Abstract
Pioglitazone ameliorates liver dysfunction in type 2 diabetes (T2D) patients with non-alcoholic fatty liver disease (NAFLD); however, its efficacy in T2D patients with alcoholic fatty liver disease (AFLD) is unclear. Here, we conducted a retrospective single-center trial investigating whether pioglitazone ameliorates liver dysfunction in T2D patients with AFLD. T2D patients (n = 100) receiving 3 months of additional pioglitazone were divided into those with or without fatty liver (FL), and those with FL were further classified into AFLD (n = 21) and NAFLD (n = 57) groups. The effects of pioglitazone were compared across groups using medical record data on body weight changes; HbA1c, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (γ-GTP) levels; and fibrosis-4 (FIB-4) index. The pioglitazone dose (mean dose: 10.6 ± 4.6 mg/day) did not affect weight gain but significantly decreased the HbA1c level in patients with or without FL (P < 0.01 and P < 0.05, respectively). The decrease in HbA1c level was significantly more pronounced in patients with FL than in those without FL (P < 0.05). In patients with FL, the HbA1c, AST, ALT, and γ-GTP levels significantly decreased after pioglitazone treatment than before (P < 0.01). The AST and ALT levels, but not the γ-GTP level, and the FIB-4 index significantly decreased after pioglitazone addition in the AFLD group, similar to that in the NAFLD group (P < 0.05 and P < 0.01, respectively). Similar effects were observed following low-dose pioglitazone treatment (≤ 7.5 mg/day) (P < 0.05) in T2D patients with AFLD and NAFLD. These results suggest that pioglitazone may be also an effective treatment option for T2D patients with AFLD.
Keywords: Pioglitazone, Alcoholic fatty liver disease, Non-alcoholic fatty liver disease, Type 2 diabetes
Introduction
Pioglitazone, a thiazolidinedione compound, has been used worldwide as an antidiabetic drug because of its ability to improve glucose and lipid metabolism by reducing insulin resistance in the adipose, liver, and muscle tissues of patients with type 2 diabetes (T2D) [1–3]. In adipose tissues, these effects are mainly initiated via the activation of peroxisome proliferator-activated receptor-γ (PPAR-γ). PPAR-γ stimulates large adipocytes to differentiate into small adipocytes, resulting in increased levels of adiponectin and decreased levels of free fatty acids, tumor necrosis factor-α, and resistin secreted by adipocytes [4, 5]. These changes in adipokines ameliorate insulin resistance in the liver and muscles by inhibiting gluconeogenesis, activating AMP-activated protein kinase, stimulating fatty acid oxidation, and inhibiting hepatic fatty acid synthesis [6, 7]. Furthermore, pioglitazone affects serum adipokine concentrations and decreases intracellular triglyceride levels in the liver and skeletal muscle of patients with T2D compared with metformin [8].
Patients with T2D have a high incidence of fatty liver, and most of these patients are diagnosed with non-alcoholic fatty liver disease (NAFLD) [9]. NAFLD is regarded as the hepatic phenotype of metabolic syndrome, and its incidence is increasing globally with the increase in the incidence of obesity and T2D [10, 11]. It is a broad disease concept that includes conditions ranging from simple steatosis to non-alcoholic steatohepatitis (NASH). NASH is characterized by hepatocyte death, inflammation, and varying degrees of interstitial fibrosis, and it can eventually progress to liver cirrhosis and hepatocellular carcinoma (HCC) [12, 13]. Alcohol is an important cause of fatty liver and the main causative factor of alcoholic cirrhosis. Additionally, the risk of liver cirrhosis increases exponentially with alcohol consumption [14–16]. The most common chronic liver disease worldwide is alcoholic liver disease, which is caused by excessive alcohol consumption; it accounts for 30% of HCC cases and HCC-specific deaths [17, 18]. As diabetes is related to an increased risk of HCC [19], the treatment of fatty liver is also crucial for patients with T2D, regardless of its cause.
Several pharmacological agents have been investigated to determine their efficacy against NAFLD and NASH [20, 21]. Multiple randomized controlled trials that examined the effect of pioglitazone on NASH have reported high-quality evidence and an additional histological improvement; however, only some of these trials included patients with diabetes [22–30]. Pioglitazone has been shown to have superior efficacy over placebo in terms of lowering the NAFLD activity score of steatosis, ballooning necrosis, and lobular inflammation [22–24, 27–29], as well as reducing fibrosis [22, 25, 26]. Furthermore, meta-analyses of the combination of rosiglitazone and pioglitazone have shown that thiazolidinedione derivatives improve liver histology and fibrosis in NASH [28, 30]; an analysis of pioglitazone alone has shown a reduction in fibrosis [26].
In a recent study limited to Asian patients with NASH, a 24-week pioglitazone treatment was well-tolerated, and it effectively improved liver histology and reduced liver steatosis [31]. Furthermore, even small doses of pioglitazone improve NAFLD in patients with T2D [32]. However, the effectiveness of pioglitazone in T2D patients with alcoholic fatty liver disease (AFLD) is unknown. AFLD, similar to NAFLD, presents a high risk of death related to liver disorder and all-cause morbidity, including that from extrahepatic malignancies [33]. Therefore, pioglitazone treatment may influence the prognosis of T2D patients with AFLD.
In this study, the effects of additional pioglitazone treatment in T2D patients with AFLD were investigated. The efficacy of the drug in improving glucose metabolism, liver function, and liver fibrosis in patients with AFLD was compared with those in patients with NAFLD according to the hemoglobin A1c (HbA1c), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl phosphatase (γ-GTP) levels and fibrosis-4 (FIB-4) index score. Pioglitazone has dose-dependent adverse effects on weight gain and edema [34], and low-dose (7.5 mg) pioglitazone may reduce adverse effects while maintaining drug efficacy [35, 36]. Therefore, we also compared the effects of additional low-dose pioglitazone on hepatic function and fibrosis in T2D patients with AFLD and those with NAFLD.
Methods
Study design and patients
In this study, 100 patients with T2D who were newly prescribed pioglitazone and received it for at least 3 months by physicians at Tokyo Teishin Hospital in Tokyo, Japan, between October 2015 and September 2020 were selected. No adverse event-related discontinuation of pioglitazone within 3 months occurred. Patients with hepatitis virus, autoimmune hepatitis, and severe renal failure (i.e., an estimated glomerular filtration rate < 30 mL/min/1.73 m2) were excluded. Fatty liver was diagnosed by radiologists using ultrasonography examination according to the criteria of the American Gastroenterology Association, on the basis of a marked increase in hepatic echogenicity, poor penetration of the posterior segment of the right lobe of the liver, and poor-to-no visualization of the hepatic vessels and diaphragm [37]. Fatty liver was also diagnosed using computed tomography examination if the liver-spleen attenuation ratio was below 1.0. Alcohol consumption habits (alcohol consumption > 30 g/day for men and > 20 g/day for women) were investigated to distinguish between NAFLD and AFLD.
This study was approved by the ethical review committee of Tokyo Teishin Hospital (approval number: 1119; approval date: January 5, 2021) and conducted according to the tenets of the Declaration of Helsinki. Informed consent was obtained using a comprehensive agreement method and opt-out. This study was registered at the University Hospital Medical Information Network (UMIN ID: UMIN000046797).
Data collection and assessment
Clinical data—including age, sex, weight, the serum levels of AST, ALT, γ-GTP, and HbA1c (according to the National Glycohemoglobin Standardization Program), platelet count, pioglitazone dosage, and history of concomitant use of antidiabetic drugs other than pioglitazone—were collected from the patient medical records. Changes in clinical parameters between pre-treatment and 3 months after pioglitazone administration were evaluated in patients with or without fatty liver and in those with AFLD or NAFLD. Changes in HbA1c levels were also compared between patients with and without fatty liver. To evaluate the efficacy of pioglitazone based on the dosage, the pioglitazone dose was divided into low dose (≤ 7.5 mg) and standard dose (≥ 15 mg).
The degree of liver fibrosis was determined using the FIB-4 index, which was validated for Japanese patients with NAFLD. The index was calculated using the following formula: age (years) × AST (IU/L) / (platelet count (109/L) × √ALT (IU/L)) [38]. The safety profile of pioglitazone was evaluated according to adverse effects within 3 months after pioglitazone administration.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Continuous and categorical variables were compared using Student’s t-test, Chi-square test, and Fisher’s exact test, as appropriate. A paired t-test was used to compare clinical data before and after pioglitazone administration. After adjusting for the baseline HbA1c level, an analysis of covariance was performed to compare the changes in HbA1c level between patients with and without fatty liver. All statistical analyses were performed using Bell Curve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). P values of < 0.05 were considered statistically significant.
Results
Patient characteristics
Patients were not administered any additional or increased doses of drugs that affect liver function other than pioglitazone during the study observation period. Of the 100 patients selected, 68 patients were male and 32 were female; the mean patient age was 66.2 ± 14.2 years. Table 1 depicts the baseline patient characteristics. Among the 78 patients with fatty liver, 21 were diagnosed with AFLD, and 57 were diagnosed with NAFLD. There were more male patients in the AFLD group than in the NAFLD group (90.4 vs. 57.8%; P < 0.05). The serum γ-GTP level was higher in the AFLD group than in the NAFLD group (120.7 ± 102.5 vs. 70.6 ± 45.9 IU/L; P < 0.05). No significant between-group difference in body weight was observed. The mean pioglitazone dose was 10.6 ± 4.6 mg, and 61% of patients received low-dose pioglitazone (≤ 7.5 mg). Patients received an average of 2.1 ± 1.1 diabetic medications other than pioglitazone. There was no significant difference in the use of diabetic medications.
Table 1.
Baseline characteristics of patients
| Total | Fatty liver ( +) | Fatty liver (−) | ||
|---|---|---|---|---|
| NAFLD | AFLD | |||
| N | 100 | 57 | 21 | 22 |
| Age (years) | 66.2 ± 14.2 | 64.4 ± 14.8 | 62.6 ± 13.9 | 73.9 ± 10.5 |
| Sex (M/F) | 68/32 | 33/24 | 19/2* | 16/6 |
| Body weight (kg) | 72.4 ± 17.1 | 74.8 ± 18.4 | 76.1 ± 12.9 | 62.6 ± 12.3 |
| BMI (kg/m2) | 26.7 ± 4.5 | 27.9 ± 4.7 | 26.9 ± 3.5 | 23.5 ± 2.9 |
| AST (IU/L) | 42.7 ± 30.0 | 48.8 ± 31.1 | 50.6 ± 29.8 | 19.2 ± 4.8 |
| ALT (IU/L) | 56.2 ± 49.2 | 66.2 ± 51.6 | 67.3 ± 49.6 | 19.5 ± 7.3 |
| γ-GTP (IU/L) | 70.8 ± 66.7 | 70.6 ± 45.9 | 120.7 ± 102.5* | 23.6 ± 10.3 |
| HbA1c (%) | 8.28 ± 1.16 | 8.24 ± 1.22 | 8.03 ± 1.27 | 8.61 ± 0.73 |
| Pioglitazone dose (mg) | 10.6 ± 4.6 | 10.8 ± 4.6 | 9.6 ± 3.3 | 10.9 ± 5.4 |
| ≤ 7.5 mg | 61 (61) | 32 (56.1) | 15 (71.4) | 14 (63.6) |
| ≥ 15 mg | 39 (39) | 25 (43.9) | 6 (28.6) | 8 (36.4) |
| Antidiabetic drugs** | ||||
| Metformin | 50 (50) | 31 (54.3) | 8 (38.0) | 11 (50) |
| DPP-4 inhibitor | 63 (63) | 35 (61.4) | 9 (42.8) | 19 (86.3) |
| GLP-1 agonist | 18 (18) | 12 (21.0) | 4 (19.0) | 2 (9.0) |
| SGLT2 inhibitor | 17 (17) | 10 (17.5) | 6 (28.5) | 1 (4.5) |
| Sulfonylurea | 31 (31) | 13 (22.8) | 4 (19.0) | 14 (63.6) |
| Glinide | 5 (5) | 4 (7.0) | 1 (4.7) | 0 (0) |
| α-GI | 11 (11) | 5 (8.7) | 1 (4.7) | 5 (22.7) |
| Insulin | 23 (23) | 13 (22.8) | 4 (19.0) | 6 (27.2) |
| Number of concomitant antidiabetic drugs | 2.1 ± 1.1 | 2.1 ± 1.2 | 1.7 ± 1.1 | 2.6 ± 0.8 |
Data are expressed as mean ± SD or n (%)
*P < 0.05 vs. NAFLD
**Patients taking several drugs are also included
Efficacy of pioglitazone on glucose intolerance and liver dysfunction in T2D patients with or without fatty liver
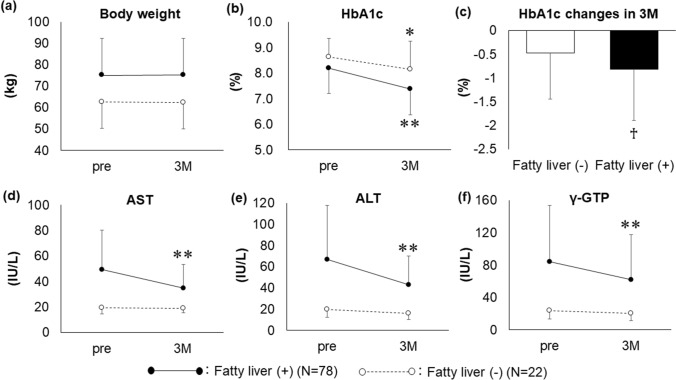
Body weight did not change after 3 months of pioglitazone treatment in patients with (N = 78) and without (N = 22) fatty liver (Fig. 1a). The HbA1c level significantly decreased from 8.18 ± 1.24 to 7.36 ± 1.08% (P < 0.01) in patients with fatty liver and from 8.61 ± 0.73 to 8.14 ± 1.10% (P < 0.05) in patients without fatty liver (Fig. 1b). However, the lowering effect was more significant in patients with fatty liver than in those without fatty liver (− 0.82 ± 1.07 vs. − 0.47 ± 0.96%; P < 0.05) (Fig. 1c). Patients with fatty liver presented a significant decrease in the AST, ALT, and γ-GTP levels from 49.3 ± 30.8 to 34.8 ± 18.4 IU/L, 66.5 ± 55.1 to 42.9 ± 27.0 IU/L, and 84.1 ± 69.7 to 62.2 ± 55.4 IU/L, respectively (P < 0.01) (Fig. 1d–f). In contrast, patients without fatty liver did not present a significant change in the AST, ALT, and γ-GTP levels (Fig. 1d–f).
Fig. 1.
Efficacy of 3 months of additional pioglitazone treatment to alleviate glucose intolerance and hepatic dysfunction in patients with type 2 diabetes with (N = 78) or without (N = 22) fatty liver. *P < 0.05 vs. pre; **P < 0.01 vs. pre; ♰P < 0.05 vs. fatty liver (−). Data are expressed as mean ± SD. 3M: after 3 months of pioglitazone treatment; pre: pre-treatment, SD: standard deviation
Efficacy of pioglitazone on glucose intolerance and liver dysfunction in T2D patients with AFLD or NAFLD
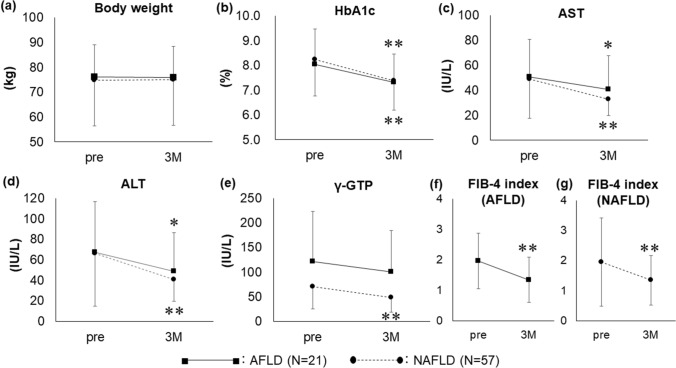
Additional pioglitazone treatment did not influence body weight changes in the AFLD (N = 21) and NAFLD (N = 57) groups (Fig. 2a). The HbA1c level significantly decreased from 8.03 ± 1.27 to 7.32 ± 1.13% (P < 0.01) in the AFLD group and from 8.24 ± 1.22 to 7.38 ± 1.06% (P < 0.01) in the NAFLD group (Fig. 2b). In the AFLD group, the AST and ALT levels significantly decreased from 50.6 ± 29.8 to 40.4 ± 27.3 IU/L (P < 0.05) and from 67.3 ± 49.6 to 48.7 ± 37.8 IU/L (P < 0.05), respectively. Although the γ-GTP level also decreased, the difference was not significant in the AFLD group. In the NAFLD group, the AST, ALT, and γ-GTP levels decreased from 48.8 ± 31.1 to 32.7 ± 13.2 IU/L, 66.2 ± 51.6 to 40.8 ± 21.3 IU/L, and 70.6 ± 45.9 to 48.1 ± 29.6 IU/L, respectively (P < 0.01) (Fig. 2c–e). Moreover, the AFLD and NAFLD groups showed a significant decrease in the FIB-4 index from 1.97 ± 0.91 to 1.35 ± 0.74 (P < 0.01) and from 1.95 ± 1.46 to 1.35 ± 0.82 (P < 0.01), respectively (Fig. 2f–g).
Fig. 2.
Efficacy of 3 months of additional pioglitazone treatment in patients with type 2 diabetes with NAFLD (N = 57) or AFLD (N = 21). *P < 0.05 vs. pre; **P < 0.01 vs. pre. Data are expressed as mean ± SD. 3M: after 3 months of pioglitazone treatment; AFLD: alcoholic fatty liver disease; NAFLD: non-fatty alcoholic liver disease; pre: pre-treatment, SD: standard deviation
Efficacy of low-dose pioglitazone on glucose intolerance and liver dysfunction in T2D patients with AFLD or NAFLD
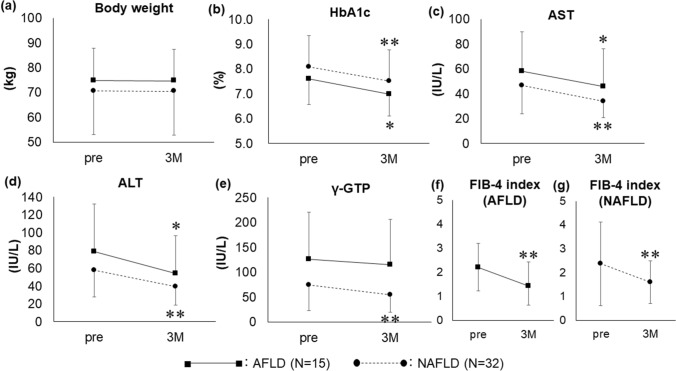
There was no significant difference in weight changes between the AFLD (N = 15) and NAFLD (N = 32) groups (Fig. 3a). In the AFLD group, the levels of HbA1c, AST, and ALT significantly decreased from 7.60 ± 1.02 to 6.98 ± 0.86%, 58.3 ± 31.7 to 45.9 ± 30.2 IU/L, and 78.5 ± 53.7 to 54.5 ± 42.3 IU/L, respectively (P < 0.05) (Fig. 3b–d). Similarly, the levels of HbA1c, AST, ALT, and γ-GTP in the NAFLD group significantly decreased from 8.09 ± 1.24 to 7.52 ± 1.23%, 46.7 ± 23.1 to 34.0 ± 13.2 IU/L, 57.7 ± 29.9 to 39.4 ± 20.4 IU/L, and 74.2 ± 51.2 to 54.2 ± 34.4 IU/L, respectively (P < 0.01) (Fig. 3b–e). Additionally, the FIB-4 index significantly decreased from 2.20 ± 0.97 to 1.43 ± 0.79 (P < 0.01) in the AFLD group and from 2.37 ± 1.75 to 1.59 ± 0.89 (P < 0.01) in the NAFLD group (Fig. 3f–g).
Fig. 3.
Efficacy of 3 months of additional pioglitazone treatment in patients with type 2 diabetes with NAFLD (N = 32) or AFLD (N = 15) who received low-dose pioglitazone (*P < 0.05 vs. pre; **P < 0.01 vs. pre). Data are expressed as mean ± SD. 3M: after 3 months of pioglitazone treatment; AFLD: alcoholic fatty liver disease; NAFLD: non-fatty alcoholic liver disease; pre: pre-treatment, SD: standard deviation
Safety of pioglitazone
Two patients who received the standard pioglitazone dose (1 with NAFLD and 1 without fatty liver) experienced edema of the lower extremities. No severe hypoglycemia or hyperglycemia was observed. Additionally, no other adverse effects related to the skin, gastrointestinal tract, urinary tract, bone metabolism, or fractures were observed during the study.
Discussion
Pioglitazone ameliorates liver dysfunction in T2D patients with NAFLD; however, its efficacy in patients with AFLD is unclear. In the current study, 3 months of additional pioglitazone treatment significantly decreased the HbA1c level and ameliorated liver dysfunction in T2D patients with AFLD, as in patients with NAFLD, although the patients were already using various antidiabetic drugs. To the best of our knowledge, this study is the first to investigate the effects of additional pioglitazone in T2D patients with AFLD.
Since pioglitazone has only a few drug interactions, and its pharmacokinetics are not significantly altered even when used in patients with liver dysfunction [39], the glucose-lowering effect in this study is mainly attributable to the additional administration of pioglitazone. Furthermore, in a comparative study of the effects of additional pioglitazone in T2D patients with or without fatty liver, the HbA1c level was more significantly decreased in those with fatty liver. Although only a few studies have compared the efficacy of pioglitazone between T2D patients with and without fatty liver, the evidence indicates that pioglitazone promotes fatty acid oxidation [1], inhibits hepatic fatty acid synthesis [40], and suppresses glycogenesis [41, 42], suggesting that the efficacy of pioglitazone in reducing hepatic insulin resistance is more pronounced in populations with fatty liver.
According to a postmarketing study of adverse drug reactions in patients with T2D in Japan, pioglitazone has fluid-retaining and liver enzyme (ALT)-lowering effects. Additionally, these effects were observed regardless of the presence or absence of a patient’s history of hepatobiliary disease [43]. However, whether T2D patients with AFLD also had alcoholic hepatitis and fatty liver is unclear. In the current study, T2D patients with fatty liver were divided into the AFLD and NAFLD groups to investigate the effects of additional pioglitazone treatment. The results showed that additional pioglitazone treatment simultaneously and significantly decreased the liver enzyme (AST and ALT) levels and the FIB-4 index in T2D patients with AFLD and NAFLD. Although these effects were not accompanied by weight changes in either group, we cannot deny the possibility that the weight gain caused by pioglitazone and the weight loss caused by routine diet and exercise therapy offset each other, resulting in no weight change. However, since pioglitazone dose was relatively low (mean of 10.6 ± 4.6 mg) in our study, and the degree of weight gain was considered to be minimum [35, 36], we consider that the weight loss caused by routine diet and exercise therapy was also minimum. The liver enzyme-reducing and histological improvement effects of pioglitazone have been widely reported in patients with NAFLD [25, 29, 44]. Additional pioglitazone treatment may have significantly lowered the liver enzyme levels and FIB-4 index in T2D patients with AFLD because the pathogenesis of AFLD is similar to that of NAFLD (i.e., from steatohepatitis to liver fibrosis and cirrhosis) [45, 46]. Because steatosis is a pathological aspect of alcoholic liver disease, the improvements in liver function owing to pioglitazone observed in our study may also reflect the alleviation of other pathological findings in alcoholic liver disease, such as inflammation and ballooning of hepatocytes, as has been reported in previous NAFLD clinical trials [20, 22, 23, 25, 26, 29].
Fluid retention, weight gain, and peripheral edema are the most frequent adverse effects of pioglitazone [43, 47–50] and are of great clinical concern. Additionally, the risk of osteoporosis and bone fracture are relatively high in an alcohol drinker [51]. Low-dose (≤ 7.5 mg) pioglitazone may prevent these problems while still having a glucose-lowering effect equivalent to that of the standard dose (15 mg) [35, 36, 52]. Therefore, we examined glucose intolerance, liver dysfunction, and FIB-4 index in T2D patients with AFLD and NAFLD who received low-dose pioglitazone (≤ 7.5 mg/day). All parameters in both groups were significantly decreased. To our knowledge, only a few studies have evaluated the effects of pioglitazone on the liver function of T2D patients with AFLD. Our study showed that additional pioglitazone had beneficial therapeutic effects in T2D patients with AFLD, even when relatively low doses were administered. This result has important clinical implications because it indicates that pioglitazone treatment may be a beneficial strategy for improving the liver function of T2D patients with AFLD.
Although the pathogenesis of AFLD is different from that of NAFLD, a new concept, metabolic dysfunction-related fatty liver disease (MAFLD), has been proposed [53–55]. MAFLD is more comprehensive than NAFLD and includes fatty liver caused by excessive alcohol intake. However, the definition of MAFLD is vague and controversial. Our results indicate that pioglitazone effectively ameliorates liver dysfunction in T2D patients with AFLD. If these results are applied to the concept of MAFLD, it may be possible to conclude that pioglitazone effectively ameliorates liver dysfunction in T2D patients with MAFLD.
Although our results are of clinical interest, this study had limitations because of its retrospective design and a small number of patients. There were only 21 patients with AFLD. However, the proportion of T2D patients with AFLD in this study was comparable to that of individuals with moderate-to-heavy alcohol consumption that developed type 2 diabetes in a population-based longitudinal study [56]. Despite the small number of patients, our study indicated statistical significance. Larger studies are needed to validate our findings in the future. Pioglitazone may have improved liver function in T2D patients with AFLD in this study by reducing alcohol consumption. Pioglitazone has the potential to suppress alcohol intake via activation of PPAR-γ receptors in the central nervous system [57]. Although we were unable to obtain data on changes in alcohol consumption after pioglitazone administration in our study, a recent clinical trial has shown that pioglitazone reduced alcohol use in patients with heavy drinking [58]. We examined the effects of alcohol consumption and type of alcohol consumed before and after pioglitazone administration, but no relationship was found. No other factors predicting the therapeutic effects of pioglitazone have been identified. Additionally, the effects of pioglitazone on AFLD without metabolic disease were not investigated in this study. Therefore, it was unclear whether the effects of pioglitazone on liver dysfunction in the patients involved in this study were attributable to AFLD improvement or metabolic improvement by pioglitazone. However, pioglitazone-induced changes in insulin resistance in adipose tissue are correlated with improvements in liver tissues with NASH [24], and metabolic residues of pioglitazone are correlated with the effects of tissue improvement [29]. Furthermore, although there were no significant differences in concomitant medications used by patients with AFLD and those with NAFLD, some effects of concomitant drug interactions could not be completely ruled out. However, it may be concluded from our findings that additional pioglitazone treatment may, directly or indirectly, decrease liver enzyme levels associated with AFLD complicated by metabolic diseases such as T2D.
The efficacy and safety of pioglitazone in this study were evaluated during 3 months of additional pioglitazone treatment; this observational period was relatively short for adequate evaluation. A longer observational period would be necessary to evaluate the long-term effect of pioglitazone. Although liver enzymes such as AST, ALT, and γ-GTP and surrogate indices such as the FIB-4 index have been used to evaluate steatohepatitis, new imaging modalities including quantitative ultrasound-based evaluation for liver steatosis and magnetic resonance imaging–proton density fat fraction (MRI-PDFF) could also be useful to evaluate steatohepatitis. For further rigorous assessments, prospective studies are needed to clarify the effect of pioglitazone on liver function in patients with AFLD and directly confirm liver histological changes caused by pioglitazone, as determined using liver biopsy, and compare these markers with our parameters. Additionally, MAFLD causes liver fibrosis independent of insulin resistance, dyslipidemia, and alcohol intake [59]. Future studies should investigate the feasibility of pioglitazone as a treatment strategy for reducing liver fibrosis in T2D patients with AFLD.
In conclusion, 3 months of additional pioglitazone administration using a low-dose strategy ameliorated liver dysfunction in patients with NAFLD. This strategy can also ameliorate liver dysfunction and glucose intolerance in T2D patients with AFLD. Overall, the results of this study indicate that pioglitazone is an effective treatment option for T2D patients with AFLD.
Acknowledgements
The authors thank the staff members of the Department of Endocrinology and Metabolism at Tokyo Teishin Hospital. The authors also thank Tokyo Teishin Hospital for financial support.
Author contributions
MA and HK analyzed data and wrote the manuscript. NT, DH, YY and HK contributed to the discussions. HK reviewed and edited the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no funding and conflicts of interest to disclose.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89(1):200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87(6):2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 3.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89(12):6068–6076. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–1792. doi: 10.1172/jci29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101(6):1354–1361. doi: 10.1172/jci1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114(2):147–152. doi: 10.1172/jci22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314(2):580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 8.Teranishi T, Ohara T, Maeda K, Zenibayashi M, Kouyama K, Hirota Y, et al. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2007;56(10):1418–1424. doi: 10.1016/j.metabol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Arase Y, Suzuki F, Ikeda K, Kumada H, Tsuji H, Kobayashi T. Multivariate analysis of risk factors for the development of type 2 diabetes in nonalcoholic fatty liver disease. J Gastroenterol. 2009;44(10):1064–1070. doi: 10.1007/s00535-009-0091-1. [DOI] [PubMed] [Google Scholar]
- 10.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10(11):627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 13.Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61(2):141–152. doi: 10.1111/j.1365-2559.2011.04145.x. [DOI] [PubMed] [Google Scholar]
- 14.Askgaard G, Grønbæk M, Kjær MS, Tjønneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015;62(5):1061–1067. doi: 10.1016/j.jhep.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29(4):437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 16.Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(10):1574–1586. doi: 10.14309/ajg.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70(2):284–293. doi: 10.1016/j.jhep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Kasuga M, Ueki K, Tajima N, Noda M, Ohashi K, Noto H, et al. Report of the Japan diabetes society/japanese cancer association joint committee on diabetes and cancer. Cancer Sci. 2013;104(7):965–976. doi: 10.1111/cas.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 21.Said A, Akhter A. Meta-analysis of randomized controlled trials of pharmacologic agents in non-alcoholic steatohepatitis. Ann Hepatol. 2017;16(4):538–547. doi: 10.5604/01.3001.0010.0284. [DOI] [PubMed] [Google Scholar]
- 22.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 24.Bell LN, Wang J, Muralidharan S, Chalasani S, Fullenkamp AM, Wilson LA, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56(4):1311–1318. doi: 10.1002/hep.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305–315. doi: 10.7326/m15-1774. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi-Suzuki M, Bril F, Kalavalapalli S, Cusi K, Frye RF. Concentration-dependent response to pioglitazone in nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;46(1):56–61. doi: 10.1111/apt.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177(5):633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawangjit R, Chongmelaxme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, et al. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): a PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore) 2016;95(32):e4529. doi: 10.1097/md.0000000000004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang JF, Dai CY, Huang CF, Tsai PC, Yeh ML, Hsu PY, et al. First-in-Asian double-blind randomized trial to assess the efficacy and safety of insulin sensitizer in nonalcoholic steatohepatitis patients. Hepatol Int. 2021;15(5):1136–1147. doi: 10.1007/s12072-021-10242-2. [DOI] [PubMed] [Google Scholar]
- 32.Della Pepa G, Russo M, Vitale M, Carli F, Vetrani C, Masulli M, et al. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Diabetes Res Clin Pract. 2021;178:108984. doi: 10.1016/j.diabres.2021.108984. [DOI] [PubMed] [Google Scholar]
- 33.Johnston MP, Patel J, Byrne CD. Causes of mortality in non-alcoholic fatty liver disease (NAFLD) and alcohol related fatty liver disease (AFLD) Curr Pharm Des. 2020;26(10):1079–1092. doi: 10.2174/1381612826666200128094231. [DOI] [PubMed] [Google Scholar]
- 34.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The pioglitazone 001 study group. Diabetes Care. 2000;23(11):1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 35.Majima T, Komatsu Y, Doi K, Shigemoto M, Takagi C, Fukao A, et al. Safety and efficacy of low-dose pioglitazone (7.5 mg/day) vs. standard-dose pioglitazone (15 mg/day) in Japanese women with type 2 diabetes mellitus. Endocr J. 2006;53(3):325–30. doi: 10.1507/endocrj.k05-067. [DOI] [PubMed] [Google Scholar]
- 36.Yanai H, Adachi H. The low-dose (7.5 mg/day) pioglitazone therapy. J Clin Med Res. 2017;9(10):821–5. doi: 10.14740/jocmr3144w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123(5):1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 38.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanefeld M. Pharmacokinetics and clinical efficacy of pioglitazone. Int J Clin Pract Suppl. 2001;121:19–25. [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 41.Shannon CE, Daniele G, Galindo C, Abdul-Ghani MA, DeFronzo RA, Norton L. Pioglitazone inhibits mitochondrial pyruvate metabolism and glucose production in hepatocytes. Febs J. 2017;284(3):451–465. doi: 10.1111/febs.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 2006;66(1):85–109. doi: 10.2165/00003495-200666010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Kawamori R, Kadowaki T, Onji M, Seino Y, Akanuma Y. Hepatic safety profile and glycemic control of pioglitazone in more than 20,000 patients with type 2 diabetes mellitus: postmarketing surveillance study in Japan. Diabetes Res Clin Pract. 2007;76(2):229–235. doi: 10.1016/j.diabres.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Kumar J, Memon RS, Shahid I, Rizwan T, Zaman M, Menezes RG, et al. Antidiabetic drugs and non-alcoholic fatty liver disease: a systematic review, meta-analysis and evidence map. Dig Liver Dis. 2021;53(1):44–51. doi: 10.1016/j.dld.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Dunn W, Shah VH. Pathogenesis of alcoholic liver disease. Clin Liver Dis. 2016;20(3):445–456. doi: 10.1016/j.cld.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Wang W, Mao M, Gao R, Shi W, Li D, et al. Similarities and differences: a comparative review of the molecular mechanisms and effectors of NAFLD and AFLD. Front Physiol. 2021;12:710285. doi: 10.3389/fphys.2021.710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edelman SV. The role of the thiazolidinediones in the practical management of patients with type 2 diabetes and cardiovascular risk factors. Rev Cardiovasc Med. 2003;4(Suppl 6):S29–37. [PubMed] [Google Scholar]
- 48.O'Moore-Sullivan TM, Prins JB. Thiazolidinediones and type 2 diabetes: new drugs for an old disease. Med J Aust. 2002;176(8):381–386. doi: 10.5694/j.1326-5377.2002.tb04461.x. [DOI] [PubMed] [Google Scholar]
- 49.Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA. Nonhypoglycemic effects of thiazolidinediones. Ann Intern Med. 2001;134(1):61–71. doi: 10.7326/0003-4819-134-1-200101020-00014. [DOI] [PubMed] [Google Scholar]
- 50.Stumvoll M, Häring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34(3):217–224. doi: 10.1080/ann.34.3.217.224. [DOI] [PubMed] [Google Scholar]
- 51.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16(7):737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 52.Satirapoj B, Watanakijthavonkul K, Supasyndh O. Safety and efficacy of low dose pioglitazone compared with standard dose pioglitazone in type 2 diabetes with chronic kidney disease: a randomized controlled trial. PLoS One. 2018;13(10):e0206722. doi: 10.1371/journal.pone.0206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 54.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 55.Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40(6):1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 56.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Effect of alcohol consumption and the presence of fatty liver on the risk for incident type 2 diabetes: a population-based longitudinal study. BMJ Open Diabetes Res Care. 2020 doi: 10.1136/bmjdrc-2020-001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69(7):642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Dieperink E, Hauser P, Dockter K, Miranda J, Evenson M, Thuras P. Reduced alcohol use in patients prescribed pioglitazone. Am J Addict. 2021;30(6):570–577. doi: 10.1111/ajad.13214. [DOI] [PubMed] [Google Scholar]
- 59.Inamine S, Kage M, Akiba J, Kawaguchi T, Yoshio S, Kawaguchi M, et al. Metabolic dysfunction-associated fatty liver disease directly related to liver fibrosis independent of insulin resistance, hyperlipidemia, and alcohol intake in morbidly obese patients. Hepatol Res. 2022;52(10):841–858. doi: 10.1111/hepr.13808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.