Abstract
Introduction
Plaque psoriasis and hidradenitis suppurativa are chronic inflammatory skin conditions with common pathogenetic pathways.
Methods
We report the case of a 38-year-old man with 15-year history of psoriasis and hidradenitis suppurativa successfully treated with tildrakizumab for both conditions. After treatment failure to adalimumab, secukinumab, and guselkumab, tildrakizumab therapy was initiated and resulted in complete remission of psoriasis and the achievement of hidradenitis suppurativa clinical response after 40 weeks, without reporting adverse events. These responses were maintained at week 52.
Conclusion
Tildrakizumab may be an effective and safe therapeutic option for concomitant psoriasis and hidradenitis suppurativa.
Keywords: Case report, Hidradenitis suppurativa, Psoriasis, Tildrakizumab
Key Summary Points
| A case of concomitant psoriasis and hidradenitis suppurativa previously unresponsive to the standard treatment is described. |
| Tildrakizumab therapy was initiated after treatment failure to adalimumab, the only biologic approved for the treatment of moderate-to-severe hidradenitis suppurativa, secukinumab, and guselkumab. |
| To the best of our knowledge, this is the first reported case on successful treatment of concomitant psoriasis and hidradenitis suppurativa with tildrakizumab. |
| Further studies in larger cohorts are required to establish conclusions that can be applied to patients with concurrent psoriasis and hidradenitis suppurativa in clinical practice. |
Introduction
Both plaque psoriasis and hidradenitis suppurativa are chronic inflammatory skin diseases sharing risk factors, such as obesity or cigarette smoking, and inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) or the interleukin (IL)-12/IL-23, and IL-17 pathways, which has been the rationale for selective targeting of these inflammatory pathways using biologics [1]. Furthermore, a positive association has been observed between psoriasis and hidradenitis suppurativa [2], and the co-occurrence of both diseases significantly impacts on patient quality of life [3, 4].
Tildrakizumab is an IL-23p19 inhibitor approved for the treatment of moderate-to-severe plaque psoriasis with demonstrated long-term effectiveness and a favorable safety profile [5, 6]. We present a case of treatment response to tildrakizumab in a patient with psoriasis and hidradenitis suppurativa previously unresponsive to standard therapies.
As this is a single case report, ethics committee approval was not required. All procedures followed were in accordance with the Helsinki Declaration of 1964 and its later amendments. The patient in this manuscript has given written informed consent to publication of his case details and images.
Case report
A 38-year-old man was referred to our dermatology department for the first time in August 2018 (at the age of 33 years) with two inflammatory dermatoses evolving concomitantly since 2008 (when he was 23 years old): severe plaque psoriasis and severe hidradenitis suppurativa. His medical history included schizophrenia (since age 16 years) and surgery for lumbar disk herniation. The patient was receiving daily treatment with olanzapine and zuclopenthixol for schizophrenia. The patient’s weight was 117 kg, and his height was 183 cm. Thus, his body mass index was 34.9 kg/m2, fulfilling the criteria for obesity. He had smoked 1–2 packs of cigarettes per day since he was 14 years and had excessive alcohol consumption (50 g/day).
Previous treatments for psoriasis, including topical corticosteroids and psoralen plus ultraviolet A irradiation, and previous treatments for hidradenitis suppurativa, including oral lymecycline and combined antibiotic therapy with clindamycin and rifampicin, were not effective. The patient had also been treated with the TNF-α inhibitor adalimumab (40 mg per week given by subcutaneous injection) for 6 months without clinical improvement in hidradenitis suppurativa, although good control of psoriasis was achieved (≥ 90% improvement in Psoriasis Area and Severity Index [PASI]).
In August 2018, the patient started treatment with methotrexate (15 mg/week), with poor results on either psoriasis or hidradenitis suppurativa. Hence, treatment with an IL-17 inhibitor (secukinumab) was initiated in October 2018, after monitoring of fecal calprotectin levels [7]. Subcutaneous secukinumab 300 mg was administered every week for 5 weeks (induction phase) followed by 300 mg every 4 weeks. In January 2019, although the psoriasis was controlled, secukinumab treatment was switched to the IL-23p19 inhibitor guselkumab owing to worsening of hidradenitis suppurativa symptoms. Guselkumab 100 mg was administered by subcutaneous injection at weeks 0 and 4, followed by a maintenance dose every 6 weeks with a 50% improvement in the overall disease condition until January 2022, when the patient had a treatment failure of hidradenitis suppurativa, presenting skin lesions on both right and left axillary and inguinal regions, with a Hurley stage of 2 and a Hidradenitis Suppurativa-Physician Global Assessment (HS-PGA) score of 4 (i.e., severe) [8, 9]. His abscess and inflammatory nodule count was 13 (3 abscesses and 10 inflammatory nodules), and his draining fistula count was 2 (Fig. 1).
Fig. 1.
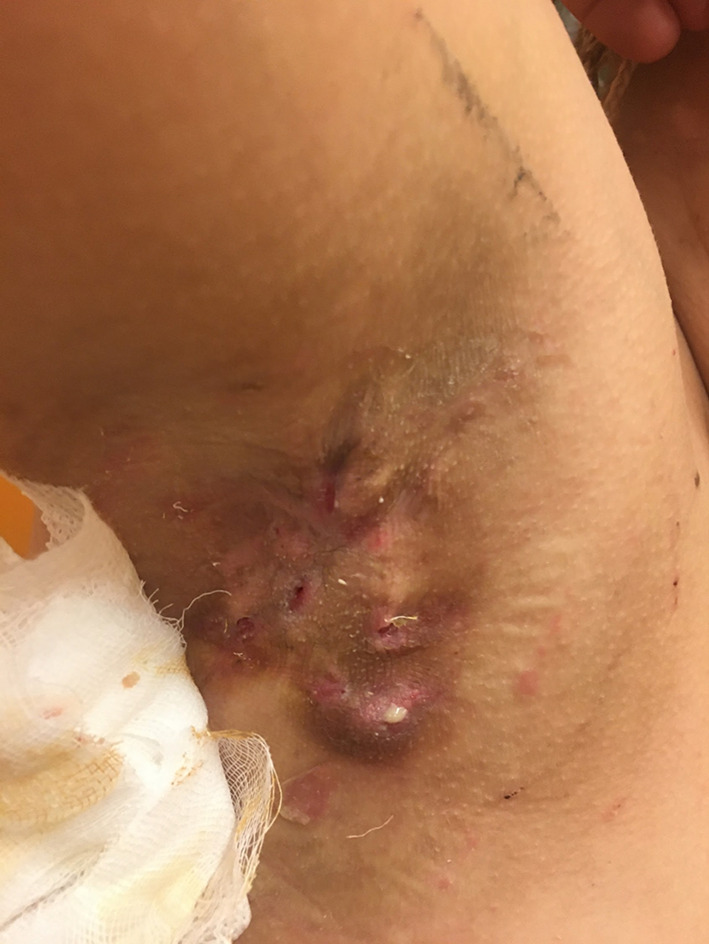
Hidradenitis suppurativa lesions in the right axillary region of patient before treatment with tildrakizumab
In February 2022, guselkumab treatment was switched to 200 mg of subcutaneous tildrakizumab every 12 weeks after the initial loading dose at weeks 0 and 4. At week 40, the Hidradenitis Suppurativa Clinical Response (HiSCR) [10, 11] was achieved, with an abscess and nodule count of 4 (4 inflammatory nodules) and without any draining fistula. The Hurley stage was 2, and the HS-PGA score was reduced to 2 (i.e., mild) (Fig. 2). A complete remission of psoriasis was also observed at week 40, with the patient achieving a PASI 100 response. These responses were maintained at week 52. No adverse events were reported during the 52-week treatment period.
Fig. 2.
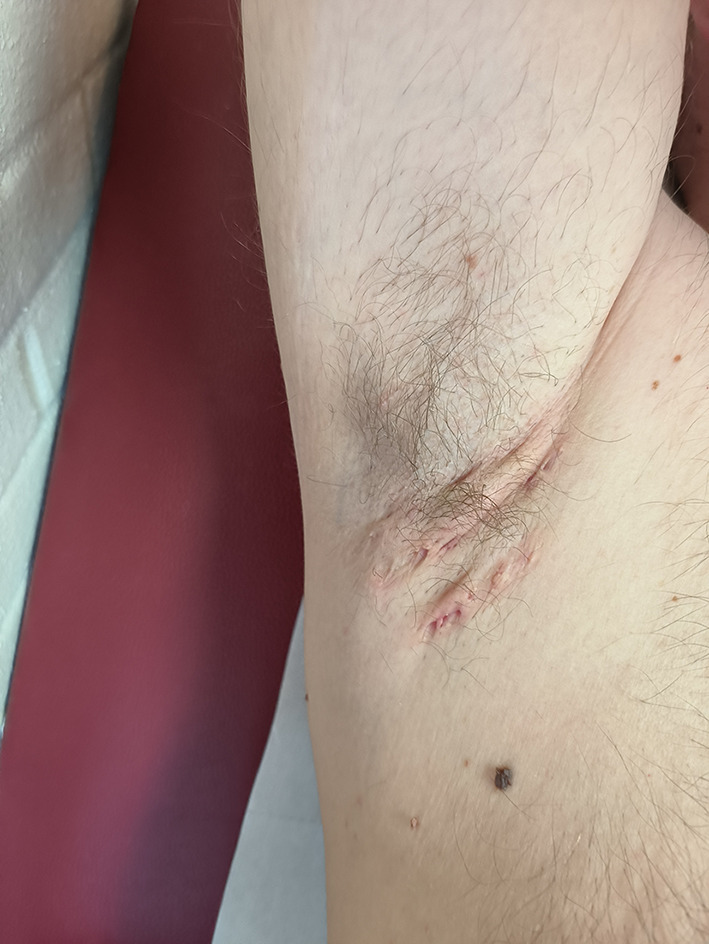
Hidradenitis suppurativa lesions in the right axillary region of the patient after 40 weeks of treatment with tildrakizumab
Discussion
We present herein the case of a patient with concomitant psoriasis and hidradenitis suppurativa. Hidradenitis suppurativa is a highly stigmatizing inflammatory disease whose pathogenesis is not fully understood, although IL-23 cytokine pathway may be involved [1]. The IL-23 axis is also implicated in the pathogenesis of psoriasis, which was reported to be associated with hidradenitis suppurativa [4]. In fact, it is estimated that 6% of patients with hidradenitis suppurativa also suffer from psoriasis [12].
The patient presented herein was initially treated with adalimumab, the only biologic approved for the treatment of moderate-to-severe hidradenitis suppurativa, and the IL-17 inhibitor secukinumab, which has recently been proved to be clinically effective in two phase III trials involving patients with moderate-to-severe hidradenitis suppurativa [13]. However, after 3 months, the treatment was switched to the IL-23p19 inhibitor guselkumab, owing to lack of efficacy, and both diseases improved drastically. Of note, clinical response to IL-23 antagonism in hidradenitis suppurativa has recently been found to be associated with male gender [14]. Despite the long-term control with guselkumab for 3 years, the treatment was switched to tildrakizumab 200 mg, owing to a relapse of hidradenitis suppurativa. To the best of our knowledge, this is the first reported case on successful use of tildrakizumab to treat concomitant psoriasis and hidradenitis suppurativa, with the patient achieving HiSCR [10, 11] and PASI 100 [15] responses after only four tildrakizumab doses. These results suggest that clinical effect of IL-23p19 inhibitors might be influenced by genetic background or gene interactions, showing a different effect depending on the anti-IL-23p19 agent [14, 16], although the use of a higher dose (200 mg) could also play a role. Nine cases of hidradenitis suppurativa successfully treated with off-label use of tildrakizumab have been described in literature [17, 18], and there is also one mention of the use of tildrakizumab as an effective treatment option in patients with PASH syndrome, which is characterized by the presence of pyoderma gangrenosum, acne, and hidradenitis suppurativa [19].
Conclusions
Tildrakizumab 200 mg may be a safe and effective alternative for patients with concurrent hidradenitis suppurativa and psoriasis even when patients switched from another IL-23p19. More studies in a larger cohort of patients are needed to validate these results.
Acknowledgements
We thank the patient for agreeing to publication of his case.
Funding
Sponsorship for this publication and Rapid Service Fee were funded by Almirall R&D, Barcelona, Spain.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Eva Mateu, PhD of TFS HealthScience. Support for this assistance was funded by Almirall R&D, Barcelona, Spain.
Author Contributions
Thomas Damsin confirms sole responsibility for the conception of the work and acquisition of data. He revised the work critically for important intellectual content and approved the version to be published.
Disclosures
Thomas Damsin has no conflicts of interest to disclose.
Compliance with Ethics Guidelines
As this is a single case report, ethics committee approval was not required. All procedures followed were in accordance with the Helsinki Declaration of 1964 and its later amendments. The patient in this manuscript has given written informed consent to publication of his case details and images.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed.
References
- 1.Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kridin K, Shani M, Schonmann Y, Fisher S, Shalom G, Comaneshter D, et al. Psoriasis and hidradenitis suppurativa: a large-scale population-based study. J Am Acad Dermatol. 2018;S0190–9622(18):32962–32971. doi: 10.1016/j.jaad.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Patel M, Cohen JM, Wright NA, Merola JF, Qureshi AA, Vleugels RA. Epidemiology of concomitant psoriasis and hidradenitis suppurativa (HS): experience of a tertiary medical center. J Am Acad Dermatol. 2015;73:701–702. doi: 10.1016/j.jaad.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 4.Gau S-Y, Preclaro IAC, Wei JC-C, Lee C-Y, Kuan Y-H, Hsiao Y-P, et al. Risk of psoriasis in people with hidradenitis suppurativa: A systematic review and meta-analysis. Frot Immunol. 2022;13:1033844. [DOI] [PMC free article] [PubMed]
- 5.Thaçi D, Piaserico S, Warren RB, Gupta AK, Cantrell W, Draelos Z, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate to severe psoriasis who respond at week 28: pooled analyses of two randomised phase 3 clinical trials (reSURFACE 1 and reSURFACE 2) Br J Dermatol. 2021;185:323–334. doi: 10.1111/bjd.19866. [DOI] [PubMed] [Google Scholar]
- 6.Tsianakas A, Schwichtenberg U, Pierchalla P, Hinz T, Diemert S, Korge B. Real-world effectiveness and safety of tildrakizumab in long-term treatment of plaque psoriasis: Results from the non-interventional, prospective, multicentre study TILOT. J Eur Acad Dermatol Venereol. 2023;37:85–92. doi: 10.1111/jdv.18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Brizzi EV, Rocco A, Babino G, Buononato D, Argenziano G, Balato A. Evaluation of the role of faecal calprotectin in the management of psoriatic patients under treatment with biologic drugs. Biomedicines. 2022;10:2968. doi: 10.3390/biomedicines10112968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian hidradenitis suppurativa foundations: part i: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76–90. doi: 10.1016/j.jaad.2019.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, Puig L. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi: 10.1177/20406223211055920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimball AB, Jemec GBE, Yang M, Kageleiry A, Signorovitch JE, Okun MM, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434–1442. doi: 10.1111/bjd.13270. [DOI] [PubMed] [Google Scholar]
- 11.Kimball AB, Sobell JM, Zouboulis CC, Gu Y, Williams DA, Sundaram M, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989–994. doi: 10.1111/jdv.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinter A, Kokolakis G, Rech J, Biermann MHC, Häberle BM, Multmeier J, et al. Hidradenitis suppurativa and concurrent psoriasis: comparison of epidemiology, comorbidity profiles, and risk factors. Dermatol Ther. 2020;10:721–734. doi: 10.1007/s13555-020-00401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball AB, Jemec GBE, Alavi A, Reguiai Z, Gottlieb AB, Bechara FG, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet. 2023;401:747–761. doi: 10.1016/S0140-6736(23)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Flora A, Kozera EK, Jepsen R, Gill K, Xu J, Frew JW. Baseline clinical, hormonal and molecular markers associated with clinical response to IL-23 antagonism in hidradenitis suppurativa: a prospective cohort study. Exp Dermatol. 2023;Online ahead of print. [DOI] [PubMed]
- 15.Balak DMW, Perez-Chada LM, Guo LN, Mita C, Armstrong AW, Bell SJ, et al. Definitions of remission in psoriasis: a systematic literature review from the National Psoriasis Foundation. J Eur Acad Dermatol Venereol. 2022;36:2291–2300. doi: 10.1111/jdv.18477. [DOI] [PubMed] [Google Scholar]
- 16.Kjaersgaard Andersen R, Clemmensen SB, Larsen LA, Hjelmborg JVB, Ødum N, Jemec GBE, et al. Evidence of gene-gene interaction in hidradenitis suppurativa: a nationwide registry study of Danish twins. Br J Dermatol. 2022;186:78–85. doi: 10.1111/bjd.20654. [DOI] [PubMed] [Google Scholar]
- 17.Kok Y, Nicolopoulos J, Howard A, Varigos G, Kern J, Dolianitis C. Tildrakizumab in the treatment of moderate-to-severe hidradenitis suppurativa. Australas J Dermatol. 2020;61:e488–e490. doi: 10.1111/ajd.13377. [DOI] [PubMed] [Google Scholar]
- 18.Kok Y, Nicolopoulos J, Dolianitis C. Tildrakizumab as a potential long-term therapeutic agent for severe Hidradenitis Suppurativa: A 15 months experience of an Australian institution. Australas J Dermatol. 2021;62:e313–e316. doi: 10.1111/ajd.13559. [DOI] [PubMed] [Google Scholar]
- 19.Kok Y, Nicolopoulos J, Varigos G, Howard A, Dolianitis C. Tildrakizumab in the treatment of PASH syndrome: A potential novel therapeutic target. Australas J Dermatol. 2020;61:e373–e374. doi: 10.1111/ajd.13285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed.


