Abstract
Environmental bisphenol compounds like bisphenol F (BPF) are endocrine-disrupting chemicals (EDCs) affecting adipose and classical endocrine systems. Genetic factors that influence EDC exposure outcomes are poorly understood and are unaccounted variables that may contribute to the large range of reported outcomes in the human population. We previously demonstrated that BPF exposure increased body growth and adiposity in male N/NIH heterogeneous stock (HS) rats, a genetically heterogeneous outbred population. We hypothesize that the founder strains of the HS rat exhibit EDC effects that were strain- and sex-dependent. Weanling littermate pairs of male and female ACI, BN, BUF, F344, M520, and WKY rats randomly received either vehicle (0.1% EtOH) or 1.125 mg BPF/l in 0.1% EtOH for 10 weeks in drinking water. Body weight and fluid intake were measured weekly, metabolic parameters were assessed, and blood and tissues were collected. BPF increased thyroid weight in ACI males, thymus and kidney weight in BUF females, adrenal weight in WKY males, and possibly increased pituitary weight in BN males. BUF females also developed a disruption in activity and metabolic rate with BPF exposure. These sex- and strain-specific exposure outcomes illustrate that HS rat founders possess diverse bisphenol-exposure risk alleles and suggest that BPF exposure may intensify inherent organ system dysfunction existing in the HS rat founders. We propose that the HS rat will be an invaluable model for dissecting gene EDC interactions on health.
Keywords: environmental exposure, endocrine disruptors, bisphenols, genetic background, metabolic rate, Rattus norvegicus
Endocrine-disrupting chemicals (EDCs) are natural or man-made compounds that mimic or interfere with normal hormonal function and may increase the risk of developing obesity and related cardiometabolic disorders (Gore et al., 2015; Heindel et al., 2022). Interactions between an individual’s genetic factors (i.e., regulation of intricate endocrine and metabolic systems) and the environment (i.e., environmental chemicals) lay the foundation for the development of complex diseases, like obesity (Tchen et al., 2022). This suggests that genetic variation may significantly influence EDC exposure outcomes.
Bisphenols are EDCs used in the manufacturing of polycarbonates and epoxy resins and in common consumer products such as water bottles, dental sealants, and thermal cash register receipt paper (Chen et al., 2016). Bisphenol F (BPF) is increasingly used as a structural analog of bisphenol A (BPA), an obesogen that is regulated in the European Union, Canada, and the United States. While BPA has an allowable daily intake (ADI) of 50 µg/kg body weight/day (USEPA, 2010), BPF is not regulated in any country and does not have an ADI for human safety. Regrettably, data validating the safety of BPA-analogs as BPA substitutes are lacking. A small-scale human biomonitoring study reported increasing BPF exposures of U.S. adults from 2000 to 2014 (Ye et al., 2015). Analysis of BPF levels in publicly available NHANES 2013–2014 data found that it is present in 66.5% of urine samples from the general U.S. population (Lehmler et al., 2018). Multiple analyses of NHANES data determined that BPF is associated with a higher risk of obesity as well as with cardiovascular disease, asthma, sleep problems, and total testosterone levels (Choi et al., 2022; Liu et al., 2019; Lu et al., 2022; Mendy et al., 2020; Zhang et al., 2022; Zhou et al., 2022).
There is a paucity of physiological and functional studies that consider genetic variation as a variable for EDC exposure outcomes. Most toxicological studies of BPF are limited to in vitro or non-mammalian models to investigate its endocrine-disrupting potential. These studies consistently demonstrate that BPF is an EDC having disruption potential like BPA, with effects on adipose, immune, neuroendocrine, thyroid, and reproductive functions (Chen et al., 2022a; Gu et al., 2022; Malaisé et al., 2020a; Michałowicz et al., 2015; Rosenfeld, 2017; Zhao et al., 2019; Zhu et al., 2018). In vivo mammalian bisphenol studies tend to be performed with isogenic or genetically undefined outbred rodents. Mouse studies have shown that BPF exposure induces hepatic dysfunction (Meng et al., 2019), immune impairment (Malaisé et al., 2020b), anxiety and depression behavioral changes (Ohtani et al., 2017), and reproductive dysfunction (Ding et al., 2022). BPF exposure studies in rats report reproductive dysfunction in outbred Sprague Dawley and Wistar strains (Fatai and Aribidesi, 2022; Ijaz et al., 2020; Kaimal et al., 2021; Ullah et al., 2018). However, using a single inbred or genetically undefined outbred model could conceal or exaggerate the dangers of EDCs and does not address the significant variability in an EDC’s effects at the population level (Festing, 1993; Richter et al., 2007). Two investigations into gene EDC interactions have been performed in humans. One candidate-based approach identified single-nucleotide polymorphisms in genes controlling oxidative stress as associated with liver function and BPA exposure (Kim et al., 2016), and a small-scale genome-wide study identified multiple loci as associated with urinary oxidative stress biomarkers and BPA and triclosan exposure (Zhong et al., 2022).
In-depth studies to identify and understand gene EDC interactions on disease risk and progression require a state-of-the-art in vivo animal model. In the 1980s, the NIH created HS rats as an outbred animal resource for experimental and selection studies (Hansen and Spuhler, 1984). Eight inbred founder strains of the HS colony (ACI/N, BN/SsN, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N), chosen based on their phenotypic differences, were systematically outcrossed to maintain genetic variation. The genetic differences between the founder strains encompass much of the rat phylogenetic tree (Saar et al., 2008). After more than 90 generations of outbreeding, each HS rat represents a genetic mosaic of the eight founders, with genetic diversity more closely representing human than other available rat models (Woods and Mott, 2017). Previous mapping studies using the HS rat have identified potential candidate genes for complex traits like addiction, behavior, kidney damage, and cardiometabolic diseases (Chitre et al., 2020; Gunturkun et al., 2022; Hong-Le et al., 2023; Keele et al., 2018, 2021; Tsaih et al., 2014). Other outbred population models, like the Collaborative Cross mice and the Diversity Outbred mice, have been leveraged in quantitative trait loci studies to identify genetic loci associated with benzene toxicity, acute ozone-induced injury, and ethanol sensitivity (Gatti et al., 2017; Parker et al., 2022; Tovar et al., 2022). No EDC exposure studies have been performed in mouse outbred populations to date.
We previously demonstrated that male HS rats exposed to BPF for 5 weeks in drinking water showed increased body weight, increased abdominal adiposity, and unexplained dehydration (Wagner et al., 2021). It is well documented that EDCs produce sex-specific responses, including BPA (Sol et al., 2020; Yang et al., 2017), so it is imperative to consider sex as a factor. Only one of the eight HS founding strains, the F344, has been exposed to bisphenols (Lejonklou et al., 2017; Manukyan et al., 2019). This leaves a critical knowledge gap in HS rat founder responses to BPF exposure and in the possible responses inherited by the HS rat population. In this study, we hypothesize that post-wean BPF exposure influences endocrine organs and whole-body metabolism in HS rat founding strains depending on sex and genetic background, demonstrating that gene EDC interactions are pivotal in exposure outcome interpretation at a population level.
Materials and methods
Animals
ACI/EurMcwi (ACI), BN/NHsdMcwi (BN), BUF/MnaMcwi (BUF), F344/StmMcwi (F344), and M520/NMcwi (M520) breeder rats were purchased from the Hybrid Rat Diversity Panel at the Medical College of Wisconsin and WKY/NCrl (WKY) breeder rats were purchased from Charles River Laboratories between 3 and 4 weeks-of-age. All breeders were maintained on ad libitum hyperchlorinated reverse-osmosis water and phytoestrogen-free Teklad 2920X diet (ENVIGO, Indianapolis, IN; <20 mg/kg isoflavones). Breeding pairs were set-up between 8 and 13 weeks-of-age. Litters were culled to six pups per litter at ear-punching at 1 week of age to normalize litter size across the six strains. Animals were housed in polysulfone micro-isolator cages with wood chip bedding on a 14:10 light/dark cycle and were provided paper huts (Bio-Huts for Rats, Certified, Bio-Serv, Flemington, NJ), paper packets (ENVIROPAK, W.F. Fisher and Son, Branchburg, NJ), and nylon bones (K3581, Bio-Serv) as environmental enrichment. Final sample sizes were 9–17 females per strain and exposure group and 10–15 males per strain and exposure group. ACI rats display renal agenesis in ∼11% of the strain, a congenital disorder that involves abnormal development of the urogenital tract (Samanas et al., 2015; Solleveld and Boorman, 1986). ACI rats with renal agenesis were removed from the study (removed three vehicle ACI males, two BPF-exposed ACI males, two vehicle ACI females, and five BPF-exposed ACI females). All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals in a protocol approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
Drinking water
BPF was administered in drinking water as oral ingestion is a major route of human exposure to bisphenols (Liao and Kannan, 2013). BPF was obtained from Angene International Limited, Nanjing, Jiangsu, China (Batch No.: AGN2017-11809-001) and had a purity >99% (Wagner et al., 2021). We previously published a study on post-weaning BPF exposure in drinking water in male rats using 1.125 mg/l BPF in 0.1% ethanol as the single exposure level, resulting in an average rat dose of 152.5 µg BPF/kg BW/day and an approximate human dose of 32 µg BPF/kg BW/day as calculated by the BW3/4 equation (USEPA, 2011; Wagner et al., 2021). Because there is no FDA ADI for BPF (the FDA ADI for BPA is 50 µg BPF/kg BW/day) and our previous study achieved an average study dose that was below a human dose of 50 µg BPF/kg BW/day, this study continued with the 1.125 mg/l BPF in 0.1% ethanol exposure paradigm previously used (Wagner et al., 2021). One exposure level was selected for this study to test the specific hypothesis that genetic background affects exposure outcomes. Briefly, the BPF dosing solution was prepared by dissolving 281.25 mg of BPF in 250 ml of ethanol (U.S.P. dehydrated ethanol; Spectrum, New Brunswick, NJ) to make a 1.125-mg/ml BPF in ethanol solution. A 1.125 mg/l in 0.1% ethanol drinking solution was prepared by diluting 10 ml of the dosing solution with 9990 ml of Barnstead water (Barnstead NANOpure Diamond Lab Water System, Barnstead Lab Water Products, Lake Balboa, CA) in a 10-l polypropylene carboy. The vehicle control drinking solution (0.1% ethanol) was prepared by combining 10 ml of the same ethanol with 9990 ml Barnstead water in a 10-l polypropylene carboy (ThermoFisher Scientific, Waltham, MA). All dosing solutions were stored at 4°C until provided to the animals.
Exposure and phenotyping protocol
Exposure
Littermate male and female rats were weaned at 3 weeks-of-age and were randomly assigned to vehicle control or BPF exposure group (Figure 1) by a random number generator (runif() function in R 4.0.2). Animals were exposed to the vehicle (0.1% ethanol) or BPF (1.125 mg/l BPF in 0.1% ethanol) in drinking water administered in glass water bottles to avoid other potential oral exposures due to leaching from plastic water bottles. All rats were singly housed and exposed through ad libitum drinking throughout the 10-week exposure and phenotyping protocol. Fluid intake was monitored weekly to estimate daily fluid intake and subsequent exposure. The intake estimates did not account for fluid bottle leak or evaporation. Since BPF exposure in children and adolescent boys is positively associated with obesity (Jacobson et al., 2019; Lehmler et al., 2018; Liu et al., 2019), exposure lasted from wean to 13 weeks-of-age (the beginning of young adulthood in rats [Sengupta, 2013]).
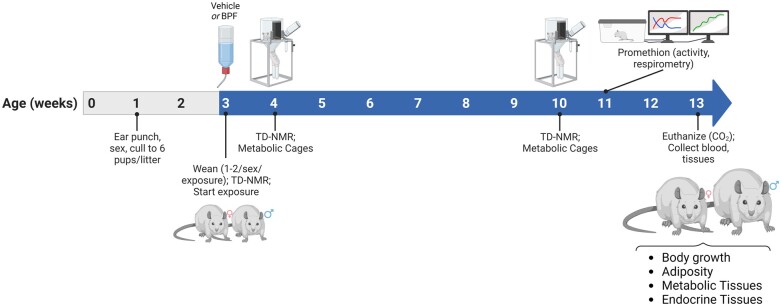
Figure 1.
Experimental design. ACI/EurMcwi (ACI), BN/NHsdMcwi (BN), BUF/MnaMcwi (BUF), F344/StmMcwi (F344), M520/NMcwi (M520), and WKY/NCrl (WKY) were set-up 8–13 weeks-of-age. Body weights of pups were tracked weekly starting at ear punching at 1 week-of-age. Litters were ear punched and culled to six pups to normalize litter size between strains. Breeders and offspring were provided ad libitum diet devoid of phytoestrogens (Teklad 2920X). One to two littermates/sex were weaned at 3 weeks-of-age and randomly assigned to Vehicle (0.1% ethanol) or 1.125 mg/l BPF in 0.1% ethanol. Animals were singly housed and glass water bottles were used. Body weight and fluid intake were monitored to calculate weekly exposure. Body composition was determined at 3, 4, and 10 weeks-of-age by TD-NMR. At 4 and 10 weeks-of-age, animals were placed in metabolic cages to assess feeding and drinking behavior and collect metabolic samples. BUF females were placed in the Promethion multiplexed metabolic phenotyping system to assess energy homeostasis at 11 weeks-of-age. Animals were euthanized at 13 weeks-of-age and blood and tissues were collected. Study end points focused on body growth, adiposity, metabolic tissues, and endocrine tissues. Created with BioRender.com.
Phenotyping
Body weight was measured weekly beginning at 1 week of age (Figure 1). Body composition was assessed at exposure start at 3 weeks-of-age, prior to metabolic cages at 4 and 10 weeks-of-age using time-domain nuclear magnetic resonance (TD-NMR; LF110, Bruker Biospin, Billerica, MA). After NMR at 4 and 10 weeks-of-age, animals were placed in metabolic cages (No. 40615, Lab Products, Inc.) and provided drinking water (either vehicle or BPF) and food (Teklad 2920X, ad libitum). After a 48-h acclimation period, 24-h urine and fecal samples were weighed and collected. Fecal samples were stored at −20°C along with a representative sample of diet. Urine samples were centrifuged at 4000 × g for 10 min, aliquoted, and stored at −80°C. Food and drinking fluid intake in the 24-h period was determined. After an overnight fast at 13 weeks-of-age, fasted blood glucose was measured from the saphenous vein in unanesthetized animals using a handheld glucometer. Animals were then euthanized by CO2 and thoracotomy. Blood samples were collected by cardiac puncture while animals were deeply anesthetized, processed into serum (BD Microtainer, SST-Amber, Franklin Lakes, NJ) and plasma (BD Microtainer, Tubes with K2E, Franklin Lakes, NJ), aliquoted, and stored at −80°C. Body length measures (nose to rump, nose to tip of tail, tibia) were determined. Tissues (liver, left ventricle of the heart, kidneys, gonads, adrenal glands, gonadal white adipose tissue [GWAT], perirenal white adipose tissue [PWAT], thymus gland, thyroid gland, hypothalamus, pituitary gland, soleus muscle, inguinal white adipose tissue [IWAT], and interscapular brown adipose tissue [BAT]) were harvested and weights collected on liver, left ventricle of the heart, kidneys, gonads, adrenal glands, GWAT, PWAT, thymus gland, thyroid gland, pituitary gland, IWAT, and BAT. Tissue samples were either snap frozen in liquid nitrogen and/or placed in RNAlater (Invitrogen, Carlsbad, CA) at −80°C for RT-qPCR.
Promethion multiplexed metabolic phenotyping
A subset of the BUF female rats (n = 9/exposure) were phenotyped at 11 weeks-of-age using a multiplexed metabolic phenotyping system (Promethion, Sable Systems International, Las Vegas, NV) at the Comprehensive Rodent Metabolic Phenotyping Core (CRMPC) at the Medical College of Wisconsin. On Monday morning, animals entered the Promethion housing and were housed continuously until Friday morning. Body composition was recorded using TD-NMR before entering and after exiting the Promethion (Reho et al., 2022). Promethion data presented were analyzed as 24-h measures from 6 am Thursday to 6 am Friday to provide maximal acclimation time of ∼72 h. Food and fluid intake, energy expenditure, and ambulation were analyzed using custom macros supplied by Sable Systems. Energy expenditure was calculated using the modified Weir equation (Weir, 1949). One female rat did not acclimate to the Promethion (fluid intake <5 g in 48 h) and was removed due to concerns over acute dehydration, reducing the vehicle BUF female sample size to n = 8.
Calorimetry
To quantitatively assess digestive efficiency and caloric absorption, bomb calorimetry was performed on fecal samples at the CRMPC as previously described (Grobe, 2017; Reho et al., 2022). Briefly, quantitative fecal samples collected in metabolic cages were desiccated in an oven and weighed prior to and after desiccation. Desiccated samples were pressed into pellets and weighed. Digestive efficiency and total daily caloric absorption were determined using a semi-microbomb calorimeter (Model 6725, Parr Instrument Co., Moline, IL) by combusting samples to completion according to the manufacturer’s protocol. Desiccated, powderized Teklad 2920X diet sample obtained at the time of metabolic cages was also analyzed by bomb calorimetry to determine total caloric density of the diet.
Urine osmolality
Urine osmolality was measured by freezing-point depression osmometry (OsmoPRO multi-sample micro-osmometer, Advanced Instruments, Norwood, MA) at the CRMPC following a published method (Wagner et al., 2021). Briefly, 24-h metabolic cage urine samples were diluted 1:4 in deionized water. System conditions were monitored by measuring a Clinitrol 290 reference solution (Advanced Instruments).
Biomarker levels
Circulating
All assays analyzed terminal blood samples obtained at tissue collection. Thyroid-stimulating hormone (TSH, ng/ml) was measured by ELISA in neat serum samples following the manufacturer’s protocol (Cat: 80564, Crystal Chem, Elk Grove Village, IL). Total triidiothyronine (total T3, ng/dl) and total thyroxine (total T4, µg/dl) were measured by radioimmunoassay in neat serum samples following the manufacturer’s protocol (tT3 kit: 06B-256447, tT4 Kit: 06B-254029, MP Biomedicals, Orangeburg, NY). Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were assayed in neat plasma samples using an LH/FSH multiplex ELISA at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core following the manufacturer protocol (EMD Millipore, Burlington, MA). Total testosterone was measured in plasma samples by LC-MS/MS following a previously published method (Raff et al., 2018). Adrenocorticotropic hormone (ACTH) and corticosterone were measured in plasma samples by radioimmunoassay following previously published methods (Raff et al., 2023).
Urine
Creatinine concentration (mg/dl) in Week 10 urine samples was determined in urine samples diluted appropriately in deionized water (Invitrogen, Carlsbad, CA) following the manufacturer protocol for the colorimetric assay (K002-H1, Arbor Assays, Ann Arbor, MI). Corticosterone concentrations (pg/ml) in Week 10 urine sample were determined in neat urine samples following the manufacturers protocol (K014-H5, Arbor Assays). Sodium and potassium were measured in Week 10 urine samples diluted in deionized water using flame atomic absorption spectroscopy (Jenway PFP7, Thermo Fisher Scientific, Waltham, MA).
Feces
Sodium and potassium were measured in Week 10 feces samples using flame atomic absorption spectroscopy (Jenway PFP7, Thermo Fisher Scientific). Feces were subjected to an ashing protocol as previously described (Titze et al., 2005) and the resulting ash was reconstituted in 10% nitric acid in deionized water.
Histological analysis
Fresh, whole adrenal glands and kidneys were fixed using 10% neutral-buffered formalin for ∼48 h and embedded in paraffin by the Children’s Research Institute Histology Core at the Medical College of Wisconsin. Embedded samples were sectioned at 4 µm. Adrenal samples were sectioned until the center of the sample was reached. Sections were stained by hematoxylin and eosin and imaged using a Hamamatsu slide scanner at the Children’s Research Institute Imaging Core at the Medical College of Wisconsin. Total area of the adrenal section and area of the adrenal medulla were assessed using the freehand region function in NDP.view 2 software (Hamamatsu, Shizuoka, Japan) in the .ndpi image files. Area of the adrenal cortex was determined by subtracting adrenal medulla area from the total adrenal section area. For each adrenal sample, the average area was calculated from three adrenal sections (WKY males n = 6/exposure). Kidney tubule damage was assessed in five random areas from the cortex and five random areas of the outer stripe of the outer medulla were visualized, imaged, and counted for each kidney. Tubular injury was determined by counting the proportion of injured-to-total tubules using cross-sectional images of the kidney at a magnification of ×20 (Regner et al., 2011). Tubular injury was defined as the presence of tubular casts, loss of brush border, flattened epithelium, and/or sloughing of tubular epithelial cells. Glomerular area was assessed using the freehand region function in NDP.view 2 software. For each kidney sample, the average glomerular area was calculated from at least 100 glomeruli throughout the entire kidney sample (BUF females n = 6/exposure).
Total RNA isolation
Whole adrenal, testes, or thyroid tissue in RNAlater was thawed on ice. One whole adrenal gland or pituitary gland, one lobe of the thyroid gland, or 30–60 mg of testes was homogenized in 1 ml of TRIzol Reagent (Invitrogen) using a Fisherbrand Bead Mill 4 Homogenizer (ThermoFisher Scientific, Waltham, MA). Homogenization was repeated, and the homogenates were cooled on ice. Samples were centrifuged at 21 100 × g for 3 min to pellet unhomogenized tissue. The supernatant was combined equally with chloroform (ThermoFisher Scientific), mixed rapidly by inversion, incubated at room temperature for ∼3 min, and centrifuged at 4°C at 12 000 × g for 15 min. Total RNA was purified from the aqueous layer following the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Samples were treated with DNase I from the RNase-Free DNase Set (Qiagen) according to the manufacturer’s protocol to remove gDNA. RNA was quantified by a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific) and was stored at −80°C.
RT-qPCR
Purified RNA (∼1 µg) was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). All RT-qPCR assays were prepared following the manufacturer protocol using Prime Time Gene Expression Master Mix gene expression assay (Integrated DNA Technologies, Coralville, IA). Experiments were conducted on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA). Primer sequences for all genes are listed in Supplementary Table 1. Genes were selected to assess thyroid hormone synthesis, testosterone synthesis, corticosterone synthesis, aldosterone synthesis, catecholamine synthesis, and lactotroph markers. Endogenous controls for gene expression normalization were: Hprt1 for adrenal and thyroid samples and Rplp0 for testes samples. Relative mRNA expression for each gene was calculated using the comparative cycle threshold (Ct) method (Pfaffl, 2004) on the average of three technical replicates per sample. PCR grade water (Invitrogen) was used as a negative control replacing the reverse transcriptase and rat universal RNA (Biochain, Newark, CA) was used as a positive control.
Calculations
To remove body weight as a confounding variable in tissue weights and drinking behavior, all absolute tissue weights and fluid intake were normalized to body weight (grams) to provide a “relative” value in units/g body weight. The corticosterone excretion rate (pg/day) was calculated by multiplying the urinary corticosterone concentration by the 24-h urine output. Urinary and fecal sodium and potassium excretion rates (mEq/day) were calculated by multiplying the sample concentration by the 24-h output value. Digestive efficiency, body weight gained between 4 and 10 weeks-of-age, food intake between 4 and 10 weeks-of-age, and energy efficiency were calculated following equations in Grobe (2017) and Reho et al. (2022) to assess energy balance. Fluid lost to urine (%) was calculated as the percentage of urine (g/day) to fluid intake in 24 h in metabolic cages. To calculate average daily BPF exposure in HS rats, the following equation was used: where T1 is time point 1, T2 is time point 2, BW is the body weight (g), and F is fluid (ml).
Statistical analysis
Outlier analysis
Outliers were removed within each sex and strain group from the raw dataset, body weight relative data, and other calculations by ROUT analysis (Motulsky and Brown, 2006) (Q = 1%) (GraphPad Prism v9.5.0 for Windows, www.graphpad.com, San Diego, CA). See Supplementary Table 2 for details regarding outlier removal and other impacts on experimental n.
ANCOVA for metabolic rate measures
To correct metabolic rate measures for covariates, such as fat-free mass, fat mass, and 24-h distance traveled fat-free mass, a generalized linear model was fit to the data. Covariates were selected after ANOVA testing of the model coefficients where F < 0.10. Estimated least squares means were extracted for the Exposure variable (Grobe, 2017). Modeling was performed in R 4.0.2 using the lsmeans package.
Comparing exposure groups
BPF exposure effect was determined in males and females separately. Comparisons between exposure groups of the same sex were performed using a two-way ANOVA and Šídák’s multiple comparisons test when all data were present or were performed using a mixed effects model and Šídák’s multiple comparisons test when there was missing data. In data generated for specific strain-sex groups, comparison between exposure groups was performed using an unpaired t test or a Welch’s unpaired t test when appropriate. RT-qPCR results were determined on the ΔΔCt values using unpaired, two-tailed t test with or without Welch’s correction when appropriate. Alpha level was 0.05 for all statistical testing. Exposure comparisons completed in GraphPad Prism v9.5.0. Data figures created in GraphPad Prism v9.5.0.
Results
Fluid intake and dosing
Weekly fluid intake was determined in all animals over the study protocol. From those data, fluid intake per day did not differ between the vehicle- and BPF-exposed animals in any sex or strain group (Supplementary Figure 1 and Supplementary Tables 3 and 4). There were strain differences in fluid intake with average daily fluid intake ranging between 11.7 and 23.5 g/day in females and 13.9 and 25.2 g/day in males (Male PStrain < 0.0001, Female PStrain < 0.0001). These differences in drinking behaviors affected the average daily BPF exposure calculated over the 10-week protocol (Supplementary Figure 2A, Supplementary Tables 5 and 6, and Table 1; PStrain < 0.0001, PSex < 0.0001, PStrain × Sex = 0.0164).
Table 1.
Average daily BPF exposure in female and male ACI, BN, BUF, F344, M520, and WKY rats during the 10-week exposure period (Mean ± SEM (N)) and the comparable human dose for each sex and strain group (mean)
| Strain | Females |
Males |
||
|---|---|---|---|---|
| Average Rat Dose (µg/kg/day) | Human Dose (BW3/4) (µg/kg/day) | Average Rat Dose (µg/kg/day) | Human Dose (BW3/4) (µg/kg/day) | |
| ACI | 169.3 ± 3.0 (11) | 31.8 | 146.9 ± 1.9 (13) | 30.2 |
| BN | 124.9 ± 2.6 (10) | 24.2 | 110.0 ± 1.8 (11) | 23.5 |
| BUF | 141.5 ± 2.3 (10) | 28.8 | 137.0 ± 3.1 (11) | 31.3 |
| F344 | 136.7 ± 4.5 (9) | 26.5 | 115.7 ± 1.8 (12) | 25.4 |
| M520 | 151.8 ± 4.1 (9) | 29.4 | 134.1 ± 2.8 (12) | 28.4 |
| WKY | 206.2 ± 6.1 (10) | 41.9 | 176.4 ± 4.8 (15) | 39.0 |
The average BPF exposure dose for female rats ranged from 124.9 to 206.2 µg/kg BW/day and for male rats ranged from 110.0 to 176.4 µg/kg BW/day. To compare the exposure doses in these animals to a human dose, the dosimetric adjustment factor equation (USEPA, 2011) was implemented using the BW3/4 equation and a human body weight of 70 kg (Supplementary Figure 2B and Table 1). The comparable human dose range for females is 24.2–41.9 µg/kg BW/day and for males is 23.5–39.0 µg/kg BW/day.
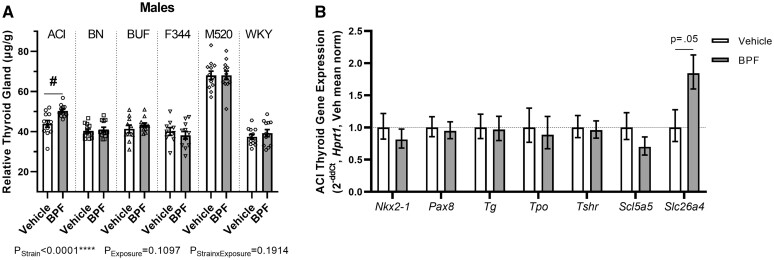
BPF affects the thyroid in ACI males
After 10 weeks of BPF exposure, relative thyroid weight was significantly increased by ∼15% in the BPF-exposed ACI males compared with vehicle ACI males (PStrain × Exposure = 0.19, pACI = 0.03) (Figure 2A and Table 2). To determine if hypothalamic–pituitary–thyroid (HPT) axis hormones had changed with the increased thyroid weight, serum levels of TSH, total T3, and total T4 were assessed in terminal blood samples. BPF exposure did not significantly alter circulating levels of TSH, total T3, or total T4 in the ACI males (Supplementary Table 10).
Figure 2.
BPF exposure effects on the thyroid in ACI males. A, At 13 weeks-of-age, thyroid glands were harvested, weighed, and adjusted for body weight. BPF-exposed ACI males had significantly increased thyroid gland tissue compared with vehicle ACI males. B, Gene expression of the TSH receptor (Tshr), important thyroid nuclear transcription factors (Nkx2-1, Pax8), thyroglobulin (Tg), thyroid peroxidase (Tpo), and critical transporters (Slc5a5, Slc26a4) (n = 9–12 per exposure group). Slc26a4 had a possible increase in expression in the BPF-exposed ACI males compared with vehicle ACI males. Unpaired, two-tailed t test with or without Welch’s correction used when appropriate for comparing vehicle and BPF-exposed ACI males only. Two-way ANOVA, Šídák’s multiple comparisons test, #p < .05.
Table 2.
Weight parameters and other phenotypes in male ACI, BN, BUF, F344, M520, and WKY rats (mean ± SEM (N))
| Phenotype | ACI |
BN |
BUF |
F344 |
M520 |
WKY |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | |
| Tissue measurements | ||||||||||||
| Adrenal glands (mg) | 38.6 ± 0.8 (12) | 39.2 ± 0.6 (13) | 50.4 ± 1.2 (10) | 50.5 ± 1.0 (10) | 37.4 ± 0.4 (10) | 37.2 ± 0.4 (11) | 45.6 ± 1.2 (12) | 45.1 ± 0.9 (12) | 38.7 ± 1.0 (12) | 37.7 ± 0.9 (12) | 48.4 ± 1.1 (13) | 51.6 ± 0.9 (14) |
| Adrenal glands (µg/g) | 178 ± 4 (12) | 181 ± 2 (13) | 205 ± 4 (10) | 205 ± 4 (11) | 110 ± 1 (10) | 109 ± 1 (11) | 159 ± 4 (12) | 157 ± 3 (12) | 163 ± 3 (12) | 159 ± 1 (12) | 160 ± 3 (13) | 173 ± 2 (14)* |
| Testes (g) | 2.2984 ± 0.0158 (12) | 2.3068 ± 0.0252 (13) | 2.9931 ± 0.0382 (9) | 3.0345 ± 0.0340 (10) | 3.0345 ± 0.0253 (10) | 3.0388 ± 0.0239 (11) | 3.0032 ± 0.0217 (12) | 2.9680 ± 0.0260 (12) | 2.5570 ± 0.0407 (12) | 2.5192 ± 0.0304 (12) | 2.5957 ± 0.0153 (15) | 2.5836 ± 0.0215 (15) |
| Testes (mg/g) | 10.599 ± 0.066 (12) | 10.659 ± 0.154 (13) | 12.171 ± 0.212 (9) | 12.557 ± 0.171 (10) | 8.944 ± 0.114 (10) | 8.905 ± 0.065 (11) | 10.432 ± 0.109 (12) | 10.333 ± 0.061 (12) | 10.757 ± 0.161 (12) | 10.652 ± 0.115 (12) | 8.645 ± 0.092 (15) | 8.682 ± 0.066 (15) |
| Thyroid gland (mg) | 9.5 ± 0.4 (12) | 11.0 ± 0.2 (12) | 9.8 ± 0.6 (9) | 9.4 ± 0.5 (11) | 14.0 ± 0.6 (10) | 14.8 ± 0.3 (11) | 11.6 ± 0.3 (12) | 11.8 ± 0.4 (12) | 16.2 ± 0.6 (12) | 16.2 ± 0.7 (12) | 11.3 ± 0.4 (12) | 11.7 ± 0.5 (12) |
| Thyroid gland (µg/g) | 43.9 ± 1.7 (12) | 50.3 ± 0.9 (13)* | 40.1 ± 1.9 (9) | 38.2 ± 1.9 (11) | 41.4 ± 1.9 (10) | 43.3 ± 1.1 (11) | 40.4 ± 1.0 (12) | 41.0 ± 1.2 (12) | 68.1 ± 2.1 (12) | 68.1 ± 2.2 (12) | 37.5 ± 1.2 (12) | 39.3 ± 1.8 (12) |
| Circulating hormones | ||||||||||||
| Plasma total testosteronea (ng/ml, Log10) | 1.9 ± 0.1 (6) | 1.9 ± 0.1 (7) | 1.2 ± 0.0 (9) | 1.4 ± 0.1 (11) | 2.3 ± 0.2 (6) | 2.1 ± 0.1 (8) | 1.5 ± 0.2 (7) | 1.5 ± 0.1 (7) | 1.7 ± 0.2 (5) | 1.7 ± 0.2 (4) | 2.1 ± 0.2 (7) | 2.0 ± 0.1 (7) |
Relative tissue weights are per gram body weight.
Total testosterone data were transformed by log10 to meet normality requirement for statistical comparison. Comparisons made between vehicle and BPF animals of the same strain done by two-way ANOVA with Šídák’s multiple comparisons test. Significant differences highlighted in bold.
p < .05.
To determine if expression of thyroid hormone synthesis genes had changed with the increased thyroid weight, gene expression of the TSH receptor (Tshr), important thyroid transcription factors (Nkx2-1, Pax8), critical transport proteins (Slc5a5, Slc26a4), thyroglobulin (Tg), and thyroid peroxidase (Tpo) were assessed in the thyroid (Figure 2B). Slc26a4 expression was increased by ∼84% in the BPF-exposed ACI males compared with the vehicle ACI males, although the difference did not reach statistical significance (pSlc26a4 = 0.05). Nkx2-1, Pax8, Tg, Tpo, Tshr, and Slc5a5 gene expression were not different between the ACI male groups.
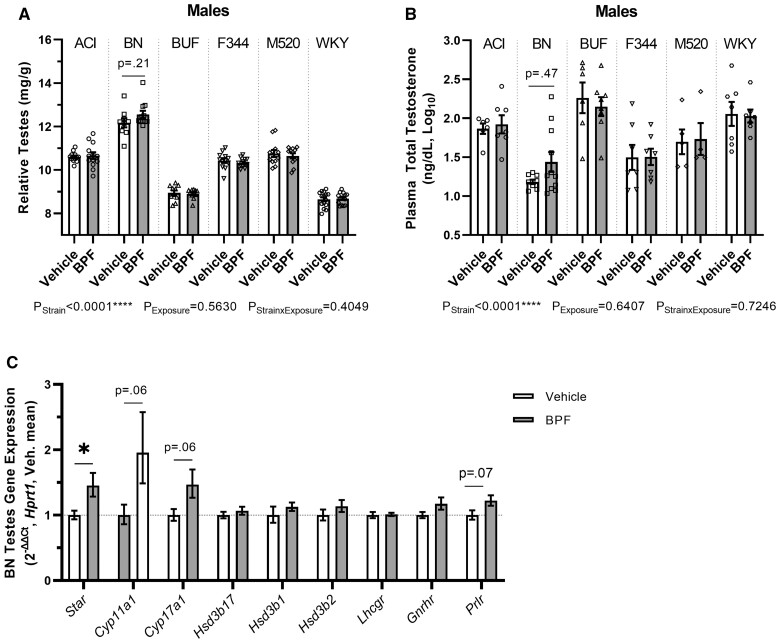
BPF affects the testes and testosterone synthesis in BN males
After 10 weeks of BPF exposure, there was a modest increase in relative testes weight (PStrain × Exposure = 0.40, pBN = 0.21, ∼3%) in BPF-exposed BN males compared with vehicle BN males (Figure 3A and Table 2). Considering the extensive literature reporting male reproductive dysfunction after BPF exposure, we measured plasma testosterone levels in terminal blood samples. Consistent with the modest increase in testes weight, there was a modest ∼22% increase in total testosterone in BPF-exposed BN males although variation was relatively high and the difference did not reach statistical significance (PStrain × Exposure = 0.72, pBN = 0.47, Figure 3B and Table 2). To determine if gonadotrophic hormones had changed, we measured plasma FSH and LH levels in terminal blood samples. BPF exposure did not affect FSH or LH levels in BN males (Figure 3C and Supplementary Table 10).
Figure 3.
BPF exposure effects on the testes in BN males. A, At 13 weeks-of-age, the testes were harvested, weighed, and adjusted for body weight. BPF-exposed BN males had possible increases in testes tissue compared with vehicle BN males. B, Total testosterone was assayed in terminal plasma samples. There was a possible increase in total testosterone in the BPF-exposed BN males compared with the vehicle BN males. C, Gene expression of enzymes in testosterone biosynthesis pathway (Star, Cyp11a1, Cyp17a1, Hsd3b1, Hsd3b2) and receptors that influence transcriptional regulation of Star (Lhcgr, Gnrhr, Prlr). There was a significant expression increase of Star and modest increases in Cyp11a1, Cyp17a1, and Prlr (n = 10–11 per exposure group). Unpaired, two-tailed t test with or without Welch’s correction used when appropriate for comparing vehicle and BPF-exposed BN males only, *p < .05. Two-way ANOVA, Šídák’s multiple comparisons test.
To ascertain if the increased testosterone in the BPF-exposed BN males was due to a change in steroidogenic capacity, gene expression of Star, the first enzyme in the steroidogenesis pathway, cell surface receptors that participate in Star transcriptional regulation (Lhcgr, Gnrhr, Prlr), and other steroidogenesis pathway enzymes (Cyp11a1, Cyp17a1, Hsd3b17, Hsd3b1, Hsd3b2) were assessed in whole testes samples (Figure 3D). Star was significantly increased by ∼45% (pStar = 0.02) and both Cyp11a1 and Cyp17a1 had increased expression by ∼96% and ∼47%, respectively, in BPF-exposed BN males compared with vehicle BN males that did not reach statistical significance (pCyp11a1 = 0.06, pCyp17a1 = 0.06). Prlr but not Lhcgr showed a possible increase in expression by ∼22% in BPF-exposed BN males compared with vehicle BN males that did not reach statistical significance (pPrlr = 0.07).
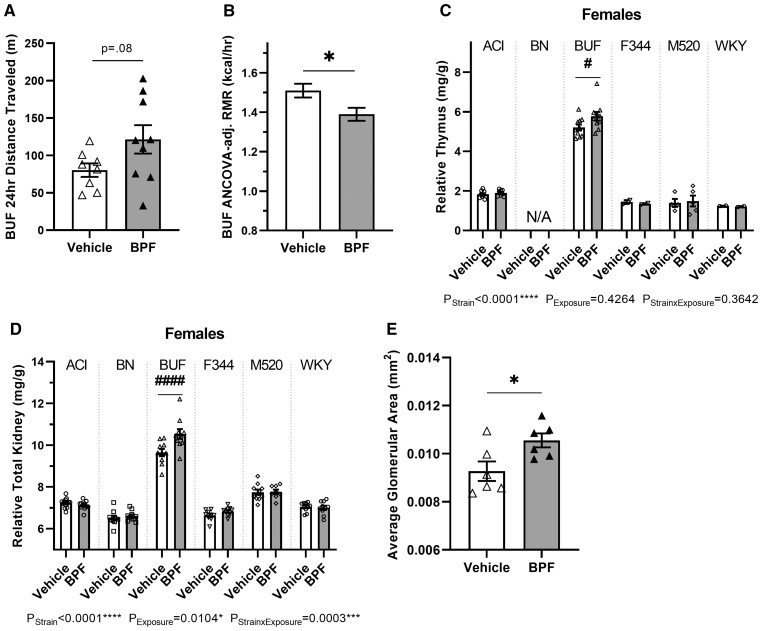
BPF affects energy homeostasis, thymus, and kidneys in BUF females
BPF-exposed BUF females showed indications of energy homeostasis imbalance without differences in body weight gained or in body composition compared with vehicle controls (Supplementary Figure 3 and Supplementary Tables 7 and 8). Metabolic cage data were analyzed from the 4- to 10-week time points to evaluate factors in energy homeostasis, like food intake and energy efficiency (body weight gained per calorie absorbed). The 24-h fecal sample collections at both time points were analyzed by bomb calorimetry to determine fecal caloric density and the results were used to calculate energy efficiency. There was a possible decrease in energy efficiency by ∼8% in the BPF-exposed BUF females compared with the vehicle BUF females that also did not reach statistical significance (PStrain × Exposure = 0.29, pBUF = 0.47) (Table 3). There was no difference in BPF-exposed BUF female 24-h food intake at either time point compared with vehicle BUF females (Supplementary Table 8). These results led to the hypothesis that BPF exposure in BUF females altered their energy expenditure.
Table 3.
Metabolic parameters and tissue phenotypes in female ACI, BN, BUF, F344, M520, and WKY rats (mean ± SEM (N))
| Phenotype | ACI |
BN |
BUF |
F344 |
M520 |
WKY |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | Vehicle | BPF | |
| Metabolic cage measurements | ||||||||||||
| Food intake Wk4–Wk10 (g) | 455.2 ± 17.2 (14) | 430.7 ± 11.9 (11) | 454.7 ± 13.0 (10) | 460.8 ± 10.9 (9) | 617.7 ± 22.2 (10) | 659.2 ± 17.5 (10) | 498.6 ± 15.7 (9) | 493.2 ± 17.2 (9) | 518.9 ± 12.5 (11) | 497.5 ± 16.8 (8) | 543.3 ± 14.8 (10) | 522.8 ± 19.8 (10) |
| Body weight gained Wk4–Wk10 (g) | 80 ± 1 (15) | 81 ± 1 (11) | 92 ± 2 (10) | 88 ± 2 (10) | 113 ± 3 (10) | 111 ± 2 (10) | 78 ± 2 (9) | 80 ± 2 (9) | 72 ± 2 (11) | 75 ± 2 (9) | 112 ± 3 (10) | 113 ± 3 (10) |
| Energy efficiency (g/kcal) | 0.205 ± 0.008 (14) | 0.215 ± 0.007 (11) | 0.191 ± 0.007 (9) | 0.196 ± 0.007 (10) | 0.206 ± 0.006 (10) | 0.189 ± 0.005 (10) | 0.210 ± 0.006 (9) | 0.205 ± 0.008 (9) | 0.160 ± 0.007 (11) | 0.176 ± 0.006 (8) | 0.235 ± 0.010 (10) | 0.237 ± 0.007 (9) |
| Tissue measurements | ||||||||||||
| Total kidney (mg) | 1054.0 ± 10.4 (15) | 1021.4 ± 13.4 (11) | 1002.1 ± 25.5 (10) | 1024.4 ± 15.7 (10) | 1971.8 ± 37.8 (10) | 2103.6 ± 41.7 (10)** | 1110.0 ± 19.8 (9) | 1105.1 ± 12.1 (9) | 1200.5 ± 21.1 (10) | 1207.8 ± 28.7 (8) | 1407.7 ± 32.5 (10) | 1405.7 ± 31.0 (10) |
| Total kidney (mg/g) | 7.2304 ± 0.0536 (15) | 7.1203 ± 0.0621 (11) | 6.5106 ± 0.1106 (10) | 6.6064 ± 0.0780 (10) | 9.6436 ± 0.1722 (10) | 10.5346 ± 0.2388 (10)*** | 6.6397 ± 0.0849 (9) | 6.8335 ± 0.0687 (9) | 7.7465 ± 0.1269 (10) | 7.7665 ± 0.1091 (8) | 7.0450 ± 0.0706 (10) | 7.0046 ± 0.1003 (10) |
| Thymus gland (mg) | 268.3 ± 9.0 (9) | 279.8 ± 8.7 (8) | – | – | 1066.1 ± 38.8 (10) | 1156.5 ± 50.5 (10) | 239.4 ± 13.8 (2) | 211.2 ± 2.1 (2) | 218.2 ± 34.9 (4) | 234.2 ± 42.5 (5) | 244.9 ± 8.2 (2) | 238.2 ± 2.4 (2) |
| Thymus gland (mg/g) | 1.823 ± 0.061 (9) | 1.899 ± 0.055 (8) | – | – | 5.206 ± 0.151 (10) | 5.774 ± 0.218 (10)* | 1.439 ± 0.096 (2) | 1.346 ± 0.038 (2) | 1.395 ± 0.204 (4) | 1.487 ± 0.275 (5) | 1.241 ± 0.012 (2) | 1.206 ± 0.021 (2) |
Relative tissue weights in mg/g are mg tissue per g body weight. Thymus weight was not assessed in the BN females (Supplementary Table 2). Comparisons made between vehicle and BPF animals of the same strain done by two-way ANOVA with Šídák’s multiple comparisons test. Significant differences highlighted in bold.
p < .05,
p < .01,
p < .0001.
To determine if BPF exposure in the BUF females affected energy expenditure, such as metabolic rate and activity levels, BUF females were observed in the Promethion multiplexed metabolic phenotyping system. BPF-exposed BUF females showed a possible increase in 24-h distance traveled by ∼51% compared with vehicle BUF females (Figure 4A, p = .08) and no change in 24-h sleep time (Table 4). There was also a modest decrease in average daily metabolic rate by ∼3% in the BPF-exposed BUF females compared with the vehicle BUF females (Table 4), and this difference remained after correction for their 24-h distance traveled, fat-free mass, and fat mass (Table 4, p = .08). Since average daily metabolic rate includes the active period and does not account for expected metabolic rate differences due to varying fat-free mass levels, resting metabolic rate (RMR) was corrected for fat-free mass. Prior to adjustment, BPF exposure significantly decreased RMR by ∼8% (Table 4, p = .01) and that difference was maintained after fat-free mass adjustment (Figure 4B and Table 4, p = .02). BPF exposure did not change 24-h food or fluid intake in the BUF females in the Promethion (Table 4).
Figure 4.
BPF exposure effects on energy balance, the thymus, and the kidneys in BUF females. A and B, At 11 weeks-of-age, BUF female energy homeostasis was assessed in the Promethion system. BPF BUF females exhibited a possible increase in 24-h distance traveled (A) compared with the vehicle BUF females. BPF-exposed BUF females also had a significant decrease in RMR after correction for fat-free mass (B). C, At 13 weeks-of-age, the thymus gland was harvested, weighed, and adjusted for body weight. BPF-exposed BUF females had significantly increased thymus gland tissue. Note that thymus weight was not assessed in BN females (Supplementary Table 2). D, At 13 weeks-of-age, kidneys were harvested, weighed, and adjusted for body weight. Total kidney weight in BPF-exposed females was significantly increased compared with vehicle BUF females. E, BUF female kidneys were fixed, embedded, sectioned, and H&E stained to measure glomerular hypertrophy (n = 6 per exposure group; glomerular area measured in 103–110 glomeruli per sample). There was significantly increased glomerular area in the BPF-exposed BUF female compared with the vehicle BUF females. Unpaired, two-tailed t test with or without Welch’s correction used when appropriate for comparing vehicle and BPF BUF females only, *p < .05. Two-way ANOVA, Šídák’s multiple comparisons test, #p < .05, ####p < .0001.
Table 4.
Energy homeostasis and kidney histology data in female BUF rats (mean ± SEM (N))
| BUF Female Phenotype | Vehicle | BPF | p-Value |
|---|---|---|---|
| 24-h food (g) | 17.42 ± 0.60 (8) | 16.92 ± 0.74 (9) | .61 |
| 24-h fluid (g) | 20.40 ± 1.23 (8) | 19.09 ± 0.90 (9) | .39 |
| 24-h sleep (h) | 14.28 ± 0.53 (8) | 13.93 ± 0.59 (9) | .67 |
| 24-h distance traveled (m) | 80.30 ± 8.91 (8) | 121.40 ± 19.01 (9) | .08* |
| Average metabolic rate (kcal/h) | 1.80 ± 0.02 (8) | 1.75 ± 0.03 (9) | .16 |
| ANCOVA-adjusted average metabolic rate (kcal/h) | 1.80 ± 0.02 (8) | 1.75 ± 0.02 (9) | .08* |
| RMR (kcal/h) | 1.53 ± 0.04 (8) | 1.38 ± 0.03 (9) | .01** |
| ANCOVA-adjusted RMR (kcal/h) | 1.51 ± 0.03 (8) | 1.39 ± 0.03 (9) | .02** |
| Kidney cortex tubule damage (%) | 26.2 ± 1.8 (6) | 28.1 ± 3.3 (6) | .62 |
| Kidney medulla tubule damage (%) | 18.9 ± 2.3 | 25.4 ± 3.4 | .15 |
| Average glomerular area (mm2) | 0.0093 ± 0.0004 (6) | 0.0106 ± 0.0003 (6) | .04** |
Comparisons made between vehicle and BPF BUF females done by unpaired, two-tailed t test or Welch’s unpaired, two-tailed t test where appropriate.
p < .10,
p < .05.
After 10 weeks, BPF exposure significantly increased relative thymus weight by ∼11% (PStrain × Exposure = 0.36, pBUF = 0.03) in BPF-exposed BUF females compared with vehicle BUF females (Figure 4C and Table 3).
Additionally, 10 weeks of BPF exposure increased relative total kidney weight by ∼10% (PStrain × Exposure = 0.0003, pBUF < 0.0001) in BPF-exposed BUF females compared with vehicle BUF females (Figure 4E and Table 3). To determine if BPF exposure induced histological differences in the BUF female kidney, a subset of kidney samples was processed for histological examination and tubule damage in the kidney cortex and medulla was assessed and glomerular area was measured. There was a significant increase in glomerular area by ∼14% in the BPF-exposed BUF females compared with the vehicle BUF females (Figure 4F, Table 4, and Supplementary Figure 5, p = .03). BPF exposure did not increase cortex or medullary tubule damage in the BUF females (Table 4).
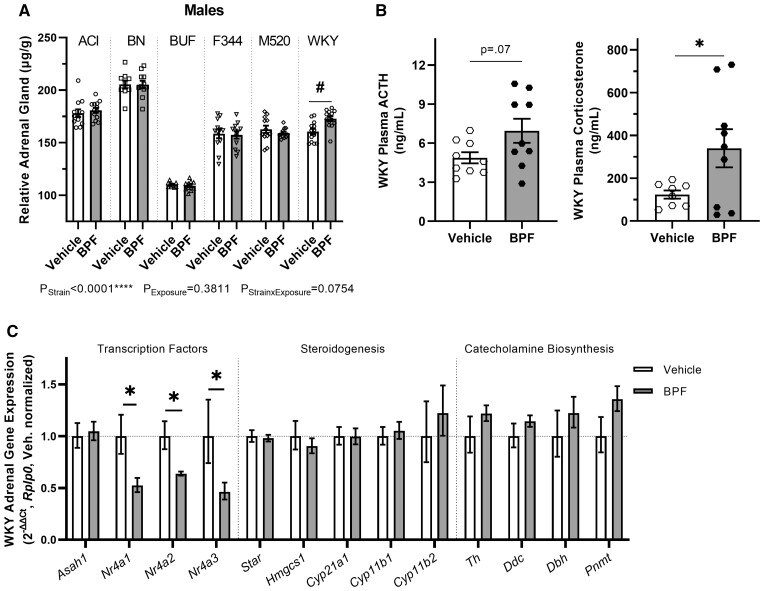
BPF affects the adrenal glands in WKY males
Ten weeks of BPF exposure significantly increased relative adrenal weight by ∼8% in the BPF-exposed WKY males compared with the vehicle WKY males (PStrain × Exposure = 0.08, pWKY = 0.01) (Figure 5A and Table 2). To determine if hypertrophy of the adrenal cortex or adrenal medulla was responsible for the increased weight, a subset of adrenal samples was processed for histological examination and the areas of the adrenal section, the adrenal cortex, and the adrenal medulla were measured (Supplementary Figure 6). There was a decrease in adrenal medulla area by ∼23% in the BPF-exposed WKY males compared with the vehicle WKY males that did not reach statistical significance (Supplementary Table 10, p = .11). Adrenal section total area and adrenal cortex area were not different between the WKY male exposure groups (Supplementary Table 10).
Figure 5.
BPF exposure effects on the adrenal glands in WKY males. A, At 13 weeks-of-age, adrenal glands were harvested, weighed, and adjusted for body weight. BPF-exposed WKY males had increased adrenal tissue compared with vehicle WKY males. B, Plasma ACTH and corticosterone levels in WKY males from terminal blood samples. BPF exposure increased both ACTH and corticosterone levels in WKY males. C, Gene expression of critical adrenal transcription factors (Asah1, Nr4a1, Nr4a2, Nr4a3), important enzymes in steroidogenesis pathways (Star, Hmgcs1, Cyp21a1, Cyp11B1, Cyp11b2) and catecholamine biosynthesis enzymes (Th, Ddc, Dbh, Pnmt) (n = 9–11 per exposure group). All three Nr4a genes had significantly reduced expression in the BPF-exposed WKY male compared with the vehicle WKY males. Unpaired, two-tailed t test with or without Welch’s correction used when appropriate for comparing vehicle and BPF-exposed WKY males only, *p < .05. Two-way ANOVA, Šídák’s multiple comparisons test, #p < .05.
To determine if the increased adrenal weight was associated with an increase in basal corticosterone secretion, urinary corticosterone and creatinine in 24-h urine samples from metabolic cages at 10 weeks-of-age were assessed. There was no difference between vehicle or BPF-exposed WKY males in urinary corticosterone concentration, excretion rate, creatinine-corrected corticosterone, urinary creatinine concentration, or creatinine excretion rate between the WKY male exposure groups (Supplementary Table 10). To ascertain if the increased weight was associated with downstream effects of aldosterone, urinary and fecal sodium and potassium were measured by flame photometry in 24-h samples collected at 10 weeks-of-age. There were no differences in concentrations or excretion rates of either sodium or potassium in urine and feces between the WKY male exposure groups (Supplementary Table 10).
Stress is an archetypal stimulus to the hypothalamic–pituitary–adrenal (HPA) axis in which ACTH from the pituitary stimulates the synthesis and release of corticosterone from the adrenal cortex (Raff et al., 2014). To assess the acute HPA axis response to a maximal stressor under BPF exposure, plasma ACTH and corticosterone were measured in blood samples collected at euthanasia. BPF-exposed WKY males showed a significant increase in plasma corticosterone levels compared with vehicle WKY males and a trend toward an increase in plasma ACTH (Figure 5B and Supplementary Table 10, pACTH = 0.07, pcorticosterone = 0.04).To determine if WKY male adrenal glands after exposure had altered expression of steroidogenic enzyme and related genes, mRNAs for Star, steroidogenesis enzymes (Hmgcs1, Hsd3b, Cyp21a1, Cyp11b1, Cyp11b2), catecholamine synthesis enzymes (Th, Ddc, Dbh, Pnmt), and important adrenal transcription factors (Nr4a1, Nr4a2, Nr4a3, Asah1) were assessed in whole adrenal gland samples (Figure 5C). BPF-exposed WKY males showed markedly decreased expression of Nr4a1, Nr4a2, and Nr4a3 by ∼48%, ∼37%, and ∼54%, respectively, compared with the vehicle WKY males (pNr4a1 = 0.01, pNr4a2 = 0.01, pNr4a3 = 0.04). Expression of Asah1, a negative regulator of the Nr4a subfamily (Lucki et al., 2012), was not differently expressed between the WKY male exposure groups. There was no difference in gene expression controlling corticosterone or aldosterone synthesis between the WKY male exposure groups.
Other BPF exposure outcomes
After 10 weeks of exposure, BPF-exposed ACI females exhibited a possible increase in relative thyroid gland weight by ∼9% compared with vehicle ACI females that did not reach statistical significance (PStrain × Exposure = 0.46, pACI = 0.24) (Supplementary Table 8). There were no differences in any measured parameter in the BPF-exposed BN female, BUF male, WKY female, F344 or M520 rats compared with vehicle controls during this study (Supplementary Figures 3 and 4 and Supplementary Tables 5–9).
Discussion
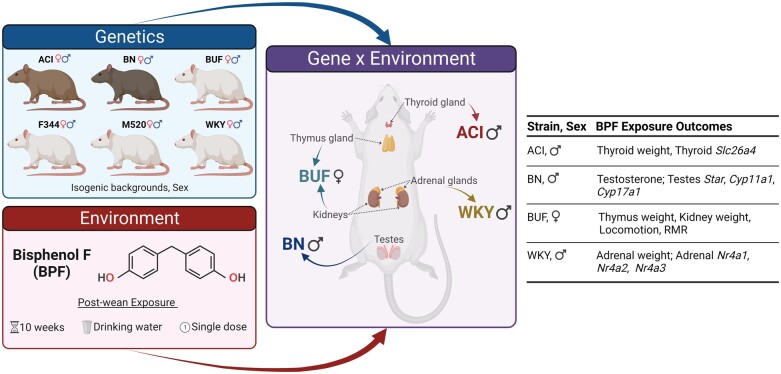
Incorporating genetic variation in toxicological and environmental health studies can provide the necessary innovation to identify precision environmental risks. We used six substrains similar to the HS rat founding strains to determine the endocrine and metabolic effect of 10 weeks post-wean BPF exposure in young-adult rats of both sexes. Our data reveal that post-wean BPF exposure has numerous sex- and strain-specific effects on the endocrine system and energy balance (Figure 6). In male rats, BPF exposure disturbed functions in the thyroid in ACI males, of the HPG axis in BN males, and of the HPA axis in WKY males. In female rats, BPF exposure increased thymus and kidney weight and decreased metabolic rate in the BUF females. We recognize the small effect size of these phenotypes; we propose that this study design allowed us to capture mechanistic features rather than long-term BPF exposure endpoints and is likely more similar to what will be experienced in human populations.
Figure 6.
Summary figure of BPF exposure outcomes in HS rat founder strains. Ten weeks of post-wean BPF exposure in drinking water induced strain- and sex-specific outcomes. These results indicate that the genetic background and sex are determinants in bisphenol exposure effects on endocrine and metabolic health. Created with BioRender.com.
The male and female rats were exposed to human comparable doses ranging from 23.5 to 41.9 µg BPF/kg/day. This dose range was due to strain- and sex-specific drinking behaviors. While the estimated dose for each rat group were not identical, all BPF exposure doses were below the current FDA ADI for BPA of 50 µg/kg/day (USEPA, 2010), making our dose relevant to human environmental health concerns. EDCs are widely known to show non-monotonic dose responses (Vandenberg, 2014). It is possible that the single BPF exposure dose design implemented in this study does not translate to the same position on the BPF dose response curve for each tested strain and sex, a limitation that warrants future studies with additional doses. However, human exposure to bisphenols is not consistent across the globe (Dodson et al., 2020; Dualde et al., 2019; Engdahl et al., 2021; Huang et al., 2022; Lu et al., 2022; Tkalec et al., 2021). Therefore, this study reflects BPF exposure outcomes from genetically and behaviorally variable groups, adding another layer of gene by EDC variability relevant to human populations. An intriguing future direction of this project is to perform BPF toxicokinetic experiments in these rat strains to determine how gene EDC interactions extend to the ADME of BPF.
Ten weeks of post-wean BPF exposure in ACI rats induced a significant increase in thyroid weight in males and an increasing trend in females. The thyroid weight change in the BPF-exposed ACI males was not associated with TSH, total T3, or total T4. Since there were no BPF exposure effects on TSH, total T3, or total T4 in the ACI males, BPF does not induce hypo or hyperthyroidism in this short exposure paradigm. In zebrafish embryo studies, BPF exposure alters whole-body TSH and thyroid hormone levels and increases thyroid hormone synthesis gene expression (Huang et al., 2016; Lee et al., 2019). Human epidemiological studies have found that urinary BPF levels in pregnant women are positively associated with free T4 in Puerto Rico (Aker et al., 2019) and free T3 in Sweden (Derakhshan et al., 2019). Thyroid hormones are metabolized by sulfotransferases and glucuronidases to promote its excretion (Emi et al., 2007; Kester et al., 2003; Richardson and Klaassen, 2010). In comparison to F344 and SD rats, ACI rats demonstrate lower T3 sulfonation activity (Gong et al., 1992), an indication of an abnormal HPT axis in ACI rats. Interestingly, Slc26a4, encoding an iodide transporter and associated with congenital hypothyroidism (Cortés and Zerón, 2019), was possibly increased in expression in the BPF-exposed ACI male thyroid, suggesting iodine accumulation in the BPF-exposed ACI males. Thyroid hyperplasia can be regulated by intracellular iodine content (Fujimoto et al., 1999; Stübner et al., 1987); however, thyroid samples were not appropriately collected for histopathological examination in this study. Future studies will determine if extending to chronic BPF exposure in aged ACI males promotes thyroid hyperplasia and thyroid disease.
Most in vivo studies report no change in testes weight with BPF exposure (Kaimal et al., 2021; Li et al., 2022; Ullah et al., 2018), which is consistent with our findings in the BPF-exposed BN males. The slight increase in plasma total testosterone is at odds with previous reports of BPF exposure in males. Sub-chronic BPF exposure (<12 weeks) studies in adult male SD and Wistar rats report decreased testosterone levels after 1, 10, 30, 50, and 100 mg BPF/kg/day (Fatai and Aribidesi, 2022). A zebrafish study reported decreased testosterone levels after sub-chronic exposure to 0.01 mg BPF/l in aquarium water (Yang et al., 2017). A chronic BPF exposure study exposing young SD male rats to 50 µg BPF/l in drinking water found that circulating testosterone levels decreased after 48 weeks of exposure (Ullah et al., 2018), indicating that the low dose exposure performed in this study may require longer exposure times to generate significant testosterone phenotypes. There are also conflicting reports of BPF associating with testosterone in humans. Analysis of NHANES data from 2011 to 2016 found that urinary BPF levels were positively associated with total testosterone and free testosterone in U.S. adult men (Zhang et al., 2022) but urinary BPF levels in reproductive-aged Chinese men were not associated with testosterone levels (Zeng et al., 2022). These conflicting reports may be rooted in genetic and/or environmental differences between the tested populations.
The BN strain is a model for primary and secondary testicular failure with low circulating testosterone levels in adulthood and a dysfunctional HPG axis (Abuelhija et al., 2012; Gruenewald et al., 1994; Syed and Hecht, 2001). The modest increase in plasma total testosterone in BPF-exposed BN males was supported by significantly increased Star and strong trends in increased Cyp11a1 and Cyp17a1 gene expression in the testes. Star expression is predominantly regulated by cAMP-dependent mechanisms through LH receptor activation (Manna et al., 2009). Lhgcr expression was not different between the BN male exposure groups, which suggest that the LH-cAMP pathway is not upregulating Star. Trophic factors, like prolactin, can also transcriptionally regulate Star in the testes. There was a possible increase in Prlr expression in the BPF-exposed BN male testes, suggesting that prolactin receptor activity may be dysregulating Star. A recent study found that male Wistar rats exposed to 30 and 50 mg/kg BPF for 28 days developed hyperprolactinemia (Fatai and Aribidesi, 2022). Prolactin levels and histopathological examination of the testes in future studies will investigate the hypothesis that BPF exposure in BN males causes increased prolactin levels and elevates testosterone synthesis.
BUF females had modestly decreased energy efficiency during the first 7 weeks of BPF exposure, meaning that the body weight gained by the females was disproportionately decreased relative to their estimated total absorbed calories, which suggests increased total energy expenditure. Further examination of the BUF females in the Promethion revealed that BPF-exposed BUF females had increased activity levels and decreased RMR after 8 weeks of BPF exposure. The decreased energy expenditure in the BPF-exposed BUF females in the Promethion is at odds with the decreased energy efficiency. These measures of energy expenditure may reflect a developmental effect of BPF or a critical shift in energy homeostasis in the BUF females. The balance in excess energy lost during activity and excess energy gained by a lower metabolic rate may explain why body weight and body composition of BPF-exposed BUF females did not change. The lack of BPF effects on body weight and composition in the BUF females is consistent with two studies in prenatally exposed and post-wean exposed female SD rats (Ijaz et al., 2020; Kaimal et al., 2021), but other studies report that BPF exposure can decrease body weight in adult female SD rats exposed to 20–500 mg BPF/kg/day (Higashihara et al., 2007; Lee et al., 2022). These inconsistencies may be due to environmental differences, genetic differences within the SD colonies, or to the exposure dose. To our knowledge, this is the first report of BPF exposure changing activity levels and metabolic rate.
Alterations in energy homeostasis in BPF-exposed BUF females may be connected to their significantly increased thymus weight. Immune activation influences whole-body metabolism and, therefore, metabolic rate (Hotamisligil, 2017; Zmora et al., 2017). The BUF rat strain is known to develop autoimmune disorders and hyperplasia of the thymus and peripheral T-cell system (Lahat et al., 1989; Matsuyama et al., 1986; Noble et al., 1976). Given that women are at higher risk for developing autoimmune disease compared with men (Kronzer et al., 2021), it is intriguing that a female group developed BPF-exposure outcomes related to the immune system. BPF has been shown in previous studies to affect immune system features, like apoptosis in RAW264.7 macrophages (Chen et al., 2022b) and increased IL-17 secretion from Th17 differentiating T-cells (Malaisé et al., 2020a). To our knowledge, this is the first report of BPF altering thymus weight. Lymphocyte profiling and thymus histopathological examination will be included in future studies of the BUF immune system after BPF exposure.
Ten weeks of BPF exposure also significantly increased kidney weight in BUF females. There was no change in fluid intake or urine output to indicate changes in hydration or kidney function. Interestingly, BUF rats are a potential model for idiopathic nephrotic syndrome because they develop focal and segmental glomerulosclerosis (Nakamura et al., 1988; Tsukaguchi et al., 2000). Two studies found no significant BPF effects on kidney weight in adult female SD rats and in female SD rats exposed gestationally (Ijaz et al., 2020; Kaimal et al., 2021). Increased kidney weight after BPF exposure was found in a subacute oral toxicity study at 100 and 500 mg BPF/kg in adult female SD rats (Higashihara et al., 2007). Urinary BPF levels in NHANES data from 2013 to 2016 indicate a significant relationship between increased BPF and decreased eGPF (Moreno-Gómez-Toledano, 2022). Our analysis showed that BPF-exposed BUF females displayed glomerular hypertrophy without tubule damage. Glomerular hypertrophy is an early phase in glomerulosclerosis development (Fogo and Ichikawa, 1991; Zamami et al., 2021), and the BUF rat kidney phenotype is preceded by renal macrophage activation and Th2 polarization (Le Berre et al., 2005). Additional studies will be conducted to assess immune infiltration and function of the BUF female kidney after BPF exposure.
Adrenal gland weight was significantly increased in WKY males after 10 weeks of BPF exposure. We did not detect differences in urinary or fecal sodium and potassium that would suggest a change in aldosterone nor was aldosterone synthase gene expression changed. There was no difference in urinary corticosterone levels in the BPF WKY males nor were corticosterone synthesis gene expression changed compared with vehicle WKY males. These results suggest that basal levels of adrenocortical hormones were unaffected by BPF exposure in WKY males. To our knowledge, this is the first report of BPF exposure altering adrenal gland weight.
All members of the Nr4a transcription factor subfamily were significantly downregulated by 40%–50% in the BPF-exposed WKY males. These transcription factors are critical regulators in the adrenal gland that control steroidogenesis (Kurakula et al., 2014) and are immediate early genes that rapidly change expression in response to external stimuli (Helbling et al., 2014). Decreased Nr4a gene expression may indicate that WKY males do not respond normally to stimuli, suggesting that BPF exposure may alter HPA axis response in WKY males. Support for this hypothesis can be found in the increased plasma ACTH and corticosterone in BPF-exposed WKY males after carbon dioxide euthanasia. These results may indicate that the BPF exposure increases the maximal secretory capacity of ACTH from the pituitary in WKY males. This was reflected in the increased corticosterone response to a supraphysiological increase in ACTH as the adrenal cortex integrates the ACTH signal from the anterior pituitary (Keller-Wood et al., 1983). An increase in maximal adrenal secretory capacity in response to ACTH as reflected in our studies may indicate a clinically significant phenomenon (Raff et al., 2014). Cognitive functions and behaviors that may indicate development of anxiety and depression have been noted after BPF exposure in gestationally exposed C57BL/6NCrSlc mice and adult male zebrafish (Kim et al., 2022; Ohtani et al., 2017). The WKY rat strain is known for its HPA axis abnormalities and depression-like behaviors, including greater immobility time in the forced swim test and avoidant behavior in social interactions (Malkesman et al., 2006; Redei et al., 2022; Rittenhouse et al., 2002). Future investigations into BPF exposure in WKY rats will incorporate behavioral assessments.
Surprisingly, there were no significant BPF effects on body weight or adiposity, the primary outcomes in our post-wean BPF exposure study in male HS rats (Wagner et al., 2021). The genetic background of the selected rat strains may be responsible for the lack of body weight and adiposity effects, a potential experimental design limitation. Of note, we were able to study six of the eight HS founder strains; the MR and WN HS rat founder strains were not studied as they are still being cryoresuscitated or are extinct, respectively. Genetic variation can occur even within an inbred strain due to incomplete inbreeding and genetic drift (Hermsen et al., 2015). The ACI, BN, F344, M520, and WKY substrains used here share 97.1%–99.9% genetic identity to the HS rat founders (RGD, 2023). The high genetic similarities between the studied substrains and the HS rat founder substrains reduce the odds that the reported BPF exposure outcomes are unique to these substrains. BUF/MnaMcwi is the most divergent substrain studied in this project and shares 73.6% genetic identity with the HS rat founder BUF/N (RGD, 2023). It is possible that BUF/MnaMcwi contains genetic loci affecting BPF exposure outcomes. The HS rat founders may individually harbor BPF exposure risk alleles for increased body weight and adiposity. It is likely that the body weight and adiposity effects were only observed in the HS rats because a mixture of HS rat founder-specific risk alleles is needed for trait development and is only available within the HS rat population.
In this study, we found that 10 weeks of post-wean BPF exposure in six inbred rat strains affected a diverse range of endocrine and metabolic measures. We report that BPF has the endocrine-disrupting potential to affect nearly all the major endocrine axes (adrenal, testes, thymus, thyroid) using a single exposure dose. The final exposure outcome was tied to the genetic make-up and sex of the exposed individual. Synthesizing the results reveals that all the major BPF exposure outcomes occurred in organs from strains with documented organ system dysfunctions, positing the working hypothesis that BPF exposure intensifies existing organ system dysfunction. Importantly, these results underscore our premise that common preclinical animal models can conceal or exaggerate the effects of BPF. This work further supports BPF exposure as an endocrine and metabolic risk factor and indicates that the HS rat will be a useful model for dissecting gene EDC interactions on health.
Supplementary Material
Acknowledgments
The authors thank Mr. Jonathan Phillips of the Raff Lab for performing the plasma testosterone assay. They thank all members of the Medical College of Wisconsin Children’s Research Institute Histology Core and Imaging Core for supporting this research as well as the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. They would also like to acknowledge Curt D. Sigmund, PhD, and Leah C. Solberg Woods, PhD, for helpful discussion.
Contributor Information
Valerie A Wagner, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
Katie L Holl, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
Karen C Clark, Cardiovascular Center, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
John J Reho, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Comprehensive Rodent Metabolic Phenotyping Core, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
Melinda R Dwinell, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Cardiovascular Center, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Rat Genome Database, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
Hans-Joachim Lehmler, Department of Occupational and Environmental Health, University of Iowa, Iowa City, Iowa 52246, USA.
Hershel Raff, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Cardiovascular Center, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Endocrine Research Laboratory, Aurora St. Luke’s Medical Center, Advocate Aurora Research Institute, Milwaukee, Wisconsin 53233, USA.
Justin L Grobe, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Cardiovascular Center, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Comprehensive Rodent Metabolic Phenotyping Core, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Department of Biomedical Engineering, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
Anne E Kwitek, Department of Physiology, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Cardiovascular Center, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Rat Genome Database, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA; Department of Biomedical Engineering, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, USA.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Funding
Our research project and scientific team were supported through funding from the University of Iowa Environmental Health Sciences Research Center (NIH P30ES005605), the National Institutes of Health Predoctoral Training Grant (T32GM008629), other National Institutes of Health grants (R01 HL134850, R01 DK133121, P01 HL084207, R01 HL064541, U24 HG010859, P01 HL149620, U24 HG010423), the Hybrid Rat Diversity Panel program (R24OD024617), and the Medical College of Wisconsin. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD Grant (R24HD102061).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abuelhija M., Weng C. C., Shetty G., Meistrich M. L. (2012). Differences in radiation sensitivity of recovery of spermatogenesis between rat strains. Toxicol. Sci. 126, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker A. M., Ferguson K. K., Rosario Z. Y., Mukherjee B., Alshawabkeh A. N., Calafat A. M., Cordero J. F., Meeker J. D. (2019). A repeated measures study of phenol, paraben and triclocarban urinary biomarkers and circulating maternal hormones during gestation in the puerto rico protect cohort. Environ. Health 18, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Kannan K., Tan H., Zheng Z., Feng Y. L., Wu Y., Widelka M. (2016). Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—a review. Environ. Sci. Technol. 50, 5438–5453. [DOI] [PubMed] [Google Scholar]
- Chen H., Li J., Zhang Y., Zhang W., Li X., Tang H., Liu Y., Li T., He H., Du B., et al. (2022a). Bisphenol F suppresses insulin-stimulated glucose metabolism in adipocytes by inhibiting IRS-1/PI3K/AKT pathway. Ecotoxicol. Environ. Saf. 231, 113201. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang Y., Li X., Zhang W., He H., Du B., Li T., Tang H., Liu Y., Li L., et al. (2022b). Transcriptome changes and potential immunotoxicity analysis in raw264.7 macrophages caused by bisphenol F. Front. Pharmacol. 13, 846562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitre A. S., Polesskaya O., Holl K., Gao J., Cheng R., Bimschleger H., Garcia Martinez A., George T., Gileta A. F., Han W., et al. (2020). Genome-wide association study in 3,173 outbred rats identifies multiple loci for body weight, adiposity, and fasting glucose. Obesity (Silver Spring) 28, 1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. Y., Lee J., Huh D. A., Moon K. W. (2022). Urinary bisphenol concentrations and its association with metabolic disorders in the US and Korean populations. Environ. Pollut. 295, 118679. [DOI] [PubMed] [Google Scholar]
- Cortés J. M. R., Zerón H. M. (2019). Genetics of thyroid disorders. Folia Med. (Plovdiv) 61, 172–179. [DOI] [PubMed] [Google Scholar]
- Derakhshan A., Shu H., Peeters R. P., Kortenkamp A., Lindh C. H., Demeneix B., Bornehag C. G., Korevaar T. I. M. (2019). Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ. Int. 133, 105123. [DOI] [PubMed] [Google Scholar]
- Ding Z. M., Chen Y. W., Ahmad M. J., Wang Y. S., Yang S. J., Duan Z. Q., Liu M., Yang C. X., Liang A. X., Hua G. H., et al. (2022). Bisphenol F exposure affects mouse oocyte in vitro maturation through inducing oxidative stress and DNA damage. Environ. Toxicol. 37, 1413–1422. [DOI] [PubMed] [Google Scholar]
- Dodson R. E., Boronow K. E., Susmann H., Udesky J. O., Rodgers K. M., Weller D., Woudneh M., Brody J. G., Rudel R. A. (2020). Consumer behavior and exposure to parabens, bisphenols, triclosan, dichlorophenols, and benzophenone-3: Results from a crowdsourced biomonitoring study. Int. J. Hyg. Environ. Health 230, 113624. [DOI] [PubMed] [Google Scholar]
- Dualde P., Pardo O., Corpas-Burgos F., Kuligowski J., Gormaz M., Vento M., Pastor A., Yusa V. (2019). Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed infants. Sci. Total Environ. 668, 797–805. [DOI] [PubMed] [Google Scholar]
- Emi Y., Ikushiro S., Kato Y. (2007). Thyroxine-metabolizing rat uridine diphosphate-glucuronosyltransferase 1a7 is regulated by thyroid hormone receptor. Endocrinology 148, 6124–6133. [DOI] [PubMed] [Google Scholar]
- Engdahl E., Svensson K., Lin P. D., Alavian-Ghavanini A., Lindh C., Rüegg J., Bornehag C. G. (2021). DNA methylation at GRIN2B partially mediates the association between prenatal bisphenol F exposure and cognitive functions in 7-year-old children in the SELMA study. Environ. Int. 156, 106617. [DOI] [PubMed] [Google Scholar]
- Fatai O. A., Aribidesi O. L. (2022). Effect of bisphenol F on sexual performance and quality of offspring in male Wistar rats. Ecotoxicol. Environ. Saf. 244, 114079. [DOI] [PubMed] [Google Scholar]
- Festing M. F. (1993). Genetic variation in outbred rats and mice and its implications for toxicological screening. J. Exp. Anim. Sci. 35, 210–220. [PubMed] [Google Scholar]
- Fogo A., Ichikawa I. (1991). Evidence for a pathogenic linkage between glomerular hypertrophy and sclerosis. Am. J. Kidney Dis. 17, 666–669. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Onodera H., Mitsumori K., Tamura T., Maruyama S., Ito A. (1999). Changes in thyroid function during development of thyroid hyperplasia induced by kojic acid in F344 rats. Carcinogenesis 20, 1567–1571. [DOI] [PubMed] [Google Scholar]
- Gatti D., French J. E., Schughart K. (2017). QTL mapping and identification of candidate genes in DO mice: A use case model derived from a benzene toxicity experiment. Methods Mol. Biol. 1488, 265–281. [DOI] [PubMed] [Google Scholar]
- Gong D. W., Murayama N., Yamazoe Y., Kato R. (1992). Hepatic triiodothyronine sulfation and its regulation by growth hormone and triiodothyronine in rats. J. Biochem. 112, 112–116. [DOI] [PubMed] [Google Scholar]
- Gore A. C., Chappell V. A., Fenton S. E., Flaws J. A., Nadal A., Prins G. S., Toppari J., Zoeller R. T. (2015). EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe J. L. (2017). Comprehensive assessments of energy balance in mice. Methods Mol. Biol. (Clifton, NJ). 1614, 123–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald D. A., Naai M. A., Hess D. L., Matsumoto A. M. (1994). The brown Norway rat as a model of male reproductive aging: Evidence for both primary and secondary testicular failure. J. Gerontol. 49, B42–B50. [DOI] [PubMed] [Google Scholar]
- Gu J., Li L., Yin X., Liang M., Zhu Y., Guo M., Zhou L., Fan D., Shi L., Ji G. (2022). Long-term exposure of zebrafish to bisphenol F: Adverse effects on parental reproduction and offspring neurodevelopment. Aquat. Toxicol. 248, 106190. [DOI] [PubMed] [Google Scholar]
- Gunturkun M. H., Wang T., Chitre A. S., Garcia Martinez A., Holl K., St Pierre C., Bimschleger H., Gao J., Cheng R., Polesskaya O., et al. (2022). Genome-wide association study on three behaviors tested in an open field in heterogeneous stock rats identifies multiple loci implicated in psychiatric disorders. Front. Psychiatry 13, 790566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C., Spuhler K. (1984). Development of the national institutes of health genetically heterogeneous rat stock. Alcohol. Clin. Exp. Res. 8, 477–479. [DOI] [PubMed] [Google Scholar]
- Heindel J. J., Howard S., Agay-Shay K., Arrebola J. P., Audouze K., Babin P. J., Barouki R., Bansal A., Blanc E., Cave M. C., et al. (2022). Obesity II: Establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 199, 115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling J. C., Minni A. M., Pallet V., Moisan M. P. (2014). Stress and glucocorticoid regulation of NR4A genes in mice. J. Neurosci. Res. 92, 825–834. [DOI] [PubMed] [Google Scholar]
- Hermsen R., de Ligt J., Spee W., Blokzijl F., Schafer S., Adami E., Boymans S., Flink S., van Boxtel R., van der Weide R. H., et al. (2015). Genomic landscape of rat strain and substrain variation. BMC Genomics 16, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashihara N., Shiraishi K., Miyata K., Oshima Y., Minobe Y., Yamasaki K. (2007). Subacute oral toxicity study of bisphenol F based on the draft protocol for the “enhanced OECD test guideline no. 407”. Arch. Toxicol. 81, 825–832. [DOI] [PubMed] [Google Scholar]
- Hong-Le T., Crouse W. L., Keele G. R., Holl K., Seshie O., Tschannen M., Craddock A., Das S. K., Szalanczy A. M., McDonald B., et al. (2023). Genetic mapping of multiple traits identifies novel genes for adiposity, lipids, and insulin secretory capacity in outbred rats. Diabetes 72, 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185. [DOI] [PubMed] [Google Scholar]
- Huang G. M., Tian X. F., Fang X. D., Ji F. J. (2016). Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 147, 188–194. [DOI] [PubMed] [Google Scholar]
- Huang H., Liang J., Tang P., Yu C., Fan H., Liao Q., Long J., Pan D., Zeng X., Liu S., et al. (2022). Associations of bisphenol exposure with thyroid hormones in pregnant women: A prospective birth cohort study in China. Environ. Sci. Pollut. Res. Int. 29, 87170–87183. [DOI] [PubMed] [Google Scholar]
- Ijaz S., Ullah A., Shaheen G., Jahan S. (2020). Exposure of BPA and its alternatives like BPB, BPF, and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats. Toxicol. Mech. Methods 30, 60–72. [DOI] [PubMed] [Google Scholar]
- Jacobson M. H., Woodward M., Bao W., Liu B., Trasande L. (2019). Urinary bisphenols and obesity prevalence among U.S. children and adolescents. J. Endocr. Soc. 3, 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimal A., Al Mansi M. H., Dagher J. B., Pope C., Varghese M. G., Rudi T. B., Almond A. E., Cagle L. A., Beyene H. K., Bradford W. T., et al. (2021). Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats. Chemosphere 276, 130118. [DOI] [PubMed] [Google Scholar]
- Keele G. R., Prokop J. W., He H., Holl K., Littrell J., Deal A., Francic S., Cui L., Gatti D. M., Broman K. W., et al. (2018). Genetic fine-mapping and identification of candidate genes and variants for adiposity traits in outbred rats. Obesity (Silver Spring) 26, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele G. R., Prokop J. W., He H., Holl K., Littrell J., Deal A. W., Kim Y., Kyle P. B., Attipoe E., Johnson A. C., et al. (2021). Sept8/SEPTIN8 involvement in cellular structure and kidney damage is identified by genetic mapping and a novel human tubule hypoxic model. Sci. Rep. 11, 2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M. E., Shinsako J., Dallman M. F. (1983). Integral as well as proportional adrenal responses to ACTH. Am. J. Physiol. 245, R53–R59. [DOI] [PubMed] [Google Scholar]
- Kester M. H., Kaptein E., Roest T. J., van Dijk C. H., Tibboel D., Meinl W., Glatt H., Coughtrie M. W., Visser T. J. (2003). Characterization of rat iodothyronine sulfotransferases. Am. J. Physiol. Endocrinol. Metab. 285, E592–E598. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Lee M. R., Hong Y. C. (2016). Modification of the association of bisphenol A with abnormal liver function by polymorphisms of oxidative stress-related genes. Environ. Res. 147, 324–330. [DOI] [PubMed] [Google Scholar]
- Kim S. S., Kim J. L., Hwang K. S., Park H. C., Bae M. A., Kim K. T., Cho S. H. (2022). Mechanism of action and neurotoxic effects of chronic exposure to bisphenol F in adult zebrafish. Sci. Total Environ. 851, 158258. [DOI] [PubMed] [Google Scholar]
- Kronzer V. L., Bridges S. L. Jr, Davis I. J. (2021). Why women have more autoimmune diseases than men: An evolutionary perspective. Evol. Appl. 14, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakula K., Koenis D. S., van Tiel C. M., de Vries C. J. (2014). Nr4a nuclear receptors are orphans but not lonesome. Biochim. Biophys. Acta 1843, 2543–2555. [DOI] [PubMed] [Google Scholar]
- Lahat N., Hirose W., Davies T. F. (1989). Enhanced induction of thyroid cell MHC class ii antigen expression in rats highly responsive to thyroglobulin. Endocrinology 124, 1754–1759. [DOI] [PubMed] [Google Scholar]
- Le Berre L., Hervé C., Buzelin F., Usal C., Soulillou J. P., Dantal J. (2005). Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in buffalo/Mna rats. Kidney Int. 68, 2079–2090. [DOI] [PubMed] [Google Scholar]
- Lee S., An K. S., Kim H. J., Noh H. J., Lee J., Lee J., Song K. S., Chae C., Ryu H. Y. (2022). Pharmacokinetics and toxicity evaluation following oral exposure to bisphenol F. Arch. Toxicol. 96, 1711–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kim C., Shin H., Kho Y., Choi K. (2019). Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 221, 115–123. [DOI] [PubMed] [Google Scholar]
- Lehmler H. J., Liu B., Gadogbe M., Bao W. (2018). Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. Adults and children: The national health and nutrition examination survey 2013–2014. ACS Omega 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejonklou M. H., Dunder L., Bladin E., Pettersson V., Ronn M., Lind L., Walden T. B., Lind P. M. (2017). Effects of low-dose developmental bisphenol A exposure on metabolic parameters and gene expression in male and female Fischer 344 rat offspring. Environ. Health Perspect. 125, 067018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li J., Shi L., Zhu Y., Tian F., Shi M., Li Q., Ge R. S. (2022). Bisphenol F blocks Leydig cell maturation and steroidogenesis in pubertal male rats through suppressing androgen receptor signaling and activating G-protein coupled estrogen receptor 1 (GPER1) signaling. Food Chem. Toxicol. 167, 113268. [DOI] [PubMed] [Google Scholar]
- Liao C., Kannan K. (2013). Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 61, 4655–4662. [DOI] [PubMed] [Google Scholar]
- Liu B., Lehmler H. J., Sun Y., Xu G., Sun Q., Snetselaar L. G., Wallace R. B., Bao W. (2019). Association of bisphenol A and its substitutes, bisphenol F and bisphenol S, with obesity in United States children and adolescents. Diabetes Metab. J. 43, 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Chen S., Jin H., Tang L., Xia M. (2022). Associations of bisphenol F and S, as substitutes for bisphenol A, with cardiovascular disease in American adults. J. Appl. Toxicol. 43, 500–507. [DOI] [PubMed] [Google Scholar]
- Lucki N. C., Bandyopadhyay S., Wang E., Merrill A. H., Sewer M. B. (2012). Acid ceramidase (ASAH1) is a global regulator of steroidogenic capacity and adrenocortical gene expression. Mol. Endocrinol. 26, 228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisé Y., Le Mentec H., Sparfel L., Guzylack-Piriou L. (2020a). Differential influences of the BPA, BPS and BPF on in vitro IL-17 secretion by mouse and human T cells. Toxicol. In Vitro 69, 104993. [DOI] [PubMed] [Google Scholar]
- Malaisé Y., Lencina C., Cartier C., Olier M., Ménard S., Guzylack-Piriou L. (2020b). Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice. Environ. Health 19, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O., Maayan R., Weizman A., Weller A. (2006). Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav. Brain Res. 175, 408–414. [DOI] [PubMed] [Google Scholar]
- Manna P. R., Dyson M. T., Stocco D. M. (2009). Regulation of the steroidogenic acute regulatory protein gene expression: Present and future perspectives. Mol. Hum. Reprod. 15, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukyan L., Dunder L., Lind P. M., Bergsten P., Lejonklou M. H. (2019). Developmental exposure to a very low dose of bisphenol A induces persistent islet insulin hypersecretion in Fischer 344 rat offspring. Environ. Res. 172, 127–136. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Yamada C., Hiai H. (1986). A single dominant susceptible gene determines spontaneous development of thymoma in BUF/Mna rat. Jpn. J. Cancer Res. 77, 1066–1068. [PubMed] [Google Scholar]
- Mendy A., Salo P. M., Wilkerson J., Feinstein L., Ferguson K. K., Fessler M. B., Thorne P. S., Zeldin D. C. (2020). Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environ. Res. 183, 108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z., Tian S., Yan J., Jia M., Yan S., Li R., Zhang R., Zhu W., Zhou Z. (2019). Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut. 247, 935–943. [DOI] [PubMed] [Google Scholar]
- Michałowicz J., Mokra K., Bąk A. (2015). Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol. In Vitro 29, 1464–1472. [DOI] [PubMed] [Google Scholar]
- Moreno-Gómez-Toledano R. (2022). Relationship between emergent BPA-substitutes and renal and cardiovascular diseases in adult population. Environ. Pollut. 313, 120106. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J., Brown R. E. (2006). Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Matsuyama M., Kojima A., Ogiu T., Kubota A., Suzuki Y., Arakawa M., Shimizu F. (1988). The effect of thymectomy on the development of nephropathy in spontaneous thymoma rats of the BUF/Mna strain. Clin. Exp. Immunol. 71, 350–352. [PMC free article] [PubMed] [Google Scholar]
- Noble B., Yoshida T., Rose N. R., Bigazzi P. E. (1976). Thyroid antibodies in spontaneous autoimmune thyroiditis in the buffalo rat. J. Immunol. 117, 1447–1455. [PubMed] [Google Scholar]
- Ohtani N., Iwano H., Suda K., Tsuji E., Tanemura K., Inoue H., Yokota H. (2017). Adverse effects of maternal exposure to bisphenol F on the anxiety- and depression-like behavior of offspring. J. Vet. Med. Sci. 79, 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. C., Philip V. M., Gatti D. M., Kasparek S., Kreuzman A. M., Kuffler L., Mansky B., Masneuf S., Sharif K., Sluys E., et al. (2022). Genome-wide association mapping of ethanol sensitivity in the diversity outbred mouse population. Alcohol. Clin. Exp. Res. 46, 941–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. (2004). Quantification strategies in real-time PCR. In: The Real-Time Pcr Encyclopedia a-z of Quantitative Pcr (Bustin S., Ed.), pp. 87–112. International University Line, La Jolla, CA. [Google Scholar]
- Raff H., , GlaeserB. L., , SzaboA., , OlsenC. M., and , Everson C. A. (2023). Sleep restriction during opioid abstinence affects the hypothalamic-pituitary-adrenal (HPA) axis in male and female rats. Stress 26, 2185864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff H., Hoeynck B., Jablonski M., Leonovicz C., Phillips J. M., Gehrand A. L. (2018). Insulin sensitivity, leptin, adiponectin, resistin, and testosterone in adult male and female rats after maternal–neonatal separation and environmental stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R12–R21. [DOI] [PubMed] [Google Scholar]
- Raff H., Sharma S. T., Nieman L. K. (2014). Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr. Physiol. 4, 739–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E. E., Udell M. E., Solberg-Woods L. C., Chen H. (2022). The Wistar Kyoto rat: A model of depression traits. Curr. Neuropharmacol. 21, 1884–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regner K. R., Nozu K., Lanier S. M., Blumer J. B., Avner E. D., Sweeney W. E. Jr,, Park F. (2011). Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB J. 25, 1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reho J. J., Nakagawa P., Mouradian G. C., Grobe C. C., Saravia F. L., Burnett C. M. L., Kwitek A. E., Kirby J. R., Segar J. L., Hodges M. R., et al. (2022). Methods for the comprehensive in vivo analysis of energy flux, fluid homeostasis, blood pressure, and ventilatory function in rodents. Front. Physiol. 13, 855054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RGD. (2023). Genetic similarity between strains in the HRDP panel and the original HS founder strains. Rat Genome Database, Medical College of Wisconsin. Accessed March 30, 2023. https://rgd.mcw.edu/wg/hrdp_panel/hrdp-to-hs-founder-strain-genetic-similarity/.
- Richardson T. A., Klaassen C. D. (2010). Role of UDP-glucuronosyltransferase (UGT) 2b2 in metabolism of triiodothyronine: Effect of microsomal enzyme inducers in Sprague Dawley and ugt 2b2-deficient Fischer 344 rats. Toxicol. Sci. 116, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse P. A., López-Rubalcava C., Stanwood G. D., Lucki I. (2002). Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology 27, 303–318. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C. S. (2017). Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 47, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar K., Beck A., Bihoreau M. T., Birney E., Brocklebank D., Chen Y., Cuppen E., Demonchy S., Dopazo J., Flicek P., et al. (2008). SNP and haplotype mapping for genetic analysis in the rat. Nat. Genet. 40, 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanas N. B., Commers T. W., Dennison K. L., Harenda Q. E., Kurz S. G., Lachel C. M., Wavrin K. L., Bowler M., Nijman I. J., Guryev V., et al. (2015). Genetic etiology of renal agenesis: Fine mapping of Renag1 and identification of kit as the candidate functional gene. PLoS ONE. 10, e0118147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P. (2013). The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 4, 624–630. [PMC free article] [PubMed] [Google Scholar]
- Sol C. M., Santos S., Duijts L., Asimakopoulos A. G., Martinez-Moral M. P., Kannan K., Jaddoe V. W. V., Trasande L. (2020). Fetal phthalates and bisphenols and childhood lipid and glucose metabolism. A population-based prospective cohort study. Environ. Int. 144, 106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solleveld H. A., Boorman G. A. (1986). Spontaneous renal lesions in five rat strains. Toxicol. Pathol. 14, 168–174. [DOI] [PubMed] [Google Scholar]
- Stübner D., Gärtner R., Greil W., Gropper K., Brabant G., Permanetter W., Horn K., Pickardt C. R. (1987). Hypertrophy and hyperplasia during goitre growth and involution in rats–separate bioeffects of TSH and iodine. Acta Endocrinol. (Copenh.) 116, 537–548. [DOI] [PubMed] [Google Scholar]
- Syed V., Hecht N. B. (2001). Selective loss of Sertoli cell and germ cell function leads to a disruption in Sertoli cell-germ cell communication during aging in the brown Norway rat. Biol. Reprod. 64, 107–112. [DOI] [PubMed] [Google Scholar]
- Tchen R., Tan Y., Boyd Barr D., Barry Ryan P., Tran V., Li Z., Hu Y. J., Smith A. K., Jones D. P., Dunlop A. L., et al. (2022). Use of high-resolution metabolomics to assess the biological perturbations associated with maternal exposure to bisphenol A and bisphenol F among pregnant African American women. Environ. Int. 169, 107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze J., Bauer K., Schafflhuber M., Dietsch P., Lang R., Schwind K. H., Luft F. C., Eckardt K. U., Hilgers K. F. (2005). Internal sodium balance in DOCA-salt rats: A body composition study. Am. J. Physiol. Renal Physiol. 289, F793–F802. [DOI] [PubMed] [Google Scholar]
- Tkalec Ž., Kosjek T., Snoj Tratnik J., Stajnko A., Runkel A. A., Sykiotou M., Mazej D., Horvat M. (2021). Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: Urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ. Int. 146, 106172. [DOI] [PubMed] [Google Scholar]
- Tovar A., Smith G. J., Nalesnik M. B., Thomas J. M., McFadden K. M., Harkema J. R., Kelada S. N. P. (2022). A locus on chromosome 15 contributes to acute ozone-induced lung injury in collaborative cross mice. Am. J. Respir. Cell Mol. Biol. 67, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaih S. W., Holl K., Jia S., Kaldunski M., Tschannen M., He H., Andrae J. W., Li S. H., Stoddard A., Wiederhold A., et al. (2014). Identification of a novel gene for diabetic traits in rats, mice, and humans. Genetics 198, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaguchi H., Yager H., Dawborn J., Jost L., Cohlmia J., Abreu P. F., Pereira A. B., Pollak M. R. (2000). A locus for adolescent and adult onset familial focal segmental glomerulosclerosis on chromosome 1q25-31. J. Am. Soc. Nephrol. 11, 1674–1680. [DOI] [PubMed] [Google Scholar]
- Ullah A., Pirzada M., Jahan S., Ullah H., Turi N., Ullah W., Siddiqui M. F., Zakria M., Lodhi K. Z., Khan M. M. (2018). Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo–pituitary–testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 121, 24–36. [DOI] [PubMed] [Google Scholar]
- USEPA. (2010). Bisphenol A Action Plan (CASRN 80-05-7). Accessed June 1, 2019. https://www.epa.gov/sites/production/files/2015-09/documents/bpa_action_plan.pdf.
- USEPA. (2011). E.P.A. Recommended use of body weight 3/4 as the default method in derivation of the oral reference dose. Risk Assessment Forum No. EPA/100/R11/0001.
- Vandenberg L. N. (2014). Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol A as a case study. Dose Response 12, 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner V. A., Clark K. C., Carrillo-Sáenz L., Holl K. A., Velez-Bermudez M., Simonsen D., Grobe J. L., Wang K., Thurman A., Solberg Woods L. C., et al. (2021). Bisphenol F exposure in adolescent heterogeneous stock rats affects growth and adiposity. Toxicol. Sci. 181, 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. (1949). New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L. C., Mott R. (2017). Heterogeneous stock populations for analysis of complex traits. Methods Mol. Biol. 1488, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Yang X., Liu J., Ren W., Chen Y., Shen S. (2017). Effects of BPF on steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish. Environ. Sci. Pollut. Res. Int. 24, 21311–21322. [DOI] [PubMed] [Google Scholar]
- Ye X., Wong L. Y., Kramer J., Zhou X., Jia T., Calafat A. M. (2015). Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ. Sci. Technol. 49, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamami R., Kohagura K., Kinjyo K., Nakamura T., Kinjo T., Yamazato M., Ishida A., Ohya Y. (2021). The association between glomerular diameter and secondary focal segmental glomerulosclerosis in chronic kidney disease. Kidney Blood Press. Res. 46, 433–440. [DOI] [PubMed] [Google Scholar]
- Zeng J. Y., Chen P. P., Liu C., Deng Y. L., Miao Y., Zhang M., Cui F. P., Lu T. T., Shi T., Yang K. D., et al. (2022). Bisphenol A analogues in associations with serum hormone levels among reproductive-aged Chinese men. Environ. Int. 167, 107446. [DOI] [PubMed] [Google Scholar]
- Zhang C., Luo Y., Qiu S., Huang X., Jin K., Li J., Yang M., Hu D., Zheng X., Jiang Z., et al. (2022). Associations between urinary concentrations of bisphenols and serum concentrations of sex hormones among US males. Environ. Health 21, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Tang Z., Xie P., Lin K., Chung A. C. K., Cai Z. (2019). Immunotoxic potential of bisphenol F mediated through lipid signaling pathways on macrophages. Environ. Sci. Technol. 53, 11420–11428. [DOI] [PubMed] [Google Scholar]
- Zhong R., He H., Jin M., Lu Z., Deng Y., Liu C., Shen N., Li J., Wang H., Ying P., et al. (2022). Genome-wide gene–bisphenol A, F and triclosan interaction analyses on urinary oxidative stress markers. Sci. Total Environ. 807, 150753. [DOI] [PubMed] [Google Scholar]
- Zhou R., Zhang L., Yan J., Sun Y., Jiang H. (2022). Association of sleep problems with urinary concentrations of personal care and consumer product chemicals: A nationally representative, population-based study. Environ. Sci. Pollut. Res. 30, 14533–14544. [DOI] [PubMed] [Google Scholar]
- Zhu M., Chen X. Y., Li Y. Y., Yin N. Y., Faiola F., Qin Z. F., Wei W. J. (2018). Bisphenol F disrupts thyroid hormone signaling and postembryonic development in Xenopus laevis. Environ. Sci. Technol. 52, 1602–1611. [DOI] [PubMed] [Google Scholar]
- Zmora N., Bashiardes S., Levy M., Elinav E. (2017). The role of the immune system in metabolic health and disease. Cell Metab. 25, 506–521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.