Abstract
Purpose
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising cancer therapeutic agent because of its tumor selectivity and its ability to induce apoptosis in cancer cells while sparing most normal cells. We evaluated whether docetaxel enhances TRAIL-mediated apoptosis in prostate cancer (PCa) cells and its mechanism.
Materials and Methods
LNCap-LN3, PC3, and DU 145 PCa cell lines were used to investigate the effects of TRAIL with docetaxel treatment (dosages, 1, 3, 5, and 10 nmol). To evaluate the mechanism, death receptor 4 (DR4), DR5, enhancer of zeste homolog 2 (EZH2) and E2F1 levels were assessed in PCa cells.
Results
Hormone-sensitive LNCap-LN3 showed apoptosis in proportion to the concentration of docetaxel. Castration-resistant PC3 and DU 145 showed no change irrespective of the docetaxel concentration. However, combinations of docetaxel (2 nM) and TRAIL (100 ng/mL) had a significant effect on apoptosis of DU 145 cells. In DU 145 cells, docetaxel reduced EZH2 and elevated expression of DR4. The decrease of EZH2 by docetaxel was correlated with the E2F1 level, which was considered as the promoter of EZH2. DZNep reduced EZH2 and elevated DR4 in all PCa cells. Additionally, DZNep-enhanced TRAIL mediated reduction of PCa cell viability.
Conclusions
Docetaxel and the EZH2 inhibitor reduced EZH2 and elevated expression of DR4 in all PCa cell lines. Docetaxel-enhanced TRAIL mediated apoptosis in PCa via elevation of DR4 through epigenetic regulation by EZH2. To improve the efficacy of TRAIL for PCa treatment, adding docetaxel or EZH2 inhibitors to TRAIL may be promising.
Keywords: Docetaxel, Enhancer of zeste homolog 2, Prostate cancer, TNF-related apoptosis-inducing ligand
INTRODUCTION
Prostate cancer (PCa) is the second most lethal disease for men in Western countries [1]. PCa rates have sharply increased in men aged 50 to 64 years with an annual change between 5% and 15% in 24 countries [2]. In as many as 10% to 50% of men with PCa, the disease will ultimately progress to an androgen independent castration-resistant prostate cancer (CRPCa) status and spread to the lymph nodes in the pelvic area and bone [3]. Docetaxel (DOC), which was approved in 2014 to treat metastatic CRPCa, has significantly improved patients’ survival and become the standard treatment [4,5]. However, the main issues with DOC are that approximately half of the cases respond, and half are resistant to DOC and patients who initially respond eventually develop resistance [6,7]. Antiandrogen synthesis therapy and anti-androgen receptors are currently used, but patients with CRPCa will eventually develop resistance to these therapies [8]. Therefore, finding a novel therapy for CRPCa is of major scientific and clinical interest to enhance and overcome the treatment limitation of DOC.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising cancer therapeutic agent because of its tumor selectivity. The advantage of TRAIL is that it induces apoptosis in cancer cells but spares most normal cells. It has been suggested that taxanes including DOC sensitize PCa cells to TRAIL-induced apoptosis [9,10,11]. As one important enzymatic subunit of polycomb-repressive complex 2 (PRC2), enhancer of zeste homolog 2 (EZH2) methylates lysine 27 of histone H3 to promote transcriptional silencing [12,13]. Elevated EZH2 expression is correlated with the development of CRPCa and resistance to DOC, although the mechanisms by which EZH2 causes PCa development is still elusive [14,15,16]. Thus, it is necessary to confirm the relationship of EZH2 with TRAIL in PCa cells resistant to DOC.
Therefore, the purpose of this study is to determine whether there is a relationship between EZH2 expression and TRAIL-induced apoptosis, and to identify relevant mediators. We hypothesized that TRAIL effectively induces apoptosis in cells with high EZH2 expression.
MATERIALS AND METHODS
1. Ethics statement
This study was approved by the Institutional Review Board (IRB) and ethics committee of Ulsan University Hospital (IRB number: NON2021-006). All animal experiments were performed in accordance with procedures approved by the Institute of Laboratory Animal Resources, University of Ulsan (Ethical code number: 032-01 [A1-0]).
2. Reagents
DOC (sanofi-aventis U.S. LLC, Bridgewater, NJ, USA), DZNep (Selleckchem, Houston, TX, USA), tazemetostat (Selleckchem), and human recombinant TRAIL (Sigma-Aldrich, St. Louis, MO, USA) were used at indicated concentrations.
3. Cell culture
PCa cell lines PC3, DU 145, and LNCaP were purchased from the Korean cell line bank. The PC3 and DU 145 cell lines were cultured in RPMI-1640 (WELGENE, Gyeongsan, Korea), and the LNCaP cell line was cultured in Eagle’s minimum essential medium (WELGENE), each supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Invitrogen). Cells were cultured at 37℃ in a humidified chamber containing 5% carbon dioxide.
4. Viability assay
At the indicated times, the CellTiter 96® Aqueous One Solution Reagent (Promega, Madison, WI, USA) was added to each well according to the manufacturer’s instructions. Then, absorbance at 490 nm (OD490) was determined for each well using the Wallac Vector 1420 Multilabel Counter (EG&G Wallac, Turku, Finland).
5. Apoptosis by Annexin V/propidium iodide analysis
Human PCa cells were seeded on a 60 mm dish, and incubated with TRAIL (100 ng/mL) and DOC (2 nM) for 24 hours, washed twice with ice-cold phosphate buffered saline (pH 7.0), and then resuspended in binding buffer (500 µL). Subsequently, 5 µL of FITC-Annexin V was added to 5 µL of propidium iodide (PI) and then incubated for 15 minutes at room temperature in the dark. The sample was analyzed using a fluorescence-activated flow cytometer (FACScan; Becton Dickinson, Franklin Lakes, NJ, USA).
6. Western blot analysis and small interfering RNA transfection
Total protein was extracted using a RIPA buffer containing proteases and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA), and protein concentration was determined using the Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were separated by electrophoresis on a 10% to 13% SDS polyacrylamide gel, and transferred to a nitrocellulose membrane (Amersham International, Little Chalfont, UK). Membranes were blocked with 5% bovine serum albumin (BSA; bioWORLD, Dublin, OH, USA) in Tris-buffered saline with tween®20 (TBST: Tech and Innovation, Chuncheon, Korea) for 1 hour at room temperature. Membranes were subsequently washed with TBST and incubated with primary antibodies to EZH2 (ab3748; Abcam, Cambridge, UK), death receptor 4 (DR4) (ab8414; Abcam), DR5 (ab47179; Abcam), phosphor-Rb (#9308; Cell Signaling, Danvers, MA, USA), Rb (#9309; Cell Signaling), E2F1 (sc-251; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (sc-47778; Sigma-Aldrich) and diluted in 5% BSA/TBST overnight at 4℃. Membranes were washed with TBST. The secondary antibody (anti-mouse or anti-rabbit immunoglobulin (IgG) HRP conjugate; Bethyl Laboratories, Montgomery, TX, USA) was diluted 2,000-fold in TBST and applied to cells for 1 hour. After washing the cells with TBST, the specific binding of antibodies was detected using an ECL kit (Thermo Fisher Scientific), following the manufacturer’s protocol. Small interfering RNA (siRNA) against human E2F1 (siE2F1) (sc-29297) and control siRNA (scRNA) (sc-37007) were purchased from Santa Cruz Biotechnology. DU145 cells (1.5 or 3×105) were transfected with each siRNA using Lipofectamine™ RNAiMAX (Invitrogen).
7. Neutralization of the DR4 receptor
TRAIL receptor inhibitors (neutralizing antibodies) and anti-human DR4 (ALX-804-297A; Enzo Life Sciences, Farmingdale, NY, USA) were always added 1 hour before the addition of TRAIL.
8. Methylation-specific polymerase chain reaction for DR4
Genomic DNA was isolated from cell lines using standard procedures. One microgram of genomic DNA was treated with sodium bisulfite using the EpiTect Bisulfite kit (Qiagen, Valencia, CA, USA). This treatment converts all unmethylated cytosines into uracil. In the subsequent methylation-specific polymerase chain reaction (MSP), all of the uracils become thymidines. Polymerase chain reaction (PCR) requires primer pairs that specifically recognize methylated or unmethylated sequences. These primers were designed in the 5’untranslated region CpG island of the published sequences. The primer sequences were as follows (5’- to -3’): DR4, CCC CTT TTC AAA ACA CCT ACA (unmethylated sense), CCT TTT CGA AAC ACC TAC GAC (methylated sense), GAA AGG TTG GGT TAA TTT TTG ATT (unmethylated antisense), and AAA GGT TGA GGT TAA TTT TCG ATC (methylated antisense). PCR amplification was performed using the EpiTect MSP kit (Qiagen). Thermal cycling conditions were as follows: 1 cycle at 95℃ for 10 minutes; 35 cycles at 94℃ for 15 seconds, 60℃ for 1 minute, and 72℃ for 30 seconds; and 1 cycle at 72℃ for 10 minutes. Each PCR (10 µL) was directly loaded onto 2% agarose gels stained with ethidium bromide (0.5 µg/mL) and directly visualized under ultraviolet light.
9. Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed using the Pierce™ Agarose ChIP Kit (Thermo Fisher Scientific). PCa cells were treated with formaldehyde and incubated to generate DNA–protein cross-linking. Then, cell lysates generated chromatin fragments and immunoprecipitated with antibodies (histone deacetylase and IgG antibodies as the control). Precipitated chromatin DNA was recovered and analyzed by real-time PCR. The PCR primers were as follows: DR4: sense, 5′-TGG TTG AGG AAC AGA AGC TGA GA-3′ and anti-sense, 5′-GGC TCC TGT TGG CTA ACC CT-3′ and DR5: sense, 5′-GCG CGG ACA GGA CCC AGA AA-3′ and anti-sense, 5′-ATC CTC CGC AAG CGC GTC CAA-3′.
10. Antitumor activity in the xenograft model
DU 145 cells were injected into the flank of 6-week-old nude (nu/nu) mice (Orient Bio, Seongnam, Korea). Prior to treatment with TRAIL and DZNep, the tumor size was measured two to three times per week until the volume reached approximately 100 mm3. The tumor volume was calculated as W2×L×0.52, where L is the largest diameter and W is the diameter perpendicular to L. After establishment of these tumor xenografts, mice were randomized into four groups of five mice per group. Mice were fed ad libitum and maintained in environments with a controlled temperature of 22℃ to 24℃ and 12-hour light and dark cycles. TRAIL (5 mg/kg) and DZNep (2 mg/kg) were administered twice per week, and an intra-tumoral injection was given as a 2-week treatment.
11. Statistical analysis
Data are expressed as mean and standard deviation. Group differences were determined using the Student t-test or Mann–Whitney U test. All statistical analyses and calculations were performed using Excel spreadsheets (Microsoft Corp., Redmond, WA, USA) and GraphPad Prism, version 5 (GraphPad Software, San Diego, CA, USA). All statistical tests were two-sided, and p-values <0.05 were considered statistically significant.
RESULTS
1. A combination of low-dose DOC and TRAIL had a significant effect on apoptosis through DR4
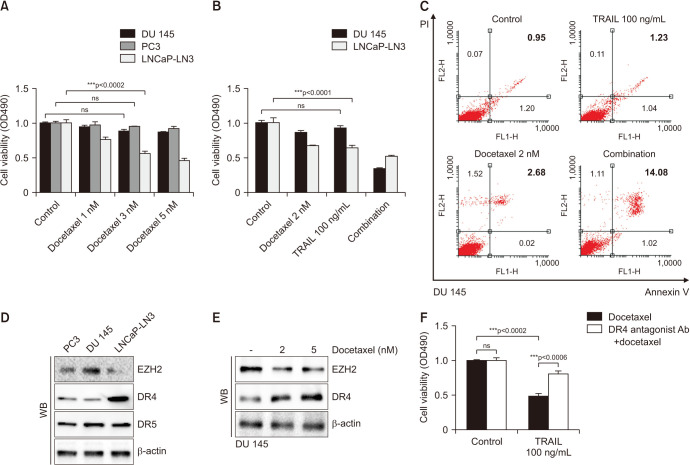
Hormone-sensitive LNCap-LN3 cells showed apoptosis in proportion to the concentration of DOC (Fig. 1A). Additionally, castration-resistant PC3 and DU 145 cells showed no change of cell viability irrespective of the DOC concentration. However, a combination of low-dose DOC (2 nmol) and TRAIL (100 ng/mL) had a significant effect on apoptosis of DU 145 cells, although TRAIL (100 ng/mL) alone did not (Fig. 1B). Flow cytometry analysis of DU 145 shows that a combination of DOC 2 nM and TRAIL 100 ng/mL increases the proportion of Annexin V(+)/PI(+) (Fig. 1C).
Fig. 1. Cell viability assay in prostate cancer cell lines after docetaxel and TRAIL treatment. Cells are exposed for 24 hours, and cell viability is determined using the MTT assay (A, B). Flow cytometry analysis of DU 145 shows that a combination of docetaxel 2 nM and TRAIL 100 ng/mL increases the proportion of Annexin V(+)/PI(+) (C). The expressions of EZH2 and DR4 in prostate cancer cell lines are shown. A WB assay is performed with specific antibodies against EZH2, DR4, DR5, and β-actin (D). After docetaxel treatment for 24 hours, the EZH2 level is decreased and the DR4 level is increased in DU 145 cells (E). The cell viability test is performed with a combination of docetaxel and TRAIL with or without the DR4 antagonist antibody (F). DR4: death receptor 4, DR5: death receptor 5, EZH2: enhancer of zeste homolog 2, ns: not significant, PI: propidium iodide, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand, WB: western blot. ***p<0.001.
To investigate why DOC (2 nmol) with TRAIL (100 ng/mL) reduced cell viability in DU 145 cells, which did not change with 10 nmol of DOC, we evaluated the mechanism of TRAIL. TRAIL functions through DR4 or DR5 in a cascade of apoptosis, and there was a high expression of the DR4 level only in hormone-sensitive LNCap-LN3 cells contrary to castration-resistant PCa cells, such as DU 145 and PC-3. Yet, there were no differences of expression of DR5 in PCa cell lines (Fig. 1C). LNCap-LN3 showed a low expression of EZH2, which is considered a marker of tumor aggressiveness in PCa, and PC3 and DU 145 cells had a high expression of EZH2. To confirm the relationship of EZH2 and TRAIL function, we assessed the change of expression of EZH2 and DR4 levels after DOC treatment. The EZH2 level showed concentration-dependent decreases, and DR4 gradually increased after DOC treatment for 24 hours in DU 145 cells (Fig. 1D). The combination of DOC (2 nmol) and TRAIL (100 ng/mL) significantly reduced cell viability of DU 145 cells, although DOC alone could not. However, treatment with the combined drugs after blocking DR4 with the DR4 antagonist antibody could not induce apoptosis (Fig. 1E). TRAIL was activated through DR4, and TRAIL, which was effective by increasing DR4 in response to DOC treatment, had no effect on the two drugs when DR4 was blocked.
2. Inhibition of EZH2 increased DR4
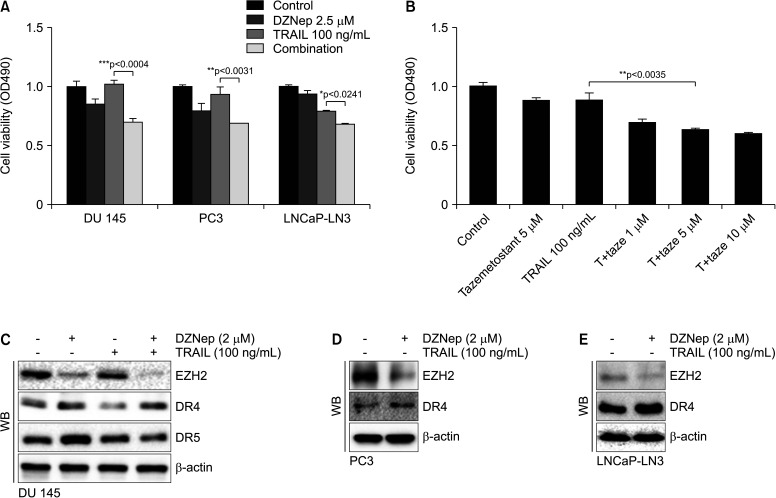
To confirm the mechanism of EZH2 and TRAIL function, the cell viability assay was evaluated after a combination of TRAIL and EZH2 inhibitors, such as DZNep or tazemetostat, were used. DZNep (2.5 µmol) mildly reduced cell viability in PC3 and DU 145 cells with a high EZH2 level, but it had almost no effect on LNCap-LN3 with a low EZH2 level (Fig. 2A). Contrary to DZNep, TRAIL (100 ng/mL) just reduced cell viability in DR4-rich LNCap-LN3 cells. A combination of DZNep (2.5 µmol) and TRAIL (100 ng/mL) significantly reduced cell viability in all PCa cell lines. A combination of TRAIL and tazemetostat also reduced DU 145 cells in proportion to the concentration of tazemetostat (Fig. 2B). DZNep reduced EZH2 expression and elevated expression of DR4 in all PCa cells (Fig. 2C-2E). The expressions of EZH2, DR4, and DR5 did not change with TRAIL alone in DU 145 cells.
Fig. 2. Cell viability assay in prostate cancer cell lines after EZH2 inhibitors and TRAIL treatment. Cells are exposed for 48 hours. Cell viability is determined using the MTT assay (A, B). A WB assay is performed with specific antibodies against EZH2, DR4, DR5, and β-actin in prostate cancer cell lines (C-E). DR4: death receptor 4, DR5: death receptor 5, EZH2: enhancer of zeste homolog 2, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand, WB: western blot. *p<0.05, **p<0.01, ***p<0.001.
3. When EZH2 is inhibited through E2F1 knockdown, TRAIL causes significant cell death
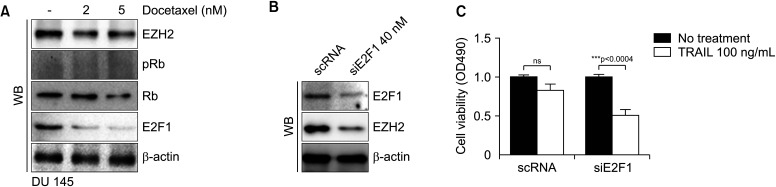
EZH2 is activated by MUC1-C through E2F1 in human cancer cells [17,18]. To determine whether DOC reduces EZH2 expression, EZH2 and E2F1 were evaluated after DOC treatment. DOC treatment for 24 hours induced concentration-dependent decreases of EZH2 and E2F1 expression in DU 145 cells (Fig. 3A). After using E2F1 knockdown in DU 145 cells, there were significant reductions of expressions of not only E2F1 but also EZH2 (Fig. 3B). Additionally, there was significant cell death after use of TRAIL (100 ng/mL) in DU 145 cells with siE2F1 (Fig. 3C). Down regulation of E2F1 deactivated EZH2 and had the same effect as EZH2 inhibitors, such as DZNep or tazemetostat.
Fig. 3. Expressions of EZH2 and E2F1 in DU 145 cells. WB assay shows decreases of EZH2 and E2F1 expressions after docetaxel treatment (A). Using knockdown of E2F1, the expressions of E2F1 and EZH2 are decreased (B), and TRAIL (100 ng/mL) treatment causes significant cell death in siE2F-1 DU 145 cells (***p<0.001) (C). DR4: death receptor 4, DR5: death receptor 5, EZH2: enhancer of zeste homolog 2, ns: not significant, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand, WB: western blot.
4. DOC or EZH2 inhibitor restored DR4 gene expression through demethylation
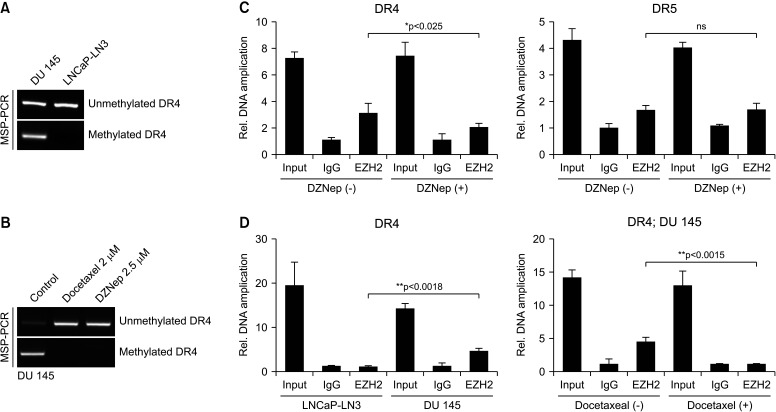
To determine why DR4 expression is low and what the gene methylation state is in castration-resistant DU 145 cells, MSP analysis was performed, and we compared castration-resistant DU 145 cells with hormone-sensitive LNCap-LN3 cells with a high DR4 level. LNCap-LN3 cells had entirely unmethylated DR4, and DU 145 cells showed both methylated and unmethylated DR4 (Fig. 4A). This finding means that gene activation of DR4 decreased and that DR4 in DU 145 cells did not function normally. After DOC or DZNep treatment for 24 hours, gene expression of DR4 recovered from the methylated to unmethylated form (Fig. 4B). Therefore, after DOC or DZNep treatment, expression of DR4 increased in DU 145 cells, and TRAIL combined with one of those treatments could induce apoptotic activity (Fig. 1, 2). To evaluate how much EZH2 is combined with the promoter lesion of the DR4 gene, a ChIP assay was performed. The EZH2 level in the promoter of the DR4 gene was also significantly lower with DZNep (2.5 µmol) treatment for 24 hours than that without in DU 145 cells (p<0.025). However, there was no difference in the EZH2 level with and without DZNep in the promoter of the DR5 gene in DU 145 cells (Fig. 4C). The EZH2 level in DU 145 cells was significantly higher than that in LNCap-LN3 cells (Fig. 4D). After DOC (2 nmol) treatment for 24 hours, the EZH2 level in the promoter of the DR4 gene was significantly lower than that without DOC in DU 145 cells.
Fig. 4. MSP analysis is performed to evaluate the methylation status (A, B). ChIP assay is used to assess the relationship of EZH2 and the DR4 promoter in LNCap-LN3 and DU 145 cells (C). After docetaxel or DZNep treatment, the relationship of EZH2 and the promoter lesion of DR4 or DR5 is evaluated (D). ChIP: chromatin immunoprecipitation, DR4: death receptor 4, DR5: death receptor 5, EZH2: enhancer of zeste homolog 2, IgG: immunoglobulin G, MSP: methylation-specific polymerase chain reaction, ns: not significant, PCR: polymerase chain reaction. *p<0.05, **p<0.01.
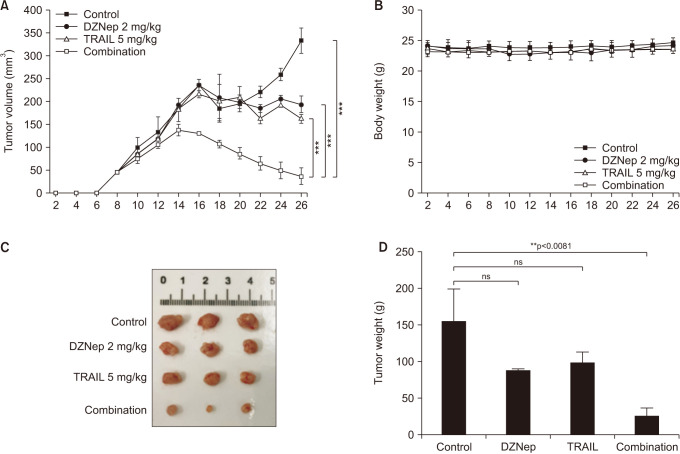
5. In the xenograft model, a combination of EZH2 inhibitor and TRAIL significantly decreased tumor volumes
To assess the effect of the EZH2 inhibitor and TRAIL in the growth of DU 145 cells in vivo, we examined whether combined treatment would be less tumorigenic in nude mice xenografts. Tumors initially appeared in all of the animals, and after 4 weeks, the tumors were extracted and size and weight were checked. The combination of DZNep (2 mg/kg) and TRAIL (0.2 mg/mL) significantly decreased the tumors compared to each treatment alone or the control (Fig. 5).
Fig. 5. Xenograft model. The combination of DZNep (2 mg/kg) and TRAIL (0.2 mg/mL) significantly decreases the tumor size compared to each treatment alone and the control (A). There are no differences in body weight between the four groups (B). After 4 weeks, the tumors are extracted and size and weight are evaluated (C, D). ns: not significant, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand. **p<0.01, ***p<0.001.
DISCUSSION
In this study, we first showed that DOC treatment decreased the EZH2 level, increased the DR4 level, and enabled apoptosis of TRAIL-resistant PCa cells through those mechanisms. Importantly, the inhibition of EZH2 by its specific inhibitors such as DZNep or tazemetostat could also re-sensitize TRAIL-resistant PCa cells by elevation of DR4 expression.
The incidence of PCa in North-East Asia is also increasing [19,20,21]. Polycomb group proteins are important epigenetic regulators for cell proliferation and differentiation and initiation and progression of cancer. Tazverik® (tazemetostat), an EZH2 inhibitor, received U. S. Food and Drug Administration (FDA) approval for treating lymphoma and epithelial sarcoma in 2020 and was generally well tolerated across patients with clinically diverse follicular lymphoma [22]. In PCa, elevated EZH2 expression is correlated with the development of CRPCa [14,15]. In CRPCa cells, phosphorylated EZH2 has been shown to act as an androgen receptor co-activator to drive AR signaling and CRPCa progression [23]. In the current study, we found that EZH2 was correlated with the promoter lesion of the DR4 gene, and the EZH2 inhibitor increased DR4 expression in all PCa cell lines. Treatment with TRAIL only did not function in TRAIL-resistant DU 145 cells, but in combination with the EZH2 inhibitor, it reduced cell viability via elevation of the DR4 level (Fig. 1) because apoptosis is induced when TRAIL binds to DR4 and DR5 on the surface of the cell [24].
Taxanes including DOC sensitize PCa cells to TRAIL-induced apoptosis [9,10,11,25]. The mechanism by which taxanes increase the TRAIL sensitivity of PCa cells is still not completely understood. Various reports were published to explain this mechanism. First, DOC enhances the PARP-1 cleavage and caspases activation by TRAIL mainly by phosphorylation of Bcl-2 by Jun N-terminal kinase activation [9]. Second, DOC sensitizes PCa cells to TRAIL-induced apoptotic synergy via endoplasmic reticulum stress and the occurrence of mitochondrial permeability [11]. Third, DOC and TRAIL exerted a synergistic inhibitory effect in both two-dimensional (2D) and 3D DU 145 spheroids [10]. DU 145 spheroids expressed lower levels of DR4 and DR5 in comparison to monolayer cells in western blot analysis of whole cell lysate.
In this study, there were lower levels of DR4 than DR5 in both DU 145 and PC3 cell lines. Contrary to previous results, with DOC treatment for 24 hours, DR4 showed a concentration-dependent increase in DU 145 cells (Fig. 1D). To confirm that DR4 is the main pathway of combined treatment of DOC (2 nM) and TRAIL (100 ng/mL), we showed that a combination of DOC and TRAIL with the DR4 antagonist could not reduce cell viability of DU 145 cells (Fig. 1E), although those without the DR4 antagonist showed a significant reduction of cell viability. There was the other evidence that DR4 is important in the function of TRAIL for DU 145 cells. DOC showed concentration-dependent decreases of EZH2 and E2F1, the promoter gene of EZH2 (Fig. 3A). The decrease in E2F1 due to treatment with DOC was also confirmed in previous head and neck cancer cell lines [26]. This change is because DNA damage causes ubiquitin/proteasome-mediated degradation of E2F1 [27]. The EZH2 inhibitor also reduced EZH2 and elevated expression of DR4 expression in all PCa cells like DOC did (Fig. 2). In the ChIP analysis, the EZH2 level correlated with the DR4 level with and without the EZH2 inhibitor. However, there was no difference in the DR5 level with and without the EZH2 inhibitor (Fig. 4). Therefore, we concluded that DOC-enhanced TRAIL mediated apoptosis in PCa via elevation of the DR4 level through epigenetic regulation by EZH2.
CONCLUSIONS
DOC treatment and the EZH2 inhibitor reduced EZH2 expression and elevated DR4 expression in all PCa cell lines. DOC treatment enhanced TRAIL-mediated apoptosis in PCa via elevation of DR4 expression through epigenetic regulation by EZH2. To improve the efficacy of TRAIL for PCa treatment, adding DOC or EZH2 inhibitors with TRAIL may be promising.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: The research was supported (in part) by the Korean Urologic Oncology Society Grant 21-03. This work was supported by the Ulsan UniversityHospital Research Grant (UUH-2021-09).
- Conceptualization: WHL, SCK, SP.
- Data curation: WHL, SHK, SHL, SCK, JML SP.
- Formal analysis: WHL, SHK, SHL, SCK, JML SP, BIC.
- Funding acquisition: SP, WHL.
- Investigation: WHL, SP, SCK, SHL, BIC.
- Methodology: WHL, SHK, SHL, SCK, JML SP, BIC.
- Project administration: SHL, SP, BIC.
- Resources: SHL, SP, BIC.
- Software: SHL, SHK, JML, SP, SCK.
- Supervision: SP, BIC.
- Validation: WHL, SCK, SHK, JHY, KHM, SHC, TK, YMK, JWP, SHL, JML, SP, BIC.
- Visualization: WHL, SCK, SHK, SHL, SP.
- Writing – original draft: WHL, SCK, SP.
- Writing review & editing: WHL, SCK, SHK, JHY, KHM, SHC, TK, YMK, JWP, SHL, JML, SP, BIC.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/FRFC8Q.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Pilleron S, Alqurini N, Ferlay J, Haase KR, Hannan M, Janssen-Heijnen M, et al. International trends in cancer incidence in middle-aged and older adults in 44 countries. J Geriatr Oncol. 2022;13:346–355. doi: 10.1016/j.jgo.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491–1498. [PubMed] [Google Scholar]
- 4.Ruiz de Porras V, Font A, Aytes A. Chemotherapy in metastatic castration-resistant prostate cancer: current scenario and future perspectives. Cancer Lett. 2021;523:162–169. doi: 10.1016/j.canlet.2021.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Sandin R, Sah J, Emir B, Mu Q, Ratiu A, et al. Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US Veterans Health Administration. Cancer Med. 2021;10:8570–8580. doi: 10.1002/cam4.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colloca G, Venturino A, Checcaglini F. Second-line chemotherapy in metastatic docetaxel-resistant prostate cancer: a review. Med Oncol. 2012;29:776–785. doi: 10.1007/s12032-011-9855-6. [DOI] [PubMed] [Google Scholar]
- 7.Reuter CW, Morgan MA, Ivanyi P, Fenner M, Ganser A, Grünwald V. Carboplatin plus weekly docetaxel as salvage chemotherapy in docetaxel-resistant and castration-resistant prostate cancer. World J Urol. 2010;28:391–398. doi: 10.1007/s00345-010-0527-5. [DOI] [PubMed] [Google Scholar]
- 8.Hou Z, Huang S, Li Z. Androgens in prostate cancer: a tale that never ends. Cancer Lett. 2021;516:1–12. doi: 10.1016/j.canlet.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Yoo J, Park SS, Lee YJ. Pretreatment of docetaxel enhances TRAIL-mediated apoptosis in prostate cancer cells. J Cell Biochem. 2008;104:1636–1646. doi: 10.1002/jcb.21729. [DOI] [PubMed] [Google Scholar]
- 10.Grayson KA, Jyotsana N, Ortiz-Otero N, King MR. Overcoming TRAIL-resistance by sensitizing prostate cancer 3D spheroids with taxanes. PLoS One. 2021;16:e0246733. doi: 10.1371/journal.pone.0246733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson KA, Hope JM, Wang W, Reinhart-King CA, King MR. Taxanes sensitize prostate cancer cells to TRAIL-induced apoptotic synergy via endoplasmic reticulum stress. Mol Cancer Ther. 2021;20:833–845. doi: 10.1158/1535-7163.MCT-20-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013;20:1147–1155. doi: 10.1038/nsmb.2669. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YA, Yu J. EZH2, an epigenetic driver of prostate cancer. Protein Cell. 2013;4:331–341. doi: 10.1007/s13238-013-2093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu X, Wang W, Li B, Cheng B, Lin K, Bai J, et al. Targeting Ezh2 could overcome docetaxel resistance in prostate cancer cells. BMC Cancer. 2019;19:27. doi: 10.1186/s12885-018-5228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajabi H, Hiraki M, Tagde A, Alam M, Bouillez A, Christensen CL, et al. MUC1-C activates EZH2 expression and function in human cancer cells. Sci Rep. 2017;7:7481. doi: 10.1038/s41598-017-07850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko YH, Roh KC, Kim BH. The national-wide incidence of prostate-specific antigen testing trend for a decade in Korea by age group. Investig Clin Urol. 2022;63:184–191. doi: 10.4111/icu.20210463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinata N, Fujisawa M. Racial differences in prostate cancer characteristics and cancer-specific mortality: an overview. World J Mens Health. 2022;40:217–227. doi: 10.5534/wjmh.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min K, Chung JW, Ha YS, Lee JN, Kim BS, Kim HT, et al. Efficacy of androgen deprivation therapy in patients with metastatic castration-resistant prostate cancer receiving docetaxel-based chemotherapy. World J Mens Health. 2020;38:226–235. doi: 10.5534/wjmh.190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raychaudhuri R, Ujjani C. Targeted therapy for relapsed/refractory follicular lymphoma: focus on clinical utility of tazemetostat. Onco Targets Ther. 2022;15:193–199. doi: 10.2147/OTT.S267011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Lee Y, Lu X, Song B, Fong KW, Cao Q, et al. Polycomb- and methylation-independent roles of EZH2 as a transcription activator. Cell Rep. 2018;25:2808–2820.e4. doi: 10.1016/j.celrep.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 25.Jaworska D, Szliszka E. Targeting apoptotic activity against prostate cancer stem cells. Int J Mol Sci. 2017;18:1648. doi: 10.3390/ijms18081648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo GH, Piechocki MP, Ensley JF, Nguyen T, Oliver J, Meng H, et al. Docetaxel induced gene expression patterns in head and neck squamous cell carcinoma using cDNA microarray and PowerBlot. Clin Cancer Res. 2002;8:3910–3921. [PubMed] [Google Scholar]
- 27.Chen D, Ming L, Zou F, Peng Y, Van Houten B, Yu J, et al. TAp73 promotes cell survival upon genotoxic stress by inhibiting p53 activity. Oncotarget. 2014;5:8107–8122. doi: 10.18632/oncotarget.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/FRFC8Q.