Abstract
Background
Autonomic dysfunction including postural orthostatic tachycardia syndrome (POTS) has been reported in individuals with post-acute sequelae of Covid-19 (PASC). However, the degree of dysautonomia in PASC has not been compared to those with POTS and healthy controls.
Methods
All participants were prospectively enrolled between 5th August 2021 and 31st October 2022. Autonomic testing included beat-to-beat hemodynamic monitoring to assess respiratory sinus arrhythmia, Valsalva ratio and orthostatic changes during a 10-minute active standing test as well as Sudomotor assessment. The Composite Autonomic Symptom Score (COMPASS-31) was used to assess symptoms and the Euroquol 5-Dimension survey (EQ-5D-5L) was used to assess health-related quality of life (HrQoL) measures.
Results
A total of 99 participants (n=33 PASC, n=33 POTS and n=33 healthy controls; median age 32 [18], 85.9% females) were included. Compared to healthy controls, the PASC and POTS cohorts demonstrated significantly reduced respiratory sinus arrhythmia (p<0.001), greater heart rate increase during 10-minute active standing test (p<0.001), greater burden of autonomic dysfunction evidenced by higher COMPASS-31 scores across all subdomains (all p<0.001) and poor HrQoL across all EQ-5D-5L domains (all p<0.001), lower median EQ-VAS (p<0.001) and lower utility scores (p<0.001). The majority (79%) of those with PASC met the internationally established criteria for POTS.
Conclusion
The prevalence of autonomic symptomology or POTS was high in those with PASC, leading to poor HrQoL and high health disutility. Autonomic testing should be routinely undertaken in those with PASC to aid diagnosis and direct appropriate management to improve health outcomes.
Trial registration
ANZCTR 12621000476831
Keywords: Post-acute sequelae of COVID-19, Long Covid, Postural orthostatic tachycardia syndrome, dysautonomia, autonomic function, orthostatic intolerance
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19), has caused over 6.9 million deaths from over 750 million infections worldwide. Acutely, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been associated with multi-system manifestations of neurological, cardiac, respiratory and immune symptomology.1., 2, 3 While most recover from acute COVID-19 infection, up to 10% of individuals seem to suffer a constellation of symptoms that persist beyond 12 weeks, including fatigue, dyspnea, chest pain, palpitations, headaches, nausea, abdominal pain, diarrhea, brain fog, and orthostatic intolerance.4 Several nomenclatures exist to describe this phenomenon including long-haul COVID-19, long-COVID and post-acute sequelae of COVID-19 (PASC). Several studies have reported a range of autonomic dysfunction amongst the PASC population with symptoms suggestive of postural orthostatic tachycardia syndrome (POTS).5 , 6
POTS is recognized as an autonomic disorder, identified by postural induced tachycardia in the absence of orthostatic hypotension.7, 8, 9 It is a heterogenous disorder, and its pathogenesis remains poorly understood. It remains unclear whether PASC related POTS is specific to the SARS-CoV-2 virus or merely representative of the frequently reported post-viral or infection related POTS.10 , 11 To date, prospective comparative studies between those with PASC and those with POTS (non PASC-related) and healthy populations have not been undertaken. In this study, we aimed to delineate the differences in autonomic and health-related quality of life parameters between these patient groups.
Methods
Consecutive PASC and POTS patients aged ≥18 years, who presented to a specialist cardiology clinic were invited to participate in this prospective comparative study. Additionally, PASC participants were simultaneously recruited through a 'long-COVID' social media support group. Healthy adults were recruited through flyers posted at our institution. All study participants were recruited between August 2021 and October 2022 and provided written informed consent. This study has institutional Human Research and Ethics Committee approval (approval number H-2021-053) and is registered with the Australian New Zealand Clinical Trials Registry (ACTRN: 12621000476831). All participants’ demographics, quality of life and health-related data were collected via electronic surveys and stored in a secure Research Electronic Data Capture (REDCap) database.
PASC was defined as persistent, unexplained symptomology with a chronicity of ≥3 months after infection with SARS-CoV-2 virus. As per the World Health Organization's Delphi consensus, those with both laboratory and physician-confirmed SARS-CoV-2 infection were included.12 All POTS participants were physician diagnosed and fulfilled the international consensus definition.13 Healthy controls were defined as absence of frequent syncope, significant neurological, cardiac, endocrine or immune disorders, alcohol or drug dependence or use of daily medications aside from oral contraceptive pill. COVID-19 infection within 90 days of study testing was an exclusion criterion.
Autonomic testing
All studies were undertaken in the morning and at post-void, fasted state. Participants were instructed to limit alcohol, nicotine and caffeine intake, and refrain from excessive fluid and salt intake for 48 hours before testing. Autonomic measurements were assessed using Finapres NOVA (Finapres Medical Systems B.V., Enschede, The Netherlands) for non-invasive continuous beat-to-beat hemodynamic monitoring. Continuous EKG tracing was recorded throughout the testing.
Participants were instructed to slowly inhale for 5 seconds and exhale over 5 seconds for a total of six cycles to determine respiratory sinus arrhythmia, which was taken as the mean difference in absolute peak to trough heart rate on inspiration and expiration during deep breathing. For the Valsalva maneuver, participants were instructed to maintain forced expiration through a mouthpiece against resistance (40mmHg) for 15 seconds. The Valsalva ratio was taken as the maximal heart rate during expiration divided by the lowest heart rate within 30 seconds. After 10-minutes of supine rest, participants were asked to move from recumbence to standing and were instructed to keep still for 10 minutes during active standing. We used SUDOSCAN (Impeto Medical, Issy-les-Moulineaux, France), a non-invasive test to detect dysfunction in small nerve fibers that innervate the sweat glands in both hands and feet.14
Symptom, quality of life and joint assessments
The Composite Autonomic Symptom Score (COMPASS-31) was used to assess autonomic symptomology across the six domains of orthostatic intolerance, secretomotor, vasomotor, gastrointestinal, bladder and pupillary motor function.15 To increase relevance, the wording of the COMPASS-31 questions were altered for the PASC cohort from "In the last year.." to "since your COVID-19 infection". The Fatigue Severity Scale-9 (FSS) was utilized to assess self-reported fatigue with maximum fatigue equivalent to a score of 63.16 Health-related quality of life (HrQoL) was assessed using the Euroquol 5-dimensional instrument (EQ-5D-5L). This instrument assesses health status across five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, using a 5-response Likert scale. Each domain of the survey has five responses ranging from no problems through to extreme problems.17 Additionally, a visual analogue scale (EQ-VAS) was used to elicit a global health scale from 0 to 100, with 100 equal to a state of full health. The Devlin et al UK dataset was utilized to apply a scoring algorithm to the EQ-5D-5L data to calculate a single index utility score; anchored on a scale from 0-1, where 0 equals a state commensurate with death and 1 with full health.17 , 18 Generalized joint hypermobility was assessed using the 5-point historical hypermobility scale. A score ≥2 was deemed to show generalized joint hypermobility as per previously validated studies.19
Statistical Analysis
All data were analyzed using SPSS statistics (version 28.0, IBM Inc, Armonk, NY, USA). Continuous data were expressed as median and interquartile range (IQR) or mean and standard deviation according to distribution. Frequencies and percentages were used for categorical variables. For normally distributed data, a one-way analysis of variance (ANOVA) was conducted for the three groups with Tukey adjusted pairwise post-hoc comparisons. Kruskal-Wallis was implemented for non-parametric distributions with Bonferroni adjustment for pairwise comparisons if the group effect was significant. For the repeated measures data (heart rate, systolic & diastolic blood pressure) a mixed effects model was created with patient ID as a random effect (to account for repeated measures) and time and group as main effects. If a significant time*group interaction was present, pairwise post-hoc comparisons with Tukey adjustment were conducted to reveal where differences were observed between groups over time. Pearson's chi-squared test was used to compare categorical data across the three groups. All tests were 2-sided and statistical significance was set at p<0.05.
Results
A total of 99 individuals completed the study (n=33 PASC, n=33 POTS & n=33 healthy controls) out of 145 recruited participants. There were n=46 withdrawals (n=35 PASC, n=10 POTS and n=1 healthy control) due to inability to completely withhold pharmacotherapy for testing (n=36), onset of new COVID-19 infection (n=5) and failure to attend for testing (n=5). The median age of all participants was 32 years old (range 18-62) and majority of participants were female (86%) and Caucasian (94%). Of the 33 PASC participants, 18 were recruited through online long-COVID support group while the remaining were consecutive patients with long-COVID referred to our specialist center. Participants with PASC were older but with shorter duration of symptoms as compared to those with POTS (Table 1 ). Co-morbid conditions of migraine, hay fever/atopy, endometriosis and asthma were more frequently seen in the POTS than PASC cohort. Additionally, those with POTS and PASC were more likely to suffer from generalized joint hypermobility than controls. Both POTS and PASC participants were less likely to be in full time employment than controls (9.1%, 27.3% and 19.2 % respectively: p<0.001). A high proportion of PASC participants (94%) reported reduced work or education attendance in the month before study testing while 67% were unable to work at all since infection.
Table 1.
Participant characteristics
| PASC (n=33) | POTS (n=33) | Controls (n=33) | P value | |
|---|---|---|---|---|
| Age, years | 37 (15) | 28 (14) | 28 (23) | 0.004 |
| Body mass index, kg/m2 | 24 (6.78) | 27 (8) | 24 (4) | 0.111 |
| Female, n (%) | 27 (81.8%) | 31 (93.9%) | 27 (81.8%) | 0.264 |
| Caucasians, n (%) | 32 (97.0%) | 32 (97.0%) | 29 (87.9%) | 0.116 |
| Tertiary educated, n (%) | 29 (87.8%) | 26 (78.8%) | 28 (84.8%) | 0.524 |
| Symptom duration, days | 175 (586) | 1095 (2738) | - | <0.001 |
| Comorbidities, n (%) | ||||
| Migraine | 12 (42.9) | 16 (48.5) | - | <0.001 |
| Hay fever/Atopy | 6 (18.2) | 15 (45.5) | - | <0.001 |
| Endometriosis | 6 (18.2) | 12 (36.4) | - | 0.003 |
| Asthma | 5 (15.2) | 8 (24.2) | - | 0.046 |
| Generalized joint hypermobility | 12 (36.4) | 25 (75.8) | 5 (15.2) | <0.001 |
Continuous values expressed as median (interquartile range). Categorical values expressed as n (%).
Onset trigger for autonomic dysfunction in the POTS cohort was post-viral (n=15), trauma (n=4) and others (n=3), while 11 participants reported gradual onset with non-identifiable trigger. At study testing, the PASC population were 326±281 days from their acute COVID-19 infection. One participant was hospitalized during acute infection but did not require oxygen or ICU admission. Headache, fever, and myalgia were the most common acute symptoms amongst those with PASC, while tachycardia, orthostatic dizziness and palpitations were the most common chronic symptoms with increasing prevalence at the time of study testing. Other common persisting symptoms are detailed in supplemental Table. Specifically, onset of orthostatic symptomology in the PASC population occurred at a median of 8 days (IQR 2.5-14.5) after acute infection with 36% reporting emergency department presentation for their non-acute symptoms.
Autonomic testing
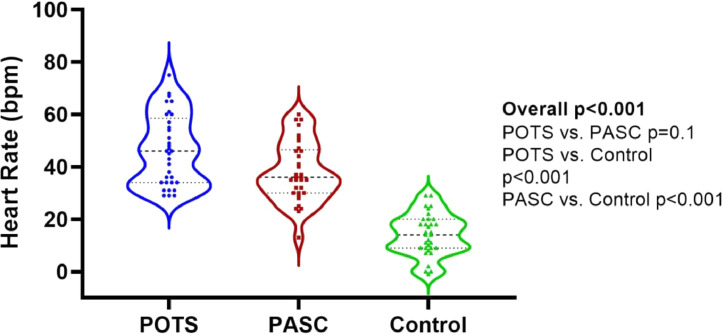
Heart rate response to deep breathing was significantly different between the three groups with lower variation in the POTS and PASC participants (Table 2 ). Additionally, this was lower in the PASC versus POTS participants (p=0.001). However, no significant differences were noted between groups in the Valsalva ratio (Table 2). Sudomotor function was different between groups in the left hand only, which were significantly lower in the PASC cohort as compared to the POTS cohort. The heart rate response during the standing test was different between groups according to time (Figure 1A ). At baseline, individuals with POTS exhibited significantly higher resting heart rate as compared to those with PASC and healthy controls (79±12 vs. 72±13 vs. 68±9 bpm, p=0.003) with no significance difference between the PASC and control groups (post-hoc p=0.054). Delta heart rate during the entire 10 minutes of standing was significantly higher in the PASC and POTS cohorts as compared to controls (Table 2 and Figure 1B )
Table 2.
Autonomic function testing
| PASC (n=33) | POTS (n=33) | Controls (n=33) | P-value | |
|---|---|---|---|---|
| Respiratory sinus arrhythmia, bpm | 9.8 (7.0-14.5)#,⁎⁎ | 14.0 (12.1-19.2)# | 17.4 (12.0-19.5) | <0.001 |
| Valsalva ratio | 1.8 (1.5-2.3) | 1.9 (1.5-2.3) | 1.7 (1.5-2.1) | 0.23 |
| Sudomotor Function, % | ||||
| Left foot | 77 (69.0-84.0) | 81 (73-84.5) | 77(70-82) | 0.24 |
| Right foot | 77 (71.5-83.5) | 82 (77.5-86.0) | 80 (72.5-83.0) | 0.15 |
| Left hand | 66 (57.0-79.0)⁎⁎ | 78 (70-83.5) | 72 (66.5-80.0) | 0.04 |
| Right hand | 70 (59-80.5) | 78 (68-83.5) | 72 (63.5-80.0) | 0.09 |
| Delta heart rate over 10-mins standing test, bpm | 36 (30-47)# | 46 (34-59)# | 15 (9-20) | <0.001 |
All values expressed as median and interquartile range.
denotes post-hoc p<0.05 versus Controls;
denotes post-hoc p<0.05 versus POTS
Figure 1A.
Mean heart rate during 10-minute active standing.
Note: error bars denote standard error of the mean.
Figure 1B.
Median delta heart rate during 10-minute standing test
Systolic blood pressure (Figure 2A ) did not differ between the groups (group p=0.12), nor did it alter differentially over time according to group (group*time p=0.14). Diastolic blood pressure (Figure 2B ) was higher in both POTs and PASC groups compared with controls (post-hoc, both p<0.03 vs. controls) but no difference was seen between the POTS and PASC groups.
Figure 2A.
Mean systolic blood pressure during 10-minute active standing test.
Note: error bars denote standard error of the mean.
Figure 2B.
Mean diastolic blood pressure during 10-minute active standing test.
Note: error bars denote standard error of the mean.
Autonomic symptomatology
Overall, median total COMPASS-31 score was higher in participants with POTS and PASC as compared to controls [51 (IQR 42-62) vs. 38 (IQR 30-49) vs. 4 (IQR 2-16), p<0.001]. Both POTS and PASC participants showed significantly higher symptom scores across all COMPASS-31 subdomains as compared to controls (Figure 3A , all p<0.001). Additionally, the severity of autonomic dysfunction was found to be higher in those with POTS as compared to PASC on post-hoc analysis for the following subdomains: vasomotor, gastrointestinal, bladder and pupillary motor (Figure 3A).
Figure 3A.
Autonomic symptoms as assessed by COMPASS-31
Health-related quality of life and fatigue severity score
Individuals with POTS and PASC experienced significantly higher level of impairment across all sub-domains of the EQ-5D-5L as compared to the control population (all p<0.001, Table 3 ). Median global health rating (EQ-VAS) was significantly lower in the POTS and PASC cohorts as compared to controls (Figure 3B ), with no difference between POTS and PASC cohorts.
Table 3.
Health-related quality of life by EQ-5D-5L
| POTS (n=33) | PASC (n=33) | Controls (n=33) | P-value | |
|---|---|---|---|---|
| Mobility | ||||
| No problems | 13 (40) | 12 (37) | 33 (100) | <0.001 |
| Slight problems | 7 (21) | 9 (27) | 0 | |
| Moderate problems | 9 (27) | 8 (24) | 0 | |
| Severe problems | 4 (12) | 4 (12) | 0 | |
| Unable to mobilize | 0 | 0 | 0 | |
| Self-Care | ||||
| No problems | 17 (52) | 21 (64) | 33 (100) | <0.001 |
| Slight problems | 7 (21) | 11 (33) | 0 | |
| Moderate problems | 7 (16) | 1 (3) | 0 | |
| Severe problems | 1 (3) | 0 | 0 | |
| Unable to care for self | 0 | 0 | 0 | |
| Usual activities | ||||
| No problems | 4 (12) | 0 | 31(94) | <0.001 |
| Slight problems | 9 (27) | 9 (27) | 2 (6) | |
| Moderate problems | 11 (33) | 9 (27) | 0 | |
| Severe problems | 5 (16) | 7 (22) | 0 | |
| Unable to do usual activities | 4 (12) | 8 (24) | 0 | |
| Pain and discomfort | <0.001 | |||
| No problems | 3 (9) | 4 (12) | 25 (76) | |
| Slight problems | 4 (12) | 14 (42) | 8 (24) | |
| Moderate problems | 15 (46) | 13 (40) | 0 | |
| Severe problems | 10 (30) | 2 (6) | 0 | |
| Extreme pain and discomfort | 1 (3) | 0 | 0 | |
| Anxiety and depression | ||||
| No problems | 11 (33) | 8 (24) | 22 (67) | <0.001 |
| Slight problems | 11 (33) | 18 (55) | 9 (27) | |
| Moderate problems | 9 (28) | 4(12) | 1 (3) | |
| Severe problems | 0 | 2 (6) | 1 (3) | |
| Extreme anxiety and depression | 2 (6) | 1 (3) | 0 |
Figure 3B.
Mean global health rating by EQ-VAS
Similarly, the median health utility score was also lower in the POTS and PASC cohorts as compared to the controls [0.60 (IQR 0.44-0.84) vs. 0.67 (IQR 0.49-0.80) vs. 1.0 (IQR 0.92-1.00), p<0.001]. Both POTS and PASC cohorts reported higher fatigue severity score than controls [58 (IQR 50-62) vs. 57 (IQR 50-63) vs. 19 (IQR 15-27), p<0.001]. The PASC population reported their health status to be at 35% (IQR 20-60) of pre-COVID-19 infection level.
Participants with PASC fulfilling POTS diagnosis
Overall, 79% (n=26) of PASC participants met the diagnostic criteria for POTS based on delta heart rate from the active standing test. Five other participants with PASC (15%) narrowly missed the 30 bpm cut-off with sustained delta heart rate between 25-29 bpm despite demonstrating postural symptoms. Two of these participants showed a transient systolic blood pressure drop of greater than 20 mmHg within the first minute of standing which resolved by the third minute.
Discussion
This study presents a detailed prospective evaluation of autonomic symptoms, function and HrQoL in those with PASC with comparison to those with POTS and healthy controls. Our principal findings (Central illustration) are as follows: 1) There is a high incidence of autonomic dysfunction in those with PASC with 79% meeting the international diagnostic criteria for POTS; 2) The PASC population demonstrated significant autonomic symptoms with increased COMPASS-31 scores across all sub-domains; 3) There was significant impairment in HrQoL involving all subdomains to result in significant health disutility; 4) The PASC cohort was older but with similar female predominance as the POTS group.
Central Illustration
Autonomic dysfunction in PASC
Several case series have highlighted the presence of autonomic dysfunction amongst those with PASC.20, 21, 22 Additionally, a recent prospective study of 24 patients with PASC showed a high incidence of autonomic dysfunction in 96% from tilt table testing.6 Another Canadian study found 73% of 70 individuals with PASC to demonstrate cardiovascular autonomic abnormalities on active standing test with 30% of these presenting with POTS phenotype.5 Our data is keeping with the aforementioned studies with a high proportion of individuals with PASC demonstrating autonomic dysfunction or POTS. The variable incidence of POTS amongst PASC cohorts could be due to disparate testing conditions, including timing of testing, continued use of rate controlling medications and shorter standing test duration.6 , 23 , 24 The comparisons with those with POTS and healthy controls in our study provide additional insights into the extent and pattern of autonomic dysfunction following SARS-CoV-2 infection. The extent of their autonomic dysfunction appears lower than those with POTS except for the orthostatic intolerance and secretomotor subdomains.
Health-related quality of life in PASC and POTS
Reduced HrQoL has been repeatedly identified in POTS cohorts.25, 26, 27 While the individuals with PASC in our study also self-report poorer HrQoL scores than the healthy controls, it appears that there is greater impact on 'usual activities' but less restriction to self-care and less severe pain and discomfort than those with POTS. Nevertheless, the HrQol in those with PASC translates into similar health utility scores to those with POTS and at lower level than those living with chronic disease including chronic kidney disease and diabetes.28 , 29 We theorize that the relatively recent decline in their health status, and its associated loss of previous social, economic, and societal engagements would have impacted on self-reported health status in the PASC population. It is also likely that the severity of orthostatic intolerance in individuals with PASC would play a large role in their poor HrQoL status as shown by others.26 , 27 Perhaps clinicians could target orthostatic intolerance management in individuals with PASC to maximize improvement in HrQoL.
COVID-19: a Trigger for Dysautonomia?
Female and Caucasian predominance (5:1) as well as post-viral trigger are commonly seen in POTS.7 , 8 These are also evident in out PASC cohort. Generalized joint hypermobility, and in particular hypermobile Ehlers Danlos syndrome, are associated with POTS. Likewise, self-reported hypermobility was higher in our PASC cohort than controls. These observations suggest that COVID-19 infection may simply be a trigger in some ‘at-risk’ individuals who may be predisposed to dysautonomia. Notably, majority of participants with PASC in this study reported mild acute COVID-19 infection and the lag in the onset of autonomic symptoms by at least two weeks from acute infection lends further support to COVID-19 as a trigger for dysautonomia. While the mechanism underlying the autonomic dysfunction in PASC remains unknown, the plausible explanations include autoimmunity, immune dysregulation and dysfunctional neurological signaling.4
Clinical Implications
Our findings of a high incidence of autonomic dysfunction resulting in poor HrQoL and reduced capability to continue usual activities and vocation in those with PASC is of clinical significance. As highlighted in a statement from the American Autonomic Society regarding long-COVID POTS, there was already a shortage of autonomic physicians familiar with managing POTS pre-COVID-19 with lengthy delays in diagnosis and treatment.30 Thus, the healthcare system is required to adapt to upscale the service provision, to increase research funding, to improve the knowledge and skills of physicians and to provide multidisciplinary integrated care to cope with the increasing PASC caseload.31
Study Limitations
The cross-sectional nature of this study limits the understanding of variance of autonomic function over time. Given the heightened consumer health literacy around autonomic dysfunction on social media sites, it is possible that individuals with autonomic symptomology may have preferentially self-selected into our study causing recruitment bias. It remains unclear if those individuals with POTS and PASC who withdrew from the study due to feeling unwell or unwillingness to withhold medications could skew our dataset.
Conclusions
This is the first comprehensive comparison of autonomic dysfunction between individuals with POTS, PASC and healthy controls. This study provides empirical evidence of the high prevalence of autonomic symptomology or POTS in those with PASC. The significant impairment in HrQOL and reduced health utility in those with PASC demands urgent attention from clinicians, healthcare services and governments to improve care in those affected.
Clinical Significance
-
Ø
Autonomic dysfunction, including postural orthostatic tachycardia syndrome (POTS), is prevalent in those with post-acute sequelae of COVID-19 (PASC).
-
Ø
The presence of autonomic dysfunction or POTS is associated with reduced health-related quality of life and poor heath utility.
-
Ø
The high and increasing caseload of PASC calls for urgent need to upskill clinicians and upscale healthcare service provisions to improve management of dysautonomia in affected individuals.
Declaration of Competing Interest
None
Footnotes
Financial support and conflict of interest disclosure: Ms Seeley is supported by an Australian Government Research Scholarship. Dr Lau reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Abbott Medical, Biotronik, Medtronic and MicroPort CRM. This work was supported by a research grant from Standing up to POTS, USA and an equipment grant from The Australian POTS Foundation. All other authors have no conflicts of interest to declare.
Author contributions:All authors contributed significantly to this work and had access to the data, with role in writing the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2023.06.010.
Appendix. SUPPLEMENTARY DATA
References
- 1.Hernandez-Romieu AC, Carton TW, Saydah S, et al. Prevalence of Select New Symptoms and Conditions among Persons Aged Younger Than 20 Years and 20 Years or Older at 31 to 150 Days after Testing Positive or Negative for SARS-CoV-2. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.47053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganesh R, Vanichkachorn GS, Munipalli B, et al. Postacute Sequelae of SARS-CoV-2 Infection-Lessons Learned From a Coordinated Health System Response. Mayo Clinic proceedings. Innovations, quality & outcomes. 2022;6:311–319. doi: 10.1016/j.mayocpiqo.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younger DS. Post-acute sequelae of SARS-CoV-2 infection (PASC): peripheral, autonomic, and central nervous system features in a child. Neurological Sciences. 2021;42:3959–3963. doi: 10.1007/s10072-021-05345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hira R, Baker JR, Siddiqui T, et al. Objective Hemodynamic Cardiovascular Autonomic Abnormalities in Post-Acute Sequelae of COVID-19. Canadian Journal of Cardiology. 2022 doi: 10.1016/j.cjca.2022.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamal SM, Landers DB, Hollenberg SM, et al. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J Am Coll Cardiol. 2022;79:2325–2330. doi: 10.1016/j.jacc.2022.03.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldon RS, Grubb BP, 2nd, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells R, Spurrier AJ, Linz D, et al. Postural tachycardia syndrome: current perspectives. Vasc Health Risk Manag. 2018;14:1–11. doi: 10.2147/VHRM.S127393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells R, Elliott AD, Mahajan R, et al. Efficacy of therapies for postural tachycardia syndrome: A systematic review and meta-analysis. Mayo Clinic Proceedings. 2018;93:1043–1053. doi: 10.1016/j.mayocp.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS) Lupus. 2015;24:1364–1369. doi: 10.1177/0961203315587566. [DOI] [PubMed] [Google Scholar]
- 11.Schofield JR, Chemali KR. Intravenous Immunoglobulin Therapy in Refractory Autoimmune Dysautonomias: A Retrospective Analysis of 38 Patients. American journal of therapeutics. 2019;26:570–582. doi: 10.1097/MJT.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 12.Organization WH. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. In: Organization WH, ed. 2021.
- 13.Sheldon RS, Grubb BP, II, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinduja A, Moutairou A, Calvet J-H. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiologie Clinique. 2021;51:193–196. doi: 10.1016/j.neucli.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruška B, Pavičić T, Pavlović I, et al. Performance of the COMPASS-31 questionnaire with regard to autonomic nervous system testing results and medication use: a prospective study in a real-life setting. Neurological Sciences. 2018;39:2079–2084. doi: 10.1007/s10072-018-3542-8. [DOI] [PubMed] [Google Scholar]
- 16.Vold JH, Gjestad R, Aas CF, et al. Validation of a three-item Fatigue Severity Scale for patients with substance use disorder: a cohort study from Norway for the period 2016–2020. Health and Quality of Life Outcomes. 2021;19:69. doi: 10.1186/s12955-021-01708-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14:133. doi: 10.1186/s12955-016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ. 2018;27:7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glans M, Humble MB, Elwin M, Bejerot S. Self-rated joint hypermobility: the five-part questionnaire evaluated in a Swedish non-clinical adult population. BMC Musculoskelet Disord. 2020;21:174. doi: 10.1186/s12891-020-3067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman BP, Khoury JA, Blair JE, Grill MF. COVID-19 Dysautonomia. Frontiers in neurology. 2021;12 doi: 10.3389/fneur.2021.624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson M, Ståhlberg M, Runold M, et al. Long-Haul Post-COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience. JACC. Case reports. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. Journal of neurology. 2022;269:587–596. doi: 10.1007/s00415-021-10735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townsend L, Moloney D, Finucane C, et al. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutt E, Vajapey R, Van Iterson EH, et al. Functional capacity and quality of life in the postural tachycardia syndrome: A retrospective cross-sectional study. Ann Med Surg (Lond) 2020;56:72–76. doi: 10.1016/j.amsu.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon J, Kim DY, Byun JI, et al. Orthostatic intolerance symptoms are associated with depression and diminished quality of life in patients with postural tachycardia syndrome. Health Qual Life Outcomes. 2016;14:144. doi: 10.1186/s12955-016-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 28.Zhou T, Guan H, Wang L, Zhang Y, Rui M, Ma A. Health-Related Quality of Life in Patients With Different Diseases Measured With the EQ-5D-5L: A Systematic Review. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.675523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeley MC, Gallagher C, Ong E, et al. Poor health-related quality of life in postural orthostatic tachycardia syndrome in comparison to a sex- and age-matched normative population. Clin Auton Res. Accepted for publication. [DOI] [PMC free article] [PubMed]
- 30.Raj SR, Arnold AC, Barboi A, et al. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res. 2021;31:365–368. doi: 10.1007/s10286-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeley MC, Lau DH. Raising the bar in postural orthostatic tachycardia syndrome research: Evidence and challenges. Auton Neurosci. 2021;233 doi: 10.1016/j.autneu.2021.102790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.