Abstract
Purpose
To evaluate the efficacy and safety of udenafil 75 mg once daily in patients with erectile dysfunction following bilateral nerve-sparing robot-assisted laparoscopic radical prostatectomy (BNS-RALP).
Materials and Methods
A multi-center, prospective, randomized, controlled, double-blind study was conducted. Among patients with localized prostate cancer with international index of erectile function-erectile function domain (IIEF-EF) score of 18 or higher before BNS-RALP, those who developed postoperative erectile dysfunction (IIEF-EF score 14 or less at 4 weeks after BNS-RALP) were enrolled. Enrolled patients were randomly assigned to the udenafil 75 mg daily group or the placebo group in a 2:1 ratio. Each subject was followed up at 8 weeks (V2), 20 weeks (V3), and 32 weeks (V4) to evaluate the efficacy and safety of udenafil.
Results
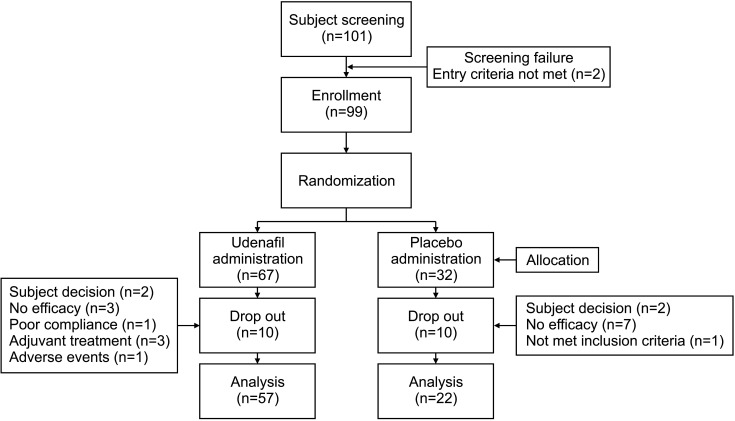
In all, 101 patients were screened, of whom 99 were enrolled. Of the 99 patients, 67 were assigned to the experimental group and 32 to the control group. Ten (14.93%) patients in the experimental group and 10 (31.25%) in the control group dropped out of the study. After 32 weeks of treatment, IIEF-EF score of 22 or higher was seen in 36.51% (23/63) of patients in the experimental group and 13.04% (3/23) patients in the control group (p=0.021). The proportion of patients with IIEF-EF improvement of 25% or more compared to the baseline was 82.54% (52/63) in the experimental group and 62.96% (17/27) in the control group (p=0.058).
Conclusions
Udenafil 75 mg once daily after BNS-RALP improved the erectile function without any severe adverse effects.
Keywords: Phosphodiesterase 5 inhibitors, Prostatectomy, Prostatic neoplasms, Rehabilitation
INTRODUCTION
Radical prostatectomy (RP) is one of the standard treatments for the patients with a localized prostate cancer whose life expectancy is at least 10 years [1]. However, inevitable complications, such as urinary incontinence and impotency, might occur after RP [2]. According to the Prostate Cancer Outcomes Study, 8.7% of patients experience urinary incontinence, and 41.9% experience sexual dysfunction after RP [3]. With an increase in the early diagnosis of prostate cancer in recent times and the increase in life expectancy, recovery of erectile function after RP has become important [4]. Although the rate of postoperative potency has significantly improved with the introduction of bilateral nerve-sparing robot-assisted laparoscopic radical prostatectomy (BNS-RALP) and the advancement in surgical techniques, a large number of patients still experience erectile dysfunction after RP [5,6]. Moreover, it has been reported that even after BNS-RALP, it can take up to 2 years for recovery of erectile function [7].
Previous studies have reported that phosphodiesterase type 5 inhibitor (PDE5i) can improve erectile function after BNS-RALP [8,9]. PDE5i prevents the degradation of cGMP by inhibiting PDE-5 and enhances the action of nitric oxide (NO) to sustain the vasodilation of the penis so that the erection is maintained [10]. However, different types of PDE5i, at different doses, are available for penile rehabilitation (PR) after BNS-RALP. A PDE5i type with optimal outcomes and best route of administration for PR after BNS-RALP has not yet been established.
Udenafil, one of the selective PDE5i, is a pyrazolopyrimidinone derivative with a molecular weight of 516.66 g/mol. The pharmacokinetic profile of udenafil confers its unique clinical properties with relatively rapid onset and a long duration of action [11]. Clinical efficacy and safety of udenafil have been evaluated in ED patients of a broad spectrum of etiologies or severity in the several trials [12]. Udenafil has higher PDE1 selectivity (selectivity ratio: 1,262), which is associated with vasodilation, flushing, and tachycardia than that of sildenafil (selectivity ratio: 41). Moreover, PDE11 selectivity of udenafil (selectivity ratio: 96) is higher than that of tadalafil (selectivity ratio: 7.1) [13]. Although its function is not yet clear, PDE11 is widely distributed in the skeletal muscle, testes, heart, prostate, kidney, liver, and pituitary [14]. Therefore, udenafil was found to be safe and well tolerated in human subjects. According Zhao et al [15], udenafil did not induce myalgias or abnormalities in color vision, which are profound side effects of tadalafil and sildenafil. Considering that patients with prostate cancer are higher average onset age than those with erectile dysfunction, udenafil, which has relative high selectivity, would be safe for PR after BNS-RALP.
This is the first study to evaluate the efficacy and safety of daily administration of udenafil 75 mg in patients who developed erectile dysfunction after BNS-RALP. In order to assess the effect of PR the patients with normal erectile function or mild erectile dysfunction before surgery were included [16]. Then international index of erectile function-erectile function domain (IIEF-EF) 22 or higher after PR was defined as a successful rehabilitation [17,18].
MATERIALS AND METHODS
1. Patients and design
A multi-center, prospective, randomized, controlled, double-blind study was performed between July 2017 and March 2021 at 7 medical institutions. The inclusion criteria were as follows: (1) subjects aged between 20 and 70 years who underwent BNS-RALP for localized prostate cancer, (2) IIEF-EF score 18 or higher before BNS-RALP (up to 6 months before), (3) IIEF-EF score 14 or less at 4 weeks after BNS-RALP (V1), (4) history of sexual intercourse with a stable consenting partner for the past 6 months and plan to continue the same during the trial period, (5) agreeable for not using other treatments for erectile dysfunction during the trial period, (6) willingness and ability to participate in this trial. The exclusion criteria were subjects who (1) required additional treatment such as radiotherapy or androgen deprivation therapy after BNS-RALP, (2) had a history of treatment for erectile dysfunction with PDE5i and injection therapy within 8 weeks before BNS-RALP, (3) had uncontrolled diabetes mellitus (HbA1C >12%), (4) had cardiopulmonary disease (including coronary disease), (5) had a spinal cord injury or history of pelvic organ surgery, (6) had an anatomical penile disorder, (7) had severe chronic renal disease or liver disease, (8) were on medication with nitrate preparations or NO.
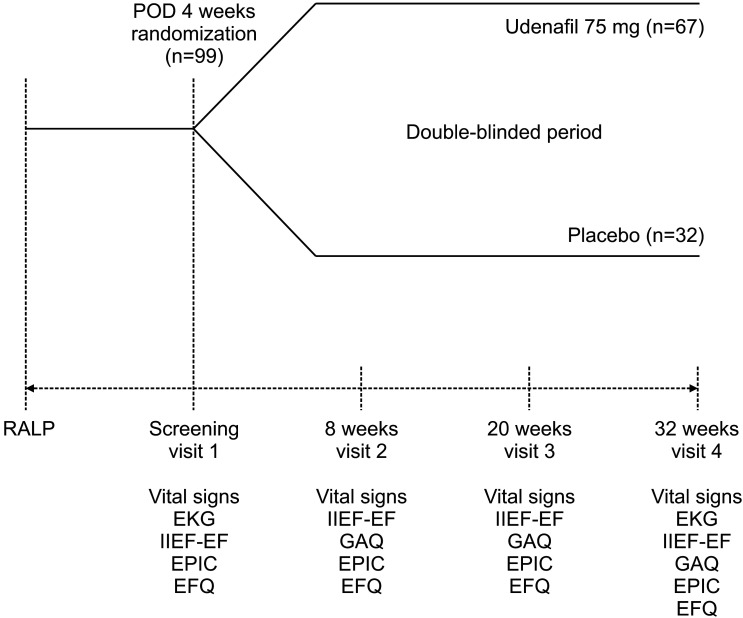
After 4 weeks of BNS-RALP, subjects who met the inclusion criteria were randomly assigned to the udenafil 75 mg daily group or the placebo group in a 2:1 ratio. Each subject was scheduled for a follow-up visit at 8 weeks (V2), 20 weeks (V3), and 32 weeks (V4) according to the planned schedule of the clinical trial for the evaluation of efficacy and safety of treatment (Fig. 1). From V1 to V4, subjects take one tablet, once a day, at the same time as possible, regardless of taking meals. The design and reporting of this study were in accordance with the criteria of the Consolidated Standards of Reporting Trials (CONSORT) statement [19]. The clinical trial is registered with Clinical Research Information Service (KCT0003093).
Fig. 1. Study design and evaluation schedule. EFQ: Erectile function questionnaire, EKG: electrocardiogram, EPIC: Expanded Prostate Cancer Index Composite, GAQ: global assessment question, IIEF-EF: international index of erectile function-erectile function domain, POD: postoperative day, RALP: robot-assisted laparoscopic radical prostatectomy.
2. Sample size
According Montorsi et al [20], the proportion of subjects with IIEF-EF score ≥22 after PR treatment was estimated to be 25%, and that of the placebo group was 14%, and the width of the 95% confidence interval for the proportion difference was assumed to be 0.38. The required number of subjects was calculated assuming allocation to the udenafil and placebo groups in a ratio of 2:1. Considering a drop-out rate of 30%, a total of 99 subjects were enrolled, 66 in the udenafil group and 33 in the placebo group. In the previous study, the mean age of enrolled patients was 57 to 58 years in each group. Considering the mean age of patients undergoing RP in Korea, it is expected that older patients will be enrolled compared to previous study. So, the IIEF-EF score was modified for enrollment.
3. Randomization and blinding
For randomization, a block randomization method for each site was followed. A randomization list was generated using statistics independent from this clinical trial. The researchers, subjects, managing pharmacists, and investigators were blinded to the treatment allocation throughout the clinical trial. The blinding was maintained by using placebo which was identical in appearance to the study drug for the entire treatment period. The investigators were asked to maintain the emergency code containing each subject’s assignment number and allocation group; this code had to be kept sealed except in emergency situations. Discontinuation of blinding was allowed in case of a serious medical emergency.
4. Assessment of efficacy and safety
The primary endpoint was the difference in the proportion of patients with IIEF-EF score ≥22 at 32 weeks after treatment. Secondary endpoints were the change in mean IIEF-EF score, the difference between two groups of global assessment question (GAQ, Has the treatment over the last 4 weeks improved your erectile function?) [21], Erectile function questionnaire (EFQ) and Expanded Prostate Cancer Index Composite (EPIC) score [22]. The EFQ consists of 6 questions (Supplement Table 1). EFQ was used for the exploratory analysis by modifying the Sexual Encounter Profile question, GAQ, and erection hardness score [23,24,25].
The safety of treatment was also evaluated. Safety assessment included blood pressure, pulse rate, 12-lead electrocardiogram, laboratory tests, and adverse events. Adverse events were evaluated by assessing their incidence and severity at every visit, and events occurring after randomization were classified as treatment-emergent adverse events (TEAEs). Adverse drug reactions were defined as drug-related TEAEs.
5. Analysis
The efficacy was evaluated by a full analysis set, based on an intention-to-treat analysis, which considered all the patients who received the study medication at least once and underwent a primary efficacy assessment at least once after randomization, including those who dropped out because of adverse effects, had poor compliance or did not participate in the follow-up. Further, all participants who took the study medication at least once after randomization were included in the safety assessment.
6. Statistical analysis
All data were recorded on standard forms. A paired t-test and dependent t-test was used to compare continuous variables. Categorical variables were analyzed using Fisher’s exact test. Statistical analyses were performed using SPSS® version 22.0 (IBM Corp., Armonk, NY, USA). A 2-tailed p-value <0.05 was considered statistically significant.
7. Ethics statement
The study was performed in accordance with the applicable laws and regulations, good clinical practices, and ethical principles as described in the Declaration of Helsinki. This is a randomized clinical trial on the second phase, registered at the Clinical Research Information Service (KCT0003093). The present study protocol was reviewed and approved by the Institutional Review Board of Samsung medical center (Reg. No. 2017-02-112). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
In all, 101 patients were screened, and 99 patients were enrolled. Of the 99 patients, 67 were assigned to the experimental group and 32 to the control group. Ten patients each in the experimental group (subject decision: 2, no efficacy: 3, poor compliance: 1, adjuvant treatment: 3, and adverse events: 1; 14.93%) and control group (subject decision: 2, no efficacy: 7, not met inclusion criteria: 1; 31.25%) dropped out of the study (Fig. 2).
Fig. 2. Flow diagram of the study.
The mean age of the subjects was 60.16±6.69 years in the experimental group and 62.41±4.63 years in the control group (p=0.091), and there was no significant difference between the two groups in terms of the baseline characteristics. Before BNS-RALP, the proportion of patients with mild erectile dysfunction (IIEF-EF <23) comprised 32.84% in the experimental group and 43.75% in the control group (p=0.271) (Table 1).
Table 1. Baseline characteristics.
| Characteristic | Experimental group (n=67) | Control group (n=32) | p-value | |
|---|---|---|---|---|
| Age, y | 60.16±6.69 | 62.41±4.63 | 0.091 | |
| Body mass index, kg/m2 | 24.39±2.28 | 24.04±2.30 | 0.497 | |
| Hypertension | 23 (34.33) | 16 (50.00) | 0.136† | |
| Diabetes mellitus | 11 (16.42) | 3 (9.38) | 0.347† | |
| Dyslipidemia | 14 (20.90) | 3 (9.38) | 0.155† | |
| Hemoglobin, g/dL | 13.83±1.17 | 14.02±1.07 | 0.505 | |
| Platelet, ×103/μL | 244.30±59.60 | 239.07±54.59 | 0.676 | |
| AST, U/L | 23.55±9.00 | 23.77±5.92 | 0.888 | |
| ALT, U/L | 23.59±15.19 | 26.30±12.96 | 0.376 | |
| BUN, mg/dL | 15.32±3.98 | 15.62±3.81 | 0.730 | |
| Creatinine, mg/dL | 0.89±0.16 | 0.85±0.11 | 0.231 | |
| Total cholesterol, mg/dL | 182.41±33.42 | 176.27±37.33 | 0.446 | |
| Total protein, g/dL | 7.45±0.50 | 7.36±0.36 | 0.339 | |
| Albumin, g/dL | 4.37±0.34 | 4.27±0.29 | 0.148 | |
| HbA1c, % | 5.67±0.65 | 5.46±0.37 | 0.053 | |
| Testosterone, ng/mL | 4.00±1.24 | 3.95±1.37 | 0.847 | |
| Prolactin, ng/mL | 10.08±4.86 | 10.04±5.10 | 0.968 | |
| IIEF-EF | 4.19±3.59 | 3.69±2.96 | 0.460 | |
| Preoperative erectile function | ||||
| Mild erectile dysfunctiona | 22 (32.84) | 14 (43.75) | 0.271† | |
| Follow-up loss | 10 (14.93) | 10 (31.25) | 0.058† | |
Values are presented as mean±standard deviation or number (%).
AST: aspartate aminotransferase, ALT: alanine aminotransferase, BUN: blood urea nitrogen, IIEF-EF: international index of erectile function-erectile function domain.
aMild erectile dysfunction: IIEF-EF <23.
Paired t-test, †Fisher’s exact test.
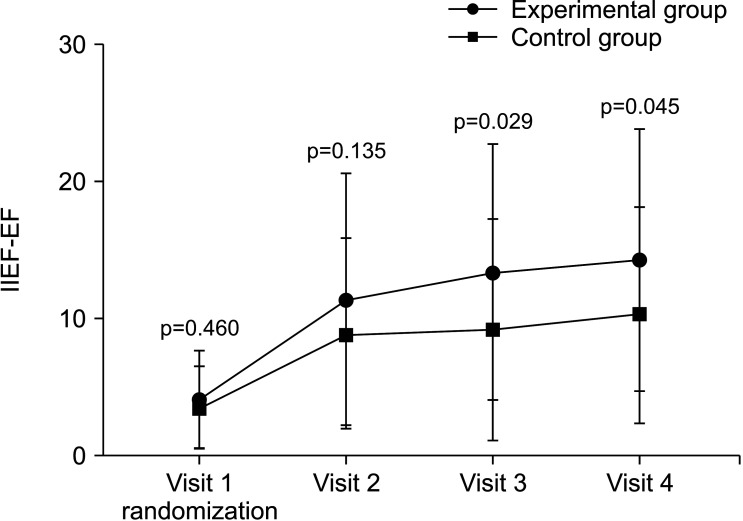
After 32 weeks of treatment, the proportion of patients with IIEF-EF scores of 22 or higher was 36.51% (23/63) in the experimental group and 13.04% (3/23) (p=0.021) in the control group. An improvement of 25% or more in the IIEF-EF score compared to the baseline was seen in 82.54% (52/63) of patients in the experimental group and 62.96% (17/27) in the control group (p=0.058). At baseline, the IIEF-EF score was 4.19±3.59 in the experimental group and 3.69±2.96 in the control group (p=0.460). At V2, the IIEF-EF score was 11.50±9.12 in the experimental group (n=66) and 9.00±6.87 in the control group (n=32) (p=0.135); at V3, the scores were 13.48±9.27 in the experimental group (n=63) and 9.32±8.03 in the control group (n=31) (p=0.029); at V4, the scores were 14.32±9.50 in the experimental group (n=63) and 10.37±7.85 in the control group (n=27) (p=0.045) (Fig. 3).
Fig. 3. Change in IIEF-EF score, student’s t-test. Visit 1: experimental group (n=67), control group (n=32). Visit 2: experimental group (n=66), control group (n=32). Visit 3: experimental group (n=63), control group (n=31). Visit 4: experimental group (n=63), control group (n=27). IIEF-EF: international index of erectile function-erectile function domain.
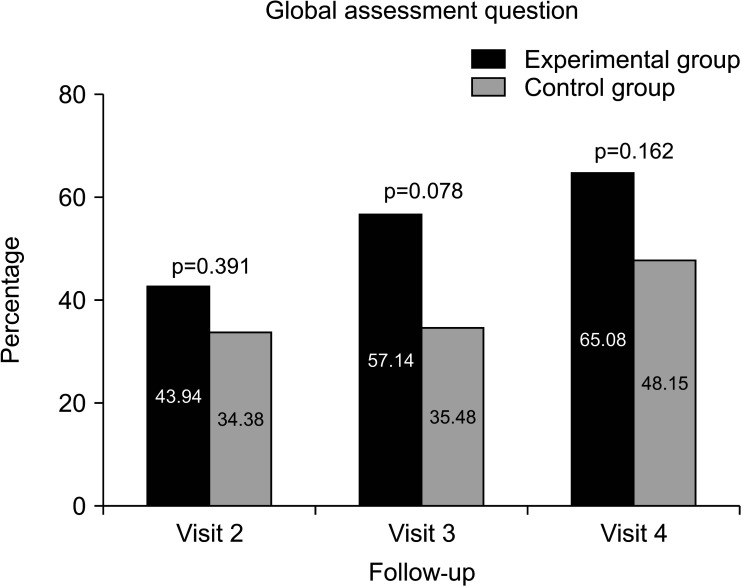
In the GAQ, the proportion of subjects who responded “yes” was 43.94% in the experimental group and 34.38% in the experimental group at V2 (p=0.391), while it was 65.08% in the experimental group (41/63) and 48.15% in the control group (13/27) (p=0.162) at V4 (Fig. 4).
Fig. 4. Global assessment question (Has the treatment over the last 4 weeks improved your erectile function?). Visit 2: experimental group (n=29/66), control group (n=11/32). Visit 3: experimental group (n=36/63), control group (n=11/31). Visit 4: experimental group (n=41/63), control group (n=13/27).
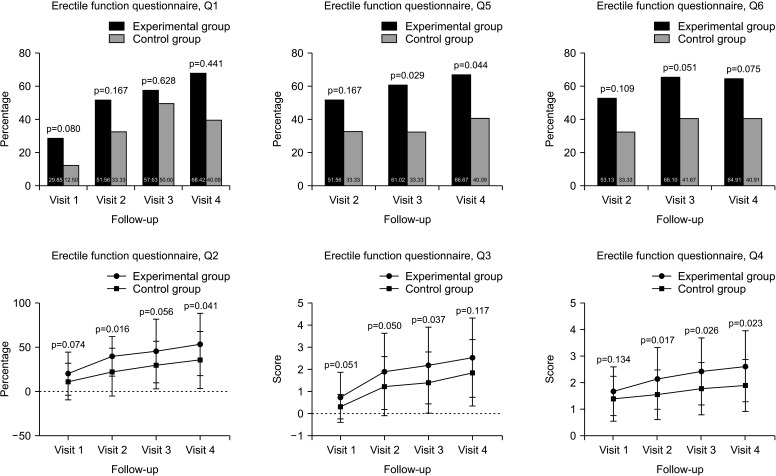
In EFQ Q1, the proportion of subjects who responded “yes” was 29.85% vs. 12.50% (p=0.080) at V1, 51.56% vs. 33.33% (p=0.167) at V2, 57.63% vs. 50.00% at V3 (p=0.628), and 68.42% vs. 40.09% at V4 (p=0.441) (experimental vs. control group, respectively). The level of erection compared to the erectile function before the diagnosis of prostate cancer (EFQ Q2) was 20.69±24.26% vs. 11.72±20.58% at V1 (p=0.074), 40.00±33.05% vs. 22.26±26.80% at V2 (p=0.016), 45.94±35.77% vs. 30.21±26.80% at V3 (p=0.056), and 53.40±34.92% vs. 35.91±32.32% at V4 (p=0.041) (experimental vs. control group, respectively). In EFQ Q3 (Are you currently able to have an erection adequate for sexual intercourse?), it was 0.76±1.13 vs. 0.34±0.55 at V1 (p=0.051), 1.92±1.71 vs. 1.26±1.32 at V2 (p=0.050), 2.19±1.73 vs. 1.42±1.38 at V3 (p=0.037), and 2.54±1.78 vs. 1.86±1.49 at V4 (p=0.117) (experimental vs. control group, respectively). In EFQ Q4 (How satisfied are you with your current sex life?), it was 1.69±0.91 vs. 1.41±0.84 at V1 (p=0.134), 2.17±1.16 vs. 1.56±0.93 at V2 (p=0.017), 2.44±1.25 vs. 1.79±0.98 at V3 (p=0.026), and 2.63±1.33 vs. 1.91±0.97 at V4 (p=0.023) (experimental vs. control group, respectively). In EFQ Q5, the proportion of subjects who responded “yes” was 51.56% vs. 33.33% at V2 (p=0.167), 61.02% vs. 33.33% at V3 (p=0.029), and 66.67% vs. 40.09% at V4 (p=0.044) (experimental vs. control group, respectively). In EFQ Q6, the proportion of subjects who responded “yes” was 53.13% vs. 33.33% at V2 (p=0.109), 66.10% vs. 41.67% at V3 (p=0.051), and 64.91% vs. 40.91% at V4 (p=0.075) (experimental vs. control group, respectively) (Fig. 5).
Fig. 5. Erectile function questionnaire. Visit 1: experimental group (n=67), control group (n=32). Visit 2: experimental group (n=64), control group (n=27). Visit 3: experimental group (n=59), control group (n=24). Visit 4: experimental group (n=57), control group (n=22).
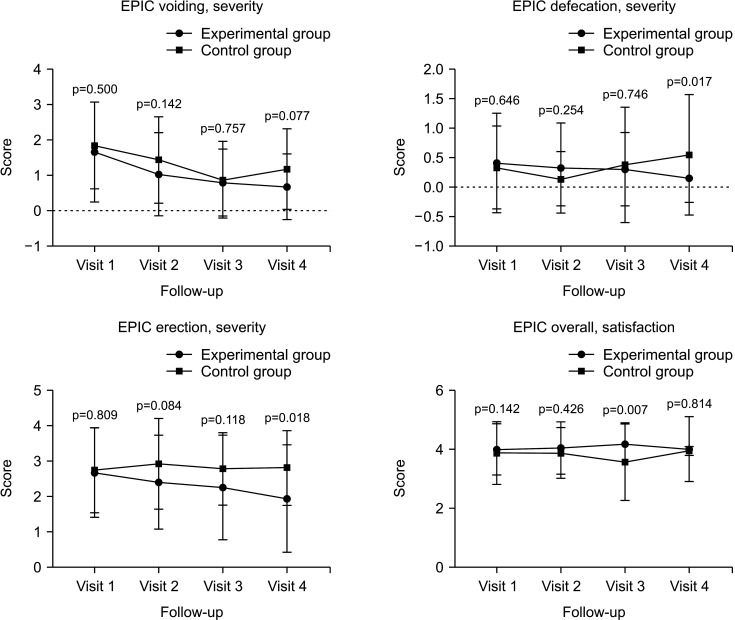
In the EPIC score, the overall problem in erectile function was 2.69±1.26 vs. 2.75±1.19 at V1 (p=0.809), 2.41±1.32 vs. 2.93±1.27 at V2 (p=0.084), 2.27±1.47 vs. 2.79±1.02 at V3 (p=0.118), and 1.96±1.51 vs. 2.82±1.05 at V4 (p=0.018) (experimental vs. control group, respectively). The overall satisfaction score was 4.01±0.86 vs. 3.88±1.04 at V1 (p=0.142), 4.05±0.88 vs. 3.89±0.85 at V2 (p=0.426), 4.19±0.71 vs. 3.58±1.28 at V3 (p=0.007), and 4.02±1.09 vs. 3.95±0.15 at V4 (p=0.814) (experimental vs. control group, respectively) (Fig. 6).
Fig. 6. Expanded Prostate Cancer Index Composite (EPIC). Visit 1: experimental group (n=67), control group (n=32). Visit 2: experimental group (n=64), control group (n=27). Visit 3: experimental group (n=59), control group (n=24). Visit 4: experimental group (n=57), control group (n=22).
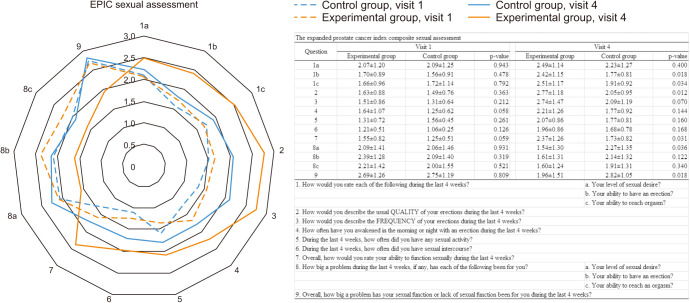
In the EPIC sexual assessment, there were no significant differences between the two groups in V1. There was no significant difference in terms of sexual desire between the two groups in V4. However, the control group showed higher score of the problems with sexual desire than the experimental group (1.54±1.30 in experimental group vs. 2.27±1.35 in control group, p=0.036) (Fig. 7). Also, higher scores were found in the experimental group for ability to erection, ability to orgasm, and erection quality than in the control group. Overall satisfaction was 2.37±1.26 in the experimental group and 1.73±0.82 in the control group (p=0.031) (Fig. 7).
Fig. 7. Expanded Prostate Cancer Index Composite (EPIC) sexual assessment.
In the results of laboratory investigations, there was no significant difference between the two groups (Supplement Table 2). Treatment-related emergent adverse events occurred in 17 subjects in the experimental group and 3 subjects in the control group. All adverse events were of mild to moderate intensity, and 4 adverse drug reactions occurred in the experimental group (hot flushing 3, dyspnea 1), all of which were mild (Table 2). In the experimental group, one patient was dropped out due to adverse effects. The patient underwent mild hot flushing and wanted to discontinue to conduct the study for this reason.
Table 2. Treatment emergent adverse events.
| Event | Experimental group (n=67) n (%) [mild/moderate/severe] |
Control group (n=32) n (%) [mild/moderate/severe] |
|
|---|---|---|---|
| Treatment emergent adverse events | |||
| Chest pain | 0 | 1 (3.13) [1/0/0] | |
| Atrial flutter | 0 | 1 (3.13) [1/0/0] | |
| Heartburn | 2 (2.99) [2/0/0] | 0 | |
| Nasopharyngitis | 1 (1.49) [1/0/0] | 0 | |
| Constipation | 2 (2.99) [1/1/0] | 1 (3.13) [1/0/0] | |
| Allergic rhinitis | 1 (1.49) [0/1/0] | 0 | |
| Insomnia | 1 (1.49) [1/0/0] | 0 | |
| Visual disorder | 1 (1.49) [1/0/0] | 0 | |
| Dizziness | 1 (1.49) [1/0/0] | 0 | |
| Dry mouth | 1 (1.49) [1/0/0] | 0 | |
| Hernia | 2 (2.99) [1/1/0] | 0 | |
| Ureter stone | 2 (2.99) [1/1/0] | 0 | |
| Anus bleeding | 1 (1.49) [1/0/0] | 0 | |
| Hematuria | 1 (1.49) [1/0/0] | 0 | |
| Leg edema | 1 (1.49) [1/0/0] | 0 | |
| Adverse drug reaction | |||
| Hot flushing | 3 (4.48) [3/0/0] | 0 | |
| Dyspnea | 1 (1.49) [1/0/0] | 0 | |
DISCUSSION
Daily administration of udenafil after BNS-RALP improved erectile function and patient satisfaction without any severe adverse effects. Moreover, according to the EPIC questionnaire, the effect of udenafil was the most prominent in the resolution of the problem with sexual function followed by the improvement in overall satisfaction and sexual function (Supplement Table 3).
Complications such as lymphocele, lower extremities edema, deep venous thrombosis, constipation, voiding dysfunctions, infection, and pain may occurred after RP [26]. Moreover, the urinary incontinence and erectile dysfunction inevitably occur after RP, although the severity can vary. Although several alternative treatment modalities such as high intensity focused ultrasound and brachytherapy have been developed to prevent these complications [27], RALP is a representative treatment for prostate cancer because of its superiority in controlling cancer and acceptable recovery rate from complications [28]. With advancements in surgical techniques, BNS-RALP was introduced to reduce the incidence of these complications, and the surgical technique has been continuously refined [29]. However, these complications have not yet been completely eliminated. Many studies have reported a decrease in potency after RP. According to a recent systematic analysis, an optimal treatment guideline for PR cannot be suggested despite several studies on PR after RP [30].
Erectile dysfunction after RP occurs owing to damage to the cavernous nerve or pudendal artery and corporeal veno-occlusive disorder [31,32]. These injuries are unavoidable despite the use of BNS-RALP. The inflammatory changes and fibrosis caused by the injury reduce the release of NO in the corpus cavernosum. As a result, a decrease in cGMP results in erectile dysfunction [33]. Therefore, PDE5i could be effective for PR after RP. According to a recently published systematic review, daily administration of sildenafil 100 mg was suggested as most effective for PR. In addition, the authors reported that on-demand use of PDE5i is not recommended as treatment for PR [34]. In the present study, udenafil 75 mg daily regimen was used. The on demand dose of udenafil is 100 mg to 200 mg. And it is recommended to use 50 mg or 75 mg of udenafil for daily use [35]. The daily use of 75 mg of udenafil, which has a high selectivity, was effective in PR without severe adverse effects in RP patients, a relatively elderly patient group.
The peak plasma concentration of udenafil is achieved 0.8 to 1.3 hours after administration, and its half-life is 9.9 to 12.1 hours [11,36]. Kim et al [37] studied factors that can predict the recovery of potency following treatment with udenafil for PR after RP. They reported high pre-operative erectile function, robotic surgery, and low stage of cancer as predictive factors for successful PR using udenafil [37]. In the present study, the efficacy of udenafil was evaluated for the first time in patients with localized prostate cancer who underwent BNS-RALP and had good erectile function before surgery, which deteriorated to poor erectile function after surgery. Following the administration of udenafil for 32 weeks after BNS-RALP, 36.51% of the patients recovered to an IIEF-EF score of 22 or higher, and the score of 82.54% of the patients had improved by 25% or more compared to that at baseline. According to Jo et al [38], early administration of 100 mg sildenafil after BNS-RALP showed an improved IIEF-5 of ≥17 at 12 months after surgery in 41.4% of the patients. The results of the present study also confirmed the recovery rate, which is not significantly different from that reported in previous studies. Padma-Nathan et al [39] reported that daily administration of 100 mg of sildenafil after BNS-RP could expect a potency for sexual activity in 29%, whereas the placebo group reported only 4%. In the present study, the proportion of subjects who responded that an erection was possible (EFQ Q1) at 32 weeks of treatment was 68.42% in the experimental group and 59.09% in the control group. For EFQ Q3 (Are you currently able to have an erection adequate for sexual intercourse?), 50.88% of subjects in the experimental group and 27.27% of those in the control group responded that it was possible in more than 50% of attempts. A previous study reported that 34% of the patients showed a spontaneous improvement in erectile function after BNS-RALP [40]. In the present study, 40% of subjects in the control group showed potency at 36 weeks after BNS-RALP; 48.15% of subjects in the control group responded that treatment improved the potency. In our study, the control group showed greater improvement in erectile function compared with that reported in previous studies. A high rate of drop-outs in the control group might have overestimated the functional outcomes and degree of satisfaction. Among patients in the control group who dropped out of the study (n=10), 7 patients withdrew owing to lack of efficacy of treatment. Although this high rate of drop-out, statistical difference between experimental group and control group might be a significant finding.
There are some limitations associated with this study. First, there no pre-operative assessment of sexual function. However, the study was conducted with normal or mild to moderated ED patients before BNS-RALP. This study had been designed to have 66 participants in the experimental group and 33 in the control group. However, 67 patients had assigned in the experimental group and 32 patients had allocated in a control group. The number of patients was relatively small, and there was a high rate of drop-outs in the control group. This might have been due to dissatisfaction with the placebo treatment. In addition, a nonvalidated EFQ was used in this study. Although the PR effect of udenafil cannot be verified through the results of this questionnaire, but it could be used as a reference. Another limitation is the lack of long-term follow-up data after treatment. Nevertheless, this is the first randomized controlled trial to show the efficacy of udenafil as a treatment for PR after BNS-RALP.
CONCLUSIONS
This first randomized controlled trial that assessed the efficacy and safety of daily administration of udenafil for the treatment of PR after BNS-RALP showed a significantly improved rate of potency and patient satisfaction without severe adverse events.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C2007662), the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (NTIS No. 9991006967) and Dong-a ST Co., Ltd., Seoul, Republic of Korea.
- Conceptualization: HA, SSJ.
- Data curation: JHC, SSJ.
- Formal analysis: JHC.
- Funding acquisition: HA, SSJ.
- Investigation: TGK, CK, GTS, SDK, JSC, HJK, HA, SSJ.
- Methodology: TGK, CK, GTS, JSC, HJK, HA, SSJ.
- Project administration: HA, SSJ.
- Resources: TGK, CK, GTS, SDK, JSC, HJK, HA, SSJ.
- Supervision: TGK, CK, GTS, SDK, JSC, HJK, HA, SSJ.
- Visualization: JHC.
- Writing – original draft: JHC.
- Writing – review & editing: TGK, CK, GTS, SDK, JSC, HJK, HA, SSJ.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time due to personal information protection policy.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220057.
Erectile function questionnaire
Results of laboratory investigations
Change of expanded prostate cancer index composite sexual assessment
References
- 1.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwin MS, Flanders SC, Pasta DJ, Stoddard ML, Lubeck DP, Henning JM. Sexual function and bother after radical prostatectomy or radiation for prostate cancer: multivariate quality-of-life analysis from CaPSURE. Cancer of the prostate strategic urologic research endeavor. Urology. 1999;54:503–508. doi: 10.1016/s0090-4295(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 3.Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the prostate cancer outcomes study. J Natl Cancer Inst. 2000;92:1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 4.Bernard B, Burnett C, Sweeney CJ, Rider JR, Sridhar SS. Impact of age at diagnosis of de novo metastatic prostate cancer on survival. Cancer. 2020;126:986–993. doi: 10.1002/cncr.32630. [DOI] [PubMed] [Google Scholar]
- 5.Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol. 2012;62:261–272. doi: 10.1016/j.eururo.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions part 2: recovery and preservation of erectile function, sexual desire, and orgasmic function. Eur Urol. 2012;62:273–286. doi: 10.1016/j.eururo.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Rabbani F, Schiff J, Piecuch M, Yunis LH, Eastham JA, Scardino PT, et al. Time course of recovery of erectile function after radical retropubic prostatectomy: does anyone recover after 2 years? J Sex Med. 2010;7:3984–3990. doi: 10.1111/j.1743-6109.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 8.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 9.Pavlovich CP, Levinson AW, Su LM, Mettee LZ, Feng Z, Bivalacqua TJ, et al. Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: results of a randomized double-blind trial with placebo. BJU Int. 2013;112:844–851. doi: 10.1111/bju.12253. [DOI] [PubMed] [Google Scholar]
- 10.Chung JH, Kang DH, Oh CY, Chung JM, Lee KS, Kim TH, et al. Safety and efficacy of once daily administration of 50 mg mirodenafil in patients with erectile dysfunction: a multicenter, double-blind, placebo controlled trial. J Urol. 2013;189:1006–1013. doi: 10.1016/j.juro.2012.08.243. [DOI] [PubMed] [Google Scholar]
- 11.Salem EA, Kendirci M, Hellstrom WJ. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs. 2006;7:661–669. [PubMed] [Google Scholar]
- 12.Cho MC, Paick JS. Udenafil for the treatment of erectile dysfunction. Ther Clin Risk Manag. 2014;10:341–354. doi: 10.2147/TCRM.S39727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouvelas D, Goulas A, Papazisis G, Sardeli C, Pourzitaki C. PDE5 inhibitors: in vitro and in vivo pharmacological profile. Curr Pharm Des. 2009;15:3464–3475. doi: 10.2174/138161209789206971. [DOI] [PubMed] [Google Scholar]
- 14.Makhlouf A, Kshirsagar A, Niederberger C. Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res. 2006;18:501–509. doi: 10.1038/sj.ijir.3901441. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Kim SW, Yang DY, Kim JJ, Park NC, Lee SW, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:380–387. doi: 10.1016/j.eururo.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–351. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim DJ, Hawksworth DJ, Hurwitz LM, Cullen J, Rosner IL, Lue TF, et al. A prospective, randomized, placebo-controlled trial of on-demand vs. nightly sildenafil citrate as assessed by Rigiscan and the international index of erectile function. Andrology. 2016;4:27–32. doi: 10.1111/andr.12118. [DOI] [PubMed] [Google Scholar]
- 18.Chiles KA, Staff I, Johnson-Arbor K, Champagne A, McLaughlin T, Graydon RJ. A double-blind, randomized trial on the efficacy and safety of hyperbaric oxygenation therapy in the preservation of erectile function after radical prostatectomy. J Urol. 2018;199:805–811. doi: 10.1016/j.juro.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32 [Google Scholar]
- 20.Montorsi F, Brock G, Stolzenburg JU, Mulhall J, Moncada I, Patel HR, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT) Eur Urol. 2014;65:587–596. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 21.Mirone V, Palmieri A, Cucinotta D, Parazzini F, Morelli P, Bettocchi C, et al. Flexible-dose vardenafil in a community-based population of men affected by erectile dysfunction: a 12-week open-label, multicenter trial. J Sex Med. 2005;2:842–847. doi: 10.1111/j.1743-6109.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee TK, Breau RH, Mallick R, Eapen L. A systematic review of expanded prostate cancer index composite (EPIC) quality of life after surgery or radiation treatment. Can J Urol. 2015;22:7599–7606. [PubMed] [Google Scholar]
- 23.Brock G, Nehra A, Lipshultz LI, Karlin GS, Gleave M, Seger M, et al. Safety and efficacy of vardenafil for the treatment of men with erectile dysfunction after radical retropubic prostatectomy. J Urol. 2003;170(4 Pt 1):1278–1283. doi: 10.1097/01.ju.0000086947.00547.49. [DOI] [PubMed] [Google Scholar]
- 24.Montorsi F, Verheyden B, Meuleman E, Jünemann KP, Moncada I, Valiquette L, et al. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol. 2004;45:339–344. doi: 10.1016/j.eururo.2003.11.010. discussion 344-5. [DOI] [PubMed] [Google Scholar]
- 25.King R, Marumo K, Paick JS, Zhang K, Shah R, Pangkahila W, et al. Satisfaction with sex and erection hardness: results of the Asia-Pacific sexual health and overall wellness survey. Int J Impot Res. 2011;23:135–141. doi: 10.1038/ijir.2011.17. [DOI] [PubMed] [Google Scholar]
- 26.Sforza S, Tellini R, Grosso AA, Zaccaro C, Viola L, Di Maida F, et al. Can we predict the development of symptomatic lymphocele following robot-assisted radical prostatectomy and lymph node dissection? Results from a tertiary referral Centre. Scand J Urol. 2020;54:328–333. doi: 10.1080/21681805.2020.1784270. [DOI] [PubMed] [Google Scholar]
- 27.Reichard C, Chapin BF. Can focal therapy replace radical therapy for prostate cancer? Against focal therapy. Eur Urol Focus. 2017;3:524–525. doi: 10.1016/j.euf.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008;179:2207–2210. doi: 10.1016/j.juro.2008.01.106. discussion 2210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Patel VR, Panaiyadiyan S, Seetharam Bhat KR, Moschovas MC, Nayak B. Nerve-sparing robot-assisted radical prostatectomy: current perspectives. Asian J Urol. 2021;8:2–13. doi: 10.1016/j.ajur.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolai M, Urkmez A, Sarikaya S, Fode M, Falcone M, Albersen M, et al. Penile rehabilitation and treatment options for erectile dysfunction following radical prostatectomy and radiotherapy: a systematic review. Front Surg. 2021;8:636974. doi: 10.3389/fsurg.2021.636974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med. 2013;10 Suppl 1:102–111. doi: 10.1111/j.1743-6109.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 32.Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010;7(4 Pt 2):1687–1698. doi: 10.1111/j.1743-6109.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 33.Mydlo JH, Viterbo R, Crispen P. Use of combined intracorporal injection and a phosphodiesterase-5 inhibitor therapy for men with a suboptimal response to sildenafil and/or vardenafil monotherapy after radical retropubic prostatectomy. BJU Int. 2005;95:843–846. doi: 10.1111/j.1464-410X.2005.05413.x. [DOI] [PubMed] [Google Scholar]
- 34.Sari Motlagh R, Abufaraj M, Yang L, Mori K, Pradere B, Laukhtina E, et al. Penile rehabilitation strategy after nerve sparing radical prostatectomy: a systematic review and network meta-analysis of randomized trials. J Urol. 2021;205:1018–1030. doi: 10.1097/JU.0000000000001584. [DOI] [PubMed] [Google Scholar]
- 35.Kang SG, Kim JJ. Udenafil: efficacy and tolerability in the management of erectile dysfunction. Ther Adv Urol. 2013;5:101–110. doi: 10.1177/1756287212470019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paick JS, Ahn TY, Choi HK, Chung WS, Kim JJ, Kim SC, et al. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med. 2008;5:2672–2680. doi: 10.1111/j.1743-6109.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim TH, Ha YS, Choi SH, Yoo ES, Kim BW, Yun SJ, et al. Factors predicting outcomes of penile rehabilitation with udenafil 50 mg following radical prostatectomy. Int J Impot Res. 2016;28:25–30. doi: 10.1038/ijir.2016.9. [DOI] [PubMed] [Google Scholar]
- 38.Jo JK, Jeong SJ, Oh JJ, Lee SW, Lee S, Hong SK, et al. Effect of starting penile rehabilitation with sildenafil immediately after robot-assisted laparoscopic radical prostatectomy on erectile function recovery: a prospective randomized trial. J Urol. 2018;199:1600–1606. doi: 10.1016/j.juro.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 39.Padma-Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, Montorsi F, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–486. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 40.Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:1063–1068. doi: 10.1016/s0360-3016(02)03030-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Erectile function questionnaire
Results of laboratory investigations
Change of expanded prostate cancer index composite sexual assessment
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time due to personal information protection policy.