Abstract
Purpose
This study investigated the risk of lower urinary tract symptoms (LUTS)/benign prostatic hyperplasia (BPH) associated with metformin use.
Materials and Methods
We used the database of Taiwan’s National Health Insurance to create 9,833 pairs of ever users and never users of metformin matched on propensity score. They were males with a new diagnosis of type 2 diabetes during 1999–2005. The incidence of LUTS/BPH was calculated from January 1, 2006 until December 31, 2011. We estimated hazard ratios by Cox regression weighted on propensity score.
Results
There were 515 incident cases in ever users after a median follow-up of 5.4 years (incidence rate: 11.24 per 1,000 person-years) and 682 cases in never users after 5.2 years (15.92 per 1,000 person-years). The hazard ratio (HR) that compared ever to never users was 0.69 (95% confidence interval [CI], 0.62–0.78). The HRs that compared ever users categorized into quartiles of cumulative duration (<19.33, 19.33–41.56, 41.57–67.17, and >67.17 mo) to never users were 1.02 (0.84–1.23), 1.01 (0.86–1.20), 0.57 (0.47–0.69), and 0.40 (0.32–0.49), respectively. For the quartiles of cumulative dose of <582.00, 582.00–1,361.00, 1,361.01–2,449.00, and >2,449.00 g, the respective HRs were 1.03 (0.85–1.24), 0.96 (0.81–1.13), 0.60 (0.49–0.72), and 0.40 (0.32–0.50). The lower risk was significant in all quartiles of defined daily dose. However, a larger daily dose was associated with a greater risk reduction. There were no significant interactions between metformin and other antidiabetic drugs. Patients who used rosiglitazone and/or pioglitazone without metformin had a significantly higher risk (HR, 1.33; 95% CI, 1.09–1.63) and a combination with metformin attenuated such an adverse impact (HR, 0.78; 95% CI, 0.66–0.91).
Conclusions
A significantly lower risk of LUTS/BPH is observed in males with type 2 diabetes who use metformin.
Keywords: Benign prostatic hyperplasia, Lower urinary tract symptoms, Metformin, National Health Insurance, Pharmacoepidemiology, Taiwan
INTRODUCTION
Lower urinary tract symptoms (LUTS) are commonly encountered in clinical practice and benign prostatic hyperplasia (BPH) is the most common cause of LUTS in men [1,2]. Surgical treatment may be necessary if the symptoms are refractory to medical therapy [3,4]. Age is an important risk factor of LUTS/BPH and the prevalence of LUTS/BPH is approximately 3% in men aged 40–49 years, which increases to >30% in men aged ≥85 years, according to a literature review of articles published between 2001 and 2013 in the UK [1].
Modifiable risk factors of LUTS/BPH include metabolic syndrome (characterized by insulin resistance and chronic inflammation with clinical components of central obesity, hyperglycemia, hypertension, and dyslipidemia), cardiovascular disease, pelvic atherosclerosis, depression, hypogonadism, diet, sedentary lifestyle, lack of exercise, hormonal imbalance, excessive alcohol intake, and cigarette smoking [5].
Metformin is the first-line treatment of type 2 diabetes mellitus (T2DM) because it has an insulin sensitizing effect and may provide pleiotropic benefits of anti-inflammation, anti-aging, anti-cancer, and even anti-microbia [6,7,8]. Metformin activates the 5’ adenosine monophosphate-activated protein kinase (AMPK) as a result of the inhibition of mitochondrial respiratory chain complex 1 [6]. Metformin also reduces the risk of nodular goiter [9], uterine leiomyoma [10], benign brain tumors [11], chronic obstructive pulmonary disease [12], inflammatory bowel disease [13], intestinal diverticula [14], hypertension [15], atrial fibrillation [16], heart failure [17], and osteoporosis/vertebral fracture [18].
Because LUTS/BPH is associated with metabolic syndrome and insulin resistance [5], it is reasonable to hypothesize a preventive role of metformin in LUTS/BPH. In this study, we investigated the risk of LUTS/BPH associated with metformin use in male patients with T2DM by using a nationwide reimbursement database of a healthcare system in Taiwan.
MATERIALS AND METHODS
1. Ethics statement
This study was approved by the Research Ethics Committee of the National Health Research Institutes (approval number: NHIRD-102-175). There was no way to contact the individuals because personal information has been de-identified before the release of the database for analyses. Therefore, informed consent is not required according to local regulations.
2. National Health Insurance
Taiwan has started to implement a compulsory healthcare system called the National Health Insurance (NHI) since March 1, 1995. More detailed descriptions of the NHI database can be seen in previously published papers [19,20]. Researchers can request for the use of the reimbursement records of the NHI for academic research.
3. Enrollment of patients
During the study period, disease diagnoses were coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The ICD-9-CM codes for the disease diagnoses used in the study are listed in Supplement Table 1. The investigated outcome included the diagnoses of LUTS together with BPH, denoted as LUTS/BPH.
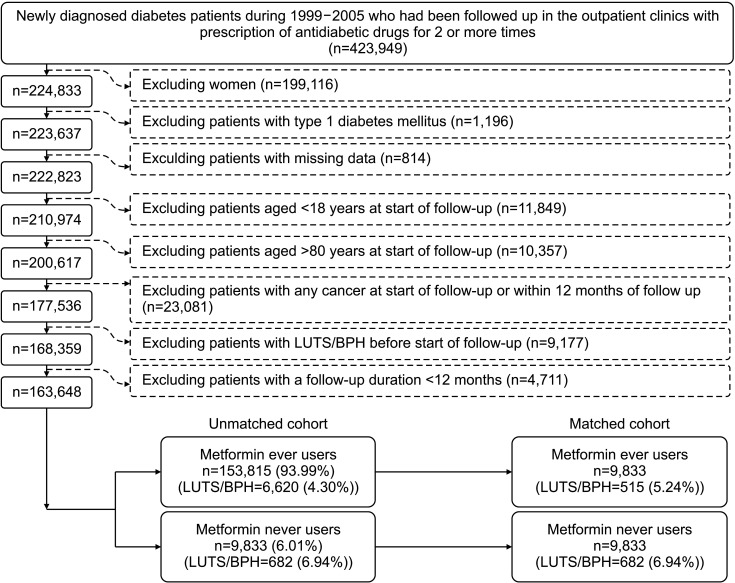
The flowchart in Fig. 1 shows the procedures followed in creating a matched cohort of ever users and never users of metformin. Firstly, we identified 423,949 patients who had a new diagnosis of diabetes mellitus from 1999 to 2005. These patients must have been prescribed antidiabetic drugs for ≥2 times at the outpatient clinics. To assure that the patients’ diabetes was newly diagnosed during 1999–2005, the patients should not have such a diagnosis during the period from 1996 to 1998. We then excluded ineligible patients as shown in the Fig. 1. Patients aged <18 years were excluded because they have very low risk of LUTS/BPH and patients aged >80 years were excluded to avoid healthy survivor effect. Patients with cancer were excluded because they might have suffered from LUTS due to their underlying cancer or the cancer-related treatment. Patients followed up for <12 months were excluded to assure the temporal correctness of cause and effect and to allow a sufficient incubation period between exposure and outcome. As a result, we identified an unmatched cohort of 9,833 never users and 153,815 ever users. We then used logistic regression to create propensity score (PS) by entering all characteristics in Table 1 as independent variables. By using the Greedy 8→1 digit match algorithm, we created a cohort of 9,833 ever users and 9,833 never users matched on PS from the unmatched cohort [20].
Fig. 1. The procedures followed in selecting a matched-pair sample for analyses. LUTS/BPH: lower urinary tract symptoms/benign prostatic hyperplasia.
Table 1. Characteristics of never and ever users of metformin.
| Variable | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Never users (n=9,833) | Ever users (n=153,815) | Standardized difference | Never users (n=9,833) | Ever users (n=9,833) | Standardized difference | ||
| Basic information | |||||||
| Age (y) | 59.41±11.85 | 55.92±11.25 | -32.48 | 59.41±11.85 | 59.59±11.27 | 2.08 | |
| Diabetes duration (y) | 4.10±2.50 | 4.66±2.33 | 24.46 | 4.10±2.50 | 4.10±2.23 | -0.37 | |
| Occupationa | |||||||
| I | 4,346 (44.20) | 72,457 (47.11) | 4,346 (44.20) | 4,396 (44.71) | |||
| II | 1,743 (17.73) | 32,197 (20.93) | 8.59 | 1,743 (17.73) | 1,718 (17.47) | -0.70 | |
| III | 1,858 (18.90) | 26,364 (17.14) | -4.55 | 1,858 (18.90) | 1,838 (18.69) | -0.49 | |
| IV | 1,886 (19.18) | 22,797 (14.82) | -12.83 | 1,886 (19.18) | 1,881 (19.13) | -0.13 | |
| Living region | |||||||
| Taipei | 3,347 (34.04) | 53,136 (34.55) | 3,347 (34.04) | 3,428 (34.86) | |||
| Northern | 1,044 (10.62) | 18,251 (11.87) | 4.19 | 1,044 (10.62) | 1,029 (10.46) | -0.47 | |
| Central | 1,669 (16.97) | 27,146 (17.65) | 1.82 | 1,669 (16.97) | 1,640 (16.68) | -0.77 | |
| Southern | 1,685 (17.14) | 24,517 (15.94) | -3.42 | 1,685 (17.14) | 1,690 (17.19) | 0.19 | |
| Kao-Ping and Eastern | 2,088 (21.23) | 30,765 (20.00) | -2.74 | 2,088 (21.23) | 2,046 (20.81) | -0.90 | |
| Major comorbidities | |||||||
| Obesity | 150 (1.53) | 5,048 (3.28) | 11.65 | 150 (1.53) | 146 (1.48) | -0.25 | |
| Dyslipidemia | 5,656 (57.52) | 102,575 (66.69) | 20.27 | 5,656 (57.52) | 5,651 (57.47) | 0.09 | |
| Hypertension | 7,175 (72.97) | 101,351 (65.89) | -16.99 | 7,175 (72.97) | 7,206 (73.28) | 0.86 | |
| Diabetes-related complications | |||||||
| Nephropathy | 2,524 (25.67) | 23,578 (15.33) | -30.02 | 2,524 (25.67) | 2,448 (24.90) | -2.20 | |
| Diabetic polyneuropathy | 1,034 (10.52) | 23,914 (15.55) | 15.75 | 1,034 (10.52) | 979 (9.96) | -1.98 | |
| Eye diseases | 967 (9.83) | 20,676 (13.44) | 11.52 | 967 (9.83) | 983 (10.00) | -0.06 | |
| Peripheral arterial disease | 1,454 (14.79) | 22,698 (14.76) | -0.82 | 1,454 (14.79) | 1,428 (14.52) | -0.91 | |
| Stroke | 2,510 (25.53) | 28,060 (18.24) | -19.99 | 2,510 (25.53) | 2,501 (25.43) | -0.27 | |
| Ischemic heart disease | 3,668 (37.30) | 48,571 (31.58) | -13.76 | 3,668 (37.30) | 3,601 (36.62) | -1.31 | |
| Hypoglycemia | 146 (1.48) | 1,220 (0.79) | -7.45 | 146 (1.48) | 121 (1.23) | -2.53 | |
| Antidiabetic drugs | |||||||
| Sulfonylurea | 7,187 (73.09) | 103,370 (67.20) | -7.67 | 7,187 (73.09) | 7,438 (75.64) | 6.30 | |
| Meglitinide | 824 (8.38) | 5,713 (3.71) | -21.93 | 824 (8.38) | 803 (8.17) | -0.97 | |
| Rosiglitazone | 262 (2.66) | 6,989 (4.54) | 10.73 | 262 (2.66) | 231 (2.35) | -2.62 | |
| Pioglitazone | 237 (2.41) | 3,996 (2.60) | 1.99 | 237 (2.41) | 240 (2.44) | -0.30 | |
| Acarbose | 1,061 (10.79) | 7,710 (5.01) | -21.93 | 1,061 (10.79) | 1,057 (10.75) | -1.25 | |
| Insulin | 893 (9.08) | 3,457 (2.25) | -33.09 | 893 (9.08) | 797 (8.11) | -5.46 | |
| Other medications | |||||||
| Aspirin | 4,707 (47.87) | 68,967 (44.84) | -7.07 | 4,707 (47.87) | 4,690 (47.70) | -0.23 | |
| Statin | 3,588 (36.49) | 62,670 (40.74) | 9.05 | 3,588 (36.49) | 3,502 (35.61) | -1.84 | |
| Fibrate | 2,650 (26.95) | 51,423 (33.43) | 14.88 | 2,650 (26.95) | 2,637 (26.82) | -0.24 | |
| Calcium channel blocker | 5,263 (53.52) | 68,361 (44.44) | -20.20 | 5,263 (53.52) | 5,276 (53.66) | 0.34 | |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 5,770 (58.68) | 83,622 (54.37) | -10.00 | 5,770 (58.68) | 5,732 (58.29) | -0.77 | |
| Selective serotonin re-uptake inhibitor | 548 (5.57) | 7,267 (4.72) | -4.49 | 548 (5.57) | 515 (5.24) | -1.50 | |
| Comorbidities that might affect exposure/outcome | |||||||
| Ocular pterygium | 336 (3.42) | 4,727 (3.07) | -1.98 | 336 (3.42) | 376 (3.82) | 2.07 | |
| Alcohol-related diagnoses | 825 (8.39) | 11,715 (7.62) | -3.61 | 825 (8.39) | 799 (8.13) | -1.30 | |
| Tobacco abuse | 237 (2.41) | 5,099 (3.32) | 5.75 | 237 (2.41) | 233 (2.37) | -0.22 | |
| Chronic obstructive pulmonary disease | 3,802 (38.67) | 55,531 (36.10) | -6.66 | 3,802 (38.67) | 3,797 (38.61) | -0.03 | |
| Pneumonia | 1,043 (10.61) | 11,908 (7.74) | -12.54 | 1,043 (10.61) | 975 (9.92) | -2.47 | |
| Gingival and periodontal diseases | 7,487 (76.14) | 122,825 (79.85) | 9.28 | 7,487 (76.14) | 7,593 (77.22) | 2.75 | |
| Hyperthyroidism | 205 (2.08) | 3,475 (2.26) | 1.15 | 205 (2.08) | 207 (2.11) | 0.11 | |
| Hypothyroidism | 72 (0.73) | 818 (0.53) | -2.71 | 72 (0.73) | 64 (0.65) | -0.87 | |
| Some disorders of the central nervous system | 1,713 (17.42) | 23,675 (15.39) | -6.54 | 1,713 (17.42) | 1,659 (16.87) | -1.43 | |
| Dementia | 427 (4.34) | 4,455 (2.90) | -9.19 | 427 (4.34) | 383 (3.90) | -2.34 | |
| Parkinson’s disease | 219 (2.23) | 1,714 (1.11) | -10.10 | 219 (2.23) | 210 (2.14) | -0.90 | |
| Episodic mood disorders | 350 (3.56) | 4,920 (3.20) | -2.81 | 350 (3.56) | 306 (3.11) | -2.45 | |
| Depressive disorder | 189 (1.92) | 2,958 (1.92) | -0.37 | 189 (1.92) | 201 (2.04) | 0.75 | |
| Nutritional deficiencies | 236 (2.40) | 2,641 (1.72) | -5.53 | 236 (2.40) | 228 (2.32) | -0.77 | |
| Human immunodeficiency virus disease | 12 (0.12) | 115 (0.07) | -1.78 | 12 (0.12) | 6 (0.06) | -2.52 | |
| Organ transplantation | 70 (0.71) | 237 (0.15) | -11.62 | 70 (0.71) | 58 (0.59) | -1.68 | |
| Psoriasis and similar disorders | 214 (2.18) | 3,828 (2.49) | 2.00 | 214 (2.18) | 195 (1.98) | -1.35 | |
| Arthropathies and related disorders | 6,036 (61.39) | 90,494 (58.83) | -5.90 | 6,036 (61.39) | 6,046 (61.49) | 0.49 | |
| Dorsopathies | 5,975 (60.76) | 95,868 (62.33) | 3.16 | 5,975 (60.76) | 5,977 (60.79) | 0.21 | |
| Urinary tract infection | 1,842 (18.73) | 26,449 (17.20) | -6.27 | 1,842 (18.73) | 1,774 (18.04) | -1.89 | |
| Urinary obstruction | 52 (0.53) | 475 (0.31) | -4.04 | 52 (0.53) | 43 (0.44) | -1.65 | |
| Calculus of kidney and ureter | 1,789 (18.19) | 29,314 (19.06) | 1.58 | 1,789 (18.19) | 1,750 (17.80) | -0.92 | |
| Calculus of lower urinary tract | 232 (2.36) | 3,600 (2.34) | -0.63 | 232 (2.36) | 246 (2.50) | 1.05 | |
| Head injury | 130 (1.32) | 1,808 (1.18) | -2.01 | 130 (1.32) | 126 (1.28) | -0.46 | |
| Osteoporosis | 801 (8.15) | 10,053 (6.54) | -7.17 | 801 (8.15) | 788 (8.01) | -0.53 | |
| Any bone fractures | 1,700 (17.29) | 26,153 (17.00) | -1.72 | 1,700 (17.29) | 1,702 (17.31) | -0.06 | |
| Atrial fibrillation | 344 (3.50) | 3,069 (2.00) | -11.48 | 344 (3.50) | 296 (3.01) | -2.83 | |
| Valvular heart disease | 759 (7.72) | 7,503 (4.88) | -13.68 | 759 (7.72) | 724 (7.36) | -1.25 | |
| Heart failure | 1,417 (14.41) | 14,263 (9.27) | -18.82 | 1,417 (14.41) | 1,357 (13.80) | -2.01 | |
| Helicobacter pylori infection | 58 (0.59) | 810 (0.53) | -1.19 | 58 (0.59) | 50 (0.51) | -1.00 | |
| Peptic ulcer | 3,287 (33.43) | 46,990 (30.55) | -7.40 | 3,287 (33.43) | 3,127 (31.80) | -3.59 | |
| Hepatitis B virus infection | 219 (2.23) | 2,923 (1.90) | -3.55 | 219 (2.23) | 220 (2.24) | -0.05 | |
| Hepatitis C virus infection | 378 (3.84) | 4,652 (3.02) | -5.26 | 378 (3.84) | 365 (3.71) | -0.77 | |
| Liver cirrhosis | 570 (5.80) | 5,570 (3.62) | -11.85 | 570 (5.80) | 550 (5.59) | -1.17 | |
| Other chronic nonalcoholic liver diseases | 767 (7.80) | 13,487 (8.77) | 3.86 | 767 (7.80) | 776 (7.89) | 0.25 | |
| Irritable bowel syndrome | 1,124 (11.43) | 15,905 (10.34) | -4.19 | 1,124 (11.43) | 1,108 (11.27) | -0.46 | |
| Noninfective enteritis and colitis | 4,032 (41.00) | 64,538 (41.96) | 1.64 | 4,032 (41.00) | 4,003 (40.71) | -0.60 | |
| Appendicitis | 150 (1.53) | 2,564 (1.67) | 1.30 | 150 (1.53) | 151 (1.54) | 0.22 | |
| Anal fissure and fistula | 295 (3.00) | 5,384 (3.50) | 3.03 | 295 (3.00) | 278 (2.83) | -1.00 | |
| Abscess of anal and rectal regions | 212 (2.16) | 4,348 (2.83) | 4.48 | 212 (2.16) | 194 (1.97) | -1.42 | |
Values are presented as mean±standard deviation or number (%).
aDefinitions of classification of occupation can be seen in “MATERIALS AND METHODS”.
4. Covariates
The following categories of variables listed in Table 1 were included as potential confounders: basic information, major comorbidities, diabetes-related complications, antidiabetic drugs, other medications and comorbidities that might affect exposure/outcome. The definitions of different categories of occupation and living region were detailed elsewhere [20]. In brief, living region was geographically classified and occupation was classified as class 1 (civil servants, teachers, employees of governmental or private businesses, professionals, and technicians), class 2 (people without a specific employer, self-employed people, or seamen), class 3 (farmers or fishermen), and class 4 (low-income families supported by social welfare or veterans).
5. Statistical analyses
We used the SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA) as a tool for statistical analyses. A 2-tailed p-value <0.05 was considered statistically significant.
We calculated the standardized difference for each variable in Table 1 and used a threshold value of >10% as an indicator of potential confounding [20].
To investigate a dose-response relationship, we calculated the cumulative duration (in months) and cumulative dose (in grams) of metformin therapy among ever users and categorized the users into 4 subgroups according to the quartiles of these indicators. We accumulated the days of metformin prescriptions from the initiation of metformin until the last prescription within the study period. The accumulated number of days was divided by 30 to express the cumulative duration as months of exposure [21]. Cumulative dose was also calculated from the database by summating the total doses of metformin in mg prescribed during the study period [21]. To investigate whether the daily dsose of metformin might affect the risk, we calculated the average defined daily dose (DDD) of metformin use per day (1 unit of DDD for metformin=2 grams).
Incidence density of LUTS/BPH was calculated for never users, ever users and ever users categorized by the quartiles of cumulative duration, cumulative dose and DDD. The numerator of the incidence was the case number of LUTS/BPH diagnosed during follow-up. The denominator was the follow-up person-years calculated from January 1, 2006 until December 31, 2011, which ended when whichever of the following events occurred first: a new diagnosis of LUTS/BPH, death or the last reimbursement record.
Cumulative incidence functions for LUTS/BPH were plotted for never users and ever users and the differences were tested by Gray’s test. Analyses were conducted with and without the exclusion of patients who had been followed up for <12 months in both the matched and the unmatched cohorts.
To reduce the potential confounding from the differences in characteristics, hazard ratios and their 95% confidence intervals were estimated by Cox regression incorporated with the inverse probability of treatment weighting using the PS [20]. Ever users and users in each quartile of cumulative duration, cumulative dose and DDD were compared to never users as the referent group. To examine whether the exclusion of patients during the matching process might lead to attrition bias, we also created, as sensitivity analyses, the above models in the unmatched cohort.
To examine the potential risk of overadjustment, we conducted as additional sensitivity analyses the following three conventional Cox models in the matched cohort: 1) unadjusted; 2) adjusted for covariates with significant p-values (<0.05) while comparing ever users to never users by Student’s t-test for continuous variables of age and diabetes duration and by chi-square test for other categorical variables; and 3) adjusted for all covariates in Table 1.
The following subgroup analyses were conducted:
I) A ge <65 years and ≥65 years.
II) Patients enrolled during 1999–2002 and during 2003–2005. This was to examine whether the findings might be affected by some unidentified confounders such as the introduction of novel antidiabetic drugs and the changes of treatment guidelines during the long follow-up duration.
III) Antidiabetic drugs prescribed by family physicians/endocrinologists and by doctors of other specialties. Diabetes patients are mainly treated by family physicians and endocrinologists in Taiwan and the prescription patterns of antidiabetic drugs might be different between family physicians/endocrinologists and doctors of other specialties.
IV) Patients who developed “symptoms involving digestive system” after enrollment and patients who did not. Gastrointestinal symptoms are common side effects of metformin that may lead to discontinuation of metformin’s prescription.
V) Patients with a diagnosis of “diabetes with ketoacidosis” and/or “diabetes with hyperosmolarity” after enrollment and patients without these diagnoses after enrollment. The clinical manifestations of diabetes ketoacidosis and/or hyperosmolarity might have been misinterpreted as LUTS.
VI) To clarify whether the use of other insulin sensitizers such as rosiglitazone and/or pioglitazone might exert a similar effect as metformin, patients were categorized into the following four subgroups: (1) patients never been treated with metformin, rosiglitazone and/or pioglitazone (as the referent group); (2) patients treated with metformin but not been treated with rosiglitazone and/or pioglitazone; (3) patients treated with rosiglitazone and/or pioglitazone but not been treated with metformin; and (4) patients having been treated with metformin and rosiglitazone and/or pioglitazone.
To investigate whether metformin might interact with other antidiabetic drugs, Cox regression models were created by entering metformin and all covariates in Table 1 as independent variables together with the interaction term of metformin and each of the antidiabetic drugs one at a time for estimating the p-value of interaction.
RESULTS
Table 1 shows the characteristics of never users and ever users. It is evident that the values of standardized difference were >10% between ever users and never users in many covariates in the unmatched cohort. However, in the matched cohort, all values were <10%, suggesting that all covariates were well matched and the risk of confounding by indication was minimal.
The cumulative incidence functions were plotted in Fig. 2. A significantly lower risk of LUTS/BPH associated with metformin use can be observed in all plots (p<0.01 by Gray’s test) in the matched cohort as well as in the unmatched cohort, disregarding whether patients followed up for <12 months had been excluded or not.
Fig. 2. Cumulative incidence functions for lower urinary tract symptoms/benign prostatic hyperplasia in never users and ever users of metformin in the matched cohort and the unmatched cohort, respectively. Plotted for the matched cohort with (A) exclusion and (B) without exclusion of patients followed up for <12 months, respectively. Plotted for the unmatched cohort with (C) exclusion and (D) without exclusion of patients followed up for <12 months, respectively. p-values by Gray’s test are all <0.01.
The incidences of LUTS/BPH and the hazard ratios in the matched cohort are shown in Table 2. There were 515 incident cases in ever users after a median follow-up of 5.4 years and 682 incident cases in never users after a median follow-up of 5.2 years. The incidence (expressed as per 1,000 person-years) in ever users was 11.24 and was 15.92 in never users. The overall risk reduction was approximately 30% with statistical significance while ever users were compared to never users. A dose-response relationship was consistently observed in terms of cumulative duration, cumulative dose and DDD. Users with a cumulative dose of more than 41.57 months (the third and fourth quartiles) or more than a cumulative dose of 1,361.01 g (the third and fourth quartiles) had a significantly lower risk. Metformin therapy in any group of the DDD showed a significantly lower risk, but a larger magnitude of risk reduction was observed with increasing DDD.
Table 2. Incidences of lower urinary tract symptoms/benign prostatic hyperplasia and hazard ratios comparing metformin exposed to unexposed groups in the matched cohort.
| Metformin use | Incident case number | Cases followed (n) | Person-years of follow-up | Incidence rate (per 1,000 person-years) | Hazard ratio | 95% confidence interval | p-value | |
|---|---|---|---|---|---|---|---|---|
| Never users | 682 | 9,833 | 42,835.45 | 15.92 | 1.00 | |||
| Ever users | 515 | 9,833 | 45,799.06 | 11.24 | 0.69 | 0.62–0.78 | <0.01 | |
| Quartiles of cumulative duration of metformin therapy (mo) | ||||||||
| Never users | 682 | 9,833 | 42,835.45 | 15.92 | 1.00 | |||
| <19.33 | 125 | 2,455 | 8,261.29 | 15.13 | 1.02 | 0.84–1.23 | 0.87 | |
| 19.33–41.56 | 175 | 2,465 | 10,835.97 | 16.15 | 1.01 | 0.86–1.20 | 0.89 | |
| 41.57–67.17 | 120 | 2,455 | 12,691.94 | 9.45 | 0.57 | 0.47–0.69 | <0.01 | |
| >67.17 | 95 | 2,458 | 14,009.87 | 6.78 | 0.40 | 0.32–0.49 | <0.01 | |
| Quartiles of cumulative dose of metformin therapy (g) | ||||||||
| Never users | 682 | 9,833 | 42,835.45 | 15.92 | 1.00 | |||
| <582.00 | 127 | 2,457 | 8,322.43 | 15.26 | 1.03 | 0.85–1.24 | 0.80 | |
| 582.00–1,361.00 | 168 | 2,457 | 10,962.03 | 15.33 | 0.96 | 0.81–1.13 | 0.61 | |
| 1,361.01–2,449.00 | 126 | 2,460 | 12,710.11 | 9.91 | 0.60 | 0.49–0.72 | <0.01 | |
| >2,449.00 | 94 | 2,459 | 13,804.49 | 6.81 | 0.40 | 0.32–0.50 | <0.01 | |
| Quartiles of defined daily dose of metformin therapya | ||||||||
| Never users | 682 | 9,833 | 42,835.45 | 15.92 | 1.00 | |||
| <0.47 | 140 | 2,458 | 10,647.28 | 13.15 | 0.83 | 0.69–0.99 | 0.04 | |
| 0.47–0.55 | 142 | 2,459 | 11,129.74 | 12.76 | 0.79 | 0.66–0.95 | 0.01 | |
| 0.56–0.73 | 127 | 2,458 | 11,957.82 | 10.62 | 0.65 | 0.54–0.79 | <0.01 | |
| >0.73 | 106 | 2,458 | 12,064.22 | 8.79 | 0.54 | 0.44–0.66 | <0.01 | |
aOne unit of defined daily dose for metformin=2 grams.
The results of the sensitivity analyses conducted in the unmatched cohort are shown in Supplement Table 2 and the results of the three conventional Cox regression models conducted in the matched cohort are shown in Supplement Table 3. All supported the robustness of the conclusion of the study.
Subgroup analyses are shown in Table 3. The significantly lower risk associated with metformin could be seen in both the younger (<65 y) and the older (≥65 y) age groups. However, the younger age group might have a larger magnitude of risk reduction (subgroup category I). Results of a lower risk associated with metformin use were consistently observed in other subgroup analyses from category II to category V. It is interesting that the use of rosiglitazone and/or pioglitazone, drugs that belong to the class of insulin sensitizers, did not similarly show a lower risk of LUTS/BPH (subgroup category VI). Patients who used rosiglitazone and/or pioglitazone without metformin would actually increase the risk (hazard ratio, 1.33; 95% confidence interval, 1.09–1.63). However, a combination with metformin might attenuate such an increased risk associated with rosiglitazone and/or pioglitazone (hazard ratio, 0.78; 95% confidence interval, 0.66–0.91).
Table 3. Subgroup analyses.
| Subgroup categories/metformin use | Incident case number | Cases followed (n) | Person-years of follow-up | Incidence rate (per 1,000 person-years) |
Hazard ratio | 95% confidence interval | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| I. Age (y) | |||||||||
| <65 | |||||||||
| Never users | 295 | 6,355 | 28,516.45 | 10.34 | 1.00 | ||||
| Ever users | 193 | 6,390 | 30,654.11 | 6.29 | 0.60 | 0.50–0.72 | <0.0001 | ||
| ≥65 | |||||||||
| Never users | 387 | 3,478 | 14,319.00 | 27.03 | 1.00 | ||||
| Ever users | 322 | 3,443 | 15,144.95 | 21.26 | 0.77 | 0.67–0.90 | 0.0006 | ||
| II. Enrollment period | |||||||||
| 1999–2002 | |||||||||
| Never users | 306 | 4,161 | 17,666.56 | 17.32 | 1.00 | ||||
| Ever users | 250 | 4,212 | 19,951.59 | 12.53 | 0.70 | 0.59–0.83 | <0.0001 | ||
| 2003–2005 | |||||||||
| Never users | 376 | 5,672 | 25,168.89 | 14.94 | 1.00 | ||||
| Ever users | 265 | 5,621 | 25,847.47 | 10.25 | 0.68 | 0.58–0.80 | <0.0001 | ||
| III. Antidiabetic drugs prescribed by family physicians or endocrinologists | |||||||||
| No | |||||||||
| Never users | 240 | 4,148 | 17,409.43 | 13.79 | 1.00 | ||||
| Ever users | 91 | 1,902 | 8,343.43 | 10.91 | 0.78 | 0.62–0.99 | 0.0478 | ||
| Yes | |||||||||
| Never users | 442 | 5,685 | 25,426.02 | 17.38 | 1.00 | ||||
| Ever users | 424 | 7,931 | 37,455.63 | 11.32 | 0.64 | 0.56–0.73 | <0.0001 | ||
| IV. Symptoms involving digestive system after enrollment | |||||||||
| No | |||||||||
| Never users | 554 | 8,644 | 37,205.98 | 14.89 | 1.00 | ||||
| Ever users | 413 | 8,480 | 39,124.65 | 10.56 | 0.70 | 0.61–0.79 | <0.0001 | ||
| Yes | |||||||||
| Never users | 128 | 1,189 | 5,629.48 | 22.74 | 1.00 | ||||
| Ever users | 102 | 1,353 | 6,674.41 | 15.28 | 0.66 | 0.51–0.86 | 0.0019 | ||
| V. Diagnosis of diabetic ketoacidosis and/or hyperosmolarity after enrollment | |||||||||
| No | |||||||||
| Never users | 661 | 9,618 | 41,961.47 | 15.75 | 1.00 | ||||
| Ever users | 491 | 9,515 | 44,340.75 | 11.07 | 0.69 | 0.62–0.78 | <0.01 | ||
| Yes | |||||||||
| Never users | 21 | 215 | 873.98 | 24.03 | 1.00 | ||||
| Ever users | 24 | 318 | 1,458.32 | 16.46 | 0.66 | 0.37–1.19 | 0.17 | ||
| VI. Use of other insulin sensitizers of rosiglitazone and/or pioglitazone | |||||||||
| None | 562 | 8,488 | 36,609.08 | 15.35 | 1.00 | ||||
| Metformin but without rosiglitazone and/or pioglitazone | 311 | 6,151 | 27,589.77 | 11.27 | 0.70 | 0.61–0.80 | <0.01 | ||
| Rosiglitazone and/or pioglitazone but without metformin | 120 | 1,345 | 6,226.37 | 19.27 | 1.33 | 1.09–1.63 | <0.01 | ||
| Metformin and rosiglitazone and/or pioglitazone | 204 | 3,682 | 18,209.29 | 11.20 | 0.78 | 0.66–0.91 | <0.01 | ||
There were no significant interactions between metformin and any other antidiabetic drugs. All p-values for the interaction terms were >0.05 (data not shown).
DISCUSSION
1. Main findings
The findings supported an overall 30% lower risk of LUTS/BPH in male patients with T2DM who had ever used metformin than patients who had never used metformin (Fig. 2, Table 2). The lower risk associated with metformin was consistently observed in all subgroup (Table 3) and sensitivity analyses (Supplement Table 2, 3).
2. Potential mechanisms
1) Improvement of insulin resistance probably not responsible
Both T2DM and LUTS/BPH share a common mechanism of insulin resistance [22] and one of the potential mechanisms of metformin is through its effect of improving insulin sensitivity [6]. However, the significantly higher risk among patients who used rosiglitazone and/or pioglitazone and metformin might mitigate such an increased risk (Table 3, subgroup category VI) did not support such a mechanism.
2) Aging
Age is a common risk factor of T2DM [23] and LUTS/BPH [1]. Metformin has anti-aging, anti-microbial and anti-inflammatory effects [6,7,8] and may inhibit the development and growth of BPH in cellular, animal and human studies [24]. Therefore, metformin may reduce the risk of LUTS/BPH via these mechanisms. However, these hypothetical mechanisms await further confirmation.
3) Hormonal effects
Hyperinsulinemia as a result of insulin resistance may lead to lower levels of total testosterone and serum sex-hormone binding globulin, which can contribute to a higher risk of LUTS [5]. In animals, testosterone exerts effects on neural tissue involved in bladder control and treatment with testosterone may improve bladder capacity, induce bladder neck smooth muscle relaxation and inhibit the contractility of detrusor muscle [5]. However, it should be noted that supraphysiological levels of testosterone may affect the urethral outlet and induce urinary retention [5].
Recent experiments have demonstrated that metformin may exert antidiuretic effects without causing hyponatremia via activation of AMPK [25]. Although not yet researched, it is theoretically possible that metformin may reduce the frequency of nocturia.
4) Nervous system
Increased sympathetic activity may increase muscle tone of the prostate and induce the micturition symptoms of LUTS [5]. Some studies suggested that metformin may alleviate sympathetic overactivity [26]. Therefore, this may also partly explain a lower risk of LUTS/BPH associated with metformin use.
Patients with cognitive impairment or dementia are at a higher risk of LUTS/BPH [27]. Our previous studies support a significantly lower risk of dementia in diabetes patients who were treated with metformin [28,29]. Therefore, metformin may lower the risk of LUTS/BPH through a beneficial effect on cognitive function and dementia.
3. Implications
There are some implications in this study. First, older men who get up for urination at night are also at risk of falling [1,22]. Therefore, reducing the frequency of nocturia may also potentially reduce the risk of falls and fractures.
Second, more patients with LUTS would seek medical help for the improvement of quality of life in recent years [30]. Therefore, LUTS is expected to increase healthcare burden and societal expenses because of the increasing proportion of aging population and the more willingness to seek medical help. Prevention of LUTS/BPH by metformin may have great clinical impacts.
Third, because an exposure to metformin use of approximately 3.5 years (or a cumulative duration of 42 mo) was required for a significant risk reduction and a higher DDD would have exerted a larger magnitude of risk reduction (Table 2), metformin should better be titrated to a maximum tolerable daily dose and be continued (if not contraindicated) even when other antidiabetic drugs were added for a better glycemic control.
4. Strengths
We have enrolled research subjects of ever users and never users who were well matched on many potential confounders (Table 1). Diagnoses of urinary tract diseases (e.g., urinary tract infection, urinary obstruction, and urinary tract stones), neurological diseases (e.g., stroke, Parkinson’s disease, and dementia) and mental disorders (e.g., episodic mood disorders and depressive disorders) that might have been potential confounders were all well matched between the two groups (Table 1).
5. Limitations
There are some limitations. First, the definitions of LUTS and BPH were based on the diagnostic codes. It has long been suggested that the definition of BPH should include parameters of prostate size, symptoms and peak urinary flow rate [2]. However, we did not have related information in the database for a more accurate definition. Furthermore, LUTS and BPH are not binomial and may change with time in varying degree of severity in relation to BPH progression, aging process, diabetes control, and many other clinical conditions. These should be considered in future investigation.
Second, because this was a ‘real-world’ retrospective cohort study, all inherent limitations associated with this kind of study design could not be excluded. However, the results seemed to be robust, because similar findings were consistently observed when analyses were conducted in the unmatched cohort (Fig. 2, Supplement Table 2), in different subgroup analyses (Table 3), with or without exclusion of patients followed up for <12 months (Fig. 2) and by using conventional Cox regression with different sets of covariates adjusted (Supplement Table 3).
Third, because biochemical data were not available, we could not investigate the impacts of glycemic control (e.g., blood glucose or hemoglobin A1c). However, because glycemic control is highly correlated to diabetes complications and other comorbidities, the balance of these diagnoses after matching (Table 1) could also have balanced the effect of glycemic control.
Fourth, because only patients with T2DM were enrolled, it is not known whether metformin may also prevent LUTS/BPH in patients with type 1 diabetes mellitus or in people without diabetes.
Fifth, we did not have information of tea or coffee drinking for adjustment because both may have diuretic effects and increase the occurrence of nocturia or LUTS.
CONCLUSIONS
The present study supports an overall 30% lower risk of LUTS/BPH associated with metformin use in male patients with T2DM in Taiwan. More research is warranted to confirm the finding in other ethnicities and to investigate metformin’s effect in non-diabetes people.
Footnotes
Conflict of Interest: The author has nothing to disclose.
Funding: The Ministry of Science and Technology of Taiwan (MOST 107-2221-E-002-129-MY3) and the Yee Fong Charity Foundation provided financial support for the study. The funders did not have any role in study design, data collection, statistical analysis, manuscript writing and decision for publication.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220133.
Disease diagnoses used in the study and their corresponding codes according to the ICD-9-CM
Incidences of lower urinary tract symptoms/benign prostatic hyperplasia and hazard ratios comparing metformin exposed to unexposed groups in the unmatched cohort
Incidences of lower urinary tract symptoms/benign prostatic hyperplasia and hazard ratios estimated by three conventional Cox regression models in the matched cohort
References
- 1.Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int. 2015;115:508–519. doi: 10.1111/bju.12745. [DOI] [PubMed] [Google Scholar]
- 2.Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6(Suppl 9):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 3.Tuccio A, Grosso AA, Sessa F, Salvi M, Tellini R, Cocci A, et al. En-bloc holmium laser enucleation of the prostate with early apical release: are we ready for a new paradigm? J Endourol. 2021;35:1675–1683. doi: 10.1089/end.2020.1189. [DOI] [PubMed] [Google Scholar]
- 4.Tuccio A, Sessa F, Campi R, Grosso AA, Viola L, Muto G, et al. En-bloc endoscopic enucleation of the prostate: a systematic review of the literature. Minerva Urol Nefrol. 2020;72:292–312. doi: 10.23736/S0393-2249.20.03706-6. [DOI] [PubMed] [Google Scholar]
- 5.Traish AM, Johansen V. Impact of testosterone deficiency and testosterone therapy on lower urinary tract symptoms in men with metabolic syndrome. World J Mens Health. 2018;36:199–222. doi: 10.5534/wjmh.180032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniar K, Moideen A, Mittal A, Patil A, Chakrabarti A, Banerjee D. A story of metformin-butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: genesis of a wonder drug? Pharmacol Res. 2017;117:103–128. doi: 10.1016/j.phrs.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Tseng CH. Metformin decreases risk of tuberculosis infection in type 2 diabetes patients. J Clin Med. 2018;7:264. doi: 10.3390/jcm7090264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng CH. Metformin and Helicobacter pylori infection in patients with type 2 diabetes. Diabetes Care. 2018;41:e42–e43. doi: 10.2337/dc17-2551. [DOI] [PubMed] [Google Scholar]
- 9.Tseng CH. Metformin reduces risk of benign nodular goiter in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2019;180:365–372. doi: 10.1530/EJE-19-0133. [DOI] [PubMed] [Google Scholar]
- 10.Tseng CH. Metformin use is associated with a lower risk of uterine leiomyoma in female type 2 diabetes patients. Ther Adv Endocrinol Metab. 2019;10:2042018819895159. doi: 10.1177/2042018819895159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CH. Metformin is associated with a lower incidence of benign brain tumors: a retrospective cohort study in patients with type 2 diabetes mellitus. Biomolecules. 2021;11:1405. doi: 10.3390/biom11101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CH. Metformin and risk of chronic obstructive pulmonary disease in diabetes patients. Diabetes Metab. 2019;45:184–190. doi: 10.1016/j.diabet.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J Crohns Colitis. 2021;15:64–73. doi: 10.1093/ecco-jcc/jjaa136. [DOI] [PubMed] [Google Scholar]
- 14.Tseng CH. Metformin reduces the risk of diverticula of intestine in Taiwanese patients with type 2 diabetes mellitus. Front Pharmacol. 2021;12:739141. doi: 10.3389/fphar.2021.739141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng CH. Metformin and risk of hypertension in Taiwanese patients with type 2 diabetes mellitus. J Am Heart Assoc. 2018;7:e008860. doi: 10.1161/JAHA.118.008860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng CH. Metformin use is associated with a lower incidence of hospitalization for atrial fibrillation in patients with type 2 diabetes mellitus. Front Med (Lausanne) 2021;7:592901. doi: 10.3389/fmed.2020.592901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng CH. Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: a retrospective cohort analysis. J Am Heart Assoc. 2019;8:e011640. doi: 10.1161/JAHA.118.011640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng CH. Metformin use is associated with a lower risk of osteoporosis/vertebral fracture in Taiwanese patients with type 2 diabetes mellitus. Eur J Endocrinol. 2021;184:299–310. doi: 10.1530/EJE-20-0507. [DOI] [PubMed] [Google Scholar]
- 19.Tseng CH. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget. 2017;8:41132–41142. doi: 10.18632/oncotarget.17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng CH. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: a retrospective cohort analysis. Diabetes Metab. 2017;43:438–445. doi: 10.1016/j.diabet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Tseng CH. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol Oncol. 2015;138:147–153. doi: 10.1016/j.ygyno.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 22.Hackett G. Should all men with type 2 diabetes be routinely prescribed a phosphodiesterase type 5 inhibitor? World J Mens Health. 2020;38:271–284. doi: 10.5534/wjmh.200027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih SR, Tseng CH. The effects of aging on glucose metabolism. Taiwan Geriatr Gerontol. 2009;4:27–38. [Google Scholar]
- 24.Tseng CH. The effect of metformin on male reproductive function and prostate: an updated review. World J Mens Health. 2022;40:11–29. doi: 10.5534/wjmh.210001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Jo CH, Kim GH. The role of vasopressin V2 receptor in drug-induced hyponatremia. Front Physiol. 2021;12:797039. doi: 10.3389/fphys.2021.797039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen JS, DiBona GF. Acute sympathoinhibitory actions of metformin in spontaneously hypertensive rats. Hypertension. 1996;27(3 Pt 2):619–625. doi: 10.1161/01.hyp.27.3.619. [DOI] [PubMed] [Google Scholar]
- 27.Chang CW, Juan YS, Yang YH, Lee HY. The relationship between lower urinary tract symptoms and severity of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2021;36:1533317521992657. doi: 10.1177/1533317521992657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng CH. Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis. 2019;10:37–48. doi: 10.14336/AD.2017.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng CH. Dementia risk in type 2 diabetes patients: acarbose use and its joint effects with metformin and pioglitazone. Aging Dis. 2020;11:658–667. doi: 10.14336/AD.2019.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu MP, Hsu YW, Weng SF, Ho CH, Wang JJ, Tong YC. Healthcare-seeking prevalence of lower urinary tract symptoms among National Health Insurance enrollees in Taiwan, 2000-2009. Urology. 2013;81:61–65. doi: 10.1016/j.urology.2012.08.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disease diagnoses used in the study and their corresponding codes according to the ICD-9-CM
Incidences of lower urinary tract symptoms/benign prostatic hyperplasia and hazard ratios comparing metformin exposed to unexposed groups in the unmatched cohort
Incidences of lower urinary tract symptoms/benign prostatic hyperplasia and hazard ratios estimated by three conventional Cox regression models in the matched cohort
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.