Abstract
Aims:
To test the hypothesis that GLP-1 receptor (GLP-1R) agonists have beneficial effects on vascular endothelial function, fibrinolysis, and inflammation through weight loss-independent mechanisms.
Materials and Methods:
Individuals with obesity and pre-diabetes were randomized to 14 weeks of the GLP-1R agonist liraglutide, hypocaloric diet, or the DPP4 inhibitor sitagliptin in a 2:1:1 ratio. Treatment with drug was double blind and placebo controlled. Measurements were made at baseline, after 2 weeks prior to significant weight loss, and after 14 weeks. The primary outcomes were measures of endothelial function: flow-mediated vasodilation (FMD), plasminogen activator inhibitor-1 (PAI-1), and urine albumin-to-creatinine ratio (UACR).
Results:
Eighty-eight individuals were studied (liraglutide N=44, diet N=22, sitagliptin N=22). Liraglutide and diet reduced weight, insulin resistance, and PAI-1, while sitagliptin did not. There was no significant effect of any treatment on endothelial vasodilator function measured by FMD. Post hoc subgroup analyses in individuals with baseline FMD below the median, indicative of greater endothelial dysfunction, demonstrated improvement in FMD by all three treatments. GLP-1R antagonism with exendin (9–39) increased fasting blood glucose but did not change FMD or PAI-1. There was no effect of treatment on UACR. Finally, liraglutide, but not sitagliptin or diet, reduced the chemokine monocyte chemoattractant protein-1 (MCP-1).
Conclusions:
Liraglutide and diet reduce weight, insulin resistance and PAI-1. Liraglutide, sitagliptin and diet do not change FMD in obese pre-diabetic individuals with normal endothelial function. Liraglutide alone lowers the pro-inflammatory and pro-atherosclerotic chemokine MCP-1, indicating that this beneficial effect is independent of weight loss.
Introduction
Glucagon-like peptide-1 receptor (GLP-1R) agonists decrease cardiovascular morbidity and mortality in patients with type 2 diabetes mellitus (T2DM).1–3 GLP-1R agonists also cause significant weight loss and have been approved as pharmacologic weight loss therapy.4 Given that weight loss improves many of the risk factors for cardiovascular disease in patients with T2DM and insulin resistance,5,6 the question remains whether the beneficial cardiovascular effects of the GLP-1R agonists are fully or partially attributable to weight loss.
Dipeptidyl peptidase 4 (DPP4) inhibitors increase endogenous GLP-1 without inducing weight loss. DPP4 inhibitors have not been shown to reduce cardiovascular mortality in T2DM.7 In addition to preventing the degradation of GLP-1, DPP4 inhibitors also prevent the degradation and formation of a variety of vasoactive peptides, rendering their actions less specific.8
To understand the weight loss versus GLP-1R-dependent effects of the GLP-1R agonist liraglutide on vascular function, we compared the effects of liraglutide to hypocaloric diet-induced weight loss and to treatment with the DPP4 inhibitor sitagliptin in a randomized controlled trial. We enrolled obese pre-diabetic individuals and measured weight, metabolic parameters, hemodynamic variables, endothelial function, fibrinolysis, and markers of inflammation at baseline, after two weeks of therapy before anticipated clinically significant weight loss, and after 14 weeks of therapy. We evaluated endothelial function using flow-mediated vasodilation (FMD) and assessed fibrinolysis by measuring plasminogen activator inhibitor-1 (PAI-1), the major inhibitor of tissue-type plasminogen activator in vivo. We also evaluated a marker of vascular inflammation by measuring the chemokine monocyte chemoattractant protein-1 (MCP-1). In addition, to assess whether the effects of treatment were GLP-1R-dependent, we studied a subset of participants after treatment with the GLP-1R antagonist exendin (9–39) and matching vehicle in a randomized, crossover design.
Materials and Methods
Detailed methods, including inclusion/exclusion criteria, screening/randomization/study procedures, and power calculations, are available in the Supplementary Appendix.
Protocol
Men and women aged 18 to 65 with obesity (BMI ≥ 30 kg/m2) and pre-diabetes were eligible. The study was approved by the Vanderbilt Institutional Review Board, registered at clinicaltrials.gov NCT03101930, and conducted according to the Declaration of Helsinki. All participants provided written informed consent.
Participants underwent a six-week run-in to optimize their medical management (Figure S1A). Participants then underwent a baseline study day for anthropometric and hemodynamic measurements, FMD, blood collection for glucose, insulin, PAI-1, MCP-1, P-selectin, and urine collection for albumin and creatinine.
Participants were then randomized in parallel to liraglutide 1.8mg/day (Novo Nordisk), sitagliptin 100mg/day (Merck and Co, Inc), or hypocaloric diet in a 2:1:1 ratio, stratified by race. Within each stratum, we used a block randomization algorithm with a block size of four. A higher proportion of participants were randomized to liraglutide to enable future investigation of individual predictors of response. Liraglutide was given on a dose escalation starting at 0.6mg/day for week 1, 1.2mg/day for week 2 and then up to the full dose of 1.8mg/day at the start of week 3. Treatment with liraglutide or sitagliptin was double blind and placebo-controlled, while treatment with diet was unblinded. Liraglutide and matching placebo were a generous gift from Novo Nordisk.
Participants were treated for a total of 14 weeks. The first 74 participants underwent four study days in a 2×2 crossover study of the GLP-1R antagonist exendin (9–39) and placebo (Figure S1B). The first two study days were conducted after two weeks of treatment to measure short-term effects. The first and second study days were separated by 48 hours and participants received a single dose infusion of placebo or exendin (9–39) on each day to assess the contribution of GLP-1R activation to any observed effects. We randomly assigned individuals to two sequences (exendin → placebo or placebo → exendin) in a 1:1 ratio using a block randomization algorithm with a block size of two. The third and fourth study days were completed after 14 weeks of treatment to measure the sustained chronic effects. The third and fourth study days were also separated by 48 hours and participants again received placebo or exendin (9–39) infusion in random order. The last 14 individuals studied did not participate in the crossover study with exendin (9–39) due to lack of drug availability, and only underwent two study days, one after 2 weeks and one after 14 weeks. Placebo vehicle was infused during each of these study days. All participants underwent repeat urine collection after 13.5 weeks at a separate visit to the Clinical Research Center.
Statistical Analyses
The primary endpoints were endothelial vascular function (FMD, UACR) and fibrinolytic function (PAI-1) after 2 weeks (prior to clinically significant weight loss) and after 14 weeks of treatment. Secondary endpoints reported here include blood pressure, heart rate, fasting insulin and glucose, MCP-1, and P-selectin. All analyses were by original assigned groups.
Descriptive statistics of patient baseline characteristics are presented as mean ± SD for continuous variables and frequencies and proportions for categorical variables. Between-group comparisons in Table 1 were performed using Kruskal–Wallis or Pearson’s chi-squared test, with pairwise comparisons made using Wilcoxon rank-sum test when the Kruskal-Wallis test was positive. To evaluate the treatment effects on variables measured pre-infusion (weight, systolic blood pressure, diastolic blood pressure, heart rate and pre-infusion PAI-1, P-selectin, and MCP-1), separate multivariable generalized least squares linear regression models were fitted using the data from pre-infusion measurements alone. Treatment (liraglutide, sitagliptin or diet), time (2- or 14-week), baseline measurement as well as interaction between treatment and time were included as independent variables. A compound symmetry structure for within-subject correlation was used. For variables measured post-infusion (fasting blood glucose, fasting insulin, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), forearm blood flow measures including FMD percent, and post-infusion PAI-1), separate multivariable generalized least squares linear regression models were fitted using data from post-infusion measurements. Treatment (liraglutide, sitagliptin or diet), infusion (vehicle or exendin (9–39)), time (2- or 14-week), baseline measurement as well as all the two-way and three-way interactions between treatment, time and infusion were included as independent variables. For each measure in the model, we examined the residuals and confirmed that they were normally distributed. For brachial artery diameter, FMD and NMD, gender was included as a covariate. Inferences on the contrasts of interest were conducted using Wald test. Estimates of change in outcome from baseline to 2- and 14-weeks were calculated based on the multivariable regression model. Spearman’s rank correlation was computed to assess the relationship between PAI-1 and HOMA-IR or MCP-1. All the analyses were performed using the statistical software R 4.1.0.
Table 1.
Baseline Participant Characteristics
| Measures | Liraglutide | Sitagliptin | Diet | Overall |
|---|---|---|---|---|
| N=44 | N=22 | N=22 | N=88 | |
|
| ||||
| Age, years | 49.8±10.1 | 52.4±10.5 | 49.2±12.5 | 50.3±10.8 |
| Gender | ||||
| Male | 29.5% (13) | 31.8% (7) | 36.4% (8) | 31.8% (28) |
| Female | 70.5% (31) | 68.2% (15) | 63.6% (14) | 68.2% (60) |
| Race | ||||
| Asian | 4.5% (2) | 0% (0) | 0% (0) | 2.3% (2) |
| Black or African American | 9.1% (4) | 13.6% (3) | 18.2% (4) | 12.5% (11) |
| White | 86.4% (38) | 81.8% (18) | 77.3% (17) | 83.0% (73) |
| More Than One Race | 0% (0) | 4.5% (1) | 4.5% (1) | 2.3% (2) |
| Ethnicity | ||||
| Hispanic or Latino | 2.3% (1) | 9.1% (2) | 4.5% (1) | 4.5% (4) |
| NOT Hispanic or Latino | 95.5% (42) | 90.9% (20) | 95.5% (21) | 94.3% (83) |
| Unknown / Not Reported | 2.3% (1) | 0% (0) | 0% (0) | 1.1% (1) |
| Weight, kg | 108.8±20.9 | 111.4±22.0 | 111.3±21.5 | 110.1±21.1 |
| BMI, kg/m2 | 38.8±6.1 | 39.9±6.0 | 38.4±5.9 | 39.0±6.0 |
| Waist Circumference, cm | 115.8±11.5 | 118.4±14.5 | 117.5±13.6 | 116.9±12.8 |
| Hip Circumference, cm | 126.2±12.7 | 126.2±12.0 | 125.7±10.9 | 126.1±11.9 |
| Fasting blood glucose, mg/dL | 96.2±10.1 | 100.2±9.4 | 96.7±11.8 | 97.4±10.4 |
| Fasting insulin, μU/mL | 21.4±13.9 | 26.9±14.0 | 18.4±7.4 | 22.3±12.8 |
| OGTT 2-hour blood glucose, mg/dL | 142.0±24.8 | 148.1±41.1 | 130.2±27.8 | 140.6±30.7 |
| HOMA-IR | 5.0±3.0 | 6.5±3.3 | 4.6±2.4 | 5.3±3.0 |
| HOMA2 | 0.41±0.25 | 0.52±0.27 | 0.36±0.15 | 0.43±0.24 |
| Hemoglobin A1c, % | 5.7±0.3 | 5.8±0.3 | 5.7±0.3 | 5.7±0.3 |
| Total cholesterol, mg/dL | 191.0±39.2 | 192.5±31.2 | 170.9±49.9 | 186.6±40.7 |
| Triglycerides, mg/dL | 122.2±51.4 | 143.3±59.6 | 115.0±67.1 | 125.9±58.0 |
| HDL-C, mg/dL | 47.5±9.6 | 45.6±11.2 | 44.1±10.9 | 46.2±10.3 |
| LDL-C, mg/dL | 119.1±31.9 | 118.2±27.0 | 111.5±35.7 | 117.0±31.4 |
| Systolic blood pressure, mmHg | 124.1±7.7 | 120.2±11.3 * | 127.7±8.3 | 124.1±9.1 |
| Diastolic blood pressure, mmHg | 77.6±9.3 | 74.7±8.7 | 77.8±7.0 | 76.9±8.6 |
| Heart rate, bpm | 64.9±7.4 | 67.2±9.0 | 63.8±8.8 | 65.2±8.2 |
| Anti-hypertensive agent use | 25.0% (11) | 36.4% (8) | 45.5% (10) | 33.0% (29) |
| FMD, % | 10.54±5.21 | 10.39±5.37 | 10.22±5.25 | 10.42±5.20 |
All measures shown as mean±SD for continuous variables and % (N) for categorical variables. OGTT indicates oral glucose tolerance test; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; HOMA2, Homeostatic Model Assessment 2. Asterisk
indicates P<0.05 versus diet using Wilcoxon rank-sum test.
Results
Participant characteristics
Participant recruitment and follow-up was completed between May 2017 to June 2021. Ninety-three individuals were randomized to treatment (liraglutide N=46, sitagliptin N=23, hypocaloric diet N=24; Figure S2). Three participants dropped out prior to receiving treatment, and two dropped out after receiving treatment but before completing any study days. Data from the remaining 88 individuals (liraglutide N=44, sitagliptin N=22, hypocaloric diet N=22) were analyzed. Seven individuals dropped out after completing study days 1 and 2 (2 liraglutide, 5 diet) and their available data were included in the analyses. Baseline characteristics were similar (Table 1).
Effect of treatment on weight and fasting metabolic measures
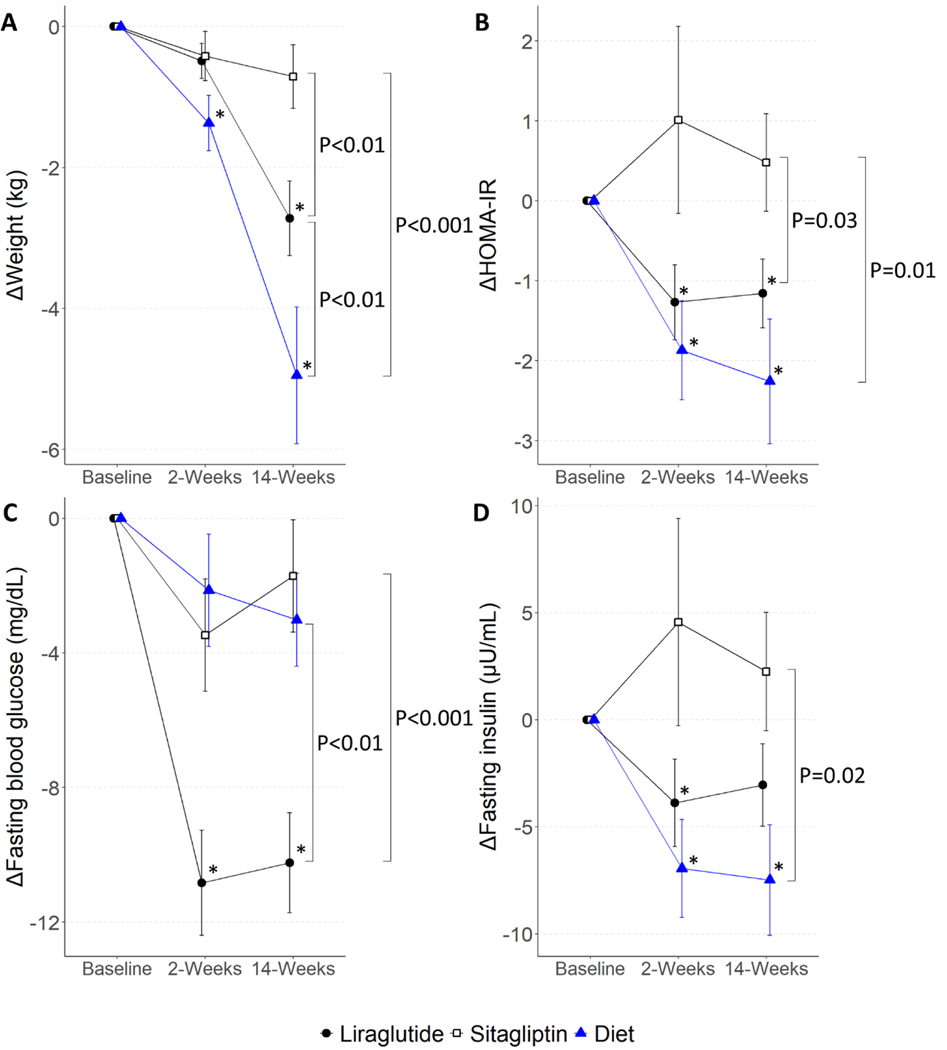
Liraglutide and diet caused weight loss, while sitagliptin did not (Figure 1A and Table 2). Weight loss in individuals in the diet arm was significant from baseline to both 2 and 14 weeks (2 weeks: difference -1.4 kg, 95% CI[−2.4, −0.3], P=0.01; 14 weeks: -4.9 kg[−6.1, −3.7], P<0.001). Individuals in the liraglutide arm lost a small amount of weight from baseline to 2 weeks (-0.5 kg[−1.3, 0.3], P=0.20), and continued to lose significant weight by 14 weeks (−2.7 kg[−3.5, −1.9], P<0.001). Hypocaloric diet-treated participants lost more weight compared to liraglutide-treated participants at 14 weeks (−2.3 kg[−3.7, −0.9], P<0.01). In addition, both diet-treated and liraglutide-treated participants lost more weight than sitagliptin-treated participants at 14 weeks (Liraglutide vs Sitagliptin −2.0 kg[−3.3, −0.6], P<0.01; Diet vs Sitagliptin −4.2 kg[−5.8, −2.6], P<0.001).
Figure 1. The Effect of Treatment on Weight, Fasting Blood Glucose, Fasting Insulin, and Insulin Resistance.
Plots show mean ± SEM for (A) weight, (B) HOMA-IR, (C) fasting blood glucose, and (D) fasting insulin at 2 and 14 weeks of treatment as change from baseline. Asterisk (*) symbols indicate P<0.05 for estimates of change from baseline, and brackets indicate difference between treatments at 14 weeks. HOMA-IR indicates Homeostatic Model Assessment of Insulin Resistance.
Table 2.
Effects of Treatment
| Liraglutide | Sitagliptin | Diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measures | Baseline | 2 weeks | 14 weeks | Baseline | 2 weeks | 14 weeks | Baseline | 2 weeks | 14 weeks |
| Weight, kg | 108.8±20.9 | 108.3±21.0 | 106.4±22.1 * † ‡ | 111.4±22.0 | 111.0±22.6 | 110.7±22.3 ‡ | 111.3±21.5 | 109.9±21.4 * | 108.6±18.1 * |
| Fasting glucose, mg/dL | 95.3±8.6 | 84.3±7.9 * † ‡ | 85.2±7.3 * † ‡ | 97.6±10.0 | 93.9±8.1 | 96.6±5.6 | 94.5±12.0 | 92.4±11.3 | 91.2±9.8 |
| Fasting insulin, μU/mL | 22.7±16.8 | 18.3±12.5 * † | 20.3±14.7 | 23.3±14.4 | 29.4±25.4 ‡ | 26.0±19.0 ‡ | 26.7±21.2 | 19.7±16.5 * | 20.3±13.7 * |
| HOMA-IR | 5.4±4.0 | 3.9±2.8 * † | 4.4±3.4 * † | 5.5±3.3 | 6.9±6.0 ‡ | 6.1±4.3 ‡ | 6.6±6.1 | 4.8±4.6 * | 4.8±3.7 * |
| Systolic BP, mmHg | 124.1±7.7 | 122.9±6.3 | 122.2±7.8 ‡ | 120.2±11.3 | 117.5±11.3 | 118.2±13.9 | 127.7±8.3 | 121.7±6.8 * | 119.7±11.1 * |
| Diastolic BP, mmHg | 77.6±9.3 | 78.3±7.7 † | 77.7±7.1 † ‡ | 74.7±8.7 | 72.3±7.8 | 73.1±9.8 | 77.9±7.0 | 76.5±5.1 | 74.8±8.3 * |
| Heart rate, bpm | 64.9±7.5 | 69.0±6.4 * † ‡ | 68.9±5.6 * † ‡ | 67.2±9.0 | 66.2±9.2 | 65.9±8.5 | 63.8±8.8 | 63.2±9.5 | 61.7±7.9 * |
| PAI-1, U/mL | 20.2±9.2 | 17.0±6.4 * | 16.9±6.4 * † | 18.5±8.6 | 16.9±6.5 | 19.5±7.0 ‡ | 18.5±8.0 | 19.4±7.5 | 15.3±5.9 * |
| MCP-1, pg/mL | 105.4±36.1 | 95.0±25.0 * | 95.2±26.9 * | 107.5±25.8 | 107.6±30.8 | 106.7±26.0 | 108.2±34.1 | 103.1±36.9 | 109.2±34.3 |
| P-selectin, ng/mL | 57.1±23.0 | 56.6±24.1 | 52.9±20.9 | 52.3±25.3 | 51.6±22.3 | 51.8±19.5 | 58.6±22.0 | 57.5±28.4 | 60.8±22.1 |
| UACR | 12.0±23.6 | ND | 10.5±14.8 | 7.9±7.6 | ND | 9.2±10.7 | 6.3±3.8 | ND | 10.1±19.4 |
|
| |||||||||
| Forearm Blood Flow Measures | |||||||||
|
| |||||||||
| Baseline diameter, mm | 3.35±0.51 | 3.25±0.47 | 3.33±0.55 | 3.51±0.62 | 3.47±0.63 | 3.47±0.66 | 3.41±0.67 | 3.44±0.74 | 3.40±0.71 |
| FMD, % | 10.54±5.21 | 11.70±5.14 | 12.01±6.18 | 10.39±5.37 | 12.18±4.56 | 11.98±4.45 | 10.22±5.25 | 10.99±5.12 | 10.73±4.80 |
| Pre-nitro diameter, mm | 3.39±0.51 | 3.14±0.40 | 3.20±0.42 | 3.82±0.65 | 3.38±0.80 | 3.68±0.72 | 3.46±0.77 | 3.55±0.85 | 3.88±0.86 |
| NMD, % | 20.39±8.59 | 22.32±8.61 | 21.60±10.07 | 19.31±6.39 | 26.57±14.19 | 21.92±8.23 | 21.05±9.92 | 20.09±9.27 | 15.44±10.91 |
All measures shown as mean±SD. HOMA-IR indicates Homeostatic Model Assessment for Insulin Resistance; PAI-1, plasminogen activator inhibitor-1; MCP-1, monocyte chemoattractant protein-1; UACR, urine albumin-to-creatinine ratio; FMD, flow-mediated dilation; Nitro, nitroglycerin; NMD, nitroglycerin-mediated dilation. ND indicates not done. Asterisk
indicates P<0.05 versus baseline; dagger
indicates P<0.05 versus sitagliptin; double dagger
P<0.05 versus diet.
Liraglutide and hypocaloric diet decreased HOMA-IR, a measure of insulin resistance, while sitagliptin did not (Figure 1B and Table 2). Diet caused the greatest decrease in HOMA-IR at both 2 and 14 weeks compared to baseline (2 weeks: −1.9[−3.1, −0.7], P<0.01; 14 weeks: −2.4[−3.7, −1.1], P<0.001). Liraglutide also decreased HOMA-IR at both 2 and 14 weeks (2 weeks: −1.3[−2.1, −0.4], P<0.01; 14 weeks: −1.2[−2.0, −0.3], P<0.01). Both diet-treated and liraglutide-treated participants had a greater decrease in HOMA-IR than sitagliptin-treated participants at 14 weeks (Liraglutide vs Sitagliptin −1.6[−3.1, −0.1], P = 0.03; Diet vs Sitagliptin −2.3[−4.1, −0.5], P=0.01).
Liraglutide decreased fasting blood glucose, while sitagliptin and hypocaloric diet did not (Figure 1C and Table 2). Liraglutide caused an early and sustained decrease in fasting blood glucose from baseline (2 weeks: −10.9 mg/dL[−13.5, −8.2], P<0.001; 14 weeks: −10.3 mg/dL[−13.0, −7.6], P<0.001). Fasting glucose in liraglutide-treated participants was significantly lower compared to sitagliptin- and diet-treated participants at 14 weeks (Liraglutide vs Sitagliptin −9.2 mg/dL[−13.9, −4.5], P<0.001; Liraglutide vs Diet −6.9 mg/dL[−11.9, −2.0], P<0.01).
Finally, diet decreased fasting insulin at both 2 and 14 weeks compared to baseline (2 weeks: −7.0 μU/mL[−11.6, −2.3], P<0.01; 14 weeks: −8.2 μU/mL[−13.3, −3.1], P<0.01; Figure 1D and Table 2). Liraglutide significantly decreased fasting insulin at 2 but not 14 weeks (2 weeks: −3.7 μU/mL[−7.1, −0.4], P=0.03; 14 weeks: −3.1 μU/mL[−6.4, 0.3], P=0.08). Sitagliptin did not alter fasting insulin at 2 or 14 weeks.
Effect of treatment on endothelial vasodilator function
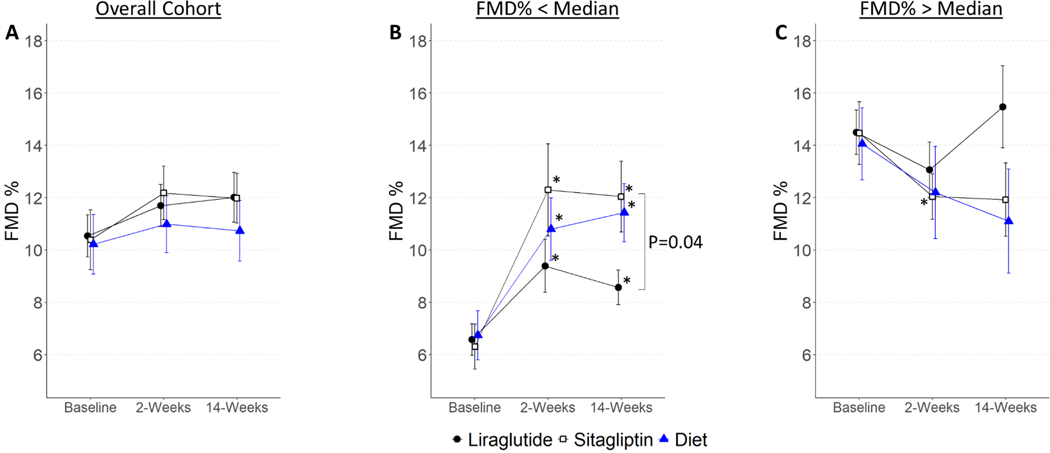
Baseline brachial artery diameter and FMD were comparable among treatment groups (Figure 2A and Table 2). Neither liraglutide, sitagliptin nor hypocaloric diet significantly changed FMD at 2 or 14 weeks compared to baseline (Liraglutide: 2 weeks: +0.70%[−0.71, 2.11], P=0.33, 14 weeks: +1.38%[−0.01, 2.78], P=0.05; Sitagliptin: 2 weeks: +1.92%[−0.05, 3.88], P=0.06, 14 weeks: +1.59%[−0.30, 3.48], P=0.10; Diet: 2 weeks: +1.24%[−0.69, 3.18], P=0.21, 14 weeks: +1.20%[−0.97, 3.38], P=0.28). There was no significant change in endothelium-independent NMD in any of the treatment groups (Table 2).
Figure 2. The Effect of Treatment on FMD.
Plots show mean ± SEM for (A) FMD in the entire cohort, (B) in subgroup with baseline FMD below median for gender, and (C) in subgroup with baseline FMD above median for gender. Asterisk (*) symbols indicate P<0.05 for estimates of change from baseline, and brackets indicate difference between treatments at 14 weeks. FMD indicates flow-mediated dilation.
In considering the cohort’s clinical characteristics, we noted that baseline endothelial function was similar to that measured in healthy subjects in our prior studies.9,10 In a post hoc exploratory analysis, we divided the cohort into those with baseline FMD below or above the median for gender, to query the effect of treatment in individuals with reduced or normal endothelial function, respectively. Baseline characteristics of these subgroups are shown in Table S1. Three individuals were missing baseline FMD and were excluded from this analysis. Individuals with lower baseline FMD, indicative of endothelial dysfunction, had increased BMI, waist circumference, fasting insulin and insulin resistance as measured by HOMA-IR and HOMA2 compared to those with higher baseline FMD. As shown in Figure 2B, in individuals with lower baseline FMD, treatment with liraglutide, sitagliptin and hypocaloric diet improved FMD after 2 and 14 weeks. Of note, FMD in individuals with normal baseline FMD did not change with any intervention, with the exception of sitagliptin treatment at 2 weeks (Figure 2C).
Additional forearm blood flow measurements are reported in Table S2. Reactive hyperemia and fold change velocity time integral (VTI), both estimates of microvascular function, increased modestly at 14 weeks by all three treatments. Changes in the microvascular ischemic response do not account for the outcomes we report. We additionally normalized FMD for shear rate using several published equations,11 and did not detect a difference.
Urine albumin-to-creatinine ratio, which is associated with vascular endothelial function and is a predictor of cardiovascular events,12–14 was not changed by treatment (Table 2). Additional summary statistics are presented in Table S3.
Effect of treatment on circulating measures of fibrinolysis and inflammation
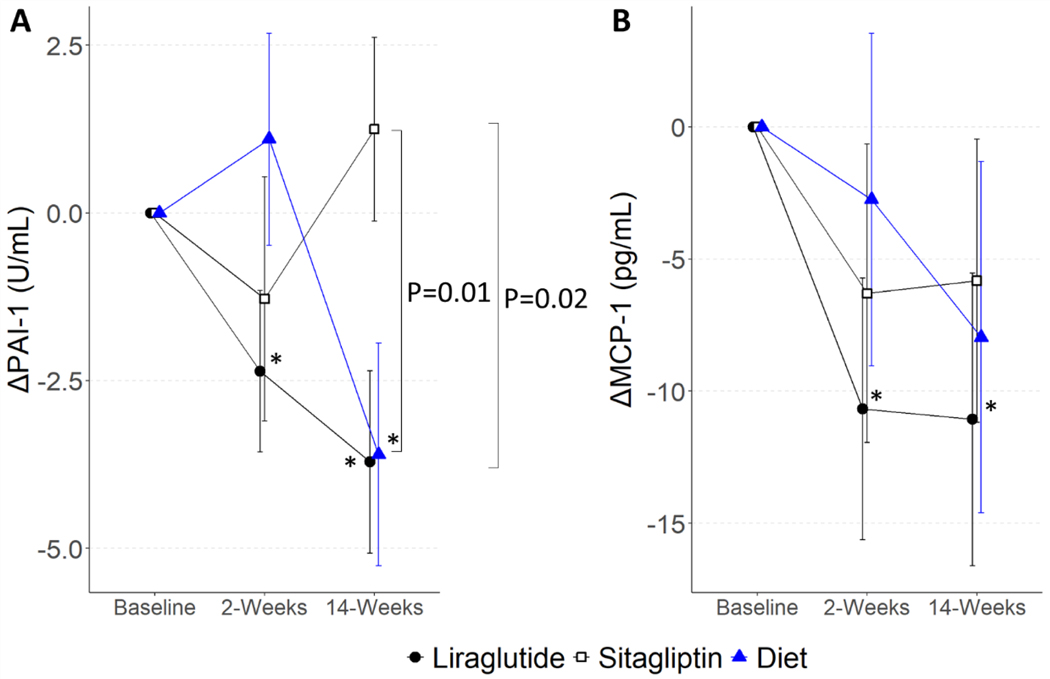
We measured concentrations of PAI-1, the major inhibitor of endogenous fibrinolysis and a marker of inflammation. Liraglutide significantly decreased PAI-1 concentration from baseline to 2 and 14 weeks (2 weeks: −2.3 U/mL[−4.1, −0.6], P<0.01; 14 weeks: −3.7 U/mL[−5.5, −2.0], P<0.001; Figure 3A and Table 2). Hypocaloric diet significantly decreased PAI-1 concentration from baseline to 14 weeks (−3.8 U/mL[−6.6, −1.0], P<0.01). Sitagliptin treatment did not affect PAI-1 concentration. In addition, PAI-1 levels were lower at 14 weeks in both the liraglutide- and diet-treated individuals compared to sitagliptin (Liraglutide vs Sitagliptin: −3.7 U/mL[−6.7, −0.6], P=0.02; Diet vs Sitagliptin: −4.8 U/mL[−8.5, −1.1], P=0.01). Finally, PAI-1 concentrations correlated with HOMA-IR both at baseline (r=0.34, P<0.01) and after treatment (Liraglutide r=0.30, P<0.01; Sitagliptin r=0.32, P<0.05; Diet r=0.49, P<0.01).
Figure 3. The Effect of Treatment on PAI-1 and MCP-1.
Plots show mean ± SEM for (A) PAI-1 and (B) MCP-1 at 2 and 14 weeks of treatment as change from baseline. Asterisk (*) symbols indicate P<0.05 for estimates of change from baseline, and brackets indicate difference between treatments at 14 weeks. PAI-1 indicates plasminogen activator inhibitor-1; MCP-1, monocyte chemoattractant protein-1.
We next measured concentrations of the circulating chemokine MCP-1. Liraglutide decreased MCP-1 at both 2 and 14 weeks (2 weeks: −10.7 pg/mL[−17.6, −3.7], P<0.01; 14 weeks: −10.7 pg/mL[−17.7, −3.7], P<0.01; Figure 3B and Table 2). Sitagliptin and hypocaloric diet did not significantly change MCP-1 levels. MCP-1 levels did not correlate with HOMA-IR (data not shown) but correlated with PAI-1 at baseline (r=0.30, P=0.01) and after treatment with liraglutide at 2 weeks (r=0.32, P<0.05), but not 14 weeks (r=0.08, P=0.60). Finally, P-selectin, a marker of platelet and endothelial activation, was unchanged by treatment (Table 2).
Effect of treatment on resting hemodynamics
Systolic blood pressure decreased significantly after hypocaloric diet (2 weeks: −6.0 mmHg[−9.4, −2.7], P<0.001; 14 weeks: −8.0 mmHg[−11.6, −4.3], P<0.001), but not with liraglutide or sitagliptin (Figure S3A). Diastolic blood pressure and heart rate also decreased significantly after 14 weeks of hypocaloric diet (DBP: −3.8 mmHg[−6.7, −0.9], P=0.01; HR: −2.9 bpm[−5.4, −0.4], P=0.02; Figure S3B). Liraglutide treatment increased heart rate at both 2 weeks (+4.0 bpm[2.4, 5.7], P<0.001) and 14 weeks (+3.7 bpm[2.1, 5.4], P<0.001; Figure S3C).
Effect of GLP-1R antagonism
To test the hypothesis that GLP-1R activation contributes to the effects of liraglutide or sitagliptin, we pre-treated participants with the GLP-1R antagonist exendin (9–39) and vehicle at 2 and 14 weeks. As shown in Figure S4A and Table S4, GLP-1R antagonism with exendin (9–39) raised fasting blood glucose in all treatment groups at both 2 and 14 weeks. Overall, exendin did not change FMD in liraglutide, sitagliptin, or diet-treated participants (Figure S4B). In the stratified analysis of those with reduced FMD, exendin decreased FMD in sitagliptin-treated individuals at 2 weeks (−3.6[−7.0, −0.3], P=0.03), but not at 14 weeks (−1.0[−4.3, 2.3], P=0.56) as compared to vehicle (Figure S4C). Finally, there was no acute effect of GLP-1R antagonism on PAI-1 concentrations in any treatment group (Table S4).
Discussion
GLP-1R agonists cause weight loss in obese individuals and reduce major adverse cardiovascular outcomes in T2DM. We performed a randomized controlled study comparing the effects of treatment with a GLP-1R agonist, hypocaloric diet-induced weight loss, and a DPP4 inhibitor in obese pre-diabetic individuals. Our aim was to dissect the weight loss-dependent and -independent effects of GLP-1R agonist treatment on measures of vascular function. We found no effect of liraglutide, diet-induced weight loss, or sitagliptin on endothelium-dependent vasodilation as measured by FMD in the overall cohort. In a post hoc analysis, liraglutide, weight loss, and sitagliptin all improved endothelial function in those individuals with reduced baseline FMD after 2 and 14 weeks. In contrast, liraglutide treatment and weight loss, but not sitagliptin treatment, significantly reduced PAI-1 after 14 weeks, and GLP-1R antagonism did not alter this effect. Finally, liraglutide treatment, but neither sitagliptin nor hypocaloric diet, reduced circulating MCP-1.
Prior studies of the effects of GLP-1R agonists on endothelial function as measured by FMD were all performed in individuals with T2DM and demonstrated mixed results. Two studies found no effect,15,16 three reported improvement in FMD,17–19 and a systematic review and meta-analysis did not find a significant effect of GLP-1R agonists on FMD.20 Interpretation of these data is limited by the modest number of and heterogeneity among published studies. Our study is the largest evaluating the effect of GLP-1R agonist treatment on FMD to date, and the first study that is controlled and blinded (to drug assignment). Our negative findings in the overall cohort agree with multiple prior studies and with the meta-analysis, suggesting that the cardiovascular benefits of GLP-1R agonists are not mediated through improved endothelial vasodilator function as measured by FMD. The lack of an acute effect of the GLP-1R antagonist on endothelium-dependent vasodilation during liraglutide, despite dramatic effects on fasting glucose, further argues against a significant contribution of improved endothelium-dependent vasodilation to the cardiovascular protective effects of GLP-1R agonists.
Notably, our overall cohort demonstrated relatively intact baseline endothelium-dependent vasodilation with a median FMD 9.91% in our group compared to values ranging from 1.6–8.9% in the prior studies.15–19 While we anticipated higher FMD in individuals with pre-diabetes as compared to those with T2DM at the outset, prior studies had demonstrated measurable endothelial dysfunction in pre-diabetic individuals, which informed our study population choice.9 Our exploratory analysis suggests that both GLP-1R activation and weight loss improve FMD in individuals with attenuated baseline FMD, and this needs to be investigated in future studies. Our findings in the subgroup with baseline FMD above median also demonstrate that improvements beyond normal do not occur, as we have shown previously.21
PAI-1 is the primary inhibitor of tissue plasminogen activator and is central to regulating fibrinolysis. PAI-1 is increased in obese22 and insulin resistant individuals,23–25 and PAI-1 levels predict incident myocardial infarction.26 PAI-1 expression is upregulated by inflammatory stimuli such as interleukin-6 and tumor necrosis factor-alpha, activation of the renin-angiotensin-aldosterone system, as well as by metabolic stimuli including hyperglycemia, hyperinsulinemia, and elevated VLDL.25 PAI-1 decreases after weight loss27 or treatment with GLP-1R agonists.28,29 In this study, liraglutide reduced PAI-1 as early as 2 weeks, with concomitant reductions in blood glucose, HOMA-IR, and MCP-1, but prior to significant weight loss. In contrast, hypocaloric diet did not reduce PAI-1 at 2 weeks, despite significant reductions in weight and HOMA-IR. This suggests that the liraglutide-induced reduction in PAI-1 at 2 weeks is not driven by weight loss or improvements in insulin resistance but may be due to improvements in hyperglycemia or inflammation. The significant correlation between PAI-1 and MCP-1 levels at 2 weeks in the liraglutide group supports this notion. By 14 weeks, reductions in PAI-1 in the liraglutide and hypocaloric diet-treated groups are likely due to a combination of the above factors including additional weight loss in both groups.
MCP-1 is a key chemokine that recruits monocytes into tissues, including the vasculature, and MCP-1 levels are increased by vascular endothelial injury, oxidative stress, and inflammatory cytokines.30,31 MCP-1 expression is higher in diseased human arteries with atherosclerotic plaques,32,33 and circulating MCP-1 levels predict restenosis after coronary angioplasty34 and are associated with histopathologic markers of plaque vulnerability.35 Furthermore, the therapeutic potential of targeting the MCP-1 pathway in cardiovascular disease has been demonstrated in animals.36 A smaller study of liraglutide 1.2 mg versus hypocaloric diet in type 2 diabetic patients for four months reported increased circulating and subcutaneous adipose tissue MCP-1 levels with liraglutide treatment, suggesting that GLP-1R agonist treatment does not improve this inflammatory parameter.37 Yet several reports have found that activation of the GLP-1R pathway reduces circulating MCP-1, although without a diet-induced weight loss comparator.38–40 In the current study, the liraglutide-induced reduction in MCP-1 by 2 weeks, prior to clinically significant weight loss, combined with failure of hypocaloric diet-induced weight loss to lower MCP-1, suggest that activation of the GLP-1 pathway specifically lowers MCP-1 in a weight-loss independent mechanism.
The current study suggests that GLP-1R-induced improvement in endothelium-dependent vasodilation does not account for the beneficial cardiovascular effects of these drugs in individuals without baseline endothelial dysfunction. This is supported by large cardiovascular trials demonstrating that the benefit of this class of agents increases with the illness severity of enrolled participants.41 In addition, demonstrating a benefit in endothelial dysfunction, a predictor of cardiovascular events and death,42 likely relies on baseline abnormal function. Reduction of PAI-1 with liraglutide as early as 2 weeks after treatment initiation, and prior to significant weight loss, suggests a role for fibrinolysis and inflammation in the cardiovascular risk improvement. In addition, the finding that MCP-1 was uniquely reduced during treatment with liraglutide may provide insight into the mechanism of cardiovascular benefit with GLP-1R agonists.
Another mechanism of cardiovascular benefit may involve platelet activation. Platelet activation plays a critical role in atherosclerosis and thrombosis, and platelets express GLP-1R. Barale et al have shown that GLP-1R agonists potentiate the anti-aggregating effects of nitric oxide in human platelets.43 We have recently reported that liraglutide attenuates thromboxane-induced platelet aggregation both in vitro and in vivo.44 Of note, MCP-1 expression is increased by platelet-derived growth factor,45 and activated platelets induce MCP-1 release in vitro.46 While we did not detect an effect of any treatment on circulating P-selectin, P-selectin reflects both platelet and endothelial cell activation.47 Thus, further studies are needed to assess the antithrombotic effects of GLP-1R agonists.
This study has several limitations. The majority of participants enrolled were women and the study was not powered to permit comparison between effects in men and women. The duration of intervention was 14 weeks, which may be too short to detect changes in our measures. Of note, prior studies of GLP-1R agonists measuring FMD ranged from 14–26 weeks.15–19 In addition, we enrolled individuals with pre-diabetes rather than overt diabetes, which may have diminished the effect size of our treatment by eliminating the hyperglycemia component of vascular dysfunction.48 We chose to enroll obese pre-diabetic individuals to study a homogenous population with baseline insulin resistance but without overt hyperglycemia to avoid confounding by concurrent anti-diabetic medications or by varying severity and duration of diabetes. As noted, the participants had normal baseline endothelium-dependent vasodilation. We completed a post hoc analysis stratified by baseline FMD below and above the median. Although not pre-specified, the finding that those with lower baseline FMD were significantly more insulin resistant than those with higher baseline FMD provides physiologic support of the analysis. However, all three interventions improved FMD in this subgroup to some extent, and clinical data has proven that only liraglutide, and not sitagliptin or equivalent weight loss through diet, improve cardiovascular outcomes.
In conclusion, in the largest study of the effect of a GLP-1R agonist on endothelium-dependent vasodilation, liraglutide, sitagliptin, and diet-induced weight loss had no effect on FMD in obese pre-diabetic individuals with normal baseline endothelial function. Liraglutide, sitagliptin and diet all improved FMD in those individuals with baseline endothelial dysfunction. Liraglutide appears to have anti-inflammatory (MCP-1 and PAI-1) and anti-thrombotic (PAI-1) effects that precede weight loss. Reduction of MCP-1 by liraglutide differentiates the effects of the GLP-1R agonist from weight loss or DPP4 inhibition. Such an anti-inflammatory effect could contribute to the unique cardiovascular benefit of GLP-1R agonists.
Supplementary Material
Acknowledgements
The authors acknowledge contributions from study nurse Patricia Wright; study dietician Dianna Olson; research assistants Sara E. Howard, Bradley Perkins, William E. Snyder, III; lab manager Anthony Dematteo.
Funding
Research reported in this publication was supported by the American Heart Association 17SFRN33520017 (M.M., J.A.B., H.N., D.M., J.R.K., C.Y., H.S., J.M.L, N.J.B), National Center for Advancing Translational Sciences 5UL1TR002243, National Institute of Diabetes and Digestive and Kidney Diseases T32DK007061 (M.M), National Institute of Allergy and Infectious Diseases U19AI095227 (K.N.C), National Heart, Lung and Blood Instituted R01HL146654 (J.D.B). This work utilized the core(s) of the Vanderbilt Diabetes Research and Training Center funded by grant DK020593 from the National Institute of Diabetes and Digestive and Kidney Disease. Novo Nordisk provided liraglutide and matching placebo.
Conflict of Interest Statement:
None unless noted below:
J.A.B.: Dr. Beckman is a consultant for JanOne, serves on a DSMB for Janssen and Novartis, and has ownership in EMX and Janacare.
J.R.K: Dr. Koethe has served as a consultant to Gilead Sciences, Merck, ViiV Healthcare, Theratechnologies and Janssen. He has also received research support from Gilead Sciences and Merck.
J.M.L: Dr. Luther has served on the advisory board for Mineralys.
N.J.B.: Dr. Brown serves on the scientific advisory board for Alnylam Pharmaceuticals. She serves as a consultant for Pharvaris Gmbh and eBioStar Tech. Dr. Brown owns equity in Abbvie and J and J Pharmaceuticals.
Footnotes
References
- 1.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. [DOI] [PubMed] [Google Scholar]
- 3.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Look ARG, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis. 2015;239(1):21–30. [DOI] [PubMed] [Google Scholar]
- 7.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA. 2018;319(15):1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson JR, Kerman SJ, Hubers SA, et al. Dipeptidyl Peptidase 4 Inhibition Increases Postprandial Norepinephrine via Substance P (NK1 Receptor) During RAAS Inhibition. J Endocr Soc. 2019;3(10):1784–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nohria A, Kinlay S, Buck JS, et al. The effect of salsalate therapy on endothelial function in a broad range of subjects. J Am Heart Assoc. 2014;3(1):e000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman JA, Hu JR, Huang S, et al. Metabolomics reveals the impact of Type 2 diabetes on local muscle and vascular responses to ischemic stress. Clin Sci (Lond). 2020;134(17):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med. 2007;167(22):2490–2496. [DOI] [PubMed] [Google Scholar]
- 14.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167(10):1226–1234. [DOI] [PubMed] [Google Scholar]
- 15.Nomoto H, Miyoshi H, Furumoto T, et al. A Comparison of the Effects of the GLP-1 Analogue Liraglutide and Insulin Glargine on Endothelial Function and Metabolic Parameters: A Randomized, Controlled Trial Sapporo Athero-Incretin Study 2 (SAIS2). PLoS One. 2015;10(8):e0135854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins ND, Cuthbertson DJ, Kemp GJ, et al. Effects of 6 months glucagon-like peptide-1 receptor agonist treatment on endothelial function in type 2 diabetes mellitus patients. Diabetes Obes Metab. 2013;15(8):770–773. [DOI] [PubMed] [Google Scholar]
- 17.Irace C, De Luca S, Shehaj E, et al. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diab Vasc Dis Res. 2013;10(1):72–77. [DOI] [PubMed] [Google Scholar]
- 18.Lambadiari V, Pavlidis G, Kousathana F, et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabetes Res Clin Pract. 2014;106(3):567–575. [DOI] [PubMed] [Google Scholar]
- 20.Batzias K, Antonopoulos AS, Oikonomou E, et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J Diabetes Res. 2018;2018:1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman JA, Liao JK, Hurley S, et al. Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low-density lipoprotein. Circ Res. 2004;95(2):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orenes-Pinero E, Pineda J, Roldan V, et al. Effects of Body Mass Index on the Lipid Profile and Biomarkers of Inflammation and a Fibrinolytic and Prothrombotic State. J Atheroscler Thromb. 2015;22(6):610–617. [DOI] [PubMed] [Google Scholar]
- 23.Vague P, Juhan-Vague I, Aillaud MF, et al. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. 1986;35(3):250–253. [DOI] [PubMed] [Google Scholar]
- 24.Juhan-Vague I, Alessi MC, Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia. 1991;34(7):457–462. [DOI] [PubMed] [Google Scholar]
- 25.Brown NJ. Therapeutic potential of plasminogen activator inhibitor-1 inhibitors. Ther Adv Cardiovasc Dis. 2010;4(5):315–324. [DOI] [PubMed] [Google Scholar]
- 26.Thogersen AM, Jansson JH, Boman K, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98(21):2241–2247. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Qamhieh HT, Wing RR, et al. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler Thromb. 1993;13(2):162–169. [DOI] [PubMed] [Google Scholar]
- 28.Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabet Med. 2008;25(9):1129–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forst T, Michelson G, Ratter F, et al. Addition of liraglutide in patients with Type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med. 2012;29(9):1115–1118. [DOI] [PubMed] [Google Scholar]
- 30.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101(Pt B):107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88(4):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yla-Herttuala S, Lipton BA, Rosenfeld ME, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88(12):5252–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cipollone F, Marini M, Fazia M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21(3):327–334. [DOI] [PubMed] [Google Scholar]
- 35.Georgakis MK, van der Laan SW, Asare Y, et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate With Plaque Vulnerability. Arterioscler Thromb Vasc Biol. 2021;41(6):2038–2048. [DOI] [PubMed] [Google Scholar]
- 36.Georgakis MK, Bernhagen J, Heitman LH, Weber C, Dichgans M. Targeting the CCL2-CCR2 axis for atheroprotection. Eur Heart J. 2022;43(19):1799–1808. [DOI] [PubMed] [Google Scholar]
- 37.Pastel E, McCulloch LJ, Ward R, et al. GLP-1 analogue-induced weight loss does not improve obesity-induced AT dysfunction. Clin Sci (Lond). 2017;131(5):343–353. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Huang J, Li J, Mao Q, He J. Effects of Liraglutide Combined with Insulin on Oxidative Stress and Serum MCP-1 and NF-kB Levels in Type 2 Diabetes. J Coll Physicians Surg Pak. 2019;29(3):218–221. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64(2):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97(1):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139(17):2022–2031. [DOI] [PubMed] [Google Scholar]
- 42.Martin BJ, Anderson TJ. Risk prediction in cardiovascular disease: the prognostic significance of endothelial dysfunction. Can J Cardiol. 2009;25 Suppl A:15A–20A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barale C, Buracco S, Cavalot F, Frascaroli C, Guerrasio A, Russo I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation. Thromb Haemost. 2017;117(6):1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cahill KN, Amin T, Boutaud O, et al. Glucagon-Like Peptide-1 Receptor Regulates Thromboxane-Induced Human Platelet Activation. JACC Basic Transl Sci. 2022;7(7):713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding M, He SJ, Yang J. MCP-1/CCL2 Mediated by Autocrine Loop of PDGF-BB Promotes Invasion of Lung Cancer Cell by Recruitment of Macrophages Via CCL2-CCR2 Axis. J Interferon Cytokine Res. 2019;39(4):224–232. [DOI] [PubMed] [Google Scholar]
- 46.Stumpf C, Raaz D, Klinghammer L, et al. Platelet CD40 contributes to enhanced monocyte chemoattractant protein 1 levels in patients with resistant hypertension. Eur J Clin Invest. 2016;46(6):564–571. [DOI] [PubMed] [Google Scholar]
- 47.Andre P. P-selectin in haemostasis. Br J Haematol. 2004;126(3):298–306. [DOI] [PubMed] [Google Scholar]
- 48.Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103(12):1618–1623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.