Abstract
According to existing studies, sedentary behavior contributes to male infertility. Both preclinical and clinical studies have investigated the association between physical exercise, semen quality, and pregnancy rates with heterogeneous results. The current review sought to examine the relationship between physical activity (PA) and male infertility, semen characteristics, and pregnancy rates. Pre-clinical studies demonstrated mixed benefits from exercise, with diet being an important consideration. Some forms of PA showed an improvement in pregnancy rates, while others did not consistently improve semen quality. Data also suggests that more intense exercise and certain types of exercise may impair male fertility. Given the limited number of randomized trials, future research is required to examine the relationship between specific forms of exercise and semen parameters along with reproductive outcomes.
Keywords: Andrology, Exercise, Infertility, Men’s health, Sports
INTRODUCTION
According to the World Health Organization’s criteria, couples are considered infertile if they are unable to conceive after a year of sexual activity [1]. Up to 50% of cases of infertility in couples involve a male element [2]. Male factor infertility (MFI) has been linked to certain modifiable risk factors, including nutrition, obesity, smoking, alcoholism, and lack of physical activity (PA) [3,4]. Exercise (EX) has a variety of documented health advantages. The risks of cardiovascular illnesses are reduced by moderate EX, which also helps prevent non-communicable chronic diseases [5]. Sedentary behavior may contribute to the rise in the incidence of infertility, according to previous studies [6]. An increasing body of research indicates men who lead sedentary lifestyles are more likely to experience late-onset male hypogonadism, which is brought on by low testosterone (T) levels, decreased libido, erectile dysfunction, and reduced sperm viability [7]. Moreover, physically active men have been shown to have a larger proportion of motile spermatozoa compared to sedentary men [8]. Additionally, it has been demonstrated that moderate EX can slow down age-related inflammatory processes [9] and DNA damage in sperm [10]. However, more extreme levels of PA have been associated with impaired semen quality [11,12]. The aim of the current review is to investigate the association between PA and male reproductive pathophysiology, semen parameters, and pregnancy rates.
PRECLINICAL STUDIES
Several studies have investigated the association between physical EX and fertility in animal models. A recent paper assessed role of swimming on the male reproductive system in isoproterenol-treated rats (used to induce testicular oxidative damage). The deleterious effects of elevated oxidative stress were successfully reduced by moderate-intensity swimming EX [13]. In contrast, Manna et al [14] observed significant swimming EX-induced oxidative stress and reproductive dysfunction in rats (experimental group: 3 hours swimming per day, 5 days a week for 4 weeks). In the experimental group, significant decreases were noted in seminiferous tubules diameter; plasma levels of T, preleptotine spermatocytes, mid-pachytene spermatocytes, and Stage 7 spermatids. Similarly, a significant decrease in epididymal sperm, testicular androgenic enzyme activities, plasma and intra-testicular T, and different types of germ cells produced during the seminiferous epithelium cycle were observed as a result of forced exhaustive swimming for eight consecutive weeks [15]. Additionally, testicular spermatogenic cells showed an increase in DNA damage induced by swimming. Importantly, alpha-lipoic acid and N-acetylcysteine supplementation protected against the spermatogenic and steroidogenic problems induced by swimming suggesting an oxidative species related mechanism.
Other studies highlighted the complex interplay between EX and fertility is influenced by diet and body habitus. The impact of various EX loads on the ability of obese male mice to reproduce, and the underlying processes were examined. A decrease in the mRNA expression of antioxidant enzymes, an increase in free radical products, an upregulation of nuclear factor kappa B and proinflammatory cytokines, an inhibition of the mRNA and protein expression of T synthases, a decrease in serum T levels and sperm quality, and an increase in sperm apoptosis were all seen in the obese group mice without an EX regimen. Both high-load and moderate-load EX decreased body fat, but only moderate-load EX effectively reduced the oxidative stress brought on by obesity, regulated the expression of nuclear factor kappa B and proinflammatory cytokines, and reversed the decline in T synthase mRNA and protein expression, serum T level, and sperm quality [16]. In contrast, the high-load EX group did not show improvements in semen quality again implying that higher EX intensity may be harmful.
Next, studies have also examined EX in the context of calorie restriction or abundance. In one study, moderate EX improved testicular function while dietary restriction impaired testicular function. Interestingly, it was discovered that when food deprivation was combined with moderate activity no change from baseline was measured. Higher T/17-estradiol ratio in the testis was connected with increased spermatogenic activity after light EX [17]. In a study by Ibáñez et al [18], male Wistar rats that were 30 days old were fed a high fat diet (35% fat) for 30 days, followed by a 40-day recovery phase on a normal fat diet. Animals began a low frequency, moderate EX program that lasted 30 days at 70 days of age. Animals under control remained sedentary. Moderate EX reversed the effects of the high fat diet, which increased the quantity of aberrant seminiferous tubule and decreased the height and width of seminiferous epithelium. Moderate and low-intensity PA can reverse adipose tissue deposition and the changes to the reproductive system.
PHYSICAL ACTIVITY EFFECTS ON SEMEN PARAMETERS
Many studies exist that examine lifestyle factors and semen quality. The literature on PA and semen quality has produced heterogeneous findings. A recent randomized controlled trial investigated indicators of male reproductive function in infertile individuals after 24 weeks of high-intensity interval training (HIIT). Infertile men (n=441) were randomized to EX or non-exercise (NON-EX) group. Proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α) and indicators of oxidative stress (reactive oxygen species [ROS], malondialdehyde [MDA], and 8-isoprostane) were lowered in seminal levels as a result of the intervention. Improvements in sperm DNA integrity, sperm metrics, and conception rates were identified [19]. Several observational studies confirmed these observations. Foucaut et al [20] carried out a multicentered case-control observational study. Four fertility clinics reported data from 79 infertile and 72 fertile men. Physical inactivity (odds ratio [OR], 2.20) and having more body fat (OR, 2.83) were associated with male infertility. Similarly, another paper investigated different type of PA in relation to seminal parameters using data from the Environment and Reproductive Health study, a prospective cohort study enrolling subfertile couples. Men in the top quartile of moderate-to-vigorous EX had sperm concentrations that were 43% higher than men in the bottom quartile (ptrend=0.04). When specific activities were examined, men in the highest categories of outdoor EX (>1.5 h/wk) and weightlifting (>2 h/wk) had sperm concentrations that were 42% and 25% higher, respectively (ptrend=0.04 and 0.02). In contrast, males who reported cycling over than 1.5 hours per week had 34% (95% confidence interval [CI], 4; 55%) lower sperm concentrations than those who did not [21]. In another study, Wise et al [11] examined data from 2,261 men attending three in vitro fertilization (IVF) clinics and reported no association between semen quality and EX. However, when examining specific types of EX, biking more than 5 h/wk was linked to lower sperm concentration (OR, 1.92) and low total motile sperm (OR, 2.05) when compared to no regular activity. Another study found that the specific type of EX matters. Here no association between various levels of general PA and the semen characteristics was observed in 454 men from infertile couples. Moreover, no correlation between running, cycling, or racquet sports and semen quality was identified. However, weightlifters had considerably lower sperm concentration (linear coefficient=-24.80) and lower total sperm counts (linear coefficient=-70.87) when compared to men that did not exercised regularly [22].
Other studies recruited men from the general population rather than infertile subjects. A recent paper investigated 207 sperm donation candidates who had a semen analysis and completed a standardized questionnaire which included data on the previous week’s PA, which was measured in metabolic equivalents (METs)-min/wk. Importantly, PA was not associated with semen parameters [23]. In contrast, a study from China investigated EX patterns and semen parameters in 746 men. Men in the highest quartiles of total and moderate-to-vigorous METs had increased total motility by 15.2% and 16.4% and progressive motility by 16.1% and 17.3%; respectively, compared to men in the lowest quartiles [24]. Finally, male road cyclists were studied to see how sixteen weeks of intense cycling affected their levels of serum ROS, MDA, superoxide dismutase (SOD), catalase, and total antioxidant capacity (TAC) [12]. The investigators reported that cycling affected also coventional semen parameters, such as semen volume, sperm concentrations, sperm motility, and number of spermatozoa, which were significantly lower than baseline (p<0.008). The levels of seminal ROS and MDA increased (p<0.008) and remained high after 30 days of recovery. The levels of seminal SOD, catalase, and TAC decreased (p<0.008) and remained low after 30 days of recovery (p<0.008). Intensive cycling training for 16 weeks had negative effects on spermatozoa, which may have an impact on male cyclists’ sperm health indices.
While the data is mixed, several studies in men suggest PA is associated with improved semen quality. However, more intense/strenuous activity may negatively affect semen parameters. In addition, there is a signal from several studies that intense cycling may negatively impact semen quality. While the mechanisms for the associations are not known, systemic inflammation (as may occur with intense EX) or scrotal heat (which may occur with prolonged cycling) have been hypothesized.
PHYSICAL ACTIVITY AND PREGNANCY RATES
While the association between PA and semen quality has been well studied, the association between PA and pregnancy rates is less well defined. However, several studies have examined the correlation between increased PA and reproductive outcomes.
A recently published prospective cohort study examined the relationship between PA and pregnancies among couples using assisted reproductive technology (ART). Of the 64 infertile couples, 31 couples underwent ART procedures, while 17 couples had an unassisted pregnancy prior to ART. Less time was reported for PA (average-73 min/wk per pair) for couples needing invasive ART treatments compared to couples who experienced spontaneous pregnancies after enrolling in the research (p=0.001). Importantly, the study did not observe a relationship between time spent engaging in PA and the likelihood of becoming pregnant [25]. In 2017, Maleki and Tartiban [26] conducted a randomized control trial to determine whether a 24-week high-intensity EX program helped infertile male patients’ reproductive health. Infertile males (n=433) were assigned to two groups: those who followed a structured EX routine (n=218) and those who did not (n=215). The pregnancy rates were assessed at baseline, at the end of weeks 12 and 24, as well as at days 7 and 30 of the recovery period. Overall, 16.5% (31/188) couples became pregnant in the EX group (OR, 6.0; 95% CI, 2.5–14.4). However, seven of the patients’ spouses (or 22.6%) miscarried. The estimated live birth rate for the EX group was 77.4% (OR, 3.3; 95% CI, 0.6–17.7).
It has been proposed that high DNA fragmentation in sperm may have detrimental effects on fertilization, embryo development, implantation, and pregnancy. Indeed, following EX intervention; changes in semen parameters, DNA integrity, inflammatory mediators, and redox state correlated with pregnancy success in infertile couples [26]. In a 2017 randomized experiment, the effects of a 24-week combined aerobic and resistance EX program on seminal markers of oxidative stress and inflammation as well as indicators of male reproductive function and reproductive success in infertile individuals were investigated. From a total of 1,296 screen infertile individuals (aged 25–40), 556 were randomized to the EX (n=278) and NON-EX (n=278) groups. Semen samples were collected before, at 12, 24, and 7 and 30 days after the intervention. Higher pregnancy rates were linked with training-induced changes in inflammation and oxidative stress state. These findings demonstrate the beneficial effects of combined aerobic and strength EX training in MFI [27]. Another 2017 study conducted by Maleki and Tartiban [27] investigated pregnancy rates after 24 weeks of moderate aerobic EX in infertile men. 1026 sedentary males (aged 25 to 40) who were seeking treatment at an infertility clinic and had a history of infertility for more than a year were examined, and 419 were randomly assigned to either the EX (n=210) or NON-EX (n=209) groups. Among couples part of the EX cohort, 70.5% (139 out of 197) achieved pregnancies (OR, 80.0; 95% CI, 32.5–646.2), of which 31 (22.3%) were unplanned. However, 4 of the patients’ spouses (2.9%) miscarried. The estimated live birth rate for the EX assigned group was 91.4% (OR, 197.0; 95% CI, 5.9–2149.6). In comparison to the non EX groups, moderate aerobic EX training was associated with an increased pregnancy rate in patients who were asthenozoospermic (OR, 186.7; 95% CI, 27.8–1255.2), asthenoteratozoospermic (OR, 59.8; 95% CI, 10.2–351.4), oligospermic (OR, 108.7; 95% CI, 17.7–667.3), oligoasthenozoospermic [28]. Similarly, another study assessed the effects of 24 weeks of resistance EX on pregnancy rates in infertile individuals. A total of 430 sedentary infertile patients (aged 25–40) were randomly assigned to the EX (n=216) and NON-EX (n=214). In this cohort of infertile patients, EX intervention significantly reduced inflammation as measured by seminal cytokines (IL-1, IL-6, IL-8, and TNF-α), oxidative stress (SOD, MDA, and 8-isoprostane), and enhanced antioxidants (SOD and catalase). Importantly, these changes correlate with significantly improved semen parameters, sperm DNA integrity, and pregnancy rate. Partner pregnancies increased by 36.2% (72 out of 199) (OR, 17.7; 95% CI, 7.7–40.7) in the EX group. The estimated live birth rate for the EX cohort was 81.9% (OR, 16.2; 95% CI, 2.4–108) as a result [29]. In 2020, Maleki and Tartibian [19] also looked at responses to 24 weeks of HIIT on indicators of male reproductive function. The EX (n=221) or NON-EX (n=220) groups were randomly assigned to infertile males (n=441). For 24 weeks, patients in the EX group underwent interval training with a 1:1 work-to-rest ratio, three times per week, at 75% to 95% of maximum oxygen intake. Rates of pregnancy were measured during baseline, week 12, week 24, and seven and thirty days later during the recovery period. 12.8% (26 out of 203) of all partner pregnancies were in the HITT cohort (OR, 4.9; 95% CI, 2.0–11.7). The overall computed live birth rate during the duration of the trial was 73.1% (OR, 9.5; 95% CI, 1.3–69.9). Further analysis revealed that the HIIT intervention resulted in increased partner pregnancies in the categories of asthenozoospermic (OR, 4.3; 95% CI, 1.0–19.0), asthenoteratozoospermic (OR, 5.3; 95% CI, 0.9–33.1), oligospermic (OR, 6.3; 95% CI, 1.0–38.8), oligoasthenozoospermic (OR, 0.9; 95% CI, 1.0–2.9). This study showed the benefits of 24 weeks of HIIT on reproductive outcomes in infertile men [19].
As previously mentioned, a recent study observed that sperm donors who engage in PA have higher-quality sperm. The paper investigated a total of 207 sperm donation candidates from a rather narrow geographic area. Moreover, semen samples from 43 approved donors were utilized in IVF and donor-assisted insemination (AID). The rates of fertilization (FR) and pregnancies (PR) were investigated. Four groups of donors were created: low, moderate, high, and extremely high PA. In the various PA groups, the AID PR was comparable. However, in IVF, both the high PA group and the very high PA group had considerably higher mean donor FRs. In IVF/intracytoplasmic sperm injection, the FRs rates were 62%, 69%, and 76% in the moderate, high, and very high PA groups. Similarly, when the mean per donor FR was calculated in each PA group, the very high PA group (72.1%; 264/366) and the high PA group (69.0%; 787/1,140) both had significantly higher FRs than the moderate PA group (63.5%; 390/614) [23].
Next, Gaskins et al [21] focused on paternal PA and its relationship with reproductive treatment outcomes. Data from 231 men seeking infertility treatment who participated in weightlifting and outdoor activities was used to investigate the relationship between sedentary or strenuous activity duration and clinical pregnancy rates or live birth rates after intrauterine insemination or ART. In this study, no evidence indicated that increasing PA led to better reproductive outcomes when used in conjunction with infertility. Finally, a recent network meta-analysis evaluated the effectiveness of EX training on MFI and observed that the top-ranking interventions for pregnancy rate were combined aerobic and resistance training (relative risk [RR], 27.81), moderate-intensity continuous training (RR, 26.67), resistance training (RR, 12.54), high-intensity continuous training (RR, 5.55), and HIIT (RR, 4.63) [30].
CONCLUSIONS
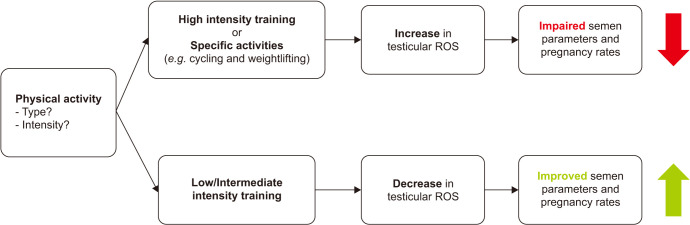
The current literature suggests an association between PA and male infertility as assessed by semen quality and limited pregnancy/ART outcomes. Pre-clinical studies demonstrated mixed benefits from EX, with diet being an important consideration. Some forms of PA showed an improvement in pregnancy rates, while others did not consistently improve semen quality. Data also suggests that more intense EX and certain types of EX may impair male fertility (Fig. 1). Given the limited number of randomized trials, future research is required to examine the relationship between specific forms of EX and semen parameters along with reproductive outcomes.
Fig. 1. The relationship between physical activity and male fertility. ROS: reactive oxygen species.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Data curation: FB, SB.
- Methodology: FB.
- Supervision: ME.
- Writing–original draft: FB, SB.
References
- 1.World Health Organization (WHO) Infertility [Internet] Geneva: WHO; 2020. [cited 2022 Sep 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/infertility. [Google Scholar]
- 2.Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603–620. doi: 10.1016/j.eururo.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J Androl. 2019;21:478–485. doi: 10.4103/aja.aja_110_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fallara G, Cazzaniga W, Boeri L, Capogrosso P, Candela L, Pozzi E, et al. Male factor infertility trends throughout the last 10 years: report from a tertiary-referral academic andrology centre. Andrology. 2021;9:610–617. doi: 10.1111/andr.12947. [DOI] [PubMed] [Google Scholar]
- 5.Lin YY, Lee SD. Cardiovascular benefits of exercise training in postmenopausal hypertension. Int J Mol Sci. 2018;19:2523. doi: 10.3390/ijms19092523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammoud AO, Meikle AW, Reis LO, Gibson M, Peterson CM, Carrell DT. Obesity and male infertility: a practical approach. Semin Reprod Med. 2012;30:486–495. doi: 10.1055/s-0032-1328877. [DOI] [PubMed] [Google Scholar]
- 7.Hammiche F, Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27:2365–2372. doi: 10.1093/humrep/des177. [DOI] [PubMed] [Google Scholar]
- 8.Vaamonde D, Da Silva-Grigoletto ME, García-Manso JM, Barrera N, Vaamonde-Lemos R. Physically active men show better semen parameters and hormone values than sedentary men. Eur J Appl Physiol. 2012;112:3267–3273. doi: 10.1007/s00421-011-2304-6. [DOI] [PubMed] [Google Scholar]
- 9.Chigurupati S, Son TG, Hyun DH, Lathia JD, Mughal MR, Savell J, et al. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199:333–341. doi: 10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Bian Y, Sun Y, Li L, Wang L, Zhao C, et al. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp Gerontol. 2013;48:869–880. doi: 10.1016/j.exger.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 11.Wise LA, Cramer DW, Hornstein MD, Ashby RK, Missmer SA. Physical activity and semen quality among men attending an infertility clinic. Fertil Steril. 2011;95:1025–1030. doi: 10.1016/j.fertnstert.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maleki BH, Tartibian B, Vaamonde D. The effects of 16 weeks of intensive cycling training on seminal oxidants and antioxidants in male road cyclists. Clin J Sport Med. 2014;24:302–307. doi: 10.1097/JSM.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 13.Osváth P, Szűcs M, Börzsei D, Szabó R, Lesi ZN, Turcsán Z, et al. Andrological aspects of exercise: moderate swimming protects against isoproterenol induced testis and semen abnormalities in rats. Antioxidants (Basel) 2022;11:436. doi: 10.3390/antiox11030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manna I, Jana K, Samanta PK. Intensive swimming exercise-induced oxidative stress and reproductive dysfunction in male wistar rats: protective role of alpha-tocopherol succinate. Can J Appl Physiol. 2004;29:172–185. doi: 10.1139/h04-013. [DOI] [PubMed] [Google Scholar]
- 15.Jana K, Dutta A, Chakraborty P, Manna I, Firdaus SB, Bandyopadhyay D, et al. Alpha-lipoic acid and N-acetylcysteine protects intensive swimming exercise-mediated germ-cell depletion, pro-oxidant generation, and alteration of steroidogenesis in rat testis. Mol Reprod Dev. 2014;81:833–850. doi: 10.1002/mrd.22354. [DOI] [PubMed] [Google Scholar]
- 16.Yi X, Tang D, Cao S, Li T, Gao H, Ma T, et al. Effect of different exercise loads on testicular oxidative stress and reproductive function in obese male mice. Oxid Med Cell Longev. 2020;2020:3071658. doi: 10.1155/2020/3071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santillo A, Giacco A, Falvo S, Di Giacomo Russo F, Senese R, Di Fiore MM, et al. Mild exercise rescues steroidogenesis and spermatogenesis in rats submitted to food withdrawal. Front Endocrinol (Lausanne) 2020;11:302. doi: 10.3389/fendo.2020.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibáñez CA, Erthal RP, Ogo FM, Peres MNC, Vieira HR, Conejo C, et al. A high fat diet during adolescence in male rats negatively programs reproductive and metabolic function which is partially ameliorated by exercise. Front Physiol. 2017;8:807. doi: 10.3389/fphys.2017.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajizadeh Maleki B, Tartibian B. High-intensity interval training modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: a randomized controlled trial. Cytokine. 2020;125:154861. doi: 10.1016/j.cyto.2019.154861. [DOI] [PubMed] [Google Scholar]
- 20.Foucaut AM, Faure C, Julia C, Czernichow S, Levy R, Dupont C ALIFERT Collaborative Group. Sedentary behavior, physical inactivity and body composition in relation to idiopathic infertility among men and women. PLoS One. 2019;14:e0210770. doi: 10.1371/journal.pone.0210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaskins AJ, Afeiche MC, Hauser R, Williams PL, Gillman MW, Tanrikut C, et al. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Hum Reprod. 2014;29:2575–2582. doi: 10.1093/humrep/deu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibañez-Perez J, Santos-Zorrozua B, Lopez-Lopez E, Irazusta J, Prieto B, Aparicio V, et al. Impact of physical activity on semen quality among men from infertile couples. Eur J Obstet Gynecol Reprod Biol. 2019;237:170–174. doi: 10.1016/j.ejogrb.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Matorras R, Navarro A, Ramos D, Malaina I, Irazusta J, Vendrell A, et al. Physical activity and sperm quality: influence in sperm donors. Reprod Biol Endocrinol. 2022;20:83. doi: 10.1186/s12958-022-00946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun B, Messerlian C, Sun ZH, Duan P, Chen HG, Chen YJ, et al. Physical activity and sedentary time in relation to semen quality in healthy men screened as potential sperm donors. Hum Reprod. 2019;34:2330–2339. doi: 10.1093/humrep/dez226. [DOI] [PubMed] [Google Scholar]
- 25.Läänelaid S, Ortega FB, Kunovac Kallak T, Joelsson L, Ruiz JR, Hreinsson J, et al. Physical and sedentary activities in association with reproductive outcomes among couples seeking infertility treatment: a prospective cohort study. Int J Environ Res Public Health. 2021;18:2718. doi: 10.3390/ijerph18052718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maleki BH, Tartibian B. High-intensity exercise training for improving reproductive function in infertile patients: a randomized controlled trial. J Obstet Gynaecol Can. 2017;39:545–558. doi: 10.1016/j.jogc.2017.03.097. [DOI] [PubMed] [Google Scholar]
- 27.Hajizadeh Maleki B, Tartibian B. Combined aerobic and resistance exercise training for improving reproductive function in infertile men: a randomized controlled trial. Appl Physiol Nutr Metab. 2017;42:1293–1306. doi: 10.1139/apnm-2017-0249. [DOI] [PubMed] [Google Scholar]
- 28.Hajizadeh Maleki B, Tartibian B. Moderate aerobic exercise training for improving reproductive function in infertile patients: a randomized controlled trial. Cytokine. 2017;92:55–67. doi: 10.1016/j.cyto.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Hajizadeh Maleki B, Tartibian B. Resistance exercise modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: a RCT. Life Sci. 2018;203:150–160. doi: 10.1016/j.lfs.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 30.Hajizadeh Maleki B, Tartibian B, Chehrazi M. Effectiveness of exercise training on male factor infertility: a systematic review and network meta-analysis. Sports Health. 2022;14:508–517. doi: 10.1177/19417381211055399. [DOI] [PMC free article] [PubMed] [Google Scholar]