Abstract
Purpose
This study assessed the outcomes of microsurgical testicular sperm extraction (mTESE) and potential preoperative predictors of sperm retrieval (SR) in patients with non-obstructive azoospermia (NOA).
Materials and Methods
Clinical data of 111 NOA patients who underwent mTESE was reviewed retrospectively. Baseline patient characteristics, including age, body mass index (BMI), testicular volumes, and preoperative endocrine levels, such as testosterone (T), follicle-stimulating hormone (FSH), serum-luteinizing hormone (LH), prolactin, sex hormone-binding globulin (SHBG), FSH/LH ratio along with T/LH ratio, were analyzed. After categorizing the patients into two groups based on SR success or failure, logistic regression analysis was performed to identify the preoperative predictors of successful SR.
Results
Sixty-eight patients had successful SR (61.3%), whereas 43 patients (38.7%) showed negative results. Failed SR group had elevated serum FSH and LH levels, whereas successful SR patients had a significantly larger testicular volume (p<0.001). Moreover, the successful group had a higher T/LH ratio (p<0.001). Multivariate logistic analysis showed that the T/LH ratio, serum FSH levels, and bilateral testicular volumes were significantly associated with successful sperm extraction.
Conclusion
In addition to traditional predictors, such as testicular volume and preoperative FSH levels, the T/LH ratio is a potential independent predictor of successful SR in infertile patients with NOA.
Keywords: Azoospermia, biomarker, hormones, sperm retrieval, spermatozoa
Graphical Abstract
INTRODUCTION
Due to infertility, approximately 15%–20% of married couples have difficulty starting a family.1,2 Male infertility factors account for half of all couple infertility cases, either as the sole cause or in combination with female factors.3 Male infertility can be caused by congenital conditions, such as Klinefelter syndrome or cryptorchidism, and external factors, such as medication, exposure to radiation or chemicals, and previous surgical histories.4 The prevalence of azoospermia is estimated to be 1% of all males and 10%–15% of infertile men.5
Azoospermia has two etiologies: obstructive azoospermia (OA) and non-obstructive azoospermia (NOA). Although normal spermatogenesis occurs in OA patients, the semen sample contains no spermatozoa due to anatomical obstruction. In comparison, NOA azoospermia is caused by testicular failure and impaired spermatogenesis.6,7 NOA is responsible for approximately 60% of the etiological causes of azoospermia.8 Microsurgical testicular sperm extraction (mTESE) is the standard retrieval method in patients with NOA due to higher successful sperm retrieval (SR) rates and less testicular damage.9 mTESE has a 45%–63% higher SR rate than conventional TESE or surgical SR methods, according to various studies.10,11 Since the vast majority of NOA patients undergoing mTESE will have successful SR, identifying biological predictors and patients with a high prospect of successful sperm extraction is crucial in fertility preservation counseling.12,13 However, more clinical studies and research are needed to identify reliable predictors of mTESE outcome.14,15
This study aimed to assess the clinical factors and other potential preoperative factors that would predict the success of SR in infertile patients with NOA.
MATERIALS AND METHODS
Patient population and study design
From March 2014 to March 2021, 111 patients with azoospermia who underwent mTESE were studied retrospectively. Clinical assessment of the patients, including medical history, physical examination, semen analyses, and hormone profile evaluation, were performed. Patients with a surgical history, such as a vasectomy or inguinal surgery, were excluded, as were those with other etiologies, such as ongoing hormonal treatments. Chromosomal studies were performed to assess the cytogenetic properties and microdeletion of the Y chromosome. Patients with abnormal karyotypes were also excluded from this study. A Prader orchidometer was used to measure testicular volumes. Previous studies have shown that 85% of the testicular volume is associated with sperm production and a decrease in testicular size correlates with impaired spermatogenesis. The testes of NOA patients have been reported to measure less than 15 cm3 in volume; therefore, patients with a testis volume greater than 15 cm3 were excluded from this study.16
An endocrine evaluation was performed 1–2 months before the mTESE procedure. After ensuring all aseptic and antiseptic precautions, blood samples were obtained from the antecubital vein between 8 am and 10 am. Separated serum was used, and tests were done within 8 hours of sample collection. The hormone profile evaluation included serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), sex hormone-binding globulin (SHBG), prolactin and testosterone (T) levels. The immune assay method was used to evaluate the patient venous blood samples. Total T levels were determined using radioimmunoassay. According to the results of a previous study, 96% of men with OA had FSH levels of 7.6 mIU/mL or less, while 89% of patients with NOA had FSH levels greater than 7.6 mIU/mL.17 Patients with FSH levels higher than 7.6 mIU/mL were included in the study cohort, and patients with a history of hypogonadotropic hypogonadism and other endocrinopathological disorders were excluded from this study. Two separate semen analyses were performed on the study population. Azoospermia was diagnosed when spermatozoa were absent in two consecutive semen samples after centrifugation and screening with an inverted microscope, according to the World Health Organization laboratory guidelines.18
The study procedure was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of CHA University School of Medicine (protocol code 2022-12-007-001) and ethics committee. Since the study data were obtained from our database, consent of participation was waivered.
mTESE surgical procedure
Participants were subjected to mTESE using a previously established technique.11 The patient was positioned supine under general anesthesia, and a transverse scrotal incision was made to deliver the larger testicle. To visualize the testicular parenchyma without disrupting the testicular vasculature, a single incision was made near the median section of the testis. The larger and whiter tubules were identified after focusing a surgical microscope at 20–25 g magnification on the seminiferous tubules. Surgical samples of testicular tissues with large and opaque tubules were obtained. Technicians at the laboratory evaluated spermatozoa from these samples. If no spermatozoa were obtained, mTESE on the contralateral testis was performed. When suitable sperm samples were recovered or when further surgery would potentially impair testicular blood supply, surgical retrieval was discontinued. When the sperm samples were deemed adequate, rapid sperm freezing was performed according to standard laboratory protocols.11
Statistical analysis
Student’s t test or chi-squared test was used to perform statistical analyses of various factors between patients who had successful and failed SR. The independent variables under consideration were binary variables and continuous variables. For statistical analysis, continuous variables were presented as mean±standard deviation, and the independent sample t test was used. Chi-squared test was used to analyze categorical variables, whereas nonparametric methods were used to compare quantitative data distribution. The association of clinical parameters with SR rates was investigated using univariate and multivariate logistic regression analyses. The optimal cutoff value for the T/LH ratio was evaluated by the receiver operating characteristic (ROC) curve analysis.
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), and a two-sided p value <0.05 was considered significant.
RESULTS
Patient demographics and baseline characteristics
Patient demographics and clinical and laboratory characteristics are presented in Table 1. The average age of the successful group was 39.9±5.4 years, whereas the mean age of the failed group was 36.7±4.7 years. The mTESE success group had a mean body mass index (BMI) of 26.3±4.2, whereas the mTESE failure group had a mean BMI of 26.5±3.1. The successful SR group included 18 (26.9%) patients with a history of diabetes mellitus (DM) and 13 (19.4%) patients with hypertension (HTN) as an underlying disorder, whereas the failure group included 7 (16.3%) patients with DM and 5 (11.6%) patients with HTN. Overall, 68 of 111 (61.3%) mTESE patients had successful sperm extraction. Although various infertility conditions were not studied independently, the overall SR rate of this study corresponded with success rates reported in the literature.11
Table 1. Patient Characteristics of Infertile Male Patients and Comparison of Factors According to Sperm Retrieval Results after mTESE.
| Success (n=68) | Failure (n=43) | p value | ||
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 39.9±5.4 | 36.7±4.7 | 0.002 | |
| Height (cm) | 173.8±7.1 | 176.7±6.8 | 0.032 | |
| Weight (kg) | 79.5±15.1 | 83.1±12.3 | 0.199 | |
| BMI (kg/m2) | 26.3±4.2 | 26.5±3.1 | 0.699 | |
| DM | 18 (26.9) | 7 (16.3) | 0.196 | |
| HTN | 13 (19.4) | 5 (11.6) | 0.282 | |
| Hormone profile | ||||
| FSH (mIU/mL) | 11.3±9.9 | 31.6±13.6 | <0.001 | |
| LH (mIU/mL) | 6.0±5.1 | 12.6±7.8 | <0.001 | |
| T (ng/mL) | 4.5±1.7 | 3.8±2.2 | 0.102 | |
| Free T (ng/mL) | 8.1±2.0 | 7.3±3.3 | 0.199 | |
| Prolactin (ng/mL) | 8.14±5.03 | 9.47±4.36 | 0.216 | |
| LH/FSH | 0.62±0.30 | 0.41±0.18 | <0.001 | |
| T/LH | 1.10±0.79 | 0.43±0.29 | <0.001 | |
| SHBG (nmol/L) | 38.17±21.46 | 41.37±22.09 | 0.678 | |
| Anatomical factors | ||||
| Volume of right testis (mL) | 11.06±5.36 | 3.25±1.83 | <0.001 | |
| Volume of left testis (mL) | 11.14±5.49 | 3.50±1.49 | <0.001 | |
| Varicocele | 5 (7.7) | 6 (14.3) | 0.273 | |
| Hydrocele | 2 (3.1) | 5 (11.9) | 0.071 | |
| Histopathology | ||||
| Focal spermatogenesis | 46 (67.6) | 1 (2.3) | 0.032 | |
| Tubular hyalinization | 9 (13.2) | 0 (0) | 0.579 | |
| Hypospermatogenesis | 9 (13.2) | 9 (20.9) | 0.494 | |
| Maturation arrest | 0 (0) | 4 (9.3) | 0.568 | |
| Sertoli cell only | 4 (5.9) | 29 (67.4) | 0.538 | |
mTESE, microsurgical testicular sperm extraction; BMI, body mass index; DM, diabetes mellitus; FSH, follicle-stimulating hormone; HTN, hypertension; LH, luteinizing hormone; T, testosterone; SHBG, sex hormone-binding globulin.
Data are presented as mean±standard deviation or n (%).
Anatomical factors
The right and left mean testicular volumes for the successful SR group were 11.06±5.36 mL and 11.14±5.49 mL, respectively, whereas the right and left testicular volumes for the negative SR subjects were 3.25±1.89 mL and 3.50±1.49 mL, respectively. A total of 18 (16.2%) of 111 patients had a history of medical conditions that had a negative impact on fertility outcomes. Five patients (7.7%) in the positive SR group had a history of varicocele, whereas 6 (14.3%) were diagnosed in the failure group. Hydroceles were found in 2 (3.1%) patients in the successful SR group and 5 (11.9%) men were diagnosed in the SR failure group. Hydrocelectomy was performed in all NOA patients while undergoing mTESE, while only one patient in the SR failure group had surgical correction of varicoceles. This study population had no history of urinary tract infections, mumps orchitis, chemotherapy, or exposure to hormonal treatments.
Hormone profiles
The serum hormone profiles of the successful and unsuccessful SR groups are shown in Table 1. Compared to the negative retrieval patients, the serum FSH levels in the successful SR group were lower (11.3±9.9 mIU/mL). Moreover, the failed SR group showed higher levels of serum LH and a lower serum concentration of T (3.8±2.2 ng/mL) compared to the successful SR group. Although there were no statistical differences in sex hormone-binding globulin (SHBG), serum prolactin, and free T levels between the comparison groups, the T/LH ratio was lower in the SR failure patients than in the successful SR study population.
Histological profiles
The majority (67.6%) of patients in the successful sperm extraction group showed histopathological findings consistent with focal spermatogenesis, whereas only 1 (2.3%) patient in the failure group had localized foci of normal testicular histopathology. Moreover, tubular hyalinization was observed in 9 (13.2%) men in the successful sperm extraction population. Both groups had nine cases of hypospermatogenesis, but the negative SR group only had 4 (9.3%) patients with maturation arrest. Sertoli cell-only histopathology was the most common in the failure group, accounting for 29 (67.4%) patients, and only 4 (5.9%) were observed in the successful SR population.
Predictors of SR success
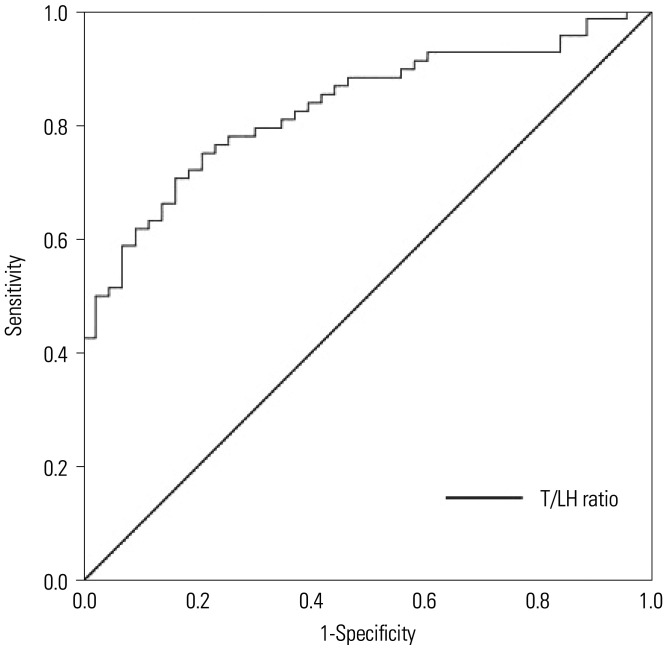
Univariate and multivariate logistic regression analyses were performed to identify preoperative predictors of SR success in azoospermia patients who underwent mTESE. As shown in Table 2, univariate analysis revealed age, serum FSH and LH levels, LH/FSH ratio, T/LH ratio, and bilateral testicular volumes as significant predictors of successful SR. In a multivariate model, serum FSH levels (p=0.009), T/LH ratio (p=0.043), and bilateral testicular volumes (p=0.004 and p=0.003, respectively) were independently associated with successful SR. The optimal cutoff value for the T/LH ratio which was obtained from the ROC analysis is presented in Fig. 1. After selecting successful SR as a state variable, the cutoff value for the T/LH ratio was 0.68, with a sensitivity of 72.1% and a specificity of 81.4% (p=0.001), with an area under the curve of 0.830.
Table 2. Predictive Factors in Infertile Male Patients for Successful Sperm Retrieval Prior to mTESE.
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 1.140 (1.044–1.245) | 0.003 | 1.120 (0.982–1.276) | 0.091 |
| BMI | 0.980 (0.886–1.084) | 0.980 | ||
| DM | 1.889 (0.714–5.000) | 0.200 | ||
| HTN | 1.830 (0.602–5.562) | 0.287 | ||
| FSH | 0.878 (0.838–0.920) | <0.005 | 0.895 (0.824–0.973) | 0.009 |
| LH | 0.839 (0.771–0.914) | <0.005 | 1.122 (0.980–1.284) | 0.096 |
| T | 1.191 (0.965–1.470) | 0.104 | ||
| Free testosterone | 1.146 (0.931–1.411) | 0.200 | ||
| SHBG | 0.994 (0.965–1.023) | 0.670 | ||
| LH/FSH | 9.376 (9.190–86.918) | 0.002 | 5.400 (0.161–180.729) | 0.346 |
| T/LH | 26.842 (6.984–103.156) | <0.005 | 6.274 (1.064–37.005) | 0.043 |
| Prolactin | 0.891 (0.701-1.332) | 0.089 | ||
| Right testis volume | 1.801 (1.401–2.316) | <0.005 | 1.545 (1.152–2.071) | 0.004 |
| Left testis volume | 1.771 (1.378–2.276) | <0.005 | 1.496 (1.152–1.942) | 0.003 |
| Varicocele | 0.280 (0.142–1.757) | 0.280 | 0.487 (0.032–7.377) | 0.604 |
| Hydrocele | 0.235 (0.043–1.272) | 0.093 | 0.061 (0.002–2.391) | 0.135 |
mTESE, microsurgical testicular sperm extraction; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; FSH, follicle-stimulating hormone; HTN, hypertension; LH, luteinizing hormone; T, testosterone; OR, odds ratio; SHBG, sex hormone-binding globulin.
Fig. 1. Receiver operating characteristic curve depicting the relationship between successful sperm retrieval and the T/LH ratio. When the T/LH ratio cutoff value was 0.68, sensitivity was 72.1% and specificity was 81.4%. T, testosterone; LH, luteinizing hormone.
DISCUSSION
Prior to the initiation of mTESE, male infertility patients with azoospermia were devised to consider donor insemination or adoption. With the advancements and development of reproductive medicine, mTESE now allows couples to have their own biological offspring when combined with intracytoplasmic sperm injection (ICSI). SR rate of mTESE is approximately 50% for men with NOA, whereas some studies have reported success rates of up to 100% for men with OA.12,19 However, since mTESE is invasive, patients who undergo the procedure are at risk of postoperative complications, such as scrotal or testicular hematoma, devascularization, and inflammation of the testis, which can lead to calcification and scar formation.20 Therefore, this study aimed to determine the predictive value of preoperative factors in NOA patients prior to surgery.
The study population’s baseline characteristics showed an age difference between the successful SR (39.9 years) and SR failure (36.7 years) groups, with the mTESE-positive study population being slightly older. The results of this study were consistent with those of Güneri, et al.,21 who found that the age of the failure and successful groups was 38 and 42 years old, respectively. A lower SR would be expected in elderly patients. As with aging, underlying diseases or comorbidities that impair testicular vascularity and cause germ cell loss are likely. However, the results of this study revealed that age and SR rates have no statistical relationship, which was consistent with the results of other previous studies.22,23
The normal range for serum FSH levels is 1.3 to 13.58 mIU/mL, whereas the mean FSH level of the successful SR patients in our study group was within normal limits (11.3±9.9 mIU/mL), serum FSH levels of the SR failure group were relatively high (31.6±13.6 mIU/mL). The right and left mean testicular volumes of the SR positive population were 11.06±5.36 mL and 11.14±5.49 mL, which were slightly smaller than normal values, and the mean bilateral testicular volumes of the SR failure group were significantly smaller (3.25±1.83 mL and 3.50±1.49 mL) than normal parameters. The results of the study revealed that patients with negative SR had elevated levels of serum FSH and LH. Although both study groups experienced a decrease in serum T levels, the differences in T levels were not statistically significant between the study population. Numerous clinical studies have examined various factors, such as serum FSH levels, T profiles, testicular volume, and pathology along with genetic factors, and study results indicate that FSH and testicular volume have a positive correlation to successful SR.24,25,26,27
Elevated serum FSH levels and small testicular volumes are associated with patients who have testicular failure and decreased spermatogenesis.28 Our results showed that patients with negative SR had higher levels of FSH and the difference was statistically significant, which were consistent with the results of other studies. Yildirim, et al.29 investigated the perioperative predictors of successful SR and concluded that FSH levels could be used as a predictor of positive SR. Moreover, bilateral testicle volumes were greater in positive SR patients than in SR failure patients. Our findings were consistent with those reported in the literature, which showed that testicular volume is positively related to successful mTESE results.13,25
The results of this study showed that serum LH levels in the SR failure group were higher than those in the positive SR group. Clinical data from a study conducted by Güneri, et al.21 also revealed that higher LH levels in the TESE-negative group were statistically significant when compared with the TESE-positive group, and a positive correlation was found between serum FSH levels and LH. Although our study results showed a higher LH value in the SR failure groups, the correlation between SR and LH levels were not statistically significant. Moreover, various studies investigating the relationship between serum LH levels and TESE outcomes have found no significant differences; therefore, the SR rates of mTESE cannot be accurately predicted based on serum LH levels.13,30,31
Additional findings of this study showed that both study groups had a lower T/LH ratio. Moreover, the T/LH ratio in the SR failure groups was significantly lower than that in the positive group (0.43±0.29 vs. 1.10±0.79). These findings support the results of previous studies that have reported a correlation between impaired spermatogenesis and a lower T/LH ratio.32 Several studies showed that male infertile patients with elevated serum LH levels can maintain normal T levels, indicating hormonal compensation due to dysfunctional Leydig cells.33,34,35 Although the underlying pathophysiology and mechanisms of male infertility and Leydig cell dysfunction are unknown, Fisher, et al.36 found that hormonal disruption during the fetal stage may affect testicular development and spermatogenesis. Therefore, a dual impairment of Leydig cells and spermatogenesis indicate congenital infertility caused by testicular dysgenesis during the fetal or infant stages. Both study groups showed a decrease in serum T levels while increasing LH levels, with the SR failure group showing a stronger tendency. Lardone, et al.37 found that testicular tissue with severely impaired spermatogenesis had larger Leydig cell clusters and a lower total volume, resulting in an altered density ratio between seminiferous tubules and Leydig cells. Although this pathological change increases intratesticular steroid levels, it is insufficient to compensate for serum T levels, implying a hormonal impairment of Leydig cells.
This study had some significant limitations, including its retrospective design and small number of patients. Since this was a single-center study, the study cohort did not fully represent the various clinical backgrounds of patients with NOA, and the results of this study may have limited applicability in the clinical setting. Further prospective and larger-scale studies are needed to fully understand the findings of this study. In addition, the relatively low serum FSH levels and a large proportion of focal spermatogenesis in the successful SR group may indicate a likelihood of the inclusion of patients with OA. A possible explanation for this discrepancy is due to the advances in mTESE. This surgical method has shown higher SR rates compared to conventional TESE. With this microsurgical approach, localizing seminiferous tubules with spermatogenesis can be achieved with higher precision and accuracy.10 Moreover, histopathology of NOA patients have been found to have localized foci of sperm production within the testicles.11 This observation has resulted in a paradigm shift to the conventional concept that the testes produces sperm in a uniform fashion, and has also led to a new understanding of focal spermatogenesis. Although testicular histology is the only definitive way to diagnose azoospermia, due to the heterogeneous nature of testicular tissue and spermatogenesis most often occurring in a focal pattern, a testicular biopsy prior to TESE is rarely used as a diagnostic tool.38,39 In the clinical setting, testicular characteristics and laboratory findings are usually adequate for the diagnosis of NOA. However, variation in the results of this study along with the aforementioned limitation further advocates the necessity of clinical investigations to define reliable predictors and prognosticators concerning SR rates in NOA patients. Moreover, this study did not examine the ICSI success rates and the associated clinical factors for pregnancy outcomes. Another missing component is the evaluation of other hormones, such as adrenal and thyroid components, which have the potential to affect fertility outcomes.
This study demonstrated that the successive SR rate of mTESE in patients with NOA was 61.3%. Preoperative serum FSH levels, bilateral testicular volumes, and the T/LH ratio were positive predictors of successful SR in NOA patients undergoing mTESE in the positive and negative SR groups. Although there are no definitive predictors of successful SR after surgical intervention, the addition of the T/LH ratio as a clinical prognosticator along with traditional factors would improve the predictive accuracy of SR outcomes in patients with azoospermia.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Tae Jin Kim and Kyo Chul Koo.
- Data curation: Tae Jin Kim.
- Formal analysis: Tae Jin Kim.
- Funding acquisition: Kyo Chul Koo.
- Investigation: Tae Jin Kim and Kyo Chul Koo.
- Methodology: Tae Jin Kim and Kyo Chul Koo.
- Project administration: Tae Jin Kim.
- Resource: Kyo Chul Koo.
- Software: Tae Jin Kim.
- Supervision: Kyo Chul Koo.
- Validation: Tae Jin Kim.
- Visualization: Tae Jin Kim and Kyo Chul Koo.
- Writing—original draft: Tae Jin Kim.
- Writing—review & editing: Tae Jin Kim and Kyo Chul Koo.
- Approval of final manuscript: all authors.
References
- 1.Klami R, Mankonen H, Perheentupa A. Successful microdissection testicular sperm extraction for men with non-obstructive azoospermia. Reprod Biol. 2018;18:137–142. doi: 10.1016/j.repbio.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Krol M, Nap A, Michels R, Veraart C, Goossens L. Health state utilities for infertility and subfertility. Reprod Health. 2019;16:47. doi: 10.1186/s12978-019-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung E, Brock GB. Cryptorchidism and its impact on male fertility: a state of art review of current literature. Can Urol Assoc J. 2011;5:210–214. doi: 10.5489/cuaj.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000Res. 2019;8:670. doi: 10.12688/f1000research.17076.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wosnitzer M, Goldstein M, Hardy MP. Review of azoospermia. Spermatogenesis. 2014;4:e28218. doi: 10.4161/spmg.28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arafat M, Har-Vardi I, Harlev A, Levitas E, Zeadna A, Abofoul-Azab M, et al. Mutation in TDRD9 causes non-obstructive azoospermia in infertile men. J Med Genet. 2017;54:633–639. doi: 10.1136/jmedgenet-2017-104514. [DOI] [PubMed] [Google Scholar]
- 7.Björndahl L, Kirkman Brown J other Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. The sixth edition of the WHO laboratory manual for the examination and processing of human semen: ensuring quality and standardization in basic examination of human ejaculates. Fertil Steril. 2022;117:246–251. doi: 10.1016/j.fertnstert.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Abdel Raheem A, Garaffa G, Rushwan N, De Luca F, Zacharakis E, Abdel Raheem T, et al. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. BJU Int. 2013;111:492–499. doi: 10.1111/j.1464-410X.2012.11203.x. [DOI] [PubMed] [Google Scholar]
- 9.Flannigan R, Bach PV, Schlegel PN. Microdissection testicular sperm extraction. Transl Androl Urol. 2017;6:745–752. doi: 10.21037/tau.2017.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco G, Scarselli F, Casciani V, De Nunzio C, Dente D, Leonardo C, et al. A novel stepwise micro-TESE approach in non obstructive azoospermia. BMC Urol. 2016;16:20. doi: 10.1186/s12894-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–135. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 12.Chan PT, Schlegel PN. Nonobstructive azoospermia. Curr Opin Urol. 2000;10:617–624. doi: 10.1097/00042307-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Tsujimura A, Matsumiya K, Miyagawa Y, Takao T, Fujita K, Koga M, et al. Prediction of successful outcome of microdissection testicular sperm extraction in men with idiopathic nonobstructive azoospermia. J Urol. 2004;172(5 Pt 1):1944–1947. doi: 10.1097/01.ju.0000142885.20116.60. [DOI] [PubMed] [Google Scholar]
- 14.Samli MM, Dogan I. An artificial neural network for predicting the presence of spermatozoa in the testes of men with nonobstructive azoospermia. J Urol. 2004;171(6 Pt 1):2354–2357. doi: 10.1097/01.ju.0000125272.03182.c3. [DOI] [PubMed] [Google Scholar]
- 15.Yücel C, Budak S, Keskin MZ, Kisa E, Kozacioglu Z. Predictive factors of successful salvage microdissection testicular sperm extraction (mTESE) after failed mTESE in patients with non-obstructive azoospermia: long-term experience at a single institute. Arch Ital Urol Androl. 2018;90:136–140. doi: 10.4081/aiua.2018.2.136. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz LI, Corriere JN., Jr Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117:175–176. doi: 10.1016/s0022-5347(17)58387-1. [DOI] [PubMed] [Google Scholar]
- 17.Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167:197–200. [PubMed] [Google Scholar]
- 18.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 19.Cissen M, Meijerink AM, D'Hauwers KW, Meissner A, van der Weide N, Mochtar MH, et al. Prediction model for obtaining spermatozoa with testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2016;31:1934–1941. doi: 10.1093/humrep/dew147. [DOI] [PubMed] [Google Scholar]
- 20.Eliveld J, van Wely M, Meißner A, Repping S, van der Veen F, van Pelt AMM. The risk of TESE-induced hypogonadism: a systematic review and meta-analysis. Hum Reprod Update. 2018;24:442–454. doi: 10.1093/humupd/dmy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Güneri Ç, Alkibay T, Tunç L. Effects of clinical, laboratuary and pathological features on successful sperm retrieval in non-obstructive azoospermia. Turk J Urol. 2016;42:168–177. doi: 10.5152/tud.2016.45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernie AM, Ramasamy R, Schlegel PN. Predictive factors of successful microdissection testicular sperm extraction. Basic Clin Androl. 2013;23:5. doi: 10.1186/2051-4190-23-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghalayini IF, Al-Ghazo MA, Hani OB, Al-Azab R, Bani-Hani I, Zayed F, et al. Clinical comparison of conventional testicular sperm extraction and microdissection techniques for non-obstructive azoospermia. J Clin Med Res. 2011;3:124–131. doi: 10.4021/jocmr542w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jezek D, Knuth UA, Schulze W. Successful testicular sperm extraction (TESE) in spite of high serum follicle stimulating hormone and azoospermia: correlation between testicular morphology, TESE results, semen analysis and serum hormone values in 103 infertile men. Hum Reprod. 1998;13:1230–1234. doi: 10.1093/humrep/13.5.1230. [DOI] [PubMed] [Google Scholar]
- 25.Kizilkan Y, Toksoz S, Turunc T, Ozkardes H. Parameters predicting sperm retrieval rates during microscopic testicular sperm extraction in nonobstructive azoospermia. Andrologia. 2019;51:e13441. doi: 10.1111/and.13441. [DOI] [PubMed] [Google Scholar]
- 26.Shrem G, Brudner Y, Atzmon Y, Michaeli M, Ellenbogen A, Shalom-Paz E. The influence of obesity, smoking, and serum follicular stimulating hormone in azoospermic patients on testicular sperm extraction-intra cytoplasmic sperm injection outcomes: a retrospective cohort study. Medicine (Baltimore) 2019;98:e14048. doi: 10.1097/MD.0000000000014048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu T, Peng L, Lin X, Li J, Xu W. Predictors for successful sperm retrieval of salvage microdissection testicular sperm extraction (TESE) following failed TESE in nonobstructive azoospermia patients. Andrologia. 2017;49:e12642. doi: 10.1111/and.12642. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa T, Fujioka H, Fujisawa M. Clinical and hormonal findings in testicular maturation arrest. BJU Int. 2004;94:1314–1316. doi: 10.1111/j.1464-410X.2004.05163.x. [DOI] [PubMed] [Google Scholar]
- 29.Yildirim ME, Koc A, Kaygusuz IC, Badem H, Karatas OF, Cimentepe E, et al. The association between serum follicle-stimulating hormone levels and the success of microdissection testicular sperm extraction in patients with azoospermia. Urol J. 2014;11:1825–1828. [PubMed] [Google Scholar]
- 30.Boitrelle F, Robin G, Marcelli F, Albert M, Leroy-Martin B, Dewailly D, et al. A predictive score for testicular sperm extraction quality and surgical ICSI outcome in non-obstructive azoospermia: a retrospective study. Hum Reprod. 2011;26:3215–3221. doi: 10.1093/humrep/der314. [DOI] [PubMed] [Google Scholar]
- 31.Ziaee SA, Ezzatnegad M, Nowroozi M, Jamshidian H, Abdi H, Hosseini Moghaddam SM. Prediction of successful sperm retrieval in patients with nonobstructive azoospermia. Urol J. 2006;3:92–96. [PubMed] [Google Scholar]
- 32.Holm M, Rajpert-De Meyts E, Andersson AM, Skakkebaek NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J Pathol. 2003;199:378–386. doi: 10.1002/path.1309. [DOI] [PubMed] [Google Scholar]
- 33.Anapliotou ML, Liparaki M, Americanos N, Goulandris N, Papaioannou D. Increased 17-OH-progesterone levels following hCG stimulation in men with idiopathic oligozoospermia and raised FSH levels. Int J Androl. 1994;17:192–198. doi: 10.1111/j.1365-2605.1994.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 34.Giagulli VA, Vermeulen A. Leydig cell function in infertile men with idiopathic oligospermic infertility. J Clin Endocrinol Metab. 1988;66:62–67. doi: 10.1210/jcem-66-1-62. [DOI] [PubMed] [Google Scholar]
- 35.Stanwell-Smith R, Thompson SG, Haines AP, Jeffcoate SL, Hendry WF. Plasma concentrations of pituitary and testicular hormones of fertile and infertile men. Clin Reprod Fertil. 1985;3:37–48. [PubMed] [Google Scholar]
- 36.Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- 37.Lardone MC, Castillo P, Valdevenito R, Ebensperger M, Ronco AM, Pommer R, et al. P450-aromatase activity and expression in human testicular tissues with severe spermatogenic failure. Int J Androl. 2010;33:650–660. doi: 10.1111/j.1365-2605.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 38.Bouker A, Halouani L, Kharouf M, Latrous H, Makni M, Marrakchi O, et al. Step-by-step loupes-mTESE in non-obstructive azoospermic men, a retrospective study. Basic Clin Androl. 2019;29:11. doi: 10.1186/s12610-019-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JK, Song SH, Jung G, Song B, Hong SK. Possibilities and limitations of using low biomass samples for urologic disease and microbiome research. Prostate Int. 2022;10:169–180. doi: 10.1016/j.prnil.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]