Abstract
Background
Cardiovascular autonomic neuropathy (CAN) is a debilitating complication of diabetes mellitus. To date, there is no systematic review on all the available drug treatments for CAN in diabetic patients, except for one review focusing on aldose reductase inhibitors.
Objective
To evaluate available drug treatment options for CAN in diabetic patients.
Methods
A systematic review was conducted with a search of CENTRAL, Embase, PubMed and Scopus from database inception till 14th May 2022. Randomised controlled trials (RCTs) of diabetic patients with CAN that investigated the effect of treatment on blood pressure, heart rate variability, heart rate or QT interval were included.
Results
Thirteen RCTs with a total of 724 diabetic patients with CAN were selected. There was a significant improvement in the autonomic indices of diabetic patients with CAN given angiotensin-converting enzyme inhibitor (ACEI) for 24 weeks (p<0.05) to two years (p<0.001), angiotensin-receptor blocker (ARB) for one year (p<0.05), single dose of beta blocker (BB) (p<0.05), omega-3 polyunsaturated fatty acids (PUFAs) for three months (p<0.05), alpha-lipoic acid (ALA) for four months (p < 0.05) to six months (p=0.048), vitamin B12 in combination with ALA, acetyl L‑carnitine (ALC), superoxide dismutase (SOD) for one year (p=0.001) and near significant improvement in the autonomic indices of diabetic patients with CAN given vitamin E for four months (p = 0.05) compared to the control group. However, there was no significant improvement in the autonomic indices of patients given vitamin B12 monotherapy (p ≥ 0.05).
Conclusion
ACEI, ARB, BB, ALA, omega-3 PUFAs, vitamin E, vitamin B12 in combination with ALA, ALC and SOD could be effective treatment options for CAN, while vitamin B12 monotherapy might be unlikely to be recommended for the treatment of CAN due to its lack of efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-023-00629-x.
Keywords: Diabetic Mellitus, Cardiovascular autonomic neuropathy, Effect, Treatment, Systematic review
Introduction
Cardiovascular autonomic neuropathy (CAN) is a severely debilitating complication of Type I and II diabetes mellitus [1]. Past studies have shown that the prevalence of CAN among diabetics is generally 20 to 36%, but it can go up to 90% due to variability in the study populations and methods used [2]. The prevalence of CAN is also higher among patients who are older or have a longer duration of diabetes [3].
The pathogenesis of CAN has not been fully elucidated, but past studies have suggested that hyperglycaemia increases inflammation and oxidative stress that cause nerve damage [4–6]. This cardiac sympathetic and parasympathetic denervation then leads to debilitating symptoms of CAN such as resting tachycardia, QT interval prolongation, non-dipping in blood pressure, orthostatic hypotension and impaired heart rate variability [7, 8]. Patients with CAN are also at a greater risk of morbidity and mortality due to the association of CAN with major cardiovascular events such as silent myocardial ischaemia, fatal arrhythmias and stroke [8, 9]. Therefore, therapeutic interventions are needed to manage the debilitating symptoms and prevent the progression of CAN.
There have been some common interventions proposed by scientific societies such as the Toronto Consensus Panel and the American Diabetes Association for the management of CAN. These interventions include glycaemic control and the use of pharmacological drug therapy such as midodrine for the treatment of orthostatic hypotension [1, 10]. However, the treatment options are limited and largely debatable due to the low level of evidence on these interventions [1, 10]. The studies cited by the scientific societies on the use of midodrine to treat orthostatic hypotension in CAN did not analyse the results of diabetic patients with CAN separately from patients with other causes of orthostatic hypotension [11, 12].
Currently, there is only one existing systematic review focusing on the use of a specific class of drugs known as aldose reductase inhibitors for the management of CAN [13]. Some aldose reductase inhibitors such as ponalrestat and tolrestat have also been withdrawn from the market due to their lack of efficacy in clinical trials or adverse effects such as fever, diarrhoea, increase in liver enzymes and deaths from fatal hepatic necrosis [14–16]. Moreover, there have been clinical trials on other drug treatment options for CAN. To date, there is no systematic review that provides a comprehensive overview of various treatment options as well as an update on the new treatment options for CAN. Hence, this systematic review aims to evaluate the effect of all the available drug treatment options on the health outcomes of diabetic patients with CAN.
Methods
This systematic review was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Search strategy
A literature search was conducted on the CENTRAL, Embase, PubMed and Scopus electronic databases to retrieve records from database inception till 14th May 2022. A search strategy was developed to identify articles relevant to the following: (i) diabetic patients with CAN, (ii) treatment for CAN, (iii) effect of the treatment. The handsearching of the reference lists of the publications was conducted to look for additional articles that could potentially be eligible. The search terms used in each database are presented in Appendix Table 1.
Study selection
Randomised controlled trials (RCT) of drug interventions that are still in production and used to treat patients diagnosed with diabetes mellitus and CAN were selected. To be included, the studies also had to report the criteria used for the diagnosis of CAN and measure at least one of the following outcomes: blood pressure, heart rate variability, heart rate or QT interval. These outcomes of interest were selected as the signs and symptoms of CAN include resting tachycardia, impaired heart rate variability, QT prolongation, orthostatic hypotension, reverse dipping and non-dipping of blood pressure due to sympathetic and parasympathetic dysfunction in diabetic patients with CAN [7, 8]. Hence, these outcomes of interest could be used to determine the effect of drug treatment on diabetic patients with CAN. The diagnosis criteria for CAN should include abnormal values for at least one of the cardiovascular autonomic reflex tests (CARTs), time or frequency-domains of heart rate variability (HRV) as these measures are used for the assessment of cardiovascular autonomic function. Studies that comprise of patients with various types of diabetic neuropathies were included if the results of the patients with CAN were analysed separately from the results of patients with other types of diabetic neuropathies. Additionally, all included studies had to be full-length articles.
Studies were excluded if they were RCTs on discontinued drugs (for example, aldose reductase inhibitors ponalrestat and tolrestat that have been discontinued in market due to their adverse effects) or reporting solely physiological (for example, arterial stiffness parameters) or biochemical (for example, levels of inflammatory advanced glycation end products) outcomes. These outcomes are valuable but may not be directly relevant to CAN symptoms due to the complex and disputed underlying mechanisms involved in the progression of CAN. Non-English reports, conference abstracts, protocols, case reports, letters, reviews and supplements were excluded. Animal studies were also excluded as they were not directly relevant to the CAN patient population.
The entire process of literature search and screening was conducted by two reviewers. After the removal of duplicate records, the preliminary screening of titles and abstracts was conducted to identify eligible records that meet the inclusion and exclusion criteria. Records that were identified to be potentially eligible were retained and their full text reports were sought for retrieval. The inclusion and exclusion criteria were then reapplied to the full text reports to assess the eligibility of the reports for inclusion into the systematic review.
Quality assessment of selected studies
The quality of the RCTs was assessed using the Cochrane Risk of Bias (RoB) tool for independent parallel-group RCT and independent crossover RCT [18]. The risk of bias in each parallel-group RCT was determined based on the judgement of the two reviewers using the RoB tool across five domains: the randomisation process, the effect of assignment to intervention, missing data, the measurement and the reporting of the outcomes. The risk of bias in each crossover RCT was also examined based on the five domains and an additional domain on the carryover effects of the treatment used in the study. The overall risk of bias was then determined for each study based on the responses in all the domains.
Data extraction and data analysis
The following data was extracted from the selected studies: (i) study design, (ii) sample size and the demographics of the study subjects, (iii) methods for the diagnosis of CAN, (iv) randomised allocation of subjects to the experimental and control groups, (v) type of treatment and dosage of treatment, (vi) duration of the study, (vii) outcome parameters measured and (viii) key findings and interpretation.
Due to the diversity of the selected studies in terms of the type, dosage and duration of treatment used, the criteria used for the diagnosis of CAN and the outcomes measured, a meta-analysis of the quantitative data was not conducted.
Results
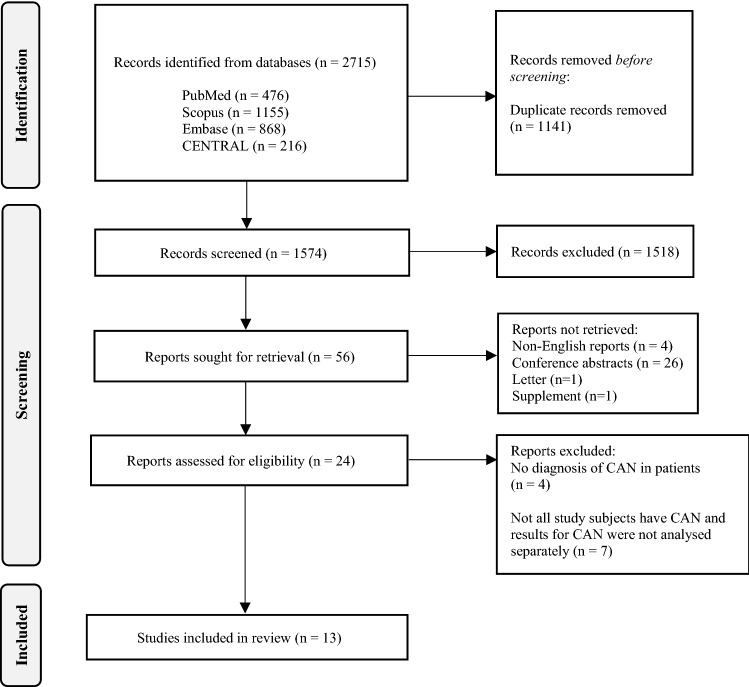
The study selection process is shown in Fig. 1. The literature search of six electronic databases yielded 2715 records related to CAN. No additional records were identified from the handsearching of the reference lists of the publications. After the removal of 1141 duplicate records, the screening of the titles and abstracts identified 56 records for full text retrieval. There were 32 reports that could not be retrieved as they were non-English reports, letter, supplement, or conference abstracts. Of the 24 reports that were retrieved, the four studies that did not diagnose the patients with CAN and the seven studies that did not analyse the results of the patients with CAN separately from those without CAN were removed. There were thirteen studies that met the selection criteria and were included in this systematic review.
Fig. 1.
Prisma Flow Chart for the process of literature search and screening
The thirteen studies measured several autonomic indices, as described in Table 1, which reflect the parasympathetic and sympathetic function of the cardiovascular system. Heart rate (HR) was used as an outcome measure in five studies [19–23]. It is a useful monitoring parameter for diabetic patients with CAN who might have elevated resting HR due to sympathetic dominance [24]. The frequency domain indices of heart rate variability (HRV) were used in six studies [19, 23, 25–28] and they were derived from the distribution of absolute or relative power in the electrocardiogram into various frequency bands [29]. The indices used were the total power (TP), very low frequency power (VLF), low frequency power (LF), high frequency power (HF) and low-to-high frequency ratio (LF/HF) [19, 23, 25–28]. The time domain indices of HRV that indicate the degree of variability in the RR intervals were measured in six studies [20, 23, 25, 27, 28, 30]. These indices were the standard deviation of RR intervals (SDRR), standard deviation of NN intervals (SDNN), standard deviation of means of NN intervals (SDANNi), the root mean square of successive difference between NN intervals (RMSSD), the percentage of differences more than 50 ms between adjacent NN intervals (pNN50) and the coefficient of variation of heart rate variability (CV) [20, 23, 25, 27, 28, 30].
Table 1.
Autonomic indices measured in the selected RCTs
| Parameters measured as outcomes | Units | Description of the parameter |
|---|---|---|
| Heart rate | bpm | Normal resting HR ranges from 60-100 bpm [31]; but elevated resting HR of 90-100 bpm could occur in diabetic patients with CAN [32] |
| Frequency-domain measures of HRV | ||
| Total power (TP) | ms2 | Sum of the VLF, LF, and HF bands from short-term ECG recordings [33] |
| Very low frequency power of heart rate variability (VLF) | ms2 | Absolute power of the VLF band (0.0033–0.04 Hz) [33] |
| Low frequency power of heart rate variability (LF) |
ms2 or NU |
Absolute power of the LF band (0.04–0.15 Hz) or Relative power of the LF band in normalised units [33] |
| High frequency power of heart rate variability (HF) |
ms2 or NU |
Absolute power of the HF band (0.15–0.40 Hz) or Relative power of HF band in normalised units [33] |
| Low-to-high frequency ratio (LF/HF) | No units | Ratio of LF to HF power [33] |
| Time-domain measures of HRV | ||
| Standard deviation of RR intervals (SDRR) | ms | Standard deviation of the RR intervals including abnormal and false beats [33] |
| Standard deviation of NN intervals (SDNN) | ms | Standard deviation of the normal RR intervals in a 24 h ECG recording [33] |
| Standard deviation of means of NN intervals (SDANNi) | ms | Mean of the standard deviations of the normal RR intervals in each of the 5-min segments of a 24 h ECG recording [33] |
| Root mean square of successive differences between NN intervals (RMSSD) | ms | Root mean square of time differences between successive normal RR heartbeats [33] |
| Percentage of differences > 50 ms between adjacent NN intervals (pNN50) | % | Percentage of adjacent normal RR intervals with difference > 50 ms [33] |
| Coefficient of variation in heart rate variability (CV) | % | Standard deviation of the RR intervals divided by the mean of RR intervals [33] |
| Cardiovascular autonomic reflex tests (CARTs) | ||
| Expiration-to-inspiration ratio (E/I) | No units | Ratio of the mean of the longest RR intervals during each expiration and the mean of the shortest intervals during each inspiration over 6 cycles of deep breathing [34] |
| Mean circular resultant of vector analysis (MCR) | No units | RR intervals obtained from deep breathing test are plotted on a time axis using vector analysis [35] |
| Valsalva ratio | No units | Ratio of the longest RR interval measured after the Valsalva manoeuvre and the shortest RR interval during the manoeuvre [36] |
| Ratio of RR intervals at 15th and 30th heartbeats after standing (30:15) | No units | Ratio of the shortest RR interval at the 15th beat and the longest RR interval at the 30th beat during a change from supine to standing position [37] |
| Orthostatic hypotension (OH) | mmHg | Decrease in SBP of 30 mmHg after a change from supine to standing position [1] |
| Blood pressure response to sustained handgrip | mmHg | Increase in the blood pressure of a subject that grips at 30% of their maximum strength for up to 5 min [37] |
| Other blood pressure parameters | ||
| Mean arterial pressure (MAP) | mmHg | Average arterial pressure throughout one cardiac cycle comprising of systole and diastole [38] |
| Systolic blood pressure (SBP) | mmHg | Highest blood pressure reading when the ventricles of the heart contract [39] |
| Diastolic blood pressure (DBP) | mmHg | Lowest blood pressure reading right before the next contraction of the ventricles of the heart [39] |
| Time index of DBP | % | Proportion of time that the DBP was above the normal reference range [40] |
| Standard deviation of DBP | mmHg | Degree of variation in the diastolic blood pressure in relation to the mean [41] |
| Diurnal index (DI) | % | Difference in mean daytime and nighttime blood pressure is divided by mean daytime blood pressure, then multiplied by 100 [40] |
HRV heart rate variability, bpm beats per minute, s seconds, ms milliseconds, ms2 milliseconds squared, ECG electrocardiogram, NU normalised units, % Percent, Hz Hertz, mmHg millimetres of mercury
Five studies also used cardiovascular autonomic reflex tests (CARTs) to assess the changes in blood pressure and heart rate variability in response to physiologic manoeuvres [20, 23, 30, 42, 43]. The autonomic indices obtained from CARTs include expiration-to-inspiration ratio (E/I), mean circular resultant of vector analysis (MCR), Valsalva ratio, orthostatic hypotension (OH), blood pressure response to sustained handgrip and the ratio of the RR intervals at the 15th and 30th heartbeat after standing (30:15) [20, 23, 30, 42, 43]. Other parameters of blood pressure (BP), apart from those measured during CARTs, were used in seven studies and can also be used to reflect the cardiovascular autonomic function in diabetic patients with CAN [19–21, 25, 42–44]. These parameters were the mean arterial pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), load of DBP, time index of DBP, standard deviation of DBP, diurnal index (DI) [19–21, 25, 42–44].
A brief overview of the twelve studies and their key findings is presented in Table 2. The enrolled patients have diabetes and CAN, but generally do not have other serious medical conditions (Table 2). While OH is one of the CARTs, the findings for OH in this systematic review have been reported under BP as it measures a decrease in SBP in response to postural change (Table 2).
Table 2.
Summary of the RCTs on various drug treatment options for diabetic patients with CAN
| First Author, Publication year, Location of study |
Study design | Experimenta /Comparator | Inclusion (I) and exclusion (E) criteria | Number of patients | Key findings* | ||||
|---|---|---|---|---|---|---|---|---|---|
| HRV, CARTs (excluding OH) | BP (including OH) | HR | Symptoms of CAN | QT Interval | |||||
| Antihypertensives–ACEI, ARB, BB | |||||||||
| Athyros et al. 1998, Greece [19] | NR, parallel-group RCT for 1 year | Quinapril 20 mg daily / Placebo |
(I) T1DM or T2DM, CAN (E) History of cardiovascular disease |
30/30 |
Significant difference in LF/HF between the 2 groups from 7:00–15:00 h, 15:00–23:00 h, 23:00–7:00 h (p < 0.001) Significant changes in all indices from baseline (quinapril): From 7:00–15:00 h: increase in HF (p < 0.05), decrease in LF/HF (p < 0.05) From 23:00- 7:00 h: increase in HF, decrease in LF and LF/HF (p < 0.001 for all three indices) Significant changes in all indices from baseline (placebo): From 7:00–15:00 h: decrease in HF (p < 0.05), increase in LF (p < 0.01) and LF/HF (p < 0.01) From 15:00–23:00 h: increase in LF/HF (p < 0.05) |
No significant changes in SBP and DBP from baseline in both groups (p ≥ 0.05) |
Significant decrease in HR (quinapril) (p < 0.01), significant increase in HR (placebo) (p < 0.05) from baseline HR was significantly lower in quinapril than placebo group (p < 0.001) |
Not studied | Not studied |
| Didangelos et al. 2006, Greece [20] | Open, parallel-group RCT for 1 year |
Quinapril 20 mg daily / Losartan 100 mg daily/ Quinapril 20 mg daily + losartan 100 mg daily |
(I) T1DM or T2DM, CAN (E) History of cardiovascular disease |
20/22/20 | Significant changes in the indices with abnormal values at baseline for all groups: increase in E/I, MCR, SDRR (all groups), Valsalva ratio (quinapril only), 30:15 (losartan only, losartan + quinapril) (p < 0.05 for all indices) | No significant change in OH, SBP and DBP from baseline in all groups (p ≥ 0.05) | Significant decrease in HR in all groups (p < 0.05) | Not studied | Not studied |
| Didangelos et al. 2017, Greece [30] | Open, parallel-group RCT for 2 years | Quinapril 20 mg daily/Placebo |
(I) T1DM or T2DM, CAN (E) Coronary artery disease |
31/32 |
Significant difference between the 2 groups for all indices except Valsalva ratio (p < 0.001 for E/I, MCR, SDRR, 30:15, p ≥ 0.05 for Valsalva ratio) Significant increase in E/I (p = 0.011), MCR (p = 0.006), SDRR (p = 0.004) from baseline (quinapril), no notable changes in Valsalva ratio, 30:15 (p ≥ 0.05 for both indices) Significant decrease in E/I (p = 0.007), MCR (p = 0.01), SDRR (p < 0.05), 30:15 (p < 0.05) from baseline (placebo), no notable changes in Valsalva ratio (p ≥ 0.05) |
Significant difference in OH between the two groups (p < 0.001) No notable changes in OH (p ≥ 0.05) from baseline (quinapril); significant increase in OH (p = 0.018) from baseline (placebo) |
Not studied | Not studied | Not studied |
| Hjortkjær et al. 2016, Denmark [21] | Double-blind crossover RCT with 12 weeks of one intervention before switching for 12 more weeks | Enalapril 20 mg in the morning and placebo at bedtime/Placebo in the morning and 20 mg enalapril at bedtime |
(I) T1DM, CAN, nocturnal BP dipping of < 10% (E) T2DM, Cardiovascular disease, kidney disease, hypersensitivity to ACEI |
13/11 | Not studied |
Significant difference in SBP dipping between the 2 groups: Mean dipping at bedtime 2.4% greater than morning (95% CI 0.03 to 4.9%, p = 0.048) Notable but not significant difference in DBP dipping between the 2 groups: mean dipping at bedtime 1.7% greater than morning (95% CI − 0.7 to 4.1%, p = 0.07) No significant difference in MAP dipping between the 2 groups: mean dipping at bedtime 2.2% greater than morning (95% CI − 0.1 to 4.5%, p = 0.17) |
No significant difference in daytime HR (p = 0.15) and nighttime HR (p = 0.34) between the 2 groups | Not studied | Not studied |
| Kontopoulos et al. 1997, Greece [25] | Double-blind, parallel-group RCT for 6 months | Quinapril 20 mg daily/Placebo |
(I) T1DM or T2DM, CAN (E) Cardiovascular disease, concurrent treatment with beta blocker or ACEI |
20/20 |
Significant difference between the 2 groups for SDNN, RMSSD, TP (p < 0.05 for three indices) and HF, LF/HF (p < 0.01 for both indices); no notable difference for LF (p ≥ 0.05); increase (quinapril) and decrease (placebo) in SDNN, RMSSD Significant increase in TP from baseline (p < 0.05) (quinapril), no notable changes in TP from baseline (p ≥ 0.05) (placebo) Significantly greater HF, RMSSD, pNN50, significantly lower LF in moderate than severe CAN (baseline: p < 0.05, at 6 months: p < 0.01) |
No significant changes in BP from baseline in both groups (p ≥ 0.05) | Not studied | Not studied | Not studied |
| Reid et al. 1987, NR [22] | Double-blind, crossover RCT with single dose interventions given in a randomised order at weekly intervals | Epanolol 200 mg/atenolol 50 mg/pindolol 5 mg/Placebo |
(I) T2DM, CAN (E) NR |
8 patients undergo all interventions in randomize-d order | Not studied | Not studied |
Significantly lower HR from 08.00–13.00 h, 14.00–23.00 h for epanolol (p < 0.05) and atenolol (p < 0.001) and from 23.00–08.00 h for atenolol (p < 0.01) than placebo Significantly higher HR for pindolol (p < 0.05) than placebo |
Not studied | Not studied |
| Antioxidants | |||||||||
| Didangelos et al. 2020, Greece [42] | Double-blind, parallel-group RCT for 1 year | SOD 10 mg, ALA 570 mg, ALC 300 mg, Vitamin B12 250mcg daily/Placebo |
(I) T2DM, CAN, DPN (E) History of cardiovascular disease including arrhythmias |
43/42 |
No significant differences in MCR (p = 0.220) and Valsalva ratio (p = 0.393) between the 2 groups Significant decrease in the MCR from baseline in the placebo group (p = 0.01) |
Significant difference in OH between the 2 groups (p = 0.001); significant decrease in OH from baseline (p = 0.001) (ALA), no notable change in OH from baseline (p = 0.06) (placebo) No significant difference in SBP (p = 0.369) and DBP (p = 0.258) between the 2 groups |
Not studied | Not studied | Not studied |
| Didangelos et al. 2021, Greece [43] | Double-blind, parallel-group RCT for 1 year | Oral dispersible tablet containing 1000 μg of methylcobala-min / Placebo |
(I) T2DM, CAN, DPN, low vitamin B12, metformin for ≥ 4 years (E) Cardiovascu-lar events, kidney disease |
44/46 |
No significant differences in MCR (p = 0.452) and Valsalva ratio (p = 0.761) between the 2 groups Significant decrease in MCR from baseline in the placebo group (p = 0.025) |
No significant difference in OH (p = 0.929), SBP (p = 0.547) and DBP (p = 0.350) between the 2 groups | Not studied | Not studied | Not studied |
|
Lee et al 2017, Korea [23] |
Double-blind, parallel-group RCT for 24 weeks | ALA 600 mg daily (12 weeks) then 1200 mg daily (12 weeks)/Placebo |
(I) T2DM, CAN (E) kidney or liver disease, cardiovascular disease including arrhythmias |
46/45 |
Notable but not significant difference in 3 indices between the 2 groups: SDNN (p = 0.06), LF/HF (p = 0.06), LF (p = 0.08) SDNN, LF/HF, LF increased in ALA group (p ≥ 0.05) but decreased in placebo group (p ≥ 0.05) |
Significant difference between the 2 groups in orthostatic SBP (p = 0.048), but no significant difference in orthostatic DBP (p = 0.286) | No significant differences between the 2 groups (p = 0.591) | Not studied | Not studied |
| Manzella et al. 2001, NR [26] | Double-blind, parallel-group RCT for 4 months | Vitamin E 600 mg daily/Placebo |
(I) T2DM, CAN (E) Respiratory rate < 10 breaths/min |
25/25 |
Near significant increase in RR interval length, TP and HF, significant decrease in LF and LF/HF (p = 0.05 for all indices) from baseline (vitamin E) No significant changes in all 5 indices from baseline (placebo) (p > 0.05) Significant negative correlation between the LF/HF and the plasma vitamin E concentration (r = –0.43, p < 0.04) |
No significant difference in MAP between the two groups (p > 0.05) | Not studied | Not studied | Not studied |
| Serhiyenko et. al 2018, Ukraine [27] | NR, parallel-group RCT for 3 months | One 1 g capsule daily (containing 90% omega-3 PUFA and 4 mg α-tocopherol acetate) with hypoglycemic treatment/ Hypoglycemic treatment |
(I) T2DM, CAN (E) NR |
21/15 |
Significant increase in SDNN, SDANNi, RMSSD, pNN50, HF, LF/HF (p < 0.05 for the five indices), LF (p < 0.01) from baseline (omega-3 PUFA and hypoglycemic treatment), no notable change in VLF (p > 0.05) No significant changes in any indices from baseline in patients given hypoglycemic treatment only (p > 0.05) |
Not studied | Not studied | Not studied | Not studied |
| Serhiyenko et al. 2019, Ukraine [44] | Open, parallel-group RCT for 3 months | One 1 g capsule daily (containing 90% omega-3 PUFA and 4 mg α-tocopherol acetate) with hypoglycemic treatment/ Hypoglycemic treatment |
(I) T2DM, CAN (E) Taken omega-3 PUFAs for 6mths before study, other causes of neuropathy, cardiovascular disease |
21/21 | Not studied |
Significant decrease in DBP, time index of DBP, standard deviation of DBP (p < 0.001 for the three indices) and load of DBP (p < 0.05) from baseline (omega-3 PUFAs and hypoglycemic treatment), no notable change in diurnal DBP index (p ≥ 0.05) No significant changes in DBP parameters from baseline at night for hypoglycemic treatment only (p ≥ 0.05) No significant changes in SBP in both groups at night (p ≥ 0.05) |
Not studied | Not studied | Not studied |
| Ziegler et al. 1997, Germany [28] | Double-blind, parallel-group RCT for 4 months | ALA 800 mg daily/Placebo |
(I) T2DM, CAN (E) Taken ALA 3 months before study, other causes of neuropathy, cardiovascular disease |
39/34 |
Significant difference in LF and RMSSD between the 2 groups (p < 0.05): both indices increased in ALA group but decreased in placebo group Notable but not significant difference in HF (p = 0.094) and CV (p = 0.097) between the 2 groups: increase in HF in ALA group but decreased in placebo group No significant difference in LF/HF between the 2 groups (p ≥ 0.05) |
No significant difference in SBP and DBP between the 2 groups (p ≥ 0.05 for both indices) | No significant difference in heart rate between the 2 groups (p ≥ 0.05) | No significant difference in the rates of CAN symptoms between the 2 groups; the rate decreased by 6.9% in ALA group, increased by 3.6% in placebo group (p ≥ 0.05) | Not studied |
HRV heart rate variability, BP blood pressure, HR heart rate, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, NR not reported by the study, RCT randomised controlled trial, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, CAN cardiovascular autonomic neuropathy, DPN diabetic peripheral neuropathy, SOD superoxide dismutase, ALA alpha-lipoic acid, ALC acetyl L-carnitine, PUFAs polyunsaturated fatty acids, ECG electrocardiogram, mL millilitres, min minute, m metre squared, TP total power of HRV, VLF very low frequency power of HRV, LF low frequency power of HRV, HF high frequency power of HRV, LF/HF low-to-high frequency ratio, OH orthostatic hypotension, DBP diastolic blood pressure, SBP systolic blood pressure, MAP mean arterial pressure, E/I expiration-to-inspiration ratio, MCR mean circular resultant of vector analysis, SDRR standard deviation of RR intervals, 30:15 ratio of RR intervals at 15th and 30th heartbeats after standing, SDNN standard deviation of NN intervals, SDANNi standard deviation of the means of all NN intervals in all 5 min segments, RMSSD root mean square of successive differences between NN intervals, pNN50 percentage of differences > 50 ms between adjacent NN intervals, CV coefficient of variation in HRV
*Significance level of 5% was used by the studies
The additional information on the diagnosis criteria for CAN used for the enrolment of patients and the baseline characteristics of the enrolled patients such as age, body mass index, glycosylated haemoglobin (HbA1c), duration of diabetes and electrocardiogram (ECG) is summarized in Appendix Table 2. The baseline characteristics were similar between the experimental and control groups. However, the diagnosis criteria for CAN varied across the different studies. As CAN could be associated with QT prolongation, QT interval was also measured in diabetic patients with CAN in five studies, but there was no abnormal ECG, conduction defects or complex arrhythmias in the diabetic patients with CAN at baseline [19, 21, 22, 25, 30]. The other studies did not report QT interval at baseline [20, 23, 26–28, 42–44].
Pharmacological treatments
Angiotensin-converting enzyme inhibitor, Angiotensin-receptor blocker
Five studies examined the use of angiotensin-converting enzyme inhibitors (ACEI) monotherapy or in combination with angiotensin-receptor blocker (ARB) for the treatment of diabetic patients with CAN [19–21, 25, 30]. In the 2006 study by Didangelos et al., diabetic patients with CAN were given either 20 mg of quinapril daily, 100 mg of losartan or a combination of quinapril and losartan daily at 20 mg and 100 mg respectively for 1 year [20]. There was a significant improvement in HR and other autonomic indices that were abnormal at baseline for all groups of patients, which were E/I, MCR and SDRR (p < 0.05 for all indices). However, there were no significant changes in OH, SBP, DBP from baseline in all groups of patients (p ≥ 0.05 for all indices) (Table 2).
In the studies by Kontopoulos et al., Athyros et al. and 2017 study by Didangelos et al., diabetic patients with CAN were given either 20 mg of quinapril daily or placebo daily for six months, one year or two years respectively [19, 25, 30]. The patients given quinapril in the study by Kontopoulos et al. experienced a significant improvement in the HRV parameters HF, LF/HF, TP, SDNN, RMSSD compared to the patients given placebo (p < 0.01 for HF, LF/HF, p < 0.05 for TP, SDNN, RMSSD). The diabetic patients with moderate CAN that were given quinapril experienced greater improvement in the in the HRV parameters HF, LF, RMSSD, pNN50 compared to diabetic patients with severe CAN (p < 0.05 between the two groups at baseline, p < 0.01 between the two groups after 6 months). Similarly, the patients given quinapril in the study by Athyros et al. experienced a significant improvement in HR (p < 0.01) and the HRV parameters HF (p < 0.05), LF (p < 0.001) and LF/HF (p < 0.05) from baseline while the patients given placebo experienced a significant deterioration in the autonomic indices from baseline (p < 0.05 for HF, LF/HF and HR, p < 0.01 for LF). However, in the studies by Kontopoulos et al. and Athyros et al., there was no significant changes in the BP from baseline in both groups of patients (p ≥ 0.05). In the 2017 study by Didangelos et al., there was also a significant improvement in HRV in the patients given quinapril, as shown by an increase in the E/I ratio, MCR and SDRR compared to the patients given placebo (p = 0.001 for the three indices). There was a significant difference in OH between the two groups (p < 0.001) as the patients given quinapril did not experience notable changes in OH from baseline (p ≥ 0.05) while the patients given placebo experienced significant deterioration in OH (p = 0.018) (Table 2).
The 24-week crossover study by Hjortkaer et al. investigated the effect of a bedtime dose versus morning dose of enalapril on the blood pressure of patients with CAN and dipping of blood pressure by < 10% [21]. Patients were given placebo in the morning and 20 mg of enalapril at night or vice versa for the initial twelve weeks. Subsequently, the time of administration of each dose was switched for another twelve weeks. The dipping in SBP was significantly greater by 2.4% in the patients given the bedtime dose of enalapril (95% confidence interval (CI) [0.03 to 4.9%], p = 0.048). Additionally, the dipping in DBP and MAP was greater by 1.7% (95% CI [− 0.7 to 4.1%], p = 0.17) and 2.2% (95% CI [− 0.1 to 4.5%], p = 0.07) respectively in patients given the bedtime dose of enalapril, but these differences did not achieve statistical significance. There was also no significant difference in daytime HR and nocturnal HR between the two groups of patients (p = 0.15 and p = 0.34 respectively) (Table 2).
Beta-blocker (BB)
There was one study by Reid et al. that investigated the use of several beta blockers for the treatment of CAN [22]. Diabetic patients with CAN were given a single dose of epanolol 200 mg, atenolol 50 mg, pindolol 5 mg and placebo in a randomised order at weekly intervals. There was a significant reduction of heart rate for patients given epanolol and atenolol compared to patients given placebo (p < 0.05 and p < 0.01 respectively). Conversely, the patients given pindolol had significantly higher heart rate than the patients given placebo (p < 0.05) (Table 2).
Alpha Lipoic Acid (ALA) monotherapy or in combination with Acetyl L-carnitine (ALC), Superoxide dismutase (SOD), Vitamin B12
Three studies examined the use of ALA in monotherapy or in combination with other antioxidants [23, 28, 42]. In the study by Ziegler et al., diabetic patients with CAN were given either 800 mg of ALA daily or placebo daily for four months [28]. There was a significant improvement in the HRV parameters LF and RMSSD in the patients given ALA compared to the patients given placebo (p < 0.05 for the two indices). The rate of the symptoms of CAN also decreased by 6.9% in the patients given ALA while it increased by 3.6% in the patients given placebo, but this difference did not achieve statistical significance (p ≥ 0.05). However, there was no significant difference in SBP and DBP between the 2 groups (p ≥ 0.05 for both indices). In the study by Lee et al., diabetic patients with CAN were given either 600 mg of ALA daily for twelve weeks followed by 1200 mg of ALA for another twelve weeks or placebo daily for 24 weeks [23]. There was a significant improvement in the orthostatic SBP (p = 0.048), but no significant changes in the orthostatic DBP and other HRV parameters in the patients given ALA compared to the patients given placebo (p ≥ 0.05 for orthostatic DBP, SDNN, RMSSD, LF, HF, LF/HF, 30:15, Valsalva ratio, E/I, handgrip blood pressure). In the 2020 study by Didangelos et al., diabetic patients with CAN were given either 570 mg of ALA in combination with 10 mg of SOD, 300 mg of ALC and 250mcg of Vitamin B12 daily or given placebo for one year [42]. There was a significant improvement in OH from baseline in the patients given drug treatment (p = 0.001) while there was no notable change in OH from baseline in the patients given placebo (p = 0.06). There was no significant difference in the other autonomic indices between the two groups (p ≥ 0.05 for MCR, Valsalva ratio, SBP, DBP) (Table 2).
Vitamin B12 monotherapy
There was one study by Didangelos et al. that investigated the effect of Vitamin B12 monotherapy on diabetic patients with CAN [43]. The patients were given either 1000 μg of Vitamin B12 daily or given placebo daily for one year. There were no significant differences in the autonomic indices between the two groups (p ≥ 0.05) (Table 2).
Vitamin E monotherapy
There was one study by Manzella et al. on the use of Vitamin E for the treatment of CAN [26]. In the study, diabetic patients with CAN were given either 600 mg of Vitamin E daily or placebo daily for four months [26]. There was a near significant improvement in all the autonomic indices measured in patients treated with Vitamin E compared to placebo (p = 0.05). Furthermore, there was also a significant negative correlation between the LF/HF ratio and the plasma vitamin E concentration (r = −0.43, p < 0.04) (Table 2). However, there was no significant difference in mean arterial blood pressure between the two groups of patients at the end of the study (p ≥ 0.05).
Omega-3 polyunsaturated fatty acids
Two studies examined the use of omega-3 PUFA for the treatment of CAN [27, 44]. In both studies, diabetic patients with CAN were given either one 1 g capsule containing approximately 90% of omega-3 PUFAs daily with hypoglycemic treatment or hypoglycemic treatment only for three months. There was a significant improvement in HRV from baseline in patients given omega-3 PUFAs (p < 0.05 for SDNN, SDANNi, pNN50, RMSSD, HF and LF/HF, p < 0.01 for LF) while there were no significant changes in any autonomic indices from baseline in the patients given only hypoglycemic treatment (p > 0.05) [27]. There was also a significant improvement in nocturnal DBP parameters in the patients given omega-3 PUFAs and hypoglycemic treatment (p < 0.05 for load of DBP, p < 0.001 for DBP, time index of DBP and standard deviation of DBP) while there were no significant changes from baseline in the patients given only hypoglycemic treatment (p ≥ 0.05) [44]. However, there were no significant changes in the nocturnal SBP in both groups of patients (p ≥ 0.05) (Table 2) [44].
Quality of selected trials
Twelve of the RCTs included in this systematic review raised some concerns of bias while one RCT raised a low risk of bias. The twelve RCTs raised some concerns of bias in at least one domain as they generally did not report the methods used for the randomised allocation of patients into the experimental and comparator groups and whether an intention-to-treat analysis was used.
Discussion
There is a paucity of RCTs on the treatment options for CAN. The results of this systematic review suggest that ACE inhibitors, ARBs, beta blockers, vitamin E, omega-3 PUFAs and ALA might be effective treatments for CAN, while vitamin B12 might be less useful for the treatment of CAN. However, further research is required for the clinical efficacy of these treatment options to be fully established.
Pharmacological treatments
The evidence on antihypertensives suggests that they could be used to improve the autonomic indices in diabetic patients with CAN. ACEI and ARB block the renin–angiotensin–aldosterone system, thereby reducing the heart rate and blood pressure which could benefit diabetic patients with CAN [32]. The use of ACEI quinapril or ARB losartan alone or in combination with one another has been reported to improve the HRV and HR of diabetic patients with CAN in the included RCTs [19, 20, 25, 30]. The use of ACEI enalapril in the study by Hjortkaer et al. also reported significant improvement in nighttime SBP dipping, but no notable improvement in DBP dipping in diabetic patients with CAN [21]. Enalapril has demonstrated its potential to treat the non-dipping of blood pressure in diabetic patients with CAN, which could reduce their risk of cardiovascular morbidity and mortality. However, more studies should be conducted to study the effect of enalapril on diabetic patients with CAN due to the mixed results on SBP and DBP dipping. The study by Kontopoulos et al. also reported that the use of quinapril in diabetic patients with moderate CAN resulted in greater improvement in autonomic indices compared to diabetic patients with severe CAN, which suggests that quinapril could have a varied effect on diabetic patients depending on their severity of CAN. Overall, the potential benefits of quinapril and enalapril suggest that there could be a class effect of ACEI in improving autonomic indices in diabetic patients with CAN. In addition, beta blockers inhibit the beta-1 receptors in the heart, which could reduce heart rate and alleviate tachycardia in diabetic patients with CAN [45]. In the study by Reid et al., the beta blockers epanolol and atenolol were reported to significantly reduce HR in diabetic patients with CAN [22]. However, the reduction of HR in diabetic patients with CAN could be due to the effects of epanolol and atenolol individually, and not due to a class effect of beta blockers as patients with CAN had significantly higher HR after receiving pindolol compared to patients who received placebo [22]. Diabetic patients with CAN might also experience an early morning surge in blood pressure due to the autonomic imbalance of the cardiovascular system [46]. Thus, a bedtime dosing of antihypertensives could also prevent an early morning surge in the blood pressure of diabetic patients with CAN when the peak in plasma drug concentration occurs four to six hours after administration [47–49]. Given the potential benefits from the pharmacokinetics and pharmacological action of antihypertensives, the administration of at least one dose of antihypertensive medication to diabetic patients with CAN at night can be considered. Furthermore, from the post-hoc analysis of the Action to Control Cardiovascular Risk in Diabetes-Blood Pressure trial, the prevalence of CAN at baseline in the diabetic patients receiving intensive blood pressure lowering therapy and standard blood pressure lowering therapy was similar at 19.9 and 19.0% respectively (p = 0.30), but the diabetic patients that received antihypertensives to lower blood pressure intensively to an average of 120.9/65.0 mmHg had lower odds of CAN compared to the diabetic patients that received antihypertensives to lower blood pressure to a less stringent average blood pressure of 133.7/70.2 mmHg (odds ratio 0.84, 95% CI 0.75–0.94, p = 0.003) [50]. Hence, the results suggest that lowering blood pressure intensively using antihypertensives in the trial such as ACEI, diuretics, beta blockers, calcium channel blockers could improve CAN in diabetic patients [51].
The use of vitamin E supplementation could have favourable effects on diabetic patients with CAN. Vitamin E is an antioxidant that scavenges for reactive oxygen species [52, 53]. It could reduce oxidative stress, which might be a contributing factor to CAN [4]. In the study by Manzella et al., there was a near significant improvement in the autonomic indices in diabetic patients with CAN given a dose of 600 mg of vitamin E daily [26]. There was also a negative correlation between LF/HF and the plasma vitamin E concentration, which could be further studied to determine the optimal dose of Vitamin E required for a concentration dependent reduction in LF/HF in diabetic patients with CAN. While the study has demonstrated the potential of vitamin E to be used for the treatment of CAN, the current evidence on vitamin E is limited as there have not been other studies on the use of vitamin E in diabetic patients with CAN. Hence, more studies are needed to establish its use for the treatment of CAN.
Omega-3 PUFAs might be an effective treatment option for CAN as they have antioxidant properties and might also slightly lower blood pressure in diabetic patients with CAN [54, 55]. Omega-3 PUFAs taken daily in a 1 g capsule together with hypoglycemic treatment significantly improved the autonomic indices in diabetic patients with CAN [27]. However, while there was a significant improvement in nocturnal DBP in the patients given omega-3 PUFAs and hypoglycemic treatment, there were no significant changes in the nocturnal SBP [44]. Hence, while the potential of omega-3 PUFAs in reducing nocturnal blood pressure has been demonstrated, more research on the effect of omega-3 PUFAs on diabetic patients with CAN needs to be conducted.
ALA might be useful for the treatment of CAN in diabetic patients. In the studies by Ziegler et al., Lee et al. and Didangelos et al., ALA used as monotherapy or in combination with other antioxidants ALC, SOD and vitamin B12 was shown to significantly improve some of the autonomic indices in diabetic patients with CAN, which were LF, RMSSD, OH [23, 28, 42]. As the diabetic patients with CAN did not experience significant changes in other autonomic indices, further research is required to determine if the effect of ALA is clinically significant in diabetic patients with CAN. Given the different doses of ALA used in the studies, more studies could also be conducted to determine if there is a dose dependent effect of ALA on diabetic patients with CAN.
Vitamin B12 could have a small beneficial effect on the autonomic indices in diabetic patients with CAN. In the 2020 study by Didangelos et al., diabetic patients with CAN that were given 250 mcg of vitamin B12, 570 mg of ALA, 10 mg of SOD, 300 mg of ALC daily experienced a significant improvement in orthostatic hypotension compared to the patients that were given placebo daily [42]. Conversely, in the 2021 study by Didangelos et al. that investigated the effect of 1000 μg of Vitamin B12 monotherapy on diabetic patients with CAN, there was no significant difference in the autonomic indices between the two groups of patients [43]. Hence, vitamin B12 might yield a small positive effect in diabetic patients with CAN when used in combination with ALA, SOD and ALC while vitamin B12 monotherapy might not be recommended for the treatment of CAN. The benefit of the combination therapy could be attributed to the use of ALA as it might improve autonomic indices in diabetic patients with CAN. Thus, more studies could be conducted to investigate the clinical significance of vitamin B12. In addition, the two studies by Didangelos et al. differed in the dose of vitamin B12 used. Further research could be conducted to determine the optimal dose of vitamin B12 to be used in the combination therapy.
CAN diagnosis criteria and monitoring parameters
Currently, there is no unanimous criteria for the diagnosis of CAN, which might delay the diagnosis and initiation of treatment for CAN in patients [1]. Furthermore, there are numerous HRV parameters that could be measured as outcomes such as the different time and frequency-domains of HRV (Table 1). As a result, there is variability in the outcomes measured in clinical trials, which makes it difficult for researchers to compare the results of the studies. Therefore, there is a need for the standardisation of the diagnosis criteria and monitoring parameters for CAN to develop common interstudy methodologies and common clinical practices for the optimal management of CAN across different healthcare institutions.
Strengths and limitations
This is the first systematic review evaluating RCTs on all the available drug treatment options for diabetic patients with CAN. This systematic review also provides insights on the potential treatment options such as omega-3 PUFAs that have yet to be mentioned in the guidelines and recommendations by the scientific societies. The review also evaluated new clinical trials on the treatment options mentioned in the guidelines and recommendations, providing an update on the effects of these treatment options.
However, the findings of this systematic review should be considered while noting its limitations. Generally, the selected RCTs investigated the effect of the drug treatment options on parameters associated with CAN such as heart rate, blood pressure and heart rate variability, but they did not investigate the effect of the drug treatment options on QT interval in diabetic patients with CAN. The selected RCTs also did not report the number of diabetic patients enrolled with mild, moderate or severe CAN as the effect of drug treatment could be affected by the severity of CAN. Hence, it is not known whether the drug treatments would only benefit certain groups of diabetic patients depending on their severity of CAN.
Generally, the selected studies also had small sample sizes and did not report the 95% confidence intervals. With small sample sizes, even large treatment effects could appear not statistically significant. Small sample sizes also reduce the power of the study, resulting in a greater risk of Type II error when there is only a slight effect of the treatment on patients and the variability in the treatment effect is large. In addition, the duration of treatment in seven studies ranged from three to six months. The duration of treatment in the studies might be sufficient to detect some improvements in diabetic patients with CAN but might be too short to determine the optimal effects and long-term effects of these treatment options on the patients. The selected RCTs also raised some concerns of bias as they did not report the methods used for the randomised allocation of patients, whether an intention-to-treat analysis was used and whether there was a loss to follow up in the study.
Conclusion
This systematic review evaluated RCTs on the available drug treatment options for diabetic patients diagnosed with CAN. The studies on ACEI, ARBs, BB, ALA, omega-3 PUFAs, vitamin E, vitamin B12 in combination with ALA, ALC and SOD have reported improvements in the autonomic indices in diabetic patients with CAN. However, there were no significant improvements in any of the autonomic indices in diabetic patients with CAN after treatment with vitamin B12 monotherapy. The results of this systematic review suggest that ACEI, ARB, BB, ALA, omega-3 PUFAs, vitamin E, vitamin B12 in combination with ALA, ALC and SOD might be effective treatments for CAN, while vitamin B12 monotherapy might not be used for the treatment of CAN due to its lack of efficacy. The diagnosis criteria and monitoring parameters for CAN should be standardised to develop common clinical practices for the optimal management of CAN. Further research should also be conducted on larger sample sizes to confirm the findings on the potential treatment options for CAN. Moreover, studies should investigate the effect of drug treatment on the QT interval in diabetic patients with CAN. As patients might suffer from multiple symptoms of CAN, future studies should also measure more than one type of outcome to determine if each treatment option could treat more than one symptom of CAN. The effects of a combination of different treatment options can also be explored in future studies to manage the different symptoms in diabetic patients with CAN.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
There is no conflict of interest by any of the authors.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jasmine KaiLi Goh, Email: jasminegohkl@gmail.com.
Leroy Koh, Email: phalk@nus.edu.sg.
References
- 1.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–653. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 2.Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. 2014;5:17–39. doi: 10.4239/wjd.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migisha R, Agaba DC, Katamba G, Kwaga T, Tumwesigye R, Miranda SL, Muyingo A, Siedner MJ. Prevalence and correlates of cardiovascular autonomic neuropathy among patients with diabetes in uganda: a hospital-based cross-sectional study. Glob Heart. 2020;15:21. doi: 10.5334/gh.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4:4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieb DC, Parson HK, Mamikunian G, Vinik AI. Cardiac autonomic imbalance in newly diagnosed and established diabetes is associated with markers of adipose tissue inflammation. Exp Diabetes Res. 2012;2012:878760. doi: 10.1155/2012/878760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46:S31–S37. doi: 10.2337/diab.46.2.S31. [DOI] [PubMed] [Google Scholar]
- 7.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33:434–441. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019;43:3–30. doi: 10.4093/dmj.2018.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko SH, Song KH, Park SA, Kim SR, Cha BY. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with type 2 diabetes mellitus: a 7-year follow-up study. Diabetes Med. 2008;25:1171–1177. doi: 10.1111/j.1464-5491.2008.02567.x. [DOI] [PubMed] [Google Scholar]
- 10.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R. Diabetic neuropathy: a position statement by the american diabetes association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. J Am Med Assoc. 1997;277:1046–1051. doi: 10.1001/jama.1997.03540370036033. [DOI] [PubMed] [Google Scholar]
- 12.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615–624. doi: 10.1056/NEJMcp074189. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Li S, Yang G, Liu H, Boden G. Efficacy and safety of aldose reductase inhibitor for the treatment of diabetic cardiovascular autonomic neuropathy: systematic review and meta-analysis. PLoS One. 2014;9:e87096. doi: 10.1371/journal.pone.0087096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldose reductase inhibitors. In: Aronson J, editor. Meyler's side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. Elsevier Science; 2015. p. 131
- 15.Tsai SC, Burnakis TG. Aldose reductase inhibitors: an update. Ann Pharmacother. 1993;27:751–754. doi: 10.1177/106002809302700616. [DOI] [PubMed] [Google Scholar]
- 16.Foppiano M, Lombardo G. Worldwide pharmacovigilance systems and tolrestat withdrawal. Lancet. 1997;349:399–400. doi: 10.1016/S0140-6736(97)80018-9. [DOI] [PubMed] [Google Scholar]
- 17.Prisma, explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J. 2020;2021(372):n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 19.Athyros VG, Didangelos TP, Karamitsos DT, Papageorgiou AA, Boudoulas H. Long-term effect of converting enzyme inhibition on circadian sympathetic and parasympathetic modulation in patients with diabetic autonomic neuropathy. Acta Cardiol. 1998;53:201–209. [PubMed] [Google Scholar]
- 20.Didangelos TP, Arsos GA, Karamitsos DT, Athyros VG, Georga SD. Effect of quinapril or losartan alone and in combination on left ventricular systolic and diastolic functions in asymptomatic patients with diabetic autonomic neuropathy. J Diabetes Complicat. 2006;20:1–7. doi: 10.1016/j.jdiacomp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Hjortkjær HØ, Jensen T, Kofoed KF, Mogensen UM, Sigvardsen PE. Nocturnal antihypertensive treatment in patients with type 1 diabetes with autonomic neuropathy and non-dipping: a randomised, placebo-controlled, double-blind cross-over trial. Br Med J. 2016;6:e012307. doi: 10.1136/bmjopen-2016-012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid W, Ewing DJ, Harry JD, Smith HJ, Neilson JM. Effects of beta-adrenoceptor blockade on heart rate and physiological tremor in diabetics with autonomic neuropathy. A comparative study of epanolol, atenolol and pindolol. Br J Clin Pharmacol. 1987;23:383–389. doi: 10.1111/j.1365-2125.1987.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Jeong SJ, Lee YC, Lee YH, Lee JE. Effects of high-dose α-lipoic acid on heart rate variability of type 2 diabetes mellitus patients with cardiac autonomic neuropathy in Korea. Diabetes Metab J. 2017;41:275–283. doi: 10.4093/dmj.2017.41.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015;6:80–91. doi: 10.4239/wjd.v6.i1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontopoulos AG, Athyros VG, Didangelos TP, Papageorgiou AA, Avramidis MJ. Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care. 1997;20:355–361. doi: 10.2337/diacare.20.3.355. [DOI] [PubMed] [Google Scholar]
- 26.Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001;73(6):1052–1057. doi: 10.1093/ajcn/73.6.1052. [DOI] [PubMed] [Google Scholar]
- 27.Serhiyenko VA, Segin VB, Serhiyenko AA. Effects of omega-3 polyunsaturated fatty acids on the circadian rhythm of heart rate variability parameters in patients with type 2 diabetes mellitus and cardiovascular autonomic neuropathy. Russ J Cardiol. 2018;23:56–6021. doi: 10.15829/1560-4071-2018-5-56-60. [DOI] [Google Scholar]
- 28.Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study) Diabetes Care. 1997;20:369–373. doi: 10.2337/diacare.20.3.369. [DOI] [PubMed] [Google Scholar]
- 29.Kiviniemi AM, Hautala AJ, Seppänen T, Mäkikallio TH, Huikuri HV. Saturation of high-frequency oscillations of R-R intervals in healthy subjects and patients after acute myocardial infarction during ambulatory conditions. Am J Physiol Heart Circ Physiol. 2004;287:H1921–H1927. doi: 10.1152/ajpheart.00433.2004. [DOI] [PubMed] [Google Scholar]
- 30.Didangelos T, Tziomalos K, Margaritidis C, Kontoninas Z, Stergiou I. Efficacy of Administration of an angiotensin converting enzyme inhibitor for two years on autonomic and peripheral neuropathy in patients with diabetes mellitus. J Diabetes Res. 2017;2017:6719239. doi: 10.1155/2017/6719239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avram R, Tison GH, Aschbacher K, Kuhar P, Vittinghoff E. Real-world heart rate norms in the Health eHeart study. NPJ Digit Med. 2019;2:58. doi: 10.1038/s41746-019-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 33.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pafili K, Trypsianis G, Papazoglou D, Maltezos E, Papanas N. Simplified diagnosis of cardiovascular autonomic neuropathy in type 2 diabetes using ewing's battery. Rev Diabet Stud. 2015;12:213–219. doi: 10.1900/RDS.2015.12.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler D, Laux G, Dannehl K, Spüler M, Mühlen H. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992;9:166–175. doi: 10.1111/j.1464-5491.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 36.Srivastav S, Jamil RT, Zeltser R. Valsalva Maneuver. In: StatPearls. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK537248. Accessed 19 Jun 2022 [PubMed]
- 37.Agashe S, Petak S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J. 2018;14:251–256. doi: 10.14797/mdcj-14-4-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeMers D, Wachs D. Physiology, Mean Arterial Pressure. In: StatPearls. StatPearls Publishing; 2021 https://www.ncbi.nlm.nih.gov/books/NBK538226. Accessed 19 Jun 2022 [PubMed]
- 39.Brzezinski WA. Blood Pressure. In: Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Butterworths; 1990. https://www.ncbi.nlm.nih.gov/books/NBK268. Accessed 19 Jun 2022 [PubMed]
- 40.Dadlani A, Madan K, Sawhney JPS. Ambulatory blood pressure monitoring in clinical practice. Indian Heart J. 2019;71:91–97. doi: 10.1016/j.ihj.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosei EA, Chiarini G, Rizzoni D. How important is blood pressure variability? Eur Heart J. 2020;22:E1–6. doi: 10.1093/eurheartj/suaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Didangelos T, Karlafti E, Kotzakioulafi E, Kontoninas Z, Margaritidis C. Efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin b12, and carnitine for 12 months in patients with diabetic neuropathy. Nutrients. 2020;12:3254. doi: 10.3390/nu12113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Didangelos T, Karlafti E, Kotzakioulafi E, Margariti E, Giannoulaki P. Vitamin B12 supplementation in diabetic neuropathy: a 1-year, randomized, double-blind. Placebo-Controlled Trial Nutrients. 2021;13:395. doi: 10.3390/nu13020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhiyenko VA, Segin VB, Serhiyenko AA. The effect of OMEGA-3 polyunsaturated fatty acids on ambulatory blood pressure monitoring parameters in patients with type 2 diabetes mellitus and cardiovascular autonomic neuropathy. In: Diabetes Mellitus. Endocrinology Research Centre; 2019. https://www.dia-endojournals.ru/jour/article/view/9630?locale=en_US. Accessed 19 Jun 2022
- 45.Farzam K, Jan A. Beta Blockers. In: StatPearls. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK532906. Accessed 19 Jun 2022
- 46.Di Gennaro F, D'Amato C, Morganti R, Greco C, Longo S. Morning blood pressure surge is associated with autonomic neuropathy and peripheral vascular disease in patients with diabetes. J Hum Hypertens. 2020;34:495–504. doi: 10.1038/s41371-019-0270-3. [DOI] [PubMed] [Google Scholar]
- 47.Vasotec label. United States: Food and Drug Administration; 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018998s076lbl.pdf. Accessed 19 Jun 2022
- 48.COZAAR. United States: Food and Drug Administration; 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020386s049lbl.pdf. Accessed 19 Jun 2022
- 49.TENORMIN. United States: Food and Drug Administration; 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018240s031lbl.pdf. Accessed 19 Jun 2022
- 50.Tang Y, Shah H, Bueno Junior CR, Sun X, Mitri J, Sambataro M, Sambado L, Gerstein HC, Fonseca V, Doria A, Pop-Busui R. intensive risk factor management and cardiovascular autonomic neuropathy in Type 2 diabetes: the ACCORD trial. Diabetes Care. 2021;44(1):164–173. doi: 10.2337/dc20-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Supplement Appendix 1 to: effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borcea V, Nourooz-Zadeh J, Wolff SP, Klevesath M, Hofmann M. Alpha-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuria. Free Radic Biol Med. 1999;26:1495–1500. doi: 10.1016/S0891-5849(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 53.Peh HY, Tan WS, Liao W, Wong WS. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther. 2016;162:152–169. doi: 10.1016/j.pharmthera.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Lepretti M, Martucciello S, Burgos Aceves MA, Putti R, Lionetti L. Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients. 2018;10(3):350. doi: 10.3390/nu10030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.