Graphical abstract
Highlights
-
•
Congenital abLAA is extremely rare.
-
•
AbLAA can occasionally present with other coexisting cardiac anomalies.
-
•
Knowledge of abLAA is necessary to fully exclude thrombus prior to cardioversion.
-
•
Inability to visualize LAA despite careful searching should raise suspicion for abLAA.
-
•
CCT is an excellent noninvasive imaging tool to visualize the LAA.
Introduction
Congenital absence of the left atrial appendage (abLAA) is extremely rare. Physiological function of the left atrial appendage (LAA) is not well understood, yet it is an important source for thromboembolism in atrial fibrillation (AF).
Case Presentation 1
A 57-year-old patient with paroxysmal AF (CHADSVASc2 score 4) presented to our emergency room with palpitations and shortness of breath. Medical history revealed apical hypertrophic cardiomyopathy and an implantable cardioverter-defibrillator that was recently placed due to ventricular tachycardia. The patient did not have a history of cardiac surgery. Upon presentation, blood pressure was 110/79 mm Hg and pulse was irregular and rapid at 144 bpm. The chest x-ray showed mild pulmonary edema. Electrocardiogram showed AF with rapid ventricular response at a heart rate of 137 (Figure 1). The patient was sent for urgent electrical cardioversion. Preprocedure transesophageal echocardiogram (TEE) could not locate the LAA (Figure 2). A three-dimensional (3D) reconstruction of the left atrium (LA) also failed to reveal the LAA or flushed thrombosis (Video 1).
Figure 1.
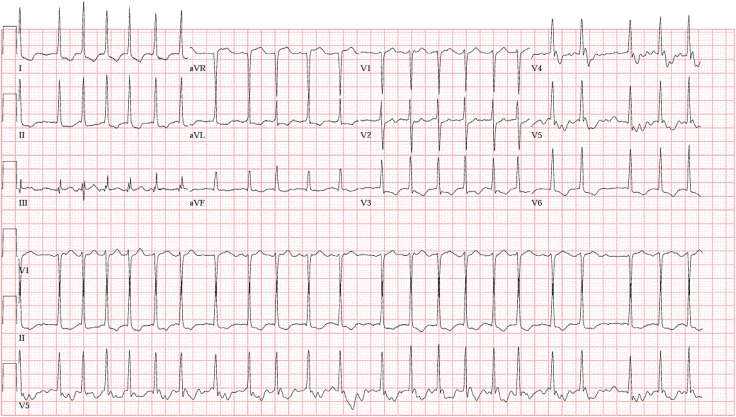
Twelve-lead electrocardiogram showed AF with rapid ventricular response at a heart rate of 137 bpm with ST depression and T-wave inversion in the lateral leads.
Figure 2.
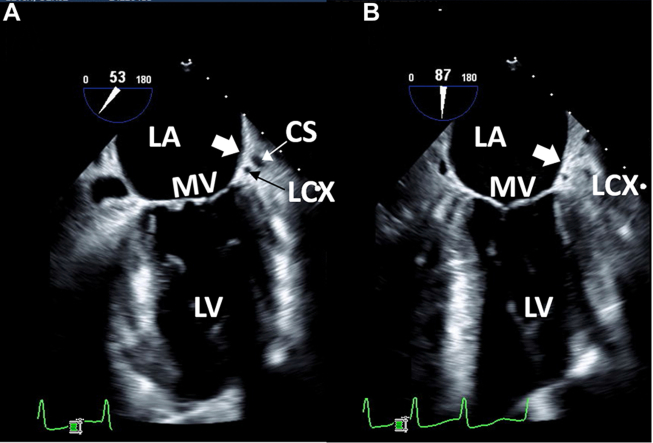
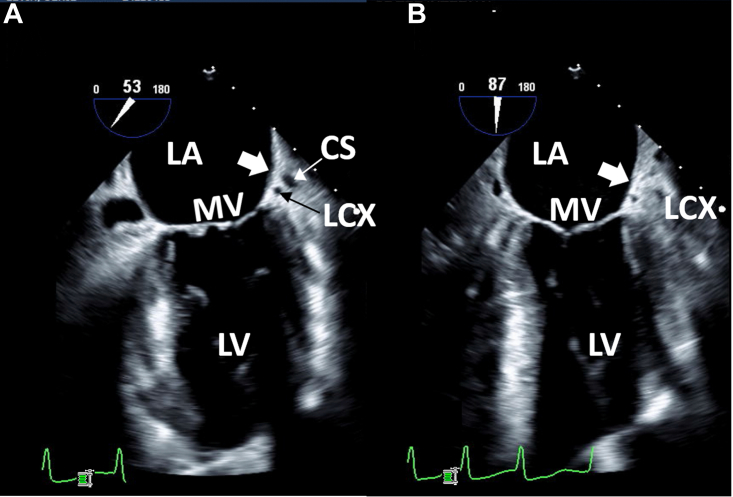
Case 1: two-dimensional TEE, midesophageal long-axis view, systolic view highlighting the mitral commissure (A; 53°) and conventional 2-chamber (B; 87°) view indicating the usual location of the LAA (thick white arrow). The CS and LCx are well visualized in the atrioventricular groove indicating the usual anatomic location of the LAA. CS, Coronary sinus; LCx, left circumflex coronary artery; LV, left ventricle; MV, mitral valve.
Considering that there was no evidence of prior open-heart surgery or percutaneous exclusion of LAA, a differential diagnosis of hypoplastic or aplastic LAA, a fully thrombosed LAA, or unusual anatomy of LAA was entertained. After echocardiographic imaging of the LA for either anomalous LAA position or unusual anatomy, abLAA was ultimately suspected. A contrast-enhanced cardiac computed tomography (CCT) scan was performed to corroborate this suspicion because abLAA is a rare presentation and it was of ultimate importance to exclude an appendage thrombus prior to an elective cardioversion.
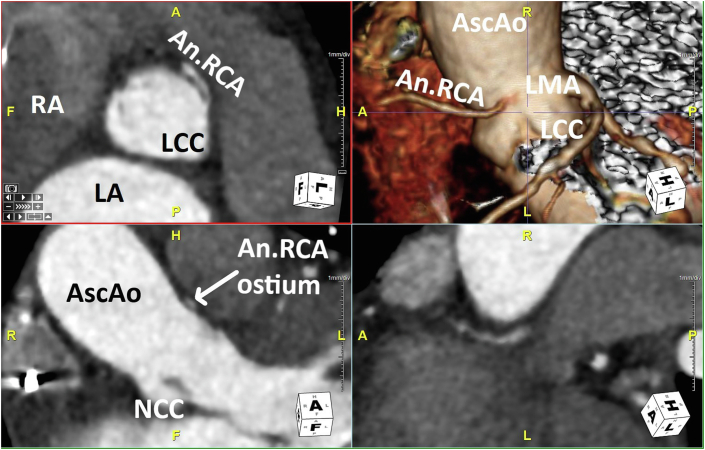
Cardiac computed tomography demonstrated no evidence of an LAA (Figure 3). Given no history of prior cardiac surgery, abLAA was presumed to be congenital. In addition, the CCT revealed an anomalous right coronary artery originating from the left coronary cusp through a slit-like ostium with an interarterial course (Figure 4). These additional findings were discussed with the referring physician, and a follow-up visit was planned for further testing.
Figure 3.
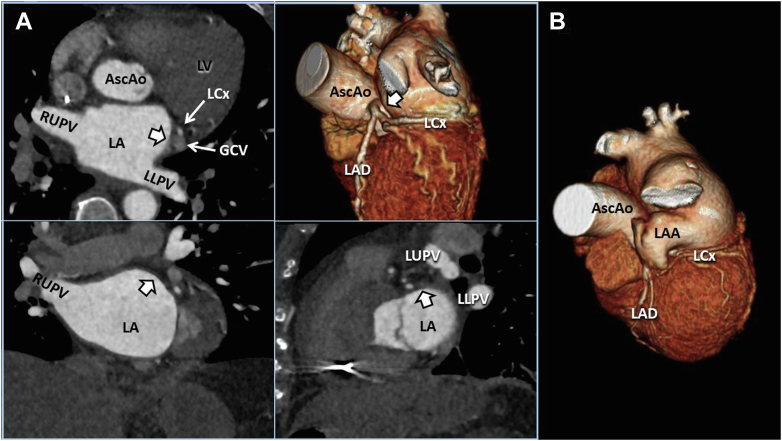
Case 1: CCT imaging demonstrates 3D cross-correlation of case 1 (A; quad panel) compared with a representative normal 3D volume-rendered example (B; right single panel). The multiplanar reformat quad display allows comprehensive demonstration of the abLAA (thick white arrow) in axial (upper left), coronal (lower left), and sagittal (lower right) orientations. AscAo, Ascending thoracic aorta; GCV, great cardiac vein; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LLPV, left lower pulmonary vein; LUPV, left upper pulmonary vein; LV, left ventricle; RUPV, right upper pulmonary vein.
Figure 4.
Case 1: CCT multiplanar reformat quad display demonstrates the anomalous RCA origin through a slit-like ostium (arises from the LCC) and interarterial course from multiple perspectives. An.RCA, Anomalous right coronary artery; AscAo, ascending thoracic aorta; LCC, Left coronary cusp; LMA, left main coronary artery; NCC, noncoronary cusp; RA, right atrium.
The patient was successfully cardioverted without complications to sinus rhythm and was continued on intravenous heparin after the procedure as bridging to oral anticoagulation. Rivaroxaban was ultimately started upon discharge.
Case Presentation 2
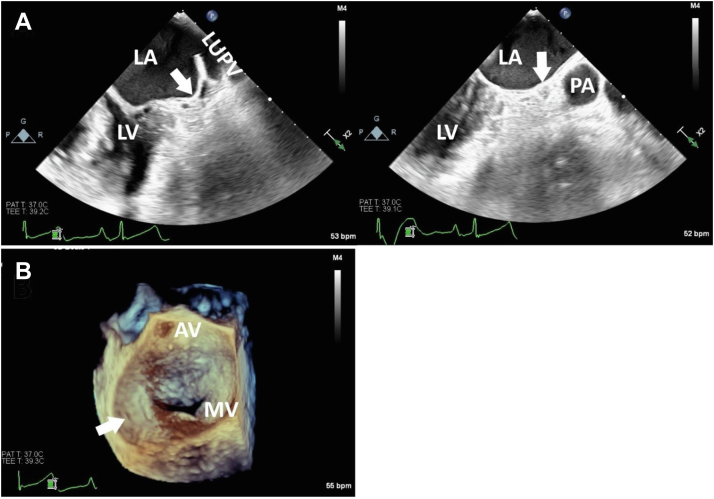
A 71-year-old patient with history of paroxysmal AF and hyperlipidemia presented with palpitations and persistent chest heaviness that started abruptly. Preliminary cardiac workup included a transthoracic echocardiography (TTE) that demonstrated severe mitral regurgitation (estimated by color flow Doppler) that was associated with rheumatic heart disease and a visually estimated left ventricular ejection fraction of 55%. Cardiovascular magnetic resonance imaging was performed and confirmed the mitral regurgitation was severe, with a regurgitant fraction of 57%. There was no mitral stenosis. A coronary angiogram showed normal coronary arteries. The patient was referred to our institution for surgical mitral valve replacement (MVR). Upon admission, a 12-lead electrocardiogram showed AF with rapid ventricular response; therefore, LAA ligation was also planned during open-heart surgery. Preoperative TEE failed to visualize the LAA (Figure 5). Congenital abLAA was visually confirmed during surgical MVR, and it is stated in the operative report as “an atretic LAA.”
Figure 5.
Case 2: two-dimensional TEE, midesophageal, long-axis systolic views at 45° (A; top left) and 90° (A; top right) demonstrates the abLAA (arrows). The TEE 3D display, surgeon’s view, demonstrates the en face view of the mitral valve from the perspective of the LA with the abLAA (arrow). AV, Aortic valve; MV, mitral valve; LUPV, left upper pulmonary vein; LV, left ventricle; PA, main pulmonary artery.
Discussion
We hereby report 2 cases of congenital abLAA. There are only 23 reported cases of congenital abLAA (Table 1). This report also demonstrates the coexistence of a congenital abnormality, specifically anomalous right coronary artery in one of the patients. Given the lack of high-risk imaging or clinical features for sudden cardiac death, no surgical intervention was recommended.1 Overall, 20% of reported cases of abLAA are associated with other cardiac congenital abnormalities, warranting careful and thorough evaluation.2, 3, 4, 5
Table 1.
| Age, years | Gender | Associated lesions | Indication | Modality | Anticoagulation | |
|---|---|---|---|---|---|---|
| Case 1∗ | 65 | F | Anomalous RCA | DCCV | TEE, CCT | DOAC |
| Case 2∗ | 71 | F | None mentioned | MVR | TEE, surgery | Warfarin |
| Case 3 | 77 | F | None mentioned | PVI | TEE, CCT | Warfarin |
| Case 4 | 78 | M | None mentioned | LAAO | TEE, CCT | P2Y12 inhibitor |
| Case 5 | 79 | M | None mentioned | Biventricular implantable cardioverter-defibrillator | TEE | ? |
| Case 6 | 54 | F | None mentioned | PVI | TEE, CCT | Yes |
| Case 7 | 70 | M | None mentioned | PVI | TEE, CCT, angio | Yes |
| Case 8 | 67 | M | None mentioned | Maze surgical ablation procedure | CCT | ? |
| Case 9 | 50 | M | None mentioned | DCCV | TEE, CCT, cardiovascular magnetic resonance imaging | Yes |
| Case 10 | 80 | M | None mentioned | PVI | TEE, CCT | DOAC |
| Case 11 | 73 | F | None mentioned | DCCV | TEE, CCT | ? |
| Case 12 | 79 | F | None mentioned | LAAO | TEE, CCT, angio | Yes |
| Case 13 | 58 | M | None mentioned | Cardiac source of emboli | TEE, CCT | ? |
| Case 14 | 62 | F | Single left-sided and 2 right-sided pulmonary veins | PVI | CCT, TEE | ? |
| Case 15 | 58 | F | None mentioned | PVI | CCT, angio | DOAC |
| Case 16 | 68 | M | None mentioned | PVI | CCT, angio | Warfarin |
| Case 17 | 76 | F | Persistent left SVC | PVI | CCT, TEE, angio | ? |
| Case 18 | 62 | M | None mentioned | PVI | CCT, TEE, angio | ? |
| Case 19 | 52 | ? | Persistent left SVC draining into the distal coronary sinus | PVI | TEE, CCT | ? |
| Case 20 | 60 | M | None mentioned | PVI | TEE, CCT, angio | ? |
| Case 21 | 57 | M | None mentioned | Wolf-Parkinson-White | TEE, CCT | ? |
| Case 22 | 3mo | M | Complex (single ventricle) | Death | Autopsy | N/A |
| Case 23 | 42 | M | None mentioned | Chest pain | CCT | N/A |
angio, Invasive LA angiography; DCCV, direct-current cardioversion; DOAC, direct oral anticoagulant; F, female; M, male; N/A, not applicable; PVI, pulmonary veins isolation; SVC, superior vena cava; Yes, anticoagulation strategy undefined; ?, not included in report.
This report.
Transthoracic echocardiography is the initial imaging study performed, frequently followed by TEE for a more detailed assessment of the cardiac valves or the LAA. This pattern was followed in both cases in this report whereby congenital abLAA was suspected; however, the diagnosis was not ascertained at that moment due to the rarity of the condition. The diagnosis was verified with CCT in the first case and with visual assessment during open-heart surgery in the second case. Anomalous coronary artery was also assessed by CCT in the first case.
The development of the LAA starts around the third week of gestational life. It is derived from the embryonic LA, whereas the remaining portion of the LA is formed from the branches of the primordial pulmonary veins.6,7 Three different scenarios can cause the LAA to be absent from its usual anatomical location (to the left of the root of the pulmonary trunk). The first scenario is congenital anomaly of right juxtaposition of the atrial appendages, which places the LAA on the right side by the right atrial appendage and ascending aorta.8 Second, spontaneous inversion of the LAA is observed, although rarely, as a complication of cardiac surgery with cardiopulmonary bypass surgery and vacuum application.9,10 This should be suspected when a new LA mass is seen on intraoperative TEE.10,11 And third is true congenital abLAA.
The LAA has attained significant importance in patients with AF since it serves as a trigger for the arrhythmia and also as the main source of thromboemboli with subsequent stroke, especially after the first 48 hours from onset.12,13 Transesophageal echocardiography is frequently used as the imaging modality to evaluate LAA thrombus prior to cardioversion given its ease of clinical setting and lack of exposure to ionizing radiation. Appropriate visualization of an existing LAA, whether previously excluded or not, is of utmost importance to rule out the presence of thrombus prior to elective cardioversion. Understanding the highly variable normal echocardiographic anatomy of the LAA (Video 2) and the appearance of the LAA after placement of an LAA occlusion device (LAAO; Video 3) or surgical clipping (Video 4) or exclusion (Video 5) is important to recognize pathology. Importantly, CCT provides excellent images of the LAA, and 3D whole-heart cine data may be diagnostic (Videos 6 and 7).
Given the lack of studies regarding the best management of anticoagulation in cases with congenital abLAA, the approach is variable, with some practitioners favoring continuation as per the patient’s CHADSVASC2 score, while others do not, arguing that the absence of the LAA stands in place for a totally excluded one (Table 1).
Conclusion
Congenital abLAA is a rare condition. A multimodality imaging approach is instrumental in arriving at the final conclusion in most cases given the usual limitations of any single modality. Other associated cardiac anomalies have been reported as per literature review. In case of congenital abLAA, anticoagulation management in AF is not addressed in the current guidelines, and the final decision will depend on the treating physician’s discretion.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
We thank Jin Chen for acquisition of images and Mario S. Beltrano Jr. for postprocessing of images.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2023.01.003.
Supplementary Data
Case 1: 3D TEE midesophageal surgeon’s view demonstrates the smooth echo texture of the upper lateral atrial wall (∗) representing the abLAA.
Representative 3D TEE example of a normal LAA from a comparison surgeon’s view demonstrates a normal ostium of the LAA (#).
Representative 3D TEE example of an LAA from a comparison surgeon’s view after placement of an LAAO device (Watchman).
Representative 3D TEE example of an LAA after surgical clipping with a residual small LAA stump (S) in a patient who underwent bioprosthetic MVR surgery. BioP MV, Bioprosthetic mitral valve.
Representative 3D TEE example of an LAA after surgical exclusion with a layer of thrombus covering the residual stump and extending into the adjacent LA wall (?). L, left lower pulmonary vein ostium; U, left upper pulmonary vein ostium.
Case 1: 3D volume-rendered CCT whole-heart image of the LA with the abLAA (thick green arrow indicates usual location of the LAA).
Three-dimensional volume-rendered CCT whole-heart image with a normal appearing and normally located LAA (arrow).
References
- 1.Jo Y., Uranaka Y., Iwaki H., Matsumoto J., Koura T., Negishi K. Sudden cardiac arrest: associated with anomalous origin of the right coronary artery from the left main coronary artery. Tex Heart Inst J. 2011;38:539–543. [PMC free article] [PubMed] [Google Scholar]
- 2.Pourafkari L., Sadeghpour A., Baghbani-Oskouei A., Savadi-Oskouei S., Pouraliakbar H., Fazelifar A.F., et al. Absent left atrial appendage: case report and review of the literature. Cardiovasc Pathol. 2020;45:107178. doi: 10.1016/j.carpath.2019.107178. [DOI] [PubMed] [Google Scholar]
- 3.Guo L.J., Ding M.Y., Sun D.D., Zhao H.Z., Pan S.Q., Zhu F. Congenital absence of left atrial appendage combined with type A Wolff-Parkinson-White syndrome diagnosed by multimodal imaging. J Clin Ultrasound. 2022;50:28–30. doi: 10.1002/jcu.23096. [DOI] [PubMed] [Google Scholar]
- 4.Vaideeswar P., Karande S. Congenital absence of the left atrial appendage. J Postgrad Med. 2020;66:108–109. doi: 10.4103/jpgm.JPGM_671_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pashun R.A., Gannon M.P., Tomassetti C., Rahmani N., Saba S.G. Congenital absence of the left atrial appendage. J Cardiovasc Comput Tomogr. 2020;14:e115–e117. doi: 10.1016/j.jcct.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Ho S.Y., Cabrera J.A., Sanchez-Quintana D. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol. 2012;5:220–228. doi: 10.1161/CIRCEP.111.962720. [DOI] [PubMed] [Google Scholar]
- 7.Beigel R., Wunderlich N.C., Ho S.Y., Arsanjani R., Siegel R.J. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251–1265. doi: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Frescura C., Thiene G. Juxtaposition of the atrial appendages. Cardiovasc Pathol. 2012;21:169–179. doi: 10.1016/j.carpath.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Fadia M., Vaideeswar P., Pandit S.P. Spontaneous inversion of left atrial appendage. Cardiovasc Pathol. 2006;15:231–232. doi: 10.1016/j.carpath.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Butter S.N., Andersen H.O., Poulsen J.B., Thyregod H.G.H. Inverted left atrial appendage mimicking a left atrial mass after surgical repair of an atrial septal defect: a case report. Eur Heart J Case Rep. 2022;21:ytac241. doi: 10.1093/ehjcr/ytac241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata K., Shigematsu S. Inverted left atrial appendage during minimal invasive mitral valve repair. Ann Card Anaesth. 2018;21:192–194. doi: 10.4103/aca.ACA_172_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T., Endo D., Matsushita S., Shimada A., Nakanishi K., Asai T., et al. Evidence and challenges in left atrial appendage management. Ann Thorac Cardiovasc Surg. 2022;28:1–17. doi: 10.5761/atcs.ra.21-00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigner M.J., Caulfield T.A., Danias P.G., Silverman D.I., Manning W.J. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med. 1997;126:615–620. doi: 10.7326/0003-4819-126-8-199704150-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case 1: 3D TEE midesophageal surgeon’s view demonstrates the smooth echo texture of the upper lateral atrial wall (∗) representing the abLAA.
Representative 3D TEE example of a normal LAA from a comparison surgeon’s view demonstrates a normal ostium of the LAA (#).
Representative 3D TEE example of an LAA from a comparison surgeon’s view after placement of an LAAO device (Watchman).
Representative 3D TEE example of an LAA after surgical clipping with a residual small LAA stump (S) in a patient who underwent bioprosthetic MVR surgery. BioP MV, Bioprosthetic mitral valve.
Representative 3D TEE example of an LAA after surgical exclusion with a layer of thrombus covering the residual stump and extending into the adjacent LA wall (?). L, left lower pulmonary vein ostium; U, left upper pulmonary vein ostium.
Case 1: 3D volume-rendered CCT whole-heart image of the LA with the abLAA (thick green arrow indicates usual location of the LAA).
Three-dimensional volume-rendered CCT whole-heart image with a normal appearing and normally located LAA (arrow).