Abstract
Background
Maternal hyperglycemia, obesity, and hypertension with gestational diabetes mellitus (GDM) are risk factors for infant complications. This study aimed to investigate maternal factors and glycemic control indicators that affect infant complications in GDM.
Methods
We conducted a retrospective cohort study including 112 mothers with GDM and their infants. Multivariate logistic regression analysis was used to investigate the variables associated with good and adverse infant outcomes. We determined the cutoff values of variables that showed a significant difference in the multivariate logistic regression analysis for predicting infant complications by performing receiver operating characteristic curve analysis.
Results
In the multivariate logistic regression analysis, prepregnancy BMI and GA in the third trimester were significantly related to good and adverse infant outcomes (adjusted odds ratios [aORs], 1.62; 95% CIs 1.17–2.25, p = 0.003 and aORs, 2.77; 95% CIs 1.15–6.64, p = 0.022, respectively). The cutoff values for prepregnancy BMI and GA in the third trimester were 25.3 kg/m2 and 13.5%, respectively.
Conclusions
The importance of weight control before pregnancy and the usefulness of GA in the third trimester to predict infant complications were suggested in this study.
Keywords: Body mass index, Gestational diabetes mellitus, Glycated albumin, Glycated hemoglobin, Infant complication
Introduction
Maternal hyperglycemia with gestational diabetes mellitus (GDM) is a risk factor for infant complications. Maternal hyperglycemia during late pregnancy leads to fetal hyperglycemia and hyperinsulinemia. Hyperinsulinemia of the fetus promotes organ growth and delays the maturation of organ function, leading to infant complications [1]. Therefore, maternal glycemic control is important to reduce the risk of infant complications in women with GDM.
Glycated hemoglobin (HbA1c) is a traditional glycemic indicator and is used in clinical diabetes practice [2]. Glycated albumin (GA) is another glycemic indicator, and it has been reported that GA is useful for monitoring the glycemic status during pregnancy [3–7]. In addition to glycemic control, prepregnancy obesity, gestational weight gain, and hypertensive disorders of pregnancy (HDP) also affect infant complications in women with diabetes [8, 9].
The association between maternal factors, such as HbA1c, prepregnancy body mass index (BMI), gestational weight gain, HDP, and infant complications, has been studied using multivariate analysis [8, 10]. Although there are some studies that suggests GA is useful in assessing glycemic control during pregnancy, only a few studies have incorporated GA into multivariate analysis models to investigate the association between maternal factors and infant complications in GDM.
Herein, we investigated the maternal factors and glycemic control indicators that affect the prognosis of infants in women with GDM.
Methods
Participants and study design
This was a single-center, retrospective cohort study conducted from January 2012 to December 2019 by reviewing patient electronic health records. This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the ethics board of our facility (approval no. S20-070, Aug 27. 2020). An opt-out consent form was published on the website of our facility. This study was exempted from the requirement for written informed consent because of its retrospective design and the fact that participants chose to participate in the study by not opting out on the university webpage, which implied their tacit consent to participate in this study. None of the participants opted out of this study as of December 1, 2021. We included 112 Japanese mothers with GDM were treated with insulin subcutaneous injection and simultaneous GA and HbA1c measurements in the second and third trimesters followed at our facility, along with their infants. GDM was diagnosed if the results of the 75-g oral glucose tolerance test met one or more of the following abnormal thresholds (equal to or greater than) according to the criteria of the International Association of Diabetes and Pregnancy Study Groups: fasting plasma glucose level, 92 mg/dL (5.1 mmol/L); 1 h plasma glucose level, 180 mg/dL (10.0 mmol/L); and 2 h plasma glucose level, 153 mg/dL (8.5 mmol/L) [11]. The exclusion criteria were mothers with complications, such as chronic systemic disease (connective tissue disease, renal disease, hyperthyroidism, and hypothyroidism), and/or those who received drugs affecting the fetus (steroid and psychotropic drugs). Mothers with GDM were followed up by an obstetrician and endocrinologist once a month. The pediatricians determined whether the infants of women with diabetes developed any postnatal complications within the first month.
Measurement and study outcomes
We retrieved clinical data from the patient electronic health records. The data included maternal age, prepregnancy BMI, gestational weight gain, HDP, mode of delivery, smoking status, gestational week, gravidity, systolic and diastolic blood pressure, glucose and pH of umbilical cord blood, infant birth weight, Apgar score (1 and 5 min after birth), infant complications, and maternal GA and HbA1c values in the second and third trimesters. HDP was defined as systolic blood pressure 140 mmHg or more and/or diastolic blood pressure 90 mmHg or more in pregnancy, in accordance with the criteria of the Japan Society for the Study of Hypertension in Pregnancy [12].
The following infant complications were defined: hypoglycemia was defined as a blood glucose level less than 1.9 mmol/L; respiratory disorders as the requirement for supplemental oxygen or an artificial respirator; hypocalcemia as a total serum calcium concentration of less than 2.0 mmol/L; polycythemia as a hematocrit value greater than 0.65/L; hyperbilirubinemia as elevated bilirubin levels necessitating phototherapy; myocardial hypertrophy as an interventricular septal thickness greater than 6 mm measured by ultrasound; and large for gestational age as body weight above the 90th percentile at birth.
GA was enzymatically measured with a liquid reagent (Lucica GA-L® Asahi Kasai Pharma Co., Tokyo, Japan) composed of ketoamine oxidase and an albumin-specific proteinase [13]. HbA1c (%) was measured using automated high-performance liquid chromatography. The HbA1c value was determined using the National Glycated Hemoglobin Standard Program.
To examine the factors that affect infant complications in women with GDM, we sorted each factor in one of the two groups: a good infant outcome and an adverse infant outcome. A good infant outcome was defined as the absence of any of the previously mentioned infant complications, whereas an adverse infant outcome was defined as the presence of one or more of the previously mentioned infant complications.
Statistical analysis
Multivariate logistic regression models were used to investigate the risk factors for infant complications in women with GDM and how they were related to potential predictors, including clinical variables. We included variables in the models based on existing knowledge of risk factors that affect infant outcomes in women with diabetes. First, we conducted a univariate analysis to identify the explanatory variables that were significantly related to infant outcomes. Thereafter, variables with a p value less than 0.1 in the univariate analysis were defined as explanatory variables to obtain a logistic regression model with the presence or absence of infant complications (good or adverse infant outcome) as a target variable.
Results are expressed as the adjusted odds ratios (aORs) with their 95% confidence intervals (CIs). We used the variance inflation factor (VIF) to confirm whether multicollinearity existed in the multivariate analysis model. For the factors that were associated with infant complications, we generated receiver operating characteristic (ROC) curves to estimate the cutoff values for predicting infant complications. All statistical data in this study were analyzed by using EZR version 1.54 software [14]. Statistical significance was set at a P value < 0.05.
Results
Baseline characteristics of study participants
We screened 112 women in this study; however, 10 were excluded (connective tissue disease, n = 1; renal disease, n = 1; hyperthyroidism, n = 4; and drugs affecting the fetus; n = 4). The final study sample comprised 102 mothers with GDM and their infants. Of the women with GDM, 50 infants (49%) developed one or more complications (Fig. 1). Table 1 showed the characteristics of the study participants. 25 (24.5%) pregnant women had a prepregnancy BMI of 25 or higher. The observed infant complications were as follows: 33 (32.3%) with hypoglycemia; 29 (28.4%) with respiratory disorders; 22 (21.5%) with hypocalcemia; 21 (20.5%) with myocardial hypertrophy; 18 (17.6%) were large for gestational age; 12 (11.7%) with hyperbilirubinemia; and 6 (5.9%) with polycythemia. All infants with hypoglycemia had elevated blood glucose levels with glucose infusion or early immediate feeding and did not experience convulsions. Two infants had respiratory disorders and were diagnosed with respiratory distress syndrome, for which they received mechanical ventilation. None of the infants with myocardial hypertrophy developed heart failure. Infants with hypocalcemia and polycythemia were asymptomatic and did not require treatment, such as intravenous calcium dosing or partial exchange transfusion. All infants with hyperbilirubinemia improved with phototherapy.
Fig. 1.
The flowchart of this study. GDM, gestational diabetes mellitus
Table 1.
Baseline characteristics of study participants
| Value, n or mean ± SD | Median | |
|---|---|---|
| Age (years) | 34.5 ± 4.4 | 35 |
| Prepregnancy aBMI (kg/m2) | 23.6 ± 2 | 23.9 |
| Previous pregnancy: yes/no | 53/49 | |
| Gestational duration (weeks) | 37.8 ± 1.7 | 37.9 |
| Gestational weight change (kg) | 8 ± 2.2 | 7.4 |
| Hypertensive disorders of pregnancy: Yes/no | 8/94 | |
| Smoking | 0 | 0 |
| Vaginal delivery | 53 | |
| Scheduled cesarean section | 30 | |
| Emergency cesarean section | 19 | |
| Systolic blood pressure (mmHg) | 115.6 ± 12.3 | 114.5 |
| Diastolic blood pressure (mmHg) | 69.4 ± 9.9 | 69 |
| bHbA1c in the second trimester (%) | 5.5 ± 0.3 | 5.6 |
| cGA in the second trimester (%) | 13.7 ± 1.5 | 13.5 |
| HbA1c in the third trimester (%) | 5.7 ± 0.3 | 5.8 |
| GA in the third trimester (%) | 13.5 ± 1.6 | 13.2 |
| Neonatal birth weight (g) | 2,869 ± 471 | 2,906 |
| Any complications in infant: yes/no | 52/50 | |
| Umbilical cord pH | 7.3 ± 0.04 | 7.3 |
| Umbilical cord blood glucose (mg/dL) | 83.7 ± 29.6 | 79 |
| Apgar score 1 min | 8 ± 0.5 | 8 |
| Apgar score 5 min | 8.8 ± 0.4 | 9 |
| Placenta (g) | 587.6 ± 108.8 | 588 |
aBody mass index (calculated as weight in kilograms divided by the square of the height in meters), bGlycated hemoglobin, cGlycated albumin. Values are expressed as the mean ± standard deviation or n
Comparison of characteristics between good infant outcomes and adverse infant outcomes in the univariate analysis
We performed a univariate analysis to identify the explanatory variables that were significantly related to infant outcomes (Table 2). The univariate analysis showed that prepregnancy BMI, gestational weight gain, HbA1c in the second trimester, GA in the second trimester, and GA in the third trimester were related to differences between a good infant outcome and an adverse infant outcome, with p values less than 0.1 (22.8 ± 1.9 vs. 24.5 ± 1.7 kg/m2, p < 0.001; 7.6 ± 1.9 vs. 8.3 ± 2 kg, p = 0.096; 5.4 ± 0.2 vs. 5.6 ± 0.4%, p = 0.035; 13 ± 0.5 vs. 14.4 ± 1.8%, p < 0.001; and 12.7 ± 0.7 vs. 14.3 ± 1.9%, p < 0.001, respectively). HbA1c showed a significant difference between good infant and adverse infant outcomes in the second trimester but not in the third trimester (5.7 ± 0.3 vs. 5.8 ± 0.4%, p = 0.197). In contrast, GA showed a significant difference between good and adverse infant outcomes in the second and third trimesters.
Table 2.
Variables associated with a good vs adverse perinatal outcome: univariable analysis
| Variable | Good outcome (n = 52) | Adverse outcome (n = 50) | P value |
|---|---|---|---|
| Age (years) | 34.5 ± 4.5 | 34.4 ± 4.4 | 0.948 |
| Prepregnancy BMI (kg/m2)a | 22.8 ± 1.9 | 24.5 ± 1.7 | < 0.001 |
| Previous pregnancy | 22 | 27 | 0.428 |
| Gestational duration (weeks) | 37.9 ± 1.4 | 37.8 ± 2 | 0.819 |
| Gestational weight change (kg) | 7.6 ± 1.9 | 8.3 ± 2 | 0.096 |
| Hypertensive disorders of pregnancy | 5 | 3 | 0.716 |
| Vaginal birth | 27 | 26 | 1 |
| Scheduled cesarean section | 15 | 15 | 1 |
| Emergency cesarean section | 9 | 10 | 1 |
| Systolic blood pressure (mmHg) | 115.9 ± 12.7 | 115.3 ± 12 | 0.794 |
| Diastolic blood pressure (mmHg) | 70.4 ± 10.6 | 68.5 ± 9 | 0.335 |
| bHbA1c in the second trimester (%) | 5.4 ± 0.2 | 5.6 ± 0.4 | 0.035 |
| cGA in the second trimester (%) | 13 ± 0.5 | 14.4 ± 1.8 | < 0.001 |
| HbA1c in the third trimester (%) | 5.7 ± 0.3 | 5.8 ± 0.4 | 0.197 |
| GA in the third trimester (%) | 12.7 ± 0.7 | 14.3 ± 1.9 | < 0.001 |
| Umbilical cord pH | 7.31 ± 0.04 | 7.30 ± 0.03 | 0.778 |
| Umbilical cord blood glucose (mg/dL) | 79.7 ± 29.9 | 87.8 ± 29.1 | 0.168 |
| Apgar score 1 min | 8 ± 0.5 | 8 ± 0.6 | 0.505 |
| Apgar score 5 min | 8.9 ± 0.4 | 8.8 ± 0.5 | 0.797 |
| Placenta (g) | 589.1 ± 114 | 586.1 ± 104.4 | 0.888 |
aBody mass index (calculated as weight in kilograms divided by the square of the height in meters), bGlycated hemoglobin, cGlycated albumin. Values are expressed as the mean ± standard deviation or n
Multivariate logistic regression analysis between good infant outcomes and adverse infant outcomes in the univariate analysis
We conducted a multivariate logistic regression analysis to identify the risk factors for infant complications in women with diabetes related to potential predictors with statistical significance in the univariate analysis (Table 3). Prepregnancy BMI and GA in the third trimester had a significant association with differences between good and adverse infant outcomes (aORs, 1.62; 95% CI 1.17–2.25, p = 0.003 and aORs, 2.77; 95% CIs 1.15–6.64, p = 0.022, respectively) in the multivariate logistic regression analysis. The VIF was used to confirm the presence of multicollinearity. None of the VIF values exceeded 10, and the mean VIF of the model was less than 5 (HbA1c in the second trimester, 1.21; prepregnancy BMI, 1.13; gestational weight gain, 1.01; GA in the second trimester, 2.71; and GA in the third trimester, 2.91), indicating no collinearity in the model.
Table 3.
Variables associated with a good vs. adverse infant outcome: Multivariable analysis
| Variable | P value | aOR [95% CI] |
|---|---|---|
| Prepregnancy BMI (kg/m2)a | 0.003 | 1.62 [1.17–2.25] |
| Gestational weight change (kg) | 0.816 | 1.03 [0.78–1.35] |
| bHbA1c in the second trimester (%) | 0.244 | 0.37 [0.54–2.1] |
| cGA in the second trimester (%) | 0.821 | 1.11 [0.45–2.7] |
| GA in the third trimester (%) | 0.022 | 2.77 [1.15–6.64] |
aBody mass index (calculated as weight in kilograms divided by the square of the height in meters), bGlycated hemoglobin, cGlycated albumin. The results are expressed as the adjusted odds ratios (aORs) and 95% confidence intervals (CIs)
The cutoff values of prepregnancy BMI and GA for predicting infant outcomes on ROC curve analyses
Prepregnancy BMI and GA in the third trimester were related to differences between good and adverse infant outcomes; therefore, we generated ROC curves to estimate the cutoff values of prepregnancy BMI and GA for predicting infant outcomes (Fig. 2). The cutoff values for prepregnancy BMI and GA in the third trimester were 25.3 kg/m2 and 13.5%, respectively. The area under the curve (AUC), sensitivity, and specificity for prepregnancy BMI and GA were moderate (prepregnancy: 0.713, 84.6, and 46%, GA: 0.797, 90.4, and 64%, respectively).
Fig. 2.
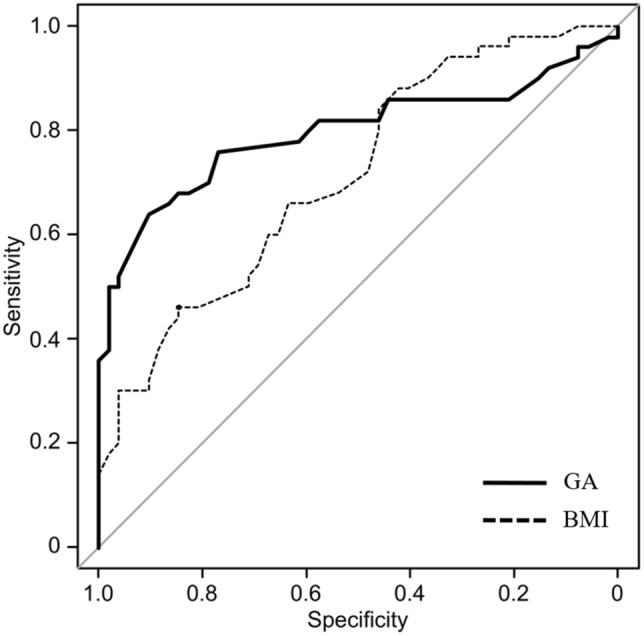
The cutoff values of prepregnancy BMI and GA in the third trimester in women with gestational diabetes mellitus for predicting perinatal outcomes via receiver operating characteristic curve analysis. BMI body mass index; GA glycated albumin
Discussion
In this study, we examined maternal factors and glycemic control indicators that affect the prognosis of infants of women with GDM. The results of this study showed two important clinical findings. First, prepregnancy BMI was related to infant complications in women with GDM. Second, GA in the third trimester was associated with a significant difference between good and adverse infant outcomes. On the contrary, HbA1c did not have a significant relationship between good and adverse infant outcomes. These results underscore the need for early prepregnancy intervention for weight control and the necessity of good glycemic control in late pregnancy to prevent adverse infant outcomes. Furthermore, GA may be a suitable glycemic control indicator in late pregnancy. However, the usefulness of GA during pregnancy has been reported in several studies, the strength of this study is that, unlike previous reports, the results were obtained through multivariate analysis.
Regarding the relationship between prepregnancy BMI and infant complications in women with diabetes, our results agree with several previous reports that showed a significant association between pregestational obesity and large for gestational age infants [8, 10, 15]. The onset of infant complications in women with diabetes is largely due to obesity-related dyslipidemia and maternal hyperglycemia [16]. Pregnant women with obesity have greater insulin resistance during pregnancy, thus inducing dyslipidemia and maternal hyperglycemia. As a result, excess glucose and lipids are supplied to the fetus, causing infant complications such as large for gestational age [17]. In the present study we only examined BMI and did not measure insulin or triglycerides and free fatty acids, which are indicators of lipids. In the future, it would be necessary to measure pre-pregnancy insulin, triglycerides, and free fatty acids and compare the association between BMI and these data.
Previous studies have reported that large for gestational age is related to mothers’ gestational weight gain and prepregnancy BMI [8, 18]. In this study, the univariate analysis showed that gestational weight gain was associated with infant complications; however, the multivariate analysis showed no significant difference. It has been reported that proper gestational weight gain is related to a good infant prognosis [19]. In this study, the mean gestational weight gain was within the same range as in the previous studies [19]. Furthermore, compared to that of previous reports, the standard deviation (SD) of gestational weight gain in our study population was small. These results might be influenced by the fact that we conducted this study in a population with a small difference in gestational weight gain.
GA in the third trimester showed a significant relationship between good and adverse infant outcomes. In contrast, HbA1c did not have a significant association between good and adverse infant outcomes. Although HbA1c is a standard glycemic indicator in clinical practice, several studies have reported that HbA1c is unsuitable for monitoring the glycemic status in pregnancy [20, 21]. HbA1c is a relatively long-term blood glucose marker reflecting the average blood glucose status for the past 1–2 months, and it is affected by iron metabolism. Specifically, HbA1c tends to be higher than the blood glucose status in late pregnancy because of iron deficiency anemia [22]. In contrast, GA represents a shorter mean blood glucose measure (the previous 2–3 weeks) than HbA1c, and it is not affected by iron metabolism [23]. Therefore, GA is useful for monitoring blood glucose in pregnancy, especially when strict blood glucose management is required in the short term and when the patient presents with iron deficiency.
It is generally known that GA levels and BMI are negatively correlated [5, 6]. Therefore, we were concerned that the correlation between GA and BMI might affect the results of our multivariate analysis. We examined the VIF of prepregnancy BMI, GA in the second trimester, and GA in the third trimester and the results were 1.13, 2.71, and 2.91, respectively. All of VIFs were less than 5, and we determined that there was no statistical evidence of multicollinearity. Koga M et al. reported that BMI was significantly negatively correlated with GA (R = − 0.221, p = 0.003) [24]. The result of the study suggests that there was a negative but relatively weak correlation between GA and BMI. Also, Huh et al. examined the direct effect of BMI on the GA/A1c ratio in structural equation modeling and reported that the effect was strong in the group with normal glucose tolerance but weak in the group with type 2 diabetes [25]. Our study subjects are GDM which is similar to type 2 diabetes. Therefore, it may be possible that the effect of BMI on GA is relatively small and, as a result, our study did not find multicolinearity.
Regarding the period during which GA was measured, the univariate analysis showed a significant association between GA in the second and third trimesters and infant outcomes. However, in the multivariate analysis, statistical significance was retained for the third trimester only. Insulin resistance in pregnant women develops in the middle of the second trimester and reaches its maximum in the first half of the third trimester [26]. In patients with GDM, good blood glucose control needs to be accomplished by < 32 weeks (third trimester) and maintained until childbirth to decrease the incidence of infant hypoglycemia and large for gestational age [27]. In other words, GA in the third trimester may be strongly related to adverse infant outcomes. However, it is important to note that not all complications are only associated with the third trimester of pregnancy. For example, respiratory distress syndrome, one of neonatal respiratory disorder, has been associated with conditions in the second trimester of pregnancy [28].
Perhaps each complication should be examined in relation to each trimester. In this study, we were unable to examine each complication due to insufficient sample size. This is an issue for future study.
The GA cutoff value in the third trimester for predicting infant outcomes according to the ROC curve was 13.5%. Li et al. reported that the frequency of birthweight ≥ 3,500 g and macrosomia increased significantly with GA levels ≥ 12.0%–12.9% in the third trimester by using the same GA assay that we used in this study [7]. In Japan, the reference intervals of GA in pregnant women with no impaired glucose tolerance are 11.5%–15.7%, and GA levels below 15.8% during pregnancy are required to reduce the frequency of infant complications [3, 29]. However, in view of our and other reports, it may be better for the GA cutoff value in the third trimester to be lower than 15.8% to prevent infant complications.
Notably, GA is easy to measure using an enzymatic method. Continuous glucose monitoring (CGM) is useful for detailed glucose monitoring, and its clinical utility has been reported for GDM [30]. However, the adoption of CGM is limited by its high cost, accompanying skin problems, and the discomfort associated with continuous wearing of the device. Suwa et al. reported that GA correlates with CGM parameters, such as average glucose, SD of glucose, and AUC for blood glucose > 180 mg/dL [31]. Therefore, the wider application of GA in glycemic monitoring is encouraged.
A few limitations to this study should be noted. First, we conducted a retrospective cohort study at a single institution. The sample size was relatively small, and there might have been selection bias. Therefore, our study results may not reflect those of the general population. Second, although potential confounding factors were adjusted for in the multivariate analysis, we could not completely exclude all confounders. For example, smoking during pregnancy has been reported to affect infant prognosis [32]. However, pregnant women who smoke were not included in this study; thus, the effects of smoking status were not investigated. A prospective study using a larger sample is needed to examine the confounding factors that could not be considered in the present study. Third, prepregnancy BMI was self-reported, and some pregnant women with obesity have been reported to underreport their body weight [33]. Therefore, information bias might have affected our study results. Fourth, all the study participants were Japanese. Although the reference interval of GA in the Japanese population is relatively similar to that in Chinese, American, and European populations [13, 34, 35]. The possibility of racial differences in GA may exist. The results of this study may not be generalizable to other races.
Conclusions
Prepregnancy BMI and GA in the third trimester are significantly related to complications in infants of women with GDM. Higher BMI in pregnant women has a negative impact on their glycemic status. In other words, it can be said that pre-pregnancy BMI affects the glycemic status of early pregnancy. Therefore, it is necessary to control blood glucose throughout the entire gestational period to prevent infant complications in GDM. However, our findings require validation in larger, better-designed studies.
Acknowledgements
The authors are grateful to the staff of our facility for their support. We thank American Journal Experts (AJE) for English language editing.
Author contributions
DS Conceptualization, Investigation, Methodology, Formal analysis, Writing-Original draft, Writing–Review & Editing. EM Investigation, Methodology, Formal analysis, Writing–Review & Editing. MM Investigation, Data curation, Formal analysis. HS Conceptualization, Methodology, Writing–Review & Editing. TK Conceptualization, Supervision, Writing–Review & Editing. KI Writing–Review & Editing, Project administration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analyzed during this study are included in this published article. The data are not publicly available due to ethical restrictions. However, the datasets are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the ethics board of Saitama Medical Center Jichi Medical University (approval no. S20-070, Aug 27. 2020). An opt-out consent form was published on the website of Saitama Medical Center Jichi Medical University. This study was exempted from the requirement for written informed consent because of its retrospective design and the fact that participants chose to participate in the study by not opting out on the Saitama Medical Center Jichi Medical University webpage, which implied their tacit consent to participate in this study. None of the participants opted out of this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med. 1994;45:245–260. doi: 10.1146/annurev.med.45.1.245. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Glycemic targets: standards of medical care in diabetes. Diabet Care. 2020;43:66–76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu I, Hiramatsu Y, Omori Y, Nakabayashi M, J.G.A. (Japan Glycated Albumin) Study Group Comparison of HbA1c and glycated albumin as a control marker for newborn complications in diabetic women in a multicentre study in Japan (Japan glycated albumin study group: study 2) Ann Clin Biochem. 2018;55:639–646. doi: 10.1177/0004563218763695. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Wei Y, Fan L, Zhao Y, Li Y, Liu Y, et al. A multicenter all-inclusive prospective study on the relationship between glycemic control markers and maternal and neonatal outcomes in pregnant women. J Matern Fetal Neonatal Med. 2021;34:3154–3161. doi: 10.1080/14767058.2019.1678139. [DOI] [PubMed] [Google Scholar]
- 5.Kohzuma T, Tao X, Koga M. Glycated albumin as biomarker: evidence and its outcomes. J Diabetes Complications. 2021;35:108040. doi: 10.1016/j.jdiacomp.2021.108040. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu I, Kohzuma T, Koga M. A proposed glycemic control marker for the future: glycated albumin. J Lab Precis Med. 2019;4:23. doi: 10.21037/jlpm.2019.05.01. [DOI] [Google Scholar]
- 7.Li HP, Wang FH, Tao MF, Huang YJ, Jia WP. Association between glycemic control and birthweight with glycated albumin in Chinese women with gestational diabetes mellitus. J Diabetes Investig. 2016;7:48–55. doi: 10.1111/jdi.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda S, Iuchi T, Goto A, Katanoda K, Iida S, Oikawa Y, et al. Weight control before and during pregnancy for patients with gestational diabetes mellitus. J. Diabetes Investig. 2019;10:1075–1082. doi: 10.1111/jdi.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury JC, Miodovnik M, LeMasters G, Sibai B. Pregnancy outcome and progression of diabetic nephropathy. What's next? J Matern Fetal Neonatal Med. 2002;11:238–244. doi: 10.1080/jmf.11.4.238.244. [DOI] [PubMed] [Google Scholar]
- 10.Usami T, Yokoyama M, Ueno M, Iwama N, Sagawa N, Kawano R, et al. Comparison of pregnancy outcomes between women with early-onset and late-onset gestational diabetes in a retrospective multi-institutional study in Japan. J. Diabetes Investig. 2020;11:216–222. doi: 10.1111/jdi.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K, Matsubara K, Nakamoto O, Ushijima J, Ohkuchi A, Koide K, et al. Outline of the new definition and classification of “hypertensive disorders of pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res Pregnancy. 2018;6:33–37. doi: 10.14390/jsshp.HRP2018-014. [DOI] [Google Scholar]
- 13.Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol. 2011;5:1455–1462. doi: 10.1177/193229681100500619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gul R, Iqbal S, Anwar Z, Ahdi SG, Ali SH, Pirzada S. Pre-pregnancy maternal BMI as predictor of neonatal birth weight. PLoS ONE. 2020;15:e0240748. doi: 10.1371/journal.pone.0240748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepercq J, Le Ray C, Godefroy C, Pelage L, Dubois-Laforgue D, Timsit J. Determinants of a good perinatal outcome in 588 pregnancies in women with type 1 diabetes. Diabetes Metab. 2019;45:191–196. doi: 10.1016/j.diabet.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 19.K.M. Rasmussen, A.L. Yaktine, 2009 Institute of Medicine. (US) and National Research Council (US). Committee to reexamine IOM pregnancy weight guidelines. Weight gain during pregnancy: reexamining the guidelines. National Academies Press, Washington (DC). US. [PubMed]
- 20.Mendes N, Alves M, Andrade R, Ribeiro RT, Papoila AL, Serrano F. Association between glycated albumin, fructosamine, and HbA1c with neonatal outcomes in a prospective cohort of women with gestational diabetes mellitus. Int J Gynaecol Obstet. 2019;146:326–332. doi: 10.1002/ijgo.12897. [DOI] [PubMed] [Google Scholar]
- 21.Hughes RC, Rowan J, Florkowski CM. Is there a role for HbA1c in pregnancy? Curr Diab Rep. 2016;16:5. doi: 10.1007/s11892-015-0698-y. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010;33:509–511. doi: 10.2337/dc09-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104. doi: 10.1016/j.cca.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Koga M, Hirata T, Kasayama S, Ishizaka Y, Yamakado M. Body mass index negatively regulates glycated albumin through insulin secretion in patients with type 2 diabetes mellitus. Clin Chim Acta. 2015;438:19–23. doi: 10.1016/j.cca.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Huh JH, Kim KJ, Lee BW, Kim DW, Kang ES, Cha BS, et al. The relationship between BMI and glycated albumin to glycated hemoglobin (GA/A1c) ratio according to glucose tolerance status. PLoS ONE. 2014;9:e89478. doi: 10.1371/journal.pone.0089478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonagra AD, Biradar SM, Murthy DKJ. Normal pregnancy- a state of insulin resistance. J Clin Diagn Res. 2014;8:01–03. doi: 10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sameshima H, Kamitomo M, Kajiya S, Kai M, Furukawa S, Ikenoue S. Early glycemic control reduces large-for-gestational-age infants in 250 Japanese gestational diabetes pregnancies. Am J Perinatol. 2000;17:371–376. doi: 10.1055/s-2000-13450. [DOI] [PubMed] [Google Scholar]
- 28.Hay WW. Care of the infant of the diabetic mother. Curr Diab Rep. 2012;12:4–15. doi: 10.1007/s11892-011-0243-6. [DOI] [PubMed] [Google Scholar]
- 29.Hiramatsu Y, Shimizu I, Omori Y, Nakabayashi M. Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J. 2012;59:145–151. doi: 10.1507/endocrj.K10E-410. [DOI] [PubMed] [Google Scholar]
- 30.Yu F, Lv L, Liang Z, Wang Y, Wen J, Lin X, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014;99:4674–4682. doi: 10.1210/jc.2013-4332. [DOI] [PubMed] [Google Scholar]
- 31.Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM) Endocr J. 2010;57:135–140. doi: 10.1507/endocrj.K09E-234. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi K, Matsuda Y, Kawamichi Y, Shiozaki A, Saito S. Smoking during pregnancy increases risks of various obstetric complications: a case-cohort study of the Japan Perinatal Registry Network database. J Epidemiol. 2011;21:61–66. doi: 10.2188/jea.JE20100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc. 2003;103:48–54. doi: 10.1053/jada.2003.50001. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Shi DB, Lv LY. The establishment of biological reference intervals of nontraditional glycemic markers in a Chinese population. J Clin Lab Anal. 2017;31:e22097. doi: 10.1002/jcla.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvin E, Warren B, He X, Sacks DB, Saenger AK. Establishment of community-based reference intervals for fructosamine, glycated albumin, and 1,5-anhydroglucitol. Clin Chem. 2018;64:843–850. doi: 10.1373/clinchem.2017.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data are not publicly available due to ethical restrictions. However, the datasets are available from the corresponding author on reasonable request.


