Graphical abstract
Keywords: Ventricular septal defect, Coarctation of the aorta, Gerbode defect
Highlights
-
•
Congenital Gerbode defect remains a rare phenomenon.
-
•
CoA can worsen shunting due to left ventricular obstruction.
-
•
Assess for coexisting congenital defects that may alter management.
Introduction
The Gerbode defect refers to a deformity in the ventricular septum that allows communication between the left ventricle (LV) and right atrium (RA). This anatomical variant was first described in an autopsy report in 1838 and named after Frank Gerbode—a surgeon who published a case series on surgical closure of the defect.1 While the incidence of acquired Gerbode defect has increased due to the rising frequency of invasive and complex cardiovascular procedures, congenital Gerbode defect remains rare and accounts for <1% of all congenital heart abnormalities.2 The embryologic development of this shunt is not fully understood but is thought to be the result of an endocardial cushion defect in addition to defective maturation of the membranous ventricular septum.3
Due to this impaired development, additional malformations are not uncommon and are seen in about one-third of cases.4 Prior reported cases of Gerbode defect have focused on the primary management of Gerbode but do not comment on how concurrent congenital abnormalities can affect its treatment. This case report describes how the finding of coarctation of the aorta (CoA) played a significant role in the management of this patient’s Gerbode defect.
Case Presentation
A 38-year-old man with a medical history of hypertension presented to our hospital with 3 days of worsening shortness of breath, abdominal distention, orthopnea, and leg swelling. They reported being diagnosed with a heart murmur as a child and were instructed to follow up with a cardiologist. However, the patient chose not to pursue consultation since they did not have any symptoms at that time. In the emergency department, blood pressure was elevated to 170/85 mm Hg, heart rate was 90 beats/min, and oxygen saturation was 100% on room air. Physical examination revealed a holosystolic 4/6 heart murmur best heard at the lower left sternal border, a palpable precordial thrill, and an elevated jugular venous pulse at 12 cm.
Laboratory results were significant for a B-type natriuretic peptide of 1,450 pg/mL and a negative troponin T. Chest x-ray showed diffuse cardiomegaly with mild central pulmonary vascular congestion. Electrocardiogram was significant for right-axis deviation, left atrial abnormality, first-degree atrioventricular block, and incomplete right bundle branch block. Transthoracic echocardiography (TTE) demonstrated a left ventricular ejection fraction of 55% to 60%, a dilated right ventricle (with a basal diameter of 5.6 cm) with visually decreased systolic function, a 1.4 cm pericardial effusion, a bicuspid aortic valve with visually mild aortic regurgitation, and color flow across the ventricular septum suggestive of a perimembranous ventricular septal defect (VSD). Color flow Doppler demonstrated continuous shunting of flow from the LV to the RA, consistent with Gerbode defect. Continuous-wave Doppler demonstrated a peak gradient of approximately 117 mm Hg (Figures 1, and 2, Videos 1, and 2).
Figure 1.
Two-dimensional TTE, parasternal short-axis view (A, B) and apical 4-chamber (C) views, without (A) and with (B, C) color flow Doppler, demonstrate the Gerbode-type perimembranous VSD (A, arrow) with systolic blood flow from the LV to the RA (B, C). LA, Left atrium; RV, right ventricle.
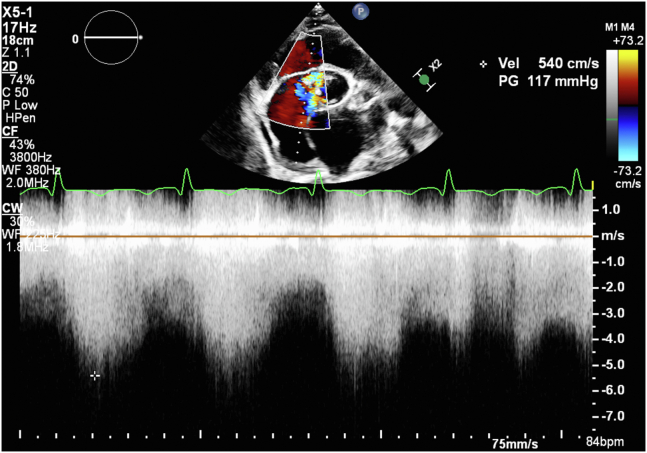
Figure 2.
Two-dimensional TTE, color flow–guided continuous-wave Doppler from the parasternal short-axis view demonstrates an increased gradient of around 117 mm Hg from the LV to the RA.
The patient was treated with intravenous diuretics for 2 days and discharged on oral diuretics with follow-up in the clinic 3 weeks later. At that time, they complained of increasing leg swelling and abdominal distention, so their diuretic dose was increased. Despite this intervention, their symptoms continued to worsen, and they required 2 additional hospitalizations for heart failure exacerbation. During the last hospitalization, left and right cardiac catheterization was performed, which demonstrated an LV end-diastolic pressure of 40 mm Hg, a step-up in oxygen saturation from the superior vena cava to RA, consistent with atrial septal defect, a pulmonary to systemic flow ratio (Qp/Qs) of 1.44:1, and CoA with a 35 to 40 mm Hg gradient across the ascending to descending aorta. After the patient’s volume status was medically optimized, they underwent angioplasty and stenting of their CoA with a 6 cm 8-Zig stent without complications and successful resolution of aortic gradient from 33 to 0 mm Hg (Figure 3). Angiography at that time showed a Qp/Qs of 1.83:1.
Figure 3.
Hemodynamic measurements made during catheterization before (black) and after (red) repair of CoA.
A week later, the patient was seen in the clinic with improvement of symptoms. The decision was made to defer management of the Gerbode defect and observe the patient over several months.
Discussion
The pathophysiology of Gerbode defect involves shunting of the blood from the LV to the RA due to the significant pressure gradient between the 2 chambers. The degree of shunting is dependent on the size of the defect and pressure difference between the LV and RA. This leads to increased flow through the right side of the heart, causing chamber dilatation and compensatory enlargement of the right heart chambers. Without correction, this process can lead to right heart failure, resulting in systemic venous congestion and ultimately left-sided heart failure from increased blood volumes resulting in left-sided chamber enlargement.
There is no universal approach to management of Gerbode defect. Yacoub et al.5 claims that all Gerbode defects of any size should be closed, while Toprak et al.6 argues that asymptomatic patients with normal ventricular volumes and pressures and small shunts can be managed conservatively with close monitoring.3 According to the American College of Cardiology/American Heart Association Task Force, adults who have a VSD with left ventricular volume overload and hemodynamically significant shunts (defined as a Qp:Qs ≥ 1.5:1) should have surgical closure of VSD as long as the pulmonary artery systolic pressure is less than 50% of systemic pressure and the pulmonary vascular resistance is less than one-third of systemic resistance.7 In practice, providers often use a Qp:Qs ratio >2:1 since that ratio was previously shown to be associated with left-sided heart failure, but the timing and type of repair depend on several factors including the magnitude of the flow and concomitant anatomic abnormalities and comorbidities.8
Our case differs from a typical Gerbode defect case since our patient had concomitant CoA as well as bicuspid aortic valve with aortic regurgitation. These abnormalities further hindered our patient’s cardiac output and significantly augmented the pressure gradient between the LV and RA, leading to greater shunting. Based on hemodynamics from his catheterization and his symptoms, it would have been reasonable to pursue Gerbode defect repair. However, given his severely elevated LV end-diastolic pressure and LV-to-RA gradient in the setting of multiple hospital admissions for decompensated heart failure, the decision was made to repair the CoA first in an attempt to improve the left ventricular afterload and diminish left-to-right shunting to improve the patient’s right heart failure before considering further interventions. Although we do not yet have long-term follow-up on the patient’s symptoms or hemodynamic profile, the repair resulted in rapid improvement in their clinical picture and has delayed the need for immediate, high-risk repair of their Gerbode defect. If the patient’s symptoms and hemodynamic profile remain stable, it is possible that correction of their Gerbode defect may be delayed even further and there may be a greater consideration for percutaneous closure. Additionally, correction of the patient’s defect will likely have a lower complication rate given the euvolemic clinical status.
Conclusion
The Gerbode defect remains a rare congenital abnormality. However, clinicians should remain aware of this phenomenon and understand its hemodynamic profile in order to provide optimal treatment. Furthermore, this case highlights the importance of conducting a full workup for coexistent congenital cardiac abnormalities since it can influence the clinical approach to such patients.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2023.01.004.
Supplementary Data
Two-dimensional TTE parasternal short-axis view, without (left) and with (right) color flow Doppler, demonstrates the perimembranous Gerbode-type VSD with systolic blood flow from the LV to the RA. A small pericardial effusion is noted.
Two-dimensional TTE apical 4-chamber view, without (left) and with (right) color flow Doppler, demonstrates the perimembranous Gerbode-type VSD with systolic blood flow from the LV to the RA. The right heart is dilated, and the right ventricular systolic function is reduced.
References
- 1.Gerbode F., Hultgren H., Melrose D., et al. Syndrome of left ventricular-right atrial shunt; successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann Surg. 1958;148:433–446. doi: 10.1097/00000658-195809000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasserman S.M., Fann J.I., Atwood J.E., et al. Acquired left ventricular–right atrial communication. Echocardiography. 2002;19:67–72. doi: 10.1046/j.1540-8175.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- 3.Saker E., Bahri G.N., Montalbano M.J., et al. Gerbode defect: a comprehensive review of its history, anatomy, embryology, pathophysiology, diagnosis, and treatment. J Saudi Heart Assoc. 2017;29:283–292. doi: 10.1016/j.jsha.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riemenschneider T.A., Moss A.J. Left ventricular-right atrial communication. Am J Cardiol. 1967;19:710–718. doi: 10.1016/0002-9149(67)90476-6. [DOI] [PubMed] [Google Scholar]
- 5.Yacoub M.H., Mansur A., Towers M., et al. Bacterial endocarditis complicating left ventricle to right atrium communication. Br J Dis Chest. 1972;66:78–82. [PubMed] [Google Scholar]
- 6.Toprak C, Kahveci G, Akpinar S, Tabakçi MM, Güler Y. Concomitant Gerbode-like defect and anterior mitral leaflet perforation after aortic valve replacement for endocarditis. Echocardiography. 2013;30:E231–E235. doi: 10.1111/echo.12259. [DOI] [PubMed] [Google Scholar]
- 7.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 8.Rajiah P., Kanne J.P. Cardiac MRI: part 1, cardiovascular shunts. Am J Roentgenol. 2011;197:W603–W620. doi: 10.2214/AJR.10.7257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE parasternal short-axis view, without (left) and with (right) color flow Doppler, demonstrates the perimembranous Gerbode-type VSD with systolic blood flow from the LV to the RA. A small pericardial effusion is noted.
Two-dimensional TTE apical 4-chamber view, without (left) and with (right) color flow Doppler, demonstrates the perimembranous Gerbode-type VSD with systolic blood flow from the LV to the RA. The right heart is dilated, and the right ventricular systolic function is reduced.