Abstract
The magnitude and quality of the germinal center (GC) response decline with age, resulting in poor vaccine-induced immunity in older individuals. A functional GC requires the co-ordination of multiple cell types across time and space, in particular across its two functionally distinct compartments: the light and dark zones. In aged mice, there is CXCR4-mediated mislocalization of T follicular helper (TFH) cells to the dark zone and a compressed network of follicular dendritic cells (FDCs) in the light zone. Here we show that TFH cell localization is critical for the quality of the antibody response and for the expansion of the FDC network upon immunization. The smaller GC and compressed FDC network in aged mice were corrected by provision of TFH cells that colocalize with FDCs using CXCR5. This demonstrates that the age-dependent defects in the GC response are reversible and shows that TFH cells support stromal cell responses to vaccines.
Subject terms: Germinal centres, Somatic hypermutation
Linterman and colleagues examine germinal center formation in older individuals. They find that aged TFH cells have dysregulated CXCR4 expression, which causes spatial mislocalization of these cells in germinal centers, impairing their ability to provide help to B cells and to promote antibody production.
Main
T cell regulation of antibody-mediated immunity is critical for health, as an enduring antibody response after vaccination or infection can generate protective immunity against subsequent infections. However, there are members of our society who are less able to generate high-titer antibody responses upon vaccination, the largest cohort being older people1. This age-dependent deficit in antibody production has been evident in the global COVID-19 vaccine rollout as, despite the success of these vaccines, older people generate lower antibody titers than younger persons2–8. Furthermore, because antibody titers decrease over time7,8, this culminates in a faster reduction in vaccine efficacy in older individuals9. This has been known for decades, but the underlying mechanism(s) remains unclear. Antibody production upon vaccination or infection can occur via two cellular pathways: the extrafollicular response, which produces an initial burst of antibodies early after antigenic challenge10; or the GC reaction, which produces memory B cells and high-affinity plasma cells with somatically mutated immunoglobulin genes that can persist long-term11. In aged mice, the extrafollicular response is intact but the formation of GCs is delayed and, once formed, is of reduced size at its peak compared with younger animals12–17. A diminished GC reaction is also observed in reactive lymph nodes (LNs) from older people compared with younger adults18 and this is reflected by circulating biomarkers of an ongoing GC response after vaccination in older people19. Therefore, understanding why the size of the GC, and its output, is impaired in aging is key to determining why older people are less capable of mounting persistent antibody responses to vaccines.
The GC is polarized into two functionally distinct regions known as the light and dark zones. GC B cells localize to the dark zone via expression of CXCR4, which facilitates their migration to the CXCL12-producing reticular stromal cells20,21. Here, GC B cells proliferate and the genes encoding the B cell receptor undergo somatic hypermutation, then selection is required to test whether the introduction of mutations has impacted the function or specificity of the B cell receptor. To undergo selection in the light zone, GC B cells exit the cell cycle and downregulate CXCR4, enabling CXCR5-dependent migration towards the CXCL13-rich FDC stromal network that defines the light zone. In the light zone, a functional B cell receptor enables GC B cells to collect antigen and present it to TFH cells22, those that successfully engage a TFH cell by presenting cognate antigen receive help, in the form of CD40L-dependent costimulation and cytokines. This protects B cells from death and induces cMyc expression, which enables re-entry into the cell cycle and promotes survival11. These GC B cells will then migrate back to the dark zone and either undergo further rounds of proliferation and mutation or exit the GC as fully differentiated memory B cells or long-lived antibody-secreting cells (ASCs).
Here, we investigated the mechanistic reasons for the suboptimal GC response in aged mice. We showed that the spatial organization of the GC is changed in aging; the FDC network failed to expand in the GC after immunization, leading to a smaller light zone, and TFH cells were dispersed throughout the GC, rather than being polarized to the light zone. In silico modeling, in vivo vaccination studies using genetically modified mice, human vaccination cohorts and cell transfers into aged mice were combined to demonstrate that the mislocalization of TFH cells is a main driver of the smaller and poorer quality GC response in aging. In our endeavor to understand how the GC response changes with age, this study identified a role for TFH cells supporting the FDC response to vaccination.
Results
GC magnitude is impaired with age in a B cell extrinsic way
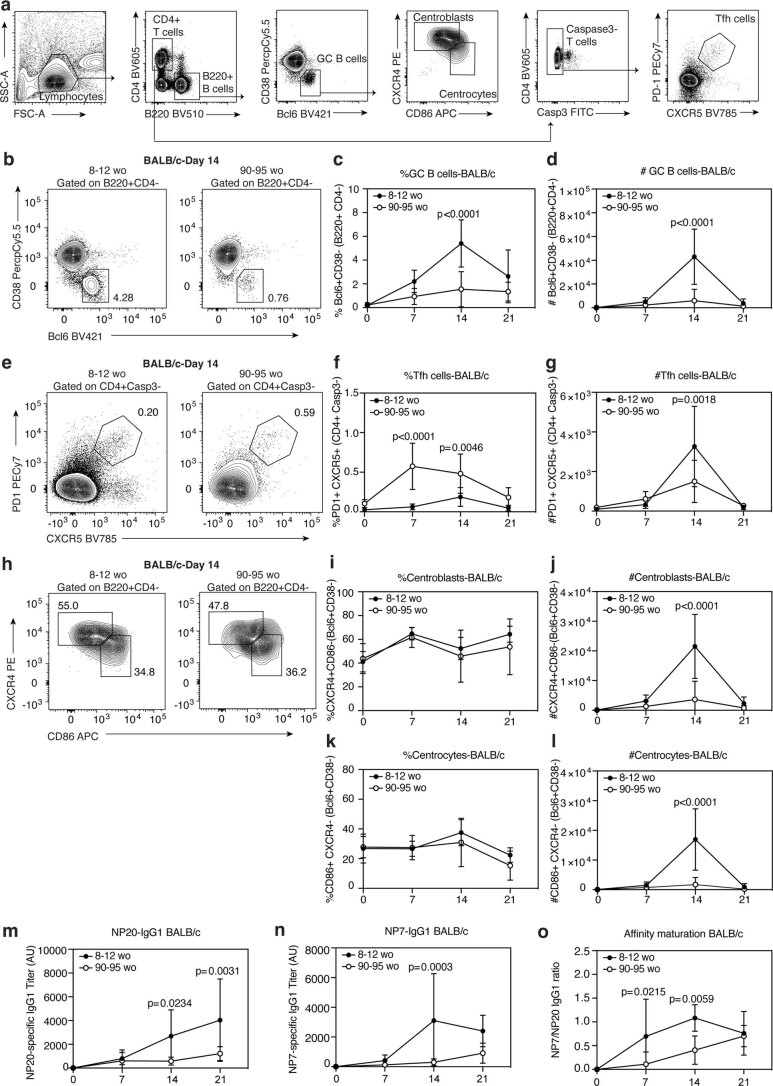
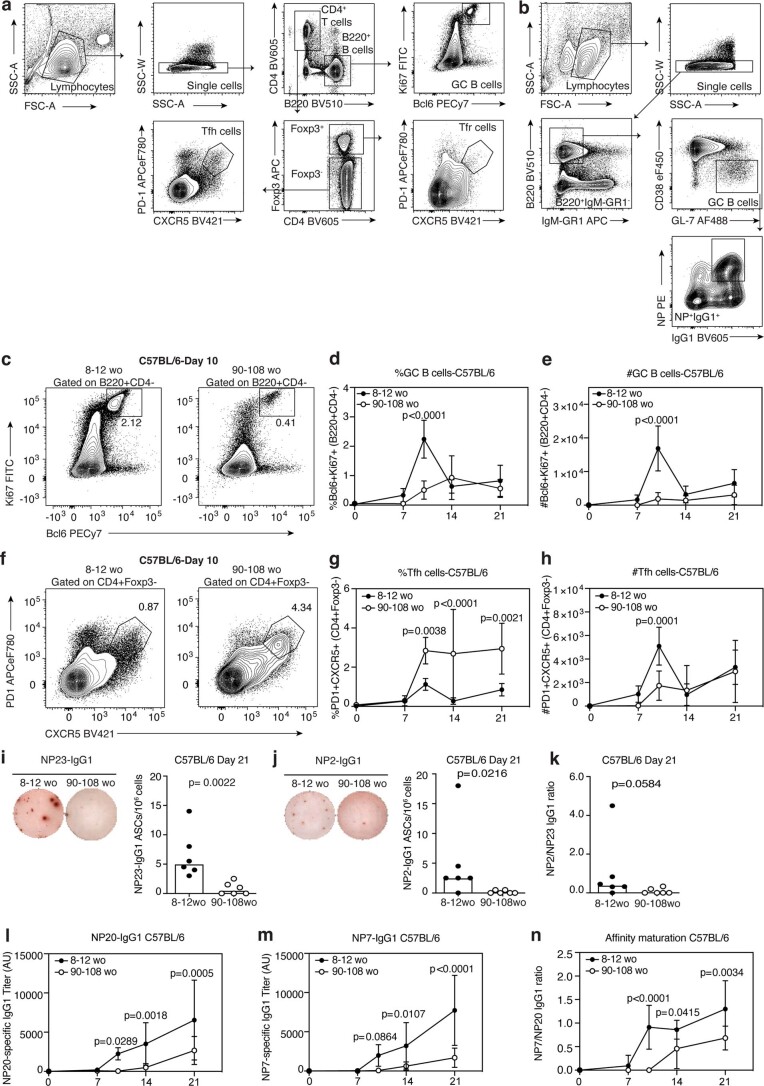
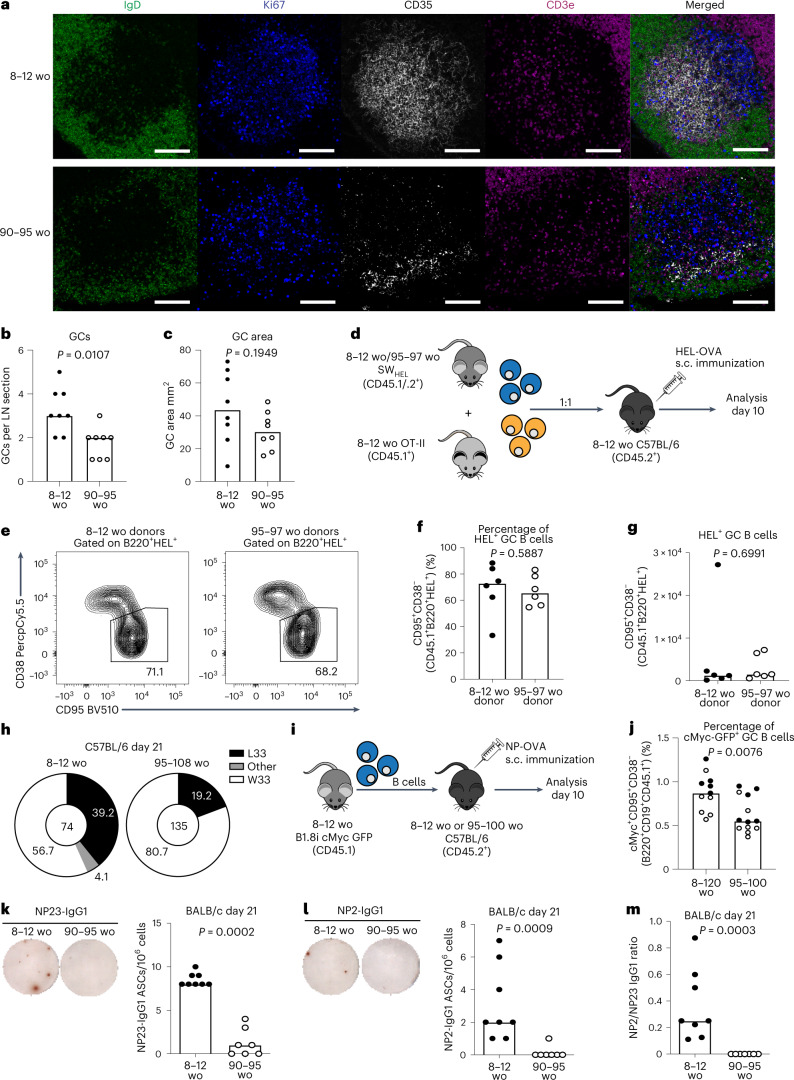
The proportion and absolute number of GC B cells were diminished at the peak of the GC response in both aged (90–108-week-old) BALB/c and C57BL/6 mice compared with younger adult (8–12-week-old) mice (Extended Data Figs. 1 and 2), consistent with previous reports13–15,23. GC formation and maintenance relies on TFH cells, which provide several B cell-supporting cues necessary throughout the GC reaction24. The proportion of TFH cells was increased with age throughout the GC reaction in both BALB/c and C57BL/6 mice, but the absolute number of TFH cells was diminished in aged mice of both strains (Extended Data Figs. 1 and 2). Comparative quantitative imaging of draining inguinal lymph node (iLN) sections showed that aged mice generated significantly fewer GCs 14 d postimmunization than adult mice (Fig. 1a–c, Supplementary Fig. 1 and Extended Data Fig. 3). This confirms that aging is associated with a poor GC response upon vaccination in two genetically different mouse strains (BALB/c and C57BL/6); however, the causal mechanism is unknown.
Extended Data Fig. 1. Kinetic analysis of GC response in aged BALB/c mice after NP-KLH/Alum immunization.
(a) Gating strategy for the analysis of GC B cells, centroblasts, centrocytes and Tfh cell populations in the iLNs of adult and aged BALB/c mice immunized with NP-KLH in alum. (b) Representative flow cytometry plots identifying GC B cells (Bcl6 + CD38−B220 + CD4-) in the inguinal lymph nodes (iLNs) of adult and aged BALB/c mice and their frequency (c) and total number (d) at the indicated timepoints post immunization. (e) Representative flow cytometry plots identifying Tfh cells (PD1 + CXCR5 + CD4 + Casp3-B220-) and their frequency (f) and total number (g) in the iLN at the indicated timepoints post immunization. (h) Representative flow cytometry plots identifying centroblasts (CXCR4hiCD86loBcl6+CD38−) and centrocytes (CD86hiCXCR4loBcl6+CD38-) and their frequency and number (i-l) at the indicated timepoints after immunization. Adult and aged BALB/c serum antibody titers of NP20 (m) and NP7 (n) specific IgG1 and antibody affinity maturation (o) as determined by the ratio of NP7/NP20 IgG1 titers, at the indicated time point post immunization. The data is representative of two independent experiments (n = 14) where each symbol represents the mean ± SD and p-values were generated by performing a two-way ANOVA with Sidak’s multiple comparisons test.
Extended Data Fig. 2. Kinetic analysis of GC response in aged C57BL/6 mice after 1W1K-NP in alum immunization.
(a) Gating strategy for the analysis of GC B cells, Tfh and Tfr populations in the iLNs of adult and aged C57BL/6 mice immunized with 1W1K-NP in alum. (b) Single-cell sorting strategy of NP + IgG1+ GC B cells from iLNs of C57BL/6 mice 21 days after immunization with 1W1K-NP in alum for VH186.2 sequencing. (c) Representative flow cytometry plots identifying GC B cells (Ki67+Bcl6+B220+CD4−) in the inguinal lymph nodes (iLNs) of adult and aged C57BL/6 mice 10 days after subcutaneous immunization with 1W1K-NP in alum and their frequency (d) and total number (e) at the indicated timepoints post immunization. (f) Representative flow cytometry plots identifying Tfh cells (PD1+CXCR5+CD4+Foxp3−B220−) in the iLNs of adult and aged C57BL/6 mice 10 days post-immunization with 1W1K-NP in alum and their frequency (g) and total number (h) at the indicated timepoints post immunization. (i-k) Representative ELISpot well images (left) and quantification (right) of bone marrow NP23 (i) and NP2 (j) specific IgG1 antibody-secreting cells (ASCs) in C57BL/6 mice 21 days after immunization. (k) Affinity maturation of bone marrow ASCs from C57BL/6 mice as determined by the ratio of NP2/NP23-specific ASCs. For (i-k) the data is representative of two independent experiments (n = 12) where each symbol represents a mouse, and the bar height represents the median. The p-values were obtained by performing an unpaired, two-tailed Mann Whitney U test. (l-n) Adult and aged C57BL/6 serum antibody titers of NP20 (l) and NP7 (m) specific IgG1 at the indicated timepoints after immunization with NP-KLH in alum and antibody affinity maturation (n) as determined by the ratio of NP7/NP20 IgG1 titers. For (l-n) antibody titers shown are displayed as arbitrary units (AU). For (c-h, l-n) The data are representative of two independent experiments (n = 16) where each symbol represents the mean ± SD and p-values were generated by performing a two-way ANOVA with Sidak’s multiple comparisons test.
Fig. 1. The GC response, and its output, is diminished in aged mice.
a, Representative confocal images of GCs at ×40 from adult and aged BALB/c mice 14 d after immunization with NP-KLH in alum; scale bars, 50 µm. LN sections were stained for IgD (green), CD35 (white), Ki67 (blue) and CD3e (magenta). b, Enumeration of GCs per LN. c, Quantification of the total GC area (n = 16). d, Experimental outline of the cotransfer of SWHEL B cells from either adult or aged donors alongside OT-II T cells from adult donors into adult C57BL/6 recipient mice in which GC formation was analyzed 10 d after immunization with HEL-OVA in alum. e, Representative flow cytometry plots identifying SWHEL-derived GC B cells (CD95+CD38−CD45.1+B220+HEL+) in recipient mice. The values next to the gates indicate the population percentage. f,g, Quantification of the frequency (f) and total number (g) of SWHEL-derived GC B cells (n = 12). h, Pie charts indicating the frequency of the affinity-inducing mutation W33L in the CDR1 region of VH186.2 sequenced from single-cell sorted NP+IgG1+ GC B cells of adult and aged C57BL/6 mice 21 d postimmunization with 1W1K-NP/alum. The values in the chart center indicate the total number of cells sequenced per group (n = 16). i, Experimental outline of the transfer of B1.8i mycGFP/GFP B cells from adult donors into adult or aged C57BL/6 recipient mice in which GC formation was analyzed 10 d after NP-OVA in alum immunization. j, Quantification of the frequency of B1.8i-derived cMyc+ GC B cells in adult or aged mice; data are pooled from two independent experiments, first experiment in black, second experiment in white (n = 23). k,l, Representative ELISpot well images (left) and quantification (right) of bone marrow NP23- (k) and NP2- (l) specific IgG1 ASCs in BALB/c mice 21 d after immunization with NP-KLH in alum. m, Affinity maturation of bone marrow ASCs from BALB/c mice as determined by the ratio of NP2/NP23-specific ASCs (n = 15). For all experiments, 2–4 experimental repeats were performed with biologically independent samples. In bar graphs, each symbol represents a mouse, and the bar height represents the median. The P values were generated by performing an unpaired two-tailed Mann–Whitney U test. ELISpot, enzyme-linked immunosorbent spot; s.c., subcutaneous; wo, weeks old.
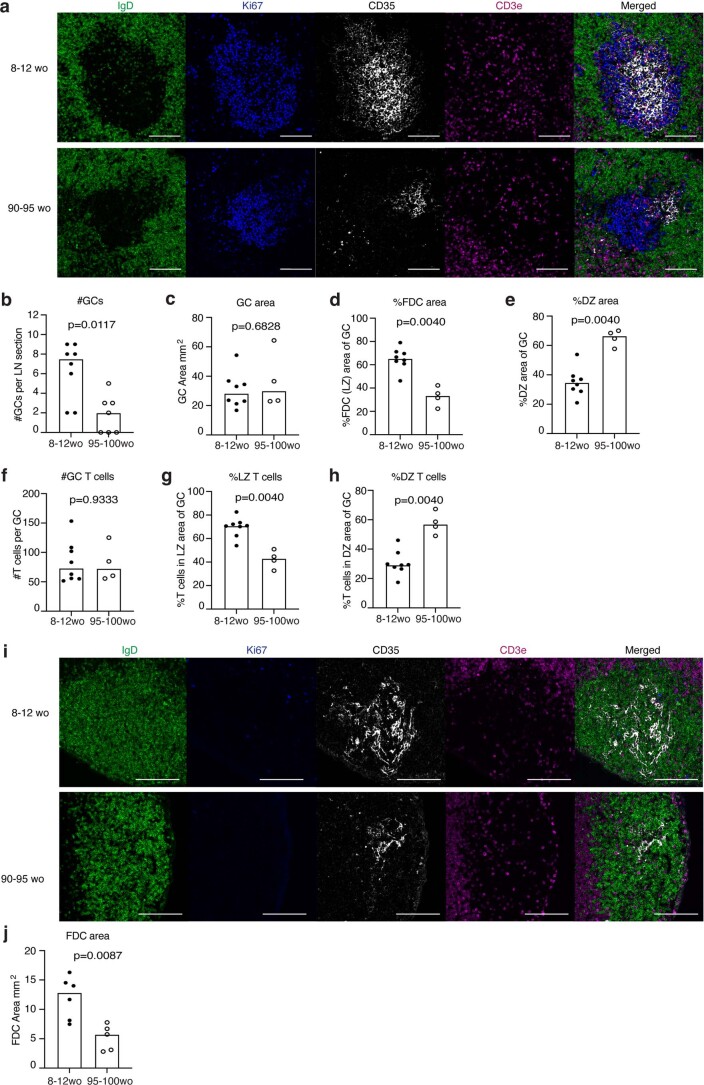
Extended Data Fig. 3. The spatial organization of the GC is disrupted in aged C57BL/6 mice.
(a) Representative confocal images of GCs at 20x from adult and aged C57BL/6 mice 12 days after immunization with NP-OVA in alum. Scale bars are 100 µm. LN sections were stained for IgD (green), CD35 (white), Ki67 (blue) and CD3 (magenta). Enumeration of GC number (b) and area (c) per LN was performed by examining 6–10 sections throughout each LN and identifying GCs as CD35+Ki67+IgD− structures. (d) Quantification of the CD35+ FDC network light zone area of the GC. (e) Quantification of the Ki67+CD35− dark zone area of the GC. (f) Quantification of the number of CD3+ T cells identified within the total Ki67+CD35+IgD− GC area. Quantification of the proportion of T cells positioned in the CD35+ FDC light zone area (g) and Ki67+CD35− dark zone (h) of the GC (n = 15). (i) Representative confocal images of GCs at 40x from unimmunized naive adult and aged C57BL/6 mice and (j) quantification of the CD35+ FDC network in the primary B cell follicle (n = 11). For (b-h, j), quantification of the GC compartments and T cell positioning was performed using an automated Cell Profiler pipeline. The data is representative of two independent experiments where each symbol on the graph represents a mouse and the bar height represents the median. The p-values were generated by performing an unpaired, two-tailed Mann Whitney U test.
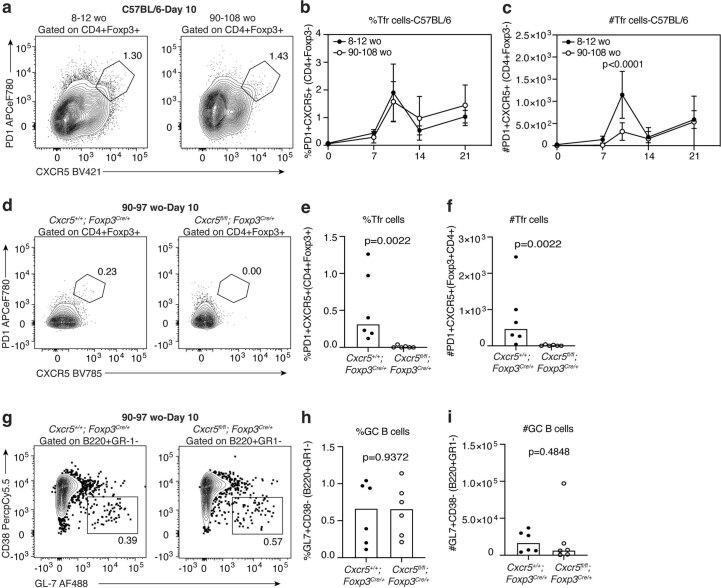
Accumulation of T follicular regulatory cells, which are key negative regulators of the GC response, has been postulated to contribute to poor GC responses in aged mice25,26, yet we found no significant differences in the proportion of T follicular regulatory cells and a reduction in their total number with age (Extended Data Fig. 4a–c). Furthermore, genetically halving the number of T follicular regulatory cells27 did not enhance the magnitude of the GC response in aged Foxp3Cre/+Cxcr5fl/fl mice (Extended Data Fig. 4d–i), indicating that the reduced GC magnitude in aging is not caused by increased T follicular regulatory cell number.
Extended Data Fig. 4. Tfr cell reduction in aged mice does not enhance GC responses.
(a) Representative flow cytometry plots identifying Tfr cells (PD1+CXCR5+CD4+Foxp3+B220−) in the iLNs of adult and aged C57BL/6 mice 10 days post-immunization with 1W1K-NP in alum. The frequency (b) and total number (c) of Tfr cells in adult and aged C57BL/6 mice was quantified at days 0, 7,10, 14 and 21 after immunization with 1W1K-NP in alum. The data is representative of two independent experiments (n = 14) where each symbol represents the mean ± SD and p-values were generated by performing a two-way ANOVA with Sidak’s multiple comparisons test. (d) Representative flow cytometry plots indicating deletion of Tfr cells in aged 90–97-week-old Foxp3creCxcr5fl/fl mice compared to aged littermate control Foxp3cre mice 10 days after immunization with NP-KLH in alum. Quantification of the frequency (e) and total number (f) of Tfr cells (n = 12). (g) Representative flow cytometry plots identifying GC B cells (GL7+CD38−B220+GR-1-) in Foxp3creCxcr5fl/fl mice 10 days after immunization with NP-KLH in alum. Quantification of the frequency (h) and total number (i) of GC B cells (n = 12). The values next to the gates on flow cytometry plots indicate the population percentage. The data is representative of two independent experiments where each symbol represents a mouse, and the bar height represents the median. The p-values were obtained by performing an unpaired, two-tailed Mann-Whitney U test.
To address whether the observed defects in GC formation could be driven by age-associated B cell-intrinsic effects, we used an adoptive transfer system of SWHEL B cells, which carry antigen specificity for hen egg lysozyme (HEL)28, from either young adult or aged SWHEL mice into young adult mice (Fig. 1d). At 10 d after immunization, both the proportion and total number of GC B cells derived from aged SWHEL donors were comparable to those derived from younger donors (Fig. 1e–g and gating strategy in Supplementary Fig. 2), implicating B cell extrinsic factors as contributors to the diminished GC formation and magnitude with age. We confirmed that there were no differences in the frequency and total number of transferred HEL-binding B cells and TFH cells derived from transferred OT-II cells from young mice between the two recipient groups (Supplementary Fig. 2). These data show that there are defects in GC formation and magnitude with age that cannot be rescued through the reduction of T follicular regulatory cell numbers and could not be accounted for by intrinsic defects of B cells in aging.
Aging impairs GC selection and affinity maturation
GC B cells increase their affinity for antigen by undergoing somatic hypermutation and selection. Sequencing of the VH186.2 heavy chain region of 4-hydroxy-3-nitrophenyl acetyl (NP)-specific GC B cells revealed that there was a near twofold reduction in the frequency of the affinity-enhancing W33L mutation in aged mice compared with younger mice (Fig. 1h). In vivo assessment of positive selection of NP-specific B cells (B1-8i-Tg) transferred from a younger adult mouse into either young or aged mice (Fig. 1i and Supplementary Fig. 2) revealed there was a lower frequency of cMyc+ GC B cells in aged recipient mice compared with younger recipients (Fig. 1j and Supplementary Fig. 2). In both aged BALB/c (Fig. 1k–m) and C57BL/6 (Extended Data Fig. 2) mice there was a clear reduction in the number of IgG1-secreting plasma cells specific for both NP23 and NP2 in the bone marrow, as well as a reduction in the ratio of high-affinity NP2-binding to NP23-binding plasma cells, compared with younger adult mice. This was accompanied by a reduction in the NP-specific antibody titers and reduced affinity maturation of serum antibodies of both aged BALB/c and C57BL/6 mice (Extended Data Figs. 1 and 2), consistent with previous reports15,29,30. These data show that both the magnitude and the quality of the GC are impaired with age and that B cells from younger adult donors do not receive positive selection signals in the aged GC as well as in younger animals.
TFH cell positioning and FDC expansion are altered in aging
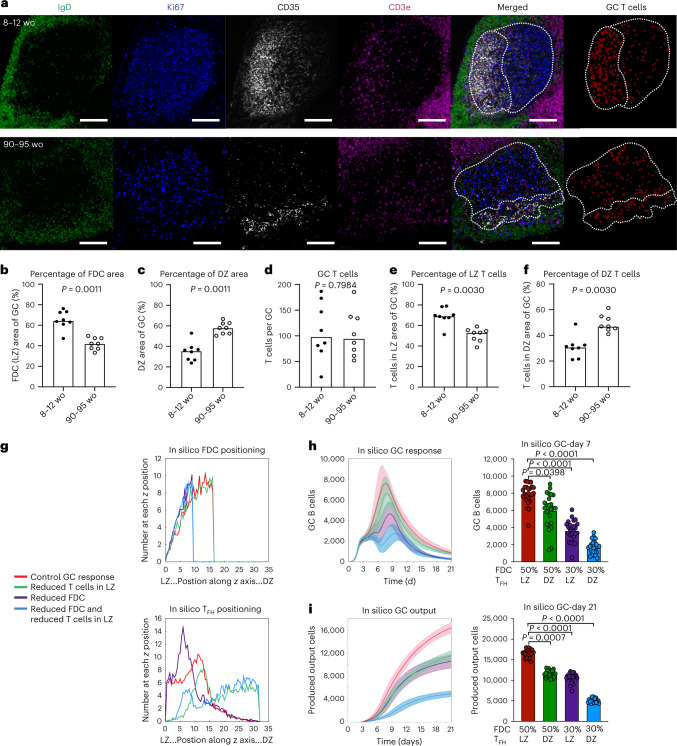
Correct structural organization of the GC allows multiple cell types to interact at the right place and at the right time. Yet, the impact of aging on the spatial organization of the GC remains unexplored. Quantitative confocal imaging of iLN sections from adult and aged mice (Fig. 2a and Supplementary Fig. 1) revealed that the area of the mesenchyme-derived FDC network within GCs of aged mice was significantly reduced compared with younger adult mice, resulting in larger dark zone areas and altered GC structure (Fig. 2b,c). The reduced FDC area was also observed in aged mice before vaccination (Extended Data Fig. 3). Despite this, the proportion of centroblasts and centrocytes as quantified by flow cytometry was not altered with age (Extended Data Fig. 1), suggesting that the structural changes observed in the GC zones are caused by the stromal compartment. The number of TFH cells per GC was comparable between adult and aged BALB/c and C57BL/6 mice (Fig. 2d), but in GCs from aged mice there was a lower proportion of TFH cells localizing to the light zone (Fig. 2e and Extended Data Fig. 3). Instead, a greater proportion of TFH cells were seen in the dark zone of aged mice (Fig. 2f). Thus, aging alters the structure of the FDC network and the spatial distribution of TFH cells. The potential outcomes of this on the GC response are twofold: (1) the reduced light zone area would reduce the amount of antigen presented to B cells and (2) the TFH cells are not closely associated with B cells that are collecting and presenting antigen in the light zone, thereby limiting the provision of TFH cell help to B cells.
Fig. 2. The spatial organization of the GC is altered in aged mice.
a, Representative confocal images of GCs at ×20 magnification from adult and aged BALB/c mice 14 d after immunization with NP-KLH in alum; scale bars, 100 µm. LN sections were stained for IgD (green), CD35 (white), Ki67 (blue) and CD3e (magenta). Representative masked images identifying GC T cells (red) generated by Cell Profiler used for enumeration (right panel). b–f, Quantification of the CD35+ FDC network light zone area (b), the Ki67+CD35− dark zone area (c) of the GC, the number of CD3+ T cells (d) within the GC area, and the proportion of T cells positioned in the CD35+ FDC light zone area (e) and Ki67+CD35− dark zone (f) of the GC. For b–f, the data are representative of four independent experiments (n = 16) where each symbol on the graph represents a mouse and the bar height represents the median. The P values were generated by performing an unpaired, two-tailed Mann–Whitney U test. g, Computational modeling of the age-associated changes to the spatial organization of the GC (control GC response in red, GC response with reduced TFH cell positioning in the light zone in green, GC response with reduced FDC network in purple and the combined effect of both defects in blue). Graphical representation of the FDC network size (top) and TFH cell positioning (bottom) within the GC compartment for the simulations performed. The units on x axes of plots are the positions of cells along the z axis in ×10 µm. h, Computational modeling of the impact on the number of GC B cells (left) with quantification at day 7 of the GC response (right). i, Computational modeling of the impact on the total number of produced output cells (left) with quantification at day 21 of the GC response (right). For h and i, lines on time-course graphs show the mean of 25 independent simulations and the shaded areas indicate the standard deviation. For bar graphs, the bar height represents the mean (n = 25). The P values indicated on the graphs were generated by performing a Kruskal–Wallis test with Dunn’s multiple comparison correction.
To disentangle the contribution of the disrupted light zone size from TFH cell localization on the GC response, and to establish whether the observed spatial changes could be causative of the poor GC responses observed with age, we performed three-dimensional spatial computational simulations31,32 of the GC. These simulations predicted that the number of GC B cells generated would be significantly impaired by either a reduction of TFH cells in the light zone or a reduction in the size of the FDC network, and even more so by the combined effect of both changes (Fig. 2g,h). Similarly, the cellular output (a combined metric of memory and plasma cells generated) of the GC was predicted to be significantly diminished by day 21 in response to the structural changes simulated, and this effect was further exacerbated by both defects simulated in combination (Fig. 2i). The simulations show that the impaired light zone area and TFH localization could explain the poor vaccine response in aged mice and support the hypothesis that impaired FDC expansion and aberrant TFH positioning synergistically contribute to the poor GC response in aging. Yet, the molecular cause(s) of these changes in spatial organization with age are unknown.
Enhanced CXCR4 localizes TFH cells to the dark zone
TFH cells preferentially localize to the light zone area of the GC using CXCR5 expression to migrate to FDC-expressed CXCL13 (ref. 33). The movement of GC B cells from the light to the dark zone is controlled by CXCR4-dependent migration to the CXCL12-expressing stromal cells in the dark zone20,21. A proportion of TFH cells located within GCs have been reported to express CXCR4 (ref. 34), and consistent with this report we observed that GC TFH cells marked by S1PR2 and low levels of CD90 have higher expression of CXCR4 than non-GC TFH cells (Extended Data Fig. 5). We hypothesized that CXCR4 may function to localize TFH cells to the dark zone and this process may be altered in aging.
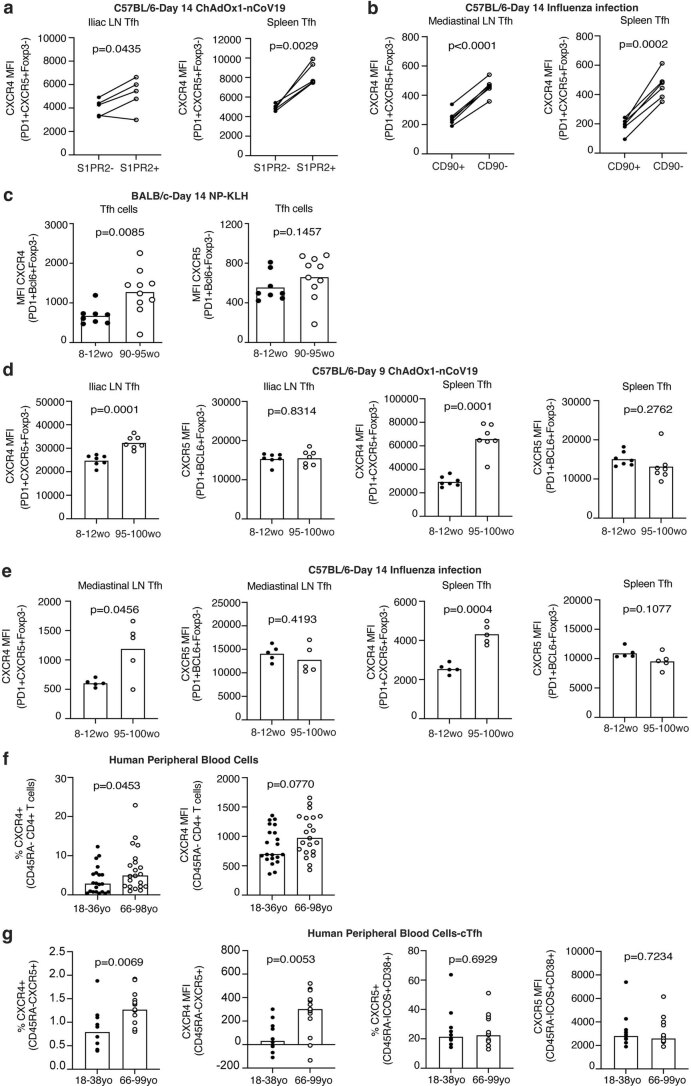
Extended Data Fig. 5. A proportion of GC Tfh cells express CXCR4 and its expression is also increased in Tfh cells from aged mice upon ChAdOx1-nCoV19 immunization and influenza infection as well as in CD4+ T cells from human peripheral blood cells.
(a) Five S1pr2ERTcre Rosa26stop-flox-RFP mice were immunized with 50uL of ChAdOx1 nCoV-19 intramuscularly, followed by oral gavage of Tamoxifen at 8- and 10-days post immunization. Dots show CXCR4 expression on S1PR2- Tfh cells and S1PR2-fatemapped GC Tfh cells 14 days after immunization (n = 10 biologically independent samples). (b) Six C57BL/6 mice were infected with influenza and CXCR4 expression assessed on CD90 + Tfh cells and CD90low GC Tfh cells 14 days after infection (n = 12 biologically independent samples). (c) Median fluorescence intensity of CXCR4 on PD1+CXCR5+Foxp3− Tfh cells and CXCR5 MFI on Bcl6+PD-1+Foxp3− Tfh cells isolated from the draining iliac of adult and aged mice 14 days after immunization with NP-KLH/Alum (n = 18). (d) Median fluorescence intensity of CXCR4 on PD1+CXCR5+Foxp3− Tfh cells and CXCR5 MFI on Bcl6+PD-1+Foxp3− Tfh cells isolated from the draining iliac LN (left) and spleen (right) of adult and aged mice 9 days after immunization with the Oxford/AstraZeneca COVID-19 vaccine candidate ChAOx1-nCoV19 (n = 14). (e) Median fluorescence intensity of CXCR4 on PD1+CXCR5+Foxp3− Tfh cells and CXCR5 MFI on Bcl6+PD-1+Foxp3− Tfh cells isolated from the draining mediastinal LN (left) and spleen (right) of adult and aged mice 14 days after infection with influenza (n = 11). (f) Flow cytometric quantification of the percentage (left) of CXCR4+ cells and CXCR4 MFI (right) from CD45RA−CD4+ T cells from human peripheral blood mononuclear cells (n = 42). (g) Flow cytometric quantification of the %CXCR4+ cells and CXCR4 MFI from CXCR5+CD45RA−CD4+ circulating Tfh cells, and %CXCR5+ cells and CXCR5 MFI on ICOS+CD38+CD45RA−CD4+ circulating Tfh cells from human peripheral blood mononuclear cells seven days after seasonal influenza vaccination (n = 34). Bar heights indicate median, each symbol represents a biological replicate and p-values were obtained by performing an unpaired, two-tailed Mann-Whitney U test. In (a-b) each symbol represents an animal, with each mouse connected by a line. P-value is from a paired non-parametric t-test.
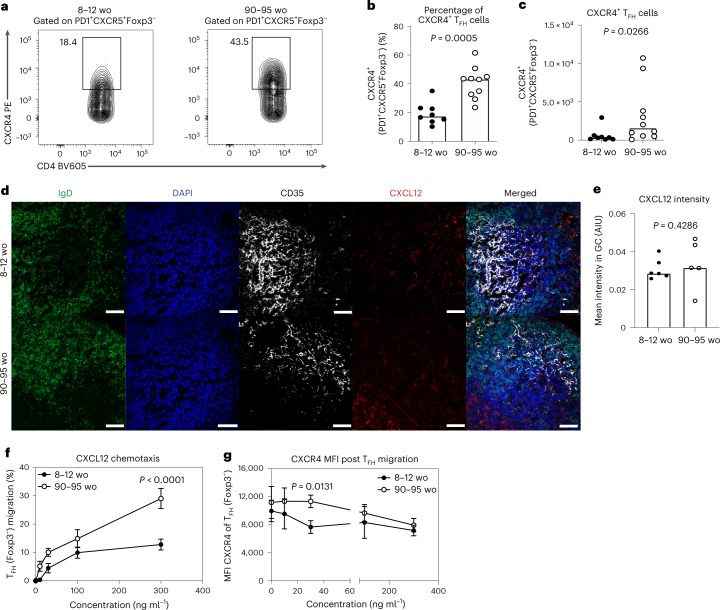
Flow cytometric analysis revealed that TFH cells from aged mice had more CXCR4+ TFH cells than younger adult mice (Fig. 3a–c), while CXCR5 expression on PD-1+Bcl6+CD4+Foxp3− TFH cells was unaltered by age (Extended Data Fig. 5). This was also observed after immunization with an adenoviral vectored COVID-19 vaccine and after influenza A virus infection (Extended Data Fig. 5). Enhanced CXCR4 expression was likewise seen in older people, with antigen-experienced CD4+ T cells from unvaccinated people over 65 yr of age having increased surface CXCR4 expression compared with 18–36-yr-old adults (Extended Data Fig. 5). The expression of CXCR4 was also higher on circulating TFH-like cells from older people 7 d after seasonal influenza vaccination compared with younger individuals, with CXCR5 expression being consistent between the age groups (Extended Data Fig. 5). Immunofluorescence staining indicated that there was comparable expression of CXCL12 within the GCs of adult and aged mice (Fig. 3d,e), suggesting that the increased dark zone positioning of TFH cells with age is likely due to an increase in the expression of CXCR4 rather than increased ligand availability in the GC dark zone. Consistent with this, the increased CXCR4 expression on TFH cells from aged mice was associated with enhanced chemotaxis towards CXCL12 in an in vitro Transwell assay (Fig. 3f). In the chemotaxis assays, TFH cells from younger mice downregulated CXCR4 after migrating to CXCL12, but this did not occur to the same extent in TFH cells from aged mice (Fig. 3g), suggesting impaired ligand-dependent internalization as the cause of enhanced CXCR4 surface expression in aging.
Fig. 3. CXCR4 expression is increased in TFH cells from aged mice.
a, Representative flow cytometry plots showing CXCR4+PD1+CXCR5+Foxp3− TFH cells in adult and aged BALB/c mice 14 d after immunization with NP-KLH in alum. Values adjacent to gates represent percentages. b,c, Quantification of the percentage (b) and total number (c) of CXCR4+PD1+CXCR5+Foxp3− TFH cells in adult and aged BALB/c mice (n = 18). Data are representative of two independent experiments. d,e, Representative ×40 confocal images (d) and quantification (e) of CXCL12 in red within the dark zone of GCs in the iLNs of adult and aged mice at day 14 postimmunization with NP-KLH in alum; IgD (green), DAPI (blue) and CD35 (white). AIU, arbitrary intensity units. n = 11 biologically independent samples. Scale bars, 50 µm. Bar heights represent the median and P values were obtained by performing an unpaired, two-tailed Mann–Whitney U test. Each symbol represents a single mouse. f,g, CXCL12 chemotaxis assays with TFH cells isolated 14 d after NP-KLH in alum immunization. f,g, Percentage of PD1+CXCR5+Foxp3− TFH cells of the total input cells that migrated to the indicated concentrations of CXCL12 (f) and median fluorescence intensity (MFI) of cell-surface CXCR4 expression (g) (n = 16). Each symbol represents the mean ± s.d. and P values are from two-way ANOVA with Sidak’s multiple comparisons test. Data are representative of two independent experiments performed with biologically independent samples. PE, phycoerythrin.
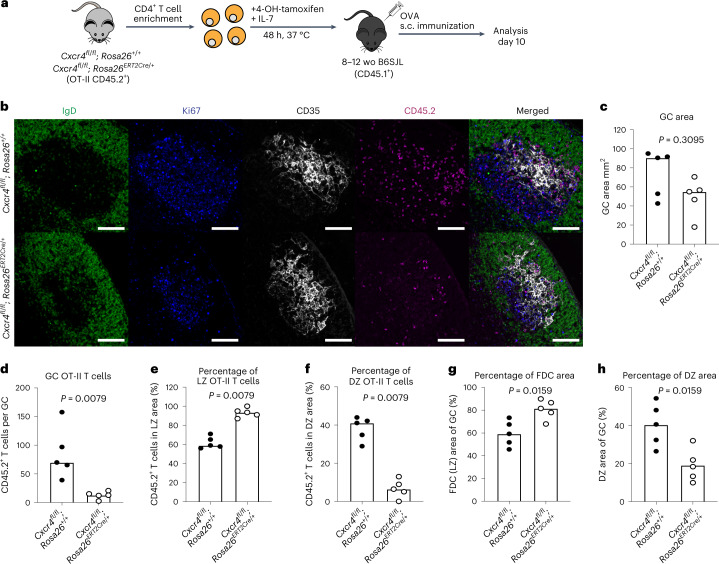
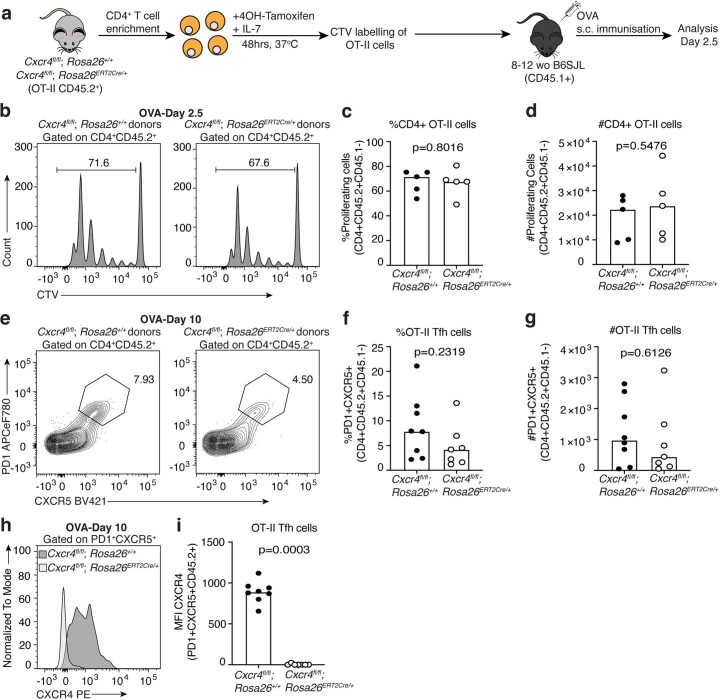
To test the functional role of CXCR4 expression in TFH localization, we used an adoptive transfer system where T cells cannot express this receptor and assessed whether the TFH cells would still localize in the dark zone. Ovalbumin (OVA)-specific (OT-II) T cells from Cxcr4fl/fl; Rosa26ERT2Cre/+ mice were isolated and treated with tamoxifen in vitro to induce deletion of Cxcr4, before being transferred into congenically distinct adult B6SJL mice (Fig. 4a). Deletion of Cxcr4 on CD4+ T cells did not alter early T cell division after activation, nor did it affect TFH cell differentiation (Extended Data Fig. 6). At 10 d after immunization, GCs had a nonsignificant trend to be smaller in recipients of CXCR4-deficient T cells (Fig. 4b,c). CXCR4-deficient TFH cells were, however, fewer in number and also enriched in the light zone, with few cells being present in the dark zone (Fig. 4d–f and Supplementary Fig. 3), showing that CXCR4 expression is necessary for dark zone positioning of TFH cells. These data demonstrate that TFH cell localization to the dark zone is actively controlled by CXCR4 expression, which is dysregulated in aging. Surprisingly, we found that the enrichment of TFH cells in the light zone increased the size of the FDC network in GCs of recipient mice, with a corresponding decrease in the dark zone (Fig. 4g,h), suggesting that T cell localization may influence the GC stroma in response to vaccination, independent of the size of the GC.
Fig. 4. CXCR4 expression determines T cell dark zone positioning.
a, Experimental outline of in vitro 4-OH-tamoxifen treatment of CD4+ T cells isolated from Cxcr4fl/fl; Rosa26ERT2Cre/+ OT-II mice that were treated for 48 h, after which the cells were transferred into adult B6SJL recipient mice. Recipient mice were immunized subcutaneously with OVA in alum and analysis was performed after 10 d. b, Representative ×20 magnification confocal images of the GCs from the iLNs of B6SJL mice that received tamoxifen-treated OT-II cells from either Cxcr4fl/fl; Rosa26+/+ or Cxcr4fl/fl; Rosa26ERT2Cre/+ mice; scale bars, 100 µm. LN sections were stained for IgD (green), CD35 (white), Ki67 (blue) and CD45.2 (magenta). c–h, Quantification of the GC area (c), the number of CD45.2+ transferred cells in the GC (d), percentages of OT-II TFH cells in the CD35+ FDC light zone area (e) and Ki67+CD35− dark zone area (f), percentage of the GC occupied by the CD35+ FDC network (g) and percentage of the GC occupied by the dark zone (h), from the iLNs of recipient B6SJL mice (n = 10). Data are representative of two independent experiments performed with biologically independent samples. In graphs, bar heights represent the median and P values were obtained by performing an unpaired, two-tailed Mann–Whitney U test. Each symbol represents a single mouse. LZ, light zone. DZ, dark zone.
Extended Data Fig. 6. Deletion of CXCR4 on T cells does not impair activation and Tfh differentiation.
(a) Experimental outline of in vitro 4-OH tamoxifen treatment of CD4+ T cells isolated from Cxcr4fl/fl; Rosa26ERT2Cre/+ OT-II mice which were treated with 200 nM of 4-OH tamoxifen for 48 hours after which the cells were labelled with cell trace violet (CTV) and transferred into adult B6SJL recipient mice. Recipient mice were immunized subcutaneously with OVA in alum and analysis was performed after 2.5 days. (b) Representative flow cytometry plots indicating CTV dilution by activated CD4+CD45.2+ T cells derived from control Cxcr4fl/fl; Rosa26ERT2+/+ (left) or Cxcr4fl/fl; Rosa26ERT2Cre/+ (right) OT-II mice. Quantification of the percentage (c) and total number (d) of proliferating CD4+CD45.2+ T cells that diluted CTV (n = 10). (e) Representative flow cytometry plots identifying PD1+CXCR5+CD4+CD45.2+ Tfh cells derived from the transferred Cxcr4fl/fl; Rosa26ERT2+/+ (left) or Cxcr4fl/fl; Rosa26ERT2Cre/+ (right) OT-II cells in the iLNs of recipient mice at day 10 after subcutaneous OVA immunization. Quantification of the percentage (f) and total number (g) of PD1+CXCR5+CD4+CD45.2+ Tfh cells derived from the transferred Cxcr4fl/fl; Rosa26ERT2+/+ or Cxcr4fl/fl; Rosa26ERT2Cre/+ OT-II cells. (h) Representative flow cytometry plot indicating the expression of CXCR4 by PD1+CXCR5+CD4+CD45.2+ Tfh cells derived from the transferred Cxcr4fl/fl; Rosa26ERT2+/+ or Cxcr4fl/fl; Rosa26ERT2Cre/+ OT-II cells. (i) Median fluorescence intensity (MFI) of CXCR4 by PD1+CXCR5+CD4+CD45.2+ Tfh cells derived from the transferred Cxcr4fl/fl; Rosa26ERT2+/+ or Cxcr4fl/fl; Rosa26ERT2Cre/+ OT-II cells (n = 15). Bar height on graphs is indicative of the median, each symbol represents a mouse and p-values were obtained by performing an unpaired, two-tailed Mann-Whitney U test. The data are representative of two independent experiments.
TFH cells in the light zone promote FDC expansion
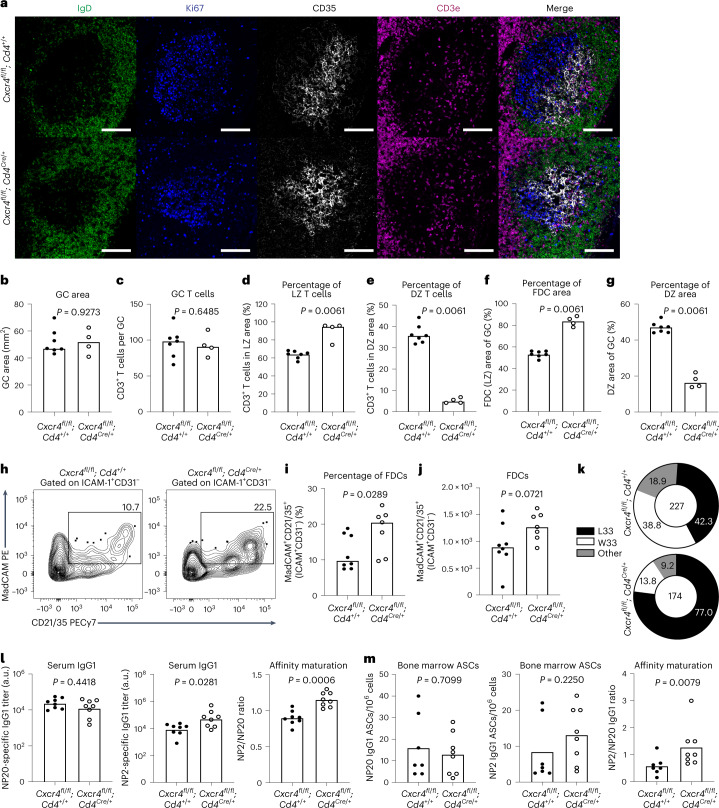
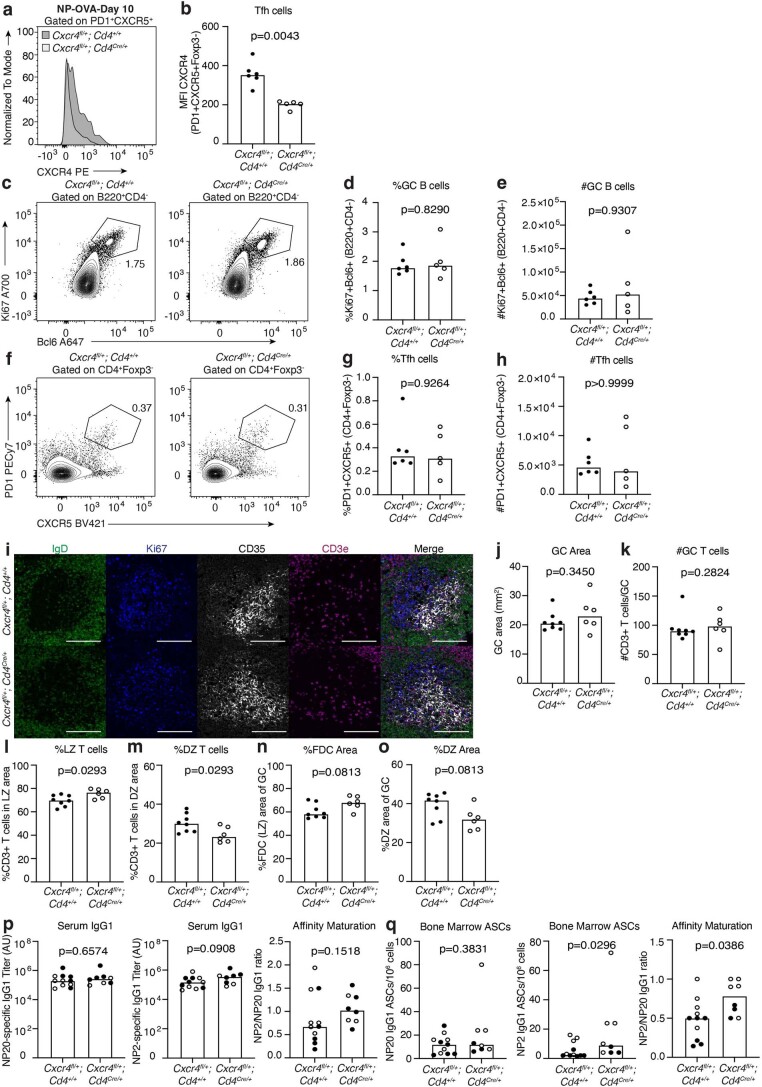
To investigate how TFH cell polarization to the light zone influences GC size and output, we immunized younger adult Cxcr4fl/fl; Cd4Cre/+ mice, which give rise to CXCR4-deficient TFH cells (Supplementary Fig. 4). At 10 d after immunization, imaging showed that GC size and TFH cell numbers were comparable in Cxcr4fl/fl; Cd4cre/+ mice and their littermate controls (Fig. 5a–c). Flow cytometric analysis showed that the percentage and total number of GC B cells and TFH cells were comparable between Cxcr4fl/fl; Cd4Cre/+ and control mice throughout the GC response at days 10, 21 and 35 postimmunizations (Supplementary Fig. 4). Despite normal numbers of GC TFH cells, the majority of CXCR4-deficient TFH cells were localized to the light zone, with less than 10% of cells in the dark zone (Fig. 5d,e and Supplementary Fig. 5). Consistent with the CXCR4-deficient OT-II transfer data (Fig. 4), we found that the enrichment of TFH cells in the light zone increased the size of the FDC network in GCs of Cxcr4fl/fl; Cd4Cre/+ mice, with a concomitant decrease in dark zone area compared with littermate control mice (Fig. 5f,g). This increase in FDC number in Cxcr4fl/fl; Cd4Cre/+ mice was confirmed by flow cytometric analysis (Fig. 5h–j and Supplementary Fig. 6). These data support a role for light zone-localized TFH cells enhancing FDC expansion upon immunization, independent of the size of the GC reaction.
Fig. 5. TFH restriction to the light zone can boost FDC expansion and alter the quality of the GC output.
a, Representative ×20 confocal images of GCs at day 10 after NP-OVA/alum immunization in the iLNs of Cxcr4fl/fl; Cd4+/+ mice (top) and Cxcr4fl/fl; Cd4Cre/+ mice (bottom); scale bar, 100 µm. LN sections were stained for IgD (green), Ki67 (blue), CD35 (white) and CD3e (magenta). b–g, Quantification of the total GC area (b), the total number of CD3+ T cells within the GC (c), and the percentage of CD3+ T cells localizing to the CD35+ light zone area (d) and the Ki67+CD35− dark zone area (e), and the CD35+ FDC light zone area (f) and the Ki67+CD35− dark zone area (g) (n = 11). h, Representative plots showing gp38+ICAM+CD31−MadCAM+CD21/35+ FDCs in adult Cxcr4fl/fl; Cd4+/+ and Cxcr4fl/fl; Cd4Cre/+ mice 10 d after immunization with NP-OVA/alum. Values adjacent to the gates represent percentages. i,j, Quantification of this population frequency (i) and total number (j) (n = 15). k, Pie charts indicating the frequency of the affinity-inducing mutation W33L in the CDR1 region of VH186.2 sequenced from NP+IgG1+ GC B cells of Cxcr4fl/fl; Cd4+/+ and Cxcr4fl/fl; Cd4Cre/+ mice at 21 d postimmunization with NP-OVA/alum. The values in the center indicate the number of cells sequenced per group (n = 11). l, Serum titers of NP20- (left) and NP2- (middle) specific IgG1 of Cxcr4fl/fl; Cd4+/+ and Cxcr4fl/fl; Cd4Cre/+ mice and antibody affinity maturation indicated by the NP2/NP20 antibody ratio (right) at 35 d postimmunization with NP-OVA (n = 16). Titers were normalized to a positive control and are displayed as arbitrary units. m, Enumeration of NP20 (left) and NP2 (middle) IgG1 ASCs and affinity maturation indicated by the ratio of NP2/NP20 ASCs (right) in the bone marrow of Cxcr4fl/fl; Cd4+/+ and Cxcr4fl/fl; Cd4Cre/+ mice at 35 d postimmunization with NP-OVA (n = 15). For all bar graphs, bar height indicates the median, each symbol represents a mouse and P values were obtained by performing an unpaired, two-tailed Mann–Whitney U test. Data are representative of two independent experiments. FDC, follicular dendritic cells. LZ, light zone. DZ, dark zone. ASCs, antibody secreting cells.
Although we did not observe an impact on GC size or GC TFH cell numbers, 21 d after NP-OVA/alum immunization there was a higher frequency of GC B cells carrying the high-affinity mutation W33L in Cxcr4fl/fl; Cd4Cre/+ mice compared with control mice (Fig. 5k). This resulted in increased affinity maturation of NP-specific IgG1 antibody in the serum of Cxcr4fl/fl; Cd4Cre/+ mice, while total titer remained unchanged (Fig. 5l). Consistent with this, the high/low affinity ratio of plasma cells from Cxcr4fl/fl; Cd4Cre/+ mice was significantly increased (Fig. 5m). We also observed increased high-affinity antibody production in heterozygous Cxcr4fl/+; Cd4Cre/+ mice, which have a mild skewing of TFH cells to the light zone (Extended Data Fig. 7). However, the effect size was not as big as in homozygous Cxcr4fl/fl; Cd4Cre/+ mice, likely due to heterozygous mice having only a ~40% reduction in expression of CXCR4 on the TFH cell’s surface. Together, these data show that TFH cell restriction to the light zone through CXCR4 deletion can enhance the expansion of the FDC network and results in higher-affinity GC responses without affecting the number of GC B cells or the area of the GC. This demonstrates that TFH cell localization and the expansion of the FDC network are entangled processes which can both be modulated by CXCR4 expression on T cells alone.
Extended Data Fig. 7. T cell-specific heterozygosity of CXCR4.
Flow cytometry plot indicating the expression of CXCR4 (a) and median fluorescence intensity (MFI) of CXCR4 (b) by PD1+CXCR5+Foxp3− Tfh cells in iLNs of Cxcr4fl/+; Cd4+/+ or Cxcr4fl/+; Cd4Cre/+ mice 10 days after NP-OVA/Alum immunisation. Flow cytometry plots (c) and quantification (d, e) of Ki67+Bcl6+ GC B cells 10 days after NP-OVA/Alum immunisation of Cxcr4fl/+; Cd4+/+ and Cxcr4fl/+; Cd4Cre/+ mice. Flow cytometry plots (f) and quantification (g, h) PD1+CXCR5+ Tfh cells 10 days after NP-OVA immunisation of Cxcr4fl/+; Cd4+/+ and Cxcr4fl/+; Cd4Cre/+ mice (n = 11). (i) Representative 20x confocal images of GCs at day 10 after NP-OVA immunisation in the iLNs of Cxcr4fl/+; Cd4+/+ mice (top) and Cxcr4fl/+; Cd4Cre/+ mice (bottom); scale bar is 100 µm. IgD (green), Ki67 (blue), CD35 (white) and CD3e (magenta). Quantification of the total GC area (j), the total number of CD3+ T cells within the GC (k), the percentage of CD3+ T cells localising to the CD35+ light zone area (l) and the Ki67+CD35− dark zone area (m), the CD35+ FDC network light zone area (n) and the Ki67+CD35− dark zone area (o) in iLNs of Cxcr4fl/+; Cd4+/+ and Cxcr4fl/+; Cd4Cre/+ mice (n = 14). (p) Serum titres of NP20 and NP2 specific IgG1 and their ratio in Cxcr4fl/+; Cd4+/+ and Cxcr4fl/+; Cd4Cre/+ mice 35 days post-immunisation with NP-OVA/Alum. (q) Enumeration of NP20 (left) and NP2 (middle) IgG1 antibody-secreting cells (ASCs) and their ratio in the bone marrow of Cxcr4fl/+; Cd4+/+ and Cxcr4fl/+; Cd4Cre/+ mice at 35 days post-immunisation with NP-OVA. Data on (p) and (q) are pooled from two independent experiments and symbol colour represents different experiments (n = 19). Bar height indicates the median, each symbol represents a mouse, and p-values from an unpaired, two-tailed Mann-Whitney U test. Data are representative of two independent experiments.
TFH restriction to the dark zone limits FDC expansion
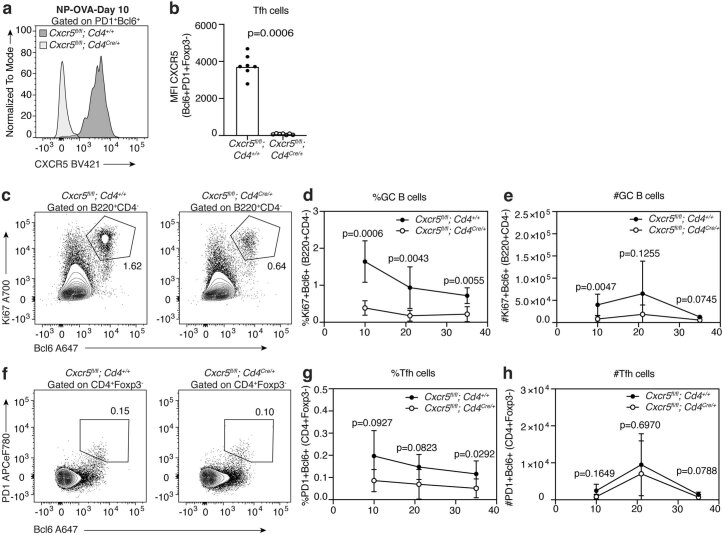
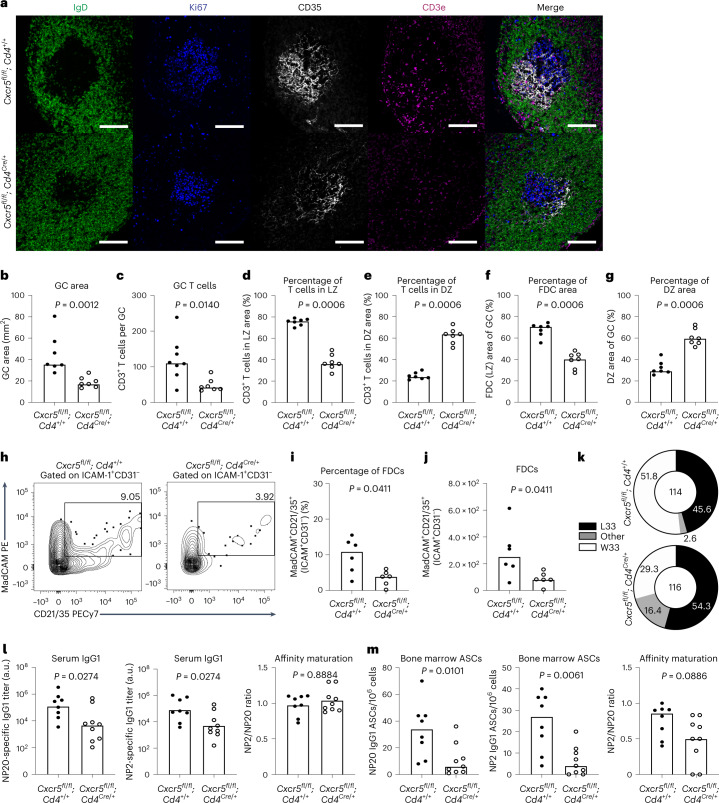
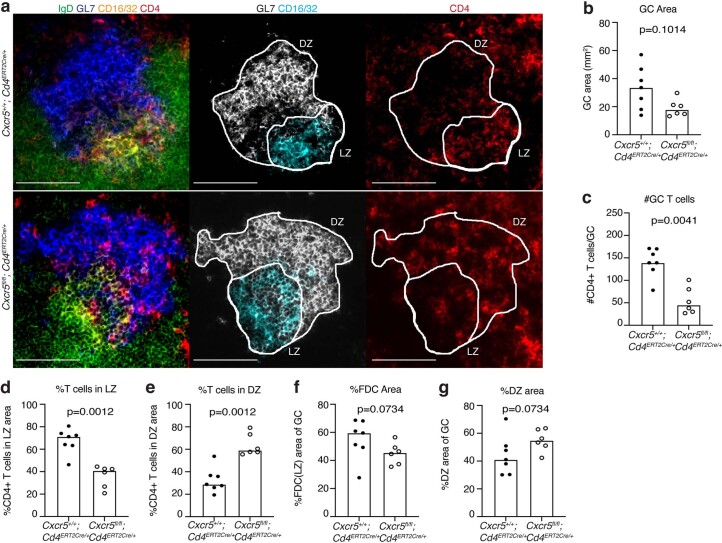
CXCR5 expression on T cells has previously been shown to localize TFH cells to the GC light zone35, the loss of which we postulated would mimic the aging GC phenotype. To test this, we generated Cxcr5fl/fl; Cd4Cre/+ mice to determine the impact of T cell-specific deletion of Cxcr5 (Extended Data Fig. 8). At 10 d after immunization, confocal microscopy showed that both GC size and the number of GC TFH cells in the iLNs of Cxcr5fl/fl; Cd4Cre/+ mice were significantly reduced compared with littermate control mice (Fig. 6a–c and Supplementary Fig. 7). Flow cytometric analysis showed a significant decrease in the percentage and number of GC B cells of Cxcr5fl/fl; Cd4Cre/+ mice throughout the GC response at days 10, 21 and 35 postimmunization (Extended Data Fig. 8). However, we found that while the proportion of TFH cells was slightly reduced in mice with T cell-specific CXCR5 deletion, there was no significant impact on the total number of Bcl6+PD-1+ TFH cells compared with control mice (Extended Data Fig. 8). By imaging, CXCR5-deficient TFH cells were predominantly localized within the GC dark zone with a significantly smaller proportion in the light zone compared with TFH cells from control mice (Fig. 6d,e). The expansion of the FDC network was significantly diminished in GCs of Cxcr5fl/fl; Cd4Cre/+ mice, resulting in a higher percentage of dark zone areas (Fig. 6f,g and Supplementary Fig. 7). This was independently validated in Cxcr5fl/fl; Cd4ERT2Cre mice, in which Cxcr5 was ablated in T cells upon tamoxifen administration on days 3 and 4 after immunization with NP-Keyhole Limpet Hemocyanin (KLH)/alum36. At 14 d after immunization, confocal microscopy confirmed the localization of CXCR5-deficient TFH cells to the dark zone and a strong trend (P = 0.07) towards a diminished FDC network in Cxcr5fl/fl; Cd4ERT2Cre mice compared with Cxcr5+/+; Cd4ERT2Cre controls (Extended Data Fig. 9). We confirmed the impaired expansion of FDCs by flow cytometric quantification (Fig. 6h–j and Supplementary Fig. 6). These data were reminiscent of the FDC reduction observed in aging and further implicated a role for TFH cell localization to the light zone in FDC expansion. Therefore, improper TFH localization to the dark zone recapitulates the smaller FDC network and smaller GCs observed in aged mice.
Extended Data Fig. 8. CD4+ T cell-specific deletion of CXCR5, flow cytometric analysis of immunized Cxcr5fl/fl; Cd4Cre/+ mice.
(a) Representative flow cytometry plot indicating the expression of CXCR5 by PD1+Bcl6+Foxp3− Tfh cells in iLNs of Cxcr5fl/fl; Cd4+/+ or Cxcr5fl/fl; Cd4Cre/+ mice 10 days after NP-OVA immunization. (b) Median fluorescence intensity (MFI) of CXCR5 by PD1+ Bcl6+Foxp3− Tfh cells of Cxcr5fl/fl; Cd4+/+ or Cxcr5fl/fl; Cd4Cre/+ mice (n = 14). Bar height on graphs is indicative of the median, each symbol represents a mouse and p-values were obtained by performing an unpaired, two-tailed Mann-Whitney U test. The data are representative of two independent experiments. (c) Representative flow cytometry plots identifying Ki67+Bcl6+ GC B cells 10 days after NP-OVA immunization of Cxcr5fl/fl; Cd4+/+ (left) and Cxcr5fl/fl; Cd4Cre/+ (right) mice; values adjacent to gates indicate percentages. Quantification of the percentage (d) and total number (e) of Ki67+Bcl6+ GC B cells at days 10, 21 and 35 after NP-OVA immunization of Cxcr5fl/fl; Cd4+/+ and Cxcr5fl/fl; Cd4Cre/+ mice. (f) Representative flow cytometry plots identifying PD1+Bcl6+ Tfh cells 10 days after NP-OVA immunization of Cxcr5fl/fl; Cd4+/+ (left) and Cxcr5fl/fl; Cd4Cre/+ (right) mice; values adjacent to gates indicate percentages. Quantification of the percentage (g) and total number (h) of PD1+Bcl6+ Tfh cells at days 10, 21 and 35 after NP-OVA immunization of Cxcr5fl/fl; Cd4+/+ and Cxcr5fl/fl; Cd4Cre/+ mice. The data is representative of two independent experiments (n = 14) where each symbol represents the mean ± SD and p-values were generated by performing a two-way ANOVA with Sidak’s multiple comparisons test.
Fig. 6. TFH restriction to the dark zone in adult mice mimics certain aspects of the aged GC response.
a, Representative ×20 confocal images of GCs at day 10 after NP-OVA/alum immunization in the iLNs of Cxcr5fl/fl; Cd4+/+ mice (top) and Cxcr5fl/fl; Cd4Cre/+ mice (bottom); scale bar, 100 µm. LN sections were stained for IgD (green), Ki67 (blue), CD35 (white) and CD3e (magenta). b–g, Quantification of the total GC area (b), the total number of CD3+ T cells within the GC (c), and the percentage of CD3+ T cells localizing to the CD35+ light zone area (d) and the Ki67+CD35− dark zone area (e), and the CD35+ FDC light zone area (f) and the Ki67+CD35− dark zone area (g) (n = 11). h, Representative plots showing gp38+ICAM+CD31−MadCAM+CD21/35+ FDCs in adult Cxcr5fl/fl; Cd4+/+ and Cxcr5fl/fl; Cd4Cre/+ mice 10 d after immunization with NP-OVA/alum. Values adjacent to the gates represent percentages. i,j, Quantification of this population frequency (i) and total number (j) (n = 15). k, Pie charts indicating the frequency of the affinity-inducing mutation W33L in the CDR1 region of VH186.2 sequenced from NP+IgG1+ GC B cells of Cxcr5fl/fl; Cd4+/+ and Cxcr5fl/fl; Cd4Cre/+ mice at 21 d postimmunization with NP-OVA/alum. The values in the center indicate the number of cells sequenced per group (n = 11). l, Serum titers of NP20- (left) and NP2- (middle) specific IgG1 of Cxcr5fl/fl; Cd4+/+ and Cxcr5fl/fl; Cd4Cre/+ mice and antibody affinity maturation indicated by the NP2/NP20 antibody ratio (right) at 35 d postimmunization with NP-OVA (n = 16). Titers were normalized to a positive control and are displayed as arbitrary units. m, Enumeration of NP20 (left) and NP2 (middle) IgG1 ASCs and affinity maturation indicated by the ratio of NP2/NP20 ASCs (right) in the bone marrow of Cxcr5fl/fl; Cd4+/+ and Cxcr5fl/fl; Cd4Cre/+ mice at 35 d postimmunization with NP-OVA (n = 15). For all bar graphs, bar height indicates the median, each symbol represents a mouse and P values were obtained by performing an unpaired, two-tailed Mann–Whitney U test. Data are representative of two independent experiments.
Extended Data Fig. 9. Imaging of GCs from Cxcr5fl/fl; Cd4ERT2Cre/+ mice after immunization.
(a) Representative confocal images of GCs at day 14 after NP-KLH/Alum immunization in the iLNs of control Cxcr5+/+; Cd4ERT2Cre/+ mice (left) and Cxcr5fl/fl; Cd4ERT2Cre/+ mice (right). Scale bars are 100 µm. LN sections were stained for IgD (green), GL7 (blue), CD16/32 (yellow) and CD4 (red). The outline of the region of interest of the GC used for analysis is outlined in white. (b) Quantification of the total area of GCs identified by immunofluorescence as GL7+CD16/32+IgD− regions in the iLNs of Cxcr5+/+; Cd4ERT2Cre/+ mice and Cxcr5fl/fl; Cd4ERT2Cre/+ mice. (c) Quantification of the total number of CD4+ T cells within the GL7+CD16/32+IgD−GC area of Cxcr5+/+; Cd4ERT2Cre/+ mice and Cxcr5fl/fl; Cd4ERT2Cre/+ mice. (d) Percentage of CD4+ T cells localizing to the CD16/32+ light zone area of the GC in Cxcr5+/+; Cd4ERT2Cre/+ mice and Cxcr5fl/fl; Cd4ERT2Cre/+ mice. (e) Percentage of CD4+ T cells localizing to the GL7+CD16/32− dark zone area of the GC in Cxcr5+/+; Cd4ERT2Cre/+ mice and Cxcr5fl/fl; Cd4ERT2Cre/+ mice. (f) Quantification of the CD16/32+ FDC network area representative of the light zone compartment of the GCs of Cxcr5+/+; Cd4ERT2Cre/+ mice and Cxcr5fl/fl; Cd4ERT2Cre/+ mice. (g) Quantification of the GL7+CD16/32− dark zone area within the GCs of Cxcr5+/+; Cd4ERT2Cre/+ mice and Cxcr5fl/fl; Cd4ERT2Cre/+ mice. Quantification was performed using ImageJ. Bar heights on graphs represent the median, each symbol represents a mouse and p-values were obtained by performing an unpaired, two-tailed Mann-Whitney U test (n = 13 biologically independent samples).
Sequencing of the VH186.2 heavy chain region of NP-specific GC B cells sorted from Cxcr5fl/fl; Cd4Cre/+ mice 21 d postimmunization showed that the frequency of the high-affinity-inducing mutation W33L was similar between Cxcr5fl/fl; Cd4Cre/+ and control mice (Fig. 6k). This was reflected in the affinity maturation of NP-specific IgG1 antibody in the serum of Cxcr5fl/fl; Cd4Cre/+ mice, which was comparable to that of control mice. However, the titers of both NP20- and NP2-specific IgG1 antibody in the serum of Cxcr5fl/fl; Cd4Cre/+ mice were significantly reduced compared with control mice (Fig. 6l). Likewise, there was only a small effect observed on the affinity ratio of bone marrow NP-specific IgG1 ASCs in Cxcr5fl/fl; Cd4Cre/+ mice compared with littermate controls, despite an evident reduction in the number of NP20- and NP2-specific IgG1 ASCs of Cxcr5fl/fl; Cd4Cre/+ mice (Fig. 6m). Taken together, these data indicate that the GC response in Cxcr5fl/fl; Cd4Cre/+ mice, which display aberrant TFH cell positioning to the dark zone, recapitulates the loss of GC magnitude and cellular output and diminished FDC expansion observed in aged mice. Therefore, the data presented support the hypothesis that aberrant TFH cell positioning contributes to poor GC responses in aging and reveal a role for TFH cell localization in the expansion of the FDC network.
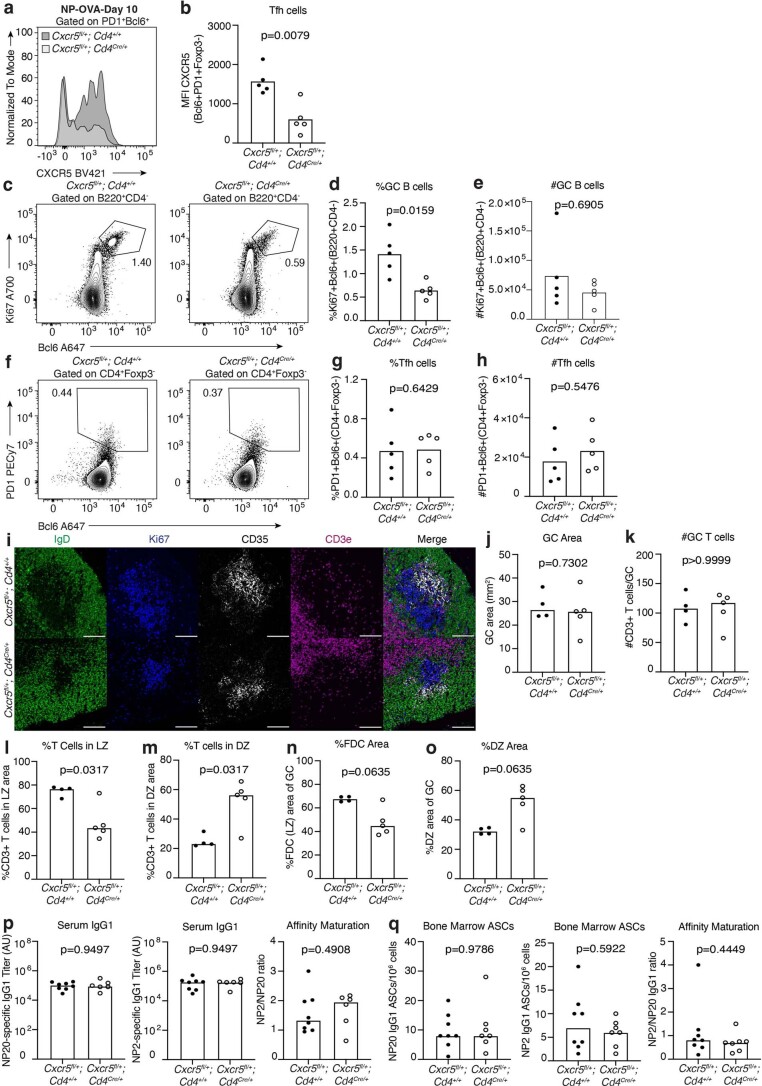
To further evaluate how TFH cell positioning towards the dark zone influences the GC, we performed similar experiments, but with mice whose T cells have only one functional allele of CXCR5, Cxcr5fl/+; Cd4Cre/+ mice. We hypothesized that TFH cells from these mice would have less surface CXCR5 expression, and an intermediate phenotype between controls and full CXCR5 T cell knockouts. At 10 d after immunization, TFH cells had a ~50% reduction in CXCR5 expression, which resulted in normal-sized GCs, and comparable numbers of GC TFH cells to control Cxcr5fl/+; Cd4+/+ mice, unlike mice that completely lack CXCR5 on their T cells. Nevertheless, heterozygosity of CXCR5 resulted in TFH cell skewing to the dark zone, and the proportion of the GC occupied by the FDC network was also diminished (Extended Data Fig. 10). This provides further evidence in support of TFH cell localization influencing the GC stroma.
Extended Data Fig. 10. T cell-specific heterozygosity of CXCR5.
Flow cytometry plot of CXCR5 (a) and its median fluorescence intensity (MFI) (b) by PD1+Bcl6+Foxp3− Tfh cells in iLNs of Cxcr5fl/+; Cd4+/+ or Cxcr5fl/+; Cd4Cre/+ mice 10 days after NP-OVA/Alum immunisation. Flow cytometry plots (c) and quantification (d, e) of Ki67+Bcl6+ GC B cells, and flow cytometry plots (f) and quantification (g, h) of PD1+Bcl6+ Tfh cells 10 days after NP-OVA immunisation of Cxcr5fl/+; Cd4+/+ and Cxcr5fl/+; Cd4Cre/+ mice (n = 11). (i) Representative 20x confocal images of GCs at day 10 after NP-OVA immunisation in the iLNs of control Cxcr5fl/+; Cd4+/+ mice (top) and Cxcr5fl/+; Cd4Cre/+ mice (bottom); scale bar is 100 µm. IgD (green), Ki67 (blue), CD35 (white) and CD3e (magenta). Quantification of the total GC area (j), the total number of CD3+ T cells within the GC (k), the percentage of CD3+ T cells localising to the CD35+ light zone area (l) and the Ki67+CD35− dark zone area (m), the CD35+ FDC network light zone area (n) and the Ki67+CD35− dark zone area (o) in iLNs of Cxcr5fl/+; Cd4+/+ and Cxcr5fl/+; Cd4Cre/+ mice (n = 14). (p) Serum titres of NP20 and NP2 specific IgG1, and their ratio of Cxcr5fl/+; Cd4+/+ and Cxcr5fl/+; Cd4Cre/+ mice 35 days post-immunisation with NP-OVA. (q) Enumeration of NP20 (left) and NP2 (middle) IgG1 antibody-secreting cells (ASCs) and affinity maturation indicated by the ratio of NP2/NP20 ASCs (right) in the bone marrow of Cxcr5fl/+; Cd4+/+ and Cxcr5fl/+; Cd4Cre/+ mice at 35 days post-immunisation with NP-OVA. Data on (p) and (q) are pooled from two independent experiments and symbol colour represents different experiments (n = 19). For all bar graphs, bar height indicates the median, each symbol represents a mouse, and p-values were obtained by performing an unpaired, two-tailed Mann-Whitney U test. Data are representative of two independent experiments.
TFH cell light zone positioning restores aged GCs and FDCs
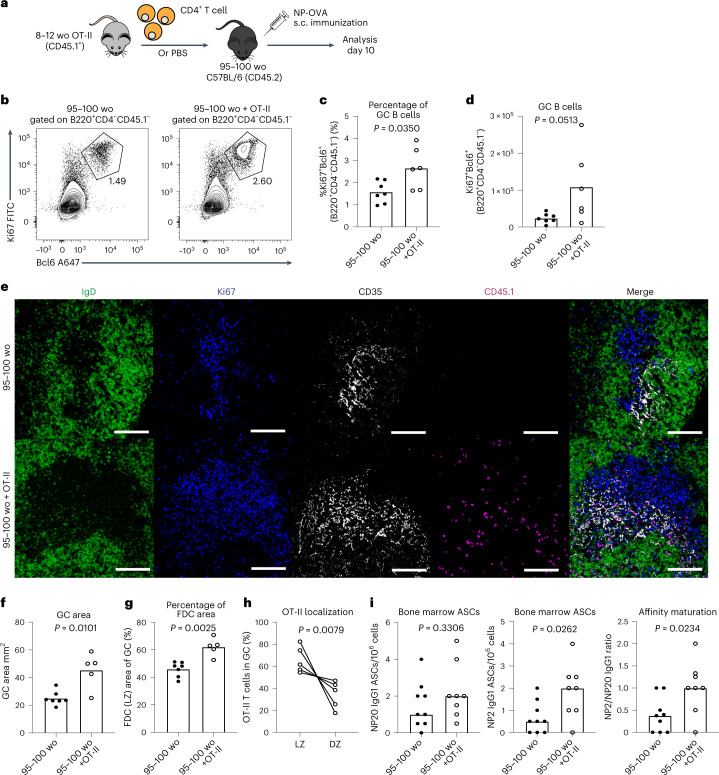
To test whether TFH cells that localize to the light zone can correct the age-associated GC defects, we transferred CD4+ OT-II T cells from young adult mice into aged mice and assessed the GC response and OT-II TFH cell positioning after NP-OVA immunization (Fig. 7a). Flow cytometry and imaging showed that supplementation of T cells boosted the GC response in aged mice (Fig. 7b–f and Supplementary Fig. 8). Quantitative imaging also revealed that the proportion of the GC occupied by the FDC network was significantly enlarged in the GCs of mice that received OT-II T cells (Fig. 7g), indicating that provision of light zone-localizing TFH cells can support the expansion of aged FDCs upon immunization. Importantly, the transferred OT-II cells correctly localized to the GC light zone (Fig. 7h), indicating that the aged GC microenvironment does not mediate aberrant TFH cell positioning to the dark zone. Of note, transfer of OT-II cells was not able to correct the delayed formation of the GC 7 d after immunization (Supplementary Fig. 8). To test whether correction of these age-dependent defects in the GC impacted humoral immunity in aging, we assessed the ability of OT-II cells to support the formation of high-affinity plasma cells. Indeed, the numbers of NP2-binding GC-derived ASCs were increased in aged mice that received OT-II cells, resulting in enhanced affinity maturation (Fig. 7i). Together, these data support that supply of light zone-localizing T cells to aged mice can expand the FDC network, increase GC size at the peak of the response and enhance GC-derived humoral immunity.
Fig. 7. T cell transfer can boost the aged GC response, FDC expansion and humoral immunity.
a, Experimental outline of the adoptive transfer of CD4+ T cells, isolated from adult OT-II mice, into aged C57BL6 recipients which were then subcutaneously immunized with NP-OVA/alum and analyzed 10 d after immunization. Control aged C57BL/6 recipient mice were injected with PBS instead of OT-II cells. b–d, Representative flow cytometry plots (b) and the percentage (c) and total number (d) of Ki67+Bcl6+ GC B cells in aged C57BL/6 mice that received either an injection of PBS (left) or CD4+ OT-II T cells (right); values adjacent to gates indicate percentage (n = 13). e, Representative confocal images of GCs at day 10 after NP-OVA immunization in the iLNs of aged C57BL/6 mice that received either an injection of PBS (top) or CD4+ OT-II T cells (bottom). Images were taken at ×20 magnification; scale bar, 100 µm. LN sections were stained for IgD (green), Ki67 (blue), CD35 (white) and CD45.1 (magenta). f–h, Quantification of the total area of GCs (f), the CD35+ FDC network area (g) and the percentage of transferred OT-II cells in the light or dark zones (h) in the iLNs of aged C57BL/6 mice that received an injection of PBS or CD4+ OT-II T cells (n = 12). i, Enumeration of NP20 (left) and NP2 (middle) IgG1 ASCs and affinity maturation indicated by the ratio of NP2/NP20 ASCs (right) in the bone marrow of aged mice that received OT-II cells or PBS at 35 d postimmunization with NP-OVA/alum (n = 17). For all bar graphs, bar height indicates the median, each symbol represents a mouse and P values were obtained by performing an unpaired, two-tailed Mann–Whitney U test. In h, P value is from a paired t-test, and individual mice are connected with a line. Data are representative of two independent experiments.
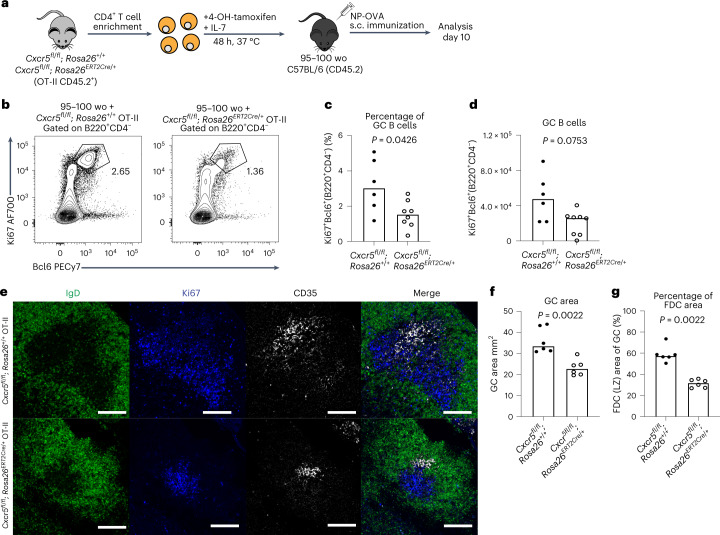
CXCR5-deficient T cells cannot rescue the aged GC response
To determine whether the observed correction of the GC and FDC response in aged mice was indeed being driven by TFH cell positioning to the light zone rather than simply the transfer of T cells from a young animal, we took advantage of the knowledge that T cells that lack CXCR5 can access the GC but are not enriched in the light zone35 (Fig. 6). CXCR5-deficient CD4+ OT-II T cells were generated by treating cells from young adult Cxcr5fl/fl; Rosa26ERT2Cre/+ mice or control OT-II cells with tamoxifen ex vivo, and then these cells were transferred into aged mice who were then immunized (Fig. 8a). At 10 d after NP-OVA immunization, the GC responses in the aged mice that received CXCR5-deficient OT-II cells were significantly reduced compared with the response in aged mice that received CXCR5-sufficient OT-II cells (Fig. 8b–d). This was also visible by confocal microscopy, with only CXCR5-sufficient OT-II cells increasing the GC area and proportion of the GC occupied by FDCs (Fig. 8e–g and Supplementary Fig. 9). Together, the data indicate that CXCR5-dependent colocalization of TFH cells with CXCL13-producing FDCs is essential for facilitating the expansion of the aged FDC network and boosting defective GC responses upon vaccination.
Fig. 8. T cell light zone positioning can boost the aged GC response and FDC expansion.
a, Experimental outline of in vitro 4-OH-tamoxifen treatment of CD4+ T cells isolated from Cxcr5fl/fl; Rosa26ERT2Cre/+ OT-II mice that were treated for 48 h, after which the cells were transferred into aged C57BL/6 recipient mice. Recipient mice were immunized subcutaneously with NP-OVA in alum and analysis was performed after 10 d. b, Representative flow cytometry plots identifying Ki67+Bcl6+ GC B cells in aged C57BL/6 mice that received either Cxcr5fl/fl; Rosa26+/+ (left) or Cxcr5fl/fl; Rosa26ERT2Cre/+ (right) CD4+ OT-II T cells; values adjacent to gates indicate percentage. c,d, Quantification of the percentage (c) and total number (d) of Ki67+Bcl6+ GC B cells in aged C57BL/6 mice that received either Cxcr5fl/fl; Rosa26+/+ or Cxcr5fl/fl; Rosa26ERT2Cre/+ OT-II cells (n = 14). e, Representative ×20 confocal images of GCs at day 10 after NP-OVA immunization in the iLNs of aged C57BL/6 mice that received an injection of either Cxcr5fl/fl; Rosa26+/+ (top) or Cxcr5fl/fl; Rosa26ERT2Cre/+ (bottom) OT-II cells; scale bar, 100 µm. LN sections were stained for IgD (green), Ki67 (blue) and CD35 (white). f,g, Quantification of the total area of GCs (f) and the CD35+ FDC network area representative of the light zone compartment within the GCs (g) of aged C57BL/6 mice that received an injection of either Cxcr5fl/fl; Rosa26+/+ (left) or Cxcr5fl/fl; Rosa26ERT2Cre/+ (right) OT-II cells (n = 12). For bar graphs, bar heights represent the median, each symbol represents a mouse and P values were obtained by performing an unpaired, two-tailed Mann–Whitney U test. Data are representative of two independent experiments.
Discussion
A functional GC response is at the heart of successful responses to vaccination. When the GC is impaired, such as in aging, vaccine efficacy is poor, leading to the need for additional vaccine doses to bolster antibody-mediated immunity. Here, we show that mislocalization of TFH cells to the CXCL12-rich dark zone of aged GCs could be accounted for by the increased expression of CXCR4 on TFH cells from aged mice and their enhanced chemotaxis towards CXCL12, as CXCR5 expression on CD4+ T cells is unaltered by age37. The increased expression of CXCR4 is a feature of aging T cells that is conserved between mice and humans38,39, suggesting a common mechanism underpinning its dysregulation. Cell-surface expression of CXCR4 is downregulated after interaction with its ligand40. Our confocal imaging of CXCL12 showed no difference in ligand expression in the GC dark zone of aged mice, which, combined with the observation that OT-II cells from young mice do not mislocalize to the GC dark zone in aged hosts, suggests that T cell-intrinsic alterations in CXCR4 internalization and/or degradation are likely responsible for its increased surface expression in aging. After binding CXCL12, CXCR4 is internalized and sorted through the endosomal compartment, where it is either recycled back to the plasma membrane or is degraded. Our data show that ligand-dependent CXCR4 internalization is defective on TFH cells from aged mice, indicating that CXCR4 proteostasis is impaired in aging, likely due to impaired ubiquitination previously described for peripheral blood T cells from older people38,39.
TFH cells are known to act within the light zone of the GC, where they provide cytokine and costimulatory signals to B cells to promote their survival and proliferation in the GC. The data presented here show that an imbalance in the light zone to dark zone distribution of TFH cells has a profound effect on GC function, and identified CXCR4 is a key controller of this distribution. This complements previous work showing that light zone polarization of TFH cells requires CXCR5 (ref. 35). By genetically modulating CXCR4 and CXCR5, we demonstrated that TFH cell positioning is important for both the magnitude and quality of the GC response. Moving TFH cells away from the light zone reduces the size of the GC and diminishes its output, consistent with a role for TFH cells in supporting the proliferation of GC B cells. By polarizing TFH cells to the light zone, the affinity of GC B cells and their progeny was increased. This was an unexpected finding as the current model of TFH cell-driven B cell selection in the GC suggests that high-affinity clones compete with each other for T cell help41, and enhancing the number of TFH cells in the light zone should relax this competition. The enhanced affinity observed would be consistent with prolonged retention of B cells and increased exposure to antigen within the GC31,42, which may be in turn regulated by the TFH cell location-dependent expansion of the FDC network we report here.
Upon immunization, FDCs increase in number, expand into the GC, upregulate the expression of various cell-surface receptors and display immune complexes on their surface that provide an antigen depot for GC B cells to access33,43. It is known that FDCs can sense danger signals directly to facilitate their response to immunization44, but to our knowledge it has not been reported that interactions with lymphocytes support the expansion of mature FDCs. Our data demonstrate that FDC expansion into the GC requires TFH cells to express CXCR5 which facilitates their colocalization with the CXCL13-producing FDCs. This prompts the question of whether the interaction is direct, or whether it occurs via additional TFH help to GC B cells which in turn promote FDC responses to vaccines. Of note, we did not observe any changes in the number of GC B cells, or their phenotype, in our Cxcr4fl/fl Cd4cre/+ mice after immunization, only an increase in the affinity of those GC B cells, indicating that B cell phenotypes are not grossly altered by TFH cell polarization to the light zone. Thus, this study brings to light a role for TFH cells in helping the GC stroma upon vaccination, but how this help is given remains to be elucidated.
Aging is a multifaceted process, and the mechanisms by which it alters the GC reaction are complex due to the number of processes required to coordinate key cellular interactions across time and space for a successful response1,45. There are three clear defects in the GC reaction with age: its formation is delayed, its size is smaller at its peak and fewer high-affinity plasma cells are produced. We and others have previously shown that the delayed formation of the GC is due to the age of the LN microenvironment, caused by a diminished response in both conventional dendritic cells and MAdCAM-1-expressing LN stromal cells. However, correction of these impairments through the use of TLR7 and TLR4 agonists could not restore the diminished size of the GC at its peak or the impaired production of high-affinity ASCs12,46–48. Here, we show that the transfer of T cells can both boost GC size at the peak of the response (day 10 postimmunization) and increase the number of high-affinity bone marrow plasma cells. However, we did not boost the GC at day 7 postimmunization simply by giving T cells alone, suggesting T cells cannot rescue the delay in GC formation. Together, these studies show that the delay in GC formation is caused by nonmigratory cells in the LN that cannot be corrected by young T cells, while the defective GC size at its peak and output of high-affinity cells are driven by CXCR4-dependent mislocalization of TFH cells. This indicates that effective strategies for enhancing vaccine responses in older people will need to concomitantly address age-dependent changes in both the microenvironment and TFH cells.
Methods
Human cohort and blood sample isolation
Healthy adults were recruited through the NIHR Bioresource before and 7 d after the seasonal influenza vaccine19,49. Samples were collected between October 2014 and February 2015, and between October and December 2016, n = 37 participants 18–36 yr old, n = 39 participants 66–98 yr old. Of the participants, 58% self-identified as female. Venous blood was collected into EDTA-coated tubes and peripheral blood mononuclear cells were isolated using Histopaque-1077 (Sigma) density gradient, then frozen in FBS supplemented with 10% dimethylsulfoxide (Sigma) and stored in liquid nitrogen before flow cytometry.
Mouse maintenance and husbandry
C57BL/6, BALB/c, OT-II TCR-Tg (ref. 50), B6SJL, SWHEL BCR-Tg (ref. 28), B1.8i BCR-Tg (ref. 51), Cd4Cre/+ (ref. 52), Rosa26ERT2Cre/+ (ref. 53), Cxcr4fl/fl (ref. 54) and Cxcr5fl/fl (ref. 55) mice were bred and maintained at the Babraham Institute Biological Support Unit; Cd4ERT2Cre/+ (ref. 56) and Cxcr5fl/fl (ref. 55) mice were bred and maintained at the Core Facility Animal Models of the Biomedical Center of LMU Munich. Mice were housed under pathogen-free conditions and were kept at an ambient temperature of ~19–21 °C with 52% relative humidity. Once weaned, mice were kept in individually ventilated cages with 1–5 mice per cage and were fed CRM (P) VP diet (Special Diet Services) ad libitum. Aged male C57BL/6 and male and female SWHEL mice were 95–108 weeks old at the start of experiments, while aged female BALB/c mice were 90–95 weeks old. All other male and female adult mice used were 8–14 weeks old.
Subcutaneous immunizations
Mice were immunized subcutaneously on both lower flanks with 100 µl of: 50 µg of NP-KLH (no. N-5060-25, Biosearch Technologies), 20 µg of 1W1K-NP (ref. 12), 50 µg of OVA (no. A5503, Sigma-Aldrich), 10 µg of OVA-HEL (no. 62970, Sigma-Aldrich; conjugated in-house using the SoluLink Protein-Protein Conjugation Kit, no. S-9010-1, TriLink BioTechnologies) or 50 µg of NP-OVA (no. N-5051-100, Biosearch Technologies) in a 1:1 ratio of PBS with Imject Alum (no. 77161, Thermo Fisher Scientific).
Flow cytometry
For mouse lymphocyte staining, single-cell suspensions from iLNs were prepared by mechanical disruption of the tissues through a 70-µm mesh in 2% FBS in PBS. For stromal cell staining, single-cell suspensions from iLNs were prepared by enzymatic digestion with 0.2 mg ml−1 Collagenase P (no. 11213865001, Sigma), 0.8 mg ml−1 Dispase II (no. 4942078001, Sigma) and 0.1 mg ml−1 DNase I (no. 10104159001, Sigma) in plain RPMI medium (no. 11875093 Gibco). The cell number and viability of samples were acquired using a CASY TT Cell Counter (Roche). For both mouse and human work, cells were stained with surface antibody stains (Supplementary Tables 1 and 2) for 30 min to 2 h at 4 °C in Brilliant stain buffer (no. 563794, BD Biosciences), then washed with 2% FBS in PBS and fixed using the Foxp3/Transcription Factor Staining Buffer Set (no. 00-5323-00, eBioscience). For intracellular staining, cells were incubated for 1 h at 4 °C with the appropriate antibodies. Samples were acquired on an LSR Fortessa (BD Biosciences) using BD FACSDiva software v.9.0 or on a Cytek Aurora Spectral Cytometer (Cytek) using SpectroFlo Software v.3.0, and analysis was done using FlowJo v.10 software (Tree Star).
Single-cell sorting for VH186.2 PCR and sequencing
Single B220+IgM-GR1-NP+IgG1+ GC B cells were single-cell sorted into 96-well plates containing 10 µl of reverse transcription lysis buffer (2 U µl−1 RNase inhibitor (no. EO0381, Thermo Fisher Scientific), 4 mM dithiothreitol (no. 43816, Sigma), 30 ng µl−1 Random Hexamers (no. SO142, Thermo Fisher Scientific), 1% NP40 and 0.2 × PBS) using an Aria III Cell Sorter (BD Biosciences). Reverse transcription, nested PCR and sequencing were performed according to a published protocol57. Briefly, complementary DNA was made from single cells then was used for nested PCR with 20 pmol of the following primers: forward, GCTGTATCATGCTCTTCTTG; and reverse, GGATGACTCATCCCAGGGTCACCATGGAGT. The PCR product was then diluted 30 times and 1 µl was used in the second round of PCR, which was prepared with the HotStar Taq DNA polymerase kit (no. 203205, QIAGEN) and 20 pmol of the following primers: forward, GGTGTCCACTCCCAGGTCCA; and reverse, CCAGGGGCCAGTGGATAGAC. The PCR product was purified using the ExoSAP-IT PCR Product Cleanup Reagent (no. 78201, Applied Biosystems) and purified samples were sent for Sanger sequencing to Source Bioscience, UK. Analysis was performed using an automated alignment pipeline in R v.4.0.4 which aligned sequences to the VH186.2 sequence, and the frequency of the affinity-inducing W33L mutation was identified for each sequence. Briefly, sequence trace analysis was automated in R, as follows. Sequence traces were read using the readsangerseq function with default parameters from the sangerseqR package58. Quality control was applied: sequences shorter than 300 nucleotides or containing more than one N base call were removed. The W33L locus was identified using matchPattern function—allowing 2 nucleotide mismatches and indels—from the Biostrings package, searching for ACCAGCTACTNNATGCACTGG in the reverse complemented sequence data. A W or L call was assigned as follows: if TNN (in the appropriate position in the nucleotide string above) was TGG, the assignment was W; if TNN was TTA or TTG, this was assigned L; any alternative sequences for TNN were assigned other. Per sample calls were exported as a .csv for downstream analysis in Prism.
Chemotaxis assays
Chemotaxis assays for CXCL12 were performed using lymphocytes isolated from draining LNs at day 14 postimmunization and washed in complete RPMI medium (no. 11875093 Gibco supplemented with 10% (v/v) FBS (no. F9665, Sigma), 1% (v/v) Penicillin-Streptomycin (no. 15140-122, Thermo Fisher Scientific), 10 mM HEPES (pH 7.2–7.5, no. 15630-056, Gibco), 0.1 mM MEM nonessential amino acid solution (no. 11140-035, Gibco), 1 mM sodium pyruvate (no. 11360-039, Gibco), 55 µM β-mercaptoethanol (no. 21985023, Thermo Fisher Scientific)). Cells were resuspended at 1 × 107 cells per ml in complete RPMI supplemented with Protease-free BSA (no. 05479-10G, Merck Life Sciences) and rested for 30 min at 37 °C, 5% CO2. Recombinant murine CXCL12 (no. 250-20A, Peprotech) was serially diluted and seeded into the bottom compartment of a 6.5-mm Transwell plate with 5.0-µm-pore polycarbonate membrane inserts (no. CLS3421, Corning Sigma-Aldrich) and plates were incubated for 15 min at 37 °C, 5% CO2. Cells were then left to migrate for 2.5 h at 37 °C, 5% CO2, after which they were collected from the bottom well and stained for flow cytometry analysis as previously described.
Tamoxifen CD4+ OT-II T cell cultures
Cell suspensions were prepared as described above under sterile conditions and were enriched for CD4+ T cells using the MagniSort Mouse CD4 T cell Enrichment Kit (no. 8804-6821-74, Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells were cultured at a concentration of 2 × 106 cells per well in complete RPMI medium containing 200 nM 4-OH-tamoxifen (no. SML1666, Sigma) and 2 ng ml−1 recombinant murine IL-7 (no. 217-17, Peprotech) for 48 h at 37 °C, 5% CO2. Following incubation, cells were washed with prewarmed RPMI medium and used for adoptive transfer.
Adoptive cell transfers
To perform adoptive T cell and B cell transfers, lymphocytes were isolated from spleens and peripheral skin-draining LNs (brachial, axial, superficial cervical and inguinal LNs) of SWHEL, B1.8i, OT-II, Cxcr4fl/fl; Rosa26ERT2Cre/+ OT-II or Cxcr5fl/fl; Rosa26ERT2Cre/+ OT-II mice. Cell suspensions were prepared as previously described under sterile conditions. For B1.8i or SWHEL B cell transfers, B cells were enriched using the MagniSort Mouse B cell Enrichment Kit (no. 8804-6827-74, Thermo Fisher Scientific), according to the manufacturer’s instructions. For OT-II cell transfers, CD4+ T cells were enriched using the MagniSort Mouse CD4 T cell Enrichment Kit (no. 8804-6821-74, Thermo Fisher Scientific), according to the manufacturer’s instructions. For transfer assessing the proliferation of OT-II cells, the CellTrace Violet Cell Proliferation Kit (no. C334557, Invitrogen) was used to stain CD4+ OT-II cells for 15 min at 37 °C. For all adoptive cell transfers, aliquots of donor cells were taken and stained to determine the proportion of antigen-specific cells by flow cytometry before transfer. For SWHEL B cell and OT-II T cell cotransfers, cells were resuspended in 2% FBS in PBS at a concentration of 2 × 106 HEL-binding B220+ B cells and 2 × 106 TCRVa2+TCRVb5+CD4+ T cells per ml and mixed at a 1:1 ratio for transfer. For CellTrace Violet-stained OT-II T cell transfers, cells were resuspended at a concentration of 5 × 106 TCRVa2+TCRVb5+CD4+ T cells per ml, and for all other OT-II transfers, cells were resuspended at a concentration of 5 × 105 TCRVa2+TCRVb5+CD4+ T cells per ml. For B1.8i B cell transfers, cells were resuspended at a concentration of 1 × 105 NP+B220+ B cells per ml. Donor cells were then injected intravenously into the tails of congenic recipient mice, and each mouse received 100 µl of cells. Recipient mice were then immunized subcutaneously with HEL-OVA, OVA or NP-OVA as described above, and iLNs were collected at the appropriate time points for flow cytometry and microscopy analysis.
ELISAs
ELISA plates (no. 456537, Thermo Fisher Scientific) were coated overnight at 4 °C with 10 µg ml−1 NP20-BSA (no. N-5050H-100, Biosearch Technologies), 2.5 µg ml−1 NP7-BSA (no. N-5050L-100, Biosearch Technologies) or 2.5 µg ml−1 NP2-BSA (no. N-5050L-100, Biosearch Technologies) in PBS. Plates were washed and blocked with 2% (w/v) BSA in PBS for 1 h at 20 °C, then washed, and sera were loaded at a starting dilution of 1:200 in 1% (w/v) BSA in PBS and titrated down the plate at a 1:4 ratio. The plates were incubated for 2 h at 20 °C and then washed. Detection of NP-specific antibodies was performed with either polyclonal goat anti-mouse IgG1 (no. ab97240, Abcam) or IgM (no. ab97230, Abcam) HRP-conjugated antibodies. The plates were developed with 100 µl per well of TMB solution (no. 421101, BioLegend) for up to 20 min, when the reaction was stopped with 50 µl per well of 0.5 M H2SO4. The absorption was measured at 450 nm using the PHERAstar FD microplate reader (BMG Labtech) with PHERAstar FSX software v.5.7.
Bone marrow enzyme-linked immunosorbent spot assays
MultiScreen-HA mixed cellulose ester plates (no. MAHAS4510, Millipore Merck) were coated with 10 µg ml−1 NP23-BSA, 10 µg ml−1 NP20-BSA or 5 µg ml−1 NP2-BSA in PBS overnight at 4 °C. Plates were then washed with PBS and blocked with complete DMEM medium (no. 41965-039, Gibco, supplemented with 10% (v/v) FBS (no. F9665, Sigma), 1% (v/v) Penicillin-Streptomycin (no. 15140-122, Thermo Fisher Scientific) and 55 µM β-mercaptoethanol (no. 21985023, Thermo Fisher Scientific)) for 1 h at 20 °C. Bone marrow cell suspensions were diluted (1:2) in complete DMEM down the plate with a starting concentration of 2 × 106 cells per well, and were incubated at 37 °C, 5% CO2 overnight. Plates were then washed with 0.05% (v/v) Tween20 PBS, PBS and H2O. Detection was performed with either anti-mouse HRP-conjugated IgG1 (no. ab97240, Abcam) or IgM (no. ab97230, Abcam) in 0.1% (w/v) BSA, 0.05% (v/v) Tween20 PBS. Plates were developed using the AEC staining kit (no. AEC101, Sigma-Aldrich). The number of ASCs was determined using a CTL ELISPOT reader (Cell Technologies) and the ImmunoSpot v.5.0 (Cellular Technology).
Immunofluorescence staining for confocal microscopy
Immunofluorescence staining for confocal imaging of GCs was done as previously described59. In brief, LN sections were stained with the primary antibodies listed in Supplementary Table 3 and the secondary antibodies listed in Supplementary Table 4. Images were acquired using the ×10, ×20 and ×40 objectives on the Zeiss 780 confocal microscope using Zen Microscopy software v.3.2-3.5. A minimum of six sections were analyzed for each GC found within each LN to capture the center-most sections of the GC. All GCs per LN sample were imaged, resulting in a total of 3–8 GCs per section, with the exception of some LNs from aged mice which did not generate GCs. Image processing was done using ImageJ and quantitative analysis was done using an automated pipeline on the Cell Profiler software v.3.19. The GC area was defined as IgD−Ki67+CD35+, the GC light zone area was defined as the IgD−CD35+ FDC network area and the GC dark zone as the Ki67+CD35−IgD− area. Using ImageJ (Fiji) v.2.0.0-rc-69/1.52p and Cell Profiler v.3.1, the number of CD3+ T cells within each compartment of the GC was identified and reported as a percentage of total T cells within the GC.
CXCL12 staining in tissue sections was performed as follows. LN sections were rehydrated with PBS and permeabilized with PBS/0.1% Triton for 30 min at 20 °C, then incubated in PBS/5% BSA/0.05% Saponin and 0.1% goat serum for 15 min at 20 °C. The purified CD35 antibody was incubated overnight at 4 °C. After washing with PBS, the AF555-conjugated secondary antibody was added for 45 min at 20 °C. Then, the CXCL12-AF647 or AF647-conjugated corresponding isotype control staining was performed along with IgD-AF488 for 1.5 h at 20 °C, followed by washes with PBS and DAPI labeling. After mounting the slides using ProLong Gold Antifade mounting medium (Thermo Fisher Scientific), the images were obtained using the LSM800 confocal microscope (Carl Zeiss).
In silico GC modeling
In silico mathematical modeling of the GC response was carried out using the agent-based model, known as hyphasma31,32 and previously calibrated to accurately describe the three-dimensional dynamics of cells in GCs from experimental datasets. Briefly, each simulated cell represents an agent with properties such as direction of movement, position and antigen receptor. The GC is modeled as a grid within a sphere, where each grid point is the size of a cell (5 µm) and has a defined concentration of the chemokines CXCL12 and CXCL13 present in the dark zone and light zone, respectively. Each simulated cell’s properties, that is, migration, position and antigen receptor, can be manipulated computationally, and it carries a polarity that defines the direction of movement and a persistence time defining the frequency of possible re-orientation in complex chemokine fields. The direction of movement follows a persistent random walk, with a turning angle predefined from experimental 2-photon microscopy46. Chemotaxis is implemented by skewing the turning angles towards the gradient of a chemokine. In each simulation, B cells first search for, and acquire, antigen from FDCs, then search for, and interact with, TFH cells to receive a proliferation signal. TFH cell help is given to those B cells with the highest amount of captured antigen, which also indicates B cell receptor affinity. The state of centroblasts and centrocytes is updated at each time point, leading to a choice of death, differentiation or recycling, subsequent to TFH cell-mediated selection. If the fate is recycling, the selected B cell becomes sensitive to CXCL12 and re-enters the cell cycle. In the model used here, the GC is seeded with a constant inflow of founder B cells, each of which divides six times60; this models the in vivo findings that 10–100 B cell clones seed a founder GC61. A full list of parameters is available in ref. 62.
To simulate the aged GC, two mechanisms have been added: the distribution of the FDCs has been relocated to a smaller area taken from measurements on aged mice, but keeping the total antigen density, and TFH cells have been given a different chemotaxis responsiveness to CXCL12. The strength of chemotaxis in silico was chosen to reproduce the changes of TFH location observed in aged mice in vivo. The chosen parameters and their original values are shown in Supplementary Table 5. Once the sensitivity was established, the biologically relevant changes that best reflect in vivo data were chosen to simulate the aged GC response. Each simulation was repeated at least 20 times and the curves, and their standard deviations, were plotted using GLE v.4.2 (Graphics Layout Engine) software
Statistics
All mouse experiments were performed 2–4 times with 4–10 mice per group, and all significant changes reported were reproducible between experimental repeats. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications12,46. Where possible, mice were randomly allocated into age- and sex-matched experimental groups by staff of the Babraham Institute Biological Services Unit. For experiments with aged mice, blinding was not possible due to overt phenotypic differences between adult and aged mice. All data points were analyzed including outliers unless there were technical errors. Any aged mice with lymphoma and/or solid tumors were also excluded from analysis. Differences between experimental groups were assessed using a two-sided nonparametric Mann–Whitney U test for unpaired comparisons, while grouped analyses were performed by a two-way analysis of variance (ANOVA) with Sidak’s multiple comparisons. Normality testing was not performed on the data before analyses because the small sample sizes used for in vivo mouse work were not well powered for normality testing. All statistical tests were performed using GraphPad Prism v.6-9 software and P values ≤ 0.05 were considered statistically significant.
Ethical approval
All research complies with the relevant ethical regulation. All mouse experimentation, except the work using Cxcr5fl/fl; Cd4ERT2Cre/+ mice, was performed in the United Kingdom with approval from the Babraham Institute Animal Welfare and Ethical Review Body, and complied with European Union and UK Home Office legislation (Home Office License P4D4AF812). Experiments with Cxcr5fl/fl; Cd4ERT2Cre/+ were done at the Core Facility Animal Models of the Biomedical Center of Ludwig Maximilian University of Munich in accordance with European regulation and federal law of Germany, and approved by the Regierung von Oberbayern. Human samples were collected in accordance with the latest revision of the Declaration of Helsinki and the Guidelines for Good Clinical Practice from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Informed consent was obtained from all participants. The samples were collected with UK local research ethics committee approval (National Research Ethics Service Committee South Central–Hampshire A, Research Ethics Committee reference 14/SC/1077), using the facilities of the National Institute for Health and Care Research Cambridge Bioresource (Research Ethics Committee reference 04/Q0108/44).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-023-01519-9.
Supplementary information
Supplementary Figs. 1–9 and Tables 1–5.
Acknowledgements
We thank C. Vinuesa and A. Liston for critical feedback on this paper. We thank the Babraham Institute Biological Support Unit staff, who performed in vivo treatments of our animals and took care of animal husbandry. We thank the staff of the Babraham Flow Cytometry and Imaging Facilities for their technical support. The National Institute for Health Research Cambridge Biomedical Research Center is a partnership between Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, funded by the National Institute for Health Research. We thank the National Institute for Health Research Cambridge Biomedical Research Center volunteers for their participation and thank staff for their contribution in coordinating the vaccinations and venipuncture. This study was supported by funding from the Biotechnology and Biological Sciences Research Council (grant nos. BB/W001578/1, BBS/E/B/000C0407, BBS/E/B/000C0427 to M.A.L.; grant no. BBSRC BB/N011740/1 to A.E.D; and the Campus Capability Core Grant to the Babraham Institute), the European Union’s Horizon 2020 research and innovation program ‘ENLIGHT-TEN’ under the Marie Sklodowska-Curie grant agreement no. 675395 to M.A.L., a grant from IdEx Université de Paris (grant no. ANR-18-IDEX-0001 to M.E.) and by an ANR PRC grant (grant no. ANR-17-CE14-0019 to K.B.). M.A.L. is an EMBO Young Investigator and a Lister Institute Prize Fellow. D.B. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Emmy Noether Programs BA 5132/1-1 and BA 5132/1-2 (grant no. 252623821 to D.B.), SFB 1054 Project B12 (grant no. 210592381 to D.B.) and Germany’s Excellence Strategy EXC2151 (grant no. 390873048 to D.B.). P.A.R. was supported by the Human Frontier Science Program (grant no. RGP0033/2015 to P.A.R.) and a PhD fellowship granted by École Normale Supérieure de Lyon. J.L.L. is supported by a National Science Scholarship (PhD) by the Agency for Science, Technology and Research, Singapore. D.L.H. received a National Health and Medical Research Council Australia Early-Career Fellowship (grant no. APP1139911). A.R.B. received a Sir Henry Wellcome Postdoctoral Fellowship (grant no. 222793/Z/21/Z). J.P.L. was a recipient of the People Program (Marie Curie Actions) of the European Union’s Seventh Framework Program (FP7/2007-2013) under REA grant agreement no. PCOFUND-GA-2013-609102.
Extended data
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Author contributions
A.S.-C. designed and performed experiments, analyzed data, constructed the figures and cowrote the paper. S.F.-B., S.I., A.R.B., E.M.W., J.L.L., L.M.C.W., R.C.J.M., A.B., I.V., D.A., J.P.L., E.J.C., D.L.H., I.C., W.S.F. and A.E.D. designed and performed experiments and analyzed data. P.A.R., M.M.-H. and A.S.-C. performed in silico modeling of the germinal center. K.B., D.B., M.E., M.M.-H., A.E.D. and M.A.L. provided supervision and contributed to experimental design and funding for this study. M.A.L. designed the study and cowrote the paper. All authors contributed intellectually to the work, and reviewed, edited and approved the paper.
Peer review
Peer review information
Nature Immunology thanks Natalia Pikor, Claire Gustafson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Laurie Dempsey, in collaboration with the Nature Immunology team.
Data availability
Source data are provided with this paper.
Code availability
The code used for in silico modeling is available at: https://gitlab.com/simm/gc/hyphasma/-/releases/Denton2020. The code used for the W33L VH186.2 sequencing analysis is available at: https://github.com/lintermanlab/W33L_caller.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41590-023-01519-9.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-023-01519-9.
References
- 1.Lee, J. L. & Linterman, M. A. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol. Lett.241, 1–14 (2022). [DOI] [PMC free article] [PubMed]
- 2.Prendecki M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier DA, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller L, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin. Infect. Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu FC, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID−19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy MN, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldridge, R. W. et al. SARS-CoV-2 antibodies and breakthrough infections in the Virus Watch cohort. Nat. Commun.13, 4869 (2022). [DOI] [PMC free article] [PubMed]
- 8.Levin EG, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrotri M, et al. Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Heal. Longev. 2022;3:e470–e480. doi: 10.1016/S2666-7568(22)00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLennan IC, et al. Extrafollicular antibody responses. Immunol. Rev. 2003;194:8–18. doi: 10.1034/j.1600-065X.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 11.Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stebegg M, et al. Rejuvenating conventional dendritic cells and T follicular helper cell formation after vaccination. eLife. 2020;9:e52473. doi: 10.7554/eLife.52473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Cayetano A, et al. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med. 2021;2:243–262.e8. doi: 10.1016/j.medj.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szakal AK, et al. Kinetics of germinal center development in lymph nodes of young and aging immune mice. Anat. Rec. 1990;227:475–485. doi: 10.1002/ar.1092270411. [DOI] [PubMed] [Google Scholar]
- 15.Kosco MH, Burton GF, Kapasi ZF, Szakal AK, Tew JG. Antibody-forming cell induction during an early phase of germinal centre development and its delay with ageing. Immunology. 1989;68:312–318. [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol. Rev. 1997;160:63–77. doi: 10.1111/j.1600-065X.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 17.Stebegg, M. et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun.10, 2443 (2019). [DOI] [PMC free article] [PubMed]
- 18.Luscieti, P., Hubschmid, T., Cottier, H., Hess, M. W. & Sobin, L. H. Human lymph node morphology as a function of age and site. J. Clin. Pathol.33, 454–461 (1980). [DOI] [PMC free article] [PubMed]
- 19.Hill DL, et al. Impaired HA-specific T follicular helper cell and antibody responses to influenza vaccination are linked to inflammation in humans. eLife. 2021;10:e70554. doi: 10.7554/eLife.70554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen CDC, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 21.Bannard O, et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39:912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenbarth SC, et al. CD4+ T cells that help B cells—a proposal for uniform nomenclature. Trends Immunol. 2021;42:658–669. doi: 10.1016/j.it.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaeser A, McGlauchlen K, Vogel LA. Aged B lymphocytes retain their ability to express surface markers but are dysfunctional in their proliferative capability during early activation events. Immun. Ageing. 2008;5:15. doi: 10.1186/1742-4933-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stebegg M, et al. Regulation of the germinal center response. Front. Immunol. 2018;9:2469. doi: 10.3389/fimmu.2018.02469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 2015;12:163–171. doi: 10.1016/j.celrep.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderleyden I, et al. Follicular regulatory T cells can access the germinal center independently of CXCR5. Cell Rep. 2020;30:611. doi: 10.1016/j.celrep.2019.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan TG, et al. B cell receptor–independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J. Exp. Med. 2003;197:845–860. doi: 10.1084/jem.20022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, et al. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J. Immunol. 2003;170:1267–1273. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y-F, Cerny J. Repertoire of antibody response in bone marrow and the memory response are differentially affected in aging mice. J. Immunol. 2002;169:4920–4927. doi: 10.4049/jimmunol.169.9.4920. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Hermann ME, Maini PK, Iber D. An analysis of B cell selection mechanisms in germinal centers. Math. Med. Biol. 2006;23:255–277. doi: 10.1093/imammb/dql012. [DOI] [PubMed] [Google Scholar]
- 32.Meyer-Hermann M, et al. A theory of germinal center B cell selection, division, and exit. Cell Rep. 2012;2:162–174. doi: 10.1016/j.celrep.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Silva-Cayetano A, Linterman MA. Stromal cell control of conventional and ectopic germinal centre reactions. Curr. Opin. Immunol. 2020;64:26–33. doi: 10.1016/j.coi.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Elsner RA, Ernst DN, Baumgarth N. Single and coexpression of CXCR4 and CXCR5 identifies CD4 T helper cells in distinct lymph node niches during influenza virus infection. J. Virol. 2012;86:7146–7157. doi: 10.1128/JVI.06904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes NM, et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 36.Alterauge D, et al. Continued Bcl6 expression prevents the transdifferentiation of established Tfh cells into Th1 cells during acute viral infection. Cell Rep. 2020;33:108232. doi: 10.1016/j.celrep.2020.108232. [DOI] [PubMed] [Google Scholar]
- 37.Webb LMC, et al. Ageing promotes early T follicular helper cell differentiation by modulating expression of RBPJ. Aging Cell. 2021;20:e13295. doi: 10.1111/acel.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cané S, Ponnappan S, Ponnappan U. Altered regulation of CXCR4 expression during aging contributes to increased CXCL12-dependent chemotactic migration of CD4+ T cells. Aging Cell. 2012;11:651–658. doi: 10.1111/j.1474-9726.2012.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cane, S., Ponnappan, S. & Ponnappan, U. Impairment of non-muscle myosin IIA in human CD4+ T cells contributes to functional deficits in the elderly. Cell. Mol. Immunol.9, 86–96 (2011). [DOI] [PMC free article] [PubMed]
- 40.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim. Biophys. Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Victora GD, Nussenzweig MC. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 42.Kuraoka M, et al. Complex antigens drive permissive clonal selection in germinal centers. Immunity. 2016;44:542–552. doi: 10.1016/j.immuni.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pikor NB, et al. Remodeling of light and dark zone follicular dendritic cells governs germinal center responses. Nat. Immunol. 2020;21:649–659. doi: 10.1038/s41590-020-0672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garin A, et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010;33:84–95. doi: 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Frasca D, Blomberg BB, Garcia D, Keilich SR, Haynes L. Age-related factors that affect B cell responses to vaccination in mice and humans. Immunol. Rev. 2020;296:142–154. doi: 10.1111/imr.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denton AE, et al. Targeting TLR4 during vaccination boosts MAdCAM-1+ lymphoid stromal cell activation and promotes the aged germinal center response. Sci. Immunol. 2022;7:eabk0018. doi: 10.1126/sciimmunol.abk0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci. Rep. 2016;6:25051. doi: 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. J. Immunol. 2010;185:6783–6794. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton AR, et al. The memory B cell response to influenza vaccination is impaired in older persons. Cell Rep. 2022;41:111613. doi: 10.1016/j.celrep.2022.111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 51.Sonoda E, et al. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/S1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 52.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/S1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 53.De Luca C, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie Y, et al. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J. Exp. Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradford, B. M., Reizis, B. & Mabbott, N. A. Oral prion disease pathogenesis is impeded in the specific absence of CXCR5-expressing dendritic cells. J. Virol.91, e00124-17 (2017). [DOI] [PMC free article] [PubMed]
- 56.Śledzińska A, et al. TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 2013;11:e1001674. doi: 10.1371/journal.pbio.1001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Natt J, Espéli M. Assessing T follicular helper cell function in vivo: antigen-specific B cell response to hapten and affinity maturation. Methods Mol. Biol. 2015;1291:87–101. doi: 10.1007/978-1-4939-2498-1_8. [DOI] [PubMed] [Google Scholar]
- 58.Hill JT, et al. Poly peak parser: method and software for identification of unknown indels using sanger sequencing of polymerase chain reaction products. Dev. Dyn. 2014;243:1632–1636. doi: 10.1002/dvdy.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fra-Bido S, Walker SA, Innocentin S, Linterman MA. Optimized immunofluorescence staining protocol for imaging germinal centers in secondary lymphoid tissues of vaccinated mice. STAR Protoc. 2021;2:100499. doi: 10.1016/j.xpro.2021.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arulraj T, Binder SC, Robert PA, Meyer-Hermann M. Synchronous germinal center onset impacts the efficiency of antibody responses. Front. Immunol. 2019;10:2116. doi: 10.3389/fimmu.2019.02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tas JMJ, et al. Visualizing antibody affinity maturation in germinal centers. Science. 2016;351:1048–1054. doi: 10.1126/science.aad3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robert PA, Rastogi A, Binder SC, Meyer-Hermann M. How to simulate a germinal center. Methods Mol. Biol. 2017;1623:303–334. doi: 10.1007/978-1-4939-7095-7_22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–9 and Tables 1–5.
Data Availability Statement
Source data are provided with this paper.
The code used for in silico modeling is available at: https://gitlab.com/simm/gc/hyphasma/-/releases/Denton2020. The code used for the W33L VH186.2 sequencing analysis is available at: https://github.com/lintermanlab/W33L_caller.