Abstract
Obesity is now recognised as a disease associated with significant morbidity and mortality. One of the most common metabolic complications of obesity is type 2 diabetes, because the two disease share similar pathophysiology. Weight loss is known to ameliorate the metabolic abnormalities underlying type 2 diabetes and improve glycemic control. A 15% or greater total body weight loss (TBWL) in patients with type 2 diabetes will have a disease-modifying effect, a result that is incomparable with other hypoglycemic-lowering interventions. Moreover, in patients with diabetes and obesity, weight loss exerts benefits beyond glycemic control and improves cardiometabolic disease risk factors and well-being. We review evidence supporting the role of intentional weight loss in managing type 2 diabetes. We suggest that many people with type 2 diabetes would benefit from an additional weight-based approach to managing their diabetes. Therefore, we proposed a weight-based treatment goal for patients with type 2 diabetes and obesity.
Keywords: Obesity, Health benefits, Quality of life, Diabetes mellitus, Weight loss
Introduction
Over the past decade, the treatment of type 2 diabetes (T2DM) has evolved and undergone significant conceptual shifts from controlling a single parameter (glucose) to multiple variables affecting disease outcomes. This shift has accelerated after the Food and Drug Administration (FDA) mandated cardiovascular (CVD) safety as an important outcome measure for all new diabetes medications in 2008/2010. The “European Medicines Agency” also indicated that novel compounds developed for T2DM need clinical trials to guarantee CVD safety. Pharmaceutical companies thus had to shift their focus away from glucose control alone to health gains instigated by better diabetes control. The American Diabetes Association (ADA) and European Association for Study of Diabetes (EASD) consensus report in 2022 recommended a patient-centred approach when treating diabetes [1]. This includes consideration of efficacy and key patient factors, which include the effect on diabetes complications such as atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), and heart failure (HF). Moreover, the guidelines also suggest that hypoglycemia risk and the effect of medication on body weight should be considered in addition to factors like cost and patient preference. However, even with these interventions, the management of diabetes mainly focuses on delaying the progression rather than reversing and remission of T2DM. Several landmark trials showed the importance of using substantial weight loss for patients with T2DM to achieve sustained remission and discontinue traditional diabetes medications associated with weight gain [2–4].
Bariatric surgery has the highest rate of sustained weight loss and has become safer with time, but scaling the treatment to millions of patients on T2DM remains challenging. Anti-obesity medications (AOM) are improving and can achieve > 15% weight loss, which has the ability to place T2DM in glycaemic remission [5] or substantially improve glycaemic control [6–10].
A weight-centric approach, instead of glucocentric, may help disrupt T2DM because it addresses the pathophysiology of diabetes rather than controlling its signs and symptoms. Ultimately, interventions can stop the disease from progressing or even reverse the disease when it has occurred. This review will evaluate the clinical evidence supporting weight loss as an integral treatment strategy for T2DM, proposes a novel framework for approaching T2DM, and explores challenges to the widespread implementation of this approach for people living with type 2 diabetes.
Type 2 diabetes and obesity are interconnected heterogeneous diseases
Obesity and T2DM are twin epidemics that share common features and complications. It remains controversial whether T2DM is a metabolic complication of the disease of obesity or whether obesity is a pathognomonic feature in some diabetes phenotypes [11]. Not all patients with obesity are at the same risk of diabetes, while not all patients with T2DM have a raised body mass index (BMI). In a population-based cohort study with more than a million patients, for an equivalent age-adjusted and sex-adjusted incidence of T2DM, BMI that signifies risk for developing T2DM, varied dramatically between ethnic groups. In White populations, a BMI of 30·0 kg/m2 signified the same risk as a BMI of 23.9 kg/m2 for South Asians, 28.1 kg/m2 for Black populations, 26.9 kg/m2 for Chinese, and 26·6 kg/m2 for Arab populations [12].
Similarly, having a high BMI could lead to other metabolic functions in some populations like non-alcoholic steatohepatitis (NASH) [11] while causing no metabolic complications in other populations [13]. The challenge is to identify those patients at high risk of developing diabetes and obesity-related complications with lower weight, fat deposition, or minimal adipose dysfunction. While race is an important parameter, this is not a modifiable factor. Body weight is, however, a modifiable risk marker for diabetes with the potential to be disease-reversing [4].
The WHO (World Health Organization) now redefines obesity as not only based on BMI but also incorporating the health risks and complications caused by excess fat. As a result, the WHO new definition of obesity as “abnormal or excessive fat accumulation that presents a risk to health” [14], and the American Association of Clinical Endocrinologists redefined obesity as an adiposity-based chronic disease (also known as ABCD) to explain its chronic nature [15]. A BMI ≥ 30 kg/m2 has relatively poor sensitivity and specificity for predicting obesity complications, suggesting that BMI can be used as a screening tool to indicate which patients should be investigated for obesity. The new definitions of obesity have higher specificity for adipose tissue disease, leading to better prioritisation of patients for advanced therapeutic interventions. Even though starting body weight is not a perfect marker to diagnose obesity, the amount of weight loss appears to be robust as a marker of treatment benefits that may be achieved [16].
A new proposed framework will focus on the quantity and quality of diabetes complications which can be addressed with substantial weight loss. Practically, health care providers need to be aware that although patients might have the same diagnoses, the appropriate intervention could be different. Moreover, targeting improvement in parameters of glycaemia such as HbA1c, Self-monitoring blood glucose (SMBG), and Continues Glucose Monitoring (CGM) alone will not lead to remission of T2DM, but rather substantial weight loss of > 15% is typically needed to achieve remission [5].
Benefits of weight loss and improving adipose tissue across the disease continuum
Adiposopathy (or sick fat) is a proposed cardiovascular disease (CVD). The concept of adiposopathy may explain the anatomical and/or functional changes to fat cells, which may indirectly increase other CVD risks. Adipose tissue volumes per se are also an integral part of the pathogenesis of diabetes [17]. Visceral adiposity will initiate the development of diabetes through decreasing β-cell volume and function, which in turn results in hyperglycemia and T2DM [18]. Patients with higher pancreatic fat deposition were at higher risk of T2DM, with each percentage increase in pancreatic fat increasing the risk for diabetes by 7% [19]. Decreasing the total fat mass percentage in patients with T2DM by 20.5% (7.49 kg) will reduce the HbA1c by 0.76% [20]. The quality of the adipose tissue function plays an important role in insulin resistance. An agent that improves adipose tissue function can reduce insulin resistance and inflammation, even if they lead to fat tissue expansion and the promotion of weight gain. One of the important mechanisms of the anti-diabetic agent (Pioglitazone) is reversing maladaptive adipose tissue remodelling [21].
Although adipose tissue quantity and quality are essential, the best way to assess and utilise it in practice is still evolving. Hence, the current valid parameter that could be used is the amount of weight loss, which is shown to be effective even at lower BMI. Because non-white races are developing diabetes with lower BMI, it is important to have more cost-effective, sensitive and specific measurements to assess adiposity, especially in those who develop T2DM at lower BMIs [12]. The Asian populations are examples of people who develop T2DM at a lower weight, but reducing weight will still have a significant impact on glycemic status. Using AOM to decrease weight in Asian patients with prediabetes and a BMI of 27 kg/m2 lead to the prevention of diabetes [22]. Furthermore, only 9% remained prediabetic after 68 weeks. In addition, reaching glycaemic remission status was achieved in more than 80% of patients with diabetes despite having BMI ≥ 27 kg/m2 pre-intervention [22]. Moreover, the macrovascular and microvascular complications of T2DM are worse in patients with class III obesity compared with patients with T2DM who have a BMI < 30 kg/m2 [23, 24]. Complications appear to be reduced in those with T2DM who achieved substantial weight loss, regardless of the starting BMI and intervention [25–27]. Recently, the ReTune study (Diabetes remission by weight loss in 'normal-weight people with type 2 diabetes) has shown that weight loss even in patients with a BMI of 24.8 ± 1.7 kg/m2) has achieved sustained remission of diabetes [28].
The recent consensus on diabetes remission defined remission as an HbA1c < 6.5% for three months without glucose-lowering medication. This definition is more straightforward than before but may need refinement in the future because with newer pharmacotherapy options that have weight loss dependent and weight loss independent effects on glycaemic control; a new definition may be required. Here we will discuss different non-invasive and invasive interventions that were able to achieve diabetes remission through focusing on weight loss.
Intensive lifestyle interventions for weight loss in people with type 2 diabetes
The lifestyle intervention could lead to diabetes remission for up to 2 years if patients respond to the treatment and the weight loss occurs early. The DiRECT study was a randomised clinical trial evaluating total diet replacement and the chance of DM remission. Remission was higher among people who maintained more than 15 kg weight loss compared to those who lost less than 5 kg (70% vs 5% remission at two years) [4]. The number of weight loss responders to the intervention remain modest around 20%, but those who do respond do very well. For the entire study population for whom 24-month data were available (n = 272), remission at 24 months was achieved by 29 of 45 participants who maintained at least 10 kg weight loss, and 14 of 20 participants who lost 15 kg or more [4].
The Look AHEAD (Action for Health in Diabetes), a long-term RCT in patients with obesity and type 2 diabetes, was aimed to achieve and maintain weight loss through decreased caloric intake and increased physical activity [29]. Over ten years, approximately 20% of participants lost at least 10% of their body weight in the first year. These weight loss responders had a 21–24% reduction in the risk of cardiovascular outcomes than those with stable weight or weight gain [25]. Also, individuals who had larger month-to-month weight losses in year one and whose weight loss was more sustained during the first year had better maintenance of weight loss over four years, independent of characteristics traditionally linked to weight loss success (p < 0.001) [30]. Approximately 11.5% of patients in the intensive lifestyle intervention had remission of T2DM within the first year of intervention, and 7% were in remission after four years [31].
Challenges of maintaining weight loss in the long term
Although substantial weight loss can be achieved with lifestyle interventions, weight loss maintenance remains a challenge [32]. There are, however, a proportion of patients who achieve weight loss and weight loss maintenance in the long term [25, 33]. This may suggest that lifestyle treatment may be an effective obesity treatment for those patients who maintain weight loss, but that lifestyle treatment may be similar to simple starvation for others who are prone to weight regain.
In those patients where lifestyle treatment only represents simple starvation, the benefit of the initial weight loss on glycaemic control is lost very quickly. Whether this yo-yo pattern of weight, as well as the yo-yo pattern on glycemia, is detrimental still needs to be determined.
Pharmacotherapy associated with weight loss in type 2 diabetes
The Food and Drug Administration has approved five anti-obesity medications: orlistat, phentermine–topiramate, naltrexone–bupropion, liraglutide 3.0 mg, and semaglutide 2.4 mg. These medications can also be used in patients with T2DM for the treatment of obesity because all have a favourable glycemic effect [6–10]. These medications have a varied response, but at least 1 in 4 patients with T2DM are able to achieve and maintain ≥ 10% weight loss, which is almost four-fold better than lifestyle treatment alone [34]. In addition, some agents have weight loss independent effects on glycemia [35].
Modern medications for T2DM, such as the sodium–glucose co-transporter 2 inhibitors (SGLT2i), also have weight loss effects, albeit less effective than the anti-obesity medications. SGLT2i alone can achieve ≥ 10% weight loss in 5.4% of patients, but combining this with phentermine results in 34.9% of participants achieving ≥ 10% weight loss [36].
The newer GLP-1 analogues are now part of the standard of care for T2DM. Semaglutide 1.0 mg dose combined with the SGLT2i, canagliflozin reduced weight by 5.1% and HbA1c by − 1.5% [37]. In addition, 15% of patients were able to achieve ≥ 10% weight loss [37]. The higher doses of Semaglutdie (2.0 mg and 2.4 mg) achieve significantly more weight loss, but the effect on glycemic control does not be much better than the 1.0 mg dose [8, 38].
The newly FDA-approved glucose-dependent insulinotropic polypeptide receptor and glucagon-like peptide-1 receptor agonist (GIP/GLP-1 RA), Tirzepatide, has shown tremendous weight loss in patients with T2DM of more than 10% in 47% of the patients, and more than 15% in quarter of the participants [39]. In addition, a HbA1c reduction of − 1.9 to − 2.3%, with more than 93% of patients achieving an HbA1c < 7% versus insulin degludec where 61% of patients achieved a HbA1c < 7% [40]. This medication and those that will follow soon are able to be scaled and thus have the ability to address obesity in patients with T2DM to disrupt diabetes.
Bariatric surgery
Bariatric surgery acts on several mechanisms to address obesity and T2DM. The primary benefit of these mechanisms is substantial weight loss which seems associated with a reduction in body fat mass set point, making weight loss straightforward and sustainable [41, 42]. The chance of diabetes remission correlates with percentage weight loss [43], but several non-weight loss-dependent mechanisms also contribute. With biliopancreatic diversion, the rate of remission of T2DM is 50% after ten years [3]. Bariatric surgery in patients with T2DM has also been associated with an increased life expectancy of 9.3 years (95% CI 7.1–11.8) [27].
The Microvascular Outcomes after Metabolic Surgery (MOMS) study included renoprotective agents such as empagliflozin and liraglutide as part of the “best medical care” for people with diabetic kidney disease (DKD), but Roux-en-Y Gastric Bypass (RYGB) was still better at achieving remission of DKD at two years [44]. This study, however, shows the benefit of combining bariatric surgery with modern diabetes medicine to thus further amplify the benefits of each modality.
Combination therapy
Combining anti-obesity medications is becoming more popular because the mechanism of the modalities appears to be complementary. The combination of sleeve gastrectomy (SG) and phentermine and topiramate extended-release in patients with a BMI of ≥ 50 kg/m.2 showed that patients with the combination therapy lost about − 38.2% after surgery, while the patients receiving surgery alone only lost − 27% [45]. The GRVITAS study, another RCT, used liraglutide 1.8 mg in combination with RYGB or SG for patients who did not achieve remission of DM. Patients who received liraglutide had better glycaemic control as well as an additional 4.2 kg weight loss compared to placebo after 26 weeks [46]
Practical considerations for making sustained weight loss a primary treatment goal of T2DM
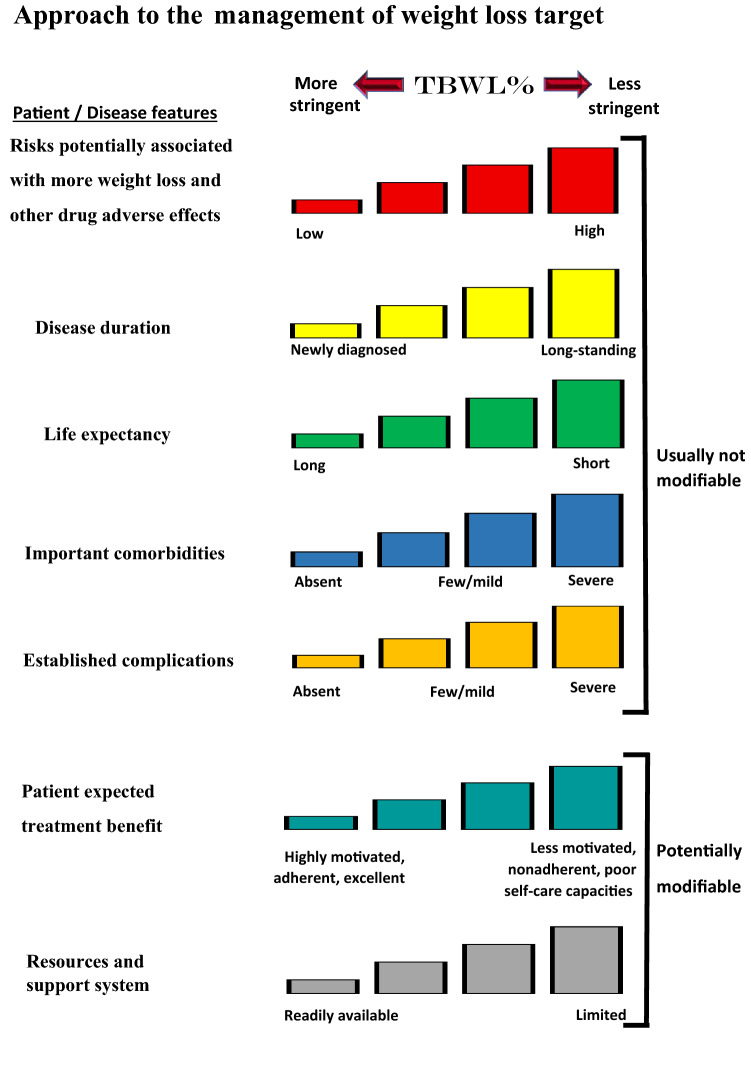
When redefining treatment goals for patients with type 2 diabetes, sustained weight loss should be considered. First, nutritional therapies, pharmacotherapies, and surgical therapies are all valid and potentially helpful options, and each can be disruptive to T2DM. BMI alone is not sufficient to select one treatment above another, but a more personalised approach is needed. Second, the patient's own expectations are important to consider, and this can be used to personalise the treatment further (Fig. 1) [47]. Third, combination therapies may be essential to optimise benefits for the patient with T2DM in the short, medium, and long term.
Fig. 1.
Modulation of the intensiveness of weight loss lowering in type 2 diabetes. Patient and disease factors are used to determine the optimal weight loss target. Characteristics and features toward the left justify more stringent efforts to decrease weight; those toward the right suggest less stringent efforts. Adapted and reprinted with permission from Silvio E. Inzucchi; Richard M. Bergenstal; John B. Buse; Michaela Diamant; Ele Ferrannini; Michael Nauck; Anne L. Peters; Apostolos Tsapas; Richard Wender; David R. Matthews, Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes; Diabetes Care 2015;38(1):140–149, https://doi.org/10.2337/dc14-2441.
Copyright 2015 by the American Diabetes Association [47]
Declarations
Conflict of interest
ClR reports grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He serves on advisory boards of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Sanofi Aventis, AstraZeneca, Janssen, Bristol-Myers Squibb, Glia, and Boehringer Ingelheim. ClR is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He is the chief medical officer and director of the Medical Device Division of Keyron since January 2011. Both of these are unremunerated positions. ClR was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. He continues to provide scientific advice to Keyron for no remuneration. The other authors declare no conflict of interest.
Compliance with Ethical Standards
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetes Care. 2022;45(11):2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397(10271):293–304. doi: 10.1016/S0140-6736(20)32649-0. [DOI] [PubMed] [Google Scholar]
- 4.Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344–355. doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- 5.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/ep161365.Gl. [DOI] [PubMed] [Google Scholar]
- 6.Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained- release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles JM, Leiter L, Hollander P, Wadden T, Anderson JW, Doyle M, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care. 2002;25(7):1123–1128. doi: 10.2337/diacare.25.7.1123. [DOI] [PubMed] [Google Scholar]
- 8.Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 10.Garvey WT, Ryan DH, Bohannon NJ, Kushner RF, Rueger M, Dvorak RV, et al. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care. 2014;37(12):3309–3316. doi: 10.2337/dc14-0930. [DOI] [PubMed] [Google Scholar]
- 11.Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/s2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 12.Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2021;9(7):419–426. doi: 10.1016/S2213-8587(21)00088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3):405–420. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization (WHO) WH: Obesity. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2021). Accessed 5th of March 2022.
- 15.Mechanick JI, Hurley DL, Garvey WT. Adiposity-based chronic disease as a new diagnostic term: the American association of clinical endocrinologists and American college of endocrinology position statement. Endocr Pract. 2017;23(3):372–378. doi: 10.4158/ep161688.Ps. [DOI] [PubMed] [Google Scholar]
- 16.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Klöting N, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99(8):E1466–E1470. doi: 10.1210/jc.2014-1074. [DOI] [PubMed] [Google Scholar]
- 18.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Chan TT, Tse YK, Lui RN-S, Wong GL-H, Chim AM-L, Kong AP-S, et al. Fatty pancreas is independently associated with subsequent diabetes mellitus development: a 10-year prospective cohort study. Clin Gastroenterol Hepatol. 2022;20(9):2014–2022.e4. doi: 10.1016/j.cgh.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, Coleman LA, Miller R, Rooks DS, Laurent D, Petricoul O, et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw Open. 2021 doi: 10.1001/jamanetworkopen.2020.33457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palavicini JP, Chavez-Velazquez A, Fourcaudot M, Tripathy D, Pan M, Norton L, et al. The insulin-sensitizer pioglitazone remodels adipose tissue phospholipids in humans. Front Physiol. 2021;12:784391. doi: 10.3389/fphys.2021.784391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206. doi: 10.1016/S2213-8587(22)00008-0. [DOI] [PubMed] [Google Scholar]
- 23.Xing Z, Pei J, Huang J, Peng X, Chen P, Hu X. Relationship of obesity to adverse events among patients with mean 10-year history of type 2 diabetes mellitus: results of the ACCORD study. J Am Heart Assoc. 2018;7(22):e010512. doi: 10.1161/JAHA.118.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/s0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Look AHEAD Research Group. Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. doi: 10.1016/S2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 27.Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830–1841. doi: 10.1016/S0140-6736(21)00591-2. [DOI] [PubMed] [Google Scholar]
- 28.Taylor R, Irvine KM, Barnes AC, Kelly TL, Clark LG, Hollingsworth KG, Al-Mrabeh A, Holman RR, Romeres D, Cobelli C. 218-LB: remission of type 2 diabetes after weight loss in “normal” weight people—the ReTUNE study. Diabetes. 2022;71(Supplement_1):218–LB. doi: 10.2337/db22-218-LB. [DOI] [Google Scholar]
- 29.Look AHEAD Research Group. Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3(3):202–15. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neiberg RH, Wing RR, Bray GA, Reboussin DM, Rickman AD, Johnson KC, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity. 2012;20(10):2048–2056. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 33.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond) 2013;37(11):1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 35.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollander P, Bays HE, Rosenstock J, Frustaci ME, Fung A, Vercruysse F, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–639. doi: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]
- 37.Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 38.Frías JP, Auerbach P, Bajaj HS, Fukushima Y, Lingvay I, Macura S, et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9(9):563–574. doi: 10.1016/S2213-8587(21)00174-1. [DOI] [PubMed] [Google Scholar]
- 39.Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. doi: 10.1016/s0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 40.Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–598. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 41.Akalestou E, Miras AD, Rutter GA, le Roux CW. Mechanisms of weight loss after obesity surgery. Endocr Rev. 2022;43(1):19–34. doi: 10.1210/endrev/bnab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurenius A, Larsson I, Melanson KJ, Lindroos AK, Lönroth H, Bosaeus I, et al. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur J Clin Nutr. 2013;67(2):168–173. doi: 10.1038/ejcn.2012.208. [DOI] [PubMed] [Google Scholar]
- 43.Sjöholm K, Pajunen P, Jacobson P, Karason K, Sjöström CD, Torgerson J, et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia. 2015;58(7):1448–1453. doi: 10.1007/s00125-015-3591-y. [DOI] [PubMed] [Google Scholar]
- 44.Cohen RV, Petry TBZ, Miras AD, Aboud CM, Johnson B, dos Santos TM, et al. Renoprotective effects of the combination of empagliflozin and liraglutide compared with Roux-en-Y gastric bypass in early-stage diabetic kidney disease: a post hoc analysis of the microvascular outcomes after metabolic surgery (MOMS) randomized controlled clinical trial. Diabetes Care. 2021;44(10):e177–e179. doi: 10.2337/dc21-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ard JD, Beavers DP, Hale E, Miller G, McNatt S, Fernandez A. Use of phentermine-topiramate extended release in combination with sleeve gastrectomy in patients with BMI 50 kg/m2 or more. Surg Obes Relat Dis. 2019;15(7):1039–1043. doi: 10.1016/j.soard.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Miras AD, Pérez-Pevida B, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–559. doi: 10.1016/s2213-8587(19)30157-3. [DOI] [PubMed] [Google Scholar]
- 47.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2014;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]