-event cognitive outcomes, such as hazard ratios and NNTs. The number needed to harm (NNH) for ARIA or death is more straightforward in this respect, but whilst NNT/NNH can be intuitive approaches for clinical decision-making, they do not communicate the context of the risk reduction/increase (e.g. an absolute risk reduction of 0.01 could be from 0.95 to 0.94, or from 0.03 to 0.02) nor the uncertainty associated with the estimate. We would therefore recommend considerable caution in interpreting clinical meaningfulness if a post-hoc responder analysis is presented using within-individual change thresholds, or when interpreting risk measures.
Safety concerns
ARIAs are a widely recognized adverse effect associated with anti-amyloid antibodies. They are mostly considered to be clinically benign and resolve either spontaneously or after a brief treatment suspension. However, a proportion of cases are symptomatic, and some individuals experience serious side-effects, especially after high-dose anti-amyloid immunotherapy (affecting around 1 in 200 treated patients).35–38 These serious symptomatic ARIA can also be associated with long-term consequences.35,37,39 Important risk factors for ARIA, in addition to drug dose, include Apolipoprotein E (APOE) ε4 carrier status (with a higher risk in APOE ε4 carriers), baseline microbleeds, antithrombotic drugs (for ARIA-H) and specific properties of the amyloid-targeting antibodies.38–41 Other factors, such as comorbid cerebrovascular disease and risk factors, autoimmune or inflammatory conditions, seizures or disorders associated with extensive white matter pathology, may also increase the risk of ARIA.41 These risk factors were identified within RCTs with strict participant exclusions regarding comorbid cardiological, neurological and auto-immune conditions.35
In order to comprehensively assess safety, regulators must have access to details of radiological severity (mild, moderate or severe), clinical severity (mild, moderate, severe, life-threatening or fatal) and clinical outcomes (resolved, resolved with sequelae, unresolved, study withdrawal, fatal or no follow-up) associated with ARIA. These results should also be published in the peer-reviewed literature in a timely manner.
A lesser-known adverse effect of anti-amyloid drugs is accelerated brain volume loss (such as enlargement of ventricular CSF volume or shrinkage of hippocampus and whole brain). These effects have been reported for lecanemab, aducanumab and donanemab (as well as many other anti-amyloid drugs).2,5,42,43 This is concerning, since outside of RCTs, acceleration of brain volume changes in Alzheimer’s disease has consistently been attributed to accelerated neurodegeneration.44 These changes have also been reported to be associated with domain-specific cognitive impairments in a prior clinical trial.44,45 Attempts have been made to explain these findings, for example, by suggesting that loss of plaque volume explains reduced brain volume, but the amount of plaque in the brain is far too small to constitute an observable change on MRIs.42 ARIA is one logical putative cause of brain volume changes, but this has not yet been investigated. A recent meta-analysis reported a correlation between the frequency of ARIA and enlargement in ventricular volume associated with exposure to monoclonal antibodies targeting brain amyloid.46
It is equally important to know which other brain regions may be affected, since only hippocampal, ventricular, and whole brain volumes are typically reported. The statistical protocol for the phase 3 trials of aducanumab pre-specified that regional brain volume changes would be explored upon stratification by ARIA and APOE ɛ4 status and listed 13 regional brain volumes for investigation, but these findings were not fully reported. There is no public evidence that the FDA reviewed the volumetric data for aducanumab before approval. Certainly, none of the FDA’s published clinical and statistical reviews comment on the brain volume changes in an otherwise extensive analysis of the other trial data. Indeed, the first report of volume changes caused by aducanumab was published nine months after the drug was approved by the FDA.2 The label for aducanumab (marketed as AduhelmTM) does not disclose that accelerated ventricular enlargement is an associated complication of this drug. Similarly, it is clear from the phase-2b trial that all doses of lecanemab caused accelerated brain volume loss relative to placebo.5 These results must be clarified and peer-reviewed as soon as possible. It is also critical that regulatory agencies consider brain volume changes associated with these drugs in their review of BLAs.
To understand the longer term effects of exposure to amyloid lowering antibodies, we recommend that all safety data from associated open-label extension studies be updated regularly and made publicly accessible, as a condition of regulatory approval, especially if the purported accrual of clinical benefits from disease-modifying therapies (DMTs) are claimed to be dependent on their long-term administration. Establishing a registry recording the management of any life-threatening symptoms related to ARIA or other adverse events is essential to inform future treatment guidelines. Regulators may also consider safety monitoring strategies such as risk minimization programmes for patients who initiate treatment with these drugs.
Generalizability of trial findings
The evaluation of clinical meaningfulness, via comparison of MCIDs and treatment-related differences in trial outcomes, together with assessment of safety concerns, relies on the generalizability of these comparisons to the clinical population that will eventually receive treatment. MCID estimates, generated from earlier clinical trials and Alzheimer’s disease research databases29,30 and previous DMT clinical trials,14,47 have recruited predominantly White and more highly educated individuals, who are younger and suffer from fewer comorbid illnesses than the more representative Alzheimer’s disease population seen in clinics. In contrast to the phase-3 trials of aducanumab that enrolled very few Black patients,2 more than 20% of the participants in North America, Europe and Asia in the CLARITY-AD trial of lecanemab were of non-White ethnicity.6 Of the US participants, 4.5% were Black and 22.5% were Hispanic. The concern about limited generalizability of findings from highly selected patient populations was underscored by the CMS’ recent decision to cover FDA approved monoclonal antibodies for the treatment of Alzheimer’s disease under ‘coverage with evidence development’ (CED). The CMS guideline for trials that are used to generate this evidence states:21
The diversity of patients included in each trial must be representative of the national population diagnosed with Alzheimer’s disease.
Besides race and ethnicity, it would also be important to ensure that the eventual product labelling of an approved drug aligns with trials’ participant inclusion and exclusion criteria, such as Alzheimer’s disease severity stage (MCI or mild dementia due to Alzheimer’s disease, etc.), presence of specific medical comorbidities and confirmation of brain amyloid positivity. The main points in this section are summarized in Box 2.
Box 2. Do reported treatment effects outweigh safety concerns?
Potential challenges
A statistically significant between-group difference at trial endpoint does not mean that the difference was clinically meaningful.
The reported MCID for early Alzheimer’s disease has been reliably estimated to be ≥1 CDR-SB point, and the between-group treatment effects at study endpoint from published clinical trials do not meet this threshold.
Emerging concerns underline rare and life-threatening ARIA with high-dose anti-amyloid monoclonal antibodies.34
The long-term clinical effects of ARIA and brain volume loss, particularly on disease progression, are unknown.
Trial data and MCID estimates have not been representative of and may not generalize to clinical populations.
Proposed approaches
Alternative, post-hoc summary statistics should be interpreted cautiously.
Long-term clinical outcomes of ARIA and brain volume reductions should be monitored by the sponsor.
All data on clinical severity and outcomes associated with ARIA should be disclosed.
Safety data from open-label extension studies should be updated regularly and publicly accessible.
Ensure a robust Risk Evaluation and Mitigation Strategy is established.
Trial data and MCID estimates from new studies that include participants who are more representative of Alzheimer’s disease patients in clinical practice are urgently needed.
Do data convincingly demonstrate disease course modification, suggesting increasing clinical benefits beyond the trials’ duration?
An emerging hypothesis, specific to DMTs in Alzheimer’s disease, is that continued treatment will lead to cumulative clinical benefit via assumed modification of underlying disease processes,48,49 e.g. a constant proportional treatment effect such as a 20–30% slowing of disease progression. This hypothesis argues that the consideration of MCID values at a trials’ primary endpoint may not represent a DMT’s ultimate treatment benefit, because this can be expected to increase with subsequent follow-up, and relative treatment effects on COA scores and/or surrogate biomarker levels at trial endpoint are assumed to predict future clinical benefit.48,49 Relatedly, comparing time to progression to an MCID level of decline between treatment groups is sometimes proposed to measure the extent to which disease progression would be delayed in the treatment group with ongoing treatment. Lecanemab has been widely reported to result in a 27% reduction in cognitive decline compared to placebo. Aside from obscuring the relatively small magnitude and uncertain clinical significance of the absolute mean difference, and omitting important information such as measures of uncertainty (e.g. 95% confidence intervals) and variability of decline, use of a relative percentage measure as a summary statistic has promoted the unsupported assumption that clinical benefit will proportionally accrue with longer term DMT treatment. These may serve to inflate perceived benefits of drug treatment, especially for lay audiences.
However, current clinical trials of anti-amyloid immunotherapies have not been designed to test for disease-modifying versus symptomatic properties. Such distinction could be addressed in future trials using delayed-start or staggered withdrawal designs, as already in Parkinson’s disease.50 Post-hoc comparisons of slopes, i.e. the progression of the primary outcome, between parallel-arm treatment groups cannot establish disease course modification. A reversible, symptomatic effect can also appear to ‘slow’ or ‘delay’ cognitive decline and lead to a difference in time-to-event. Hence, claims of disease modification by these drugs do not invalidate the relevance of MCIDs. Further, evaluation of clinical benefits on the basis of any post-hoc analyses and as yet unavailable randomized data would involve significant uncertainty. We would therefore urge caution in the interpretation of any analyses of clinical meaningfulness that rely on low-level evidence-based assumptions of disease modification.
The relatively small between-group treatment effects of currently approved amyloid-lowering agents, which lie below empirically derived MCID estimates, has contributed to ambiguity in what constitutes a ‘clinically meaningful’ effect. Alternative trial designs may help to clarify whether treatment effects were clinically meaningful within individuals, considering baseline disease severity and CDR-SB domain(s) affected, and whether any relationship can be established between treatment duration and disease course modification. The main points in this section are summarized in Box 3.
Box 3. Do the data convincingly demonstrate disease course modification, suggesting that increasing clinical benefits beyond the duration of the trials are likely?
Potential challenges
Post-hoc comparisons of slopes, i.e. the progression of the primary outcome, between parallel-arm treatment groups cannot establish disease course modification.
A reversible, symptomatic effect can also appear to ‘slow’ or ‘delay’ cognitive decline and lead to a difference in time-to-event.
Proposed approaches
Alternative trial designs may be needed to clarify whether clinically meaningful within-individual treatment effects or disease course modification have occurred, e.g. delayed-start or staggered withdrawal designs.
Simple assumptions of disease course modification should not invalidate the relevance of MCIDs.
Conclusion
The advent of a new class of licenced drugs for the treatment of Alzheimer’s disease, particularly with the promise of disease course modification, represents an exciting and long-anticipated milestone for patients, caregivers and their doctors. However, with the limited data that have been made available from licencing clinical trials, questions over biases due to unblinding and differential drop out, as well as concerns about safety and clinical and cost-effectiveness remain. These must be addressed by regulators and payors when making approval decisions and by clinicians and patients when treatments are licenced. Hope and desperation for a treatment are not good reasons to drive such impactful decisions. We propose that attention to these three pertinent questions can guide an informed and evidence-based consideration of this important class of drugs.
Acknowledgements
We are grateful to Ms. Batool Rizvi, University of California Irvine (UCI) for creating the graphical abstract.
Abbreviations
- Aβ =
amyloid-beta
- ADAS-Cog14 =
14-item Alzheimer’s Disease Assessment Scale-Cognitive
- ARIAs =
amyloid-related imaging abnormalities
- ARIA-E =
ARIA with cerebral oedema or effusion
- ARIA-H =
ARIA with microhaemorrhages or haemosiderosis
- BLAs =
Biologics License Applications
- CDR-SB =
Clinical Dementia Rating Scale Sum of Boxes
- COA =
clinical outcome assessment
- CMS =
(US) Centers for Medicare and Medicaid Services
- DMT =
disease-modifying therapy
- FDA =
(US) Food and Drug Administration
- ICER =
Institute for Clinical and Economic Review
- iADRS =
integrated Alzheimer's Disease Rating Scale
- MCI =
mild cognitive impairment
- MCID =
minimal clinically important difference
- NNH =
number needed to harm
- NNT =
number needed to treat
- RCT =
randomized controlled trial.
Contributor Information
Kathy Y Liu, Division of Psychiatry, University College London, London W1T 7NF, UK.
Nicolas Villain, AP-HP.Sorbonne Université, Institut de la Mémoire et de la Maladie d’Alzheimer, Département de Neurologie, Hôpital Pitié-Salpêtrière, 75013 Paris, France; Sorbonne Université, Institut national de la Santé et de la Recherche Medical (INSERM) U1127, Centre National de la Recherche Scientifique (CNRS) 7225, Institut du Cerveau—ICM, 75013 Paris, France.
Scott Ayton, Melbourne Dementia Research Centre, Florey Institute of Neuroscience and Mental Health, The University of Melbourne, Parkville, VIC 3052, Australia.
Sarah F Ackley, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA 94158, USA.
Vincent Planche, Univ. Bordeaux, CNRS, UMR 5293, Institut des Maladies Neurodégénératives, F-33000 Bordeaux, France; Centre Mémoire Ressources Recherches, Pôle de Neurosciences Cliniques, CHU de Bordeaux, F-33000 Bordeaux, France.
Robert Howard, Division of Psychiatry, University College London, London W1T 7NF, UK.
Madhav Thambisetty, Clinical and Translational Neuroscience Unit, Laboratory of Behavioral Neuroscience, National Institute on Aging, Baltimore, MD 21224, USA.
Funding
Supported in part by the Intramural Research Program (IRP) of the National Institute on Aging. R.H. is supported by the National Institute for Health Research University College Hospital London Biomedical Research Centre. K.Y.L. is supported by the UK Medical Research Council (MR/S021418/1).
Competing interests
Independent of this work, N.V. received research support from Fondation Bettencourt-Schueller, Fondation Servier, Union Nationale pour les Intérêts de la Médecine (UNIM), Fondation Claude Pompidou, Fondation Alzheimer and Fondation pour la Recherche sur l’Alzheimer; travel grant from the Movement Disorders Society, Merz-Pharma, UCB Pharma, and GE Healthcare SAS; is an unpaid local principal investigator or sub-investigator in NCT04241068 and NCT05310071 (aducanumab, Biogen), NCT04437511 (donanemab, Eli-Lilly), NCT05463731 (remternetug, Eli-Lilly), NCT04592341 (gantenerumab, Roche), NCT03887455 (lecanemab, Eisai), NCT03352557 (gosuranemab, Biogen), NCT03828747 and NCT03289143 (semorinemab, Roche), NCT04619420 (JNJ-63733657, Janssen—Johnson & Johnson), NCT04374136 (AL001, Alector), NCT04592874 (AL002, Alector), NCT04867616 (bepranemab, UCB Pharma), NCT04777396 and NCT04777409 (semaglutide, Novo Nordisk); and has given unpaid lectures in symposia organized by Eisai and the Servier Foundation. During the past 3 years, V.P. was a local unpaid investigator or sub-investigator for the following trials: Evoke and Evoke + (NCT04777396, NCT04777409, NovoNordisk), Tango and Embark (NCT03352527, NCT04241068, Biogen), Lucidity (NCT03446001, TauRx Pharmaceuticals), Autonomy (NCT04619420, Janssen), Green Memory (NCT04520412, Green Valley Pharmaceuticals) and INFRONT-3 (NCT04374136, Alector). He received research grants from Fondation Bettencourt-Schueller (CCA-Inserm-Bettencourt) and from Fondation PSP-France. M.T. served as member of FDA Peripheral and Central Nervous System (PCNS) advisory committee evaluating Biologics License Application (BLA) 761178 for aducanumab solution for intravenous infusion for the treatment of Alzheimer’s disease. This service was not remunerated and was performed as part of his official duties as an NIH employee. The other authors do not have any relevant disclosures.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- 1. Dunn B, Stein P, Cavazzoni P. Approval of aducanumab for Alzheimer disease-the FDA's perspective. JAMA Intern Med. 2021;181(10):1276–1278. [DOI] [PubMed] [Google Scholar]
- 2. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. [DOI] [PubMed] [Google Scholar]
- 3. Salloway S, Chalkias S, Barkhof F, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terry M. Additional Deaths of Patients Taking Aduhelm Spark Renewed Concern. BioSpace 2022. Accessed 13 February 2023.https://www.biospace.com/article/additional-patient-deaths-in-patients-receiving-biogen-s-aducanumab/.
- 5. Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-abeta protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9–21. [DOI] [PubMed] [Google Scholar]
- 7. Couzin-Frankel J, Piller C. Alzheimer's drug stirs excitement-and concerns. Science. 2022;378(6624):1030–1031. [DOI] [PubMed] [Google Scholar]
- 8. Reish NJ, Jamshidi P, Stamm B, et al. Multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-PA for stroke. N Engl J Med. 2023;388(5):478–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691–1704. [DOI] [PubMed] [Google Scholar]
- 10. Ostrowitzki S, Lasser RA, Dorflinger E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salloway S, Farlow M, McDade E, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease. Nat Med. 2021;27(7):1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genentech Provides Update on Phase III GRADUATE Program Evaluating Gantenerumab in Early Alzheimer’s Disease. Genentech Press Releases. Published November 13 2022. Accessed 16 June 2023. https://www.gene.com/media/press-releases/14974/2022-11-13/genentech-provides-update-on-phase-iii-g.
- 13. Meglio M. Gantenerumab Fails to Meet End Points in Initial GRADUATE Study Data. 2022;5. Accessed 16 June 2023. https://cdn.sanity.io/files/0vv8moc6/neurolive/8c29929a167f2d6d31beb594b69abcc37f8ba398.pdf/NeurologyLive_December2022_FINAL.pdf.
- 14. Canevelli M, Rossi PD, Astrone P, Consorti E, Vanacore N, Cesari M. “Real world” eligibility for aducanumab. J Am Geriatr Soc. 2021;69(10):2995–2998. [DOI] [PubMed] [Google Scholar]
- 15. Robinson JC. Why is aducanumab priced at $56,000 per patient? Lessons for drug-pricing reform. N Engl J Med. 2021;385(22):2017–2019. [DOI] [PubMed] [Google Scholar]
- 16. Whittington MD, Campbell JD, Rind D, Fluetsch N, Lin GA, Pearson SD. Cost-effectiveness and value-based pricing of aducanumab for patients with early Alzheimer disease. Neurology. 2022;98(9):e968–e977. [DOI] [PubMed] [Google Scholar]
- 17. Dhruva SS, Ramachandran R, Ross JS. Medicare's national coverage determination for aducanumab—A one-off or a pragmatic path forward? N Engl J Med. 2022;387(17):1539–1541. [DOI] [PubMed] [Google Scholar]
- 18. Mahase E. Alzheimer's disease: FDA approves lecanemab amid cost and safety concerns. BMJ. 2023;380:73. [DOI] [PubMed] [Google Scholar]
- 19. Lin GA, Whittington MD, Wright A, Agboola F, Herron-Smith S, Pearson SD, Rind DM. Beta-Amyloid Antibodies for Early Alzheimer’s Disease: Effectiveness and Value; Evidence Report. Institute for Clinical and Economic Review. https://icer.org/assessment/alzheimers-disease-2022/#timeline.
- 20. Feuerstein A . FDA Rejects Lilly’s Bid for Accelerated Approval for its Alzheimer’s Drug. Accessed 13 February 2023.https://www.statnews.com/2023/01/19/fda-rejects-lillys-bid-for-accelerated-approval-for-its-alzheimers-drug/.
- 21.Centers for Medicare & Medicaid Services. Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease. Published April 7, 2022. Accessed 16 June 2023. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305.
- 22. Ackley SF, Zimmerman SC, Brenowitz WD, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: Instrumental variable meta-analysis. BMJ. 2021;372:n156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jutten RJ, Sikkes SAM, Van der Flier WM, et al. Finding treatment effects in Alzheimer trials in the face of disease progression heterogeneity. Neurology. 2021;96(22):e2673–e2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gleason A, Ayton S, Bush AI. Unblinded by the light: Amyloid-related imaging abnormalities in Alzheimer's clinical trials. Eur J Neurol. 2021;28(1):e1. [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration. Aduhelm (aducanumab) FDA CLINICAL REVIEW. Published online June 7, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761178Orig1s000MedR_Redacted.pdf.
- 26.US Food and Drug Administration. FDA Statistical Review and Evaluation. Published June 22, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761178Orig1s000StatR_Redacted.pdf.
- 27. Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer's disease assessment scale-cognitive subscale (ADAS-cog): Modifications and responsiveness in pre-dementia populations. A narrative review. J Alzheimers Dis. 2018;63(2):423–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu KY, Schneider LS, Howard R. The need to show minimum clinically important differences in Alzheimer's disease trials. Lancet Psychiatry. 2021;8(11):1013–1016. [DOI] [PubMed] [Google Scholar]
- 29. Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement (N Y). 2019;5:354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lansdall CJ, McDougall F, Butler LM, et al. Establishing clinically meaningful change on outcome assessments frequently used in trials of mild cognitive impairment due to Alzheimer's disease. J Prev Alzheimers Dis. 2023;10(1):9–18. [DOI] [PubMed] [Google Scholar]
- 31. U.S. Food and Drug Administration . Patient-focused drug development public workshop guidance 4 discussion document. Published December 6, 2019. Accessed 19 April 2021. https://www.fda.gov/media/132505/download.
- 32. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferreira G, Maher CG, et al. Problems with responder analysis in clinical trials: In response to Korownyk . BMJ. 2021;372:m4825. [Google Scholar]
- 34. Senn S. Statistical pitfalls of personalized medicine. Nature. 2018;563(7733):619–621. [DOI] [PubMed] [Google Scholar]
- 35. Villain N, Planche V, Levy R. High-clearance anti-amyloid immunotherapies in Alzheimer's disease. Part 1: Meta-analysis and review of efficacy and safety data, and medico-economical aspects. Rev Neurol (Paris). 2022;178(10):1011–1030. [DOI] [PubMed] [Google Scholar]
- 36. Brashear HR, Ketter N, Bogert J, Di J, Salloway SP, Sperling R. Clinical evaluation of amyloid-related imaging abnormalities in bapineuzumab phase III studies. J Alzheimers Dis. 2018;66(4):1409–1424. [DOI] [PubMed] [Google Scholar]
- 37. VandeVrede L, Gibbs DM, Koestler M, et al. Symptomatic amyloid-related imaging abnormalities in an APOE epsilon4/epsilon4 patient treated with aducanumab. Alzheimers Dement (Amst). 2020;12(1):e12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-Related imaging abnormalities and beta-amyloid-targeting antibodies: A systematic review. JAMA Neurol. 2022;79(3):291–304. [DOI] [PubMed] [Google Scholar]
- 39. Barakos J, Purcell D, Suhy J, et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with anti-amyloid beta therapy. J Prev Alzheimers Dis. 2022;9(2):211–220. [DOI] [PubMed] [Google Scholar]
- 40. Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat Rev Neurol. 2020;16(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cummings J, Rabinovici GD, Atri A, et al. Aducanumab: Appropriate use recommendations update. J Prev Alzheimers Dis. 2022;9(2):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ayton S. Brain volume loss due to donanemab. Eur J Neurol. 2021;28(9):e67–e68. [DOI] [PubMed] [Google Scholar]
- 43. Thambisetty M. Anti-Amyloid Drugs and the Mystery of Treatment-Associated Brain Shrinkage. Accessed 13 February 2023. https://www.statnews.com/2022/11/28/anti-amyloid-drugs-treatment-associated-brain-shrinkage/.
- 44. Hua X, Hibar DP, Lee S, et al. Sex and age differences in atrophic rates: An ADNI study with n=1368 MRI scans. Neurobiol Aging. 2010;31(8):1463–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Apostolova LG, Babakchanian S, Hwang KS, et al. Ventricular enlargement and its clinical correlates in the imaging cohort from the ADCS MCI donepezil/vitamin E study. Alzheimer Dis Assoc Disord. 2013;27(2):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alves F, Kallinowski P, Ayton S. Accelerated brain volume loss caused by anti-beta-amyloid drugs: A systematic review and meta-analysis. Neurology. 2023;100(20):e2114–e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manly JJ, Glymour MM. What the aducanumab approval reveals about Alzheimer disease research. JAMA Neurol. 2021;78(11):1305–1306. [DOI] [PubMed] [Google Scholar]
- 48. Assuncao SS, Sperling RA, Ritchie C, et al. Meaningful benefits: A framework to assess disease-modifying therapies in preclinical and early Alzheimer's disease. Alzheimers Res Ther. 2022;14(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Petersen RC, Aisen PS, Andrews JS, et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement. 2023. doi: 10.1002/alz.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahlskog JE, Uitti RJ. Rasagiline, Parkinson neuroprotection, and delayed-start trials: Still no satisfaction? Neurology. 2010;74(14):1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.