Abstract
Purpose
To evaluated the efficacy and safety of gelatinized Maca (Lepidium meyenii) for eugonadal patients with late onset hypogonadism symptoms (LOH).
Materials and Methods
Participants were instructed to receive 1,000 mg of Maca or placebo, two pills at a time, three times per day for 12 weeks before food intake. To evaluate the efficacy of the drug, Aging Males’ Symptoms scale (AMS), Androgen Deficiency in the Aging Males (ADAM), International Prostate Symptom Score (IPSS), and International Index of Erectile Function (IIEF) questionnaires, serologic tests (total testosterone and free testosterone, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglyceride), body weight, and waist circumference were assessed at 4 and 12 weeks after treatment.
Results
A total of 80 participants were enrolled and randomly assigned to Maca treated group (n=41) or the placebo group (n=39). AMS, IIEF, and IPSS were significantly (p<0.05) improved in Maca treated group than in the placebo group. ADAM positive rate was also significantly (p<0.0001) decreased in Maca treated group.
Conclusions
Maca may be considered an effective and safe treatment for eugonadal patients with late onset hypogonadism symptoms.
Keywords: Herbal medicine, Hypogonadism, Lepidium, Physiological, Sexual dysfunction
INTRODUCTION
Late-onset hypogonadism (LOH) is a characterized by clinical symptoms of androgen deficiency and low testosterone levels with advancing age. Symptoms of LOH include loss of muscle mass, increased body fat, anemia, osteoporosis, depressed mood, decreased vitality, sweating, hot flushes, loss of libido, and erectile dysfunction (ED). Among them, sexual symptoms and ED and reduced libido are the most important symptoms related to LOH [1].
Some of the guidelines for treating LOH focus on the testosterone substitution for a combination symptoms of testosterone deficiency and low serum testosterone levels. However, there has been no agreement on threshold levels signifying low testosterone. On the other hand, prescribed testosterone products have potential to increase the risk of not only prostate cancer but also heart attacks and strokes [2]. The US Endocrine Society Practice Guideline on testosterone treatment (2018) recommends clinicians to offer testosterone treatment on an individualized basis after explicitly discussing potential risks and benefits associated with such testosterone treatment [3]. However, previous studies have reported that LOH-related symptoms are not significantly correlated with serum total testosterone level [4,5,6,7]. And there remains unmet need for many middle aged and older eugonadal patients suffering from LOH-related symptoms who are not eligible for testosterone treatment. Therefore, there is a need to develop products derived from natural herbal medications that have fewer side effects and are easy to take.
Lepidium meyenii, a plant with a common name of Maca, which belongs to Brassicaceae family, grows at over 4,000 m in Peru. It has been consumed worldwide for centuries as a dietary supplement of its nutritional and medicinal properties. And previous studies have proved various biological effects of Maca in the field of male reproductive functions [8,9,10,11,12]. In particular, a study that improved sexual desire without effecting testosterone concentration in adult men could be an advantage in LOH treatment [11]. Also there were no cases of serious adverse events in studies with Maca. It comes in yellow, red and black varieties and previous study suggested that black Maca has prior effect in increasing sperm count compared to yellow or red Maca [13]. Additionally, the existing Maca products are mostly in powder form and had poor compliance to administration. In order to increase compliance with dose, we performed clinical trial with tablet product.
So in this study, looking forward to male reproductions of black Maca as described above with improved compliance with tablet product, we evaluated the efficacy and safety of gelatinized Maca for eugonadal patients with LOH-related symptoms.
MATERIALS AND METHODS
1. Ethical approval
This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea, Seoul St. Mary’s Hospital (IRB No: KC20HIDV0061). Subjects participated in the trial after fully understanding about the purposes, schedule, risks, and benefits of this study. Written informed consent was obtained from subjects before screening. This study was also approved by the Clinical Research Information Service of South Korea (CRIS No: Clinical Research Information Service Identifier: KCT0006415), which is registered in World Health Organization (WHO) International Clinical Trials Registry Platform.
2. Study design
This study conducted a double-blind, randomized, parallel-group, placebo-controlled clinical trial at two hospitals (The Catholic University of Korea, Seoul St. Mary’s Hospital, Korea, Korea University Guro Hospital, Korea) in accordance to the Declaration of Helsinki and ICH Guideline for Good Clinical Practice. Subjects were recruited prospectively and consecutively between February 2020 and February 2021. The number of subjects was estimated from a previous study using plant-derived herbal medicine which evaluated Aging Males’ Symptoms scale (AMS) differences between two groups [14]. With a power of 80% at a significance level of 0.05, a total of 39 subjects were required for each group, and the number of subjects to be enrolled was 88 for appropriate efficacy evaluation considering the dropout rate of 10%. A study assistant randomly allocated patients via block randomization using a table of random numbers. The patients were recruited from outpatients at the hospital. Enrolled patients visited the outpatient department of urology at each hospital for follow-ups, and two urologists (SWK, DGM) assumed overall responsibility for this process. Eligible subjects were randomized to receive investigational products (1,000 mg Maca or placebo) two tablets at a time, three times per day for 12 weeks before food intake according to a previous report [15]. Subjects were assessed at 4 and 12 weeks after treatment.
3. Inclusion and exclusion criteria
Patients met following criteria were included in the study: men aged over 40 years and total scores on the AMS questionnaire ≥27. Subjects with following medical conditions were excluded from the study: prostate specific antigen (PSA) ≥4.0 ng/mL, history or diagnosis of prostate cancer, severe obstructive sleep apnea, total serum testosterone <2.0 ng/mL in the morning, subjects who had taken testosterone replacement therapy (TRT) within 6 months before randomization, any clinically significant abnormal hematological or biochemical findings (anemia: hemoglobin less than 10 g/dL, leukocytosis: white blood cell >10.0×109/L, neutropenia: absolute neutrophil counts<1.0×109/L and etc.) or physical findings (quadriplegia, tetraplegia and etc.) or unstable or untreated endocrine disorders (diabetes, hyper or hypothyroidism, cushing syndrome and etc.), hepatic failure of 2.5 times higher than normal limits of aspartate transaminase/alanine trasferase, or renal disease with a serum creatinine value higher than 2.5 mg/dL. Those with psychiatric diseases, history or presence of other disorders such as clinically significant cardiovascular, cerebrovascular or hematological disorders were also excluded depending on the opinion of the investigator. Finally, 41 subjects were enrolled for the Maca treatment group and 39 men were recruited for the control group.
4. Investigational products
Each Maca capsule (1,000 mg/capsule) contained 833 mg Maca gelatinized powder. The placebo capsule contained lactose mixed powder at the same dose, shape, and color. Both capsules were manufactured at Easter B&F good manufacturing practice site in Seoul, Korea. Release criteria for Maca gelatinized powder were as follows: content of n-benzyl-hexadecanamide should be between 115 and 175 µg/g, contents of cadmium and total mercury should be less than 0.3 mg/kg, contents of total arsenic and plumbum should be less than 0.5 mg/kg, and the absence of coliform bacteria. Investigational products were labeled and coded for blinding following Good Clinical Practice standards. The total daily Maca dose was approximately 5 g (six capsules).
5. Outcome measures
The primary efficacy was evaluated with the change of AMS score from baseline. Secondary efficacy measurements were the change of subjective questionnaires including Androgen Deficiency in the Aging Males (ADAM), the International Index of Erectile Function (IIEF), and International Prostate Symptom Score (IPSS), serum levels of total testosterone, free testosterone, total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglyceride, body weight, and waist circumference. Included safety assessments were blood laboratory tests (PSA, hematology, clinical biochemistry), urinalysis, vital sign (heart rate and blood pressure), and adverse events.
6. Statistical analysis
All statistical analyses were performed using SAS software version 9.4 (SAS institute, Cary, NC, USA). Continuous variables were represented as mean±standard deviation and paired t-test were used to evaluate primary and secondary outcomes. Difference was considered significant when p-value was less than 0.05.
RESULTS
1. Patients characteristics
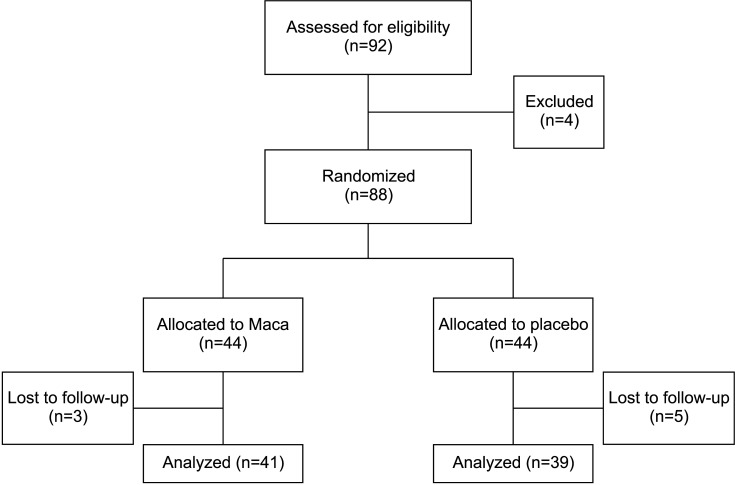
A total of 88 patients were recruited and randomly assigned to Maca treated group (n=44) or placebo group (n=44). Among them 3 patients (2 patients were reluctant to take the clinical drug continuously and 1 patient stopped visiting the clinic for no reason) from the Maca treated group and 5 patients (3 patients were reluctant to take the testing drug and 2 patients wanted to take different treatment such as TRT) from place group were lost to follow-up (Fig. 1). So total 80 men were enrolled at the end of the study. At baseline, there were no significant changes in questionnaire scores or clinical parameters between the two groups except serum total testosterone, serum free testosterone, serum total cholesterol, and serum LDL-C (Table 1).
Fig. 1. Flow diagram of study participants. Maca: Lepidium meyenii.
Table 1. Baseline characteristics of patients.
| Characteristic | Maca (n=41) | Placebo (n=39) | p-value |
|---|---|---|---|
| Age (y) | 65.02±6.80 | 65.74±8.68 | |
| Height (cm) | 167.31±6.31 | 168.04±6.01 | 0.6012 |
| Body weight (kg) | 69.09±9.31 | 69.47±7.92 | 0.8424 |
| Waist circumference (cm) | 87.76±6.97 | 87.88±7.09 | 0.9389 |
| AMS | 45.61±9.22 | 47.77±10.11 | 0.3210 |
| ADAM | 29 (70.7) | 32 (82.1) | 0.2344 |
| IIEF | 33.17±16.43 | 31.33±16.33 | 0.6175 |
| IPSS | 13.93±6.40 | 13.18±7.06 | 0.6211 |
| PSA | 1.31±0.92 | 1.55±1.15 | 0.2700 |
| Serum total testosterone (ng/mL) | 4.40±1.64 | 5.46±1.88 | 0.0086 |
| Serum free testosterone (pg/mL) | 7.41±1.78 | 8.78±1.99 | 0.0018 |
| Serum total cholesterol (mg/dL) | 161.66±32.42 | 179.97±35.42 | 0.0181 |
| Serum HDL-C (mg/dL) | 49.37±10.60 | 50.49±11.36 | 0.6491 |
| Serum LDL-C (mg/dL) | 88.63±28.78 | 103.51±31.54 | 0.0304 |
| Serum triglyceride (mg/dL) | 110.27±68.36 | 119.85±65.98 | 0.5259 |
Values are presented as mean±standard deviation or number (%).
Maca: Lepidium meyenii, AMS: Aging Males’ Symptoms scale, ADAM: Androgen Deficiency in the Aging Males, IIEF: International Index of Erectile Function, IPSS: International Prostate Symptom Score, PSA: prostate specific antigen, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol.
p-value indicates the paired t-test.
2. Primary efficacy outcomes
1) Aging Males’ Symptoms scale questionnaire
AMS total scores were improved at 4 weeks in both Maca treated group and placebo group (p<0.0001 and p=0.0251, respectively). However, after 12 weeks, AMS total score showed significant improvement only in the Maca treated group by -10.66 from the baseline (p<0.0001), not in the placebo group (p=0.5007). Based on changes from baseline, significant differences of AMS total scores were found at 12 weeks after treatment between the two groups (p<0.0001) (Table 2).
Table 2. Time courses of quetionnaire scores related symptoms of patients.
| Variable | Visit (wk) | Maca (n=41) | p-valuea | Placebo (n=39) | p-valuea | p-valueb |
|---|---|---|---|---|---|---|
| AMS total score, mean | 0 | 45.61±9.22 | 47.77±10.11 | |||
| 4 | 38.56±11.48 | <0.0001 | 44.85±10.96 | 0.0251 | 0.014 | |
| 12 | 34.95±11.21 | <0.0001 | 48.62±9.97 | 0.5007 | <0.0001 | |
| ADAM score positive (%) | 0 | 29 (70.7) | 32 (82.1) | |||
| 4 | 12 (29.3) | 7 (18.0) | 0.2344 | |||
| 12 | 23 (56.1) | 2 (5.1) | <0.0001 | |||
| IIEF score, mean | 0 | 33.17±16.43 | 31.33±16.33 | |||
| 4 | 39.46±17.45 | 0.0241 | 28.69±14.89 | 0.0153 | 0.0025 | |
| 12 | 47.17±18.60 | <0.0001 | 26.00±16.41 | 0.049 | <0.0001 | |
| IPSS total score, mean | 0 | 13.93±6.40 | 13.18±7.06 | |||
| 4 | 12.34±6.82 | 0.0134 | 13.41±6.48 | 0.6807 | 0.0373 | |
| 12 | 11.59±6.64 | 0.0025 | 14.59±6.68 | 0.0221 | 0.0001 |
Values are presented as mean±standard deviation.
Maca: Lepidium meyenii, AMS: Aging Males’ Symptoms scale, ADAM: Androgen Deficiency in the Aging Males, IIEF: International Index of Erectile Function, IPSS: International Prostate Symptom Score.
aCompared within groups (paired t-test).
bCompared between groups (adjusted baseline).
3. Secondary efficacy outcomes
1) Androgen Deficiency in the Aging Males questionnaire
In Maca treated men, ADAM positive rate decreased from 70.7% to 29.3% at 4 weeks and to 56.1% at 12 weeks. At end of the study, Maca treated group showed statistically significant improvement in androgen deficiency symptoms compared to the placebo group (p<0.0001) (Table 2).
2) International Index of Erectile Function
Total IIEF scores of Maca treated men showed statistically significant improvements at 4 weeks (6.29±17.19, p=0.0241) and 12 weeks (14.00±18.40, p<0.0001). However, there was no improvement in total IIEF score in the placebo group. Instead subjects in the placebo group showed decreases of total IIEF scores (Table 2). The change of total IIEF score showed significant difference between the two groups at the end of study (p<0.0001).
3) International Prostate Symptom Score
Total IPSS scores of subjects in Maca treated group showed statistically significant improvements at 4 weeks (-1.59±3.92, p=0.0134) and 12 weeks (-2.34±4.65, p=0.0025). On the other hand, total IPSS scores showed no improvements in the placebo group (Table 2). There was a statistically significant difference in the change of IPSS total score between the two groups at the end of study (p=0.0001).
4) Testosterone, free testosterone and PSA
Total testosterone and free testosterone levels were evaluated in morning blood samples. In Table 3, The placebo group showed a decrease in serum total testosterone level. However, there was no statistically significant difference in serum total testosterone level between the two groups (Maca-treated and placebo) (p=0.444). There was no significant difference in the change of free testosterone level either between the two groups (p=0.9184). PSA level, also did not show significant change before and after the treatment between two groups (p=0.3127).
Table 3. Diffences of clinical parameters after treatment.
| Variable | Visit (wk) | Maca (n=41) | p-valuea | Placebo (n=39) | p-valuea | p-valueb |
|---|---|---|---|---|---|---|
| Body weight (kg) | 0 | 68.88±9.49 | 69.19±8.01 | |||
| 12 | 69.30±9.27 | 0.1009 | 70.01±8.00 | 0.0127 | 0.1871 | |
| Waist circumference (cm) | 0 | 87.76±6.97 | 87.88±7.09 | |||
| 12 | 88.72±8.11 | 0.0269 | 88.57±6.88 | 0.0277 | 0.603 | |
| PSA (ng/mL) | 0 | 1.31±0.92 | 1.55±1.15 | |||
| 12 | 1.35±1.14 | 0.1509 | 1.45±0.97 | 0.1580 | 0.0569 | |
| Serum testosterone (ng/mL) | 0 | 4.40±1.64 | 5.46±1.88 | |||
| 12 | 4.41±1.76 | 0.9526 | 4.73±1.66 | 0.0156 | 0.444 | |
| Serum free testosterone (pg/mL) | 0 | 7.54±1.84 | 8.74±1.98 | |||
| 12 | 7.59±2.06 | 0.5295 | 8.33±2.74 | 0.3051 | 0.9184 | |
| Serum total cholesterol (mg/dL) | 0 | 161.66±32.42 | 179.97±35.42 | |||
| 12 | 159.44±37.31 | 0.3382 | 183.08±37.50 | 0.3441 | 0.1607 | |
| Serum HDL-C (mg/dL) | 0 | 49.37±10.60 | 50.49±11.36 | |||
| 12 | 48.51±9.58 | 0.3676 | 49.33±12.25 | 0.3127 | 0.9429 | |
| Serum LDL-C (mg/dL) | 0 | 88.63±28.78 | 103.51±31.54 | |||
| 12 | 89.44±33.26 | 0.6897 | 106.62±31.53 | 0.2595 | 0.3937 | |
| Serum triglyceride (mg/dL) | 0 | 110.27±68.36 | 119.85±65.98 | |||
| 12 | 96.59±40.42 | 0.1412 | 135.44±82.70 | 0.0878 | 0.0047 |
Values are presented as mean±standard deviation.
Maca: Lepidium meyenii, PSA: prostate specific antigen, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol.
aCompared within groups (paired t-test).
bCompared between groups (adjusted baseline).
5) Lipid profile
There were decreases of total cholesterol, HDL-cholesterol, and triglyceride levels but increases of LDL-cholesterol levels in Maca-treated group, although these changes were not clinically significant (p=0.3382, p=0.3676, p=0.1412, and p=0.6897, respectively). In the placebo group, there were increases of total cholesterol, LDL-cholesterol, triglyceride levels but decreases of HDL-cholesterol levels. These changes were not significant either (p=0.3441, p=0.2595, p=0.0878, and p=0.3127, respectively). There were no significant differences in changes of lipid profiles between the two groups except for serum triglyceride (p=0.0047).
6) Body weight and waist circumference
Body weight and waist circumference showed increases in both Maca-treated and placebo groups. However, they showed no significant differences between the two groups (p=0.1871 and p=0.603, respectively).
4. Safety assessment
Two participants in Maca-treated group complained about gastro-intestinal disorders. However, they showed mild symptoms and continued to participate in the study. There was no other treatment related severe side effects recorded. Nor there were clinically significant changes in laboratory tests or vital signs.
DISCUSSION
Since TRT has limitation for LOH patients with prostate cancer, cardiovascular disease, or polycythemia because of its risk, clinicians should carefully consider benefits and risks of TRT and choose the optimal indication for it [2]. Moreover, eugonadal patients will benefit more if there is an alternative therapy with fewer side effects.
Maca has been consumed worldwide for centuries and because of its nutritional and therapeutic properties of hypocotyls, it is considered as a super food [16]. A previous regional study suggested that Maca lowered interleukin-6 levels and improved general health of Peruvians living on high altitude [17]. In past animal experiments, there have been studies that positively affect male reproductive health, such as ED [18], post ejaculatory latency [10], premature ejaculation and libido [19]. Although they did not find the exact pharmacological mechanism, there was a study that improved sperm count in epididymis by acting as an anti-oxidant [20]. Cicero et al [21] also found that Hexanic Maca improved the sexual performance of rats more effectively and commented glucocinolates as the reason. While these past studies may give reasons why Maca has shown positive effects of this study, further research on the mechanism is needed in the future.
Previous clinical studies also have reported various biological effects of Maca, such as fertility-enhancing, reduction in glucose levels, energizing, neuroprotective, memory enhancement, anti-fatigue, anti-osteoporosis, and antioxidant activities [22,23]. Gonzales-Arimborgo et al [24] had conducted a 12-week study to compare active treatment with different doses of Maca with a placebo. The regression analysis resulted that Maca had a positive effect on sexual desire at 8 weeks and 12 weeks after treatment. Its effect was not due to changes in depression, anxiety, serum testosterone, or serum estradiol levels [24]. Similarly, in this study, Maca treatment improved LOH related symptoms without causing significant changes in laboratory tests (blood chemistry and urinalysis) or vital signs. Adverse events were also minimal and patients recovered without additional treatment. Most of all, the Maca had no effects on PSA levels.
In Maca treated group, IIEF also improved in week 4 and 12, while placebo turned worse. This result is similar to the study of Zenico et al [12]. In current study, not only questionnaires of sexual functions but also IPSS showed significant improvement compared to placebo group. Among the clinical trials using Maca, it is hard to find a study confirming improvement in IPSS levels. Which makes this study unique and confirming that Maca can have a positive effect on urination symptoms as well as male reproductive function.
A study in Japan has proven that a traditional herbal medicine called Kampo is an effective and safe treatment for LOH. Approved by Japanese traditional Food and Drug Administration, it is used widely in urology clinics and covered by medical insurance [25]. Additionally, Lee et al [26] have demonstrated that total IIEF and AMS scores are improved by Ojayeonjonghwan treatment, thus helping patients with LOH symptoms. In Korea, some of herbal medicines are classified as health functional foods instead of specialty medicines as is preferable to use for improving LOH symptoms without critical effects on human body or having risks of developing prostate cancer. Likewise, various studies are being conducted around the world to improve LOH symptoms through herbal medications that have fewer side effects and are easy to take.
Maca, is also called as “Peruvian ginseng” or “Plant Viagra”, and it has been consumed worldwide as a health functional food, enhancer of male fertility [27], aphrodisiac [15], immune-stimulant [28], and anabolic or hormonal balancer [29]. Additionally, taking Maca regularly is known to slow the disease progression of chronic neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and etc [30]. Pino-Figueroa et al [31] have suggested that lipophilic constituents of Maca as fatty acid amide hydrolase inhibitors might have effects on neurotransmissions in the central nervous system. Another study has proven that Maca can prevent cardiac ischemic dysfunction via activation of NOS pathways [32]. However, the mechanism of Maca on LOH remains clear. Therefore, elucidating the therapeutic mechanism of Maca on LOH is needed in the future.
Considering the above biological benefits of Maca, if it is possible to treat patients with LOH who do not require testosterone replacement with natural products instead of drugs, it can be expected that worries of side effects will be reduced. As far as we know, this is the first randomized clinical trial to discover the efficacy of Maca with official questionnaires for LOH evaluation with IIEF and IPSS.
This study has some limitations. The follow-up duration was 12 weeks, which is a short study period. Thus, longer period of research is needed in the future to evaluate long term effects of Maca. Also, participants were all Koreans in this study. Thus, outcomes might differ for different ethnic groups. Finally, we evaluated the total scores of AMS only. Analysis of each domain would be beneficial to making clear of changes in symptoms, especially physical and psychological symptoms of LOH. IIEF and IPSS scores could have also be analyzed by of each domain or sub-scores for deeper analysis. In addition, it will be the future task to find out the pharmacological mechanism as mentioned above.
CONCLUSIONS
Maca improved AMS, IIEF, IPSS and ADAM positive rates significantly, showing clinically effective for treating LOH related symptoms in eugonadal men. It was easy to take and showed no severe adverse events. Further studies with long term follow-up and elucidate the pharmacological mechanisms, it may be a promising alternative therapy for those who cannot obtain testosterone treatment due to side effects.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (Project Number: RS-2022-00140928).
- Conceptualization: DGM, WJB, SWK.
- Data curation: SHJ.
- Formal analysis: DS.
- Funding acquisition: DGM, WJB, SWK.
- Investigation: DGM, SWK.
- Methodology: DS.
- Project administration: DGM, SWK.
- Resources: STA, HJP, HJC, SHH.
- Software: GQZ, JP, Supervision.
- Validation: DS.
- Visualization: IP, WJT, KJH.
- Writing – original draft: DS.
- Writing – review & editing: All authors.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/Z05QVA.
References
- 1.Corona G, Krausz C. Late-onset hypogonadism a challenging task for the andrology field. Andrology. 2020;8:1504–1505. doi: 10.1111/andr.12917. [DOI] [PubMed] [Google Scholar]
- 2.Shin YS, Park JK. The optimal indication for testosterone replacement therapy in late onset hypogonadism. J Clin Med. 2019;8:209. doi: 10.3390/jcm8020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieschlag E. Late-onset hypogonadism: a concept comes of age. Andrology. 2020;8:1506–1511. doi: 10.1111/andr.12719. [DOI] [PubMed] [Google Scholar]
- 4.Christ-Crain M, Mueller B, Gasser TC, Kraenzlin M, Trummler M, Huber P, et al. Is there a clinical relevance of partial androgen deficiency of the aging male? J Urol. 2004;172:624–627. doi: 10.1097/01.ju.0000135303.96496.ee. [DOI] [PubMed] [Google Scholar]
- 5.Kang S, Park HJ, Park NC. Serum total testosterone level and identification of late-onset hypogonadism: a community-based study. Korean J Urol. 2013;54:619–623. doi: 10.4111/kju.2013.54.9.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YC, Hwang TI, Chiang HS, Yang CR, Wu HC, Wu TL, et al. Correlations of androgen deficiency with clinical symptoms in Taiwanese males. Int J Impot Res. 2006;18:343–347. doi: 10.1038/sj.ijir.3901417. [DOI] [PubMed] [Google Scholar]
- 7.Miwa Y, Kaneda T, Yokoyama O. Correlation between the aging males' symptoms scale and sex steroids, gonadotropins, dehydroepiandrosterone sulfate, and growth hormone levels in ambulatory men. J Sex Med. 2006;3:723–726. doi: 10.1111/j.1743-6109.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 8.Shin BC, Lee MS, Yang EJ, Lim HS, Ernst E. Maca (L. meyenii) for improving sexual function: a systematic review. BMC Complement Altern Med. 2010;10:44. doi: 10.1186/1472-6882-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng BL, He K, Kim CH, Rogers L, Shao Y, Huang ZY, et al. Effect of a lipidic extract from lepidium meyenii on sexual behavior in mice and rats. Urology. 2000;55:598–602. doi: 10.1016/s0090-4295(99)00549-x. [DOI] [PubMed] [Google Scholar]
- 10.Cicero AF, Bandieri E, Arletti R. Lepidium meyenii Walp. improves sexual behaviour in male rats independently from its action on spontaneous locomotor activity. J Ethnopharmacol. 2001;75:225–229. doi: 10.1016/s0378-8741(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales GF, Córdova A, Vega K, Chung A, Villena A, Góñez C, et al. Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia. 2002;34:367–372. doi: 10.1046/j.1439-0272.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 12.Zenico T, Cicero AF, Valmorri L, Mercuriali M, Bercovich E. Subjective effects of Lepidium meyenii (Maca) extract on well-being and sexual performances in patients with mild erectile dysfunction: a randomised, double-blind clinical trial. Andrologia. 2009;41:95–99. doi: 10.1111/j.1439-0272.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales GF, Nieto J, Rubio J, Gasco M. Effect of black maca (Lepidium meyenii) on one spermatogenic cycle in rats. Andrologia. 2006;38:166–172. doi: 10.1111/j.1439-0272.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 14.Park HJ, Lee KS, Lee EK, Park NC. Efficacy and safety of a mixed extract of Trigonella foenum-graecum seed and Lespedeza cuneata in the treatment of testosterone deficiency syndrome: a randomized, double-blind, placebo-controlled clinical trial. World J Mens Health. 2018;36:230–238. doi: 10.5534/wjmh.170004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales GF, Córdova A, Vega K, Chung A, Villena A, Góñez C. Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol. 2003;176:163–168. doi: 10.1677/joe.0.1760163. [DOI] [PubMed] [Google Scholar]
- 16.Valerio LG, Jr, Gonzales GF. Toxicological aspects of the South American herbs cat's claw (Uncaria tomentosa) and maca (Lepidium meyenii) : a critical synopsis. Toxicol Rev. 2005;24:11–35. doi: 10.2165/00139709-200524010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gonzales GF, Gasco M, Lozada-Requena I. Role of maca (Lepidium meyenii) consumption on serum interleukin-6 levels and health status in populations living in the Peruvian Central Andes over 4000 m of altitude. Plant Foods Hum Nutr. 2013;68:347–351. doi: 10.1007/s11130-013-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi HR, Lee MJ, Lee SJ, Park HJ, Song JY, Kim IS, et al. Effect of Lepidium spp. (maca) extract on the improvement of sexual function in rats induced erectile dysfunction with ethanol. Korean J Food Nutr. 2018;31:328–334. [Google Scholar]
- 19.Lentz A, Gravitt K, Carson CC, Marson L. Acute and chronic dosing of Lepidium meyenii (Maca) on male rat sexual behavior. J Sex Med. 2007;4:332–339. doi: 10.1111/j.1743-6109.2007.00437.x. discussion 339-40. [DOI] [PubMed] [Google Scholar]
- 20.Gasco M, Aguilar J, Gonzales GF. Effect of chronic treatment with three varieties of Lepidium meyenii (maca) on reproductive parameters and DNA quantification in adult male rats. Andrologia. 2007;39:151–158. doi: 10.1111/j.1439-0272.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 21.Cicero AF, Piacente S, Plaza A, Sala E, Arletti R, Pizza C. Hexanic maca extract improves rat sexual performance more effectively than methanolic and chloroformic maca extracts. Andrologia. 2002;34:177–179. doi: 10.1046/j.1439-0272.2002.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Leitão Peres N, Cabrera Parra Bortoluzzi L, Medeiros Marques LL, Formigoni M, Fuchs RHB, Droval AA, et al. Medicinal effects of Peruvian maca (Lepidium meyenii): a review. Food Funct. 2020;11:83–92. doi: 10.1039/c9fo02732g. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Hu B, Hua H, Liu C, Cheng Y, Guo Y, et al. Macamides: a review of structures, isolation, therapeutics and prospects. Food Res Int. 2020;138:109819. doi: 10.1016/j.foodres.2020.109819. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales-Arimborgo C, Yupanqui I, Montero E, Alarcón-Yaquetto DE, Zevallos-Concha A, Caballero L, et al. Acceptability, safety, and efficacy of oral administration of extracts of black or red maca (Lepidium meyenii) in adult human subjects: a randomized, double-blind, placebo-controlled study. Pharmaceuticals (Basel) 2016;9:49. doi: 10.3390/ph9030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano T, Imao T, Takemae K. Clinical efficacy of Japanese traditional herbal medicine (Kampo) in patients with late-onset hypogonadism. Aging Male. 2010;13:166–173. doi: 10.3109/13685530903536684. [DOI] [PubMed] [Google Scholar]
- 26.Lee KW, Bae SR, Jeong HC, Choi JB, Choi SW, Bae WJ, et al. A randomized, controlled study of treatment with ojayeonjonghwan for patients with late onset hypogonadism. Aging Male. 2020;23:264–271. doi: 10.1080/13685538.2018.1480599. [DOI] [PubMed] [Google Scholar]
- 27.Abarikwu SO, Onuah CL, Singh SK. Plants in the management of male infertility. Andrologia. 2020;52:e13509. doi: 10.1111/and.13509. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Zhu F. Chemical composition and health effects of maca (Lepidium meyenii) Food Chem. 2019;288:422–443. doi: 10.1016/j.foodchem.2019.02.071. [DOI] [PubMed] [Google Scholar]
- 29.He Y, Sun Q, Zhang X, Bao X, Wang Y, Rasheed M, et al. Authentication of the geographical origin of maca (Lepidium meyenii Walp.) at different regional scales using the stable isotope ratio and mineral elemental fingerprints. Food Chem. 2020;311:126058. doi: 10.1016/j.foodchem.2019.126058. [DOI] [PubMed] [Google Scholar]
- 30.Patel SS, Raghuwanshi R, Masood M, Acharya A, Jain SK. Medicinal plants with acetylcholinesterase inhibitory activity. Rev Neurosci. 2018;29:491–529. doi: 10.1515/revneuro-2017-0054. [DOI] [PubMed] [Google Scholar]
- 31.Pino-Figueroa A, Vu H, Kelley CJ, Maher TJ. Mechanism of action of Lepidium meyenii (maca): an explanation for its neuroprotective activity. Am J Neuroprotection Neuroregeneration. 2011;3:87–92. [Google Scholar]
- 32.Colareda GA, Matera SI, Bayley M, Ragone MI, Flores ML, Córdoba OL, et al. Lepidium meyenii (maca) and soy isoflavones reduce cardiac stunning of ischemia-reperfusion in rats by mitochondrial mechanisms. J Tradit Complement Med. 2021;11:471–480. doi: 10.1016/j.jtcme.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/Z05QVA.