Abstract
Background
Myrica nagi is popular in unani and ayurveda. Chemical constituents like myricetin isolated from its fruit has been shown to exert beneficial effects against cardiovascular disease, cancer, inflammatory conditions, and metabolic disorders.
Objectives
This study aimed to elucidate the anti-obesity effect of the methanolic extract of M. nagi (MEMN) using in vivo animal models of obesity induced by gold thioglucose or a high-fat diet.
Materials and methods
The obese mice were treated or untreated with MEMN for 8 weeks. Thereafter, feed intake, Lee index, and body mass index (BMI); biochemical parameters such as lipid profile, liver enzymes and specific biomarkers of obesity, including insulin, leptin, adiponectin, free fatty acids (FFA), monocyte chemoattractant protein (MCP)-1, and resistin, were recorded. The weight and histopathology of organs and fat tissue were examined to validate the effectiveness of the extract.
Results
MEMN administration at various doses significantly reduced the induced weight gain, feed intake, BMI, and Lee index. Adipose tissue decreased as the MEMN dose increased. MEMN attenuated liver enzyme activity, decreased lipid, leptin, MCP-1, resistin, and FFA levels, and increased adiponectin levels. It also increased protection of liver cells and decreased accumulation of mesenteric fat.
Conclusions
MEMN supplementation decreased weight and improved obesity serum/plasma lipid biomarker, insulin, leptin, adiponectin, MCP-1, and resistin levels. The weight-reducing activity of MEMN may be mediated by decreased gastrointestinal fat absorption and modulation of inflammation associated signaling pathways, leading to reduced adipose inflammation associated with energy expenditure.
Keywords: Obesity, Methanolic extract, Myrica nagi, High-fat diet, Gold thioglucose
Highlights
-
•
Methanolic extract of Myrica nagi (MEMN) reduced body weight of mouse models.
-
•
GTG- and HFD-induced mouse obesity model used to analyze MEMN.
-
•
MEMN improved obesity-related serum and plasma biomarkers and lipid profile.
-
•
Effects of MEMN were likely mediated by AMPK and TLR) signaling pathways.
1. Introduction
Obesity affects at least 300 million people globally. The World Health Organization (WHO) describes obesity as the accumulation of excess fat in the body [1]. Even though adult obesity is not as prevalent in the country, India is ranked second in childhood obesity worldwide [2]. The primary cause of obesity is an imbalance between energy intake and expenditure [[3], [4]]. Clinically, an individual with a body mass index (BMI) > 40 is considered obese. Obesity is also related to clinical symptoms, such as troubled sleep, apnea, shortness of breath, gallstones, high blood pressure and cholesterol levels, and joint pain [5].
Generally, an increase in the amount of adipose tissue causes an individual to become overweight. The primary cellular feature of obesity is the enlargement of adipocytes, which initiates various inflammatory molecular mechanisms [6]. Dysregulation of peptides, such as leptin, adiponectin, resistin, plasminogen activator inhibitor-1, omentin, amylin, and obestatin, in adipose tissues is also known to lead to obesity [7].
In gold thioglucose (GTG)-induced obesity, the initial ventromedial hypothalamic lesion increases the secretion of neuropeptide Y, agouti-related peptide, and melanocyte-stimulating hormone, which stimulate appetite, accompanied by a rapid increase in insulin-like peptide 5, ghrelin, cholecystokinin, peptide YY, glucagon-like peptide 1, and leptin in the blood, promoting food intake [8]. Excessive food intake impairs molecular mechanisms mediated by, inflammatory adipokines, and AMP-activated protein kinase (AMPK) pathway in preadipocytes [[9], [10], [11]]. Adipokines, such as leptin, visfatin, adiponectin, resistin, monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor (TNF)-α, are secreted by adipose tissue, which has been recognized as the largest endocrine organ [[12], [13], [14], [15], [16], [17]].
Low-grade inflammation plays a critical role in obesity and inflammatory agents, such as macrophages, cytokines, and chemokine-secreting leukocytes, infiltrate adipose tissue [18]. In obesity, levels of pro-inflammatory cytokines and C-reactive protein are markedly increased, while obesity-stimulated increases in the MCP-1 and resistin levels reduce the secretion of adiponectin [6].
Measures to diminish calorie intake or increase calorie expenditure are often proposed when addressing weight gain; however, in serious cases, surgery may be required. Few anti-obesity drugs are currently approved by the US Food and Drug Administration, and the available ones, such as orlistat, phentermine, and topiramate, present adverse effects [19,20]. Indeed, most anti-obesity drugs have been withdrawn from the market because of adverse effects during long-term therapy. Gene therapy, which aims to increase lipolysis and energy expenditure to restore energy homeostasis, has proven efficacious in adiposity [21,22]. Various pathways and proteins targeted in the treatment of obesity include PPAR-γ, PPAR-α, β-adrenergic receptors, glucagon-like peptide-1, brain-derived neurotrophic factor, fibroblast growth factor-21, adipokines, hypoxia-inducible factor, the AMPK pathway, and glucose transporter 2 [11,21,23,24]. Hence, anti-obesity approaches have focused on these and related pathways.
Herbal medicines are considered safer than conventional chemical agents, as well as efficacious and cost-effective, making them increasingly popular as possible treatments against obesity. The plant M. nagi Thunb (family Myricaceae) possesses medicinal properties owing to which it has been popular in unani and ayurveda. Phytochemical analysis of the leaves has detected carbohydrates, alkaloids, saponins, tannins, flavonoids, sterols, and triterpenoids [25]. Traditionally, the plant fruit has been used as a carminative, antiulcer agent, and a cure for bleeding piles [26,27]. It has also been used as a stimulant [27], and against indigestion and anorexia [26,28]. Chemical constituents like myricetin isolated from the fruit has been shown to exert beneficial effects against cardiovascular disease, cancer, inflammatory conditions, and metabolic disorders. Egg albumin-induced allergy tests of the ethyl acetate and aqueous extracts of M. nagi have shown degranulation and stabilization of mast cells, which are important mediators of inflammatory reactions, including anaphylaxis and allergies [29].
Gas chromatography mass spectrometry (MS) and phytochemical fingerprinting were used to investigate the antioxidant activity of M. nagi [30]. Liquid chromatography (LC)-MS of its leaf extract revealed the presence of 21 compounds with potential anti-obesity activity [31]. Particularly, molecular docking analysis has shown that myricanol could act as an AMPK activator that inhibits lipid accumulation by suppressing adipogenic factors like leptin adiponectin [32]. Accordingly, M. nagi extract could serve for the treatment of obesity via various anorexigenic pathways.
In the present study, we aimed to investigate the anti-obesity effect of the methanolic extract of M. nagi (MEMN) and its potential underlying mechanisms using GTG- and high-fat diet (HFD)-induced obesity mouse models.
2. Materials and Methods
2.1. Materials
The chemicals and reagents used in this study were of analytical grade. Cellulose powder, concentrated HCl, dl-methionine, ethanol, FeCl3, sucrose, heptane, and n-butanol were from S.D. Fine Chemicals Ltd. (Mumbai, India). The total cholesterol (TC), serum glutamate-pyruvate transaminase (SGPT), serum glutamate-oxalate transaminase (SGOT), triglyceride (TG), and creatinine measurement kits were obtained from Span Diagnostics (Surat, India); the glucose and total protein measurement kits were obtained from Beacon Diagnostics (Navsari, India). Glucose was obtained from Avecon Health Care Pvt. Ltd. (Parwanoo, India) and the fasting insulin chemiluminescent immunoassay kit was from Diagnostic Automation Inc (Woodland Hills, CA, USA). The leptin enzyme-linked immunosorbent assay (ELISA), and MCP-1 and plasma resistin quantitation kits were from Ray Bio Rat (Norcross, GA, USA). A rat adiponectin Assay Max ELISA kit was obtained from Assay pro (St. Charles, MO, USA).
2.2. Authentication of collected plants
M. nagi fruits were harvested from Mandi District, Himachal Pradesh, India, in June–July 2017. Fruit samples were authenticated by NISCAIR, Delhi, India (Ref. No. NISCAIR/RHMD/Consult/2017/3102-51-4; NISCAIR, 1959). The plant name has been verified on http://www.theplantlist.org.
2.3. Plant extract preparation and phytochemical analysis
Freshly collected fruits of M. nagi were dried in the shade, pulverized to obtain a coarse powder, passed through a number 40 sieve, weighed (500 g), and extracted with hot methanol as the solvent using a Soxhlet apparatus. Finally, the extract was dried using a rotary vacuum evaporator. Primary phytochemical screening was carried out on the extract to determine presence of phytoconstituents [33].
2.4. Acute oral toxicity study
Acute oral toxicity was assessed according to the procedure outlined in the 2001 Organization for Economic Co-operation and Development (OECD) guideline no. 423 (acute toxic class method) [34]. The extract did not exhibit any degree of toxicity even at 2000 mg/kg, the maximum limit dose indicated by the guideline; hence, we selected doses of 100, 200, and 400 mg/kg, corresponding to 1/20, 1/10, and 1/5, respectively, of the limit dose.
2.5. Animal care
Swiss albino (male and female) mice, weighing 20–25 g, were housed in polypropylene cages and provided a diet of standard mouse pellets and water ad libitum. The cages were maintained at 23 ± 5 °C and 40–60% humidity, under a 12 h light/dark cycle. Experiments were conducted according to ethical norms approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Institutional Animal Ethics committee (RIP/IAEC/2016–2017/09 and RIP/IAEC/2018–19/01).
2.6. HFD-induced mouse model and treatment
HFD was purchased from Research Diets, New Brunswick, USA diet code D12492 and comprised the ingredients listed in Table 1 [35,36]. The mice were fed HFD for 16 weeks [[37], [38]], and MEMN treatment was initiated after 8 weeks of beginning HFD. HFD was administered daily in the morning with water and each animal's weight was compared before and 8 weeks post feeding. Weight gain was monitored, and the experiment was continued for another 8 weeks, during which the obese mice were treated orally (p.o.) with MEMN or orlistat [39]. The dose and diet administration schedule of the various groups was as follows: group I (normal control), 0.9% NaCl (normal saline [NS]), 10 mL/kg, p.o.; group II (induced), HFD from day 1 to day 112; group III (standard drug-treated), orlistat (10 mg/kg, p.o., suspended in NS) from day 56 to day 112 plus HFD; and groups IV–VI (MEMN treatment), 100, 200, and 400 mg/kg MEMN, respectively, from day 56 to day 112 plus HFD.
Table 1.
High-fat diet formulationa.
| Ingredients | Quantity (g/kg) |
|---|---|
| Casein | 200 |
| Cysteine, L | 3 |
| Lodex 10 (Corn Starch) | 260 |
| Sucrose | 164 |
| Solka Flox Cellulose | 50 |
| Lard | 245 |
| Soyabean Oil USP | 25 |
| Choline Bitartrate | 2 |
| Vitamin Mixture | 1 |
| Mineral Mixture | 50 |
| Dye | 0.05 |
2.7. GTG-induced obesity model
The GTG-induced mouse model of obesity was established as indicated previously [39]. GTG causes necrosis of the ventromedial portion of the hypothalamus, leading to obesity [40,41]. Young healthy Swiss albino mice of either sex weighing 30–40 g were used for the pre-clinical study and were randomly grouped for the experiment. Each mouse was marked differently with dye for individual identification. The mice were kept in different cages for 5 days prior to the experiment to acclimatize to laboratory conditions. The extract and standard drug were prepared in the indicated vehicles prior to administration. NS was used as the vehicle for preparing MEMN [42] and orlistat, which was administered at 10 mg/kg [43]. The test and standard drugs were administered p.o. for 8 weeks using an oral feeding cannula. The mice were administered GTG (300 mg/kg) once intraperitoneally (i.p.) at 4 weeks of age and were subsequently monitored for 12–18 weeks to ensure development of obesity, as well as to measure their lipid profiles and weight. After 8 weeks, the animals were treated as follows: group I (normal, standard diet, 0.5 mL NS, p.o.); group II (induced), GTG, 300 mg/kg i.p.; group III, GTG, 300 mg/kg i.p. plus orlistat, 10 mg/kg; groups IV–VI (treatment), GTG, 300 mg/kg, i.p. plus MEMN (100, 200, and 400 mg/kg, p.o., respectively).
2.8. In vivo pharmacological evaluation
The in vivo pharmacological effect was evaluated using previously reported methods [44] for the following parameters: body weight, feed intake, BMI, Lee index. The BMI was calculated as body weight (g)/length (cm2) [45], and the Lee index was calculated as cube root of body weight (g)/nasoanal length (cm) [46].
2.9. Biochemical studies
After treating both mouse obesity models for 8 weeks, the animals were starved for 12 h, anesthetized, and then euthanized. The liver and fat tissue were excised and weighed, whereas blood samples were collected through a heart puncture, placed in separate sterilized dry centrifugation tubes, allowed to stand for 30 min at 37 °C, and centrifuged. The supernatant containing clear serum was collected and the following biochemical parameters were assessed: TG, TC, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), SGOT, SGPT, blood glucose, insulin, leptin, adiponectin, resistin, and MCP-1. Histological analysis and fat pad isolation were also performed, and all biomarkers were estimated using ELISA kits following the manufacturer's instructions.
2.10. Fat pad isolation
The mesenteric fat pad was harvested from each mouse starting from the duodenum and stripping the intestine by pulling gently up to the end of the colon. The left epididymal pad was removed and pulled up after making a midline incision, and the epididymis was visible after turning the pad over. The “distal epididymal fat pad”, which is thinner than the epididymis, was cut immediately above the latter. Perirenal fat was removed by first pulling the kidneys toward the midline to reveal the division between the retroperitoneal and perirenal pads, so that the latter could be removed [47].
2.11. Histopathological examination
The liver, fat pads (Mesenteric, Epididymal and Perirenal) were harvested and weighed using an electronic balance, and then stored in 10% neutral buffered formalin. The organs liver, Mesenteric fat pads were appropriately processed, sectioned, stained with hematoxylin (0.6% w/v) for 15 min and eosin (1% w/v) for 2 min, and finally examined under a microscope at 10 × magnification.
2.12. Statistical analysis
Data analysis was performed using GraphPad Prism 8.2.0 software (La Jolla, CA, USA) and Graph Pad In stat and values are expressed as the means ± standard error of mean (SEM, n = 8). Data were analyzed using one-way analysis of variance, followed by Tukey's Cramer multiple comparison test. Significant levels were set at p < 0.05. Figures drawn in excel and graph pad prism.
3. Results
3.1. Phytochemical analysis
Standard phytochemical tests showed that MEMN contained triterpenoids, flavonoids, alkaloids, carbohydrates, saponins, and steroids.
3.2. In vivo toxicity studies in mice
Acute toxicity testing in mice was conducted by administering MEMN at a dose of 2 g/kg according to the OECD Test Guideline 423 [34]. The level of toxicity was determined during 14 days of treatment and no adverse reactions were observed. Doses of 100, 200, and 400 mg/kg were selected for subsequent experiments.
3.3. HFD-and GTG induced obesity model
3.3.1. Effect of MEMN on body weight
The effect of MEMN on body weight was monitored at week 8, and 16. After 8 weeks of HFD and GTG all groups of mice showed weight gain of 25–30% compared with that of group I (control) mice. Notably, all treatment groups (III orlistat, IV, V, and VI MEMN) showed a reduction in body weight at week 16 post HFD and GTG (i.e., after 8 weeks of the treatment), while group II showed a continuous increase (Table 2 a, b).
Table 2.
Effect of methanolic extract of Myrica nagi (MEMN) on body weight (gm) in a high-fat diet (HFD) and GTG mouse model.
| a | ||||||
|---|---|---|---|---|---|---|
| Week | Control | Inducer (HFD) | Standard (10 mg/kg) | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| WK-00 | 20.83 ± 0.17 | 20.75 ± 0.20 | 20.83 ± 0.15 | 20.49 ± 0.11 | 21.98 ± 0.14 | 21.16 ± 0.12 |
| WK-08 | 22.16 ± 0.20 | 28.83 ± 0.49 | 24.86 ± 0.75 | 26.56 ± 0.21 | 25.83 ± 0.19 | 25.89 ± 0.24 |
| WK-16 | 26.66 ± 0.16 | 36.69 ± 0.30### | 22.67 ± 1.10∗∗∗ | 23.56 ± 0.52∗∗∗ | 23.83 ± 0.51∗∗∗ | 23.91 ± 0.54∗∗∗ |
| b | |||
|---|---|---|---|
| TREATMENT | WEEK 0 (Initial) | WEEK 8 (Mice observed for 8 weeks) (Final) | WEEK 16 (Mice treated for 8 weeks) (After treatment) |
| Normal Control | 18.68 ± 0.89 | 20.68 ± 0.55 | 21.00 ± 0.73 |
| Gold thioglucose (300 mg/kg) | 18.11 ± 0.85 | 30.43 ± 1.85 | 40.54 ± 1.47### |
| GTG + Orlistat (10 mg/kg) | 20.61 ± 0.34 | 30.09 ± 1.10 | 31.98 ± 0.09∗∗∗ |
| GTG + MEMN (100 mg/kg) | 20.65 ± 1.09 | 30.16 ± 0.56 | 32.78 ± 0.24∗∗∗ |
| GTG + MEMN (200 mg/kg) | 18.85 ± 1.45 | 32.16 ± 1.21 | 33.56 ± 1.19∗∗∗ |
| GTG + MEMN (400 mg/kg) | 20.92 ± 1.20 | 30.58 ± 1.21 | 33.55 ± 0.78∗∗∗ |
Methanolic extract of M. nagi., MEMN; GTG., Gold thio glucose; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, #When compared with normal control group, ∗when compared with inducer group.
3.3.2. Effect of MEMN on feed intake
The feed intake of all six groups was monitored at weeks 0, 8, and 16 post HFD and GTG. Compared to the control group that was fed a standard diet, the feed intake was higher in all treatment groups, particularly in the HFD and GTG group. A dose of 400 mg/kg MEMN effectively suppressed appetite more than the 100 and 200 mg/kg doses (Table 3).
Table 3.
Effect of methanolic extract of Myrica nagi (MEMN) on feed intake in a high-fat diet (HFD) and GTG model.
| a | ||||||
|---|---|---|---|---|---|---|
| Week | Control | Inducer (HFD) | Standard (10 mg/kg) | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| 00 | 2.25 ± 0.04 | 2.75 ± 0.02 | 2.62 ± 0.02 | 2.62 ± 0.02 | 2.25 ± 0.02 | 2.5 ± 0.02 |
| 08 | 2.87 ± 0.06 | 3.87 ± 0.04 | 4.00 ± 0.04 | 4.25 ± 0.05 | 4.25 ± 0.05 | 4.3 ± 0.06 |
| 16 | 2.91 ± 0.04 | 4.98 ± 0.05 | 3.25 ± 0.06 | 3.99 ± 0.05 | 3.95 ± 0.06 | 3.25 ± 0.08 |
| b | ||||||
|---|---|---|---|---|---|---|
| Week | Control | Inducer (GTG) | Orlistat (10 mg/kg) | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| 0 | 2.5 ± 1.01 | 2.75 ± 0.57 | 2.62 ± 1.37 | 2.62 ± 0.66 | 2.25 ± 1.3 | 2.5 ± 0.2 |
| 8 | 3.11 ± 0.52 | 3.87 ± 0.71 | 4 ± 1.2 | 4.25 ± 0.17 | 4.26 ± 1.22 | 4.37 ± 0.23 |
| 16 | 3.12 ± 1.1 | 5 ± 0.94 | 3.25 ± 0.94 | 3.78 ± 1.02 | 3.62 ± 1.25 | 3.5 ± 0.67 |
Values estimated in mean ± SEM (n = 8).
3.3.3. Effect of MEMN on BMI
Mouse BMI was calculated at week 0, 8, and 16 post HFD and GTG. A continuous increase in BMI was observed for groups II, III, IV, V, and VI during the initial 8 weeks compared with that in the control group. Then, from weeks 8–16, the BMI of the treatment groups gradually decreased compared with that of the HFD group (Table 4).
Table 4.
Effect of methanolic extract of Myrica nagi (MEMN) on BMI (g/cm2) in a high-fat diet (HFD), GTG model.
| a | ||||||
|---|---|---|---|---|---|---|
| Week | Control | Inducer (HFD) | Standard (10 mg/kg) | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| WK-00 | 0.251 ± 0.08 | 0.253 ± 0.01 | 0.24 ± 0.09 | 0.231 ± 0.07 | 0.256 ± 0.07 | 0.241 ± 0.09 |
| WK-08 | 0.251 ± 0.09 | 0.441 ± 0.01 | 0.414 ± 0.09 | 0.395 ± 0.08 | 0.403 ± 0.09 | 0.394 ± 0.01 |
| WK-16 | 0.299 ± 0.09 | 0.573 ± 0.01### | 0.337 ± 0.09 ∗∗∗ | 0.322 ± 0.06 ∗∗∗ | 0.33 ± 0.06 ∗∗∗ | 0.319 ± 0.09 ∗∗∗ |
| b | ||||||
|---|---|---|---|---|---|---|
| Week | Control group | GTG | Orlistat | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| 0 | 0.227 ± 0.006 | 0.257 ± 0.006 | 0.251 ± 0.005 | 0.256 ± 0.004 | 0.253 ± 0.005 | 0.256 ± 0.004 |
| 8 | 0.226 ± 0.006 | 0.374 ± 0.008 | 0.375 ± 0.005 | 0.377 ± 0.005 | 0.374 ± 0.005 | 0.377 ± 0.006 |
| 16 | 0.227 ± 0.006 | 0.498 ± 0.006### | 0.404 ± 0.004∗∗∗ | 0.468 ± 0.004∗∗∗ | 0.428 ± 0.003∗∗∗ | 0.411 ± 0.005∗∗∗ |
Methanolic extract of M. nagi., MEMN; GTG., Gold thio glucose; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, #When compared with normal control group, ∗when compared with inducer group.
3.3.4. Effect of MEMN on the Lee index
The Lee index of group II exhibited significant difference compared with that of group I. Then, from weeks 8–16, the Lee index of the treatment groups gradually decreased compared with that of the HFD and GTG group (Table 5).
Table 5.
Effect of methanolic extract of Myrica nagi (MEMN) on the Lee index ∛g/cm of a high-fat diet (HFD), GTG model.
| a | ||||||
|---|---|---|---|---|---|---|
| Week | Control | Inducer (HFD) | Standard (10 mg/kg) | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| WK-00 | 0.400 ± 0.04 | 0.295 ± 0.09 | 0.309 ± 0.06 | 0.304 ± 0.06 | 0.308 ± 0.04 | 0.315 ± 0.05 |
| WK-08 | 0.302 ± 0.06 | 0.313 ± 0.09 | 0.324 ± 0.07 | 0.314 ± 0.04 | 0.319 ± 0.06 | 0.331 ± 0.08 |
| WK-16 | 0.303 ± 0.08 | 0.326 ± 0.09### | 0.335 ± 0.05∗∗∗ | 0.325 ± 0.03∗∗∗ | 0.332 ± 0.04∗∗∗ | 0.34 ± 0.04∗∗∗ |
| b | ||||||
|---|---|---|---|---|---|---|
| Weeks | Control | GTG | Orlistat | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| 0 | 301.23 ± 0.009 | 305.76 ± 0.001 | 303.19 ± 0.009 | 305.13 ± 0.007 | 304.02 ± 0.009 | 305.2 ± 0.009 |
| 8 | 306.88 ± 0.007 | 346.63 ± 0.001 | 346.69 ± 0.008 | 347.49 ± 0.008 | 346.55 ± 0.007 | 347.34 ± 0.007 |
| 16 | 313.33 ± 0.008 | 381.2 ± 0.009### | 355.49 ± 0.007∗∗∗ | 373.31 ± 0.007∗∗∗ | 362.26 ± 0.006∗∗∗ | 357.63 ± 0.006∗∗∗ |
Methanolic extract of M. nagi., MEMN; GTG., Gold thio glucose; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, #When compared with normal control group, ∗when compared with inducer group.
3.3.5. Effect of MEMN on the lipid profile
Lipid profiles were analyzed in all groups at the end of week 16 by quantifying TC, TG, HDL, and LDL levels. As shown in Table 6, group II exhibited a high level of all parameters except HDL. In contrast, TC, TG, and LDL levels were significantly lower (p < 0.01) in groups III, IV, V, and VI than in group II, whereas HDL was significantly higher (p < 0.01) (Table 6).
Table 6.
Effect of methanolic extract of Myrica nagi (MEMN) on the lipid profile in a high-fat diet (HFD), GTG model. TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
| a | ||||
|---|---|---|---|---|
| Treatment | Cholesterol | Triglyceride | HDL | LDL |
| Normal Control | 149.37 ± 2.65 | 99.09 ± 1.94 | 47.702 ± 1.93 | 45.343 ± 2.89 |
| HFD | 285.73 ± 1.75### | 134.62 ± 1.92### | 36.308 ± 1.3### | 135.13 ± 1.42### |
| HFD + Orlistat (10 mg/kg, p.o.) | 166.61 ± 2.75∗∗∗ | 122.058 ± 1.16∗∗∗ | 40.013 ± 1.45∗∗∗ | 59.95 ± 1.3∗∗∗ |
| HFD + MEMN (100 mg/kg, p.o.) | 179.01 ± 1.61∗∗∗ | 119.08 ± 2.88∗∗∗ | 35.41 ± 2.44ns | 70.41 ± 2.27∗∗∗ |
| HFD + MEMN (200 mg/kg, p.o.) | 171.95 ± 1.91∗∗∗ | 116.905 ± 1.76∗∗∗ | 35.85 ± 0.27 ns | 65.30 ± 1.64∗∗∗ |
| HFD + MEMN (400 mg/kg, p.o.) | 155.92 ± 1.92∗∗∗ | 110.657 ± 1.53∗∗∗ | 40.72 ± 1.36∗∗∗ | 52.63 ± 2.09∗∗∗ |
| b | |||||
|---|---|---|---|---|---|
| Groups | Cholesterol (TC) | Triglycerides | HDLs | LDLs | VLDL |
| Control | 87.91 ± 1.54 | 132.31 ± 1.47 | 33.42 ± 2.68 | 28.02 ± 0.78 | 26.46 ± 1.78 |
| Gold thioglucose (300 mg/kg, i.p.) | 141.7 ± 0.85### | 223.54 ± 2.82### | 24.12 ± 2.53### | 72.87 ± 1.31### | 44.7 ± 2.04## |
| GTG + Orlistat (10 mg/kg, p.o.) | 111.87 ± 4.16∗∗∗ | 141.68 ± 1.38∗∗∗ | 32.12 ± 2.35∗ | 51.41 ± 1.44∗∗ | 28.33 ± 1.45∗∗ |
| GTG + MEMN (100 mg/kg, p.o.) | 123.34 ± 0.88∗∗∗ | 157.42 ± 1.45∗∗∗ | 28.07 ± 2.88∗ | 63.79 ± 1.83∗∗ | 31.48 ± 1.37∗∗ |
| GTG + MEMN (200 mg/kg, p.o.) | 125.5 ± 2.21∗∗∗ | 152 ± 1.16∗∗∗ | 29.69 ± 1.41∗∗ | 65.41 ± 1.51∗∗ | 30.4 ± 1.48∗∗ |
| GTG + MEMN (400 mg/kg, p.o.) | 115.3 ± 2.18∗∗∗ | 142.87 ± 0.96∗∗∗ | 32.17 ± 2.08∗∗ | 54.55 ± 0.60∗∗ | 28.57 ± 1.41∗∗ |
Methanolic extract of M. nagi., MEMN; GTG., Gold thio glucose; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, #When compared with normal control group, ∗when compared with inducer group.
3.3.6. Effect of MEMN on liver function
A significant decrease (p < 0.01) in both ALP and SGPT levels was noted in all test groups compared with group II. MEMN treatment significantly reduced (p < 0.01) liver enzyme levels compared to those of group II (Table 7).
Table 7.
Effect of methanolic extract of Myrica nagi (MEMN) on liver enzymes in a high-fat diet (HFD), GTG model.
| a | ||
|---|---|---|
| Group | SGPT U/L | ALP U/L |
| Control | 32.79 ± 0.71 | 224.25 ± 4.69 |
| HFD | 81.21 ± 1.8### | 422.12 ± 0.95### |
| Orlistat (10 mg/kg) | 73.06 ± 0.98∗ | 373.06 ± 2.92∗∗∗ |
| MEMN (100 mg/kg) | 70.04 ± 1.25∗ | 370.04 ± 2.35∗∗∗ |
| MEMN (200 mg/kg) | 64.25 ± 0.99∗∗ | 364.25 ± 1.68∗∗∗ |
| MEMN (400 mg/kg) | 43.18 ± 0.82∗∗∗ | 343.18 ± 2.29∗∗∗ |
| b | ||
|---|---|---|
| Groups/Treatment | ALP (U/L) | SGPT (U/L) |
| Normal Control | 37.33 ± 1.202 | 31.33 ± 1.202 |
| Gold thioglucose (300 mg/kg, i.p.) | 68.66 ± 2.028## | 65.66 ± 1.764## |
| GTG + Orlistat (10 mg/kg, p.o.) | 42.66 ± 2.333∗∗ | 41.66 ± 0.881∗∗ |
| GTG + MEMN (100 mg/kg, p.o.) | 58.33 ± 1.453∗∗ | 56 ± 0.577∗∗ |
| GTG + MEMN (200 mg/kg, p.o.) | 46.66 ± 1.202∗∗ | 46 ± 1.732∗∗ |
| GTG + MEMN (400 mg/kg, p.o.) | 45.69 ± 1.202∗∗ | 44.20 ± 1.512∗∗ |
SGPT, serum glutamate-pyruvate transaminase; ALP, Alkaline phosphatase. Methanolic extract of M. nagi., MEMN; GTG., Gold thio glucose; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, #When compared with normal control group, ∗when compared with inducer group.
3.3.7. Effect of MEMN on organ weight
The effect of MEMN on the weight of the liver was evaluated. As shown in Table 8, organ was significantly heavier (p < 0.01) in group II than in control animals. In comparison, the treatment groups showed nearly normal weight and a significant decrease (p < 0.01) in comparison to that of group II (Table 8).
Table 8.
Effect of methanolic extract of Myrica nagi (MEMN) on kidney and liver weight in a high-fat diet (HFD) model.
| a | ||||||
|---|---|---|---|---|---|---|
| Organ | Control (I) | Inducer (II) | Standard (III) | MEMN (100 mg/kg) (IV) | MEMN (200 mg/kg) (V) | MEMN (400 mg/kg) (VI) |
| Liver(gm) | 0.916 ± 0.113 | 1.56 ± 0.119### | 1.01 ± 0.101∗∗∗ | 1.42 ± 0.11ns | 1.36 ± 0.05∗∗ | 1.26 ± 0.112∗∗∗ |
| b | ||
|---|---|---|
| Groups | Treatment | Liver (gm) |
| I | Normal Control | 0.82 ± 0.10 |
| II | Gold thioglucose (300 mg/kg, i.p.) | 1.76 ± 0.22### |
| III | GTG + Orlistat (100 mg/kg, p.o.) | 1.12 ± 0.08∗∗∗ |
| IV | GTG + MEMN (100 mg/kg, p.o.) | 1.48 ± 0.09∗∗∗ |
| V | GTG + MEMN (200 mg/kg, p.o.) | 1.38 ± 0.14∗∗∗ |
| VI | GTG + MEMN (400 mg/kg, p.o.) | 1.15 ± 0.05∗∗∗ |
Methanolic extract of M. nagi., MEMN; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, #When compared with normal control group, ∗when compared with inducer group.
3.3.8. Effect of HFD and MEMN on obesity-related parameters
The effect of a HFD on various obesity-related parameters, such as insulin, leptin, adiponectin, free fatty acids, MCP-1, and resistin, was monitored in the six groups (Table 9). For all parameters, the levels were significantly higher (p < 0.01) in group II compared to those in the control, indicating a considerable effect of the HFD. Mice treated with 100, 200, and 400 mg/kg MEMN exhibited a significant decrease (p < 0.01) in all parameters compared to that in the HFD mice, confirming the effectiveness of the extract. Only adiponectin was significantly lower (p < 0.01) in group II than in the control animals, whereas, in the MEMN-treated groups, it appeared significantly higher (p < 0.01) than that in group II. Importantly, in nearly all cases, 400 mg/kg MEMN restored the parameters to nearly normal levels.
Table 9.
Effect of methanolic extract of Myrica nagi (MEMN) on a. insulin b. adiponectin c. free fatty acids (FFA) d. monocyte chemoattractant protein (MCP)-1 e. resistin f. leptin in a high-fat diet (HFD) model.
| Control | HFD | Orlistat | MEMN 100 mg/kg |
MEMN 200 mg/kg |
MEMN 400 mg/kg |
|
|---|---|---|---|---|---|---|
| Insulin (μU/mL) | 34.2 ± 2.53 | 57.6 ± 5.5### | 32.6 ± 2.56∗∗ | 45.9 ± 3.12b | 38.2 ± 2.36∗∗ | 32.45 ± 4.5∗∗ |
| Glucose (mg/dL) | 101.6 ± 1.05 | 183.5 ± 1.2### | 97 ± 1.5∗∗∗ | 131 ± 1.95∗∗ | 128.83 ± 1.89∗∗∗ | 127.82 ± 1.47∗∗∗ |
| Adiponectin (μg/mL) | 10.12 ± 0.89 | 3.23 ± 0.45### | 7.45 ± 0.85∗∗∗ | 6.22 ± 0.78∗∗∗ | 7.64 ± 0.89∗∗∗ | 8.89 ± 0.52∗∗∗ |
| FFA((mmol/L) | 0.41 ± 0.05 | 0.89 ± 0.04### | 0.68 ± 0.04b | 0.52 ± 0.06∗∗ | 0.44 ± 0.09∗∗ | 0.41 ± 0.03∗∗ |
| MCP-1 (pg/mL) | 22.2 ± 1.2 | 46.1 ± 1.02### | 26.5 ± 1.3∗∗ | 40.8 ± 1.5∗∗ | 38.5 ± 2.1∗∗ | 22.4 ± 1.4∗∗ |
| Plasma resistin (pg/mL) | 215.6 ± 9.56 | 520.39 ± 21.5### | 220.5 ± 19.8∗∗ | 435.6 ± 13.5∗∗ | 330.39 ± 21.9∗∗ | 244.56 ± 14.8∗∗ |
| Plasma leptin levels (ng/mL) | 2.2 ± 0.7348 | 18.7 ± 2.939### | 6.98 ± 2.67∗∗∗ | 8.18 ± 2.53∗∗∗ | 7.5 ± 2.4∗∗∗ | 6.05 ± 2.425∗∗∗ |
Methanolic extract of M. nagi., MEMN; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, # When compared with normal control group, ∗ when compared with inducer group.
3.3.9. Effect of MEMN on fat pad weight
Assessment of the mesenteric and perirenal fat weight revealed that mice fed a HFD, GTG (group II) exhibited a higher fat pad weight than the control animals (group I) (Table 10). Groups treated with MEMN or orlistat (groups III, IV, V, and VI) exhibited lower fat pad weight than that of group II.
Table 10.
Effect of methanolic extract of Myrica nagi (MEMN) on mesenteric fat pad and perirenal fat in a high-fat diet (HFD) model.
| a | ||||||
|---|---|---|---|---|---|---|
| Control | Inducer | Standard | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) | |
| Mesenteric Fat (gm) | 0.301 ± 1.76 | 1.823 ± 1.45### | 0.923 ± 1.89∗∗∗ | 1.6 ± 1.56∗∗ | 1.413 ± 1.4∗∗∗ | 1.215 ± 1.62∗∗∗ |
| b | ||||||
|---|---|---|---|---|---|---|
| Fat Pad Weight (gm) | Control | GTG | Orlistat | MEMN (100 mg/kg) | MEMN (200 mg/kg) | MEMN (400 mg/kg) |
| Mesenteric Fat | 0.56 ± 0.10 | 1.45 ± 0.10### | 0.92 ± 0.10∗∗∗ | 1.4 ± 0.10∗∗∗ | 1.31 ± 0.10∗∗∗ | 1.28 ± 0.10∗∗∗ |
| Epididymal Fat | 1.31 ± 1.54 | 2.85 ± 1.59### | 1.83 ± 1.45∗∗∗ | 2.51 ± 1.24∗∗∗ | 2.5 ± 1.54∗∗∗ | 2.2 ± 1.54∗∗∗ |
| Perirenal Fat | 0.61 ± 1.76 | 0.97 ± 1.45### | 0.81 ± 1.89∗∗∗ | 0.91 ± 1.56∗∗∗ | 0.89 ± 1.44∗∗∗ | 0.86 ± 1.62∗∗∗ |
Methanolic extract of M. nagi., MEMN; GTG., Gold thio glucose; p.o., per oral. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001, #p < 0.05, ##p < 0.01, # When compared with normal control group, ∗ when compared with inducer group.
3.3.10. Histopathological evaluation HFD model
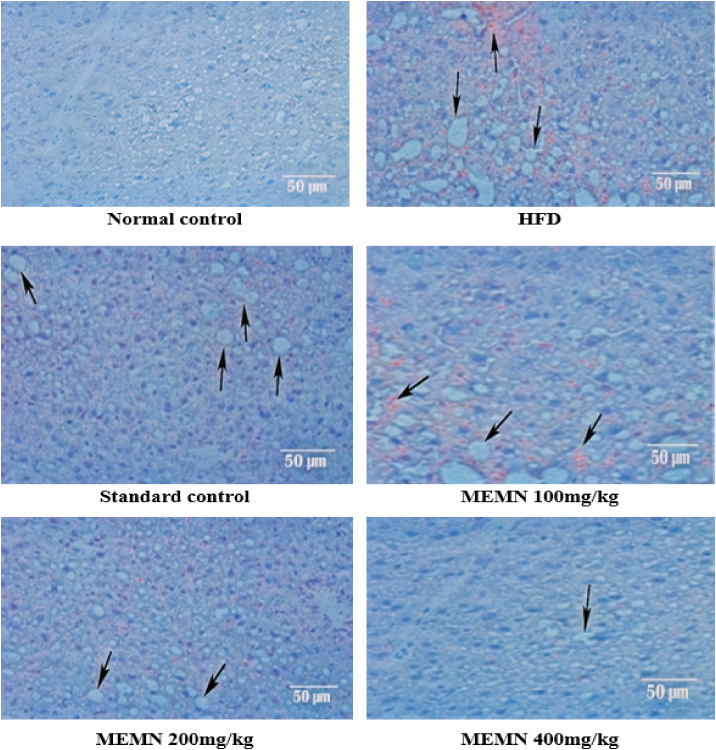
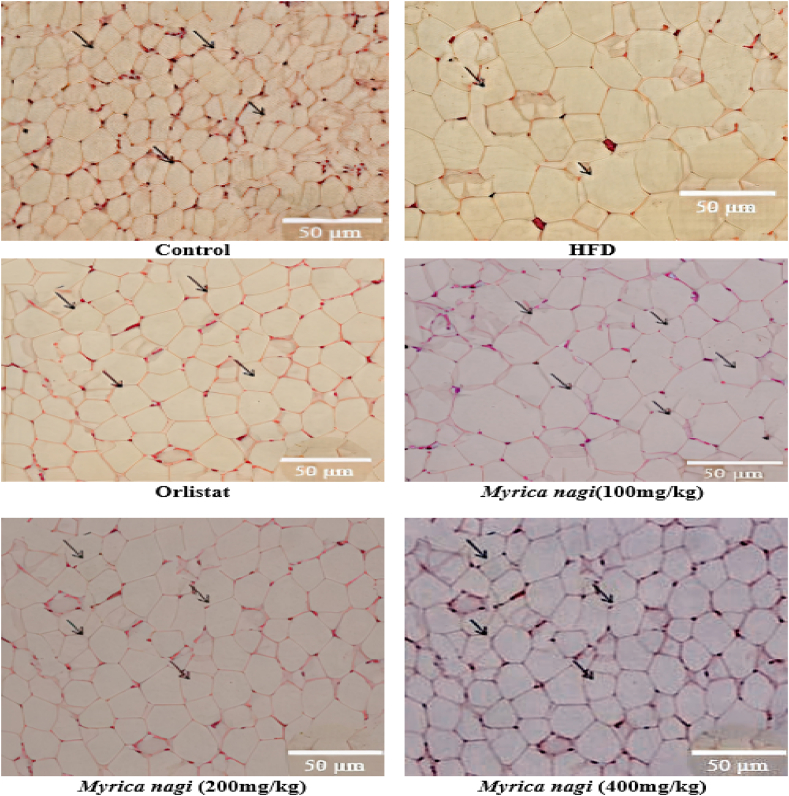
A histopathological examination of the liver and mesenteric fat showed that hepatocytes were normal in the control group, while microvesicular fatty changes and cellular necrosis were detected in the liver cells of the induced group. Remarkable hepatoprotection was observed in the standard drug group, whereas the treatment groups showed some degree of protection, with few foamy-looking cells. In the mesenteric fat pad of normal control mice, uniform spherical cells with a normal shape and structure could be seen, whereas in the induced group, decreased membrane integrity and accumulation of fat were observed. A remarkable reduction in cell size was also observed in the standard drug-treated group; a phenomenon also noted in the treatment groups (Fig. 1, Fig. 2).
Fig. 1.
Histopathological changes in mouse liver of a high-fat diet (HFD) model 100× Magnification.
Fig. 2.
Histopathological changes in mouse mesenteric fat in a high-fat diet (HFD) model 100× magnification.
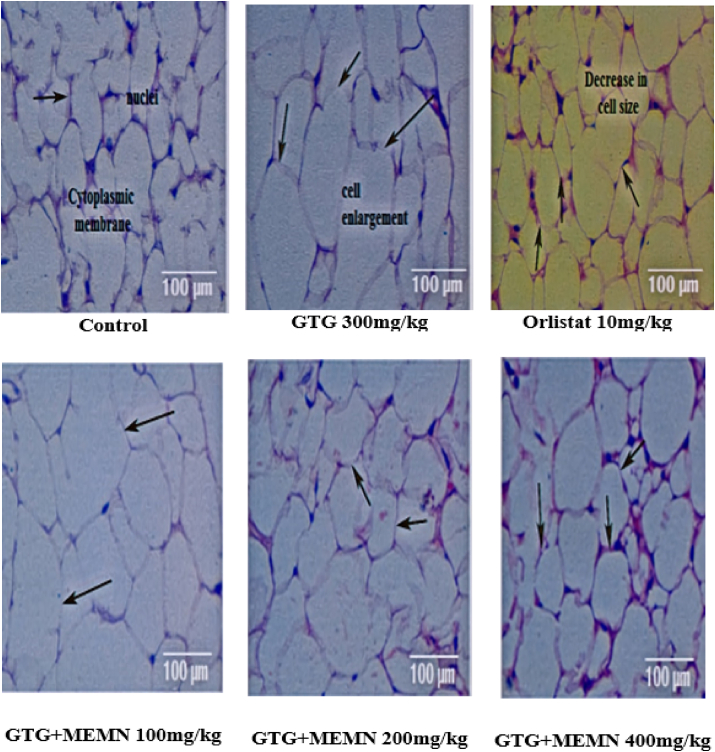
3.3.11. Histopathological analysis GTG model
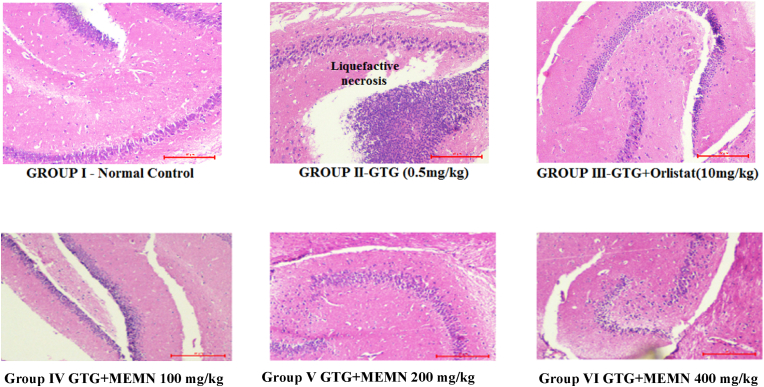
Histopathological analysis of adipose, liver, and brain tissue showed only minor damage, with an acceptable range of toxicity that did not affect any cellular functions. Specifically, only GTG-treated mouse adipose tissue samples exhibited increased cell size, whereas those from orlistat-treated mice showed decreased cell size. The GTG + MEMN 400 mg/kg group displayed less cellular damage and a similar cell size as the control group (Fig. 3). This result confirmed a strong effect of MEMN and orlistat on GTG-treated mice.
Fig. 3.
Histopathological analysis of adipose tissue in a gold thioglucose (GTG) model 100x.
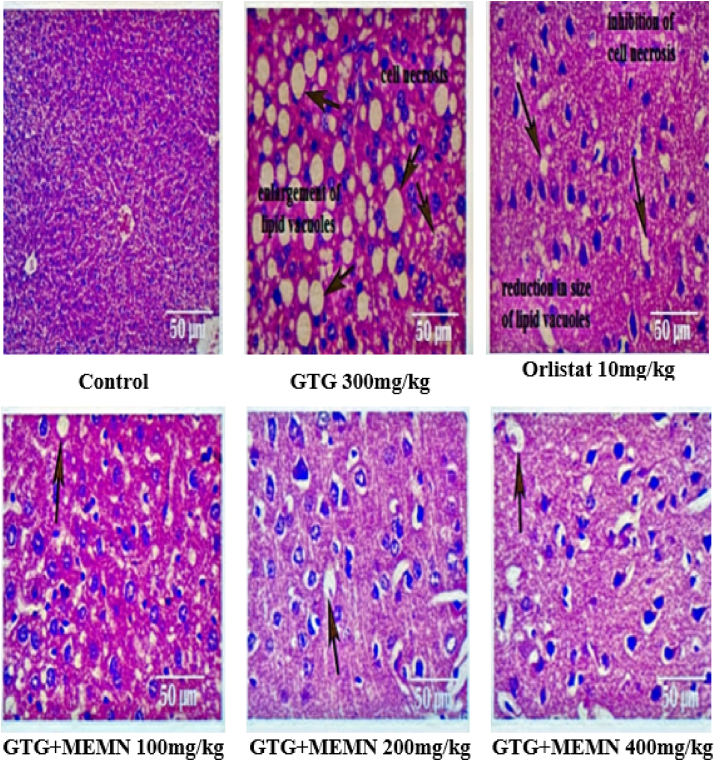
In the case of the liver, GTG induction resulted in more tissue damage and extremely low cell proliferation than those in control mice (Fig. 4), whose cells were tightly packed and healthy. Mice in the GTG group were characterized by enlarged lipid vacuoles and micro vesicular fatty changes leading to necrosis of hepatocytes, whereas orlistat-treated mice showed a reduction in cell size that protected cells from necrosis. MEMN treatment groups also exhibited varying degrees of hepatoprotection, with few foamy cells and less micro vesicular fatty change.
Fig. 4.
Histopathological analysis of liver tissue in a gold thioglucose (GTG) model. 100x.
In brain tissue samples, cells appeared healthy and alive in the control group, but detached from the surface and liquefied in the GTG group, leading to liquefactive necrosis (Fig. 5). All remaining groups exhibited a pattern similar to that in the control group and, hence, looked substantially better than the GTG group.
Fig. 5.
Histopathological evaluation of brain tissue in a gold thioglucose (GTG) model 100x.
4. Discussion
Our study demonstrates that administration of GTG and HFD could successfully generate mouse obesity models and that treatment with MEMN led to excellent control of fat deposition in these two experimental models. Currently, numerous herbal preparations are used to treat various diseases and health problems. Here, we selected a medicinal plant with known beneficial health effects, to determine whether a natural product could be used to treat obesity.
MEMN was prepared using methanol as the solvent. Its phytochemical analysis revealed the presence of six compounds, including amino acids, carbohydrates, flavonoids, saponins, and glycosides. As demonstrated in our previous study, M. nagi extract contains high levels of flavonoids, phenolics, and tannins [48]. A previous LC-MS study showed the presence of various phenolic compounds, including myricanone, myricetin, gallic acid, myricanol, and Β-sitosterol acid, which are effective in reducing body fat [31]. These results are consistent with those of the present study on the effectiveness of MEMN in reducing obesity.
Predominant phenolic compounds in MEMN, such as myricetin and gallic acid, might be responsible for the anti-obesity effects observed in our study. Phenolics have been shown to hinder adipocyte multiplication and TG accumulation, while enhancing lipolysis and leading to a decrease in adipocyte size and number. Myricetin administration to HFD-induced obese mice has been shown to significantly reduce body weight, and insulin [49]. Following previous in vitro findings, additional in vivo studies were conducted and the initial toxicological analysis of MEMN showed that it was not cytotoxic in mice.
The GTG-induced obesity model is an established tool for investigating the pathogenesis of hypothalamic-related obesity. In the present study, GTG administration induced hyperphagia and severe obesity in mice, leading to the development of a diabetic state [50]. The mechanism underlying the establishment of this model is based on the damage GTG causes to the hypothalamus, increasing the levels of TGs, TC, LDL, and VLDL, while decreasing that of HDL [51].
The HFD model is one of the best setups for investigating the pathogenesis of obesity. The binding of saturated fat to receptors, such as Toll-like receptor 2 (TLR2) and TLR4, on microglia, which protect the hypothalamus, leads to the release of cytokines. These include TNF-α, interleukin (IL)-1β, and IL-6, which then damage the neurons that control hunger and thermogenesis [52]. HFD-induced hypothalamic inflammation and altered gut microflora lead to the establishment of one of the most efficient models characterized by elevated plasma levels that can be attributed to high levels of TC, TGs, LDL, and VLDL but concomitantly low HDL levels [53]. HFD also leads to increased glucose levels in mice [54], and all these factors favor the use of this model to investigate lipid levels in mice. Hence, the HFD model was used to advance our understanding of the pharmacological properties of M. nagi in obesity. In our present study, administration of HFD for 16 weeks led to obesity, dyslipidemia, obesity-induced inflammation, hepatic imbalance, and even affected the weight of various organs. This was indicated by weight gain as well as increased feed consumption, BMI, Lee index, temperature, blood lipids, and hepatic markers.
Our study confirmed an increase in adipokines produced by adipose tissues, such as resistin, leptin, and MCP-1, in obese mice. The increase in other adipokines leads to decreased secretion of adiponectins [55]. MCP-1 is considered an inflammatory biomarker and is upregulated in both hereditary and diet-induced corpulence [56]. Secretion of pro-inflammatory cytokines such as MCP-1 induces macrophage accumulation and adipocyte dysfunction [57,58].
The results of liver, brain, and fat histological examinations indicate a marked difference in the efficacy of phenolics in M. nagi, as the 400 mg/kg dose exhibited more potent protective effect than the untreated HFD- and GTG-induced groups. Adipose tissue increases with the number of cells and amount of stored lipids, eventually leading to weight gain [59]. MEMN administration reduced the number of epididymal adipocytes and hepatocytes, decreasing the fat mass compared to that in the untreated model. This observation suggests that our extract might inhibit adipogenesis and lipid accumulation.
The results obtained with the various doses of MEMN were compared with the results of the control groups. Monitoring of metabolic and physical changes showed elevated levels of all parameters in the GTG group, whereas they were efficiently controlled in all MEMN-treated mice. MEMN exhibited anti-obesity, hepatoprotective, and hyperlipidemic activities, while also lowering liver enzymes levels. The hormone leptin supports the nourishment and homeostasis of tissues, such as the liver and muscles [59]. In our study, HFD and GTG induction of obesity increased the leptin levels, in accordance with the results of a previous study [60]. Adiponectin stimulates food intake and decreases energy consumption [61]. In the current study, MEMN promoted the production of leptin but inhibited that of adiponectin, suggesting that phytochemicals in MEMN modulated levels of adiponectin and leptin.
Nonetheless, to confirm the pharmacological activity of MEMN in lowering cholesterol transport and blood glucose and related increased lipogenesis, the findings observed in vitro and in animal models must be eventually extrapolated to clinical studies in humans.
5. Conclusion
Taken together, our findings support and encourage the use of herbal extracts for treating obesity, particularly because they are thought to possess fewer side-effects than conventional chemical agents, while exhibiting potent pharmacological activity. Numerous studies have reported that herb-based drugs show fewer side-effects than allopathic drugs. M. nagi extract has a broad range of applications as it contains numerous bioactive components with a wide therapeutic set of targets. The findings of this study suggest that MEMN has potent activity even at low doses and, thus, could offer an effective alternative for the treatment or prophylaxis of obesity. From above study it was also found that M. nagi extract has a potential to reduce the obesity effects through different biochemical and body parameters as compared to obese group. From the present findings and supported literature concluded that MEMN have safe beneficial effect on reducing obesity state of mice. The data of our study promotes the use of herbal extracts as potent agents to alter various adipokines levels and reduce inflammation associated obesity.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Authors have no conflict of interest to declare.
Acknowledgements
The authors wish to thank the Director and Management of Rayat Institute of Pharmacy, SBS Nagar, Punjab.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 2.Roth C.L., Jain V. Rising obesity in children: a serious public health concern. Indian J Pediatr. 2018;85(6):461–462. doi: 10.1007/s12098-018-2639-7. [DOI] [PubMed] [Google Scholar]
- 3.Shiwaku K., Anuurad E., Enkhmaa B., Nogi A., Kitajima K., Shimono K., et al. Overweight Japanese with body mass indexes of 23.0–24.9 have higher risks for obesity-associated disorders: a comparison of Japanese and Mongolians. Int J Obes Relat Metab Disord. 2004;28:152–158. doi: 10.1038/sj.ijo.0802486. [DOI] [PubMed] [Google Scholar]
- 4.Romieu I., Dossus L., Barquera S., Blottière H.M., et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., et al. Obesity management task force of the European association for the study of obesity., 2015. European guidelines for obesity management in adults. Obes. Facts. 2015;8:402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadowaki T., Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 7.Löhn M., Dubrovska G., Lauterbach B., Luft F.C., Gollasch M., Sharma A.M. Periadventitial fat releases a vascular relaxing factor. Faseb J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 8.Proietto J. Chemical messengers: how hormones make us hungry and full. Univ. Melbourne Conversation Acad. Rigour. 2015;3:1001–1080. [Google Scholar]
- 9.Laurencikiene J., Rydén M. Liver X receptors and fat cell metabolism. Int J Obes. 2012;36:1494–1502. doi: 10.1038/ijo.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiomi Y., Yamauchi T., Iwabu M., Okada-Iwabu M., Nakayama R., Orikawa Y., et al. A novel peroxisome proliferator-activated receptor (PPAR) α agonist and PPARγ antagonist, Z-551, ameliorates high-fat diet-induced obesity and metabolic disorders in mice. J Biol Chem. 2015;290:14567–14581. doi: 10.1074/jbc.M114.622191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaoka I. Modification of core body temperature by amino acid administration. Asia Pac J Clin Nutr. 2008;17:309–311. doi: 10.1021/jf903355q. [DOI] [PubMed] [Google Scholar]
- 12.Bulcão C., Ferreira S.R., Giuffrida F.M., Ribeiro-Filho F.F. The new adipose tissue and adipocytokines. Curr Diabetes Rev. 2006;2:19–28. doi: 10.2174/157339906775473617. [DOI] [PubMed] [Google Scholar]
- 13.Antuna-Puente B., Feve B., Fellahi S., Bastard J.P. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Bastard J.P., Maachi M., Lagathu C., Kim M.J., Caron M., Vidal H., et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 15.de Souza Batista C.M., Yang R.Z., Lee M.J., Glynn N.M., Yu D.Z., Pray J., et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 16.Klöting N., Berndt J., Kralisch S., Kovacs P., Fasshauer M., Schön M.R., et al. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun. 2006;339:430–436. doi: 10.1016/j.bbrc.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Yang R.Z., Lee M.J., Hu H., Pray J., Wu H.B., Hansen B.C., et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 18.Vinolo M.A., Rodrigues H.G., Festuccia W.T., Crisma A.R., Alves V.S., Martins A.R., et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab. 2012;303:E272–E282. doi: 10.1152/ajpendo.00053.2012. [DOI] [PubMed] [Google Scholar]
- 19.Adnyana I.K., Sukandar E.Y., Yuniarto A., Finna S. Anti-obesity effect of the pomegranate leaves ethanol extract (Punica granatum L.) in high-fat diet induced obese mice. Int J Pharm Pharmaceut Sci. 2014;6:626–631. [Google Scholar]
- 20.Haslam D., Sattar N., Lean M. ABC of obesity--time to wake up. BMJ. 2006;333:640–642. doi: 10.1136/bmj.333.7569.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao M., Liu D. Gene therapy for obesity: progress and prospects. Discov Med. 2014;17:319–328. [PubMed] [Google Scholar]
- 22.Kandasamy A.D., Sung M.M., Boisvenue J.J., Barr A.J., Dyck J.R. Adiponectin gene therapy ameliorates high-fat, high-sucrose diet-induced metabolic perturbations in mice. Nutr Diabetes. 2012;2:e45. doi: 10.1038/nutd.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharitonenkov A. FGFs and metabolism. Curr Opin Pharmacol. 2009;9:805–810. doi: 10.1016/j.coph.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Madsbad S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes Metabol. 2014;16:9–21. doi: 10.1111/dom.12119. [DOI] [PubMed] [Google Scholar]
- 25.Panthari P., Kharkwal H., Kharkwal H., Joshi D.D. Myrica nagi: a review on active constituents, biological and therapeutic effects. Int J Pharm Pharmaceut Sci. 2012;4:38–42. [Google Scholar]
- 26.Kirtikar K.R., Basu B.D. 2nd ed. vol. III. International book distributors; New Delhi: 1999. p. 1699. (Indian medicinal plants). [Google Scholar]
- 27.Nadkarni K.M. 3rd ed. Popular Book Depot; Mumbai: 2002. Indian materia medica; p. 871. [Google Scholar]
- 28.Ayurvedic Pharmacopoeia of India . vol. III. Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homeopathy; New Delhi: 2007. pp. 90–96. (Part 1). [Google Scholar]
- 29.Patel T., Dudhpejiya A., Sheath N. Anti-inflammatory activity of Myrica nagi Linn. bark. Ancient Sci Life. 2011;30:100–103. [PMC free article] [PubMed] [Google Scholar]
- 30.Kar P., Chakraborty A.K., Dutta S., Bhattacharya M., Chaudhuri T.K., Sen A. Fruit juice of silverberry (Elaeagnus) and bayberry (Myrica) may help in combating against kidney dysfunctions. Clin. Phytosci. 2019;5:22. doi: 10.1186/s40816-019-0117-z. [DOI] [Google Scholar]
- 31.Middha S.K., Goyal A.K., Bhardwaj A., Kamal R., Lokesh P., Prashanth H.P., et al. In silico exploration of cyclooxygenase inhibitory activity of natural compounds found in Myrica nagi using LC-MS. Symbiosis. 2016;70:169–178. doi: 10.1007/s13199-016-0417-8. [DOI] [Google Scholar]
- 32.Shen S., Liao Q., Feng Y., Liu J., Pan R., Lee S.M., et al. Myricanol mitigates lipid accumulation in 3T3-L1 adipocytes and high fat diet-fed zebrafish via activating AMP-activated protein kinase. Food Chem. 2019;270:305–314. doi: 10.1016/j.foodchem.2018.07.117. [DOI] [PubMed] [Google Scholar]
- 33.Kokate C.K., Purohit A.P., Gokhale S.B. Nirali Prakashan; 2001. Pharmacognosy by CK kokate; pp. 181–183. [Google Scholar]
- 34.OECD . OECD Publishing; Paris: 2001. Guidelines for the testing of chemicals. Test guideline 423: acute oral toxicity – acute toxic class method. [Google Scholar]
- 35.Lee J.S., Lee M.K., Ha T.Y., Bok S.H., Park H.M., Jeong K.S., et al. Supplementation of whole persimmon leaf improves lipid profiles and suppress body weight gain in rats fed high fat diet. Food Chem Toxicol. 2006;44:1875–1883. doi: 10.1016/j.fct.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Vasselli J.R., Weindruch R., Heymsfield S.B., Pi-Sunyer F.X., Boozer C.N., Yi N., et al. Intentional weight loss reduces mortality rate in a rodent model of dietary obesity. Obes Res. 2005;13:693–702. doi: 10.1038/oby.2005.78. [DOI] [PubMed] [Google Scholar]
- 37.Panchal S.K., Ward L., Brown L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr. 2013;52:559–568. doi: 10.1007/s00394-012-0358-9. [DOI] [PubMed] [Google Scholar]
- 38.Seyedan A., Mohamed Z., Alshagga M.A., Koosha S., Alshawsh M.A. Cynometra cauliflora Linn. attenuates metabolic abnormalities in high-fat diet-induced obese mice. J Ethnopharmacol. 2019;236:173–182. doi: 10.1016/j.jep.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Boucher J., Quilliot D., Pradères J.P., Simon M.F., Grès S., Guigné C., et al. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 2005;48:569–577. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goyal R.K., Kadnur S.V. Beneficial effects of Zingiber officinale on gold thioglucose induced obesity. Fitoterapia. 2006;77:160–163. doi: 10.1016/j.fitote.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Minigh J. In: xPharm: the comprehensive pharmacology reference. Enna S.J., Bylund D.B., editors. Elsevier; Amsterdam: 2007. Gold salts; pp. 1–4. [Google Scholar]
- 42.Chirmade D., Vadnere G., Chirmade H. Assessment of phytochemical and hypolipidemic activity of roots of Inula racemosa Hook. World J Pharmaceut Res. 2015;4:589–603. [Google Scholar]
- 43.Ji W., Zhao M., Wang M., Yan W., Liu Y., Ren S., et al. Effects of canagliflozin on weight loss in high-fat-induced obese mice. PLoS One. 2017;12:559–571. doi: 10.1371/journal.pone.0179960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanarek R.B., Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr. 1982;112:1546–1554. doi: 10.1093/jn/112.8.1546. [DOI] [PubMed] [Google Scholar]
- 45.Novelli E.L.B., Diniz Y.S., Galhardi C.M., Ebaid G.M.X., Rodrigues H.G., Mani F., et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–119. doi: 10.1258/002367707779399518. https://doi.org/10.1258%2F002367707779399518. [DOI] [PubMed] [Google Scholar]
- 46.Bernardis L.L. Prediction of carcass fat, water and lean body mass from Lee's “nutritive ratio” in rats with hypothalamic obesity. Experientia. 1970;26:789–790. doi: 10.1007/bf02232553. [DOI] [PubMed] [Google Scholar]
- 47.Mann A., Thompson A., Robbins N., Blomkalns A.L. Localization, identification, and excision of murine adipose depots. JoVE. 2014;94 doi: 10.3791/52174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prashar Y., Patel N.J. An in vitro approach to evaluate the anti-adipogenic effect of Myrica nagi Thunb. Fruit extract on 3T3-L1 adipocyte cell line. Obes Med. 2020;18:100228. doi: 10.1016/j.obmed.2020.100228. [DOI] [Google Scholar]
- 49.Su H.M., Feng L.N., Zheng X.D., Chen W. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. J Zhejiang Univ - Sci B. 2016;17:437–446. doi: 10.1631/jzus.B1600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karasawa H., Takaishi K., Kumagae Y. Obesity-induced diabetes in mouse strains treated with gold thioglucose: a novel animal model for studying β-cell dysfunction. Obesity. 2011;19:514–521. doi: 10.1038/oby.2010.171. [DOI] [PubMed] [Google Scholar]
- 51.Yu S., François M., Huesing C., Münzberg H. The hypothalamic preoptic area and body weight control. Neuroendocrinology. 2018;106:187–194. doi: 10.1159/000479875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D.E., et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods S.C., Seeley R.J., Rushing P.A., D'Alessio D., Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 54.Ikemoto S., Thompson K.S., Takahashi M., Itakura H., Lane M.D., Ezaki O. High fat diet-induced hyperglycemia: prevention by low level expression of a glucose transporter (GLUT4) minigene in transgenic mice. Proc Natl Acad Sci U S A. 1995;92:3096–3099. doi: 10.1073/pnas.92.8.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu Y.H., Ginsberg H.N. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2015;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 56.Bai Y., Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev. 2015;16:127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang Y.E., Kim J.M., Joung K.H., Lee J.H., You B.R., Choi M.J., et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K.H., Park Y. Food components with anti-obesity effect. Annu Rev Food Sci Technol. 2011;2:237–257. doi: 10.1146/annurev-food-022510-133656. [DOI] [PubMed] [Google Scholar]
- 60.Frederich R.C., Hamann A., Anderson S., Löllmann B., Lowell B.B., Flier J.S. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 61.Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H., et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metabol. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]