Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned coprimary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
Initial results from the phase III JAVELIN Bladder 100 trial (ClinicalTrials.gov identifier: NCT02603432) showed that avelumab first-line (1L) maintenance plus best supportive care (BSC) significantly prolonged overall survival (OS) and progression-free survival (PFS) versus BSC alone in patients with advanced urothelial carcinoma (aUC) who were progression-free after 1L platinum-containing chemotherapy. Avelumab 1L maintenance treatment is now a standard of care for aUC. Here, we report updated data with ≥ 2 years of follow-up in all patients, including OS (primary end point), PFS, safety, and additional novel analyses. Patients were randomly assigned 1:1 to receive avelumab plus BSC (n = 350) or BSC alone (n = 350). At data cutoff (June 4, 2021), median follow-up was 38.0 months and 39.6 months, respectively; 67 patients (19.5%) had received ≥2 years of avelumab treatment. OS remained longer with avelumab plus BSC versus BSC alone in all patients (hazard ratio, 0.76 [95% CI, 0.63 to 0.91]; 2-sided P = .0036). Investigator-assessed PFS analyses also favored avelumab. Longer-term safety was consistent with previous analyses; no new safety signals were identified with longer treatment duration. In conclusion, longer-term follow-up continues to show clinically meaningful efficacy benefits with avelumab 1L maintenance plus BSC versus BSC alone in patients with aUC. An interactive visualization of data reported in this article is available.
INTRODUCTION
In the phase III JAVELIN Bladder 100 trial, avelumab first-line (1L) maintenance plus best supportive care (BSC) significantly prolonged overall survival (OS) and progression-free survival (PFS) versus BSC alone in patients with advanced urothelial carcinoma (aUC) who were progression-free after 1L platinum-containing chemotherapy.1 The results led to the approval of avelumab 1L maintenance in various countries and its inclusion as standard of care in international treatment guidelines with level 1 evidence.2-4 We report updated trial data with ≥2 years of follow-up in all patients (19.5 additional months from the initial analysis).
METHODS
Study Design
The design of JAVELIN Bladder 100 (ClinicalTrials.gov identifier: NCT02603432) has been described previously.1 Eligible patients had locally advanced or metastatic urothelial carcinoma and were progression-free after 4-6 cycles of 1L chemotherapy (cisplatin and/or carboplatin plus gemcitabine). After a 4-10–week interval from last chemotherapy dose, patients were randomly assigned 1:1 to receive avelumab plus BSC (avelumab arm) or BSC alone (control arm), stratified by visceral/nonvisceral metastatic disease site at chemotherapy initiation and response/stable disease with chemotherapy. Treatment continued until patient withdrawal, confirmed progression, unacceptable toxicity, or other criteria for discontinuation occurred.
End Points
The primary end point was OS, assessed from random assignment in the overall and PD-L1+ (Ventana SP263 assay) populations. Secondary end points included PFS and objective response per RECIST version 1.1 by investigator assessment and safety. Statistical methodology is reported in the Data Supplement (online only). Because the trial met its objective in the initial analysis (data cutoff: October 21, 2019),1 updated analyses are considered exploratory, and all P values are descriptive.
The trial was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines, defined by the International Council of Harmonisation. All patients provided written consent. The Protocol (online only), amendments, and informed consent forms were approved by an institutional review board or independent ethics committee at each trial site.
RESULTS
Patients
Overall, 700 patients were randomly assigned to the avelumab (n = 350) or control (n = 350) arm; 189 of 328 (57.6%) and 169 of 300 (56.3%) patients evaluable for PD-L1 status had PD-L1+ tumors, respectively. Baseline characteristics were balanced between arms (Table 1).
TABLE 1.
Baseline Characteristics
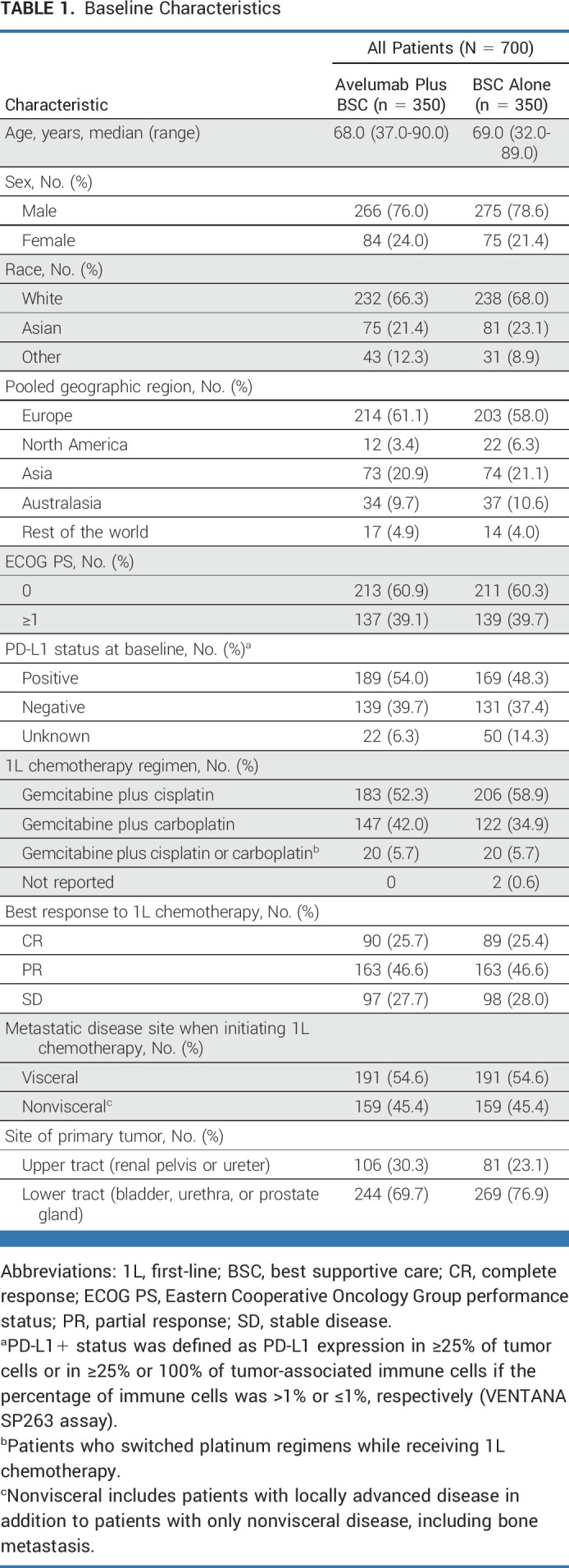
At data cutoff (June 4, 2021), the median follow-up in the avelumab and control arms was 38.0 and 39.6 months (≥2 years in all patients) and treatment was ongoing in 43 (12.3%) and 10 (2.9%) patients, respectively. Reasons for treatment discontinuation are presented in the Data Supplement. The median duration of avelumab treatment (defined as treatment exposure until data cutoff without adjustment for ongoing treatment/censoring) was 5.8 months (range, 0.5-49.7); 67 patients (19.5%) received ≥2 years of avelumab treatment.
Efficacy
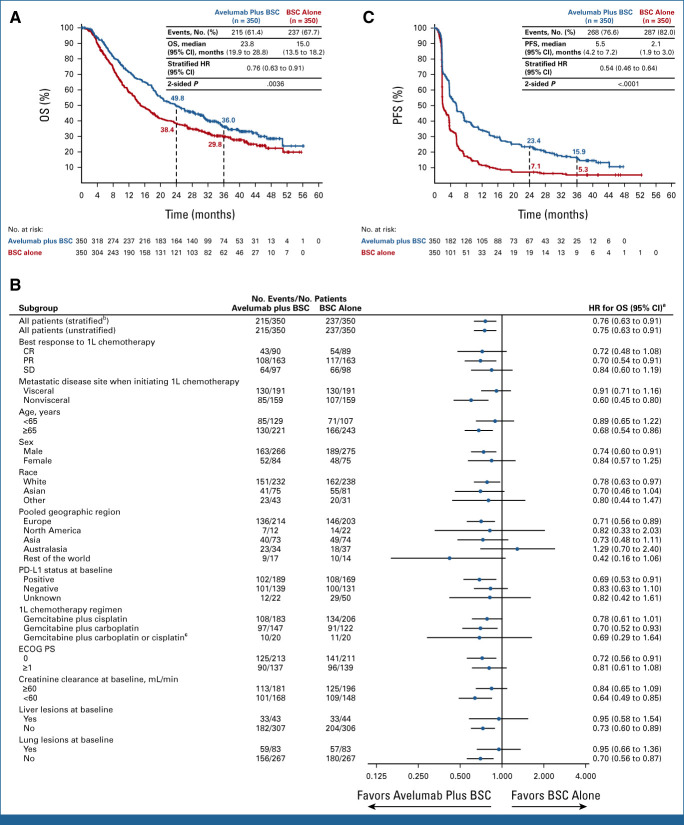
In the overall population, OS was prolonged with avelumab versus control (Fig 1A); the median OS was 23.8 months (95% CI, 19.9 to 28.8) versus 15.0 months (95% CI, 13.5 to 18.2), respectively (hazard ratio [HR], 0.76 [95% CI, 0.63 to 0.91]; 2-sided P = .0036); 2-year OS rates were 49.8% (95% CI, 44.3 to 55.0) versus 38.4% (95% CI, 33.2 to 43.7), respectively. OS analyses also favored avelumab across subgroups, including those defined by chemotherapy regimen and best response to chemotherapy (Fig 1B and Data Supplement). To account for variability between geographic regions, subgroup data were analyzed using an empirical Bayesian shrinkage estimator; using this method, HRs for geographic subgroups were similar (Data Supplement).5,6 Restricted mean survival time (prespecified analysis) showed a benefit with avelumab versus control (Data Supplement).
FIG 1.
Efficacy analyses showing (A) Kaplan-Meier estimate of OS in the overall population, (B) forest plot of OS subgroup analysis, and (C) Kaplan-Meier estimate of investigator-assessed PFS in the overall population. (B) Unless otherwise stated, all analyses are unstratified, and analyses in subgroups with unreported or unknown creatinine clearance or 1L chemotherapy regimen are not shown because of the small number of patients in these subgroups. aHRs and CIs were calculated using a Cox proportional hazards model. bStratified by best response to 1L chemotherapy (CR or PR v SD) and metastatic disease site when initiating 1L chemotherapy (visceral v nonvisceral). cPatients who switched platinum regimens while receiving 1L chemotherapy. 1L, first-line; BSC, best supportive care; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease.
Investigator-assessed PFS was also prolonged with avelumab versus control (Fig 1C and Data Supplement). The median PFS was 5.5 months (95% CI, 4.2 to 7.2) versus 2.1 months (95% CI, 1.9 to 3.0), respectively (HR, 0.54 [95% CI, 0.46 to 0.64]; 2-sided P < .0001); 2-year PFS rates were 23.4% (95% CI, 18.9 to 28.3) versus 7.1% (95% CI, 4.5 to 10.4), respectively.
Rates of investigator-assessed confirmed objective response and disease control were higher with avelumab versus control (Data Supplement). Among responders, the median duration of response was 28.4 months (95% CI, 15.9 to 42.3) in the avelumab arm (n = 50) and 26.9 months (95% CI, 4.4 to not estimable) in the control arm (n = 14). Among all randomly assigned patients, the restricted mean duration of response was 2.8 months (95% CI, 1.5 to 4.0) longer with avelumab versus control (Data Supplement).7,8
Subsequent Therapy
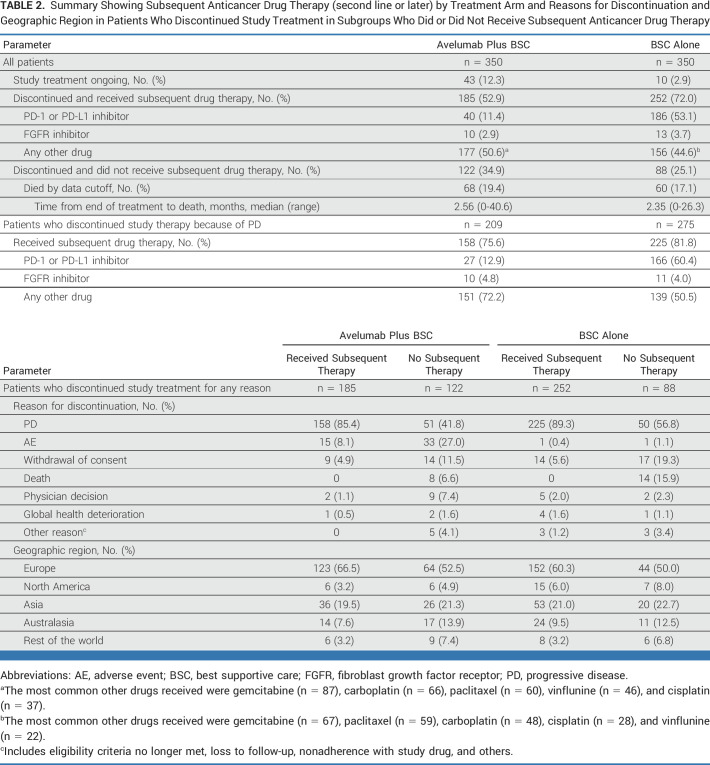
Subsequent anticancer drug therapy (second-line or later) was received by 185 patients (52.9%) in the avelumab arm and 252 (72.0%) in the control arm, including a PD-1/PD-L1 inhibitor in 40 (11.4%) and 186 (53.1%), respectively (Table 2). In patients who discontinued study therapy because of progressive disease, 158 of 209 (75.6%) in the avelumab arm versus 225 of 275 (81.8%) in the control arm received a subsequent anticancer drug therapy, including a PD-1/PD-L1 inhibitor in 27 (12.9%) versus 166 (60.4%).
TABLE 2.
Summary Showing Subsequent Anticancer Drug Therapy (second line or later) by Treatment Arm and Reasons for Discontinuation and Geographic Region in Patients Who Discontinued Study Treatment in Subgroups Who Did or Did Not Receive Subsequent Anticancer Drug Therapy
In patients with no subsequent anticancer therapy, 36 of 122 patients in the avelumab arm and 10 of 88 in the control arm were confirmed alive at data cutoff.
Safety
In avelumab-treated patients (n = 344), treatment-emergent adverse events (AEs) of any grade (treatment-related or -unrelated) occurred in 338 (98.3%), including grade ≥3 AEs in 185 (53.8%; Data Supplement). In patients with ≥12 months of avelumab treatment (n = 118), any-grade AEs occurred after ≥12 months in 102 (86.4%), including grade ≥3 AEs in 56 (47.5%). The most common AEs are shown in the Data Supplement.
In all avelumab-treated patients, any-grade treatment-related AEs (TRAEs) occurred in 269 (78.2%), including grade ≥3 TRAEs in 67 (19.5%). The most common TRAEs at the initial analysis1 and additional TRAEs with longer-term follow-up are shown in the Data Supplement. Any-grade TRAEs occurred after ≥12 months in 59 of 118 patients (50.0%), including grade ≥3 TRAEs in 14 (11.9%; Data Supplement). TRAEs occurring after ≥12 months led to discontinuation of avelumab in 12 patients (10.2%) and death in one patient (attributed to immune-mediated nephritis by investigator). Any-grade immune-related AEs occurred after ≥12 months in 27 patients (22.9%), including grade ≥3 immune-related AEs in 5 (4.2%).
Digital Dashboard
An interactive visualization of data reported in this article is available.9
DISCUSSION
Longer-term results from JAVELIN Bladder 100 continue to show prolonged OS and PFS with avelumab 1L maintenance plus BSC versus BSC alone in the overall population and across various subgroups.1 To our knowledge, JAVELIN Bladder 100 remains the only phase III trial to report significant improvement in OS in the 1L setting in patients with aUC since trials that established the efficacy of platinum-containing chemotherapy.1,10-13
OS was prolonged with avelumab despite 72.0% of patients in the control arm receiving subsequent anticancer drug therapy, including PD-1/PD-L1 inhibitors (53.1%). In real-world clinical practice, only 30%-40% of patients are able to receive second-line therapy14-17; although more patients may receive subsequent therapy in the maintenance setting, a significant proportion do not receive subsequent therapy even in studies where crossover is available.18,19 In addition, in this global trial population, anti–PD-1/PD-L1 inhibitors may not have been available for second-line therapy in some countries. The HR point estimate for OS in this longer-term analysis was closer to 1 compared with the initial analysis, which may have been influenced by subsequent therapy. However, the CIs for both analyses substantially overlap, indicating that OS benefits with avelumab 1L maintenance remained consistent with additional follow-up.
The results confirm the long-term safety profile of avelumab 1L maintenance, with 19.5% of patients receiving ≥2 years of treatment and a low overall rate of discontinuation because of TRAEs (10.2%). No new safety signals were identified. Rates of TRAEs occurring after ≥12 months were modest versus rates of overall TRAEs, and most were low grade, suggesting that long-term avelumab treatment is feasible and manageable. Previously reported patient-reported outcomes from this trial also indicated tolerability.20 This is particularly important because patients with aUC tend to be older and have associated comorbidities.21
In conclusion, longer-term results from JAVELIN Bladder 100 further support the recommendation of avelumab 1L maintenance as standard of care for patients with aUC that has not progressed with 1L platinum-containing chemotherapy, with level 1 evidence.2-4
ACKNOWLEDGMENT
The authors thank the patients and their families, the investigators, coinvestigators, and study teams at each of the participating centers.
Thomas Powles
Honoraria: AstraZeneca, Eisai, Gilead Sciences, Merck & Co (Kenilworth, NJ), Novartis, Pfizer, Roche Laboratories Inc, Astellas Pharma, BMS GmbH & Co. KG, Exelixis, Incyte, Ipsen, Seattle Genetics, the healthcare business of Merck KGaA (Darmstadt, Germany), Johnson & Johnson/Janssen, Mashup Ltd
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Ipsen, Pfizer, Novartis, Seattle Genetics, Roche, Exelixis, Merck & Co (Kenilworth, NJ), the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma, Johnson & Johnson, Eisai, Mashup Ltd, Incyte
Research Funding: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen, Merck & Co (Kenilworth, NJ), Novartis, Pfizer, Seattle Genetics, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma, Johnson & Johnson, Eisai
Travel, Accommodations, Expenses: Pfizer, Merck & Co (Kenilworth, NJ), AstraZeneca, Roche, Ipsen
Se Hoon Park
Honoraria: the healthcare business of Merck KGaA (Darmstadt, Germany), Pfizer, Ono Pharmaceutical
Consulting or Advisory Role: Janssen Oncology
Research Funding: Ono Pharmaceutical, Sanofi
Begoña P. Valderrama
Honoraria: Bristol Myers Squibb/Medarex, Roche, Ipsen, EUSA Pharma, Pfizer, Astellas Pharma, Bayer, the healthcare business of Merck KGaA (Darmstadt, Germany)
Consulting or Advisory Role: Bristol Myers Squibb/Medarex, Merck & Co (Kenilworth, NJ), Astellas Pharma, AstraZeneca, Novartis, Bayer
Travel, Accommodations, Expenses: the healthcare business of Merck KGaA (Darmstadt, Germany), Pfizer
Howard Gurney
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Merck & Co (Kenilworth, NJ), AstraZeneca, Janssen-Cilag, Pfizer, Roche, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma
Speakers' Bureau: the healthcare business of Merck KGaA (Darmstadt, Germany), AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Anders Ullén
Consulting or Advisory Role: the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma
Research Funding: the healthcare business of Merck KGaA (Darmstadt, Germany) (Inst)
Yohann Loriot
Consulting or Advisory Role: Janssen (Inst), Astellas Pharma, Roche, AstraZeneca, Merck & Co (Kenilworth, NJ) (Inst), Seattle Genetics, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical, Loxo/Lilly, Pfizer, the healthcare business of Merck KGaA (Darmstadt, Germany)
Research Funding: Janssen Oncology (Inst), Merck & Co (Kenilworth, NJ) (Inst), AstraZeneca (Inst), Exelixis (Inst), Incyte (Inst), Pfizer (Inst), Nektar (Inst), Sanofi (Inst), Seattle Genetics (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), the healthcare business of Merck KGaA (Darmstadt, Germany) (Inst), Taiho Pharmaceutical (Inst), Basilea (Inst), BMS (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, Merck & Co (Kenilworth, NJ), AstraZeneca, Seattle Genetics
Srikala S. Sridhar
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen (Inst), Bayer (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Merck & Co (Kenilworth, NJ) (Inst), Pfizer (Inst), Seattle Genetics (Inst), Gilead Sciences (Inst)
Research Funding: Bayer (Inst), Janssen (Inst), Pfizer (Inst)
Cora N. Sternberg
Consulting or Advisory Role: Bayer, Merck & Co (Kenilworth, NJ), Pfizer, Roche, Incyte, AstraZeneca, the healthcare business of Merck KGaA (Darmstadt, Germany), Medscape, UroToday, Astellas Pharma, Genzyme, Immunomedics, Foundation Medicine, Bristol Myers Squibb/Medarex, IMPAC Medical Systems, Amgen, Gilead Sciences, Janssen Oncology
Joaquim Bellmunt
Stock and Other Ownership Interests: Rainier Therapeutics
Honoraria: UpToDate
Consulting or Advisory Role: Pierre Fabre, Astellas Pharma, Pfizer, the healthcare business of Merck KGaA (Darmstadt, Germany), Genentech, Novartis, AstraZeneca/MedImmune, Bristol Myers Squibb
Research Funding: Millennium (Inst), Sanofi (Inst), Pfizer (Inst), the healthcare business of Merck KGaA (Darmstadt, Germany)
Patents, Royalties, Other Intellectual Property: UpToDate Bladder Cancer
Travel, Accommodations, Expenses: Pfizer, Merck & Co (Kenilworth, NJ), Ipsen
Jeanny B. Aragon-Ching
Honoraria: Bristol Myers Squibb, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Scientific and Medical Affairs, Inc, Pfizer
Consulting or Advisory Role: Algeta/Bayer, Dendreon, AstraZeneca, Janssen Biotech, Sanofi, the healthcare business of Merck KGaA (Darmstadt, Germany), AstraZeneca/MedImmune, Bayer, Merck & Co (Kenilworth, NJ), Seattle Genetics, Pfizer, Immunomedics, Amgen, AVEO, Pfizer/Myovant, Exelixis
Speakers' Bureau: Astellas Pharma, Janssen-Ortho, Bristol Myers Squibb, Seattle Genetics/Astellas
Travel, Accommodations, Expenses: Dendreon, Algeta/Bayer, Bristol Myers Squibb, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma
Jing Wang
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Bo Huang
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Robert J. Laliberte
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Alessandra di Pietro
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Honoraria: Pfizer
Petros Grivas
Consulting or Advisory Role: Merck & Co (Kenilworth, NJ), Bristol Myers Squibb, AstraZeneca, the healthcare business of Merck KGaA (Darmstadt, Germany), Seattle Genetics, Pfizer, Janssen, Mirati Therapeutics, Exelixis, Roche, Genentech, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma, Regeneron, Astellas Pharma, Guardant Health, Urogen Pharma, Gilead Sciences, Silverback Therapeutics, BostonGene, Fresenius Kabi, Lucence, PureTech, G1 Therapeutics, AADi, CG Oncology, Strata Oncology, ImmunityBio
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Bavarian Nordic (Inst), Bristol Myers Squibb (Inst), Debiopharm Group (Inst), Merck & Co (Kenilworth, NJ) (Inst), QED Therapeutics (Inst), GlaxoSmithKline (Inst), Mirati Therapeutics (Inst), the healthcare business of Merck KGaA (Darmstadt, Germany) (Inst), G1 Therapeutics (Inst), Gilead Sciences (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 ASCO Genitourinary Cancers Symposium, San Francisco, CA, February 17-19, 2022.
SUPPORT
Supported by Pfizer and the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945). Medical writing support was provided by Jamie Ratcliffe of Clinical Thinking and was funded by Pfizer and the healthcare business of Merck KGaA, Darmstadt, Germany.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
AUTHOR CONTRIBUTIONS
Conception and design: Srikala S. Sridhar, Cora N. Sternberg, Joaquim Bellmunt, Jeanny B. Aragon-Ching, Bo Huang, Robert J. Laliberte, Alessandra di Pietro, Petros Grivas
Administrative support: Bo Huang, Robert J. Laliberte
Provision of study materials or patients: Thomas Powles, Se Hoon Park, Claudia Caserta, Begoña P. Valderrama, Howard Gurney, Anders Ullén, Yohann Loriot, Srikala S. Sridhar, Cora N. Sternberg, Joaquim Bellmunt, Jeanny B. Aragon-Ching, Bo Huang, Robert J. Laliberte, Petros Grivas
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas Powles
Honoraria: AstraZeneca, Eisai, Gilead Sciences, Merck & Co (Kenilworth, NJ), Novartis, Pfizer, Roche Laboratories Inc, Astellas Pharma, BMS GmbH & Co. KG, Exelixis, Incyte, Ipsen, Seattle Genetics, the healthcare business of Merck KGaA (Darmstadt, Germany), Johnson & Johnson/Janssen, Mashup Ltd
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Ipsen, Pfizer, Novartis, Seattle Genetics, Roche, Exelixis, Merck & Co (Kenilworth, NJ), the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma, Johnson & Johnson, Eisai, Mashup Ltd, Incyte
Research Funding: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen, Merck & Co (Kenilworth, NJ), Novartis, Pfizer, Seattle Genetics, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma, Johnson & Johnson, Eisai
Travel, Accommodations, Expenses: Pfizer, Merck & Co (Kenilworth, NJ), AstraZeneca, Roche, Ipsen
Se Hoon Park
Honoraria: the healthcare business of Merck KGaA (Darmstadt, Germany), Pfizer, Ono Pharmaceutical
Consulting or Advisory Role: Janssen Oncology
Research Funding: Ono Pharmaceutical, Sanofi
Begoña P. Valderrama
Honoraria: Bristol Myers Squibb/Medarex, Roche, Ipsen, EUSA Pharma, Pfizer, Astellas Pharma, Bayer, the healthcare business of Merck KGaA (Darmstadt, Germany)
Consulting or Advisory Role: Bristol Myers Squibb/Medarex, Merck & Co (Kenilworth, NJ), Astellas Pharma, AstraZeneca, Novartis, Bayer
Travel, Accommodations, Expenses: the healthcare business of Merck KGaA (Darmstadt, Germany), Pfizer
Howard Gurney
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Merck & Co (Kenilworth, NJ), AstraZeneca, Janssen-Cilag, Pfizer, Roche, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma
Speakers' Bureau: the healthcare business of Merck KGaA (Darmstadt, Germany), AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Anders Ullén
Consulting or Advisory Role: the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma
Research Funding: the healthcare business of Merck KGaA (Darmstadt, Germany) (Inst)
Yohann Loriot
Consulting or Advisory Role: Janssen (Inst), Astellas Pharma, Roche, AstraZeneca, Merck & Co (Kenilworth, NJ) (Inst), Seattle Genetics, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical, Loxo/Lilly, Pfizer, the healthcare business of Merck KGaA (Darmstadt, Germany)
Research Funding: Janssen Oncology (Inst), Merck & Co (Kenilworth, NJ) (Inst), AstraZeneca (Inst), Exelixis (Inst), Incyte (Inst), Pfizer (Inst), Nektar (Inst), Sanofi (Inst), Seattle Genetics (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), the healthcare business of Merck KGaA (Darmstadt, Germany) (Inst), Taiho Pharmaceutical (Inst), Basilea (Inst), BMS (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, Merck & Co (Kenilworth, NJ), AstraZeneca, Seattle Genetics
Srikala S. Sridhar
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen (Inst), Bayer (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Merck & Co (Kenilworth, NJ) (Inst), Pfizer (Inst), Seattle Genetics (Inst), Gilead Sciences (Inst)
Research Funding: Bayer (Inst), Janssen (Inst), Pfizer (Inst)
Cora N. Sternberg
Consulting or Advisory Role: Bayer, Merck & Co (Kenilworth, NJ), Pfizer, Roche, Incyte, AstraZeneca, the healthcare business of Merck KGaA (Darmstadt, Germany), Medscape, UroToday, Astellas Pharma, Genzyme, Immunomedics, Foundation Medicine, Bristol Myers Squibb/Medarex, IMPAC Medical Systems, Amgen, Gilead Sciences, Janssen Oncology
Joaquim Bellmunt
Stock and Other Ownership Interests: Rainier Therapeutics
Honoraria: UpToDate
Consulting or Advisory Role: Pierre Fabre, Astellas Pharma, Pfizer, the healthcare business of Merck KGaA (Darmstadt, Germany), Genentech, Novartis, AstraZeneca/MedImmune, Bristol Myers Squibb
Research Funding: Millennium (Inst), Sanofi (Inst), Pfizer (Inst), the healthcare business of Merck KGaA (Darmstadt, Germany)
Patents, Royalties, Other Intellectual Property: UpToDate Bladder Cancer
Travel, Accommodations, Expenses: Pfizer, Merck & Co (Kenilworth, NJ), Ipsen
Jeanny B. Aragon-Ching
Honoraria: Bristol Myers Squibb, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Scientific and Medical Affairs, Inc, Pfizer
Consulting or Advisory Role: Algeta/Bayer, Dendreon, AstraZeneca, Janssen Biotech, Sanofi, the healthcare business of Merck KGaA (Darmstadt, Germany), AstraZeneca/MedImmune, Bayer, Merck & Co (Kenilworth, NJ), Seattle Genetics, Pfizer, Immunomedics, Amgen, AVEO, Pfizer/Myovant, Exelixis
Speakers' Bureau: Astellas Pharma, Janssen-Ortho, Bristol Myers Squibb, Seattle Genetics/Astellas
Travel, Accommodations, Expenses: Dendreon, Algeta/Bayer, Bristol Myers Squibb, the healthcare business of Merck KGaA (Darmstadt, Germany), Astellas Pharma
Jing Wang
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Bo Huang
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Robert J. Laliberte
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Alessandra di Pietro
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Honoraria: Pfizer
Petros Grivas
Consulting or Advisory Role: Merck & Co (Kenilworth, NJ), Bristol Myers Squibb, AstraZeneca, the healthcare business of Merck KGaA (Darmstadt, Germany), Seattle Genetics, Pfizer, Janssen, Mirati Therapeutics, Exelixis, Roche, Genentech, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma, Regeneron, Astellas Pharma, Guardant Health, Urogen Pharma, Gilead Sciences, Silverback Therapeutics, BostonGene, Fresenius Kabi, Lucence, PureTech, G1 Therapeutics, AADi, CG Oncology, Strata Oncology, ImmunityBio
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Bavarian Nordic (Inst), Bristol Myers Squibb (Inst), Debiopharm Group (Inst), Merck & Co (Kenilworth, NJ) (Inst), QED Therapeutics (Inst), GlaxoSmithKline (Inst), Mirati Therapeutics (Inst), the healthcare business of Merck KGaA (Darmstadt, Germany) (Inst), G1 Therapeutics (Inst), Gilead Sciences (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Powles T, Park SH, Voog E, et al. : Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218-1230, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Cathomas R, Lorch A, Bruins HM, et al. : The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol 81:95-103, 2022 [DOI] [PubMed] [Google Scholar]
- 3.Powles T, Bellmunt J, Comperat E, et al. : Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33:244-258, 2022 [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer v1. 2023. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf [Google Scholar]
- 5.Quan H, Li M, Shih WJ, et al. : Empirical shrinkage estimator for consistency assessment of treatment effects in multi-regional clinical trials. Stat Med 32:1691-1706, 2013 [DOI] [PubMed] [Google Scholar]
- 6.US Food & Drug Administration : Impact story: Using innovative statistical approaches to provide the most reliable treatment outcomes information to patients and clinicians, 2019. https://www.fda.gov/drugs/regulatory-science-action/impact-story-using-innovative-statistical-approaches-provide-most-reliable-treatment-outcomes
- 7.Huang B, Tian L, Talukder E, et al. : Evaluating treatment effect based on duration of response for a comparative oncology study. JAMA Oncol 4:874-876, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Tian L: Utilizing restricted mean duration of response for efficacy evaluation of cancer treatments. Pharm Stat 21:865-878, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical Trials Dashboard: JAVELIN Bladder 100 trial: Results after ≥2 years of follow-up, 2023. https://clinical-trials.dimensions.ai/javelin/
- 10.Galsky MD, Arija JAA, Bamias A, et al. : Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395:1547-1557, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Powles T, van der Heijden MS, Castellano D, et al. : Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 21:1574-1588, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Powles T, Csőszi T, Özgüroğlu M, et al. : Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol 22:931-945, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Bristol Myers Squibb Provides Update on CheckMate-901 Trial Evaluating Opdivo (Nivolumab) Plus Yervoy (Ipilimumab) as First-Line Treatment for Patients With Unresectable or Metastatic Urothelial Carcinoma. Bristol Myers Squibb, 2022. https://news.bms.com/news/corporate-financial/2022/Bristol-Myers-Squibb-Provides-Update-on-CheckMate‐‐901-Trial-Evaluating-Opdivo-nivolumab-Plus-Yervoy-ipilimumab-as-First-Line-Treatment-for-Patients-with-Unresectable-or-Metastatic-Urothelial-Carcinoma/default.aspx [Google Scholar]
- 14.Cheeseman S, Thompson M, Sopwith W, et al. : Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front Oncol 10:167, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niegisch G, Gerullis H, Lin SW, et al. : A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J Cancer 9:1337-1348, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richters A, Mehra N, Meijer RP, et al. : Utilization of systemic treatment for metastatic bladder cancer in everyday practice: Results of a nation-wide population-based cohort study. Cancer Treat Res Commun 25:100266, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Simeone JC, Nordstrom BL, Patel K, et al. : Treatment patterns and overall survival in metastatic urothelial carcinoma in a real-world, US setting. Cancer Epidemiol 60:121-127, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Powles T, Huddart RA, Elliott T, et al. : Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol 35:48-55, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Galsky MD, Mortazavi A, Milowsky MI, et al. : Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J Clin Oncol 38:1797-1806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivas P, Kopyltsov E, Su PJ, et al. : Patient-reported outcomes from JAVELIN Bladder 100: Avelumab first-line maintenance plus best supportive care versus best supportive care alone for advanced urothelial carcinoma. Eur Urol 83:320-328, 2023 [DOI] [PubMed] [Google Scholar]
- 21.Stensland KD, Galsky MD: Current approaches to the management of bladder cancer in older patients. Am Soc Clin Oncol Ed Book e250-e256, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.