Abstract
Introduction
Management of hidradenitis suppurativa (HS) often requires a combined medical/procedural approach. Biologics are frequently reserved for severe cases after irreversible tissue damage has occurred. We evaluated the association between consistent biologic use and the need for procedural interventions, systemic medications, and healthcare utilization.
Methods
UNITE, a 4-year, global, prospective, observational, HS disease registry, documented the natural history, diagnostic/treatment patterns, and clinical outcomes of HS. Patients aged 12 years or more, with active HS were enrolled between October 2013 and December 2015 and evaluated every 6 months for 48 months at 73 sites across 12 countries (data cutoff December 2019). Proportions of patients requiring different HS procedures, systemic medications, and healthcare utilization were assessed during the 6-month periods before, during, and after biologic initiation for 12 weeks or more (i.e., consistent use).
Results
There were 63 instances of initiation of consistent biologic use (adalimumab [81%], infliximab [16%], and ustekinumab [3%]) in 57 patients. Patients’ mean age was 40 years, 58% were female, and 53%/47% had Hurley stage II/III disease, respectively. Fewer patients required surgical/procedural interventions and systemic medications for the 6-month period during/6-month period after biologic initiation versus the 6-month period before biologic initiation, including intralesional corticosteroid injections (22%/14% vs 24%), incision and drainage (I&D) by physician (10%/10% vs 17%), I&D by patient (10%/10% vs 14%), surgical excision (8%/10% vs 11%), deroofing (5%/2% vs 5%), systemic antibiotics (43%/41% vs 54%), and systemic immunosuppressants (10%/6% vs 13%). Fewer patients required hospital admission for HS (17%/13% vs 21%) or emergency department visits for HS (8%/8% vs 16%) during the 6-month periods in which consistent biologics use started and continued versus the 6-month period before consistent biologic use.
Conclusion
Following initiation of consistent biologic use (12 weeks or more), fewer patients required acute procedural interventions, systemic medications, and healthcare utilization, supporting the importance of early biologic initiation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00954-8.
Keywords: Biologics, Hidradenitis suppurativa, Registry
Key Summary Points
| Why carry out this study? |
| For patients with hidradenitis suppurativa (HS), biologics are often reserved for severe disease after irreversible damage has occurred. |
| There is a need to understand how consistent treatment (12 weeks or more) with biologics affects the requirement for medical and surgical interventions. |
| What was learned from the study? |
| Using data from UNITE, a 4-year, global HS disease registry, this study showed that fewer patients required procedural interventions (steroid injections, drainage of boils, surgery), emergency department visits, hospital admissions, and visits to healthcare providers for HS following consistent treatment with biologics. |
| In UNITE, a reduced need for certain procedures and healthcare requirements was observed in patients initiating consistent treatment with biologics, suggesting that biologics should be started earlier. Additional research is warranted to confirm these findings. |
Introduction
Hidradenitis suppurativa (HS) is a painful, chronic, inflammatory skin disease characterized by recurrent nodules, abscesses, cutaneous tunnels, and scarring [1]. HS carries a high comorbidity burden and has debilitating effects on patient quality of life and work productivity [2, 3]. The estimated prevalence of HS is 0.1–1% of the general population, and HS disproportionately affects women versus men and black versus white individuals [1, 4, 5].
Management of HS often requires a combined medical and surgical approach. Generally, topical or systemic antibiotics are first-line therapies, although evidence for efficacy is limited [6–8]. Biologics (e.g., tumor necrosis factor [TNF] inhibitors) are often reserved for patients with severe disease who have experienced irreversible tissue damage [6, 8]. Some biologics not approved for the treatment of HS may also be safe and effective [9]. Intralesional corticosteroid injections and incision and drainage (I&D) are used to manage acutely painful lesions but are associated with high recurrence rates [6, 8]. Surgical procedures (e.g., deroofing, excision) are often required to remove tunnels and scar tissue; however, extensive surgery can be associated with postoperative morbidity (infection, bleeding, and contracture) [6, 8, 10]. Although biologics have been shown to be safe and effective alone and in combination with surgery [11–14], a recent cross-sectional analysis revealed low utilization of TNF inhibitors for the treatment of HS [15]. Furthermore, information on how consistent treatment with biologics affects medical and procedural interventions is limited.
UNITE is a 4-year, global, prospective, observational, HS disease registry documenting the natural history, diagnostic and treatment patterns, and clinical outcomes of patients with HS [16]. A recent analysis of UNITE demonstrated that HS is a highly progressive disease; the majority of the 574 enrolled patients experienced lesion or scar spread or Hurley stage progression over a 4-year period [17]. Disease progression occurred most frequently in patients with more severe, long-standing disease, highlighting the importance of early management to minimize the debilitating consequences of disease progression [17].
The objective of this analysis of the UNITE registry was to evaluate the association between consistent biologic use and the need for procedural interventions, systemic medications, and healthcare utilization.
Methods
UNITE enrolled patients (aged 12 years or more) with active HS (defined by the presence of inflammatory HS lesions) and no previous or current participation in an adalimumab clinical study or registry [16]. Patients were enrolled between October 2013 and December 2015 from 73 sites across 12 countries in North America, Europe, and Australia and received treatment per routine clinical practice [16]. Patients were evaluated every 6 months for up to 48 months (data cutoff December 2019).
This analysis included patients who reported newly initiated biologic use for HS with 12 weeks or more of use (i.e., consistent use) in the past 6 months. All patients or their legal guardians signed an informed consent form that was approved by an institutional review board or ethics committee according to local law. A list of all study sites and information on ethics committees who approved this study is provided in Supplemental Table 1.
Endpoints
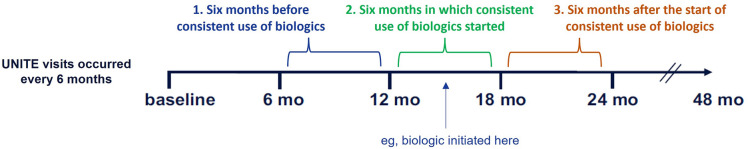
Data were compared between three 6-month periods: the period before initiation of consistent use of biologics (period 1), period during which the consistent use of biologics was initiated (period 2), and period after the initiation of consistent biologics use (period 3; Fig. 1).
Fig. 1.
UNITE visits and assessment periods. The proportions of patients requiring procedures, systemic medications, and healthcare utilization for hidradenitis suppurativa were assessed during three 6-month periods between UNITE visits: period 1, before initiation of consistent biologics use; period 2, during initiation of consistent biologics use; and period 3, after initiation of consistent biologics use
The assessed endpoints included the proportions of patients requiring surgical/procedural interventions (including intralesional corticosteroid injections, I&D by physician, I&D by patient, excision, deroofing, laser therapy, and photodynamic therapy), proportions of patients with systemic medication use (antibiotics, immunosuppressants, pain medications, hormonal agents, and retinoids), and the proportions of patients requiring any hospital bed use, hospital admission, emergency department (ED) visit, or visits to dermatologists, any healthcare providers (HCPs), primary care physicians (PCPs), or surgeons for HS.
Statistical Analysis
All baseline demographics and disease characteristics were summarized by descriptive statistics. Mean (SD) was reported for continuous variables and number and proportion for categorical variables.
Results
Of the 594 patients with HS enrolled in UNITE who provided baseline data [16], postbaseline data were available for 574 patients, including 62 adolescents. Of these, 57 reported 63 instances of initiation of consistent biologic use. Their mean age was 40 years, 58% were female, and 53% and 47% had Hurley stage II and III disease, respectively (Table 1). Biologic initiations were adalimumab (51 [81%]), infliximab (10 [16%]), and ustekinumab (2 [3%]).
Table 1.
Baseline patienta characteristics and demographics
| Characteristic | Overall populationb N = 57 |
|---|---|
| Age, median (range), years | 40 (14–76) |
| < 18 years | 3 (5) |
| Female | 33 (58) |
| Race | |
| White | 45 (79) |
| Black or African American | 11 (19) |
| American Indian or Alaska native | 1 (2) |
| Body mass index | |
| Normal | 6 (11) |
| Overweight | 12 (21) |
| Obese | 39 (68) |
| Tobacco use | |
| Current | 28 (49) |
| Former | 12 (21) |
| Never | 17 (30) |
| Hurley stage | |
| II | 30 (53) |
| III | 27 (47) |
| No. of initiations of consistent biologics use | 63 |
| Biologic | |
| Adalimumab | 51 (81) |
| Infliximab | 10 (16) |
| Ustekinumab | 2 (3) |
aPatients who initiated consistent biologics use and made consecutive visits before and after initiation
bAll data are number (%) unless otherwise noted
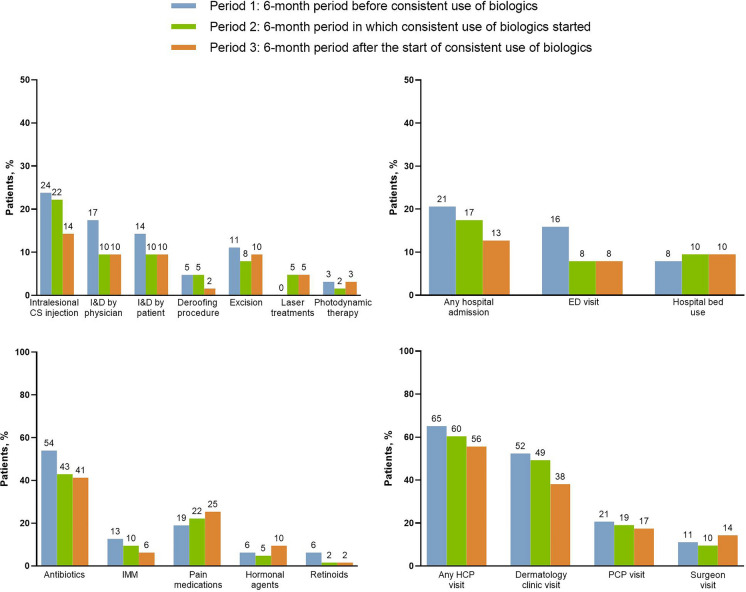
Fewer patients required surgical/procedural interventions during periods 2 and 3 versus period 1, including for intralesional corticosteroid injections, I&D by physician, I&D by patient, deroofing, and excision (Fig. 2). This trend was more pronounced for procedures tied to acute disease flares, including intralesional corticosteroids and I&D procedures, compared with those more likely to be planned for chronic disease, such as deroofing and excision. No difference was observed for photodynamic therapy between the periods, whereas laser treatments increased from period 1 (none) to periods 2 and 3 (5% each).
Fig. 2.
Proportions of patients requiring procedural/surgical interventions, systemic medications, and healthcare utilization before, during, and after initiation of consistent use of biologics. Data are based on 63 initiations of biologics. ED emergency department, HCP healthcare provider, IMM immunosuppressants, PCP primary care physician
Similarly, the number of any hospital admissions, ED visits, or any HCP, dermatologist, or PCP visits for HS was lower during period 2 versus period 1, with continued declines or maintenance noted during period 3 (Fig. 2). In contrast, hospital bed use or surgeon visits increased from period 1 to period 3.
The use of systemic antibiotics and immunosuppressants declined during periods 2 and 3 compared with period 1 (Fig. 2). However, the use of pain medications and hormonal agents increased from period 1 to period 3.
Overall, the results were generally similar when assessing only adalimumab initiations (Fig. 3).
Fig. 3.
Proportions of patients requiring procedural/surgical interventions, systemic medications, and healthcare utilization before, during, and after initiation of consistent use of adalimumab. Data are based on 51 initiations of adalimumab. ED emergency department, HCP healthcare provider, IMM immunosuppressants, PCP primary care physician
Discussion
In UNITE, after initiation of biologics for 12 weeks or more in patients with active HS, fewer acute procedures, systemic medications, hospitalizations, ED visits, or HCP visits were required during the 6 months in which the biologic was initiated and the 6 months following initiation compared with the 6 months before initiation. Notably, ED visits halved from period 1 to period 2 and 3. An analysis including only adalimumab initiations yielded similar results. These findings suggest that consistent biologic use reduces the need for acute episodic interventions and invasive surgeries, supporting the importance of early initiation.
Although HS is a chronic disease with a remitting-relapsing course and frequent painful flares [1], for many patients it is managed reactively, with acute procedural/surgical interventions without medications that address the underlying disease. Poorly controlled disease results in accumulation of tissue damage, often requiring costly and invasive procedures. As demonstrated previously in UNITE, the majority of patients experienced disease progression over 4 years despite routine clinical care [17].
Our findings also suggest that sustained biologic treatment helps address the underlying disease process and therefore reduces the need for acute interventions. Furthermore, biologics may be useful in reducing disease progression when used appropriately as part of a comprehensive management plan [11–13]. Currently, only one biologic, the TNFα inhibitor adalimumab, is approved by the US Food and Drug Administration and European Medicines Agency for the treatment of moderate to severe HS. Several other biologics have shown beneficial effects in exploratory studies of limited duration and small sample sizes [7]. Although most concomitant medication use decreased during the 6-month period of biologic initiation and following the initiation, the use of pain and hormonal medications increased from period 1 to period 3. The reasons for the increases are unknown, but they may be related to use of these medications as an adjunctive therapy in combination with biologics and, in the case of pain medications, for the treatment of unpredictable pain during HS flares, which is a common feature of the disease. Hospital bed use and surgeon visits increased from period 1 to period 3; however, these visits could reflect preplanned surgeries for long-standing disease or suggest the need of surgery in patients with advanced end-stage disease for whom the use of biologics might have limited success.
This analysis included patients who were treated in routine clinical practice with no specific restrictions. Because of this, concomitant therapies could have confounded the results; however, this patient population also gives a better representation of real-world clinical practice. Main limitations of this analysis included a lack of statistical testing and comparisons between the groups. Other limitations included small sample size, possible biases in patient selection, recall of information, inconsistent reporting, and measurement errors. Additionally, if a patient discontinued a biologic after 12 weeks of use, they were still included in the analysis because consistent use of biologics was defined as 12 weeks or more of use. Furthermore, patients who stay in registries may be self-selecting for favorable effectiveness. Additional studies are needed to confirm the findings of our analysis.
The main strengths of this study included that UNITE was the first and, at the time of completion, largest global real-world HS registry, making the results generalizable to clinical practice. In addition, patients were compared before and after biologic initiation, controlling for confounding that could result from comparing separate cohorts with individual differences.
Conclusions
Overall, these results demonstrate that initiation and consistent use of biologics could result in fewer procedures, reduced systemic medication use, and reduced utilization of healthcare resources compared with before initiation. Early initiation of biologics may be beneficial to improve the disease trajectory in patients with moderate to severe HS.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
AbbVie Inc. funded this study and the Rapid Service Fee and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Maria Hovenden, Ph.D., and Janet Matsuura, Ph.D., of ICON (Blue Bell, PA) and was funded by AbbVie Inc.
Author Contributions
Concept and planning of the work described: Kassim Rahawi, Marília Oliveira, Christopher Sayed, Yinghui Duan. Analysis of the data: Kassim Rahawi, Michael Lane, Yinghui Duan, Marília Oliveira. Interpretation of the data: Kassim Rahawi, Michael Lane, Yinghui Duan, Marília Oliveira, Christopher J. Sayed. Drafting of the manuscript: Kassim Rahawi, Marília Oliveira, Christopher J. Sayed. Critical revision of the manuscript: Kassim Rahawi, Ahmad Z. Amin, Marília Oliveira, Christopher J. Sayed. All authors read and approved the final version and agree to be accountable for part (Ahmad Z. Amin, Yinghui Duan) or all (Christopher J. Sayed, Kassim Rahawi, Michael Lane, Marília Oliveira) aspects of the work.
Prior Presentation
The results of this study were previously presented at the 6th Annual Symposium on Hidradenitis Suppurativa Advances, September 24–26, 2021.
Disclosures
Marília Oliveira was a full-time employee of AbbVie at the time of this study and may hold AbbVie stock or stock options. She is currently affiliated with Novant Health Matthews Medical Center and Novant Health Presbyterian Medical Center, Matthews, NC. Kassim Rahawi was a full-time employee of AbbVie at the time of this study and manuscript development and may hold AbbVie stock or stock options. Yinghui Duan and Michael Lane are full-time employees of AbbVie and may own stock or stock options. Ahmad Z. Amin received consulting fees from AbbVie, Bristol Myers Squibb, Dermavant, and Eli Lilly and honoraria as a speaker for AbbVie, Amgen, Dermavant, Eli Lilly, Janssen, Pfizer, Regeneron, Sanofi/Genzyme, and UCB. Christopher J. Sayed received consulting fees from AbbVie, Alumis, Novartis, Sonoma Biotherapeutics, and UCB; honoraria as a speaker for AbbVie and Novartis; participated on an advisory board for AbbVie, Incyte, InflaRx, Novartis, and UCB; and is a directing member of the Hidradenitis Suppurativa Foundation (unpaid). His institution receives funding for his work as an investigator for AbbVie, Chemocentryx, InflaRx, Incyte, Novartis, and UCB.
Compliance with Ethics Guidelines
All patients or their legal guardians signed an informed consent form that was approved by an institutional review board or ethics committee according to local law.
Data Availability
The data sets generated during and/or analyzed during the current study are available on reasonable request using the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Footnotes
Affiliations for Marília Oliveira and Kassim Rahawi were correct at the time of the study.
References
- 1.Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.Garg A, Neuren E, Cha D, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol. 2020;82(2):366–376. doi: 10.1016/j.jaad.2019.06.1301. [DOI] [PubMed] [Google Scholar]
- 3.Reddy S, Strunk A, Garg A. Comparative overall comorbidity burden among patients with hidradenitis suppurativa. JAMA Dermatol. 2019;155(7):797–802. doi: 10.1001/jamadermatol.2019.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178(4):917–924. doi: 10.1111/bjd.16101. [DOI] [PubMed] [Google Scholar]
- 6.Orenstein LAV, Nguyen TV, Damiani G, Sayed C, Jemec GBE, Hamzavi I. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236(5):393–412. doi: 10.1159/000507323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91–101. doi: 10.1016/j.jaad.2019.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization—systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33(1):19–31. doi: 10.1111/jdv.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montero-Vilchez T, Pozo-Roman T, Sanchez-Velicia L, Vega-Gutierrez J, Arias-Santiago S, Molina-Leyva A. Ustekinumab in the treatment of patients with hidradenitis suppurativa: multicenter case series and systematic review. J Dermatolog Treat. 2022;33(1):348–353. doi: 10.1080/09546634.2020.1755008. [DOI] [PubMed] [Google Scholar]
- 10.Rompel R, Petres J. Long-term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg. 2000;26(7):638–643. doi: 10.1046/j.1524-4725.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 11.Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]
- 12.Bechara FG, Podda M, Prens EP, et al. Efficacy and safety of adalimumab in conjunction with surgery in moderate to severe hidradenitis suppurativa: the SHARPS randomized clinical trial. JAMA Surg. 2021;156(11):1001–1009. doi: 10.1001/jamasurg.2021.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanmugam VK, Mulani S, McNish S, Harris S, Buescher T, Amdur R. Longitudinal observational study of hidradenitis suppurativa: impact of surgical intervention with adjunctive biologic therapy. Int J Dermatol. 2018;57(1):62–69. doi: 10.1111/ijd.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvador-Rodriguez L, Cuenca-Barrales C, Arias-Santiago S, Molina-Leyva A. Neoadjuvant biologic therapy in the surgical management of patients with hidradenitis suppurativa: a cohort study. Acta Derm Venereol. 2020;100(16):adv00257. doi: 10.2340/00015555-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orenstein LAV, Wright S, Strunk A, Garg A. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84(5):1399–1401. doi: 10.1016/j.jaad.2020.07.108. [DOI] [PubMed] [Google Scholar]
- 16.Prens EP, Lugo-Somolinos AM, Paller AS, et al. Baseline characteristics from UNITE: an observational, international, multicentre registry to evaluate hidradenitis suppurativa (Acne Inversa) in clinical practice. Am J Clin Dermatol. 2020;21(4):579–590. doi: 10.1007/s40257-020-00504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimball AB, Sayed C, Duan Y, Gamelli A, Oliveira M, Sobell JM. Assessment of the natural history of hidradenitis suppurativa over 4 years: results from the HS UNITE registry. American Academy of Dermatology (AAD) 2021 VMX Event; 2021 April 23–25; Virtual.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available on reasonable request using the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.