Abstract
Exposure to ozone causes decrements in pulmonary function, a response associated with alterations in lung lipids. Pulmonary lipid homeostasis is dependent on the activity of peroxisome proliferator activated receptor gamma (PPARγ), a nuclear receptor that regulates lipid uptake and catabolism by alveolar macrophages (AMs). Herein, we assessed the role of PPARγ in ozone-induced dyslipidemia and aberrant lung function in mice. Exposure of mice to ozone (0.8 ppm, 3 h) resulted in a significant reduction in lung hysteresivity at 72 h post exposure; this correlated with increases in levels of total phospholipids, specifically cholesteryl esters, ceramides, phosphatidylcholines, phosphorylethanolamines, sphingomyelins, and di- and triacylglycerols in lung lining fluid. This was accompanied by a reduction in relative surfactant protein-B (SP-B) content, consistent with surfactant dysfunction. Administration of the PPARγ agonist, rosiglitazone (5 mg/kg/day, i.p.) reduced total lung lipids, increased relative amounts of SP-B, and normalized pulmonary function in ozone-exposed mice. This was associated with increases in lung macrophage expression of CD36, a scavenger receptor important in lipid uptake and a transcriptional target of PPARγ. These findings highlight the role of alveolar lipids as regulators of surfactant activity and pulmonary function following ozone exposure and suggest that targeting lipid uptake by lung macrophages may be an efficacious approach for treating altered respiratory mechanics.
Keywords: ozone, pulmonary lipids, macrophage, PPARγ
Inhalation of ozone causes decrements in lung function in both healthy and susceptible populations including children, the elderly, and individuals with chronic lung disease (Holm and Balmes, 2022). Lung function is dependent on pulmonary surfactants which dynamically regulate surface tension, thereby reducing the amount of energy required to expand the lungs during inspiration and preventing alveolar collapse on expiration (Agassandian and Mallampalli, 2013; Olmeda et al., 2017; Wright and Dobbs, 1991). Many lung diseases are associated with surfactant abnormalities, but their role in pulmonary dysfunction caused by ozone is unknown (Milad and Morissette, 2021).
Pulmonary surfactant consists of a complex mixture of surfactant proteins (SPs) and lipids including phospholipids, triglycerides, cholesterols, and fatty acids. Phosphatidylcholine is the major surface-active component of the lung lining comprising >80% of surfactant lipids; it primarily exists in the disaturated form, dipalmitoylphosphatidylcholine (DPPC) (Batenburg, 1992; Holm et al., 1996). SPs include hydrophobic SP-B and SP-C, which are key in maintaining alveolar surface tension and lung function and the hydrophilic collectins, SP-A and SP-D, which regulate inflammatory macrophage responses (Agassandian and Mallampalli, 2013;Agudelo et al., 2020; Castillo-Sánchez et al., 2022; Ikegami and Jobe, 1998; Ross et al., 2002). Surfactant homeostasis is maintained by alveolar epithelial type II (ATII) cells, which produce and recycle pulmonary lipids, and alveolar macrophages (AMs), which phagocytize and degrade them (Agudelo et al., 2020; Gurel et al., 2001; Hussell and Bell, 2014). Clearance of lipids by AMs and to a lesser extent, production by ATII cells, is dependent on the nuclear receptor, peroxisome proliferator activated receptor gamma (PPARγ) (Baker et al., 2010; Schachtrup et al., 2008). Activation of PPARγ by ligands such as fatty acids and eicosanoids transcriptionally regulates expression of genes involved in lipid transport and metabolism (Chawla et al., 2001; Chinetti et al., 2001; Liu et al., 2021; Tontonoz et al., 1998).
In the present studies, we analyzed the role of PPARγ in ozone-induced dysregulation of lipid homeostasis and pulmonary function using the thiazolidinedione PPARγ agonist, rosiglitazone. We hypothesized that by activating PPARγ, rosiglitazone would restore ozone-induced alterations in extracellular lipids, surfactant homeostasis, and decrements in pulmonary function, and indeed this is what we observed. This work demonstrates that lung lipid homeostasis is disrupted following ozone exposure, a response linked to decrements in respiratory mechanics, and suggests that restoring lipid handling may effectively limit lung toxicity.
Materials and methods
Animals and exposures
C57BL/6J mice (12 weeks; The Jackson Laboratories, Bar Harbor, ME) were housed in filter-top microisolation cages and provided food and water ad libitum. Female mice were used for our studies as they have been shown to be more sensitive to ozone than male mice (Cabello et al., 2015). All animals received humane care in compliance with the institution’s guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Mice were exposed to air or ozone (0.8 ppm, 3 h) in a whole-body Plexiglas chamber. Ozone was generated from oxygen gas via an ultraviolet light generator and mixed with air as previously described (Fakhrzadeh et al., 2002, 2004, 2008; Francis et al., 2020). The ozone concentration inside the chamber was continuously monitored using an InDevR Photometric ozone analyzer (2B Technologies, Broomfield, CO). Mice were treated i.p. with rosiglitazone (5 mg/kg; Adipogen Life Sciences, San Diego, CA) or vehicle control (1 mg/kg) daily beginning 24 h prior to air or ozone exposure. Animals were euthanized 72 h post-exposure by i.p. injection of ketamine (135 mg/kg) and xylazine (30 mg/kg) (Covetrus, Dublin, OH).
Bronchoalveolar lavage (BAL) fluid collection and analysis
Lung cells and cell-free lung lining fluid were collected by bronchoalveolar lavage as previously described (Francis et al., 2020). For preparation of large aggregate fractions for phospholipid quantitation, lung lining fluid was centrifuged at 20 000 × g for 1 h at 4°C. Pellets containing lipid-rich large aggregates were resuspended in 25 µl of saline. Inorganic phosphates were then extracted and measured as previously described (Bligh and Dyer, 1959; Massa et al., 2014).
Measurement of pulmonary mechanics
Mice were anesthetized with ketamine (115 mg/kg) and xylazine (30 mg/kg) and connected to a SCIREQ flexiVent (Montreal, Canada) via a tracheal cannula. Respiratory mechanics were assessed at positive end expiratory pressures (PEEPs) of 1, 3, and 6 cm H2O (Groves et al., 2012; Massa et al., 2014). Data from the impedance spectra were fit to a single-compartment model to calculate elastance (Ers) and a constant phase model, to calculate tissue damping (G) and tissue elastance (H). Lung hysteresivity was calculated as the ratio of G/H. The PV Loop Salazar Knowles Equation was used to estimate quasi-static compliance (Cst). Data were analyzed using flexiWare Version 8.1 software.
Western blotting
For analysis of SP-D, cell-free lung lining fluid proteins (2 μl BAL/well) were separated on NuPAGE 4%–12% Bis-Tris Gels (Thermo Fisher Scientific). SP-B was analyzed in large aggregate fractions of lung lining fluid (1.5 µg phosphate/well). Proteins were transferred to polyvinylene difluoride membranes, and non-specific binding blocked by incubating the membranes with 10% milk in T-TBS (0.5% Tween 20 in Tris-buffered saline) for 45 min at RT. Membranes were then incubated with rabbit polyclonal anti-SP-D antibody (DU117, 1:10 000; a gift from A. Pastva, Duke University) or rabbit polyclonal anti-SP-B antibody (gift from M.F. Beers, University of Pennsylvania) overnight at 4°C. Membranes were washed and incubated for 1 h at room temperature with HRP-conjugated secondary antibody (1:5000, 1% non-fat milk in tris-buffered saline with 0.5% Tween 20, (T-TBS); Bio-Rad, Hercules, CA). Bands were visualized using an Amersham ECL Prime Western Blotting System (Millipore Sigma, Burlington, MA). Band intensities were quantified using ImageJ software. Data were normalized to the Air + Veh group within each blot and then averaged across 3 different experiments.
Ultra-high performance liquid chromatography-mass spectrometry lipidomics
Deuterated 1-palmitoyl-2-palmitoyl-d31-sn-glycero-3-phosphocholine (DPPC, 32 µg; Avanti Polar Lipids, Alabaster, AL) was added to total large aggregate BAL fractions for absolute quantitation of lipid species. Lipids were extracted following the lipid tert-butyl methyl ether (MTBE) extraction method (Matyash et al., 2008). The upper layer was aliquoted (80 µl), dried, and resuspended in 2 ml of 1:1 MeOH:IPA for analysis by ultra-high performance liquid chromatography (UHPLC)-mass spectrometry at the Rutgers Cancer Institute of New Jersey Metabolomics Core following a chromatography protocol adapted from Chen et al. (2020). Full scan mass spectrometry analysis was performed on a Thermo Q Exactive PLUS with a HESI source under positive mode. Relative quantitation of select lipids was performed by back-calculation based on the average ion intensities of deuterated-DPPC and normalizing to 32 µg spike-in. Raw and processed data are available as Supplementary Files and https://doi.org/10.5061/dryad.4f4qrfjh1 (last accessed May 30, 2023).
Immunohistochemistry
Inflation fixation was performed by instilling up to 1 ml of 3.5% paraformaldehyde in PBS into the lungs through a tracheal cannula; the lungs were then removed and fixed in 3.5% paraformaldehyde. After 24 h at 4°C, the lungs were transferred to 70% ethanol and paraffin embedded. Tissue sections (5 µm) were processed for immunohistochemistry as previously described (Francis et al., 2020). Sections were incubated for 2 h at RT with serum to block nonspecific binding. This was followed by overnight incubation at 4°C in a humidified chamber with primary antibodies (Supplementary Table 1) or the appropriate IgG controls diluted in blocking buffer. Binding was visualized using an avidin-biotinylated enzyme complex with 3,3′-diaminobenzidine (DAB) as the substrate.
Flow cytometry
Lung cells were suspended in 1 ml PBS and then incubated with Invitrogen eBioscience Fixable Viability Dye eFluor 780 (Thermo Fisher Scientific) for 30 min at 4°C in the dark. An additional 1 ml of PBS was added, and the cells centrifuged (400 × g, 6 min, RT), resuspended in 100 µl of flow cytometry staining buffer (5% FBS, 0.02% sodium azide in PBS), and then incubated with TruStain FcX (anti-mouse CD16/32) antibody (BioLegend, San Diego, CA) for 10 min at 4°C to block nonspecific binding. This was followed by incubation with fluorophore-conjugated antibodies (Supplementary Table 2) or isotype controls for 30 min at 4°C in the dark. Cells were washed with 1 ml of staining buffer, centrifuged (400 × g, 6 min, RT), and processed for intracellular staining of arginase-1 using the Intracellular Fixation & Permeabilization Buffer Set according to the manufacturer’s directions (Thermo Fisher Scientific). Cells were then analyzed on a Beckman Coulter Gallios flow cytometer (Indianapolis, IN). Data were analyzed using Beckman Coulter Kaluza Analysis 2.0 flow cytometry software.
RT-qPCR
Total RNA was extracted from lung cells by the phenol-chloroform extraction method using TRIzol RNA Isolation Reagent (Thermo Fisher Scientific). Purified RNA was resuspended in 15 µl RNAsecure RNase Inactivation Reagent (Thermo Fisher Scientific) and stored at −80°C until analysis. RNA (1 µg) was reverse transcribed using the qScript cDNA Synthesis Kit (Quantabio, Beverly, MA) following the manufacturer’s guidelines. qPCR was performed using 10 ng of template mixed with primers for Cd36 (Mm00432403_m1, Thermo Fisher Scientific), Gapdh (Mm99999915_g1, Thermo Fisher Scientific), Msr1 (Mm00446214_m1, Thermo Fisher Scientific), or Scarb1 (Mm00450234_m1, Thermo Fisher Scientific), and TaqMan Universal PCR Master Mix (ThermoFisher Scientific), following the manufacturer’s recommended cycling parameters on an Applied Biosystems QuantStudio 6 Flex qPCR machine (Thermo Fisher Scientific). Target gene CT values were normalized to Gapdh as it exhibited the lowest coefficient of variation, and its expression did not vary with treatment. Fold changes were calculated relative to Air + Vehicle controls using the ΔΔCT method.
Statistical analysis
PRISM Version 9.2.0 (GraphPad Software, La Jolla, CA) was used for statistical analyses. Grubb’s test was used to identify individual outliers. Lipidomic data were analyzed by Student’s t test; all other data were analyzed using 2-way ANOVA followed by Tukey’s multiple comparisons test. Differences in absolute levels of select phosphatidylcholine species were determined using 1-way ANOVA followed by Tukey’s multiple comparisons test. A p value of ≤ .05 was considered statistically significant.
Results
Rosiglitazone blunts ozone-induced alterations in total phospholipids and SP-B levels in lung lining fluid
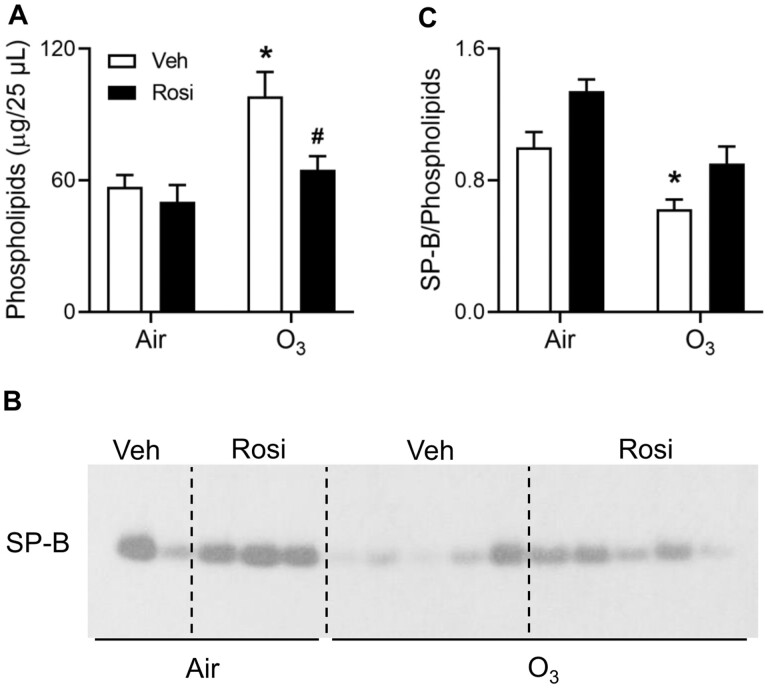
Respiratory mechanics are dependent on pulmonary surfactant and proper surfactant function requires a balance between levels of SP-B and phospholipids in the lung lining fluid (Glasser et al., 2001; Nambiar et al., 2021; Serrano and Pérez-Gil, 2006). A significant increase in total phospholipids in lung lining fluid was observed in ozone-exposed mice relative to air (Figure 1A); this resulted in a reduction in the relative amount of SP-B to total phospholipids (Figures 1B and 1C). Treatment of mice with rosiglitazone abrogated the effects of ozone on lung lipids, resulting in an increase in the ratio of SP-B to total phospholipids toward control levels.
Figure 1.
Effects of rosiglitazone (Rosi) on ozone (O3)-induced alterations in lung phospholipids and SP-B. Lung lining fluid was collected 72 h after exposure of mice to Air + Vehicle (Veh), Air + Rosi, O3 + Veh, or O3 + Rosi. A, Total phospholipids were analyzed in lung lining fluid. B, Equal amounts of phospholipids were loaded into each well of a denaturing gel. SP-B levels were assessed by western blotting. Each lane represents one mouse; data shown are representative of 3 independent experiments. C, Western blots were quantified using ImageJ. Band intensities were normalized to the Air + Veh group within individual blots and then averaged across the blots; data are expressed relative to Air + Veh. Bars, mean ± SE (n = 7–16 mice). Data were analyzed by 2-way ANOVA followed by Tukey’s multiple comparisons test. *Significantly different (p < .05) from Air + Veh. #Significantly different (p < .05) from O3 + Veh.
Rosiglitazone suppresses ozone-induced dyslipidemia
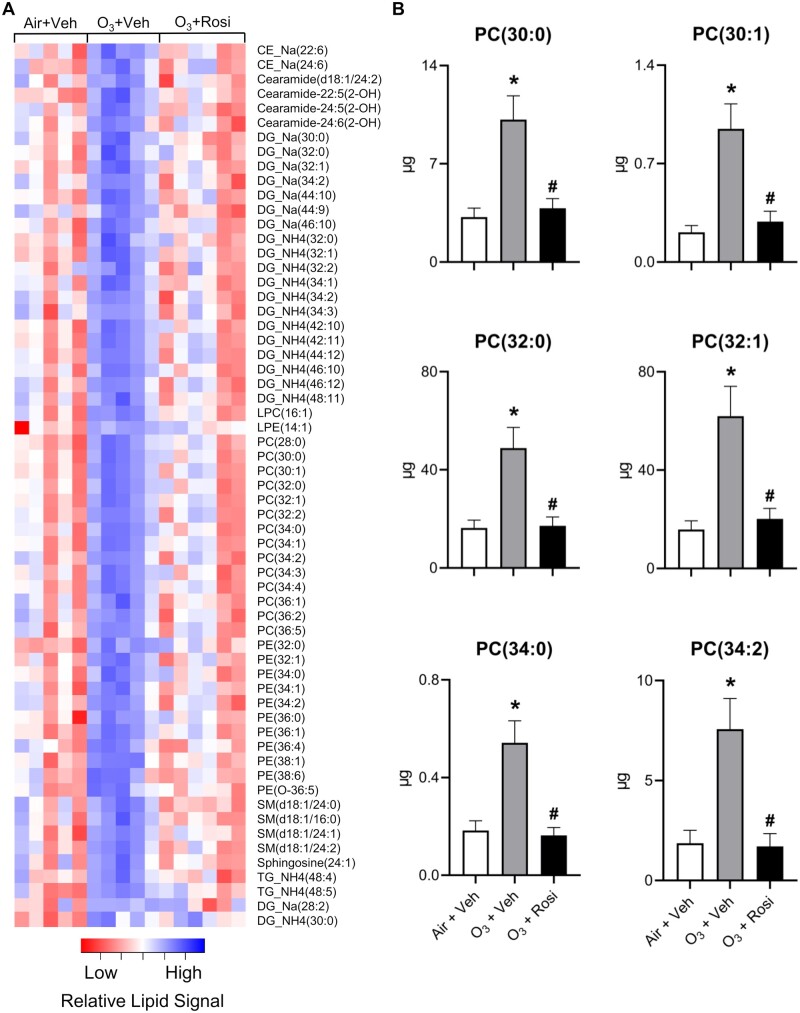
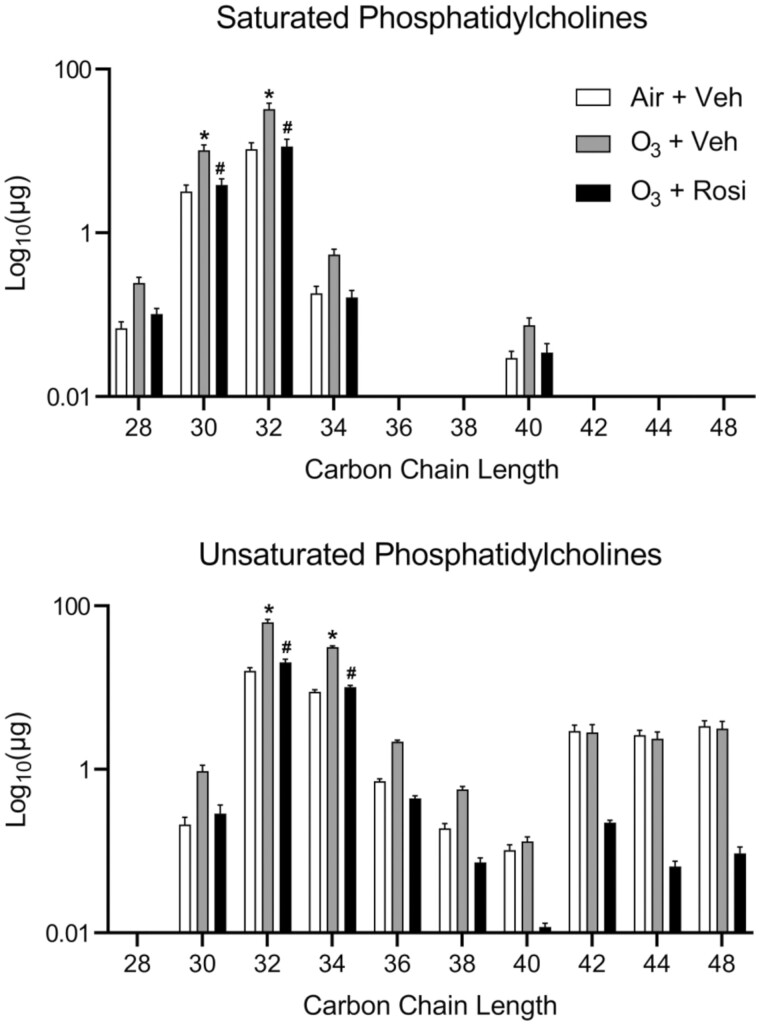
The ability of pulmonary surfactant to dynamically regulate surface tension is reliant on both the absolute amount of phospholipids, particularly phosphatidylcholine species, and the degree of unsaturation in their carbon chains (Walther et al., 2005). To identify changes in the amount and composition of lipids in lung lining fluid, we performed a lipidomic analysis. Since we did not observe differences in total phospholipids between air-exposed vehicle and air-exposed rosiglitazone treated mice (Figure 1A), we limited our analysis to ozone-exposed mice treated with vehicle control or rosiglitazone and air-exposed mice treated with vehicle. After ozone exposure, 61 lipid species were identified that increased including various cholesteryl esters, ceramides, phosphorylethanolamines, sphingomyelins, di- and triacylglycerols and all major phosphatidylcholine species, including 1,2-dipentadecanoyl-sn-glycero-3-phosphocholine [PC(30:0)], 1-dodecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine [PC(30:1)], 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine [PC(32:0)], 1-tetradecanoyl-2-(11Z-octadecenoyl)-sn-glycero-3-phosphocholine [PC(32:1)], 1-decanoyl-2-tetracosanoyl-sn-glycero-3-phosphocholine [PC(34:0)], 1-hexadecanoyl-2-(10E, 12Z-octadecadienoyl)-sn-glycero-3-phosphocholine [PC(34:2)] (Figures 2A and 2B). These results suggest that the increase in total phospholipids in the lung after ozone exposure is due to a general elevation in all lipids rather than individual lipid species. It should be noted, however, that these are steady-state measurements; thus, the dynamic status of the lipids is unknown. Increases in total phosphatidylcholine levels were associated with greater amounts of 32 and 34 carbon chain length species that contained at least 1 double bond in the fatty acid tail, as well as saturated 30 and 32 carbon chain length phosphatidylcholines (Figure 3). Treatment of ozone-exposed mice with rosiglitazone reduced levels of unsaturated phosphatidylcholines in lung lining fluid to levels detected in air-exposed mice treated with vehicle control.
Figure 2.
Rosi suppresses O3-induced alterations in lung lipids. Lipidomic profiles in lung lining fluid were assessed 72 h after exposure of mice to Air + Vehicle (Veh), O3 + Veh, or O3 + Rosi by non-targeted mass spectrometry as described in the Materials and methods section. A, Heatmap of lipid species that exhibited significant differences in ion intensities between Air + Veh and O3 + Veh groups, as determined by Student’s t test (p < .05). Colors indicate mean-centered Log10(Ion Intensities). B, Absolute quantitation of phosphatidylcholine species including 1,2-dipentadecanoyl-sn-glycero-3-phosphocholine [PC(30:0)], 1-dodecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine [PC(30:1)], 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine [PC(32:0)], 1-tetradecanoyl-2-(11Z-octadecenoyl)-sn-glycero-3-phosphocholine [PC(32:1)], 1-decanoyl-2-tetracosanoyl-sn-glycero-3-phosphocholine [PC(34:0)], 1-hexadecanoyl-2-(10E, 12Z-octadecadienoyl)-sn-glycero-3-phosphocholine [PC(34:2)] based on deuterated-PC(32:0) spike-in. Bars, mean ± SE (n = 5–6). Data were analyzed by 1-way ANOVA followed by Tukey’s multiple comparisons test. *Significantly different (p < .05) from Air + Veh. #Significantly different (p < .05) from O3 + Veh.
Figure 3.
Effects of Rosi on O3-induced alterations in phosphatidylcholine species. Total amounts of saturated and unsaturated phosphocholine species were assessed in lung lining fluid 72 h after exposure of mice to Air + Vehicle (Veh), O3 + Veh, or O3 + Rosi by non-targeted mass spectrometry as described in the Materials and methods section. Absolute phosphatidylcholine levels were calculated based on deuterated-PC(32:0) spike-in. Bars, mean ± SE (n = 5–6). Data were analyzed by 2-way ANOVA followed by Tukey’s multiple comparisons test. *Significantly different (p < .05) from Air + Veh. #Significantly different (p < .05) from O3 + Veh.
Effects of rosiglitazone on ozone-induced decrements in lung function
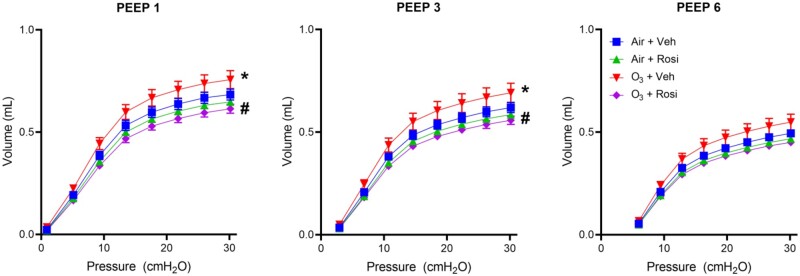
As rosiglitazone restored ozone-induced dyslipidemia, we next analyzed its effects on ozone-induced alterations in pulmonary function. Consistent with previous studies (Francis et al., 2020), ozone caused a leftward shift in the expiratory limb of the pressure volume (PV) loop, indicating a loss of elastic recoil properties (Figure 4 and Table 1) (Eidelman et al., 1989). This shift was most pronounced at PEEP = 1 cm H2O, where a significant reduction in lung hysteresivity was observed (Table 1). In ozone-exposed mice, rosiglitazone administration caused an increase in both lung elastance and hysteresivity; this was associated with a decrease in quasi-static compliance (Table 1). Rosiglitazone had no effect on lung function in air-exposed mice.
Figure 4.
Effects of Rosi on O3-induced alterations in respiratory mechanics. Pulmonary function was analyzed using a SCIREQ flexiVent system 72 h after exposure of mice to Air + Vehicle (Veh), Air + Rosi, O3 + Veh, or O3 + Rosi. PV loops were generated at PEEPs of 1, 3, and 6 cm H2O. Data are mean ± SE (n = 4). Data were analyzed by 2-way ANOVA. *Significantly different (p < .05) from Air + Veh. #Significantly different (p < .05) from O3 + Veh.
Table 1.
FlexiVent parameters
| PEEP | Group | G/H | Ers | Cst |
|---|---|---|---|---|
| 1 | Air + Veh | 0.19 ± 0.01 | 39.85 ± 1.87 | 0.06 ± 0.00 |
| Air + Rosi | 0.17 ± 0.01 | 40.97 ± 1.10 | 0.06 ± 0.00 | |
| O3 + Veh | 0.15 ± 0.01* | 34.04 ± 2.32 | 0.07 ± 0.01 | |
| O3 + Rosi | 0.18 ± 0.01 | 43.48 ± 2.08# | 0.06 ± 0.00# | |
| 3 | Air + Veh | 0.18 ± 0.01 | 37.07 ± 1.93 | 0.06 ± 0.00 |
| Air + Rosi | 0.17 ± 0.01 | 38.37 ± 0.91 | 0.05 ± 0.00 | |
| O3 + Veh | 0.17 ± 0.01 | 31.22 ± 2.66 | 0.06 ± 0.00 | |
| O3 + Rosi | 0.18 ± 0.01 | 40.63 ± 2.11# | 0.05 ± 0.00 | |
| 6 | Air + Veh | 0.21 ± 0.01 | 35.74 ± 1.79 | 0.07 ± 0.00 |
| Air + Rosi | 0.19 ± 0.00 | 37.26 ± 0.83 | 0.06 ± 0.00 | |
| O3 + Veh | 0.20 ± 0.01 | 30.60 ± 2.85 | 0.07 ± 0.01 | |
| O3 + Rosi | 0.20 ± 0.01 | 39.49 ± 2.13# | 0.06 ± 0.00 |
Pulmonary function was measured 72 h following ozone exposure using a Scireq Flexivent. Data are mean ± SE (n = 4).
Significantly different (p < .05) from Air + Veh as determined by 1-way ANOVA followed by Tukey’s multiple comparisons test. #Significantly different (p < .05) from O3 + Veh as determined by 2-way ANOVA followed by Tukey’s multiple comparisons test.
Abbreviations: PEEP, positive end expiratory pressure; O3, ozone; Veh, vehicle; Rosi, rosiglitazone; G/H, hysteresivity; Ers, elastance (cmH2O/ml); Cst, quasi-static compliance (ml/cmH2O).
Effects of rosiglitazone on ATII cell function
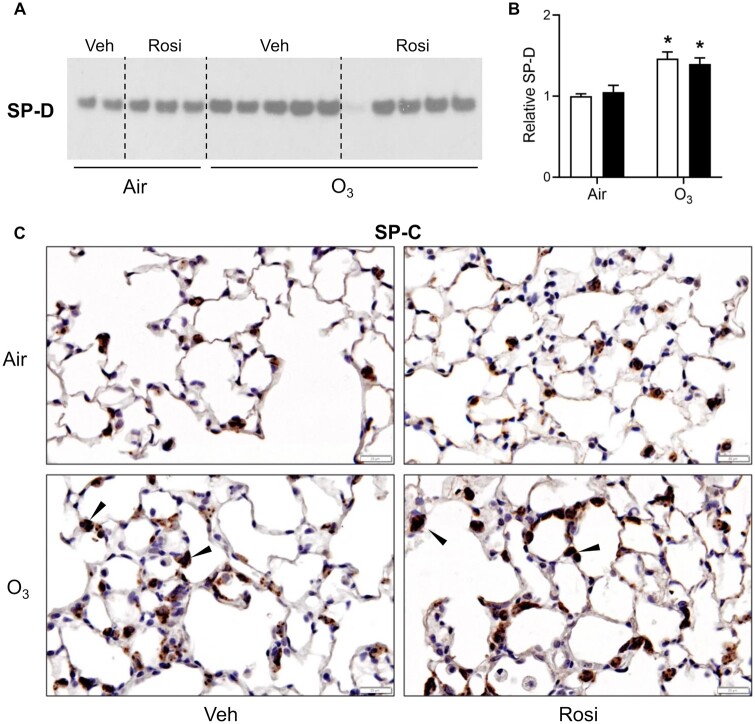
In line with altered ATII cell function in response to lung injury (Beers et al., 1992; Crouch et al., 2000; Sunil et al., 2018), we found that ozone caused a significant increase in SP-D in lung lining fluid; increases in pro-SP-C were also detected in ATII cells in histologic sections in areas adjacent to sites of injury. Administration of rosiglitazone had no effect on ozone-induced increases in SP-D or pro-SP-C (Figure 5).
Figure 5.
Effects of Rosi on O3-induced alterations in SP-D and pro-SP-C. A, SP-D levels were assessed 72 h after exposure of mice to Air + Vehicle (Veh), Air + Rosi, O3 + Veh, or O3 + Rosi by western blotting. A, Representative gel from 3 independent experiments is shown. Each lane represents one mouse. B, Blots were quantified using ImageJ. Band intensities were normalized to the Air + Veh group within individual blots and then averaged across the blots; data are expressed relative to Air + Veh. Bars, mean ± SE (n = 7–10 mice). Data were analyzed by 2-way ANOVA followed by Tukey’s multiple comparisons test. *Significantly different (p < .05) from Air + Veh. C, Tissue sections collected 72 h after exposure were stained with antibody to pro-SP-C. Binding was visualized using a peroxidase DAB substrate kit. One representative section from 4 mice/group is shown (original magnification, ×400). Arrows indicate ATII cells positively staining for pro-SP-C.
Effects of rosiglitazone on inflammatory macrophages responding to ozone
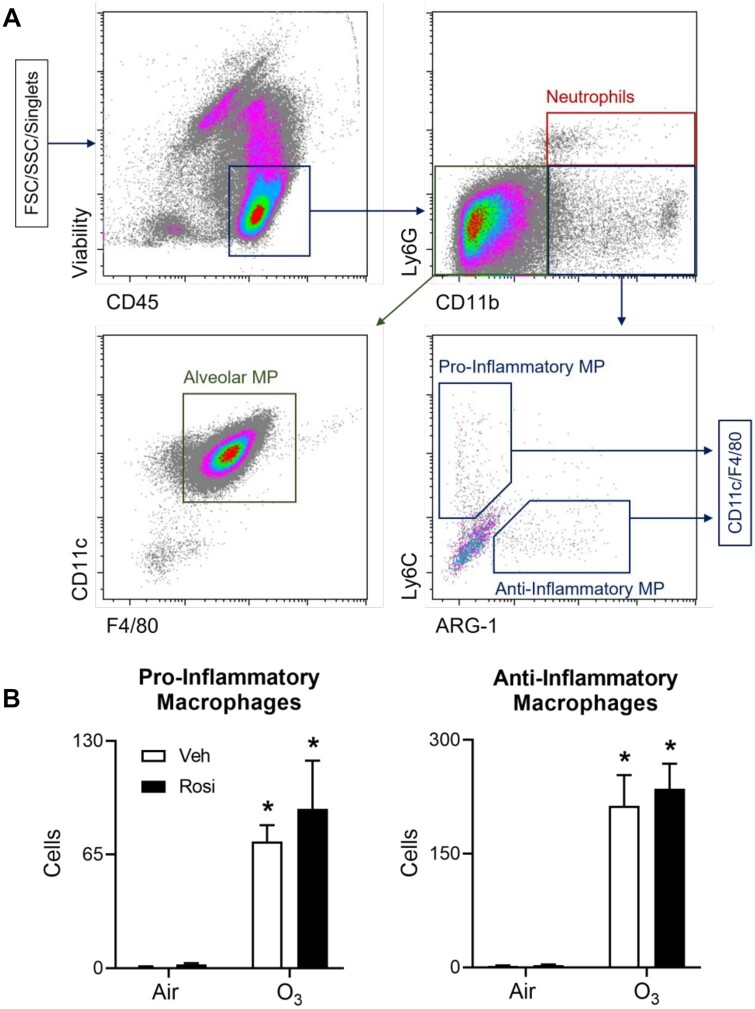
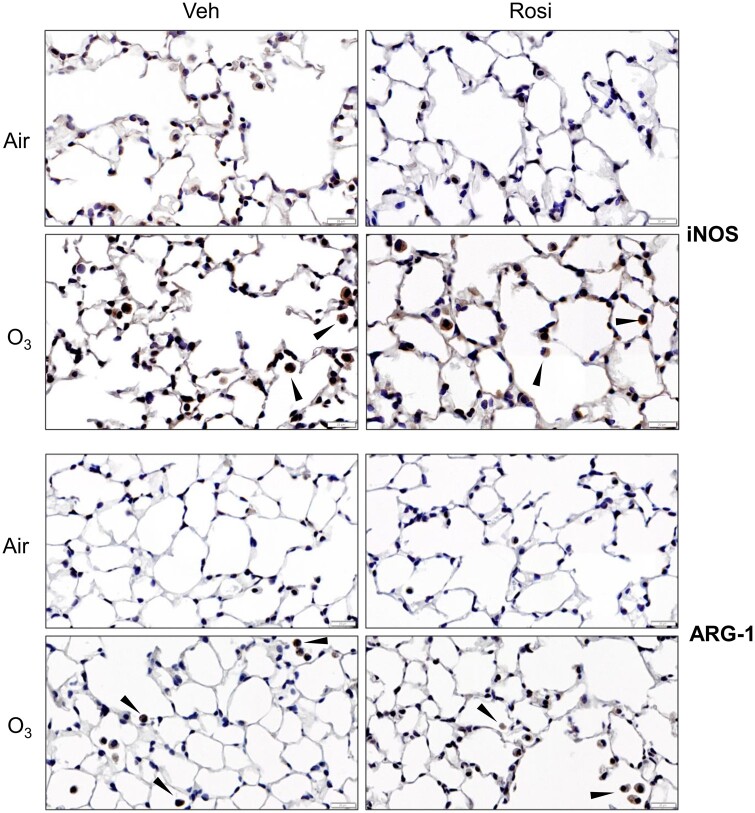
Recent studies have demonstrated that increased lipid turnover in macrophages is associated with an anti-inflammatory phenotype (Huang et al., 2014; Lugg et al., 2022). To determine whether the ability of rosiglitazone to restore lung lining lipid homeostasis is due to changes in macrophage phenotype, we used techniques in flow cytometry. After ozone exposure, significant increases in CD45+ pro-inflammatory (Ly6G-F4/80+CD11c+CD11b+Ly6Chi) and anti-inflammatory (Ly6G-F4/80+CD11c+CD11b+ARG-1+) macrophages were detected in lung lining fluid, with no effect on Ly6G+CD11b+ neutrophils (Figure 6). This was associated with upregulation of macrophage inducible nitric oxide synthase (iNOS) and arginase-1 (ARG-1), pro- and anti-inflammatory macrophage activation markers, respectively, in histologic sections (Figure 7 and Supplementary Table 3). Rosiglitazone had no effect on numbers of pro- or anti-inflammatory macrophages or on ozone-induced changes in expression of iNOS or ARG-1; neutrophils were also unaffected by rosiglitazone (data not shown).
Figure 6.
Effects of Rosi on inflammatory macrophage accumulation in the lung following ozone exposure. Cells, collected from lung lining fluid 72 h after exposure of mice to Air + Vehicle (Veh), Air + Rosi, O3 + Veh, or O3 + Rosi, were analyzed by flow cytometry. A, After exclusion of debris and doublets, cells were sequentially analyzed for expression of CD45, CD11b, Ly6G, CD11c, F4/80, Ly6C, and Arg-1. Viable cells were defined as neutrophils (CD45+Ly6G+CD11b+), resident AMs (CD45+Ly6G-CD11b-CD11c+F4/80+), and pro- (CD45+Ly6G-CD11b+Ly6ChiARG-1-CD11c+F4/80+) or anti- (CD45+Ly6G-CD11b+Ly6CloARG-1+CD11c+F4/80+) inflammatory macrophages. B, Numbers of pro- and anti-inflammatory macrophages. Bars, mean ± SE (n = 4–10). Data were analyzed by 2-way ANOVA followed by Tukey’s multiple comparisons test. *Significantly different (p < .05) from Air + Veh. Abbreviation: MP, macrophage.
Figure 7.
Effects of Rosi on O3-induced increases in expression of iNOS and ARG-1. Lung sections, prepared 72 h after exposure of mice to Air + Vehicle (Veh), air + Rosi, O3 + Veh, or O3 + Rosi, were stained with antibody to iNOS (upper panels) or ARG-1 (lower panels). Binding was visualized using a peroxidase DAB substrate kit. One representative section from 4 mice per group is shown (original magnification, ×400). Arrows indicate positively staining macrophages.
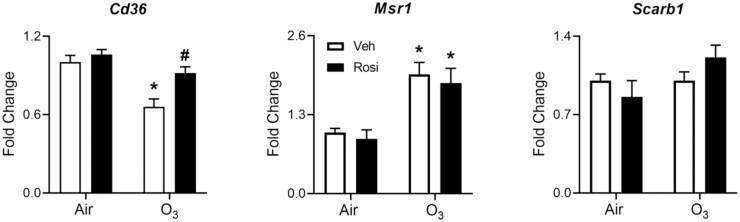
We next analyzed the effects of rosiglitazone on macrophage expression of genes involved in lipid uptake. Whereas ozone exposure caused a significant reduction in expression of the lipid scavenger receptor Cd36 in lung macrophages, expression of macrophage scavenger receptor 1 (Msr1) was increased (Figure 8). Conversely, ozone had no effect on expression of Scarb1, a scavenger receptor for high-density lipoprotein (Figure 8). Ozone-induced downregulation of Cd36 was blunted by rosiglitazone, with no effect on Msr1 or Scarb1 (Figure 8). Of note, we did not observe differences in expression of PPARγ or other transcriptional targets of PPARγ including the lipid efflux transporters, ATP-binding cassette transporter A1 (Abca1), or Abcg1 (data not shown).
Figure 8.
Effects of Rosi on O3-induced alterations in expression lipid scavenger receptors. Lipid scavenger receptor mRNA expression was assessed in macrophages collected 72 h after exposure of mice to Air + Vehicle (Veh), air + Rosi, O3 + Veh, or O3 + Rosi. Target gene CT values were normalized to Gapdh and fold changes calculated relative to Air + Vehicle controls using the ΔΔCT method. Bars, mean ± SE (n = 4). Differences between ΔCT values were analyzed by 2-way ANOVA followed by Tukey’s multiple comparisons test. *Significantly different (p < .05) from Air + Veh. #Significantly different (p < .05) from O3 + Veh.
Discussion
Lipids and particularly phospholipids in the lung lining fluid are critical for maintaining surface activity of pulmonary surfactant and minimizing the work of breathing (Serrano and Pérez-Gil, 2006). The present studies demonstrate that administration of rosiglitazone, a member of the thiazolidinedione class of antidiabetic drugs known to modulate lung lipids by promoting AM differentiation and lipid scavenging activity (Gorki et al., 2022; Mirakaj et al., 2014), restored ozone-induced alterations in lung lining lipids and surfactant activity. We speculate that this is due to increases in the lipid uptake capacity of AMs as administration of rosiglitazone blunted ozone-induced decreases in Cd36 expression in these cells, a response correlated with improved pulmonary function. These findings suggest that targeting lung lipid influx and efflux in macrophages may be an efficacious approach for treating altered respiratory mechanics caused by ozone.
Treatment of mice with ozone was associated with a significant increase in total phospholipid levels in the airspace; this led to a reduction in the relative amount of SP-B to total phospholipids, an indication of surfactant dysfunction (Massa et al., 2017). Administration of rosiglitazone to ozone-exposed mice reduced lipid levels in the lung lining fluid, and as a consequence, the relative amount of SP-B to total phospholipids. SP-B is critical for maintaining the surface activity of pulmonary surfactant by sustaining film stability at the most compressed states (Cochrane and Revak, 1991; Schürch et al., 2010). It dynamically regulates surface tension by facilitating rapid phospholipid insertion into the air–liquid interface on inspiration and then removal to large aggregates on expiration (Ross et al., 2002). Disruption in the balance between total phospholipids and SP-B decreases the efficiency of phospholipid incorporation into the monolayer and removal to large aggregates increasing the work of breathing (Serrano and Pérez-Gil, 2006; Massa et al., 2017). Previous studies demonstrated that acute ozone inhalation alters the surface activity of pulmonary surfactant by impairing hydrophobic SP function, a response attributed to interference with the surface film by inflammatory proteins (Currie et al., 1998a,b; Nachtman et al., 1986; Putman et al., 1997). In contrast, our results indicate a central role for lung lipids in the actions of ozone. Thus, ozone causes a disruption in the balance of SP-B to phospholipids which results in reduced pulmonary efficiency, and these responses are restored by rosiglitazone.
The control of surface tension is also affected by constituent lipids within the lung lining fluid (Serrano and Pérez-Gil, 2006; Wang et al., 1996). Our lipidomic analysis revealed an overall increase in various lipid species after ozone exposure; of note was the increase in the number of phosphatidylcholine species possessing an unsaturation in their carbon chain. Rosiglitazone blunted both responses and restored the quantity of lipid species, along with the degree of unsaturation in phosphatidylcholines to air control levels. Previous studies have demonstrated that increases in specific lipid species reflect differences in precursor availability (eg, intracellular fatty acids or stored triglycerides) (Brandsma et al., 2018). It remains to be determined whether the general increase in levels of all lipid species in the airspace is due to altered production and/or clearance or to changes in specific precursor availability, and whether there are treatment-specific effects of rosiglitazone on baseline lung lipidomic profiles in air-exposed mice.
Changes in the composition of pulmonary surfactants are known to influence respiratory mechanics particularly at low PEEPs where the lung is more prone to atelectasis. Following ozone exposure, a significant reduction in lung hysteresivity was observed, most prominently at PEEP = 1 cm H2O; this was blunted by rosiglitazone. Lung hysteresivity reflects the mechanical coupling of energy dissipative forces and tissue elastic properties at low PEEPs and is dependent on surfactant function (Glasser et al., 2001). Unsaturated phospholipids affect surfactant function by increasing the fluidity of the surface film by permitting structural transformations associated with the transfer of surface-active molecules from cells to the air–liquid interface; thus, increases in the total amount and degree of unsaturated phosphatidylcholines facilitate the readsorption of surface-active molecules during expansion of the lung. This renders surfactant more effective in reducing surface tension which increases pulmonary compliance and reduces elastance (Lopez-Rodriguez and Pérez-Gil, 2014; Massa et al., 2017; Nachtman et al., 1986). Rosiglitazone was found to significantly reduce quasi-static compliance and increased pulmonary elastance at PEEP = 1 cm H2O in ozone-exposed mice indicating a normalization of pulmonary function. This may be a consequence of restoring the balance between total phospholipids and SP-B, which increases the efficiency of phospholipid incorporation into the monolayer and removal to large aggregates. Alternatively, rosiglitazone may act by reducing the number of phosphatidylcholine species containing an unsaturation in the carbon chain (Massa et al., 2017; Serrano and Pérez-Gil, 2006).
ATII cells synthesize pulmonary lipids and SPs and are thus critical in maintaining surfactant homeostasis (Agudelo et al., 2020). Whereas SP-D is a pulmonary collectin released by ATII cells in response to lung injury to modulate the inflammatory response (Crouch et al., 2000; Sunil, 2018), SP-C works with SP-B to regulate pulmonary surface tension by facilitating lipid adsorption into the air–liquid interface and reducing the work of breathing (Sehlmeyer et al., 2020). Our findings that levels of SP-D and pro-SP-C increased indicate that ATII cell activity is augmented after ozone exposure. This is supported by earlier studies demonstrating that ozone-induced injury results in hyperplasia and hypertrophy of ATII cells, increases in pro-SP-C expression, and inflammatory activation (Prokhorova et al., 1998; Punjabi et al., 1994). Rosiglitazone had no effect on the response of ATII cells to ozone indicating that their function is preserved. As saturated phosphatidylcholines and SP-C are concomitantly released by ATII cells, it is likely that the increase in BAL phospholipids after ozone exposure is due, in part, to their increased production by ATII cells (Ikegami and Jobe, 1998). The fact that administration of rosiglitazone had no effect on SP-D or SP-C in ozone-exposed mice suggests that PPARγ activation does not reduce phospholipid levels by regulating ATII cell surfactant synthesis.
In line with our previous studies, exposure to ozone caused significant increases in numbers of activated pro- and anti-inflammatory macrophages in the lung (Francis et al., 2020; Sunil et al., 2012, 2015). Rosiglitazone had no effect on numbers of these cells or on their expression of activation markers, indicating that its ability to restore lipid homeostasis is independent of the phenotype or function of inflammatory macrophages responding to ozone-induced injury. This is in accord with previous studies showing that PPARγ activation does not alter anti-inflammatory marker expression on mature macrophages in atherosclerotic lesions (Bouhlel et al., 2007). Importantly, while phenotypic and functional activation of lung macrophages was not affected by rosiglitazone, expression of lipid scavenger receptor Cd36 was upregulated. The Cd36 promoter contains a binding site for PPARγ/RXRα heterodimers, and rosiglitazone has been shown to increase DPPC uptake in human AMs by upregulating Cd36 (Aronoff et al., 2007; Dodd et al., 2016; Röszer, 2017; Röszer et al., 2011). Impairment of AM phagocytosis and metabolism of pulmonary surfactant results in an accumulation of lipids in the alveolar space (Trapnell et al., 2003). We speculate that the ability of rosiglitazone to restore lipid homeostasis after ozone exposure is due, at least in part, to specific upregulation of Cd36 in AMs. This is supported by our findings that macrophage expression of the lipid scavenger receptor Msr1, which is not a transcriptional target of PPARγ, was unaffected by rosiglitazone. The lack of effect of rosiglitazone on Scarb1 may be due to liver-specific actions of PPARγ (Ahmed et al., 2009). Mechanisms underlying changes in Cd36 expression in lung macrophages after ozone exposure are unknown. Ozone has been shown to upregulate tumor necrosis factor alpha (TNFα) in macrophages, a negative regulator of PPARγ (Fakhrzadeh et al., 2004; Francis et al., 2020; Zhang et al., 1996). Further studies are required to determine if TNFα generated following ozone exposure plays a role in regulating PPARγ activity.
In summary, inhalation of ozone causes dyslipidemia in the lung, a response associated with increased surfactant production by ATII cells and downregulation of Cd36 expression in AMs; this results in a failure to clear excess lung lipids, disruption of lipid composition, surfactant dysfunction, and impaired pulmonary mechanics. We speculate that by activating PPARγ, rosiglitazone upregulates Cd36 in macrophages leading to a restoration of lung lipid homeostasis and improved pulmonary function. Taken together, these studies suggest that targeting processes controlling pulmonary surfactant may represent an effective approach to mitigating lung diseases caused by exposure to pulmonary oxidants.
Supplementary Material
Acknowledgments
The authors thank Nicole Wasseff for assistance in conducting the experiments and collecting data and Eric Chiles for help in writing the methods for the lipidomic analysis.
Contributor Information
Ley Cody Smith, Department of Pharmaceutical Sciences, University of Connecticut School of Pharmacy, Storrs, Connecticut 06269, USA; Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Andrew J Gow, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Elena Abramova, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Kinal Vayas, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Changjiang Guo, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Jack Noto, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Jack Lyman, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Jessica Rodriquez, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Benjamin Gelfand-Titiyevskiy, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Callum Malcolm, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Jeffrey D Laskin, Department of Environmental and Occupational Health and Justice, School of Public Health, Rutgers University, Piscataway, New Jersey 08854, USA.
Debra L Laskin, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, New Jersey 08854, USA.
Supplementary data
Supplementary data are available at https://doi.org/10.5061/dryad.4f4qrfjh1.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the National Institutes of Health grants ES032473, ES030984, ES007148, ES004738, ES033698, ES005022, HL086621, and S10OD026876, and the Rutgers Center for Lipid Research, New Jersey Institute for Food, Nutrition and Health.
References
- Agassandian M., Mallampalli R. K. (2013). Surfactant phospholipid metabolism. Biochim. Biophys. Acta 1831, 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo C. W., Samaha G., Garcia-Arcos I. (2020). Alveolar lipids in pulmonary disease. A review. Lipids Health Dis. 19, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R. A. M., Murao K., Imachi H., Yu X., Li J., Wong N. C. W., Ishida T. (2009). Human scavenger receptor class B type 1 is regulated by activators of peroxisome proliferators-activated receptor-gamma in hepatocytes. Endocrine 35, 233–242. [DOI] [PubMed] [Google Scholar]
- Aronoff D. M., Serezani C. H., Carstens J. K., Marshall T., Gangireddy S. R., Peters-Golden M., Reddy R. C. (2007). Stimulatory effects of peroxisome proliferator-activated receptor-gamma on Fcgamma receptor-mediated phagocytosis by alveolar macrophages. PPAR Res. 2007, 52546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. D., Malur A., Barna B. P., Ghosh S., Kavuru M. S., Malur A. G., Thomassen M. J. (2010). Targeted PPARγ deficiency in alveolar macrophages disrupts surfactant catabolism. J. Lipid Res. 51, 1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batenburg J. J. (1992). Surfactant phospholipids: Synthesis and storage. Am. J. Physiol. 262, L367–L385. [DOI] [PubMed] [Google Scholar]
- Beers M. F., Wali A., Eckenhoff M. F., Feinstein S. I., Fisher J. H., Fisher A. B. (1992). An antibody with specificity for surfactant protein C precursors: Identification of pro-SP-C in rat lung. Am. J. Respir. Cell Mol. Biol. 7, 368–378. [DOI] [PubMed] [Google Scholar]
- Bligh E. G., Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Bouhlel M. A., Derudas B., Rigamonti E., Dievart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. (2007). PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Goss V. M., Yang X., Bakke P. S., Caruso M., Chanez P., Dahlén S.-E., Fowler S. J., Horvath I., Krug N., et al. ; U-BIOPRED Study Group. (2018). Lipid phenotyping of lung epithelial lining fluid in healthy human volunteers. Metabolomics 14, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N., Mishra V., Sinha T., DiAngelo S. L., Chroneos Z. C., Ekpa N. A., Cooper T. K., Caruso C. R., Silveyra P. (2015). Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1150–L1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Sánchez J. C., Cerrada A., Conde M., Cruz A., Perez-Gil J. (2022). Compositional, structural and functional properties of discrete coexisting complexes within bronchoalveolar pulmonary surfactant. Biochim. Biophys. Acta Biomembr. 1864, 183808. [DOI] [PubMed] [Google Scholar]
- Chawla A., Boisvert W. A., Lee C. H., Laffitte B. A., Barak Y., Joseph S. B., Liao D., Nagy L., Edwards P. A., Curtiss L. K., et al. (2001). A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7, 161–171. [DOI] [PubMed] [Google Scholar]
- Chen L., Vasoya R. P., Toke N. H., Parthasarathy A., Luo S., Chiles E., Flores J., Gao N., Bonder E. M., Su X., et al. (2020). HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology 158, 985–999.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti G., Lestavel S., Bocher V., Remaley A. T., Neve B., Torra I. P., Teissier E., Minnich A., Jaye M., Duverger N., et al. (2001). PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 7, 53–58. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D. (1991). Pulmonary surfactant protein B (SP-B): Structure–function relationships. Science 254, 566–568. [DOI] [PubMed] [Google Scholar]
- Crouch E., Hartshorn K., Ofek I. (2000). Collectins and pulmonary innate immunity. Immunol. Rev. 173, 52–65. [DOI] [PubMed] [Google Scholar]
- Currie W. D., van Schaik S., Vargas I., Enhorning G. (1998a). Breathing and pulmonary surfactant function in mice 24 h after ozone exposure. Eur. Respir. J. 12, 288–293. [DOI] [PubMed] [Google Scholar]
- Currie W. D., van Schaik S. M., Vargas I., Enhorning G. (1998b). Ozone affects breathing and pulmonary surfactant function in mice. Toxicology 125, 21–30. [DOI] [PubMed] [Google Scholar]
- Dodd C. E., Pyle C. J., Glowinski R., Rajaram M. V. S., Schlesinger L. S. (2016). CD36-mediated uptake of surfactant lipids by human macrophages promotes intracellular growth of Mycobacterium tuberculosis. J. Immunol. 197, 4727–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman D. H., Ghezzo H., Kim W. D., Hyatt R. E., Cosio M. G. (1989). Pressure–volume curves in smokers. Comparison with alpha-1-antitrypsin deficiency. Am. Rev. Respir. Dis. 139, 1452–1458. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L., Laskin J. D., Laskin D. L. (2002). Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am. J. Respir. Cell Mol. Biol. 26, 413–419. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L., Laskin J. D., Laskin D. L. (2004). Ozone-induced production of nitric oxide and TNF-alpha and tissue injury are dependent on NF-kappaB p50. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L279–L285. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L., Laskin J. D., Laskin D. L. (2008). Regulation of caveolin-1 expression, nitric oxide production and tissue injury by tumor necrosis factor-alpha following ozone inhalation. Toxicol. Appl. Pharmacol. 227, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M., Guo G., Kong B., Abramova E. V., Cervelli J. A., Gow A. J., Laskin J. D., Laskin D. L. (2020). Regulation of lung macrophage activation and oxidative stress following ozone exposure by farnesoid X receptor. Toxicol. Sci. 177, 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser S. W., Burhans M. S., Korfhagen T. R., Na C. L., Sly P. D., Ross G. F., Ikegami M., Whitsett J. A. (2001). Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc. Natl. Acad. Sci. U.S.A 98, 6366–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorki A.-D., Symmank D., Zahalka S., Lakovits K., Hladik A., Langer B., Maurer B., Sexl V., Kain R., Knapp S. (2022). Murine ex vivo cultured alveolar macrophages provide a novel tool to study tissue-resident macrophage behavior and function. Am. J. Respir. Cell Mol. Biol. 66, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. M., Gow A. J., Massa C. B., Laskin J. D., Laskin D. L. (2012). Prolonged injury and altered lung function after ozone inhalation in mice with chronic lung inflammation. Am. J. Respir. Cell Mol. Biol. 47, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel O., , IkegamiM., , ChroneosZ. C., and , Jobe A. H. (2001). Macrophage and type II cell catabolism of SP-A and saturated phosphatidylcholine in mouse lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1266–L1272. [DOI] [PubMed] [Google Scholar]
- Holm B. A., Wang Z., Egan E. A., Notter R. H. (1996). Content of dipalmitoyl phosphatidylcholine in lung surfactant: Ramifications for surface activity. Pediatr. Res. 39, 805–811. [DOI] [PubMed] [Google Scholar]
- Holm S. M., Balmes J. R. (2022). Systematic review of ozone effects on human lung function, 2013 through 2020. Chest 161, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. C.-C., Everts B., Ivanova Y., O’Sullivan D., Nascimento M., Smith A. M., Beatty W., Love-Gregory L., Lam W. Y., O’Neill C. M., et al. (2014). Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T., and , Bell T. J. (2014). Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 14, 81–93. [DOI] [PubMed] [Google Scholar]
- Ikegami M., Jobe A. H. (1998). Surfactant protein metabolism in vivo. Biochim. Biophys. Acta 1408, 218–225. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhang H., Li Y., Zhang Y., Bian Y., Zeng Y., Yao X., Wan J., Chen X., Li J., et al. (2021). S100A4 enhances protumor macrophage polarization by control of PPAR-γ-dependent induction of fatty acid oxidation. J. Immunother. Cancer 9, e002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez E., Pérez-Gil J. (2014). Structure–function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta 1838, 1568–1585. [DOI] [PubMed] [Google Scholar]
- Lugg S. T., Scott A., Parekh D., Naidu B., Thickett D. R. (2022). Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 77, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa C. B., Scott P., Abramova E., Gardner C., Laskin D. L., Gow A. J. (2014). Acute chlorine gas exposure produces transient inflammation and a progressive alteration in surfactant composition with accompanying mechanical dysfunction. Toxicol. Appl. Pharmacol. 278, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa C. B., Groves A. M., Jaggernauth S. U., Laskin D. L., Gow A. J. (2017). Histologic and biochemical alterations predict pulmonary mechanical dysfunction in aging mice with chronic lung inflammation. PLoS Comput. Biol. 13, e1005570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V., Liebisch G., Kurzchalia T. V., Shevchenko A., Schwudke D. (2008). Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad N., Morissette M. C. (2021). Revisiting the role of pulmonary surfactant in chronic inflammatory lung diseases and environmental exposure. Eur. Respir. Rev. 30, 210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V., Mutz C., Vagts D., Henes J., Haeberle H. A., Husung S., König T., Nöldge-Schomburg G., Rosenberger P. (2014). Rosiglitazone dampens pulmonary inflammation in a porcine model of acute lung injury. Inflammation 37, 1102–1110. [DOI] [PubMed] [Google Scholar]
- Nachtman J. P., Hajratwala B. R., Moon H. L., Gross K. B., Wright E. S. (1986). Surface-tension measurements of pulmonary lavage from ozone-exposed rats. J. Toxicol. Environ. Health 19, 127–136. [DOI] [PubMed] [Google Scholar]
- Nambiar S., Clynick B., How B. S., King A., Walters E. H., Goh N. S., Corte T. J., Trengove R., Tan D., Moodley Y. (2021). There is detectable variation in the lipidomic profile between stable and progressive patients with idiopathic pulmonary fibrosis (IPF). Respir. Res. 22, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda B., , Martínez-CalleM., and , Pérez-Gil J. (2017). Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat. 209, 78–92. [DOI] [PubMed] [Google Scholar]
- Prokhorova S., Patel N., Laskin D. L. (1998). Regulation of alveolar macrophage and type II cell DNA synthesis: Effects of ozone inhalation. Am. J. Physiol. 275, L1200–L1207. [DOI] [PubMed] [Google Scholar]
- Punjabi C. J., Laskin J. D., Pendino K. J., Goller N. L., Durham S. K., Laskin D. L. (1994). Production of nitric oxide by rat type II pneumocytes: Increased expression of inducible nitric oxide synthase following inhalation of a pulmonary irritant. Am. J. Respir. Cell Mol. Biol. 11, 165–172. [DOI] [PubMed] [Google Scholar]
- Putman E., Liese W., Voorhout W. F., van Bree L., van Golde L. M., Haagsman H. P. (1997). Short-term ozone exposure affects the surface activity of pulmonary surfactant. Toxicol. Appl. Pharmacol. 142, 288–296. [DOI] [PubMed] [Google Scholar]
- Ross M., Krol S., Janshoff A., Galla H.-J. (2002). Kinetics of phospholipid insertion into monolayers containing the lung surfactant proteins SP-B or SP-C. Eur. Biophys. J. 31, 52–61. [DOI] [PubMed] [Google Scholar]
- Röszer T., Menéndez-Gutiérrez M., Lefterova M. I., Alameda D., Núñez V., Lazar M. A., Fischer T., Ricote M. (2011). Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J. Immunol. 186, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röszer T. (2017). Transcriptional control of apoptotic cell clearance by macrophage nuclear receptors. Apoptosis 22, 284–294. [DOI] [PubMed] [Google Scholar]
- Schachtrup C., Malcharek S., Haitsma J. J., Lachmann B., Owada Y., Binas B., Kondo H., Rüstow B., Galla H.-J., Spener F. (2008). Activation of PPARgamma reverses a defect of surfactant synthesis in mice lacking two types of fatty acid binding protein. Biochim. Biophys. Acta 1781, 314–320. [DOI] [PubMed] [Google Scholar]
- Schürch D., Ospina O. L., Cruz A., Pérez-Gil J. (2010). Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophys. J. 99, 3290–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer K., Ruwisch J., Roldan N., Lopez-Rodriguez E. (2020). Alveolar dynamics and beyond—the importance of surfactant protein C and cholesterol in lung homeostasis and fibrosis. Front. Physiol. 11, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A. G., Pérez-Gil J. (2006). Protein–lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids 141, 105–118. [DOI] [PubMed] [Google Scholar]
- Sunil V. R., Patel-Vayas K., Shen J., Laskin J. D., Laskin D. L. (2012). Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol. Appl. Pharmacol. 263, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil V. R., Vayas K. N., Cervelli J. A., Ebramova E. V., Gow A. J., Goedken M., Malaviya R., Laskin J. D., Laskin D. L. (2018). Protective role of surfactant protein-D against lung injury and oxidative stress induced by nitrogen mustard. Toxicol. Sci. 166, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil V. R., Francis M., Vayas K. N., Cervelli J. A., Choi H., Laskin J. D., Laskin D. L. (2015). Regulation of ozone-induced lung inflammation and injury by the β-galactoside-binding lectin galectin-3. Toxicol. Appl. Pharmacol. 284, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Nagy L., Alvarez J. G., Thomazy V. A., Evans R. M. (1998). PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Whitsett J. A., Nakata K. (2003). Pulmonary alveolar proteinosis. N. Engl. J. Med. 349, 2527–2539. [DOI] [PubMed] [Google Scholar]
- Walther F. J., Hernández-Juviel J. M., Gordon L. M., Waring A. J., Stenger P., Zasadzinski J. A. (2005). Comparison of three lipid formulations for synthetic surfactant with a surfactant protein B analog. Exp. Lung Res. 31, 563–579. [DOI] [PubMed] [Google Scholar]
- Wang Z., Hall S. B., Notter R. H. (1996). Roles of different hydrophobic constituents in the adsorption of pulmonary surfactant. J. Lipid Res. 37, 790–798. [PubMed] [Google Scholar]
- Wright J. R., and , Dobbs L. G. (1991). Regulation of pulmonary surfactant secretion and clearance. Annu. Rev. Physiol. 53, 395–414. [DOI] [PubMed] [Google Scholar]
- Zhang B., Berger J., Hu E., Szalkowski D., White-Carrington S., Spiegelman B. M., Moller D. E. (1996). Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol. Endocrinol. 10, 1457–1466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.