Abstract
Background
Despite a large proportion of the population having been vaccinated and infected, Singapore had SARS-CoV-2 waves driven by the BA.5 and XBB sublineages of the omicron (B.1.1.529) variant. Data on the protective immunity against medically attended, symptomatic reinfections with omicron BA.4, BA.5, and XBB conferred by previous SARS-CoV-2 infections and vaccinations are scarce. We therefore aimed to derive information from Singapore's experience as one of the first countries with an XBB-driven wave.
Methods
For this retrospective national cohort study, we used information from official databases of the Ministry of Health of Singapore to assess hybrid immunity (obtained from previous infection and vaccination) against medically attended, symptomatic BA.4 and BA.5 reinfections from Oct 1, 2022, to Nov 1, 2022, and medically attended, symptomatic XBB reinfections from Oct 18, 2022, to Nov 1, 2022, among Singapore citizens and permanent residents aged at least 18 years. All individuals with acute respiratory symptoms who presented at any health-care facility in Singapore between the stated dates were tested for SARS-CoV-2. Individuals were grouped into SARS-CoV-2-naive, pre-omicron, omicron BA.1, and omicron BA.2 groups according to their previous infection status. Data were also stratified by time from first infection to analyse the waning of immunity. Incidence rate ratios (IRRs) were measured by generalised linear Poisson regressions, with SARS-CoV-2-naive individuals as the reference group, and protective immunity was calculated as one minus the risk ratio multiplied by 100.
Findings
2 456 791 individuals were included in the study, contributing 53·1 million person-days of observation for the SARS-CoV-2-naive group, 3·4 million person-days for the pre-omicron group, 6·6 million person-days for the BA.1 group, and 13·7 million person-days for the BA.2 group between Oct 1, 2022, and Nov 1, 2022. Compared with SARS-CoV-2-naive individuals, first infections with pre-omicron variants did not confer protection against reinfection with BA.4 or BA.5 (IRR 0·87 [95% CI 0·73–1·05] for pre-omicron infection with booster vaccination) or XBB (IRR 1·29 [1·23–1·35] for pre-omicron infection with booster vaccination). Previous BA.2 infection with booster provided the greatest protection against reinfection, but this was lower against reinfection with XBB (protective immunity 51%; 95% CI 49–53) than against reinfection with BA.4 or BA.5 (78%; 74–82). Protection conferred by previous BA.2 infection against XBB reinfection waned faster over time from first infection (from 74% [72–75] at 3–6 months to 49% [47–52] at 7–8 months) than protection against BA.4 or BA.5 reinfection (from 87% [82–90] at 3–6 months to 74% [66–80] at 7–8 months).
Interpretation
Protection against XBB reinfection conferred by a previous omicron infection with vaccination was lower and waned faster than protection against BA.4 or BA.5 reinfection, which is indicative of the greater immune evasiveness of the XBB sublineage. Although severe COVID-19 is uncommon, populations remain vulnerable to future reinfection waves from emerging SARS-CoV-2 variants despite high rates of vaccination and infection, as reflected by substantially higher reinfection rates during Singapore's XBB wave than during the previous BA.5-driven wave. Policy makers could consider emerging public health interventions, such as omicron-adapted bivalent vaccines, to maintain population immunity against COVID-19.
Funding
None.
Introduction
Since the omicron (B.1.1.529) variant of SARS-CoV-2 was first detected in November, 2021, it has expanded into more than 100 sublineages and led to numerous waves of infection globally. In Singapore, the omicron variant was first detected on Dec 2, 2021, and quickly overtook delta (B.1.617.2) to become the predominant variant by January, 2022. The omicron sublineages BA.1 and BA.2 caused the largest wave of COVID-19 cases in Singapore to date, with more than 26 000 new cases recorded on Feb 22, 2022 alone.1 Following detection of the first local cases of the omicron sublineages BA.4 and BA.5 in May, 2022, omicron BA.5 subsequently superseded BA.2 as the predominant circulating strain in Singapore and reached a peak of 16 870 recorded cases on July 13, 2022.1 Less than 3 months later, Singapore had another surge in cases driven by the XBB sublineage, a recombinant of BA.2.10.1 and BA.2.75 that was first detected in India in August, 2022.2 By mid-October, 2022, XBB had surpassed BA.5 as the predominant omicron sublineage in Singapore, with reinfections comprising a larger share of daily cases during the XBB wave than during the previous BA.5-driven wave.
Research in context.
Evidence before this study
We searched PubMed for articles published between database inception and Oct 30, 2022, using the keywords (“COVID-19” OR “SARS-CoV-2”) AND (“previous*” OR “prior*” OR “reinfection*”) AND (“BA.5” OR “XBB”), with no language restrictions. We identified four observational studies that reported protection from previous infection or hybrid immunity against reinfection with the BA.5 sublineage of the omicron (B.1.1.529) variant of SARS-CoV-2. Data from Qatar and Portugal show that previous SARS-CoV-2 infection had a protective effect against reinfection with BA.5, with previous BA.1 and BA.2 infection conferring the greatest protection. A study in Denmark found that previous omicron infection in individuals who had received three doses of a COVID-19 vaccine provided strong protection against reinfection with BA.5 and BA.2. We found no real-world studies that investigated protection conferred by infection or vaccination against reinfection with XBB.
Added value of this study
This study uses comprehensive national data to estimate the protection that infection and vaccination confer against medically attended, symptomatic reinfection with BA.4, BA.5, and XBB in Singapore—one of the first countries to have an XBB-driven wave. Our findings indicate that a previous pre-omicron infection conferred no or minimal protection against BA.4, BA.5, or XBB reinfection, whereas protection from an earlier omicron infection against XBB reinfection was lower and waned faster than protection against BA.4 or BA.5 reinfection. Among individuals with a previous BA.1 or BA.2 infection, those who received a booster dose of an mRNA COVID-19 vaccine had greater protection against XBB reinfection than those who received only the primary vaccination series.
Implications of all the available evidence
Our findings highlight that XBB has greater immune-evasion capabilities than BA.5 and, although severe COVID-19 is uncommon, populations remain vulnerable to reinfection waves from emerging SARS-CoV-2 variants despite high rates of vaccination and infection. Countries should maintain vigilance against COVID-19, especially if new SARS-CoV-2 variants are on the horizon. Emerging public health tools such as bivalent vaccines could have an important role in maintaining population immunity against COVID-19.
By November, 2022, 92% of Singapore's population had received the primary COVID-19 vaccine series and 80% had received a booster dose under the COVID-19 National Vaccination Programme, and by October, 2022, an estimated 75% of the population had been infected by SARS-CoV-2, based on seroprevalence of anti-N antibodies and modelling studies.3 Assessing the immunity conferred by previous infections and vaccination against reinfections with emerging variants and sublineages is therefore important to guide public health measures and vaccination policy recommendations.
Previous studies4, 5, 6 found that infection with an earlier omicron sublineage conferred strong protection against reinfection with BA.4 or BA.5, whereas infection with pre-omicron variants provided lower protection. Singapore was one of the first countries to have an XBB-driven wave and information on this omicron sublineage is scarce. An in-vitro study7 of emerging omicron sublineages found that, of all those tested, XBB had among the greatest capability to evade the humoral immune system; however, real-world data are sparse. Here we aimed to assess the protective immunity that previous SARS-CoV-2 infections and vaccinations confer against medically attended, symptomatic reinfections with omicron sublineages BA.4, BA.5, and XBB.
Methods
Study design and participants
In this retrospective cohort study among citizens and permanent residents of Singapore aged at least 18 years, we used the incidence of medically attended, symptomatic omicron BA.4 and BA.5 infections between Oct 1, 2022, and Nov 1, 2022, and medically attended, symptomatic XBB infections between Oct 18, 2022, and Nov 1, 2022, to evaluate the immunity conferred by previous infection and vaccination. Data were collected from official databases maintained by the Ministry of Health of Singapore, including national records of all vaccines administered and confirmed SARS-CoV-2 infections and reinfections. The study was conducted under the Infectious Diseases Act, Singapore, to support policy decision making and the public health response to COVID-19; a separate ethics review by an institutional review board was therefore not required.
Individuals who received two doses of the BNT162b2 vaccine (tozinameran; Pfizer-BioNTech) or the mRNA-1273 vaccine (elasomeran; Moderna) were considered fully vaccinated with the primary series, and those who received three doses were considered boosted. Reinfection was defined as a positive test at least 90 days after the last positive test in the same individual.8 Individuals who received non-mRNA vaccines or second booster doses or who had multiple reinfection episodes were excluded.
SARS-CoV-2 testing with PCR or rapid antigen tests was conducted on all individuals presenting to any health-care facility in Singapore with acute respiratory symptoms (cough, runny nose, sore throat, or fever), and all positive cases were required by law to be notified to the Ministry of Health of Singapore under the Infectious Diseases Act. As Singapore detected its first omicron case on Dec 1, 2021, all infections before this date were classified as pre-omicron. For all infections after Dec 1, 2021, the variant and sublineage were established by whole-genome sequencing or S-gene target failure status where available. Corresponding to the timing of infection waves driven by different variants and sublineages in Singapore, infections with S-gene target failure between Jan 16, 2022, and April 30, 2022, were assumed to be BA.1, whereas those after April 30, 2022, were assumed to be BA.4 or BA.5. Infections without S-gene target failure before Jan 16, 2022, were assumed to be pre-omicron, whereas those between Jan 16, 2022, and July 31, 2022, were assumed to be BA.2. As XBB became the predominant omicron sublineage from Oct 18, 2022, all subsequent infections without S-gene target failure were assumed to be XBB.
For first infection or reinfection episodes in which whole-genome sequencing or S-gene target failure status were not available, we imputed the variant and sublineage. During time periods in which at least 75% of cases sequenced systematically under the national genomic surveillance programme for COVID-19 belonged to a particular predominant variant, imputation followed the predominant variant. For time periods during which no variant was predominant, regression imputation was conducted with a logistic regression model on the basis of the calendar date of reporting and additional demographic variables (age, sex, ethnicity, and housing type) as predictors (see appendix pp 1–3), similar to a previous study.9
Statistical analysis
We used generalised linear Poisson regression models to estimate the incidence rate ratios (IRRs) of omicron BA.4, BA.5, and XBB reinfections, with individuals who were SARS-CoV-2-naive serving as the reference group. Models were adjusted for age, sex, ethnicity, housing type (as a proxy for socioeconomic status), force of infection over time (by adjusting for calendar date in the model),10, 11 vaccination status, time since last vaccine dose, and time since previous infection if the individual had a previous infection. When counting person-time, we accounted for the varying nature of vaccination status and infection status over time; the same individual could contribute person-time to multiple groups in different time periods. Generalised linear Poisson regressions can overcome overdispersion,12 and 95% CIs were calculated on the basis of Wald statistics. Sensitivity analysis was conducted using the same set of Poisson regressions on a matched cohort of SARS-CoV-2-naive individuals to previously infected individuals in a 2:1 ratio, matched in terms of age, sex, ethnicity, housing type, vaccination status, and time since last vaccine dose. We also stratified the data by time from infection to analyse the waning of immunity. Protective immunity was calculated as one minus the relative risk, then multiplied by 100 to express as a percentage. The analysis was done with Stata (version 17).
Role of the funding source
There was no funding source for this study.
Results
2 456 791 individuals were included in the study, contributing 76·8 million person-days of observation between Oct 1, 2022, and Nov 1, 2022: 53·1 million person-days for the SARS-CoV-2-naive group, 3·4 million person-days for the pre-omicron group, 6·6 million person-days for the BA.1 group, and 13·7 million person-days for the BA.2 group (table 1 ). A combined total of 3784 cases of BA.4 and BA.5 were recorded between Oct 1, 2022, and Nov 1, 2022, comprising 3298 first infections and 486 reinfections. 46 753 cases of XBB were recorded between Oct 18, 2022, and Nov 1, 2022, of which 36 510 were first infections and 10 243 were reinfections. 47 (1·43%) individuals with BA.4 or BA.5 first infections and two (0·41%) individuals with BA.4 or BA.5 reinfections had severe disease (defined as requiring oxygen supplementation, admission to an intensive care unit, or death); for XBB, 76 (0·21%) individuals with first infections and 22 (0·21%) individuals with reinfections had severe disease.
Table 1.
Demographic characteristics of the study cohort by SARS-CoV-2 infection status
| Naive |
Previous SARS-CoV-2 infection |
|||||
|---|---|---|---|---|---|---|
| Pre-omicron | Omicron BA.1 | Omicron BA.2 | ||||
| Total person-days at risk | 53 076 623 | 3 401 554 | 6 630 233 | 13 672 314 | ||
| Sex | ||||||
| Male | 25 733 252 (48·5%) | 1 885 610 (55·4%) | 3 232 398 (48·8%) | 6 439 779 (47·1%) | ||
| Female | 27 343 371 (51·5%) | 1 515 944 (44·6%) | 3 397 835 (51·2%) | 7 232 535 (52·9%) | ||
| Age | ||||||
| 18–29 years | 10 421 695 (19·6%) | 541 136 (15·9%) | 1 423 147 (21·5%) | 2 944 900 (21·5%) | ||
| 30–39 years | 9 769 215 (18·4%) | 549 097 (16·1%) | 1 367 503 (20·6%) | 2 967 412 (21·7%) | ||
| 40–49 years | 10 421 147 (19·6%) | 639 017 (18·8%) | 1 224 951 (18·5%) | 2 749 153 (20·1%) | ||
| 50–59 years | 9 652 118 (18·2%) | 735 782 (21·6%) | 1 048 480 (15·8%) | 2 145 454 (15·7%) | ||
| 60–69 years | 7 479 150 (14·1%) | 549 078 (16·1%) | 919 581 (13·9%) | 1 720 247 (12·6%) | ||
| 70–79 years | 3 701 641 (7·0%) | 261 173 (7·7%) | 445 820 (6·7%) | 809 971 (5·9%) | ||
| ≥80 years | 1 631 657 (3·1%) | 126 271 (3·7%) | 200 751 (3·0%) | 335 177 (2·5%) | ||
| Ethnicity | ||||||
| Chinese | 39 604 433 (74·6%) | 2 301 609 (67·7%) | 4 622 623 (69·7%) | 10 234 558 (74·9%) | ||
| Malay | 4 894 985 (9·2%) | 405 054 (11·9%) | 590 065 (8·9%) | 1 031 784 (7·5%) | ||
| Indian | 6 831 074 (12·9%) | 586 455 (17·2%) | 1 223 950 (18·5%) | 1 994 768 (14·6%) | ||
| Other | 1 746 131 (3·3%) | 108 436 (3·2%) | 193 595 (2·9%) | 411 204 (3·0%) | ||
| Housing type | ||||||
| Public housing | 41 628 614 (78·4%) | 2 921 241 (85·9%) | 5 652 796 (85·3%) | 11 158 499 (81·6%) | ||
| One-room or two-room | 2 503 212 (4·7%) | 263 284 (7·7%) | 358 208 (5·4%) | 515 145 (3·8%) | ||
| Three-room | 8 815 253 (16·6%) | 740 921 (21·8%) | 1 223 899 (18·5%) | 2 157 894 (15·8%) | ||
| Four-room | 17 018 306 (32·1%) | 1 141 921 (33·6%) | 2 341 347 (35·3%) | 4 764 092 (34·8%) | ||
| Five-room | 13 291 843 (25·0%) | 775 115 (22·8%) | 1 729 342 (26·1%) | 3 721 368 (27·2%) | ||
| Private housing | 11 448 009 (21·6%) | 480 313 (14·1%) | 977 437 (14·7%) | 2 513 815 (18·4%) | ||
| Vaccination status | ||||||
| Unvaccinated or partially vaccinated (0 or 1 dose) | 1 842 779 (3·5%) | 134 615 (4·0%) | 63 887 (1·0%) | 117 811 (0·9%) | ||
| Completed primary series | 1 664 476 (3·1%) | 489 732 (14·4%) | 662 962 (10·0%) | 782 089 (5·7%) | ||
| Boosted | 49 569 368 (93·4%) | 2 777 207 (81·6%) | 5 903 384 (89·0%) | 12 772 414 (93·4%) | ||
| <5 months | 968 400 (1·8%) | 635 667 (18·7%) | 586,002 (8·8%) | 298 991 (2·2%) | ||
| 5 to <6 months | 1 017 757 (1·9%) | 702 958 (20·7%) | 246 892 (3·7%) | 125 004 (0·9%) | ||
| 6 to <7 months | 1 502 689 (2·8%) | 381 553 (11·2%) | 105 409 (1·6%) | 148 235 (1·1%) | ||
| 7 to <8 months | 2 989 459 (5·6%) | 357 374 (10·5%) | 103 830 (1·6%) | 544 544 (4·0%) | ||
| 8 to <9 months | 7 469 571 (14·1%) | 238 093 (7·0%) | 558 990 (8·4%) | 1 747 912 (12·8%) | ||
| ≥9 months | 35 621 492 (67·1%) | 461 562 (13·6%) | 4 302 261 (64·9%) | 9 907 728 (72·5%) | ||
| Time since infection | ||||||
| 3 to <6 months | NA | .. | .. | 4 791 699 (35·0%) | ||
| 6 to <7 months | NA | .. | .. | 2 893 593 (21·2%) | ||
| 7 to <8 months | NA | .. | .. | 5 372 227 (39·3%) | ||
| <8 months | NA | .. | 2 102 381 (31·7%) | .. | ||
| ≥8 months | NA | .. | 4 527 852 (68·3%) | 614 795 (4·5%) | ||
| <12 months | NA | 1 708 251 (50·2%) | .. | .. | ||
| ≥12 months | NA | 1 693 303 (49·8%) | .. | .. | ||
Data are n or n (%).
Individuals who were infected with a pre-omicron variant (regardless of vaccination status) or who had a BA.1 or BA.2 infection but were not fully vaccinated did not have protection against reinfection with BA.4, BA.5, or XBB (table 2 ). Compared with SARS-CoV-2-naive individuals, the IRR of BA.4 or BA.5 infection was 0·87 (95% CI 0·73–1·05) and of XBB infection was 1·29 (1·23–1·35) among those who had a previous pre-omicron infection and had received a booster.
Table 2.
IRR and protective immunity of previous SARS-CoV-2 infection and vaccination against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfection
|
Omicron BA.4 or BA.5 |
Omicron XBB |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-days at risk | Number of cases | Incidence per million person-days | IRR* (95% CI) | Protective immunity† (95% CI) | Person-days at risk | Number of cases | Incidence per million person-days | IRR* (95% CI) | Protective immunity† (95% CI) | ||
| SARS-CoV-2-naive | 53 076 623 | 3298 | 62·1 | 1·00 (reference value) | Reference | 24 498 337 | 36 510 | 1490 | 1·00 (reference value) | Reference | |
| Pre-omicron | |||||||||||
| Unvaccinated or partially vaccinated (0 or 1 dose) | 134 615 | 7 | 52·0 | 2·00 (0·90 to 4·43) | .. | 62 426 | 56 | 897 | 2·81 (2·11 to 3·75) | .. | |
| Completed primary series | 489 732 | 20 | 40·8 | 1·08 (0·66 to 1·79) | .. | 226 313 | 313 | 1383 | 1·82 (1·58 to 2·08) | .. | |
| Boosted | 2 777 207 | 134 | 48·2 | 0·87 (0·73 to 1·05) | 13% (−5 to 27) | 1 277 769 | 2369 | 1854 | 1·29 (1·23 to 1·35) | .. | |
| Omicron BA.1 | |||||||||||
| Unvaccinated or partially vaccinated (0 or 1 dose) | 63 887 | 5 | 78·3 | 2·77 (1·10 to 6·99) | .. | 29 701 | 23 | 774 | 2·43 (1·59 to 3·72) | .. | |
| Completed primary series | 662 962 | 12 | 18·1 | 0·45 (0·24 to 0·83) | 55% (17 to 76) | 308 506 | 243 | 788 | 0·97 (0·84 to 1·13) | 3% (−13 to 16) | |
| Boosted | 5 903 384 | 146 | 24·7 | 0·39 (0·33 to 0·46) | 61% (54 to 67) | 2 738 726 | 3034 | 1108 | 0·70 (0·68 to 0·73) | 30% (27 to 32) | |
| Omicron BA.2 | |||||||||||
| Unvaccinated or partially vaccinated (0 or 1 dose) | 117 811 | 6 | 50·9 | 2·07 (0·88 to 4·87) | .. | 55 004 | 28 | 509 | 1·80 (1·22 to 2·66) | .. | |
| Completed primary series | 782 089 | 6 | 7·7 | 0·20 (0·09 to 0·47) | 80% (53 to 91) | 365 077 | 214 | 586 | 0·78 (0·67 to 0·91) | 22% (9 to 33) | |
| Boosted | 12 772 414 | 150 | 11·7 | 0·22 (0·18 to 0·26) | 78% (74 to 82) | 5 969 416 | 3963 | 664 | 0·49 (0·47 to 0·51) | 51% (49 to 53) | |
IRR=incidence rate ratio.
Adjusted for age, sex, ethnicity, housing type, calendar date, vaccination status of SARS-CoV-2-naive group, days since last vaccine dose, and days since previous infection.
Negative protective immunity estimates are not presented.
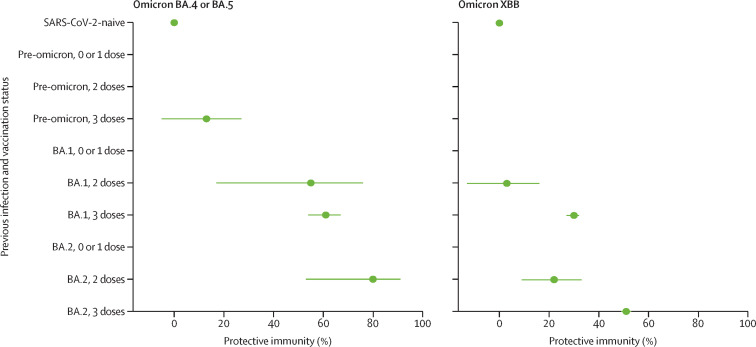
Against BA.4 and BA.5 reinfection, hybrid immunity from a first infection with BA.1 and primary vaccination series gave a protective immunity of 55% (95% CI 17–76), similar to that obtained by a previous BA.1 infection with booster (61%; 54–67; figure 1 ). Among individuals with a previous BA.2 infection, protection against BA.4 or BA.5 reinfection was also similar for those who completed the primary vaccination series (80%; 53–91) and those who received a booster (78%; 74–82).
Figure 1.
Protective immunity from previous infection and vaccination against medically attended, symptomatic omicron BA.4, BA.5, and XBB reinfection
Error bars are 95% CIs. Protective immunity is adjusted for age, sex, ethnicity, housing type, calendar date, vaccination status of SARS-CoV-2-naive group, days since last vaccine dose, and days since previous infection. Negative protective immunity estimates are not presented.
Protection against XBB reinfection was lower than protection against BA.4 or BA.5 reinfection across all combinations of hybrid immunity. Against XBB reinfection, previous BA.1 infection and primary series vaccination conferred no or minimal protection, whereas hybrid immunity from a BA.1 infection and a booster afforded slight protection (30%; 27–32). Protective immunity from a previous BA.2 infection and primary series vaccination was 22% (9–33), whereas a BA.2 infection with booster conferred the greatest protection (51%; 49–53) against reinfection with XBB.
When data were stratified by time from first infection, the waning of immunity against BA.4, BA.5, and XBB reinfection was observed (table 3 ). Previous infections with pre-omicron variants were ineffective at reducing the risk of BA.4, BA.5, or XBB reinfection. A first BA.1 infection less than 8 months previously provided protective immunity of 65% (95% CI 57–72) against reinfection with BA.4 or BA.5, which declined to 49% (35–59) at 8 months or more after first infection (figure 2 ). Protective immunity conferred by a previous BA.1 infection against XBB reinfection was 40% (95% CI 32–47) less than 8 months after infection and declined to 27% (23–31) at 8 months or more. A previous BA.2 infection within 6 months provided a protective immunity of 87% (82–90) against BA.4 or BA.5 reinfection, which decreased slightly to 74% (66–80) at 7–8 months after infection. In comparison, protection conferred by a previous BA.2 infection against XBB reinfection was lower within 6 months of first infection (74%, 72–75) and waned at a faster rate, decreasing to 49% (47–52) at 7–8 months and 37% (32–43) at 8 months or more from infection.
Table 3.
IRR and protective immunity of previous SARS-CoV-2 infection against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfection, stratified by time from first infection
|
Omicron BA.4 or BA.5 |
Omicron XBB |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-days at risk | Number of cases | Incidence per million person-days | IRR* (95% CI) | Protective immunity† (95% CI) | Person-days at risk | Number of cases | Incidence per million person-days | IRR* (95% CI) | Protective immunity† (95% CI) | ||
| SARS-CoV-2-naive | 53 076 623 | 3298 | 62·1 | 1·00 (reference value) | Reference | 24 498 337 | 36 510 | 1490 | 1·00 (reference value) | Reference | |
| Pre-omicron | |||||||||||
| 3 to <12 months | 1 708 251 | 98 | 57·4 | 0·86 (0·70 to 1·06) | 14 (−6 to 30) | 594 500 | 1107 | 1862 | 1·35 (1·26 to 1·43) | .. | |
| ≥12 months | 1 693 303 | 63 | 37·2 | 1·03 (0·80 to 1·33) | .. | 972 008 | 1631 | 1678 | 1·34 (1·27 to 1·41) | .. | |
| Omicron BA.1 | |||||||||||
| 3 to <8 months | 2 102 381 | 90 | 42·8 | 0·35 (0·28 to 0·43) | 65 (57 to 72) | 194 201 | 223 | 1148 | 0·60 (0·53 to 0·68) | 40 (32 to 47) | |
| ≥8 months | 4 527 852 | 73 | 16·1 | 0·51 (0·41 to 0·65) | 49 (35 to 59) | 2 882 732 | 3077 | 1067 | 0·73 (0·70 to 0·76) | 27 (24 to 30) | |
| Omicron BA.2 | |||||||||||
| 3 to <6 months | 4 791 699 | 45 | 9·4 | 0·13 (0·10 to 0·18) | 87 (82 to 90) | 2 033 272 | 850 | 418 | 0·26 (0·25 to 0·28) | 74 (72 to 75) | |
| 6 to <7 months | 2 893 593 | 56 | 19·4 | 0·20 (0·16 to 0·26) | 80 (74 to 84) | 928 954 | 617 | 664 | 0·42 (0·39 to 0·45) | 58 (55 to 61) | |
| 7 to <8 months | 5 372 227 | 61 | 11·4 | 0·26 (0·20 to 0·34) | 74 (66 to 80) | 2 819 806 | 2236 | 793 | 0·51 (0·48 to 0·53) | 49 (47 to 52) | |
| ≥8 months | 614 795 | 0 | 0 | .. | .. | 607 465 | 502 | 826 | 0·63 (0·57 to 0·68) | 37 (32 to 43) | |
IRR=incidence rate ratio.
Adjusted for age, sex, ethnicity, housing type, calendar date, vaccination status, and days since last vaccine dose.
Negative protective immunity estimates are not presented.
Figure 2.
Protective immunity of previous infection against medically attended, symptomatic omicron BA.4, BA.5, and XBB reinfection over time from previous infection
Error bars are 95% CIs. Protective immunity is adjusted for age, sex, ethnicity, housing type, calendar date, vaccination status, and days since last vaccine dose. Negative protective immunity estimates are not presented.
A sensitivity analysis with a matched cohort design gave similar estimates of protective immunity by previous infection and vaccination status, and by previous infection stratified by time from infection, as those in our primary analysis (appendix pp 6–7).
Discussion
Our estimates of protection against BA.4 or BA.5 reinfection conferred by a previous BA.1 or BA.2 infection and at least two doses of COVID-19 vaccine ranged from 55% to 80%, similar to the results of earlier studies evaluating protection by infections against BA.4 or BA.5 reinfections in populations with similarly high vaccination coverage. A study in Qatar, with a test-negative design, estimated that an earlier omicron infection provided a protective immunity of 76·2% (95% CI 66·4–83·1) against symptomatic BA.4 or BA.5 reinfection,4 whereas a cohort study from Portugal reported that a protective immunity of 76·8% (76·5–77·1) was provided by previous BA.1 or BA.2 infection against BA.5 reinfection.5
We found that individuals with a previous infection who were unvaccinated or partially vaccinated (ie, had received only one dose of the primary vaccine series) were not protected against reinfection with BA.4, BA.5, or XBB regardless of the variant of the first infection, indicating that natural immunity alone is insufficient. Hybrid immunity from an earlier omicron infection and vaccination consistently conferred lower protection against reinfection with XBB than with BA.4 or BA.5, supporting early data and in-vitro studies suggesting that XBB is more immune-evasive than BA.5.8 Against reinfection with XBB, a previous BA.1 infection and primary series vaccination did not confer protection, previous BA.1 infection and a booster or previous BA.2 infection and primary series vaccination provided modest protection, and previous BA.2 infection and a booster was most protective.
Our findings suggest that previous omicron infection with primary series vaccination with an mRNA vaccine provides adequate protection against BA.4 or BA.5 reinfection. However, protection by a previous omicron infection and a booster dose could be insufficient against XBB reinfection, and a second booster dose might be recommended, especially for people who are vulnerable to severe COVID-19, such as those who are older13 or immunocompromised.14
We found that infection with pre-omicron variants also did not confer any protection against BA.4, BA.5, or XBB reinfection regardless of vaccination status, suggesting that little hybrid immunity is generated by pre-omicron infections against reinfections with omicron sublineages. For individuals infected during the delta wave or before, bivalent vaccines that target wild-type SARS-CoV-2 and the omicron variant could be recommended to maintain immunity during this phase of the pandemic while the omicron variant is dominant.
In the time-stratified analysis, we found that the protection conferred by a previous BA.2 infection against XBB reinfection was lower within 6 months of first infection and waned faster over time than protection conferred against reinfection with BA.4 or BA.5. As the largest COVID-19 wave in Singapore was predominantly driven by the BA.2 sublineage, and XBB emerged several months after BA.4 and BA.5, the faster waning of natural immunity from a previous BA.2 infection against XBB meant that the population was more susceptible to XBB reinfection. Together with the greater immune escape of XBB from hybrid immunity, this led to substantially higher proportions of reinfections during the XBB wave (about 20% of daily cases) than during the BA.5 wave (about 5% of daily cases) in Singapore (appendix p 8). Nevertheless, severe outcomes of reinfections were rare in our cohort, and we were therefore unable to analyse protection conferred by hybrid immunity against severe BA.4, BA.5, or XBB reinfection. The low rates of severe disease in our cohort, in which a high proportion of individuals were vaccinated, also suggest that current vaccines continue to provide strong protection against severe outcomes from new omicron sublineages.
On the basis of modelling and seroprevalence studies of anti-N antibodies, about 75% of the population of Singapore had been infected by SARS-CoV-2 by mid-October, 2022. This high prevalence of infection makes our findings applicable. However, uncertainty surrounding the true prevalence of SARS-CoV-2 infection in a population still exists, as seroconversion varies widely among individuals and non-mRNA vaccines can induce anti-N antibodies.
The key strength of this study is the use of official national health-care administrative data on infections and vaccinations, which is comprehensive and minimises recall bias. However, several limitations exist. First, as whole-genome sequencing or S-gene target failure screening was not done in all cases, variant types could have been misclassified during imputation. Second, owing to incomplete case ascertainment, some individuals who were classified as infection-naive could have had a previous infection episode. Finally, although we adjusted for possible demographic, socioeconomic, and time confounders, residual confounding from comorbidities or other factors that affect susceptibility to reinfections or health-care-seeking behaviour might still be present.
Our study shows that infection with an earlier omicron variant and vaccination provided hybrid immunity against medically attended symptomatic BA.4, BA.5, and XBB reinfections, but protection against XBB was lower and waned faster, explaining the substantially higher reinfection rates observed in Singapore during the XBB-driven wave than during the BA.5 wave. Despite high rates of vaccination and infection, populations remain vulnerable to future waves of reinfection from emerging SARS-CoV-2 variants and sublineages with progressively greater immune-escape capabilities. Reassuringly, severe outcomes in BA.4, BA.5, and XBB reinfections are uncommon. New omicron-adapted bivalent vaccines could have a key role in maintaining immunity against COVID-19 while the omicron variant is predominant, and their effectiveness against reinfections will require evaluation.
Data sharing
The databases with individual-level information used for this study are not publicly available due to personal data protection. Deidentified data can be made available for research, subject to approval by the Ministry of Health of Singapore. All inquiries should be sent to the corresponding author.
Declaration of interests
We declare no competing interests.
Contributors
CYT and CJC searched the literature and interpreted the data. DP, VJL, BO, KBT, and DCL conceived the study. DP and KBT analysed the data. VJL, BO, and KBT provided supervision. CYT, CJC, and DP wrote the manuscript, which was critically revised by DCL. All authors had full access to all data in the study and take responsibility for the decision to submit for publication. DP and KBT directly accessed and verified the underlying data reported in the manuscript.
Supplementary Material
References
- 1.Ministry of Health Singapore COVID-19 statistics. https://www.moh.gov.sg/covid-19/statistics
- 2.Global Initiative on Sharing Avian Influenza Data Genomic epidemiology of SARS-CoV-2 with subsampling focused globally over the past 6 months. https://gisaid.org/phylodynamics/global/nextstrain
- 3.Ministry of Health Singapore Opening remarks by Minister for Health Mr Ong Ye Kung at the MOH press conference to update on the COVID-19 situation on 15 October 2022. Oct 15, 2022. https://www.moh.gov.sg/news-highlights/details/opening-remarks-by-minister-for-health-mr-ong-ye-kung-at-the-moh-press-conference-to-update-on-the-covid-19-situation-on-15-october-2022
- 4.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Protective effect of previous SARS-CoV-2 infection against omicron BA.4 and BA.5 subvariants. N Engl J Med. 2022;387:1620–1622. doi: 10.1056/NEJMc2209306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malato J, Ribeiro RM, Leite PP, et al. Risk of BA.5 infection among persons exposed to previous SARS-CoV-2 variants. N Engl J Med. 2022;387:953–954. doi: 10.1056/NEJMc2209479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen CH, Friis NU, Bager P, et al. Risk of reinfection, vaccine protection, and severity of infection with the BA.5 omicron subvariant: a nation-wide population-based study in Denmark. Lancet Infect Dis. 2023;23:167–176. doi: 10.1016/S1473-3099(22)00595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Jian F, Wang J, et al. Imprinted SARS-CoV-2 humoral immunity induces convergent omicron RBD evolution. Nature. 2022 doi: 10.1038/s41586-022-05644-7. published online Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CY, Chiew CJ, Pang D, et al. Vaccine effectiveness against delta, omicron BA.1, and BA.2 in a highly vaccinated Asian setting: a test-negative design study. Clin Microbiol Infect. 2023;29:101–106. doi: 10.1016/j.cmi.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMenamin ME, Nealon J, Lin Y, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22:1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melliana A, Setyorini Y, Eko H, Rosi S, Purhadi The comparison of generalized Poisson regression and negative binomial reression methods in overcoming overdispersion. Int J Sci Technol Res. 2013;2:255–258. [Google Scholar]
- 13.Skarbinski J, Wood MS, Chervo TC, et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread omicron (B.1.1.529) and delta (B.1.617.2) variant circulation in northern California: a retrospective cohort study. Lancet Reg Health Am. 2022;12 doi: 10.1016/j.lana.2022.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singson JRC, Kirley PD, Pham H, et al. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19—COVID-NET, 10 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:878–884. doi: 10.15585/mmwr.mm7127a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The databases with individual-level information used for this study are not publicly available due to personal data protection. Deidentified data can be made available for research, subject to approval by the Ministry of Health of Singapore. All inquiries should be sent to the corresponding author.