Abstract
Introduction
To evaluate the efficacy and safety of lidocaine patches in Chinese patients with postherpetic neuralgia (PHN).
Methods
Patients were randomized to receive lidocaine patches or placebo every day for 4 weeks. Efficacy endpoints included the decrease of analogue scale score (VAS) value at week 4, 2 and 1 and the percentage of patients that achieved a 30% decrease of VAS value. Safety analyses were conducted as well.
Results
Two hundred forty Chinese patients were randomized. At week 1, lidocaine patch-treated patients had a higher clinical response versus placebo, and at week 4, the mean (SD) decreases of VAS value compared to the baseline were 14.01 (14.35) in the treatment group and 9.36 (12.03) in the placebo group (p = 0.0088). Overall, the safety profile in the treatment group was consistent with that observed in the placebo group [adverse event (AE) incidence rate: 33.33% versus 37.29%, p = 0.5857].
Conclusions
Lidocaine patches resulted in improved clinical response versus placebo in the treatment of PHN patients and were well tolerated.
Keywords: Lidocaine patch, Postherpetic neuralgia, Clinical study, Efficacy, Safety
Key Summary Points
|
The authors believe that 5% lidocaine patch is a kind of topical analgesic with good efficacy and safety in treatment of postherpetic neuralgia (PHN). We conducted a randomized, placebo-controlled, multicenter clinical study to verify this and obtained a reliable result. Considering placebo has efficacy in decreasing the analogue scale score (VAS) as well, the result should be evaluated carefully. Since the study was compliant with good clinic practice (GCP) and good design, the article shows the efficacy and safety data of the 5% lidocaine patch and placebo, strictly following the evidence-based medicine method (randomized, placebo-controlled, double blind and multicenter). As a topical analgesic medicine, the greatest challenge in development is to prove a significant difference between the treatment medicine and placebo, and here we present a reliable efficacy result showing that the mean (SD) decreases of VAS value compared to the baseline were 14.01 (14.35) in the treatment group and 9.36 (12.03) in the placebo group (p = 0.0088), with a good safety result as well. This will be useful as a reference for other similar research. |
Introduction
Postherpetic neuralgia (PHN) is defined as pain lasting ≥ 3 months after the healing of herpes zoster (HZ) rash in the European and US guidelines or consensus [1–3]. The clinical manifestations are neuron dysfunction, ectopic discharge, and peripheral and central sensitization, resulting in pain [4–7]. As a common complication of HZ, it seriously affects patients' sleep and quality of life [8, 9].
Systemic painkillers include anticonvulsant drugs (such as pregabalin and gabapentin) and tricyclic antidepressants (such as amitriptyline and nortriptyline) that can help alleviate related pain. Patients with severe pain can consider opioids such as morphine, tramadol and oxycodone. Systemic medication often restricts clinical use because of intolerable systemic adverse reactions [10]. Topical drugs are easy to use and can act locally on painful sites without causing systemic adverse reactions and drug interactions. The widely used topical analgesics are lidocaine and capsaicin [11].
In 1999, FDA approved 5% lidocaine patch for the treatment of PHN. It is a targeted peripheral analgesic. As a non-selective sodium channel inhibitor, it can affect the generation and conduction of nerve impulses, stabilize the nerve membrane and reduce activity of pain receptors. On the other hand, about 3 ± 2% of the maximum recommended dose of lidocaine is systemically absorbed, so the amount of lidocaine can act on local pain relief without anesthetic effect and reduce the risk of systemic toxicity and potential drug interactions with stable release [1, 12].
Methods
Study Design
The study was a Phase 3, randomized, double-blinded, placebo-controlled, multicenter study of lidocaine patches in Chinese patients with PHN. The study was approved by the clinical trial ethics committee of Peking University First Hospital, strictly in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki regarding medical research in humans. Written informed content was obtained from each subject prior to enrollment. The register number in www.chinadrugtrials.org.cn is CTR20182203. The reported analysis was prespecified in the protocol and includes 240 patients recruited from 12 sites in China between 26 December 2018 and 31 August 2020. Eligible patients were randomized to receive lidocaine 700 mg/patch (10 × 14 cm) every day for 4 weeks. All participants provided written informed consent, and no personally identifiable information was used in this study.
Patients
Inclusion Criteria
Patients included adults aged 18 (included)—80 (included) years with moderate to severe neuropathic pain [analogue scale score (VAS) with a mean of ≥ 40 mm for at least 5 days] symptoms on the trunk, limbs or lower neck associated with PHN for > 3 months after the crusting of the acute HZ rash. On the first day of the screening period and the first day of the treatment period, VAS value had to be ≥ 40 mm and in the screening period the 17-item version of the Hamilton Depression Scale (HAMD) scored ≤ 17 points. All subjects understood the content of the trial and signed an informed consent form voluntarily.
Exclusion Criteria
These included: (1) patients with a damaged skin surface at the site of PHN; (2) patients who had undergone destructive nerve block or neurosurgical ablation for the treatment of HZ-related pain; (3) patients who had received minimally invasive interventional therapy or physical therapy for PHN within 1 week before screening, including but not limited to neurointerventional techniques, neuroregulatory techniques, acupuncture therapy, etc.; (4) patients who were being treated with drugs containing local anesthetic ingredients or who were using traditional Chinese medicine for pain relief; (5) patients who were currently using gabapentin or pregabalin to treat PHN before screening and were unwilling to discontinue medication; (6) patients being treated with class I antiarrhythmic drugs (such as tacanyl and mexiletine); (7) patients undergoing treatment with opioids and glucocorticoids; (8) patients who had received treatment for severe cardiopulmonary disease, such as angina pectoris, congestive heart failure, bundle branch block or arrhythmia (including implantation of cardiac pacemakers), within 3 months before screening and whose condition was not stable; (9) patients with malignant blood diseases and other related malignant tumors; (10) patients with liver and renal insufficiency, the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) being 1.5 times higher than the upper limit of normal values and/or serum creatinine (SCr) level being higher than the upper limit of the normal value; (11) patients having a related medical history or currently suffering from severe mental illness, epilepsy or other brain or mental state disorders that affected their ability to self-evaluate; (12) in addition to HZ pain, there were other causes of pain in the HZ area, such as compressive neuropathy (spinal stenosis), fibromyalgia, etc., or before the diagnosis of PHN, there were other painful diseases in the local area which would affect the evaluation of pain sensation in this trial; (13) there were other serious pain disorders that, according to the judgment of the researcher, might confuse the subject's self-evaluation of pain caused by PHN; (14) the presence of other neurological diseases (such as cognitive impairment) that might affect the evaluation of PHN or the ability of the subject to complete the diary card; (15) patients with allergies or contraindications to any of the drugs or excipients permitted in the regimens such as lidocaine (including other amides), NSAIDs and acetaminophen; (16) difference in VAS value between any two records during the screening was > 30 mm; (17) subjects who were breast-feeding or pregnant, or wer planning a pregnancy (or a partner has a pregnancy plan) during the trial; (18) patients who had a history of alcohol or drug abuse; (19) patients who had participated in or were participating in other clinical trials within 1 month before screening; (20) patients who were judged by the investigator to be unsuitable for inclusion.
Early Disengagement Mechanism
After randomization, if the medication was used for ≥ 7 days, and PHN was not alleviated or even worsened, the subject was allowed to end the trial in advance and complete the group exit safety inspection and effectiveness information collection, being then considered to have completed the trial.
Study Procedures
At the first week of screening period, all subjects used the placebo once a day. During the 4-week double-blind trial period, eligible subjects were randomly assigned to the treatment group or placebo group in a 1:1 ratio. Subjects in the treatment group used a lidocaine patch (lidocaine 700 mg, 10 × 14 cm) once a day, and subjects in the placebo group use a placebo patch (lidocaine 0 mg, 10 × 14 cm). The patches are applied to the affected area (where the pain is obvious) no more than 12 h. To ensure the consistency of the evaluation results, it is recommended to start the application before 12:00 a.m., using a maximum of three patches per day.
Efficacy and safety assessments were made at baseline, weeks 1, 2 and 4. Each morning, the subjects evaluated the VAS value of pain over the past 24 h, and this was marked on the subject’s daily pain diary card. During each visit, the subject evaluated the VAS value of the pain in the past week. All scores were expressed as 0–100.
Efficacy Outcomes
Efficacy endpoint in this analysis was the mean value of VAS score at the fourth week of medication compared to those during the screening period (using the VAS score from the subject's daily pain diary card). Other endpoints in this analysis included the percentage of mean value of VAS for the fourth week of medication decreasing ≥ 30% compared to the screening period.
Safety Outcomes
The occurrence of adverse events (AEs) and serious AEs was evaluated over 4 weeks of treatment, including vital signs, laboratory tests (blood routine, urine routine, liver function, kidney function, 12 lead electrocardiogram), pregnancy tests and skin irritation symptoms.
Skin irritation and allergic reaction were evaluated referring to "Guidance for industry: Skin and sensitization testing of generic transdermal drug products by FDA" using the Hill Top Research, Inc., scoring method [13].
Statistical Analysis
The statistical description of measurement data included number of cases, mean, standard deviation, median, maximum, minimum and p value, and the statistical description of counting data included frequency distribution, composition ratio, statistics and p values. Efficacy endpoints were calculated using SAS9.4 with per protocol set (PPS) and full analysis set (FAS). Safety analyses were calculated with safety analysis set (SS).
Results
Disposition of Subjects and their Demographic Characteristics
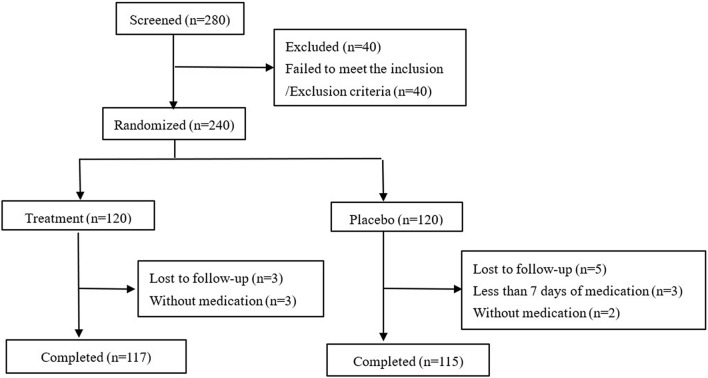
Of the 280 Chinese patients screened in the overall population, 240 were randomized. Of those, 120 received lidocaine patches and 120 received placebo. The number of patients who discontinued the study was similar between treatment groups and placebo groups (3:5, p = 0.4386), considering the balance in size due to the randomization ratio (Fig. 1). One hundred twenty subjects in both thetreatment group and placebo group were included in FAS, 114 subjects in the treatment group and 110 in the placebo group were included in PPS, and 117 subjects in the treatment group and 118 in the placebo group were included in SS.
Fig. 1.
Disposition of subjects
Demographics and baseline disease characteristics were balanced across the treatment group and placebo group. Enrolled patients had a mean (SD) VAS of 62.07 (11.67) in the treatment group and 62.18 (11.57) in the placebo group, and the mean (SD) ages were 66.50 (8.49) and 68.00 (7.61) years, respectively; 65/120 (54.17%) of patients were male in the treatment group and 61/120 (50.83%) in the placebo group. 101/120 (84.17%) and 97/120 (80.83%), respectively. (Table 1).
Table 1.
Demographic and baseline characteristics (FAS)
| Placebo group (n = 120) | Treatment group (n = 120) | p value | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 61 (50.83%) | 65 (54.17%) | 0.6983 |
| Female | 59 (49.17%) | 55 (45.83%) | |
| Age (years), mean (SD) | 68.00 (7.61) | 66.50 (8.49) | 0.2243 |
| Height (cm), mean (SD) | 163.93 (8.24) | 163.37 (7.66) | 0.5917 |
| Wight (kg), mean (SD) | 64.43 (11.31) | 63.18 (11.41) | 0.3267 |
| PHN location, n (%) | |||
| Limbs | 12 (10.00%) | 10 (8.33%) | 0.6962 |
| Trunk | 97 (80.83%) | 101 (84.17%) | |
| Lower neck | 1 (0.83%) | 3 (2.50%) | |
| Limbs and trunk | 8 (6.67%) | 5 (4.17%) | |
| Limbs and lower neck | 0 (0.00%) | 0 (0.00%) | |
| Trunk and lower neck | 2 (1.67%) | 1 (0.83%) | |
| Limbs, trunk and lower neck | 0 (0.00%) | 0 (0.00%) | |
| HAMD (score), mean (SD) | 4.91 (3.37) | 5.26 (3.48) | 0.4895 |
| VAS (mm), mean (SD) | 62.18 (11.57) | 62.07 (11.67) | 0.6286 |
Clinical Efficacy Endpoints
Higher responses were observed with treatment group versus placebo group as early as week 1. At week 4, the mean (SD) decreases of VAS value compared to the baseline were 14.01 (14.35) in the treatment group and 9.36 (12.03) in the placebo group (p = 0.0088), 9.72 (10.79) versus 6.80 (9.34) (p = 0.0363) and 5.68 (7.73) versus 3.09 (6.19) (p = 0.0045) at week 2 and week 1, respectively, in FAS, and were similar in PPS (Table 2).
Table 2.
Summary of VAS value (FAS and PPS)
| FAS | PPS | ||||
|---|---|---|---|---|---|
| Placebo group (n = 120) | Treatment group (n = 120) | Placebo group (n = 110) | Treatment group (n = 114) | ||
| Baseline | Mean (SD) | 62.18 (11.57) | 62.07 (11.67) | 61.42 (11.27) | 62.14 (10.56) |
| z | 0.22 | 0.64 | |||
| p | 0.8249 | 0.5199 | |||
| 95% CI | − 2.84, 3.07 | − 3.60, 2.15 | |||
| Week 1 | Mean (SD) | 59.09 (12.72) | 56.39 (12.65) | 58.46 (12.47) | 56.19 (11.63) |
| Decrease at week 1 | Mean (SD) | 3.09 (6.19) | 5.68 (7.73) | 2.95 (6.27) | 5.95 (7.83) |
| z | 2.84 | 3.29 | |||
| p | 0.0045 | 0.0010 | |||
| 95% CI | − 4.37, − 0.81 | − 4.87, − 1.13 | |||
| Week 2 | Mean (SD) | 55.39 (14.07) | 52.35 (14.04) | 54.49 (13.76) | 51.94 (13.10) |
| Decrease at week 2 | Mean (SD) | 6.80 (9.34) | 9.72 (10.79) | 6.93 (9.65) | 10.20 (10.85) |
| z | 2.09 | 2.42 | |||
| p | 0.0363 | 0.0154 | |||
| 95% CI | − 5.49, − 0.36 | − 5.98, − 0.56 | |||
| Week 4 | Mean (SD) | 52.83 (15.09) | 48.06 (16.95) | 51.96 (14.73) | 47.41 (16.25) |
| Decrease at week 4 | Mean (SD) | 9.36 (12.03) | 14.01 (14.35) | 9.46 (12.24) | 14.73 (14.35) |
| z | 2.62 | 2.98 | |||
| p | 0.0088 | 0.0028 | |||
| 95% CI | − 8.02, − 1.28 | − 8.79, − 1.76 | |||
Thirty percent decrease of VAS value was achieved by more patients receiving lidocaine patches at week 4 versus placebo (34.17% versus 15.83%, p = 0.0009) in FAS; this was similar in PPS (Table 3).
Table 3.
Summary of 30 percent decrease of VAS value (FAS and PPS)
| FAS | PPS | ||||
|---|---|---|---|---|---|
| Placebo group (n = 120) | Treatment group (n = 120) | Placebo group (n = 110) | Treatment group (n = 114) | ||
| 30 percent decrease of VAS value | Not achieved (N, %) | 101 (84.17%) | 79 (65.83%) | 93 (84.55%) | 73 (64.04%) |
| Achieved (N, %) | 19 (15.83%) | 41 (34.17%) | 17 (15.45%) | 41 (35.96%) | |
| χ2 | 11.10 | 12.76 | |||
| p | 0.0009 | 0.0004 | |||
Safety
The frequencies of AE and adverse drug reaction (ADR) were not significantly different between the treatment and placebo group during the 4 weeks of administration (Table 4). Thirty-nine (33.33%) experienced 105 AEs after the administration of lidocaine patches, and 44 (37.29%) experienced 104 AEs after the administration of placebo. The common AEs were attributed to reaction at the administration area (8.55% versus 6.78%) and skin disease (8.55% versus 5.93) including erythema, pruritus rash, skin irritation, rash, dry skin, papules and pruritus (Table 5). Twenty-seven (23.08%) experienced 78 drug-related AEs as ADR after the administration of lidocaine patches, and 22 (18.64%) experienced 58 ADRs after the administration of placebo. No death, serious AE or serious drug-related AE occurred during the study.
Table 4.
Summary of safety information (SS)
| Placebo group (n = 118) | Treatment group (n = 117) | ||||||
|---|---|---|---|---|---|---|---|
| Time | Subject | Incidence rate (%) | Time | Subject | Incidence rate (%) | p | |
| AE | 104 | 44 | 37.29 | 105 | 39 | 33.33 | 0.5857 |
| ADR | 58 | 22 | 18.64 | 78 | 27 | 23.08 | 0.4259 |
Table 5.
Details of AEs in safety information (SS)
| Placebo group (n = 118) | Treatment group (n = 117) | p | |||||
|---|---|---|---|---|---|---|---|
| Time | Subject | Incidence rate (%) | Time | Subject | Incidence rate (%) | ||
| Reaction at the administration area | 38 | 10 | 8.47 | 18 | 10 | 8.55 | 1.0000 |
| Skin disease | 11 | 9 | 7.63 | 42 | 10 | 8.55 | 0.8158 |
| Laboratory examination | 13 | 6 | 5.08 | 5 | 4 | 3.42 | 0.7485 |
| Gastrointestinal system diseases | 10 | 8 | 6.78 | 5 | 4 | 3.42 | 0.3751 |
| Infectious diseases | 7 | 6 | 5.08 | 5 | 5 | 4.27 | 1.0000 |
| Nervous system disease | 6 | 5 | 4.24 | 7 | 6 | 5.13 | 0.5390 |
| Respiratory system disease | 3 | 2 | 1.69 | 4 | 2 | 1.71 | 1.0000 |
| Cardiovascular disease | 5 | 5 | 4.24 | 4 | 4 | 3.42 | 0.4461 |
| Metabolic and nutritional diseases | 2 | 2 | 1.69 | 4 | 3 | 2.56 | 0.6835 |
| Urinary system diseases | 4 | 4 | 3.39 | 3 | 3 | 2.56 | 1.0000 |
| Hepatobiliary diseases | 1 | 1 | 0.85 | 2 | 2 | 1.71 | 0.6218 |
| Immune system diseases | 1 | 1 | 0.85 | 2 | 2 | 1.71 | 0.6218 |
| Ear diseases | 0 | 0 | 0.00 | 2 | 2 | 1.71 | 0.2468 |
| Musculoskeletal diseases | 2 | 2 | 1.69 | 0 | 0 | 0.00 | 0.4979 |
| Eye diseases | 1 | 1 | 0.85 | 1 | 1 | 0.85 | 1.0000 |
| Blood and lymphatic system | 0 | 0 | 0.00 | 1 | 1 | 0.85 | 0.4979 |
Discussion
The annual incidence rate of PHN in the population is 3.9–42.0/100,000. The incidence rate and prevalence of HZ and PHN gradually increase with age. About 65% of patients with HZ > age 60 will have PHN, and 75% of those > age 70 will have PHN. PHN patients have a variety of pain properties, which can feel like burning, electric shock, knife cut, needles or tears and can also be dominated by one or multiple types of pain [14].
The purpose of PHN treatment is to effectively control pain as soon as possible, alleviate accompanying sleep and emotional disorders, and improve quality of life. After the effective dose of recommended drug treatment, it should still be maintained for at least 2 weeks instead of stopping the drug immediately and evaluation of effectiveness and adverse reactions made. Decreases of VAS value ≥ 30% are usually considered clinically effective [15]. The first-line treatment of PHN can be calcium channel regulators (pregabalin and gabapentin), tricyclic antidepressants (amitriptyline) and lidocaine patch.
Lidocaine is an amide-based local anesthetic that can temporarily block the conduction of nerve fibers and has an anesthetic effect, raising the pain threshold and alleviating local pain. It has the characteristics of quick function, high penetration and diffusion ability, long action time, reduced irritation and low toxicity in clinical practice. Rainer et al. [3] investigated the short- and long-term efficacy and safety of 5% lidocaine plaster in the treatment of elderly PHN. Mean average pain intensity improved in the ≥ 70-year-old patients by − 2.1 (SD 2.1) vs. − 2.5 (SD 2.0) for < 70-year-old patients after 4 weeks, by − 1.4 (SD 1.8) vs. − 1.7 (SD 1.3) after 8 weeks and by − 1.5 (SD 1.9) vs. − 2.7 (SD 2.2) after 12 months. Aiping et al. [16] enrolled patients with PHN for > 1 month and treated with with a lidocaine patch for 4 weeks. The average decreases of VAS in the treatment and placebo groups were 20.71 (19.07) and 8.04 (14.25), respectively. In this study, we enrolled the patients with PHN for > 3 months with lidocaine patch treatment for 4 weeks. The average decreases of VAS in the treatment and placebo groups were 14.01 (14.35) and 9.36 (12.03), respectively. Due to the different disease durations of PHN subjects, there were differences in efficacy outcomes in the treatment group, but efficacy outcomes in the placebo group were similar.
Lidocaine patch is used for neuropathic pain with good risk/benefit ratio, safety, tolerance and continuous effectiveness in long-term treatment. The tolerance to lidocaine patch was better than that to pregabalin, an oral administration drug (incidence rate of AE: 5.8% versus 41.2%, p < 0.0001) [12]. Navez et al. [17] enrolled a total of 394 subjects for whom the skin-related incidence rate of ADR was 16.5%, and the main reactions were erythema, pruritus, pain, irritation, rash and dermatitis at 6.3%, 2.8%, 2.0%, 1.8%, 1.8% and 1.5%, respectively. In this study, the skin-related incidence rate of AE was 17.01% (20/117), and the main reactions were erythema (5.13%, 6/117), pruritus (5.13%, 6/117), burning sensation (2.56%, 3/117), rash (2.56%, 3/117), pain (1.71%, 2/117) and irritation (1.71%, 2/117). Pruritus occurred in addition to irritation in one patient, and pain occurred in addition to irritation in another. Whether or not it occurred at the administration site, all skin-related AEs were considered drug related. Considering that only one patient withdrew because of rash and another patient withdrew because of a papule in the treatment group, we believe that the skin-related ADRs were tolerable and similar to the literature reports.
Lidocaine patch for external use in the treatment of PHN has been supported by the consensus of clinical practice guidelines worldwide. It is also widely used for other peripheral neuropathic pain, such as diabetes-related peripheral neuropathic pain, postoperative or post-traumatic neuropathic pain, and it has even been reported to have effect on local nociceptive pain [18]. Nadia et al. [19] reported the efficacy and safety of 5% lidocaine patch and 8% capsaicin patch in the treatment of diabetes patients with peripheral neuropathic pain. The decreases of average daily pain score were 21.1% and 40.7% after 24 weeks treatment of lidocaine patch and capsaicin patch, respectively.
This research, however, is subject to two limitations. First, the study focused on validating the efficacy and safety in PHN. More studies will be conducted to further confirm the effectiveness of the wide use of lidocaine patches in neuropathic pain. Second, the subject number and enrollment were limited. The baseline VAS value is about 60, which might result in the best efficacy. There will neither be no pain relief due to a low baseline nor will the placebo effect be too good because of a high baseline. This ensured that a significant difference could be achieved between the lidocaine patch and placebo. However, baseline levels are uncertain in the real world. We expect that more data will be collected for wide use in future real-world studies.
Conclusions
Lidocaine patch is safe, effective and well tolerated in the treatment of PHN as a first-line treatment drug. In the future, more exploration should be conducted to evaluate the application prospects of lidocaine patch in the treatment of other pathological pain.
Acknowledgements
We thank the participants of the study, the investigator team and clinical CRO team for helpful management. The authors are grateful to the associate editor and the reviewers for their useful feedback that improved this paper.
Funding
The Rapid Service Fee is funded by the sponsor, Hainan Huiyuantang Pharmaceutical Co., Ltd.
Medical Writing and/or Editorial Assistance
Clinical Research Center, China State Institute of Pharmaceutical Industry.
Authors’ Contributions
Aiping Wang: Investigator; Hang Li: Principal Investigator; Zhihong Xie: Investigator; lingfeng Li: Investigator; Xian Jiang: Investigator; Qing Guo: Investigator; Fengming Hu: Investigator; Jianzhong Zhang: Investigator; Yong Cui: Investigator; Yangfeng Ding: Investigator; Hong Fang: Investigator; Xiuping Han: Investigator; Shuping Guo: Investigator; Junlong Wang: Review & Editing; Na Ni: Writing - Original Draft.
Disclosures
All authors (Aiping Wang, Hang Li, Zhihong Xie, lingfeng Li, Xian Jiang, Qing Guo, Fengming Hu, Jianzhong Zhang, Yong Cui, Yangfeng Ding, Hong Fang, Xiuping Han, Shuping Guo, Junlong Wang, and Na Ni) have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the clinical trial ethics committee of Peking University First Hospital, strictly in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki regarding medical research in humans. Written informed content was obtained from each subject prior to the enrollment. The register number in www.chinadrugtrials.org.cn is CTR20182203.
Data Availability
The data that support the findings of this study are available on request from the corresponding author (Hang Li), upon reasonable request.
References
- 1.Binder A, Bruxelle J, Rogers P, et al. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post-herpetic neuralgia results of a double-blind, placebo-controlled, multinational efficacy and safety trial. Clin Drug Investig. 2009;29(6):393–408. doi: 10.2165/00044011-200929060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hans G, Sabatowski R, Binder A, et al. Efficacy and tolerability of a 5%lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study. Curr Med Res Opin. 2009;25(5):1295–1305. doi: 10.1185/03007990902901368. [DOI] [PubMed] [Google Scholar]
- 3.Sabatowski R, Bösl I, König S, et al. Treatment of postherpetic neuralgia with 5% lidocaine medicated plaster in elderly patients—subgroup analyses from three European clinical trials. Curr Med Res Opin. 2017;33(3):595–603. doi: 10.1080/03007995.2016.1277990. [DOI] [PubMed] [Google Scholar]
- 4.Yuguang H, Jianguo X. Clinical diagnosis and treatment of neuropathic pain. People’s Medical Publishing House Co. LTD; 2010. pp. 309–324. [Google Scholar]
- 5.Expert group on diagnosis and treatment of neuropathic pain. Consensus on diagnosis and treatment of neuropathic pain. Chinese Journal of Pain Medicine, 2013, 19(12): 705–710.
- 6.Johnson RW, Wasner G, et al. Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother. 2007;7(11):1581–1595. doi: 10.1586/14737175.7.11.1581. [DOI] [PubMed] [Google Scholar]
- 7.Zhiqi Z. Herpes zoster pain: basic and clinical. Chin J Pain Med. 2014;6(20):369–375. [Google Scholar]
- 8.Amato F, Duse G, Consoletti L, et al. Efficacy and safety of 5% lidocaine-medicated plasters in localized pain with neuropathic and/or inflammatory characteristics: an observational, real-world study. Eur Rev Med Pharmacol Sci. 2017;21(18):4228–4235. [PubMed] [Google Scholar]
- 9.Johnson RW, Rice ASC. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 10.de León-Casasola OA, Mayoral V. The topical 5% lidocaine medicated plaster in localized neuropathic pain: a reappraisal of the clinical evidence. J Pain Res. 2016;9(1):67–79. doi: 10.2147/JPR.S99231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AJ. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31(4):235–243. doi: 10.3344/kjp.2018.31.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickering G, Martin E, Tiberghien F, et al. Localized neuropathic pain: an expert consensus on local treatments. Drug Des Dev Ther. 2017;11:2709–2718. doi: 10.2147/DDDT.S142630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA. Guidance for industry: Skin irritation and sensitization testing of generic transdermal drug products [J/OL]. 2000-03-02.
- 14.Shengyuan Y, You W, Qi W, et al. Consensus of Chinese experts on the diagnosis and treatment of postherpetic neuralgia. Chin J Pain Med. 2016;22(3):161–167. [Google Scholar]
- 15.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Aiping W, Xiaowen W, Junying Z, et al. Clinical study on the efficacy and safety of lidocaine gel patch in the treatment of postherpetic neuralgia. J Pract Dermatol. 2015;8(3):181–184. [Google Scholar]
- 17.Navez ML, Monella C, Bösl I, et al. 5% Lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther. 2015;4(1):1–15. doi: 10.1007/s40122-015-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haijuan Z, Pengmei L, Bifa F. Application of lidocaine topical patch in pain treatment. Chin J Pain Med. 2021;27(11):845–850. [Google Scholar]
- 19.Nadia H, Amira SAS, Farideh AJ, et al. The efficacy and safety profile of capsaicin 8% patch versus 5% Lidocaine patch in patients with diabetic peripheral neuropathic pain: a randomized, placebo-controlled study of south Asian male patients. J Diabetes Metab Disord. 2021;20(1):271–278. doi: 10.1007/s40200-021-00741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (Hang Li), upon reasonable request.