Abstract
Purpose
To investigate the association between skin advanced glycation end-products (AGEs) levels, blood antioxidative vitamin and carotenoid concentrations, and severe erectile dysfunction (ED) in community-dwelling men.
Materials and Methods
This cross-sectional study used the 5-Item International Index of Erectile Function to identify 335 community-dwelling men with ED. The accumulation of skin AGEs was assessed noninvasively by measuring skin autofluorescence. Background-adjusted multivariable logistic regression analyses using the inverse probability of treatment weighting method were performed to evaluate the effects of AGEs, vitamins, and carotenoids on severe ED. Moreover, multiple linear regression analyses were performed to assess the association between skin AGEs levels and serum carotenoid concentrations.
Results
The median age of study participants was 57 years. Of the 335 men, 289 (86.3%) and 46 (13.7%) were classified into the mild/moderate and severe ED groups, respectively. Multivariable analyses revealed that skin AGEs levels, blood vitamins C and E, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, total lycopene, and cis-lycopenes concentrations were significantly associated with severe ED, whereas all-trans lycopene concentrations were not. In the multiple linear regression analyses, serum zeaxanthin concentrations were negatively and significantly correlated with skin AGEs levels.
Conclusions
Higher skin AGEs levels and lower blood antioxidative vitamin and carotenoid concentrations were significantly associated with severe ED. Serum zeaxanthin levels were negatively and significantly correlated with skin AGEs levels, suggesting the possible effects of zeaxanthin on ED by decreasing tissue AGEs levels.
Keywords: Carotenoids; Erectile dysfunction; Glycation end products, advanced; Vitamins; Zeaxanthins
INTRODUCTION
Erectile dysfunction (ED) is defined as the consistent inability to attain or maintain sufficient penile erection for satisfactory sexual intercourse [1] and negatively impacts the quality of life (QOL) [2]. Because men with severe ED have poorer QOL than those with mild/moderate ED [3], it is essential to identify the risk factors for severe ED.
Advanced glycation end-products (AGEs) are formed in an irreversible reaction between glucose and proteins, lipids, and nucleic acids via a non-enzymatic glycation pathway [4]. AGEs formation contributes to the pathologic cross-linking of collagen, leading to endothelial dysfunction, vascular thickening, and eventually atherosclerosis [5]. Several studies have investigated the role of AGEs in the etiology of ED. However, because many of these studies were conducted in patients with diabetes mellitus (DM) or used rat models of DM [6,7,8,9], the association between AGEs formation and ED in community-dwelling men remains unclear.
Vitamins and carotenoids are well-known antioxidants. Since oxidative stress is thought to play a crucial role in the pathogenesis of ED [10], vitamins and carotenoids may have preventive effects against ED. However, no studies have investigated the association between these antioxidants and ED in humans. Moreover, it remains unclear whether these antioxidants contribute to the decreased AGEs formation in human tissues.
Thus, this cross-sectional study aimed to investigate the association between skin AGEs levels, blood antioxidative vitamin and carotenoid concentrations, and ED severity in community-dwelling men.
MATERIALS AND METHODS
1. Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The data of community-dwelling men were collected from the Iwaki Health Promotion Project, and the study was approved by the ethics review board of the Hirosaki University Graduate School of Medicine (authorization no. 2014–377). The project was designed to prevent lifestyle-related diseases, promote health, and extend the lifespan of the residents of Hirosaki City (Iwaki District) in collaboration with Hirosaki City, Hirosaki University, and the Aomori Prefecture General Screening Centre. All the participants voluntarily participated in the project and provided written informed consent.
2. Participant selection
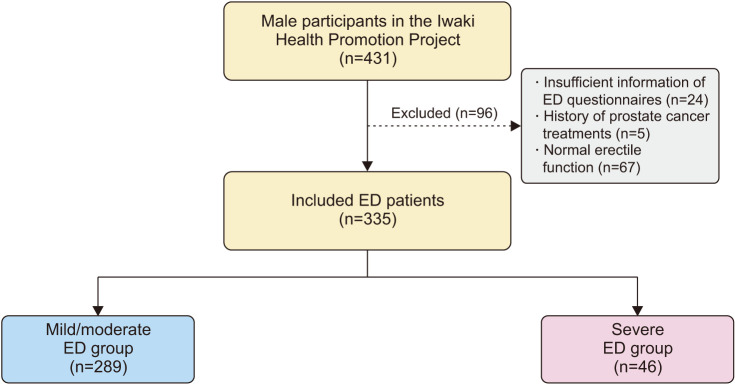
Of the 1,113 Japanese participants in the Iwaki Health Promotion Project 2015, 431 men were selected, excluding 682 women. Of those, 96 were excluded based on the following exclusion criteria: (1) insufficient information on ED questionnaires; (2) history of prostate cancer treatments, such as radical prostatectomy, radiation therapy, and androgen-deprivation therapy, because these therapies affect erectile function [11,12]; and (3) normal erectile function (5-Item International Index of Erectile Function [IIEF-5] score ≥22). Ultimately, 335 community-dwelling men with ED were included in the analysis (Fig. 1).
Fig. 1. Participant selection. Number of included and excluded participants. ED: erectile dysfunction.
3. Evaluation of variables
The following variables were analyzed: age, body mass index, hypertension (HTN), dyslipidemia, DM, cardiovascular disease (CVD), smoking status, current habitual drinking, education level, medications (β-blockers, calcium [Ca]-blockers, thiazide diuretics, and spironolactone), mental health status, laboratory values, ankle-brachial index (ABI), and brachial-ankle pulse wave velocity (baPWV). HTN was defined as taking antihypertensive drugs and/or having a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg. Dyslipidemia was defined as taking lipid-lowering drugs and/or having a low-density lipoprotein cholesterol level ≥140 mg/dL, triglyceride level ≥150 mg/dL, and/or high-density lipoprotein cholesterol level <40 mg/dL. DM was defined as taking glycemic control drugs and/or having a history of type 2 DM. Blood samples were collected in the morning. Payne’s formula was used to calculate adjusted serum Ca levels. Renal function was evaluated by estimated glomerular filtration rate (eGFR) using a modified version of the abbreviated Modification of Diet in Renal Disease Study formula for Japanese participants [13]. ABI and baPWV were measured using a volume-plethysmographic apparatus (Form PWV/ABI; Omron COLIN Co. Ltd., Tokyo, Japan). The averages of the right and left ABI and baPWV values were used in the analyses. Mental health status was evaluated using the 36-Item Short-Form Health Survey Mental Component Summary. Scores were added and transformed into 0 to 100 scales, with higher values indicating better health status [14]. Smoking status was classified as never, former, or current smoker.
4. Evaluation of erectile dysfunction
ED was assessed using the IIEF-5. The IIEF-5 scores were interpreted as follows: no ED (22–25), mild ED (17–21), mild-to-moderate ED (12–16), moderate ED (8–11), and severe ED (5–7). Based on this, participants with ED were divided into the mild/moderate (IIEF-5 score 8–21) and severe ED (IIEF-5 score ≤7) groups (Fig. 1).
5. Evaluation of advanced glycation end-products
The accumulation of skin AGEs can be assessed noninvasively by measuring the skin autofluorescence. Skin autofluorescence was measured as previously described [15]. Briefly, the amount of skin AGEs accumulation was evaluated using an AGE reader (Diagnoptics Technologies B.V., Groningen, The Netherlands). Skin autofluorescence was measured on the volar side of the forearm, approximately 10 cm below the elbow. The AGEs reader illuminated a skin surface of 4 cm2 with ultraviolet light wavelengths between 300 and 420 nm (peak intensity 370 nm). The emitted and reflected light intensities were measured using a spectrometer in the range of 300 to 600 nm. Skin autofluorescence was calculated as the average intensity of the emitted light (wavelengths between 420 and 600 nm) divided by the average intensity of reflected light (wavelengths between 300 and 420 nm), multiplied by 100. Skin autofluorescence was expressed in arbitrary units (AUs).
6. Measurement of blood vitamin and carotenoid concentrations
The blood antioxidants levels were measured by KAGOME CO., LTD. (Nagoya, Japan). The plasma concentrations of ascorbic acid (vitamin C) were assayed using a commercially available kit (R01K02; Shima Laboratories Co., Ltd., Tokyo, Japan). The serum concentrations of α-tocopherol (vitamin E) and carotenoids (lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, total lycopene [sum of all-trans lycopene and cis-lycopenes]) were quantified by a high-performance liquid chromatograph using a photodiode array detector (Prominence LC-30AD/Nexera X2 SPPD-M30A; SHIMADZU Corp., Kyoto, Japan) after extraction by organic solvents as previously described [16].
7. Statistical analysis
SPSS version 24.0 (IBM Corp., Armonk, NY, USA), R 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria), and GraphPad Prism 5.03 (GraphPad Software, San Diego, CA, USA) were used for statistical analyses. Quantitative variables are expressed as medians with interquartile ranges. Categorical variables were compared using the Fisher’s exact test or chi-squared test. Differences in quantitative variables were analyzed using the Mann–Whitney U-test. Correlations between variables were analyzed using Spearman’s rank correlation coefficient. The optimal cutoff values of AGEs, vitamins, and carotenoids for severe ED were calculated using the receiver operating characteristic curve. Univariable and multivariable logistic regression analyses were performed to investigate the factors associated with severe ED. Reweighting of the affected and unaffected groups was performed using the inverse probability of treatment weighting (IPTW) method to emulate a propensity score-matched population [17]. Background-adjusted multivariable logistic regression analyses using the IPTW method were performed to evaluate the effects of AGEs, vitamins, and carotenoids on severe ED. IPTW is an increasingly popular method used to address confounding factors and achieve a balanced distribution of confounders across the treatment groups. The following variables were included in the IPTW method to emulate a propensity score-matched population: age, HTN, dyslipidemia, DM, CVD, smoking status, current habitual drinking, eGFR, plasma testosterone level, and baPWV. Moreover, multiple linear regression analyses were performed with skin AGEs levels as dependent variable and age and serum carotenoid concentrations as independent variables. Statistical significance was set at p<0.05.
RESULTS
1. Background of participants
The study participants’ median age and IIEF-5 scores were 57 years and 15, respectively. Of the 335 men with ED, 289 (86.3%) and 46 (13.7%) were classified into the mild/moderate and severe ED groups, respectively (Fig. 1).
Age, the prevalence of DM, history of CVD, number of β-blocker users, mental health status, renal function, plasma total cholesterol level, and baPWV values were significantly different between the two groups (Table 1).
Table 1. Participant background characteristics.
| Characteristic | All (n=335) | Mild/moderate ED group (n=289) | Severe ED group (n=46) | p-value | |
|---|---|---|---|---|---|
| Age (y) | 57 (43–66) | 54 (41–65) | 66 (62–77) | <0.001* | |
| Body mass index (kg/m2) | 23 (22–26) | 23 (22–26) | 24 (21–25) | 0.978 | |
| Hypertension | 155 (46.3%) | 128 (44.3%) | 27 (58.7%) | 0.069 | |
| Dyslipidemia | 114 (34.0%) | 96 (33.2%) | 18 (39.1%) | 0.432 | |
| Diabetes mellitus | 41 (12.2%) | 31 (10.7%) | 10 (21.7%) | 0.034* | |
| Cardiovascular disease | 34 (10.1%) | 24 (8.3%) | 10 (21.7%) | 0.005* | |
| Smoking status | 0.176 | ||||
| Never | 125 (37.3%) | 103 (35.6%) | 22 (47.8%) | ||
| Former | 110 (32.8%) | 95 (32.9%) | 15 (32.6%) | ||
| Current | 100 (29.9%) | 91 (31.5%) | 9 (19.6%) | ||
| Current habitual drinking | 227 (67.8%) | 196 (67.8%) | 31 (67.4%) | 0.954 | |
| Post-high school education | 82 (24.5%) | 76 (26.3%) | 6 (13.0%) | 0.052 | |
| Medications | |||||
| β-blockers | 9 (2.7%) | 5 (1.7%) | 4 (8.7%) | 0.023* | |
| Calcium-blockers | 43 (12.8%) | 35 (12.1%) | 8 (17.4%) | 0.342 | |
| Thiazide diuretics | 10 (3.0%) | 8 (2.8%) | 2 (4.3%) | 0.633 | |
| SF-36 MCS | 51 (45–56) | 50 (44–56) | 53 (49–59) | 0.010* | |
| Laboratory blood test | |||||
| eGFR (mL/min/1.73 m2) | 81 (71–88) | 81 (73–90) | 73 (63–83) | <0.001* | |
| Total cholesterol (mg/dL) | 204 (180–225) | 206 (183–228) | 193 (167–217) | 0.016* | |
| LDL-cholesterol (mg/dL) | 116 (97–134) | 117 (97–134) | 104 (91–135) | 0.171 | |
| Triglyceride (mg/dL) | 96 (71–147) | 97 (71–154) | 85 (61–118) | 0.055 | |
| Testosterone (ng/dL) | 614 (467–753) | 610 (467–753) | 633 (461–762) | 0.943 | |
| Ankle-brachial index | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | 0.308 | |
| baPWV | 1,500 (1,315–1,792) | 1,479 (1,291–1,767) | 1,653 (1,495–1,926) | 0.001* | |
| IIEF-5 score | 15 (11–19) | 16 (12–20) | 6.0 (5.0–7.0) | <0.001* | |
Values are presented as median (interquartile range) or number (%).
ED: erectile dysfunction, SF-36 MCS: 36-Item Short Form Health Survey Mental Component Summary, eGFR: estimated glomerular filtration rate, LDL: low density lipoprotein, baPWV: brachial-ankle pulse wave velocity, IIEF: International Index of Erectile Function.
*Statistically significant p<0.05.
2. Association between skin advanced glycation end-products levels and the severity of erectile dysfunction
Spearman’s rank correlation test showed a significant negative correlation between skin AGEs levels and IIEF-5 scores (ρ=−0.355, p<0.001; Fig. 2A). The median skin AGEs levels in all men with ED were 2.0 AU. Skin AGEs levels in the severe ED group were significantly higher than those in the mild/moderate ED group (2.2 vs. 1.9 AU, respectively, p<0.001; Fig. 2B). The optimal cutoff value of the skin AGEs level for severe ED was 2.01 AU. Men with skin AGEs level ≥2.01 AU had a significantly higher prevalence of severe ED than those with skin AGEs level <2.01 AU (25.0% vs. 5.3%, respectively, p<0.001; Fig. 2C). In the univariable analyses, age, DM, CVD, eGFR, and baPWV were significantly associated with severe ED (Table 2). In the multivariable analysis, skin AGEs levels were significantly associated with severe ED (odds ratio [OR]: 2.417, 95% confidence interval [CI]: 1.027–5.688, p=0.043; Supplement Table). Similarly, in the multivariable analysis using the IPTW method, skin AGEs levels were significantly associated with severe ED (OR: 2.563, 95% CI: 1.040–6.312, p=0.042; Table 3).
Fig. 2. Association between skin advanced glycation end-products (AGEs) levels and the severity of erectile dysfunction (ED). (A) The correlation between skin AGEs levels and the 5-Item International Index of Erectile Function (IIEF-5) scores was analyzed using Spearman’s rank correlation coefficient. (B) Skin AGEs levels were compared between the two groups using the Mann–Whitney U-test. (C) The prevalence of severe ED was compared between men with lower and higher skin AGEs levels using the chi-squared test. AU: arbitrary unit.
Table 2. Univariable analyses for severe erectile dysfunction.
| Variable | Factor | p-value | Odds ratio | 95% CI |
|---|---|---|---|---|
| Age | Continuous | <0.001* | 1.099 | 1.063–1.136 |
| Body mass index | Continuous | 0.822 | 0.989 | 0.895–1.092 |
| Hypertension | Positive | 0.071 | 1.787 | 0.951–3.360 |
| Dyslipidemia | Positive | 0.433 | 1.292 | 0.681–2.453 |
| Diabetes mellitus | Positive | 0.038* | 2.312 | 1.046–5.112 |
| Cardiovascular disease | Positive | 0.007* | 3.067 | 1.357–6.934 |
| Smoking status | Current | 0.105 | 0.529 | 0.245–1.143 |
| Current habitual drinking | Positive | 0.954 | 0.981 | 0.505–1.905 |
| eGFR | Continuous | <0.001* | 0.965 | 0.945–0.985 |
| Testosterone level | Continuous | 0.586 | 0.998 | 0.999–1.001 |
| baPWV | Continuous | 0.015* | 1.001 | 1.000–1.002 |
CI: confidence interval, eGFR: estimated glomerular filtration rate, baPWV: brachial-ankle pulse wave velocity.
*Statistically significant p<0.05.
Table 3. Multivariable analyses for severe erectile dysfunction using the inverse probability of treatment weighting method.
| Variable | Factor (cutoff value) | p-value | Odds ratio | 95% CI |
|---|---|---|---|---|
| AGEs | ≥2.01 AU | 0.042* | 2.563 | 1.040–6.312 |
| Vitamin C | ≥3.58 μg/mL | 0.047* | 0.382 | 0.149–0.980 |
| Vitamin E | ≥1.59 μg/mL | <0.001* | 0.090 | 0.027–0.306 |
| Lutein | ≥0.24 μg/mL | 0.004* | 0.368 | 0.188–0.718 |
| Zeaxanthin | ≥0.04 μg/mL | 0.015* | 0.388 | 0.181–0.830 |
| β-cryptoxanthin | ≥0.06 μg/mL | 0.029* | 0.429 | 0.201–0.915 |
| α-carotene | ≥0.08 μg/mL | 0.002* | 0.354 | 0.185–0.676 |
| β-carotene | ≥0.24 μg/mL | <0.001* | 0.284 | 0.143–0.564 |
| Total lycopene | ≥0.19 μg/mL | 0.007* | 0.341 | 0.156–0.745 |
| All-trans lycopene | ≥0.07 μg/mL | 0.076 | 0.510 | 0.243–1.071 |
| Cis-lycopenes | ≥0.14 μg/mL | 0.004* | 0.240 | 0.091–0.630 |
CI: confidence interval, AGEs: advanced glycation end-products, AU: arbitrary unit.
*Statistically significant p<0.05.
3. Association of blood vitamin and carotenoid concentrations with the severity of erectile dysfunction
Table 4 summarizes blood vitamin and carotenoid concentrations. Plasma vitamin C, serum vitamin E, zeaxanthin, α-carotene, total lycopene, all-trans lycopene, and cis-lycopenes concentrations in the severe ED group were significantly lower than those in the mild/moderate ED group, whereas serum lutein, β-cryptoxanthin, and β-carotene concentrations were not significantly different between the two groups (Table 4). The optimal cutoff values of vitamin and carotenoid concentrations for severe ED are shown in Table 3. Men with lower blood vitamins C and E, zeaxanthin, α-carotene, total lycopene, all-trans lycopene, and cis-lycopenes concentrations had a significantly higher prevalence of severe ED than those with higher concentrations. In contrast, the prevalence of severe ED was not significantly different between lower and higher serum lutein, β-cryptoxanthin, and β-carotene concentrations (Fig. 3). In the multivariable analyses, vitamin C (OR: 0.264, p=0.006), vitamin E (OR: 0.173, p=0.005), lutein (OR: 0.416, p=0.021), zeaxanthin (OR: 0.397, p=0.030), β-cryptoxanthin (OR: 0.434, p=0.044), α-carotene (OR: 0.282, p=0.001), β-carotene (OR: 0.217, p<0.001), total lycopene (OR: 0.394, p=0.028), and cis-lycopenes (OR: 0.297, p=0.019) concentrations were significantly associated with severe ED, whereas all-trans lycopene concentrations were not (OR: 0.520, p=0.094; Supplement Table). Similarly, in the multivariable analyses using the IPTW method, vitamin C (OR: 0.382, p=0.047), vitamin E (OR: 0.090, p<0.001), lutein (OR: 0.368, p=0.004), zeaxanthin (OR: 0.388, p=0.015), β-cryptoxanthin (OR: 0.429, p=0.029), α-carotene (OR: 0.354, p=0.002), β-carotene (OR: 0.284, p<0.001), total lycopene (OR: 0.341, p=0.007), and cis-lycopenes (OR: 0.240, p=0.004) concentrations were significantly associated with severe ED, whereas all-trans lycopene concentrations were not (OR: 0.510, p=0.076; Table 3).
Table 4. Comparisons of blood vitamin and carotenoid concentrations between groups (unit: µg/mL).
| Variable | All | Mild/moderate ED group | Severe ED group | p-value |
|---|---|---|---|---|
| Vitamin C | 4.7 (3.9–5.7) | 4.8 (3.9–5.7) | 4.1 (2.9–5.6) | 0.032* |
| Vitamin E | 13.7 (11.9–16.6) | 14.0 (12.0–16.8) | 13.0 (11.2–14.8) | 0.008* |
| Lutein | 0.29 (0.22–0.39) | 0.29 (0.22–0.40) | 0.28 (0.21–0.38) | 0.525 |
| Zeaxanthin | 0.06 (0.05–0.08) | 0.06 (0.05–0.08) | 0.05 (0.04–0.07) | 0.012* |
| β-cryptoxanthin | 0.09 (0.07–0.14) | 0.09 (0.07–0.14) | 0.10 (0.06–0.14) | 0.699 |
| α-carotene | 0.09 (0.07–0.15) | 0.10 (0.07–0.15) | 0.07 (0.06–0.12) | 0.019* |
| β-carotene | 0.24 (0.13–0.38) | 0.24 (0.13–0.38) | 0.21 (0.13–0.35) | 0.680 |
| Total lycopene | 0.19 (0.12–0.29) | 0.20 (0.13–0.30) | 0.14 (0.09–0.19) | <0.001* |
| All-trans lycopene | 0.07 (0.04–0.11) | 0.07 (0.05–0.11) | 0.05 (0.03–0.08) | <0.001* |
| Cis-lycopenes | 0.11 (0.08–0.18) | 0.12 (0.08–0.19) | 0.08 (0.06–0.12) | <0.001* |
Values are presented as median (interquartile range).
ED: erectile dysfunction.
*Statistically significant p<0.05.
Fig. 3. Comparison of the prevalence of severe erectile dysfunction (ED) between lower and higher blood vitamin and carotenoid concentrations. (A-J) The prevalence of severe ED was compared between men with lower and higher blood vitamin and carotenoid concentrations using the Fisher’s exact test or chi-squared test.
4. Correlation of blood vitamin and carotenoid concentrations with skin advanced glycation end-products levels
Spearman’s rank correlation tests showed significant negative correlations of serum α-carotene, zeaxanthin, total lycopene, cis-lycopenes, and all-trans lycopene concentrations with skin AGEs levels (ρ=-0.124, p=0.029 [Fig. 4C]; ρ=-0.114, p=0.044 [Fig. 4F]; ρ=-0.238, p<0.001 [Fig. 4H]; ρ=-0.265, p<0.001 [Fig. 4I]; ρ=-0.185, p=0.001 [Fig. 4J]), whereas blood vitamins C and E, β-carotene, lutein, and β-cryptoxanthin levels were not negatively and significantly correlated with skin AGEs levels (Fig. 4A, 4B, 4D, 4E, 4G). In the multiple linear regression analyses, only serum zeaxanthin concentrations were negatively and significantly correlated with skin AGEs levels (βstd=-0.099, p=0.032; Table 5).
Fig. 4. Correlations between blood vitamin and carotenoid concentrations and skin advanced glycation end-products (AGEs) levels. (A-J) Correlations between blood vitamin and carotenoid concentrations and skin AGEs levels were analyzed using Spearman’s rank correlation coefficient. AU: arbitrary unit.
Table 5. Multiple linear regression analyses of serum carotenoid concentrations for skin advanced glycation end-products levels.
| Variable | Unstandardized coefficient | Standardized coefficient | t | p-value | ||
|---|---|---|---|---|---|---|
| B | SE | β | ||||
| Zeaxanthin | -1.929 | 0.894 | -0.099 | -2.158 | 0.032* | |
| Constant | 1.122 | 0.100 | 11.21 | <0.001* | ||
| Age | 0.018 | 0.001 | 0.576 | 12.56 | <0.001* | |
| α-carotene | -0.257 | 0.173 | -0.068 | -1.483 | 0.139 | |
| Constant | 1.032 | 0.085 | 12.08 | <0.001* | ||
| Age | 0.018 | 0.001 | 0.580 | 12.59 | <0.001* | |
| Total lycopene | -0.063 | 0.171 | -0.018 | -0.368 | 0.713 | |
| Constant | 1.021 | 0.104 | 9.807 | <0.001* | ||
| Age | 0.018 | 0.002 | 0.574 | 11.82 | <0.001* | |
| All-trans lycopene | -0.073 | 0.431 | -0.008 | -0.169 | 0.866 | |
| Constant | 1.007 | 0.100 | 10.11 | <0.001* | ||
| Age | 0.018 | 0.001 | 0.578 | 12.12 | <0.001* | |
| Cis-lycopenes | -0.126 | 0.270 | -0.023 | -0.468 | 0.640 | |
| Constant | 1.028 | 0.105 | 9.783 | <0.001* | ||
| Age | 0.018 | 0.002 | 0.572 | 11.68 | <0.001* | |
SE: standard error.
*Statistically significant p<0.05.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the association between skin AGEs levels, blood vitamin and carotenoid concentrations, and ED severity in community-dwelling men. The results of the present study revealed that higher skin AGEs levels and lower blood vitamin and carotenoid concentrations were significantly associated with severe ED. Moreover, significant negative correlations between serum zeaxanthin concentrations and skin AGEs levels suggests the possible effects of zeaxanthin on ED by decreasing tissue AGEs levels. These results may broaden our understanding of the etiology of severe ED and open new therapeutic avenues for severe ED.
The present study showed a significant association between skin AGEs levels and ED severity in community-dwelling men. One possible mechanism that may explain these results is reduced cellular nitric oxide (NO) bioavailability and altered synthesis of endothelial NO synthase (eNOS). In in vitro experiments, AGEs quenched NO and diminished eNOS activity through receptor-mediated phosphorylation of serine residues in eNOS [18,19]. Another mechanism is oxidative stress, a well-known risk factor for ED. AGEs directly enhance the synthesis of free radicals and reactive oxygen species [20]. In addition, AGEs allow monocyte migration into the vessel wall, modify intercellular adhesion and extracellular matrix by increasing collagen cross-linking, and increase fat accumulation in the vessel wall [21]. Moreover, our results are consistent with previous studies that have demonstrated the therapeutic effects of aminoguanidine, a selective AGEs inhibitor, and ALT-711, a cross-link breaker of AGEs, on erectile function in rat models of DM [6,7,8]. On the other hand, minimal evidence about the association between AGEs and ED is available in humans. Kouidrat et al. [9] have measured skin autofluorescence, a noninvasive marker of AGEs, in patients with DM and showed a significant negative correlation between skin autofluorescence and the IIEF-5 score. In addition, Seftel et al. [22] have demonstrated that AGEs in diabetic human penile tissue were elevated compared with non-diabetic ones. Although these studies suggest possible effects of AGEs formation on ED in diabetic men, the effects of AGEs that accumulate in the context of normal aging on the development of ED remain unclear. Jiaan et al. [23] have investigated the presence of AGEs in penile tissue specimens obtained at autopsy or at the time of insertion of a penile prosthesis and revealed that AGEs in penile tissue increased exponentially with age. However, they could not evaluate the association between AGEs accumulation and erectile function. Further studies are required to clarify the association between AGEs levels and ED in humans.
Although the present study demonstrated a significant association between skin AGEs levels and ED severity, it remains unclear whether lowering AGEs levels can contribute to the prevention of severe ED and restore erectile function. The evidence is lacking in humans to answer this question. However, several studies using animal models may be helpful for understanding the association. Gurbuz et al. [24] have reported that chronic administration of sildenafil decreased penile tissue AGEs levels and improved erectile function in a rat model of chronic renal failure. Moreover, Cartledge et al. [7] have reported that aminoguanidine, a selective AGEs inhibitor, reversed the impairment in neuronal and endothelial NO-mediated penile smooth muscle relaxation in a rat model of DM. In addition, Lin and Gou [25] have reported that Panax notoginseng saponin, a traditional Chinese herbal medicine, decreased AGEs expression in corpora cavernosa tissues and increased intracavernous pressure / mean arterial pressure ratio in diabetic ED rats. Although these studies suggest potential effects of lowering AGEs levels on improving erectile function, no evidence is available in humans. Further studies are warranted to address this issue.
Although vitamins and carotenoids are well-known antioxidants and oxidative stress is thought to be one of the major causes of ED, only vitamin E has been investigated as a potential synergistic effector with a phosphodiesterase type 5 inhibitor in humans and animal models [26,27]. Moreover, little is known about the effects of carotenoids on AGEs accumulation in humans. Our results demonstrated that not only vitamin E, but also vitamin C and carotenoids were significantly associated with severe ED in community-dwelling men. Notably, serum zeaxanthin concentrations were significantly correlated with skin AGEs levels, suggesting the possible effects on ED by decreasing tissue AGEs levels. Although previous studies have demonstrated the effects of carotenoids on AGEs accumulation in in-vitro experiments or diabetic rat models [28,29,30], no evidence in humans has been presented. Thus, the results in the present study may broaden our understanding of the association between vitamins, carotenoids, AGEs, and ED severity. However, the direct mechanism that can explain the correlation between serum zeaxanthin concentrations and skin AGEs levels remains unclear. In addition, serum zeaxanthin concentrations were lowest among the carotenoids assessed in the present study, indicating a need for further validation study.
We observed a significant association between serum cis-lycopenes concentrations and severe ED, whereas the association between all-trans lycopene concentrations and severe ED was marginal. These results may be due to the different biological activities of cis and trans isomers of lycopene. Müller et al. [31] have demonstrated that cis-lycopenes showed a higher antioxidant capacity than all-trans lycopene. Moreover, a higher bioavailability of cis-lycopenes compared with all-trans lycopene may also affect the results [32]. However, the exact differences in biological effects on ED remain unclear. Further studies are warranted to elucidate the different roles of lycopene isomers in ED.
This study had several limitations. First, its cross-sectional nature prevents us from determining cause-and-effect associations. It may also involve selection bias and other unmeasurable confounding factors that cannot be controlled. Second, this study included a relatively small number of men with severe ED. Third, it has been reported that regression estimates with IPTW methods tended to reject the null hypothesis frequently because of inflated sample sizes [33]. Fourth, penile color Doppler ultrasonography for differentiating between vascular and non-vascular causes of ED was not assessed. Finally, the measurement of skin AGEs levels and blood vitamin and carotenoid concentrations at a single point in time may not accurately reflect the average levels over a prolonged period.
CONCLUSIONS
Higher skin AGEs levels and lower blood vitamin and carotenoid concentrations were significantly associated with severe ED. Serum zeaxanthin concentrations were negatively and significantly correlated with skin AGEs levels, suggesting the possible effect on ED by decreasing tissue AGEs levels. These results may broaden our understanding of the etiology of severe ED.
Acknowledgements
The authors would like to thank all participants, the data collection team of the Iwaki Health Promotion Project 2015, and the staff of the Department of Social Medicine, Hirosaki University, for their contribution to this study. The authors also thank Editage (www.editage.com) for the English language editing.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by a Grant-in-Aid for Scientific Research (Nos. 19H05556 and 20K09517) from the Japan Society for the Promotion of Science and the Japan Science and Technology Agency, Center of Innovation Program, Hirosaki (no. JPMJCE1302).
- Conceptualization: NF.
- Data curation: MI, TI, HS, MM.
- Formal analysis: NF.
- Funding acquisition: SH, CO.
- Investigation: NF.
- Methodology: NF.
- Project administration: NF.
- Supervision: TM, KI, CO.
- Validation: SH, TY, YH.
- Writing – original draft: NF.
- Writing – review & editing: all authors.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time due to legal, ethical reasons, and personal information protection policy.
Supplementary Material
Supplementary material can be found via https://doi.org/10.5534/wjmh.220154.
Multivariable logistic regression analyses for severe erectile dysfunction
References
- 1.NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Sánchez-Cruz JJ, Cabrera-León A, Martín-Morales A, Fernández A, Burgos R, Rejas J. Male erectile dysfunction and health-related quality of life. Eur Urol. 2003;44:245–253. doi: 10.1016/s0302-2838(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 3.Hwang TI, Lo HC, Tsai TF, Chiou HY. Association among hypogonadism, quality of life and erectile dysfunction in middle-aged and aged male in Taiwan. Int J Impot Res. 2007;19:69–75. doi: 10.1038/sj.ijir.3901480. [DOI] [PubMed] [Google Scholar]
- 4.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- 5.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. Erratum in: Diabetologia 2002;45:293. [DOI] [PubMed] [Google Scholar]
- 6.Usta MF, Bivalacqua TJ, Yang DY, Ramanitharan A, Sell DR, Viswanathan A, et al. The protective effect of aminoguanidine on erectile function in streptozotocin diabetic rats. J Urol. 2003;170(4 Pt 1):1437–1442. doi: 10.1097/01.ju.0000077557.45582.f3. [DOI] [PubMed] [Google Scholar]
- 7.Cartledge JJ, Eardley I, Morrison JF. Advanced glycation end-products are responsible for the impairment of corpus cavernosal smooth muscle relaxation seen in diabetes. BJU Int. 2001;87:402–407. doi: 10.1046/j.1464-410x.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 8.Gurbuz N, Gurkan R, Ender Caylan A, Surmen Usta S, Usta MF. The therapeutic effect of ALT-711 on erectile function in rats treated with high-level AGEs (advanced glycation end products) containing diet. Int J Impot Res. 2022;34:222–228. doi: 10.1038/s41443-021-00417-8. Erratum in: Int J Impot Res 2022;34:231. [DOI] [PubMed] [Google Scholar]
- 9.Kouidrat Y, Zaitouni A, Amad A, Diouf M, Desailloud R, Loas G, et al. Skin autofluorescence (a marker for advanced glycation end products) and erectile dysfunction in diabetes. J Diabetes Complications. 2017;31:108–113. doi: 10.1016/j.jdiacomp.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27:335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 11.Gaither TW, Awad MA, Osterberg EC, Murphy GP, Allen IE, Chang A, et al. The natural history of erectile dysfunction after prostatic radiotherapy: a systematic review and meta-analysis. J Sex Med. 2017;14:1071–1078. doi: 10.1016/j.jsxm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Candy B, Jones L, Williams R, Tookman A, King M. Phosphodiesterase type 5 inhibitors in the management of erectile dysfunction secondary to treatments for prostate cancer: findings from a Cochrane systematic review. BJU Int. 2008;102:426–431. doi: 10.1111/j.1464-410X.2008.07668.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CC, Shen MY, Chang KC, Wang GJ, Liu SH, Chang CT. Skin autofluorescence is associated with rapid renal function decline in subjects at increased risk of coronary artery disease. PLoS One. 2019;14:e0217203. doi: 10.1371/journal.pone.0217203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshima S, Sakamoto H, Ishiguro Y, Terao J. Accumulation and clearance of capsanthin in blood plasma after the ingestion of paprika juice in men. J Nutr. 1997;127:1475–1479. doi: 10.1093/jn/127.8.1475. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26:1654–1670. doi: 10.1177/0962280215584401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu B, Ji Y, Yao K, Cao YX, Ferro A. Inhibition of human endothelial cell nitric oxide synthesis by advanced glycation end-products but not glucose: relevance to diabetes. Clin Sci (Lond) 2005;109:439–446. doi: 10.1042/CS20050183. [DOI] [PubMed] [Google Scholar]
- 20.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 21.Neves D. Advanced glycation end-products: a common pathway in diabetes and age-related erectile dysfunction. Free Radic Res. 2013;47 Suppl 1:49–69. doi: 10.3109/10715762.2013.821701. [DOI] [PubMed] [Google Scholar]
- 22.Seftel AD, Vaziri ND, Ni Z, Razmjouei K, Fogarty J, Hampel N, et al. Advanced glycation end products in human penis: elevation in diabetic tissue, site of deposition, and possible effect through iNOS or eNOS. Urology. 1997;50:1016–1026. doi: 10.1016/S0090-4295(97)00512-8. [DOI] [PubMed] [Google Scholar]
- 23.Jiaan DB, Seftel AD, Fogarty J, Hampel N, Cruz W, Pomerantz J, et al. Age-related increase in an advanced glycation end product in penile tissue. World J Urol. 1995;13:369–375. doi: 10.1007/BF00191219. [DOI] [PubMed] [Google Scholar]
- 24.Gurbuz N, Kol A, Ipekci T, Ates E, Baykal A, Usta MF. Chronic administration of sildenafil improves erectile function in a rat model of chronic renal failure. Asian J Androl. 2015;17:797–801. doi: 10.4103/1008-682X.146973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin F, Gou X. Panax notoginseng saponins improve the erectile dysfunction in diabetic rats by protecting the endothelial function of the penile corpus cavernosum. Int J Impot Res. 2013;25:206–211. doi: 10.1038/ijir.2013.19. [DOI] [PubMed] [Google Scholar]
- 26.Kondoh N, Higuchi Y, Maruyama T, Nojima M, Yamamoto S, Shima H. Salvage therapy trial for erectile dysfunction using phosphodiesterase type 5 inhibitors and vitamin E: preliminary report. Aging Male. 2008;11:167–170. doi: 10.1080/13685530802273624. [DOI] [PubMed] [Google Scholar]
- 27.De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J Androl. 2004;25:830–836. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 28.Figueiredo ID, Lima TFO, Inácio MD, Costa MC, Assis RP, Brunetti IL, et al. Lycopene improves the metformin effects on glycemic control and decreases biomarkers of glycoxidative stress in diabetic rats. Diabetes Metab Syndr Obes. 2020;13:3117–3135. doi: 10.2147/DMSO.S265944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabrez S, Al-Shali KZ, Ahmad S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: a novel approach to halt the AGE-RAGE axis menace. Biofactors. 2015;41:372–381. doi: 10.1002/biof.1238. [DOI] [PubMed] [Google Scholar]
- 30.Manabe Y, Takii Y, Sugawara T. Siphonaxanthin, a carotenoid from green algae, suppresses advanced glycation end product-induced inflammatory responses. J Nat Med. 2020;74:127–134. doi: 10.1007/s11418-019-01354-z. [DOI] [PubMed] [Google Scholar]
- 31.Müller L, Goupy P, Fröhlich K, Dangles O, Caris-Veyrat C, Böhm V. Comparative study on antioxidant activity of lycopene (Z)-isomers in different assays. J Agric Food Chem. 2011;59:4504–4511. doi: 10.1021/jf1045969. [DOI] [PubMed] [Google Scholar]
- 32.Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW., Jr Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J Nutr. 1999;129:1176–1181. doi: 10.1093/jn/129.6.1176. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariable logistic regression analyses for severe erectile dysfunction
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time due to legal, ethical reasons, and personal information protection policy.