Abstract
Multiple paradigms indicate that the physical environment can influence spontaneous and learned behavior. In rodents, context-dependent behavior is putatively supported by the prefrontal cortex and the medial temporal lobe. A preponderance of the literature has targeted the role of the hippocampus. In addition to the hippocampus proper, the medial temporal lobe also comprises parahippocampal areas, including the perirhinal and postrhinal cortices. These parahippocampal areas directly connect with multiple regions in the prefrontal cortex. The function of these connections, however, is not well understood. This article first reviews the involvement of the perirhinal, postrhinal, and prefrontal cortices in context-dependent behavior in rodents. Then, based on functional and anatomical evidence, we suggest that perirhinal and postrhinal contributions to context-dependent behavior go beyond supporting context representation in the hippocampus. Specifically, we propose that the perirhinal and postrhinal cortices act as a contextual-support network that directly provides contextual and spatial information to the prefrontal cortex. In turn, the perirhinal and postrhinal cortices modulate prefrontal input to the hippocampus in the service of context-guided behavior.
Keywords: perirhinal cortex, postrhinal cortex, prefrontal cortex, hippocampus, context memory, occasion setting
Overview of the parahippocampal region and context-dependent behavior
Since the case of HM, research on the neural bases of memory has focused on structures in the medial temporal lobe. The medial temporal lobe includes the hippocampal formation (dentate gyrus, hippocampus proper, subiculum) and the parahippocampal region. The parahippocampal region includes the perirhinal cortex (PER), the parahippocampal cortex, the lateral and medial areas of the entorhinal cortex (LEA and MEA), the presubiculum, and the parasubiculum (Burwell & Witter, 2002; Scharfman et al., 2000). The homologies between primate and rodent for the medial temporal lobe are well established, and complete since the definition of the rodent postrhinal cortex (POR), homolog of the primate parahippocampal cortex (Burwell & Witter, 2002; Burwell et al., 1995). This review will primarily focus on rodent studies, with occasional single-cell recording studies in monkeys. However, patterns of anatomical connection and functions of the areas discussed are largely well-preserved across rodents, non-human primates, and humans.
The PER is located near the rhinal fissure and comprises the ventrally located area 35 and the dorsally located area 36 (Burwell, 2001). The patterns of cortical and subcortical connections differ substantially for areas 35 and 36, though the functional difference between them is not well-known. The POR lies dorsal to the extension of the rhinal fissure, bordering the caudal boundary of the PER. The PER and POR provide most of the cortical input to the hippocampus, both directly to CA1 and subiculum and indirectly through sub-areas of the entorhinal cortex (Figure 1). Reciprocal connections also exist between the PER and POR, with the heavier projection originating from the POR (Burwell & Amaral, 1998b). The PER and POR receive both polymodal associational and unimodal associational input. The PER receives sensory associational information of all modalities, and the POR receives most input from cortical areas devoted to visuo-spatial processing (Burwell & Amaral, 1998a). Whereas PER efferents terminate mostly in frontal and temporal regions, the POR projects more heavily to caudal cortical regions including visual, visuo-spatial, and, to a lesser extent, auditory cortices (Agster & Burwell, 2009).
Figure 1.
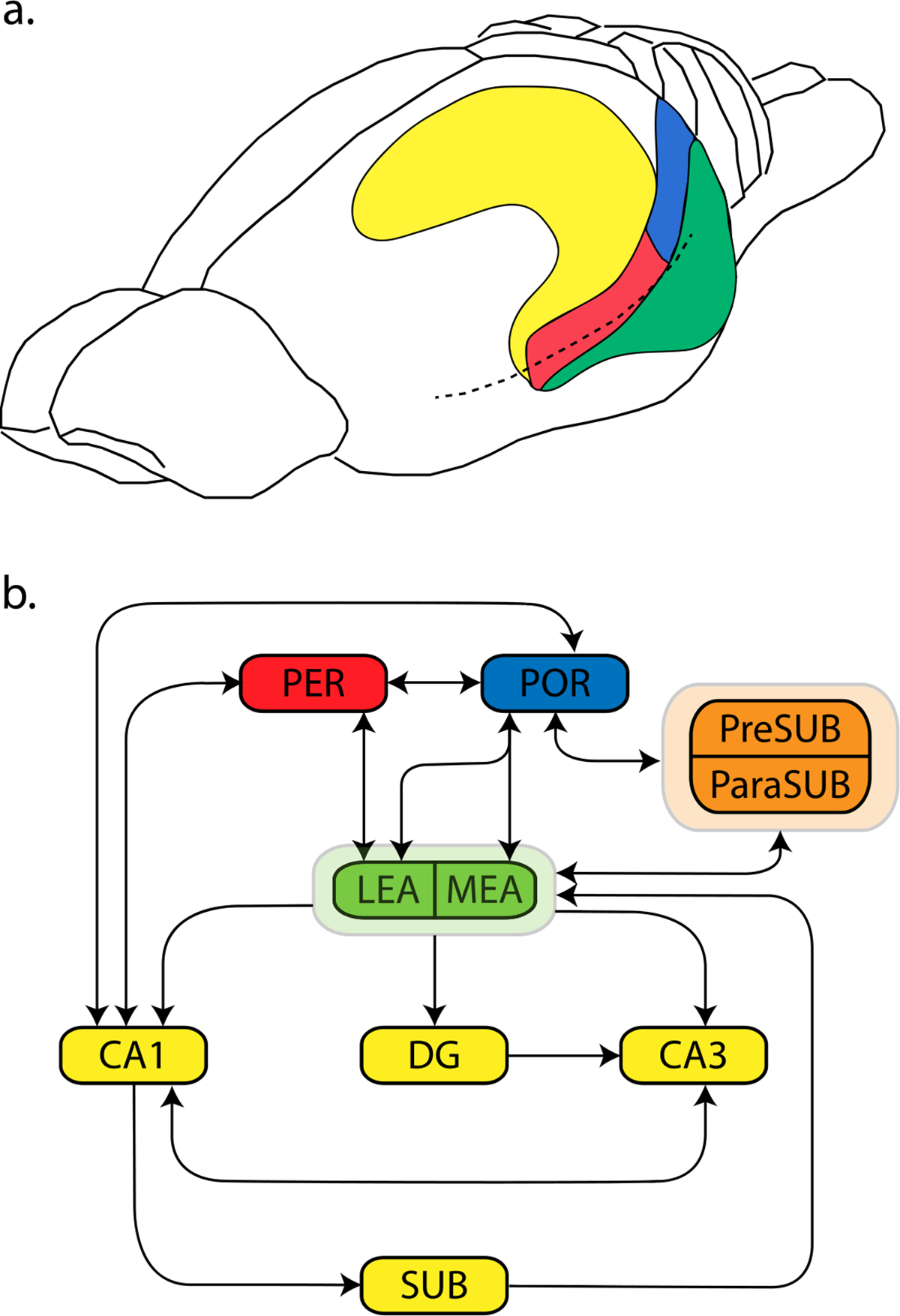
The perirhinal and postrhinal cortices in rodents. A) The perirhinal cortex (PER, red) and the postrhinal cortex (POR, blue). B) A schematic showing simplified connections within the medial temporal lobe. Entorhinal cortex is shown in green, the hippocampal formation in yellow, and the pre- and parasubiculum are in orange. Abbreviations: LEA: lateral entorhinal area. MEA: medial entorhinal area. DG: dentate gyrus. SUB: subiculum. PreSUB: presubiculum. ParaSUB: parasubiculum. CA1 and CA3: subfields of the hippocampus.
Functions of the PER and POR were described by the “Binding of items and contexts” (BIC) model (Diana et al., 2007; Eichenbaum et al., 2012; Eichenbaum et al., 2007) such that information about items and objects (i.e. “what”) is processed by the PER and lateral entorhinal area, while contextual information (i.e. “where”) is processed by the POR and medial entorhinal area. The hippocampus binds item information from the PER with context information form the POR for event memory (Figure 2). This model also proposes that medial temporal lobe structures contribute to different processes of recognition memory. Familiarity-based recognition relies on an intact PER, and it does not require involvement of the POR or hippocampus. In contrast, recollection-based recognition involves recalling the item together with the context in which it occurred, requiring contribution from the PER, POR and hippocampus.
Figure 2.

The “binding of items and contexts” (BIC) model (Eichenbaum et al., 2007). This is a model of one aspect of relational memory, the association of an item with a particular context. Item could be, for example, an object, odor, pattern, or prior experience of a stimulus. Abbreviations: HPC: hippocampus. LEA: lateral entorhinal area. MEA: medial entorhinal area. PER: perirhinal cortex. POR: postrhinal cortex.
One important function of the medial temporal lobe is processing context. In a broad sense, context encompasses a wide range of internal and external stimuli that are not directly and imminently related to an action or its goal. One important aspect of context is the external physical environment. Understanding how the physical environment controls behavior is a longstanding theme in psychology and neuroscience. Even though there is no clear-cut distinction between contextual and non-contextual stimuli, context differs from discrete signals or cues in that it often consists of sensory information from multiple modalities that are temporally continuous and spatially diffuse. For example, in an experimental setting, events (such as electric shocks) may be preceded by an LED light or an auditory tone, which are localized unimodal stimuli with clear temporal onsets and termination. In contrast, the context of the experimental environment typically remains constant throughout each session, and it often contains multi-modal physical elements, including visual features, background noise, texture of the floor, odors, etc.
Context guides behavior either via direct associations or by indirectly modulating associations between other stimuli. The Rescorla-Wagner model proposes that context can be incorporated into stimulus-stimulus or stimulus-response associations in the same way as an explicit, discrete stimulus; hence, context itself can acquire either positive or negative associative strength and directly elicit behavioral changes through its associations (Rescorla & Wagner, 1972; Wagner & Rescorla, 1972). Alternatively, context may control behavior indirectly through modulating lower-order associative relations in a hierarchical organization; in other words, it “sets the occasion” in which certain associations apply while others do not (Fraser & Holland, 2019; Trask, Thrailkill, et al., 2017). For example, the same cue may signal the arrival of food pellets in one context and an impending electric shock in another. The context acts as an “occasion-setter” that disambiguates the meaning of the cue and allows the subject to respond accordingly.
Though its role in context learning is not as well-studied as the medial temporal lobe, the prefrontal cortex is also frequently implicated in context-guided behavior. The prefrontal cortex is responsible for executive functions in primates and rodents (Dalley et al., 2004; Logue & Gould, 2014; Sharpe et al., 2019). Even though the precise homologies are still subject to ongoing debate, the prefrontal regions in rodents have been proposed based on both anatomical characteristics and functional similarity to the primate prefrontal cortex (Dalley et al., 2004; Hwang et al., 2018). Here, discussion of the rodent prefrontal cortices will include the medially located prelimbic (PL) and infralimbic (IL) cortices, orbitofrontal cortex (OFC), and secondary motor cortex (MOs, also called the medial precentral cortex, medial agranular cortex, dorsomedial prefrontal cortex, second frontal area, and frontal orienting field). The PL and IL in rodents are often collectively referred to as the medial prefrontal cortex (mPFC). Some studies explicitly target the PL or IL, whereas others investigate the mPFC as a whole and involve both the PL or IL via lesioning or inactivation. In the former case, the specific area will be used when discussing the finding, whereas in the latter case, the more general term of mPFC will be used. All these prefrontal areas are reciprocally connected with the PER and POR to various degrees (Agster & Burwell, 2009; Burwell & Amaral, 1998a; Hwang et al., 2018). In the following sections, we will examine studies showing the involvement of the PER/POR and prefrontal areas in rodent context-dependent behavioral paradigms. Then we will review anatomical evidence on the connections between the PER/POR and prefrontal cortices. Finally, we propose possible roles of these connections as part of the cortico-cortical network supporting context-dependent behavior.
The Roles of PER/POR and PFC in context-modulated behavior
Conditioning, Extinction, and Renewal
Classic associative learning paradigms have provided valuable insight into the neural circuits of learning and memory, including the effects of context on conditioned responses. Existing evidence indicates crucial roles for the PER and POR in associating fear with the environmental context. Pre-training or post-training damage to the PER or POR leads to reduced freezing to the context in both signaled and unsignaled Pavlovian fear conditioning (Bucci et al., 2000; Corodimas & LeDoux, 1995; Kholodar-Smith et al., 2008; Lindquist et al., 2004). PER or POR damage also produces deficits in context discrimination after one of the contexts is paired with shocks (Bucci et al., 2002). Furthermore, PER or POR lesions conducted even 100 days after training still lead to freezing deficits during the context test, indicating ongoing roles for the PER and POR in processing remote contextual fear memory (Burwell et al., 2004). In comparison, the hippocampus is typically necessary for recalling recent but not remote contextual fear memory (Maren et al., 1997; Winocur et al., 2013). These deficits produced by PER or POR lesions cannot be explained by impairment in associating simple stimuli, because lesioning either area has no effect on tone-elicited freezing (Bucci et al., 2000; Lindquist et al., 2004), or eye-blink responses in delayed eyeblink conditioning (Suter et al., 2013). Interestingly, disconnecting the PER and POR via unilateral cross-lesioning does not impair contextual fear conditioning, whereas it does impair another contextual memory task that will be discussed later, context-guided object recognition (Heimer-McGinn et al., 2017).
Even though lesioning either area impairs contextual fear conditioning, contributions from the PER and POR are likely to be different. Consistent with the BIC model, the PER likely processes and integrates multi-modal or complex sensory information about environmental elements, whereas the POR processes the spatial layout of the environment based on input from other visuospatial areas. In one series of experiments, a combination of auditory cue, context, and time of day (“what-where-when”) jointly determined whether or not electric shocks were delivered (Iordanova et al., 2009). For example, a tone signaled shock only if it was presented in a chamber with dotted walls in the morning. Permanent lesions of the PER reduce freezing under the “what-where-when” and “what-where”, but not the “what-when” combinations, suggesting that the PER critically contributes to processing the spatial environment but not temporal information of events hours apart (Iordanova et al., 2009). Other studies support the role of PER in processing complex stimuli in associative learning, particularly when stimuli are composed of temporally discontinuous units (Kent & Brown, 2012). For example, lesioning the PER leads to impaired fear conditioning to a complex auditory stimulus, while leaving conventional tone-signaled conditioning intact (Kholodar-Smith et al., 2008; Lindquist et al., 2004). Similarly, inactivating the PER during extinction reduces freezing to a discontinuous visual CS during both extinction and retrieval (Potter et al., 2020). It should be noted that suppressing the PER during extinction also decreases freezing to a continuous visual cue during the session, which indicates that PER is part of the preferred circuit regardless of the nature of the CS, and other structures can fully compensate for the loss of PER during conditioning unless the CS is discontinuous (Potter et al., 2020). In comparison, the POR is involved in processing visuospatial information of the context, possibly through interaction with the retrosplenial cortex. Neurons projecting from the retrosplenial cortex to POR show increased c-fos level after fear conditioning, and disconnecting these two cortices diminishes conditioned freezing during context testing (Robinson et al., 2012).
Among prefrontal areas, the PL and IL in the mPFC are frequently shown to be involved in context-dependent associative learning paradigms. Their specific contributions are still under debate and likely vary between Pavlovian and instrumental conditioning as compared with appetitive and aversive conditioning. However, existing evidence suggests that the PL is generally needed for acquiring and expressing excitatory associations, whereas IL involvement is more frequently seen during acquisition of inhibitory associations (e.g. in extinction). In fear conditioning, the PL is not necessary for acquiring tone-shock association when no trace interval exists between the tone and the shock (Corcoran & Quirk, 2007; Gilmartin & Helmstetter, 2010; Zelikowsky et al., 2013). Whether the PL is critical for the acquisition of contextual fear depends on whether the shocks are explicitly signaled by a discrete cue. In unsignaled (or “foreground”) contextual fear conditioning, inactivating the PL does not lead to reduced fear responses during subsequent re-exposure to the context (Corcoran & Quirk, 2007; Santos et al., 2017). In comparison, during signaled (or “background”) contextual fear conditioning, context-shock association is blocked if the PL is temporarily suppressed (Gilmartin & Helmstetter, 2010; Gilmartin et al., 2013), but not if it is lesioned pre-training (Zelikowsky et al., 2013). PL functioning is also required when there is a short gap between context exposure and shock delivery (Santos et al., 2017). Together, lesion and inactivation studies suggest that the PL possibly maintains the contextual representation when the context-shock association cannot be established at the exact moment of shock delivery, because either context exposure and shocks are temporally discontinuous (Santos et al., 2017) or there are more potent cues (e.g. tones) that overshadow the context for fear association (Gilmartin & Helmstetter, 2010; Gilmartin et al., 2013). Increased activity in the PL is indeed observed after contextual fear conditioning and after testing, suggesting that some neurons in the PL encode the context while others process the context-shock association (Zelikowsky et al., 2014). Similarly, the level of early-growth-response gene 1 (Egr-1) after training elevates in animals who have been pre-exposed to the training context, compared to those with pre-exposure to a different context, presumably because the former utilizes the PL to retrieve a representation of the context formed during pre-exposure to establish the context-shock association (Chakraborty et al., 2016). It should be noted, though, that some studies show that pre-training or post-training lesion of the PL does not affect discrimination between a shock context and a no-shock context (Kim et al., 2013; Zelikowsky et al., 2013). Instead, contextual discrimination deficits are present after pre-training damage to the IL, another area in the mPFC (Zelikowsky et al., 2013). The role of the IL in context-dependent fear is less well studied, though evidence suggests that it may serve as part of the circuit as well. For example, similar to the PL, Egr-1 level in the IL is also increased after shock training with context pre-exposure (Chakraborty et al., 2016).
Context-induced renewal refers to the phenomenon in which extinguished conditioned responses re-appear after changes in context (Bouton & Bolles, 1979). The key component in any renewal procedure is re-exposure to a context distinct from the one in which extinction takes place; any switch in context causes return of the previously extinguished response (Trask, Thrailkill, et al., 2017). In the commonly used “ABA” renewal procedure, the subject undergoes conditioning in context A, extinction in context B, and finally re-testing in context A. Context renewal has been observed for both Pavlovian and instrumental conditioning; however, their underlying mechanisms may be different. The dominant view on Pavlovian context renewal is that the extinction context acts as a negative occasion-setter that signals the negative contingency between the conditioned stimulus (CS) and the unconditioned stimulus (US) (Bouton, 2004; Trask, Thrailkill, et al., 2017). Since this occasion setting property is limited to the extinction context, re-testing in any other context prevents the retrieval of extinction memory and the CR re-appears because of the original excitatory CS-US association. In contrast, the context directly inhibits the CR during extinction of instrumental responses, and context renewal is due to the absence of such inhibitory association between the non-extinction context and the CR (Trask, Thrailkill, et al., 2017).
As with other types of contextual learning, both the medial temporal lobe and prefrontal cortices are involved in context-induced renewal. Among medial temporal lobe structures, existing research indicates a crucial role of the hippocampus in contextual fear renewal (Ji & Maren, 2007). Since the PER and POR are critically involved in processing contextual fear, however, it can be expected that they are also required for context renewal as well; future research is needed to confirm this. Within the prefrontal region, the orbitofrontal cortex (OFC) and the mPFC have been implicated as part of the context-renewal circuit. Inactivating the OFC attenuates context renewal of alcohol-seeking behavior and increased fos expression in the OFC is observed after the renewal session (Bianchi et al., 2018). Temporarily inactivating the lateral—but not medial—OFC impairs context renewal of cocaine-seeking (Lasseter et al., 2009), and the connection between lateral OFC and basolateral amygdala is necessary for this effect (Lasseter et al., 2011). The functional differentiation between the medial and lateral portions of the OFC in context renewal is interesting, as will be discussed in the following sections, because the lateral OFC is more heavily connected with the PER and POR than the medial OFC (Hwang et al., 2018), and certain forms of spatial mapping have been observed in the lateral OFC (Farovik et al., 2015; Feierstein et al., 2006).
The mPFC also contributes to context renewal. Suppressing either the PL or IL diminishes renewal of a food-seeking response in its original acquisition context, i.e. in ABA renewal (Eddy et al., 2016), but PL inactivation has no effect when the renewal test is conducted in a third, novel context, i.e. in ABC renewal (Trask, Shipman, et al., 2017). This suggests that the PL particularly supports instrumental responding in the context where initial acquisition occurs, possibly through retrieving context-dependent action-outcome associations (Trask, Shipman, et al., 2017). The PL plays an important role in Pavlovian context renewal as well, even though it does not involve the same associative structures as instrumental conditioning. Pre-training PL damage causes similar levels of freezing when animals are tested in extinction and renewal context, abolishing the context renewal effect (Zelikowsky et al., 2013). In another study, pre-training lesions or inactivation of the PL diminished context renewal without affecting fear response during extinction (Sharpe & Killcross, 2015). Moreover, the absence of renewal is accompanied by deficits in orienting responses to the CS. Therefore, the impairment is at least partially due to attentional deficits towards the CS during re-testing. Since this procedure purposefully limits direct fear conditioning to the context (e.g. by using extended context pre-exposure and long inter-stimulus intervals), it promotes the use of contextual cues as an occasion-setter over direct associations with the shock. Overall, these studies provide evidence that the PL is able to utilize context information to activate lower-order stimulus-stimulus associations.
To summarize, evidence from several commonly used associative learning procedures shows that the PER, POR, mPFC, and OFC are typically not required for delayed conditioning to simple cues, whereas they play crucial roles when responses are elicited or modulated by the spatial context. Specifically, as proposed by the BIC model (Diana et al., 2007; Eichenbaum et al., 2012; Eichenbaum et al., 2007), the PER and POR process non-spatial and spatial sensory input from various cortical and subcortical sources to efficiently construct context representations. Under certain circumstances, these representations may require maintenance or retrieval by the mPFC for associative learning. Moreover, when stimulus-stimulus or response-outcome relationships change after initial training (e.g. via context-specific extinction), the mPFC and OFC may use the context to re-structure existing associations and allow subjects to respond according to updated stimulus contingencies. The PL and IL in the mPFC, though differing in their specific contribution, both are capable of utilize context information to guide behavior.
Object recognition and discrimination
Object recognition and discrimination provide another set of rodent assays in which behaviors can be guided by their physical context. These assays can be categorized into two groups. One group is collectively referred to as context-dependent spontaneous object exploration (SOR) tasks. These tasks take advantage of the rodent’s natural tendency to explore novel objects and do not require reward deliveries or lengthy training sessions. The other group comprises biconditional discrimination tasks in which rodents are trained to discriminate objects to obtain rewards. In this second group, whether an object is associated with a reward is determined by object identity in combination with its spatial environment; in other words, the object-reward contingencies reverse when the objects are presented in a different context. Successfully obtaining rewards in such biconditional discrimination tasks requires the ability to not only discriminate objects but also acquire and retrieve context-specific response rules and then respond flexibly in different contexts.
Context-dependent SOR tasks include the contextual SOR and the object-in-place (OIP) task, whereas the standard SOR is often employed as a control task. In contextual SOR (Figure 3B), the animal undergoes two sampling phases where it is placed in two different physical contexts to explore two pairs of identical objects, then during the test phase it is put into one of the sample contexts with one object from each pair. Normal rats preferentially explore the object previously not appearing in the test context; in other words, more time is spent exploring the novel object-context pairing. Since this procedure involves multiple sampling phases, it is important to counter-balance the order in which objects and contexts are explored, either across subjects or by conducting multiple trials. Otherwise, results during the test phase may be confounded by the recency effect, in which case the rat explores an object more because it is the least recently encountered or it explores an object less because it is the most recent (Tam et al., 2015). In the OIP task (Figure 3C), the subject explores multiple different objects during sampling, but during testing two of the objects switch places while others are not moved; innate preference is directed towards the objects appearing in novel locations. Whereas the standard SOR can be accomplished through simply encoding each object’s familiarity, performance in contextual SOR and OIP depends on the recollection of associations between objects and the location or spatial context in which they occur (Barker & Warburton, 2011).
Figure 3.

Variants of the spontaneous object recognition task. A) Standard SOR task: healthy animals are expected to spend more time exploring the triangle over the hexagon during test. B) Contextual SOR: the subject should show preference for the triangle if tested in the dotted context and for the hexagon if tested in the checkered context. The order in which objects and contexts are presented should be counter-balanced (Heimer-McGinn et al., 2017). Therefore, in contextual SOR, subjects as a group have an equal level of familiarity and recency with each object and context at testing, but one of the object-context pairing is novel. C) Object-in-place task: preferential exploration is expected for the square and the hexagon over the triangle and the circle.
The PER is well-established as an important area for recognition memory (Eichenbaum et al., 2007; Suzuki & Naya, 2014; E. Warburton & M. W. Brown, 2015). Neurons in the monkey PER respond to specific objects and their activity decreases with repeated exposure, signaling increased familiarity (Xiang & Brown, 1998). Lesioning or inactivating the PER typically disrupts performance in standard object recognition tasks (Barker et al., 2007; Hannesson et al., 2004; Norman & Eacott, 2004, 2005; Winters & Bussey, 2005), though there have been exceptions (Jo & Lee, 2010). There are multiple possible explanations for the inconsistency. First, spared recognition memory may be observed if PER lesions are incomplete. It has been shown that the degree of SOR deficits are positively correlated with lesion sizes in the PER, particularly in its caudal portion (Albasser et al., 2009). Since it can be reasonably assumed that visual memory is one of the primary aspects of memory assessed in most object recognition procedures (typically conducted in the light), this is consistent with the fact that unimodal visual input mostly targets caudal area 36 of PER, whereas rostral PER mostly receives auditory, somatosensory and tactile information (Burwell & Agster, 2008; Burwell & Amaral, 1998a). Second, it has been proposed that the PER may serve a perceptual function in addition to its role in mnemonic processes (Bussey et al., 2002, 2005). The idea is that the PER combines various elements of an object into one configural representation, therefore it is particularly important for discriminating objects that cannot be easily distinguished based on individual perceptual elements, e.g. when they have highly overlapping features. Therefore, intact object recognition memory after PER damage may also be due to the relatively low perceptual feature overlap between objects. Despite the null results of recognition test after PER lesions, the majority of evidence is in accordance with the view that the PER plays a fundamental role in object recognition.
It is perhaps not surprising, then, that the PER critically contributes to procedures involving associating objects with their location or context in addition to memorizing objects themselves. Bilateral lesions of the PER reduce preferential exploration of objects whose locations have been switched in the OIP task (Barker et al., 2007). Another study utilized a biconditional object discrimination task (Jo & Lee, 2010). In a multi-arm maze, food is hidden under object A and not B in one arm while it is under object B and not A in another arm on other trials. Therefore, successful discrimination requires associating objects with the context (arm) where they are rewarded. Post-training lesion of the PER disrupts the learned object-context associations before subjects gradually regain the pre-surgical level of accuracy. However, subjects are unable to discriminate new object-context pairings involving new objects, suggesting that the PER is necessary for the initial encoding of novel object-context associations. Simple object discrimination was not affected by PER lesions (Jo & Lee, 2010).These results were confirmed by another study using a similar procedure. Subjects with PER damage performed significantly worse compared to pre-lesion levels and revert to a side bias, preferentially selecting objects on a particular side, regardless of object identity and spatial context (Hernandez et al., 2017).
Like the PER, the POR is also required for contextual object recognition tasks. POR damage impairs the ability to form object-context and object-place pairings without negatively affecting standard SOR (Norman & Eacott, 2005). This is consistent with single-cell recording evidence that some POR neurons selectively respond to object-place conjunctions, i.e. when an object appears in a certain location but not when the same object appears in other locations or other objects appear in the same location (Furtak et al., 2012). This object-place encoding likely directly depends on PER-POR interaction, because animals with PER-POR disconnection are significantly impaired in both two-dimensional and three-dimensional versions of the contextual SOR task (Heimer-McGinn et al., 2017). Object location conjunctive coding emerges in the hippocampus as animals learn to associate particular stimuli to places where they have differential consequences (Komorowski et al., 2009). Interestingly, object location conjunctive coding appears in the POR before it appears in the hippocampus (Estela, 2020). This is consistent with our proposal that object-location conjunctive coding in the hippocampus signifies associative learning, whereas object-location conjunctive coding in the postrhinal is a signature of representations of local contexts including the spatial layout of items and features of the context.
There is also evidence that the POR monitors the local environment and helps direct attention towards motivationally relevant events by communicating with cortices known for visuospatial attention, such as the posterior parietal cortex (Agster & Burwell, 2009; Burwell & Amaral, 1998a). For example, POR lesions in rodents impair attentional orienting towards a light signal paired with food delivery (Bucci & Burwell, 2004), and spatial firing correlates in the POR remap when local and distal cues are moved (Burwell & Hafeman, 2003). In another study, neurons in the POR and surrounding visual association cortices selectively responded to food cues (Burgess et al., 2016). This preference disappeared after satiation, suggesting that visual association areas are updated when the motivational value of visual cues in the environment changes. It is further shown that different ensembles of POR neurons encode cue identity and motivational value (Ramesh et al., 2018), and ensembles encoding both the cue and the reward undergo preferential reactivation after training that strengthens their connectivity through hippocampal-related consolidation process (Sugden et al., 2020). Collectively, these results provide evidence for considerable item and cue encoding in the POR and that the POR is involved in monitoring cues in the environment and updating changes in cue motivational value and spatial location.
Prefrontal cortex in rodents is not required to detect and explore novel objects (Barker et al., 2007; Ennaceur et al., 1997; Hannesson et al., 2004). However, the mPFC is necessary for biconditional object discrimination tasks (Hernandez et al., 2017; Lee & Solivan, 2008) and for discriminating object-place and object-context pairings in OIP tasks (Barker et al., 2007; Cross et al., 2013). The core factor shared by these tasks is the requirement to integrate object appearance with its spatial attributes, i.e. location or context. The fact that the loss of mPFC functioning impairs these tasks points to the idea that the mPFC critically contributes to object recognition only if it involves binding and retrieving the object together with the context of its prior appearance, i.e. via recollection-based recognition, not when the decision is based on familiarity judgement alone. Another reason why the mPFC supports biconditional discrimination may be related to its well-known role of enabling flexible decision-making. Since the object-reward contingency varies across contexts, the reward cannot be obtained above chance level by always selecting the same object or any object on the same side across all contexts. Evidence supporting this idea comes from the control condition in which rewards are associated with locations instead of objects (“location-in-place”, e.g. rewards are always on the left side inside context A, regardless of what object is on the left). Under this condition, mPFC inactivation initially disrupts performance but it quickly rebounds to pre-inactivation level, possibly due to subjects switching towards an egocentric strategy to obtain rewards (Lee & Solivan, 2008). The need for mPFC for flexible action selection, however, does not apply to OIP, because it does not involve acquiring and applying complex response rules that necessitate flexible action selection across trials. Finally, it has been proposed that although primarily a spatial-object memory tasks, the OIP in fact contains a significant temporal element due to the need to accurately and separately represent memories of objects and their layout into distinct instances (Aggleton & Nelson, 2020). Although this idea has yet been fully investigated, it does fit well with a known function of the mPFC to reduce interference when retrieving past memories, particularly through coordinating with the hippocampus (Eichenbaum, 2017).
In summary, context-dependent object recognition depends on the functional integrities of the PER and POR as well as the mPFC. Together with previously mentioned context-dependent tasks, the results of these studies are summarized in Table 1. A cortico-hippocampal-cortical network proposes that the PER is specifically involved in processing and storing object information, whereas the hippocampus is responsible for providing spatial information regarding where the object appears, and finally the mPFC integrates them to form object-place associations (E. C. Warburton & M. W. Brown, 2015). However, this model does not take into account the direct contribution of the POR. The fact that PER-POR disconnection impairs contextual SOR (Heimer-McGinn et al., 2017) and observations of object-location conjunctive coding in the POR (Furtak et al., 2012) suggest that the parahippocampal region itself does contain certain forms of contextual representation. Blocking PER-POR communication likely reduces the fidelity of context representations in the parahippocampal region as well as in the hippocampus, leading to disrupted performance in contextual object recognition, even though less-detailed “gist-like” representations may still exist that are sufficient for less demanding contextual tasks, such as contextual fear conditioning (Heimer-McGinn et al., 2017).
Table 1.
Lesion and inactivation studies of common contextual and non-contextual tasks
| Manipulation | OR | cxtSOR | OiP | BOD | cxtFC | FCs | FCc | CR |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| PER | ||||||||
| pre-training | ✓✓✓× | ✓ | ✓ | ✓✓✓✓✓ | ×××× | ✓✓ | ||
| post-training | ✓ 1 | ✓✓✓ | ✓ 2 | |||||
| inactivation | ✓✓ | ✓ | ✓ | ✓ | ||||
| POR | ||||||||
| pre-training | ✓ | ✓✓ | ×× | |||||
| post-training | ✓✓ | |||||||
| mPFC | ||||||||
| pre-training | ××× | ✓✓ | ✓✓ | |||||
| inactivation | × | ✓✓ | ✓✓×× | ××× | ✓✓✓× 3 | |||
| OFC | ||||||||
| inactivation | ✓✓ | |||||||
| Disconnection | ||||||||
| PER-mPFC | ✓ | ✓ | ||||||
| PER-POR | × | ✓ | × | |||||
Summary of rodent studies involving the PER, POR, and mPFC in common contextual and non-contextual tasks after pre-training lesion, post-training lesion, or temporary inactivation.
: deficit.
: no deficit. CR: context renewal. OR: object recognition (including spontaneous object recognition and trained object discrimination. cxtSOR: contextual spontaneous object recognition. OiP: object-in-place. BOD: biconditional object discrimination. cxtFC: contextual fear conditioning. FCs: signaled fear conditioning where the CS is a simple stimulus, e.g. pure tone. FCc: signaled fear conditioning where the CS is complex or discontinuous.
Although the surgery group recovered to normal levels of performance after initially dropping post-surgery, they were severely impaired when learning new object-place associations (Jo & Lee, 2010).
There was significant damage to the auditory association cortex dorsal to the PER, which might explain reduced fear response to the auditory CS (Corodimas & LeDoux, 1995).
Deficits were observed in ABA renewal but not ABC renewal (Trask, Shipman, et al., 2017).
Anatomical connections between prefrontal cortices and the perirhinal/postrhinal cortex
In the rodent brain, there are both direct and indirect pathways by which prefrontal and parahippocampal areas communicate. Even though direct parahippocampal connections with prefrontal cortices are relatively weak compared to its connections with association and sensory cortices or within the parahippocampal region (Agster & Burwell, 2009; Burwell & Amaral, 1998a), direct reciprocal connections do exist between the PER/POR and multiple prefrontal areas, including the medially located PL and IL cortices, multiple subregions of the OFC, and the secondary motor cortex (MOs). Indirect prefrontal-parahippocampal communication is possibly mediated via the nucleus reuniens of the thalamus, which is reciprocally connected with the PER/POR (Agster et al., 2016; Tomás Pereira et al., 2016). Importantly, the pattern of reciprocal prefrontal-parahippocampal pathways differs from that between prefrontal cortices and the hippocampus. This section will mainly focus on direct prefrontal afferents and parahippocampal efferents based on Agster and Burwell (2009), Burwell and Amaral, (1998a), and Hwang, Willis, and Burwell (2018). Results reported by these studies are mostly consistent with others (Delatour & Witter, 2002; Kondo & Witter, 2014; Reep et al., 1987; Reep et al., 1990). As a comparison, the anatomical connections between prefrontal cortices and the hippocampus will be briefly reviewed as well (Figure 4).
Figure 4.

Anatomical connections between the prefrontal cortices, the hippocampus, and parahippocampal cortices. Abbreviations: ENT: entorhinal cortex. HPC: hippocampus. MOs: secondary motor cortex. NRe: nucleus reuniens. lOFC: the lateral, ventral, and ventrolateral portions of the orbitofrontal cortex. mOFC: medial orbitofrontal cortex. PER: perirhinal cortex. mPFC: medial prefrontal cortex. POR: postrhinal cortex. The shape of arrows indicates the strength of the connection. For parahippocampal cortices, the strength of an afferent connection is determined by the ratio of the number of retrograde-labeled cells in the origin over the number of all labeled cells. For other connections, the strength reflects the density of labeling. In articles where data is split into multiple sub-regions, the results across sub-regions are averaged (Agster & Burwell, 2009; Agster et al., 2016; Barreiros et al., 2021; Burwell & Amaral, 1998a; Delatour & Witter, 2002; Hwang et al., 2018; Jay & Witter, 1991; Kerr et al., 2007; Kondo & Witter, 2014; McKenna & Vertes, 2004; Murphy & Deutch, 2018; Reep et al., 1987; Reep et al., 1990; Reep et al., 1984; Tomás Pereira et al., 2016; Vertes et al., 2006).
Both area 35 and 36 of the PER project to the entire rostrocaudal extent of both PL and IL. Area 36 projects to the PL and IL more strongly than area 35, with heavier projection originating from the rostral to mid-rostrocaudal sites of area 36. Area 35 sends strong projection to the mid-rostrocaudal portion of the PL. Both areas send axons to the medial, lateral, and ventrolateral portions of the OFC, though area 36 has more efferents compared to area 35. These projections originate more from the rostral and mid-rostrocaudal levels, and they arrive at rostral levels of all orbitofrontal subareas. Area 35 and 36 also project to the MOs, though relatively weaker than to other PFC areas. The projections originate from the entire rostrocaudal extent of both areas, with the rostral regions preferentially targeting rostral MOs. In terms of afferent connections, prefrontal areas project more strongly to area 35 than area 36. The orbitofrontal regions combined provide around half of the prefrontal output to the two PER areas, with the majority arising from the lateral or ventrolateral OFC. The MOs provides most of the remaining prefrontal output to the PER. Lastly, for medial prefrontal regions, the IL provides stronger input to the PER than the PL, and for both regions, input to the PER is stronger from the rostral levels.
The POR exhibits a different pattern of prefrontal connection compared to area 35 or 36 of the PER. Overall, the POR projects to prefrontal areas less strongly than the PER. The strongest projections originate from the caudal POR and terminate in rostral levels of both ventrolateral OFC and MOs. The entire rostrocaudal extent of POR projects to the MOs. The POR projection to the IL and PL are weak overall. The MOs provides the most input to the POR among all prefrontal regions; stronger projection originates from the mid-rostrocaudal and the most caudal levels of the MOs. The orbital regions provide the second most prefrontal output, mostly from the ventrolateral portion. Output from mPFC to the POR is small.
In comparison, connectivity between the hippocampus and prefrontal areas is limited in two ways. First, hippocampal efferents terminate in the medial but not lateral or the most dorsal portions of prefrontal cortices (Hoover & Vertes, 2007; Jay & Witter, 1991; Vertes et al., 2007). The areas to which the hippocampus does not project, i.e. the lateral and ventrolateral OFC and the MOs, not only receive efferents from the PER and POR but also account for most of the prefrontal input to these two cortices. Second, whereas both direct and indirect pathways exist between the prefrontal and parahippocampal regions, the mPFC lacks direct projection to the hippocampus and the connection is relayed via the nucleus reuniens instead (Vertes et al., 2007).
Parahippocampal-prefrontal communication supports context-dependent behavior
Widespread reciprocal connectivity and mutual involvement in multiple context-guided tasks suggest that parahippocampal areas, such as the PER and POR, may directly provide contextual information to the prefrontal cortices. Despite the functional evidence and well-described connections, existing research has focused more on the hippocampus as the primary source of contextual input to prefrontal areas. For example, connections between the mPFC and the hippocampus were shown to be necessary for context-dependent renewal following fear conditioning (Orsini et al., 2011; Wang et al., 2016). Another series of studies provided evidence that dorsal hippocampus and mPFC are involved in working memory versions of a biconditional discrimination task in which texture and the visual appearance of the floor in a maze guides spatial responses (Hallock et al., 2013; Hallock & Griffin, 2013). Moreover, inactivation of midline thalamic nuclei that provide an indirect pathway between the hippocampus and medial prefrontal cortex also impaired performance on the task (Urban et al., 2014).
Although prefrontal-hippocampal interaction indeed plays essential roles in some forms of contextual memory, such interactions, alone, seem insufficient to support all aspects of context-dependent behavior. Based on anatomical and functional evidence, we propose a more distributed prefrontal to medial temporal lobe circuit that also involves interaction and coordination between the parahippocampal and the prefrontal regions (Figure 5). Typical examples of context-dependent behavior that this model supports include paradigms reviewed in previous sections. However, it should also account for any behavior that requires encoding, storing, and retrieving memory for diffuse external stimuli (as opposed to simple cues or signals). Another example of a procedure that this model may support is the conditioned place preference for modeling substance abuse (Bardo & Bevins, 2000). Moreover, it should be noted that although the proposed model is based on research that operationally defines “context” as the spatial environment, there has been evidence that many of these regions are also involved for processing the temporal aspects of context. For example, the hippocampus, mPFC, and the PER are all required in tasks where subjects need to encode and retrieve the order in which events occur (Allen et al., 2020; Barker et al., 2007; Hannesson et al., 2004). Thus, although this review focuses on the spatial context, the same network may also be responsible for processing a broader range of information that constitutes the “what-where-when” aspects of episodic-like memory.
Figure 5.

Schematics of information flow between the PFC, PER-POR and HPC. Some areas or connections are not included for simplicity, such as the entorhinal cortex and the nucleus reuniens.
In this model (Figure 5), the PER and POR form a cortical contextual-support network that provide context information to both the hippocampus and the prefrontal cortices for executive control. Instead of emerging in the hippocampus, basic context representations are already present at the PER-POR level, most likely in the POR where object-location conjunctive coding has been observed. These representations can be passed onto the hippocampus either directly to the CA1 or indirectly through the entorhinal cortex, finally reaching the hippocampus to support associative learning, some forms of contextual learning, and episodic memory. Although context representations are necessary for a number of hippocampal dependent functions, they can also be utilized directly by prefrontal cortices to support context guided behavior. Contextual information facilitates cue-outcome and reward value encoding in the OFC, while also enabling efficient updating in response to varying contextual demands. In return, goal-related input from the OFC arrives at the PER and POR and it may modify place-field firing patterns in the hippocampus. Contextual input can also be utilized by the MOs to accurately encode similar actions or actions in sequence, which supports efficient action selection, planning, and execution. Though the mPFC does receive direct projections from the hippocampus and projects back via the nucleus reuniens, the PER and POR may provide additional contextual input that the mPFC uses to modulate other associations via occasion-setting mechanisms. Consistent with this view, context-activated ensembles in the mPFC show activity patterns distinct from those in the hippocampus: ensembles in the mPFC are more differentiated between successive exposure to the same context, whereas hippocampal representations are separated more in terms of distinct locations within the same environment (Hyman et al., 2012). This suggests that the mPFC relies on sources in addition to the hippocampus to maintain different forms of context representations that are suitable for coping with varying task demands.
Prefrontal-perirhinal connections are known to play important roles in object recognition and discrimination tasks with contextual demands but not simple object recognition (Barker et al., 2007; Barker & Warburton, 2009; Hernandez et al., 2017). These studies either involve creating permanent lesions or employ procedures that cannot be separated into distinct phases of acquisition and recognition, making the specific function of these connections difficult to pin down. It is possible that the mPFC facilitates retrieval of memories containing information about familiar objects as well as the context in which they occurred, therefore it is required for recollection-based recognition to retrieve object-context pairings; the mPFC is not critically involved for simple object recognition, which may be solved by the PER and familiarity-based process alone. Alternatively, the mPFC may utilize contextual input to modulate retrieval of object information in the PER during recognition, eventually affecting neuronal firing pattern in the hippocampus (Navawongse & Eichenbaum, 2013; Place et al., 2016). To explore these possibilities, future research may employ techniques that silence the mPFC-PER pathway in a direction-specific manner. It has already been demonstrated that information flows of opposite directions take place between the mPFC and hippocampus during the sampling and retrieving phases of a context-guided object recognition task (Place et al., 2016). Beyond object recognition, mPFC-PER connectivity may also underlie the context renewal effect. We might speculate that disconnecting these areas will reduce conditioned responding during re-exposure to the acquisition context, and this effect may be strongest when temporary disconnection occurs during re-exposure, compared to during extinction.
In addition to mPFC connections, the PER and POR also have substantial connectivity with the OFC, particularly its lateral and ventrolateral portion (Delatour & Witter, 2002; Hwang et al., 2018; Kondo & Witter, 2014). The PER and POR are well positioned to provide contextual input to support the OFC in goal-oriented decision-making, and they may rely on projections from the OFC for cue-outcome associations and reward values. The OFC is proposed to house a task space for goal-oriented tasks as well as monitoring and updating the current location in that space (Bradfield & Hart, 2020; Wilson et al., 2014). The lateral and ventrolateral OFC contains detailed spatial mapping of goals, actions, and other task-related context information (Farovik et al., 2015; Feierstein et al., 2006). These types of mapping loosely resemble the “cognitive map” in the hippocampus (Wikenheiser & Schoenbaum, 2016). Since the lateral and ventrolateral portion of the OFC receive no direct projection from the hippocampus (Jay & Witter, 1991), the parahippocampal region is a prime source for providing both spatial and non-spatial information that is represented by the OFC.
Jointly, PER and POR may provide information to the OFC about external environmental stimuli to be integrated with internal cues to aid goal-directed behavior. Specifically, the POR may communicate with the OFC to support and update spatial mapping containing reward locations and other task-relevant information. Inhibiting orbitofrontal neurons that receive projections from the POR indeed leads to deficits in detecting object location change (Qi et al., 2019). The PER is capable of efficiently combining and monitoring reward features and transmit them to the OFC. Since the values of all relevant features of a reward need to be computed and integrated before optimal decisions are reached, having accurate input from the PER may be useful not only for maintaining choice strategies but also for flexibly adjusting behavior if reward properties change. A recent study supports this speculative role of the PER in maintaining reward representation: bilateral PER inactivation leads to decreased choice stability over two rewards with different magnitudes and less successful exploitation during a dynamic delay discounting task (Kreher et al., 2019). In turn, since the OFC has been shown to project outcome signals and influence other cortical areas (Banerjee et al., 2020; Liu et al., 2020), it may modify firing patterns of PER or POR neurons regarding outcome expectancy in goal-directed behavior, eventually altering spatial mapping in the hippocampus (Hok et al., 2007). In mice, the POR and its surrounding regions contain ensembles selectively encoding the identity or the motivational value of visual cues (Burgess et al., 2016; Ramesh et al., 2018), and hippocampal-induced reactivation produces learning-related changes in their connectivity (Sugden et al., 2020). Future research is required to show that such mapping in the POR indeed relies upon input from the OFC, perhaps during spatial reward tasks in which spatial correlates have been detected in the POR (Burwell & Hafeman, 2003). After the POR establishes spatial mapping containing information about reward locations, partial or complete re-mapping may occur if reward location moves or reward value changes, e.g. via devaluation. Such re-mapping among POR neurons may directly result from value update signals originating from the OFC, in which case re-mapping will not occur if OFC input has been blocked.
So far, no study has directly examined the involvement of the secondary motor cortex (MOs) in classic context-dependent tasks; however, since the MOs is among the prefrontal areas most heavily connected to both the PER and POR (Hwang et al., 2018; Reep et al., 1987; Reep et al., 1990), it may be expected to contribute significantly to some aspects of context-dependent behavior. The MOs is characterized by expansive cortico-cortical connections with the primary motor cortex, the somatosensory, visual, auditory, retrosplenial and parahippocampal cortices (Reep et al., 1987; Reep et al., 1990), and it is proposed to be involved in selecting, planning, and executing voluntary actions based on sensory input (Barthas & Kwan, 2017). The primate analogue of the rodent MOs is not well established; possible candidates include the supplementary motor area (Reep et al., 1987; Reep et al., 1990) and the dorsolateral prefrontal cortex (Hwang et al., 2018). MOs neurons encode and discriminate upcoming choice actions during action planning and persist throughout its execution (Olson et al., 2020). The choice signals occur in the MOs earlier than the primary motor cortex or other brain areas (Sul et al., 2011). In addition, the presence of value signals in the MOs suggests that it may be involved in certain decision-making processes as well (Sul et al., 2011). Both single-unit and population coding of rewarded choices exist in the MOs during an auditory discrimination task with probabilistic outcomes, though it does not encode the magnitude of rewards (Siniscalchi et al., 2019). Although it has been proposed that areas such as the retrosplenial and posterior parietal cortices are the primary sources of spatial information to the MOs (Olson et al., 2020), the anatomical evidence suggests that the MOs may rely on the PER and POR for contextual input as well. Encoding similar actions differently based on their context may facilitate efficient action selection according to variable situational demand. Contextual information may even be required to encode different instances of the same action separately, particularly when the outcome is determined by a sequence of actions, such as navigating a maze that contains multiple left and right turns (Olson et al., 2020).
To conclude, although hippocampal-mPFC communication is critical for some forms of context-dependent behavior, there is substantial evidence for cooperation between prefrontal areas and other medial temporal lobe structures. On the one hand, due to their mutual connectivity with multiple prefrontal areas, parahippocampal areas such as the PER and POR may provide context information directly to the prefrontal cortex in addition to supporting context associations in the hippocampus. On the other hand, prefrontal areas may send important information about values and actions back to the PER and POR and eventually to the hippocampus, serving as another route of prefrontal-hippocampal interaction. Examining the functional connections between prefrontal areas and parahippocampal cortices, particularly the PER and POR, will contribute importantly to our understanding of learning and memory in general.
HIGHLIGHTS.
Perirhinal and postrhinal cortices are important for context-modulated behavior
Both regions project directly to the prefrontal cortex and to the hippocampus
These perirhinal and postrhinal projections certainly carry information about context
This input is likely used for prefrontal-hippocampal interactions in context learning
Funding
This work was supported by the National Institute of Mental Health grant R01MH108729 and the National Science Foundation Award IOS-1656488 to Rebecca D. Burwell.
Footnotes
Declarations of interest
The authors have no conflicts of interest or competing interests to declare.
References
- Aggleton JP, & Nelson AJD (2020). Distributed interactive brain circuits for object-in-place memory: A place for time? Brain Neurosci Adv, 4, 2398212820933471. 10.1177/2398212820933471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, & Burwell RD (2009). Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus, 19(12), 1159–1186. 10.1002/hipo.20578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, Tomás Pereira I, Saddoris MP, & Burwell RD (2016). Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. II. efferents. Hippocampus, 26(9), 1213–1230. 10.1002/hipo.22600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Davies M, Futter JE, & Aggleton JP (2009). Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci, 123(1), 115–124. 10.1037/a0013829 [DOI] [PubMed] [Google Scholar]
- Allen LM, Lesyshyn RA, O’Dell SJ, Allen TA, & Fortin NJ (2020). The hippocampus, prefrontal cortex, and perirhinal cortex are critical to incidental order memory. Behav Brain Res, 379, 112215. 10.1016/j.bbr.2019.112215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Parente G, Teutsch J, Lewis C, Voigt FF, & Helmchen F (2020). Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature, 585(7824), 245–250. 10.1038/s41586-020-2704-z [DOI] [PubMed] [Google Scholar]
- Bardo MT, & Bevins RA (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl), 153(1), 31–43. 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, & Warburton EC (2007). Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. Journal of Neuroscience, 27(11), 2948–2957. 10.1523/JNEUROSCI.5289-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, & Warburton EC (2009). Critical role of the cholinergic system for object-in-place associative recognition memory. Learning & Memory, 16(1), 8–11. 10.1101/lm.1121309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, & Warburton EC (2011). When is the hippocampus involved in recognition memory? Journal of Neuroscience, 31(29), 10721–10731. 10.1523/JNEUROSCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiros IV, Panayi MC, & Walton ME (2021). Organization of Afferents along the Anterior–posterior and Medial–lateral Axes of the Rat Orbitofrontal Cortex. Neuroscience, 460, 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthas F, & Kwan AC (2017). Secondary motor cortex: where ‘sensory’meets ‘motor’in the rodent frontal cortex. Trends in neurosciences, 40(3), 181–193. 10.1016/j.tins.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi PC, de Oliveira PEC, Palombo P, Leão RM, Cogo-Moreira H, da Silva Planeta C, & Cruz FC (2018). Functional inactivation of the orbitofrontal cortex disrupts context-induced reinstatement of alcohol seeking in rats. Drug and alcohol dependence, 186, 102–112. 10.1016/j.drugalcdep.2017.12.045 [DOI] [PubMed] [Google Scholar]
- Bouton ME (2004). Context and behavioral processes in extinction. Learning & Memory, 11(5), 485–494. 10.1101/lm.78804 [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979). Contextual control of the extinction of conditioned fear. Learning and motivation, 10(4), 445–466. 10.1037//0097-7403.5.4.368 [DOI] [Google Scholar]
- Bradfield LA, & Hart G (2020). Rodent medial and lateral orbitofrontal cortices represent unique components of cognitive maps of task space. Neuroscience & Biobehavioral Reviews, 108, 287–294. 10.1016/j.neubiorev.2019.11.009 [DOI] [PubMed] [Google Scholar]
- Bucci DJ, & Burwell RD (2004). Deficits in Attentional Orienting Following Damage to the Perirhinal or Postrhinal Cortices [Article]. Behavioral neuroscience, 118(5), 1117–1122. 10.1037/0735-7044.118.5.1117 [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, & Burwell RD (2000). Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral neuroscience, 114(5), 882. 10.1037//0735-7044.114.5.882 [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, & Burwell RD (2002). Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behavioral neuroscience, 116(3), 479–488. 10.1037/0735-7044.116.3.479 [DOI] [PubMed] [Google Scholar]
- Burgess CR, Ramesh RN, Sugden AU, Levandowski KM, Minnig MA, Fenselau H, Lowell BB, & Andermann ML (2016). Hunger-dependent enhancement of food cue responses in mouse postrhinal cortex and lateral amygdala. Neuron, 91(5), 1154–1169. 10.1016/j.neuron.2016.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell R, & Agster K (2008). Anatomy of the hippocampus and the declarative memory system. 10.1016/B978-012370509-9.00117-0 [DOI] [Google Scholar]
- Burwell RD (2001). Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. Journal of Comparative Neurology, 437(1), 17–41. 10.1002/cne.1267 [DOI] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998a). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Journal of Comparative Neurology, 398(2), 179–205. [DOI] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998b). Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. Journal of Comparative Neurology, 391(3), 293–321. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, & Jutras MJ (2004). Perirhinal and postrhinal contributions to remote memory for context. In (Vol. 24, pp. 11023–11028). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, & Hafeman DM (2003). Positional firing properties of postrhinal cortex neurons. Neuroscience, 119(2), 577–588. 10.1016/s0306-4522(03)00160-x [DOI] [PubMed] [Google Scholar]
- Burwell RD, & Witter MP (2002). Basic anatomy of the parahippocampal region in monkeys and rats [Chapter]. Oxford University Press. 10.1093/acprof:oso/9780198509172.003.0003 [DOI] [Google Scholar]
- Burwell RD, Witter MP, & Amaral DG (1995). Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus, 5(5), 390–408. 10.1002/hipo.450050503 [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2002). Perirhinal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience, 15(2), 365–374. 10.1046/j.0953-816x.2001.01851.x [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2005). The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. The Quarterly Journal of Experimental Psychology Section B, 58(3–4), 269–282. 10.1080/02724990544000004 [DOI] [PubMed] [Google Scholar]
- Chakraborty T, Asok A, Stanton M, & Rosen J (2016). Variants of contextual fear conditioning induce differential patterns of Egr-1 activity within the young adult prefrontal cortex. Behavioural brain research, 302, 122–130. 10.1016/j.bbr.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Quirk GJ (2007). Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience, 27(4), 840–844. 10.1523/JNEUROSCI.5327-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corodimas KP, & LeDoux JE (1995). Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behavioral neuroscience, 109(4), 613. 10.1037//0735-7044.109.4.613 [DOI] [PubMed] [Google Scholar]
- Cross L, Brown MW, Aggleton JP, & Warburton EC (2013). The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learning & Memory, 20(1), 41–50. 10.1101/lm.028266.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, & Robbins TW (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews, 28(7), 771–784. 10.1016/j.neubiorev.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Delatour B, & Witter M (2002). Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. European Journal of Neuroscience, 15(8), 1400–1407. 10.1046/j.1460-9568.2002.01973.x [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in cognitive sciences, 11(9), 379–386. 10.1016/j.tics.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Eddy MC, Todd TP, Bouton ME, & Green JT (2016). Medial prefrontal cortex involvement in the expression of extinction and ABA renewal of instrumental behavior for a food reinforcer. Neurobiology of learning and memory, 128, 33–39. 10.1016/j.nlm.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci, 18(9), 547–558. 10.1038/nrn.2017.74 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, & Lipton P (2012). Towards a functional organization of episodic memory in the medial temporal lobe. Neuroscience & Biobehavioral Reviews, 36(7), 1597–1608. 10.1016/j.neubiorev.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annu. Rev. Neurosci, 30, 123–152. 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, & Aggleton JP (1997). Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental brain research, 113(3), 509–519. 10.1007/pl00005603 [DOI] [PubMed] [Google Scholar]
- Estela VJ (2020). Neural correlates of early- and late-stage learning in the hippocampus and postrhinal cortex [Neural correlates of early- and late-stage learning in the hippocampus and postrhinal cortex, Brown University]. Providence, RI. [Google Scholar]
- Farovik A, Place RJ, McKenzie S, Porter B, Munro CE, & Eichenbaum H (2015). Orbitofrontal cortex encodes memories within value-based schemas and represents contexts that guide memory retrieval. Journal of Neuroscience, 35(21), 8333–8344. 10.1523/JNEUROSCI.0134-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, & Mainen ZF (2006). Representation of spatial goals in rat orbitofrontal cortex. Neuron, 51(4), 495–507. 10.1016/j.neuron.2006.06.032 [DOI] [PubMed] [Google Scholar]
- Fraser KM, & Holland PC (2019). Occasion Setting. In (Vol. 133, pp. 145–175). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, & Burwell RD (2012). Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron, 76(5), 976–988. 10.1016/j.neuron.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, & Helmstetter FJ (2010). Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning & Memory, 17(6), 289–296. 10.1101/lm.1597410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, & Helmstetter FJ (2013). NR2A-and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learning & Memory, 20(6), 290–294. 10.1101/lm.030510.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, & Griffin AL (2013). Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiol Learn Mem, 100, 108–116. 10.1016/j.nlm.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Hallock HL, & Griffin AL (2013). Dynamic coding of dorsal hippocampal neurons between tasks that differ in structure and memory demand. Hippocampus, 23(2), 169–186. 10.1002/hipo.22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson D, Vacca G, Howland J, & Phillips A (2004). Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behavioural brain research, 153(1), 273–285. 10.1016/j.bbr.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Heimer-McGinn VR, Poeta DL, Aghi K, Udawatta M, & Burwell RD (2017). Disconnection of the perirhinal and postrhinal cortices impairs recognition of objects in context but not contextual fear conditioning. Journal of Neuroscience, 37(18), 4819–4829. 10.1523/JNEUROSCI.0254-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Reasor JE, Truckenbrod LM, Lubke KN, Johnson SA, Bizon JL, Maurer AP, & Burke SN (2017). Medial prefrontal-perirhinal cortical communication is necessary for flexible response selection. Neurobiology of learning and memory, 137, 36–47. 10.1016/j.nlm.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JW, & Burwell RD (2014). Perirhinal and postrhinal functional inputs to the hippocampus. In Space, time and memory in the hippocampal formation (pp. 55–81). Springer. 10.1007/978-3-7091-1292-2_3 [DOI] [Google Scholar]
- Hok V, Lenck-Santini P-P, Roux S, Save E, Muller RU, & Poucet B (2007). Goal-related activity in hippocampal place cells. Journal of Neuroscience, 27(3), 472–482. 10.1523/JNEUROSCI.2864-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function, 212(2), 149–179. 10.1007/s00429-007-0150-4 [DOI] [PubMed] [Google Scholar]
- Hwang E, Willis BS, & Burwell RD (2018). Prefrontal connections of the perirhinal and postrhinal cortices in the rat. Behavioural brain research, 354, 8–21. 10.1016/j.bbr.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, & Seamans JK (2012). Contextual encoding by ensembles of medial prefrontal cortex neurons. Proceedings of the National Academy of Sciences, 109(13), 5086–5091. 10.1073/pnas.1114415109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Burnett DJ, Aggleton JP, Good M, & Honey RC (2009). The role of the hippocampus in mnemonic integration and retrieval: complementary evidence from lesion and inactivation studies. European Journal of Neuroscience. [DOI] [PubMed] [Google Scholar]
- Jay TM, & Witter MP (1991). Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. Journal of Comparative Neurology, 313(4), 574–586. 10.1002/cne.903130404 [DOI] [PubMed] [Google Scholar]
- Ji J, & Maren S (2007). Hippocampal involvement in contextual modulation of fear extinction. Hippocampus, 17(9), 749–758. 10.1002/hipo.20331 [DOI] [PubMed] [Google Scholar]
- Jo YS, & Lee I (2010). Perirhinal cortex is necessary for acquiring, but not for retrieving object–place paired association. Learning & Memory, 17(2), 97–103. 10.1101/lm.1620410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, & Brown TH (2012). Dual functions of perirhinal cortex in fear conditioning. Hippocampus, 22(10), 2068–2079. 10.1002/hipo.22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, & Burwell RD (2007). Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus, 17(9), 697–708. 10.1002/hipo.20315 [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith D, Allen T, & Brown T (2008). Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behavioral neuroscience, 122(5), 1178. 10.1037/a0012902 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim N, Kim HT, & Choi J-S (2013). The prelimbic cortex is critical for context-dependent fear expression. Frontiers in behavioral neuroscience, 7, 73. 10.3389/fnbeh.2013.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, & Eichenbaum H (2009). Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci, 29(31), 9918–9929. 10.1523/JNEUROSCI.1378-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, & Witter MP (2014). Topographic organization of orbitofrontal projections to the parahippocampal region in rats. Journal of Comparative Neurology, 522(4), 772–793. 10.1002/cne.23442 [DOI] [PubMed] [Google Scholar]
- Kreher MA, Johnson SA, Mizell JM, Chetram DK, Guenther DT, Lovett SD, Setlow B, Bizon JL, Burke SN, & Maurer AP (2019). The perirhinal cortex supports spatial intertemporal choice stability. Neurobiol Learn Mem, 162, 36–46. 10.1016/j.nlm.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, & Fuchs RA (2009). Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. European Journal of Neuroscience, 30(7), 1370–1381. 10.1111/j.14609-568.2009.06906.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, & Fuchs RA (2011). Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology, 36(3), 711–720. 10.1038/npp.2010.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, & Solivan F (2008). The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learning & Memory, 15(5), 357–367. 10.1101/lm.902708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist DH, Jarrard LE, & Brown TH (2004). Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. Journal of Neuroscience, 24(14), 3610–3617. 10.1523/JNEUROSCI.4839-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Deng J, Zhang Z, Zhang ZY, Sun YG, Yang T, & Yao H (2020). Orbitofrontal control of visual cortex gain promotes visual associative learning. Nat Commun, 11(1), 2784. 10.1038/s41467-020-16609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, & Gould TJ (2014). The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacology Biochemistry and Behavior, 123, 45–54. 10.1016/j.pbb.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, & Fanselow MS (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural brain research, 88(2), 261–274. 10.1016/s0166-4328(97)00088-0 [DOI] [PubMed] [Google Scholar]
- McKenna JT, & Vertes RP (2004). Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol, 480(2), 115–142. 10.1002/cne.20342 [DOI] [PubMed] [Google Scholar]
- Murphy MJM, & Deutch AY (2018). Organization of afferents to the orbitofrontal cortex in the rat. J Comp Neurol, 526(9), 1498–1526. 10.1002/cne.24424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navawongse R, & Eichenbaum H (2013). Distinct pathways for rule-based retrieval and spatial mapping of memory representations in hippocampal neurons. Journal of Neuroscience, 33(3), 1002–1013. 10.1523/JNEUROSCI.3891-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, & Eacott M (2004). Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural brain research, 148(1–2), 79–91. 10.1016/s0166-4328(03)00176-1 [DOI] [PubMed] [Google Scholar]
- Norman G, & Eacott M (2005). Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral neuroscience, 119(2), 557. 10.1037/0735-7044.119.2.557 [DOI] [PubMed] [Google Scholar]
- Olson JM, Li JK, Montgomery SE, & Nitz DA (2020). Secondary motor cortex transforms spatial information into planned action during navigation. Current Biology, 30(10), 1845–1854. e1844. 10.1016/j.cub.2020.03.016 [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, & Maren S (2011). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci, 31(47), 17269–17277. 10.1523/JNEUROSCI.4095-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, & Eichenbaum H (2016). Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nature neuroscience, 19(8), 992. 10.1038/nn.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter NM, Calub CA, & Furtak SC (2020). Perirhinal cortex inactivation produces retrieval deficits in fear extinction to a discontinuous visual stimulus. Behavioral neuroscience, 134(2), 144. 10.1037/bne0000351 [DOI] [PubMed] [Google Scholar]
- Qi X, Du ZJ, Zhu L, Liu X, Xu H, Zhou Z, Zhong C, Li S, Wang L, & Zhang Z (2019). The Glutamatergic Postrhinal Cortex–Ventrolateral Orbitofrontal Cortex Pathway Regulates Spatial Memory Retrieval. Neuroscience bulletin, 35(3), 447–460. 10.1007/s12264-018-0325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh RN, Burgess CR, Sugden AU, Gyetvan M, & Andermann ML (2018). Intermingled ensembles in visual association cortex encode stimulus identity or predicted outcome. Neuron, 100(4), 900–915. e909. 10.1016/j.neuron.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep R, Corwin J, Hashimoto A, & Watson R (1987). Efferent connections of the rostral portion of medial agranular cortex in rats. Brain research bulletin, 19(2), 203–221. 10.1016/0361-9230(87)90086-4 [DOI] [PubMed] [Google Scholar]
- Reep R, Goodwin G, & Corwin J (1990). Topographic organization in the corticocortical connections of medial agranular cortex in rats. Journal of Comparative Neurology, 294(2), 262–280. 10.1002/cne.902940210 [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, & Watson RT (1984). Afferent connections of medial precentral cortex in the rat. Neuroscience letters, 44(3), 247–252. 10.1016/0304-3940(84)90030-2 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Wagner A (1972). A theory of Pavlovian conditioning : Variations in the effectiveness of reinforcement and nonreinforcement. Classical conditioning II: Current research and theory, 64–99. [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, & Bucci DJ (2012). Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. Journal of Neuroscience, 32(35), 12076–12086. 10.1523/JNEUROSCI.2814-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos TB, Kramer-Soares JC, Favaro VM, & Oliveira MGM (2017). Involvement of the prelimbic cortex in contextual fear conditioning with temporal and spatial discontinuity. Neurobiology of learning and memory, 144, 1–10. 10.1016/j.nlm.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Witter MP, & Schwarcz RE (2000). The parahippocampal region: Implications for neurological and psychiatric diseases. New York Academy of Sciences. [PubMed] [Google Scholar]
- Sharpe M, & Killcross S (2015). The prelimbic cortex uses contextual cues to modulate responding towards predictive stimuli during fear renewal. Neurobiology of learning and memory, 118, 20–29. 10.1016/j.nlm.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, Stalnaker T, Schuck NW, Killcross S, Schoenbaum G, & Niv Y (2019). An integrated model of action selection: distinct modes of cortical control of striatal decision making. Annual review of psychology, 70, 53–76. 10.1146/annurev-psych-010418-102824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalchi MJ, Wang H, & Kwan AC (2019). Enhanced population coding for rewarded choices in the medial frontal cortex of the mouse. Cerebral Cortex, 29(10), 4090–4106. 10.1093/cercor/bhy292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden AU, Zaremba JD, Sugden LA, McGuire KL, Lutas A, Ramesh RN, Alturkistani O, Lensjø KK, Burgess CR, & Andermann ML (2020). Cortical reactivations of recent sensory experiences predict bidirectional network changes during learning. Nature neuroscience, 23(8), 981–991. 10.1038/s41593-020-0651-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul JH, Jo S, Lee D, & Jung MW (2011). Role of rodent secondary motor cortex in value-based action selection. Nature neuroscience, 14(9), 1202. 10.1038/nn.2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter EE, Weiss C, & Disterhoft JF (2013). Perirhinal and postrhinal, but not lateral entorhinal, cortices are essential for acquisition of trace eyeblink conditioning. Learning & Memory, 20(2), 80–84. 10.1101/lm.028894.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, & Naya Y (2014). The perirhinal cortex. Annual review of neuroscience, 37, 39–53. 10.1146/annurev-neuro-071013-014207 [DOI] [PubMed] [Google Scholar]
- Tam SK, Bonardi C, & Robinson J (2015). Relative recency influences object-in-context memory. Behav Brain Res, 281, 250–257. 10.1016/j.bbr.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás Pereira I, Agster KL, & Burwell RD (2016). Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. I. afferents. Hippocampus, 26(9), 1189–1212. 10.1002/hipo.22603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Shipman ML, Green JT, & Bouton ME (2017). Inactivation of the prelimbic cortex attenuates context-dependent operant responding. Journal of Neuroscience, 37(9), 2317–2324. 10.1523/JNEUROSCI.3361-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Thrailkill EA, & Bouton ME (2017). Occasion setting, inhibition, and the contextual control of extinction in Pavlovian and instrumental (operant) learning. Behavioural processes, 137, 64–72. 10.1016/j.beproc.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban KR, Layfield DM, & Griffin AL (2014). Transient inactivation of the medial prefrontal cortex impairs performance on a working memory-dependent conditional discrimination task. Behav Neurosci, 128(6), 639–643. 10.1037/bne0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, & Rodriguez JJ (2006). Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol, 499(5), 768–796. 10.1002/cne.21135 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, & Leranth C (2007). Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain research bulletin, 71(6), 601–609. 10.1016/j.brainresbull.2006.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, & Rescorla R (1972). Inhibition in Pavlovian conditioning: Application of a theory. Inhibition and learning, 301–336. [Google Scholar]
- Wang Q, Jin J, & Maren S (2016). Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats. Neurobiology of learning and memory, 134, 38–43. 10.1016/j.nlm.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E, & Brown MW (2015). Neural circuitry for rat recognition memory. Behavioural brain research, 285, 131–139. 10.1016/j.bbr.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, & Brown MW (2015). Neural circuitry for rat recognition memory. Behav Brain Res, 285, 131–139. 10.1016/j.bbr.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, & Schoenbaum G (2016). Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nature Reviews Neuroscience, 17(8), 513–523. 10.1038/nrn.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, & Niv Y (2014). Orbitofrontal cortex as a cognitive map of task space. Neuron, 81(2), 267–279. 10.1016/j.neuron.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Sekeres MJ, Binns MA, & Moscovitch M (2013). Hippocampal lesions produce both nongraded and temporally graded retrograde amnesia in the same rat. Hippocampus, 23(5), 330–341. 10.1002/hipo.22093 [DOI] [PubMed] [Google Scholar]
- Winters BD, & Bussey TJ (2005). Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience, 25(1), 52–61. 10.1523/JNEUROSCI.3827-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J-Z, & Brown M (1998). Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology, 37(4–5), 657–676. 10.1016/s0028-3908(98)00030-6 [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, & Fanselow MS (2013). Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proceedings of the National Academy of Sciences, 110(24), 9938–9943. 10.1073/pnas.1301691110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, & Fanselow MS (2014). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. Journal of Neuroscience, 34(25), 8462–8466. 10.1523/JNEUROSCI.3624-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


