Abstract
Maintaining the integrity of crucial fiber tracts allows functional preservation and improved recovery in patients with glioma resection. Diffusion tensor imaging (DTI) and intraoperative subcortical mapping (ISM) are commonly required for pre- and intraoperative assessment of white matter fibers. This study investigated differences of clinical outcomes in glioma resection aided by DTI or ISM. A comprehensive literature retrieval of the PubMed and Embase databases identified several DTI or ISM studies in 2000–2022. Clinical data, including extent of resection (EOR) and postoperative neurological deficits, was collected and statistically analyzed. Heterogeneity was regressed by a random effect model and the Mann–Whitney U test was used to test statistical significance. Publication bias was assessed by Egger test. A total of 14 studies with a pooled cohort of 1837 patients were included. Patients undergoing DTI-navigated glioma surgery showed a higher rate of gross total resection (GTR) than ISM-assisted surgical resection (67.88%, [95% CI 0.55—0.79] vs. 45.73%, [95% CI 0.29—0.63], P = 0.032). The occurrence of early postoperative functional deficit (35.45%, [95% CI 0.13—0.61] vs. 35.60% [95% CI 0.20—0.53], P = 1.000), late postoperative functional deficit (6.00%, [95% CI 0.02—0.11] vs. 4.91% [95% CI 0.03—0.08], P = 1.000) and severe postoperative functional deficit (2.21%, [95% CI 0—0.08] vs. 5.93% [95% CI 0.01—0.16], P = 0.393) were similar between the DTI and ISM group, respectively. While DTI-navigation resulted in a higher rate of GTR, the occurrence of postoperative neurological deficits between DTI and ISM groups was comparable. Together, these data indicate that both techniques could safely facilitate glioma resection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10143-023-02058-5.
Keywords: Glioma, Diffusion tensor imaging, Intraoperative subcortical mapping, Extent of resection, Postoperative neurological deficit
Introduction
Glioma, the most prevalent primary intracranial tumor, exhibits invasive behavior, facilitating infiltration of tumor tissue into the cerebral cortex and subcortical fiber tracts. This feature poses significant challenges to the safe and effective resection of the lesion and contributes to high mortality and morbidity [1, 2]. Neurosurgeons strive to achieve maximal safe resection during glioma surgery while balancing functional preservation with tumor removal [3, 4]. In cases where gliomas are located in eloquent areas, intraoperative brain mapping, and neuronavigation systems can significantly aid in achieving a better surgical dissection along the functional-oncological boundary [5, 6]. Intraoperative surgical mapping has been proposed as a standard of care in the neurosurgical treatment of glioma. Further, the preservation of subcortical components and protection of the cerebral cortex is crucial for ensuring a favorable functional prognosis or neural plasticity, particularly in the case of deep-seated gliomas.
Intraoperative subcortical stimulation mapping (ISM) and diffusion tensor imaging (DTI) navigation can be used in combination to better preserve subcortical structures during glioma surgery [7-9]. ISM, considered the gold standard for subcortical identification, provides superior intraoperative neurological function monitoring, which improves the extent of tumor resection and reduces the likelihood of neurological dysfunction [10-12]. Despite its superior ability to distinguish function-related fiber tracts from white matter, ISM is subject to several significant drawbacks, including patient cooperation, operator experience, and extensive prior knowledge of fiber tracts. To address these issues, DTI is often incorporated into neuronavigation systems to support preoperative assessment of the relationship between fiber tracts and the tumor and intraoperative fiber tract localization. In practice, DTI merged into a neuronavigation system can support preoperative assessment of the relationship between the fiber tracts and tumor and intraoperative localization of fiber tracts [13-15]. However, the accuracy of intraoperative fiber tract localization can be impacted by brain edema and intraoperative brain shift during neuronavigation, as well as the anatomical seed location and fractional anisotropy threshold settings [16]. Considering the benefits and limitations of each approach, advanced neurosurgery centers commonly use both techniques in combination to achieve optimal fiber tract preservation.
The widespread use of ISM is limited by its dependence on surgical experience, which has led to questions about the safety of DTI and whether it can serve as a reproducible substitute for ISM. Few studies have compared the extent of tumor resection and prognosis achieved with DTI versus ISM [17, 18]. This is the first study employs a meta-analysis methodology to examine the impact of ISM and DTI neuronavigation on the extent of lesion resection and postoperative neurological function.
Methods
Study design
This study was carried out according to the PRISMA guidelines for systematic reviews and meta-analysis [19-21]. Using the PICO criteria (P: patient, I: intervention, C: control, O: outcome) and PRISMA checklist [19, 20], a key question was identified: do patients suffering from glioma have a more significant percentage of gross total resection (GTR) and lower postoperative neurological deficits when receiving DTI versus ISM-assisted surgery? This study was registered to the INSPLAY registry for systematic review and meta-analysis (registration number: INPLASY202180013, https://doi.org/10.37766/inplasy2021.8.0013).
Data sources and search strategy
The PubMed and Embase databases were accessed and used to identify relevant studies published from January 1st, 2000, to December 31st, 2022. The combination of keywords and index terms created search strategies. Three key terms were used to identify relevant studies published in the search period: “glioma”, “intraoperative subcortical mapping”, and “diffusion tensor imaging” (detailed search strategy was showed in appendix 1 and 2). In addition to those databases, additional studies were included by citation search.
Study selection and quality assessment
Case series studies, observational studies, and randomized controlled trials (RCT) were assessed for inclusion. Included studies should meet the following criteria: (i) studies published in English; (ii) patients were pathologically diagnosed with glioma (WHO grades 1–4); (iii) DTI or ISM was used in glioma surgery; (iv) providing information on the extent of lesion resection or assessment of neurological deficits. Literatures not related to search strategy, case reports, systematic reviews, meta-analyses, studies of pediatric neurosurgery, studies of non-supratentorial glioma, and studies with a patient cohort with less than 20 cases were excluded from the current analysis. Literature screening and selection were divided into two stages. First, investigators conducted the literature search according to the above search strategy. Second, the selected studies were validated by reviewing the abstract and intensive reading of the full text. Two investigators repeated this process. Additionally, two investigators independently performed the statistical analyses of included studies. Lastly, to verify the selection of the included literature, all of the selected studies were scored independently and subsequently reviewed by two authors (Y.L. and J.G.) using the Newcastle–Ottawa Scale (NOS) [22]. Each study was scored by population, comparability, and outcome measures. The NOS scored a full 9, with ≥ 5 meeting the inclusion criteria. Controversial studies were decided after consensus by co-authors.
Data extraction and outcome measurement
The data from the included studies were extracted by investigators (Y.L. and J.G.). Next, the extracted data were checked independently by two authors. Data extraction was performed according to DECiMAL guidelines for meta-analysis [22]. The data extracted from the included literature was divided into basic (author, publication time, study type, and assistive technology for surgery) and clinical data (e.g., population, gender ratio, location and volume of tumor, and postoperative neurological condition). System data extraction was performed with customized sheets.
This meta-analysis aimed to assess the extent of tumor resection and its relationship to postoperative neurological deficit. For the extent of tumor resection, a 100% resection rate with no obvious lesion detected was regarded as a “gross total resection” [23, 24]. Postoperative neurological deficit evaluation was based on the criteria published by De Witt Hamer et al. [24, 25]. In brief, neurological deficits were divided into early (< 3 months) and late (> 3 months) deficits after tumor resection [25]. Additionally, postoperative neurological deficits were divided into severe (muscle strength grades 1–3, aphasia, hemianopia, and vegetative state) and non-severe (other types of neurological deficits) deficits [25]. All eligible publications that aligned with these criteria were included in this work.
Statistical analysis
All statistical analysis was performed using the meta [26] and metafor [27] packages deployed in R (version 4.0.3) and R studio (version 1.4.1106). The mean value and 95% confidence intervals (95% CI) of the primary outcome were calculated. The Mann–Whitney U test was used to evaluate whether the group difference was statistically significant (IBM SPSS 26). Due to the heterogeneity of the included studies, the random effect model was used for analysis. Differences with a P value < 0.05 were considered significant. Egger’s test was used to detect publication bias, and T and P values were used as the analysis results. A P value exceeding the 0.05 value indicated no publication bias.
Results
Overview of the included studies
A total of 1744 relevant articles were obtained from PubMed and Embase databases (Appendix 3). Duplicated studies were deleted. After reading through the full text and re-screening, 14 papers were retained. Of those 14 studies, 1 was an RCT, and the remaining studies were observational. All of the studies were published from 2003 to 2020.
Research characteristics and quality evaluation
Eight ISM and 6 DTI studies were incorporated into the current analysis. From these studies, a total of 1837 patients underwent surgical resection, ranging from 20 to 702 cases in each study. Included patients had an average age of 48.5 years, and 56.7% were male. All patients were diagnosed with glioma by histopathology. Regarding assistive technology, 389 patients were divided into the DTI group, and the other 1448 patients were placed into the ISM group. Because there were no included studies have clear compared of the scope of tumor resection in high-grade and low-grade glioma, it is hard to conduct subgroup analysis about extent of resection in different tumor grades. No studies which assessed the combination of the two techniques were included (summary information of included studies were shown in Table 1).
Table 1.
Characteristics of included studies
| Study | Country/ Publication time |
No. of cases | DTI/ ISM |
Study design | Data indicator | Quality assessment |
|---|---|---|---|---|---|---|
| Angel Aibar-Duran J [28] | Spain/2020 | 20 | DTI | Cohort | abcd | High-quality |
| Bello L [36] | Italy/2007 | 88 | ISM | Case series | abcd | Medium-quality |
| Castellano A [40] | Italy/2012 | 73 | DTI | Case series | b | High-quality |
| Mo Cho J [29] | Korea/2014 | 103 | DTI | Case series | abc | High-quality |
| D'Andrea G [30] | Italy/2016 | 27 | DTI | Case series | abd | High-quality |
| Duffau H [31] | France/2003 | 103 | ISM | Case series | abcd | Medium-quality |
| Duffau H [32] | France/2008 | 115 | ISM | Case series | ac | High-quality |
| Han SJ [37] | America/2018 | 702 | ISM | Case series | bcd | High-quality |
| Ius T [38] | Italy/2012 | 73 | ISM | Case series | bc | High-quality |
| Javadi SA [17] | Germany/2017 | 20 | ISM | Case series | abcd | High-quality |
| Evren KG [39] | America/2004 | 294 | ISM | Case series | bc | High-quality |
| Ohue S [33] | Japan/2015 | 49 | DTI | Case series | abd | High-quality |
| Raabe A [34] | Switzerland/2014 | 53 | ISM | Case series | abcd | High-quality |
| Wu JS [35] | China/2007 | 118 | DTI | RCT | abd | High-quality |
DTI, Diffusion tensor imaging; ISM, Intraoperative subcortical mapping; Cohort, Cohort studies; Case series, Observational case series studies; RCT, Randomized controlled trials; a, Percentage of gross total resection (% GTR); b, Percentage of postop-early neurological deficits; c, Percentage of postop-late neurological deficits; d, Percentage of postop-severe neurological deficits
Comparison of GTR ratio
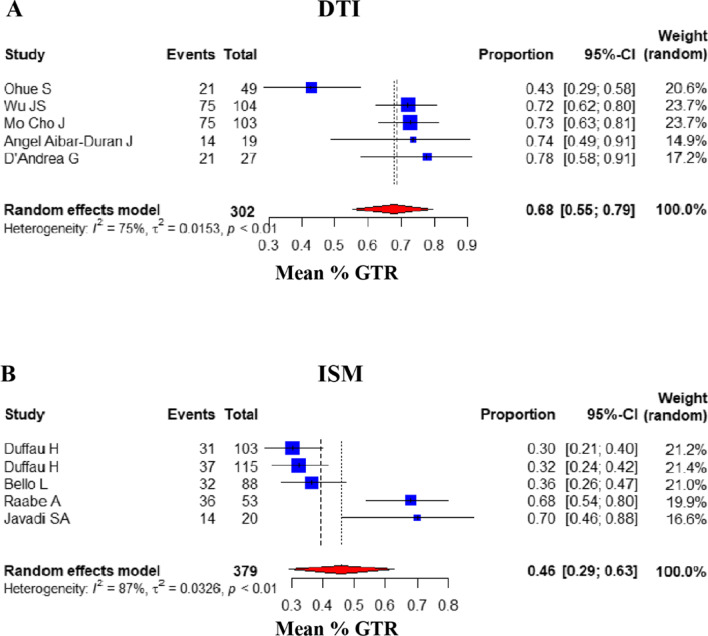
Of the 14 articles assessed, 10 reported the percentage of GTR of glioma (DTI group: 5, ISM group: 5) [7, 17, 28-35]. In this study, the mean percentage of GTR between DTI and ISM groups was assessed, and the 95% confidence intervals were calculated. The overall mean percentage of GTR for patients undergoing DTI-assisted surgery was 67.88% [95% CI: (0.5526, 0.7936)], compared with those of the ISM group (45.73%) [95% CI: (0.2914, 0.6282)]. The result of Mann–Whitney U test showed P value was 0.032. In consequence, the results of data analysis showed that the DTI group had a significantly higher GTR rate than the ISM group (Fig. 1).
Fig. 1.
Comparison of GTR. Forest plot for pooled comparison of GTR between DTI and ISM group (random effect model). A DTI-navigated group vs. B ISM-assisted group. Comparison of GTR reveals that DTI group has higher ratio of GTR ratio, and the contrast between groups has reach statistically significant difference. Estimates of relative risk are presented by blue boxes, the area of which is proportional to the number of cases in each study. Ninety-five percent confidence intervals are indicated by a red diamond and horizontal lines
Comparison of postoperative neurological deficits
Postoperative early deficits
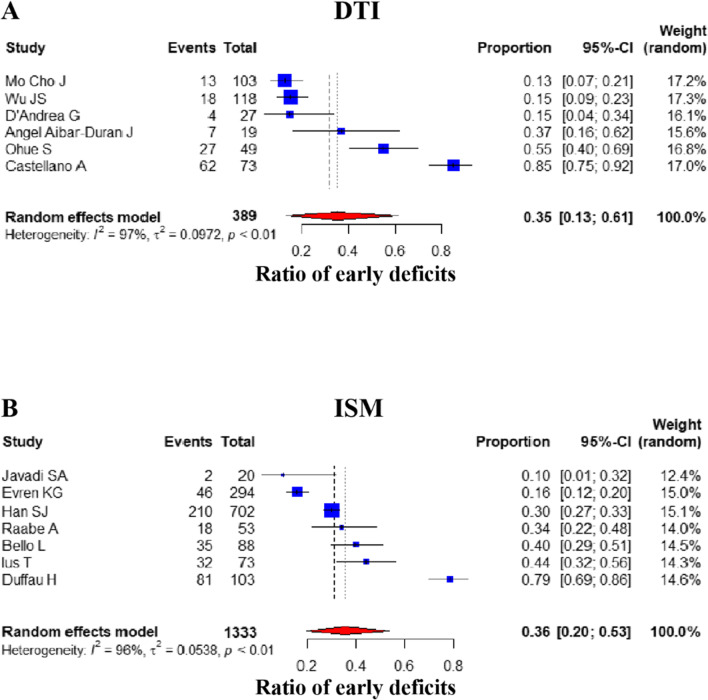
The occurrence of early postoperative neurological deficits was assessed and compared proportionally in 13 studies (DTI group: 6, ISM group: 7) [17, 28-31, 33-40]. The overall random effect pooled prevalence in the DTI group (35.45%) [95% CI: (0.1325, 0.6142)] was similar to the ISM group (35.60%) [95% CI: (0.1958, 0.5342)] (P value = 1.000, Mann–Whitney U test) (Fig. 2).
Fig. 2.
Comparison of early postoperative deficits. Forest plot for pooled comparison of postoperative early deficits between DTI and ISM group. A DTI-navigated group vs. B ISM-assisted group. Comparison of early postoperative deficits reveals that DTI group has little difference that of ISM group, the contrast between groups has not reach statistically significant difference. Estimates of relative risk are presented by blue boxes, the area of which is proportional to the number of cases in each study. Ninety-five percent confidence intervals are indicated by a red diamond and horizontal lines
Postoperative late deficits
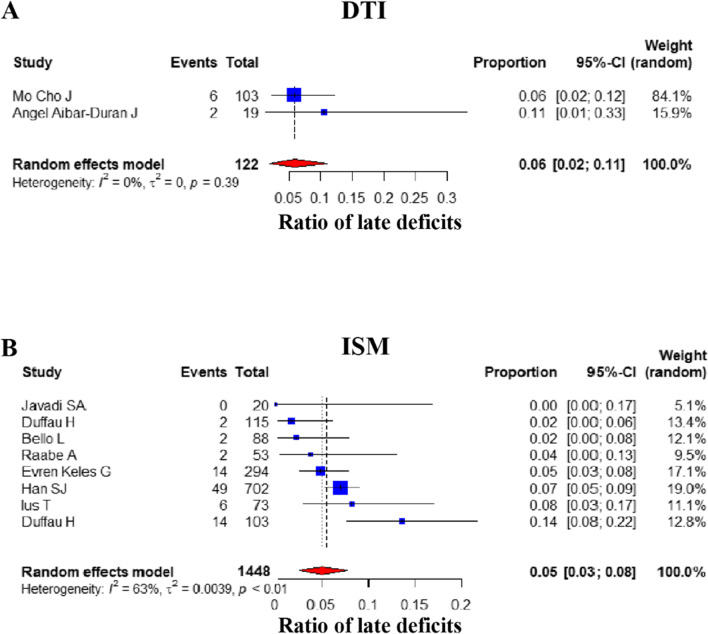
Next, the occurrence of late postoperative deficits was assessed and compared proportionally in 10 studies (DTI group: 2, ISM group: 8) [17, 28, 29, 31, 32, 34, 36-39]. The overall random-effects pooled prevalence of late postoperative deficits were similar between the DTI (6.00%) [95% CI: (0.02, 0.11)] and the ISM groups (4.91%) [95% CI: (0.0262, 0.0778)] (P value = 1.000, Mann–Whitney U test) (Fig. 3).
Fig. 3.
Comparison of late postoperative deficits. Forest plot for pooled comparison of postoperative late deficits between DTI and ISM group. A DTI-navigated group vs. B ISM-assisted group. Comparison of late postoperative deficits reveals that DTI group has slightly lower ratio of late postoperative deficits, and there is no statistically significant difference between groups. Estimates of relative risk are presented by blue boxes, the area of which is proportional to the number of cases in each study. Ninety-five percent confidence intervals are indicated by a red diamond and horizontal lines
Postoperative severe deficits
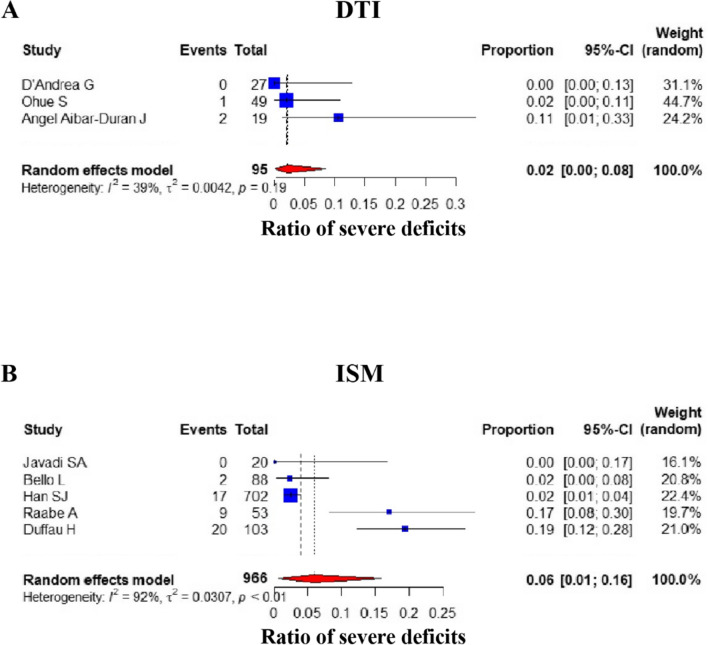
Of the 14 studies assessed, severe postoperative neurological deficits, as defined by the criteria mentioned above, were identified in 8 separate studies (DTI group: 3, ISM group: 5) [17, 28, 30, 31, 33, 34, 36, 37]. The frequency of patients with severe postoperative neurological deficits was 2.21% [95%CI: (0, 0.0845)] and 5.93% [95% CI: (0.0068, 0.1585)] in the DTI and ISM groups, respectively. In summary, for deficit severity, the proportion of the DTI group was slightly lower than that of the ISM group; however, this difference was not statistically significant (P value = 0.393, Mann–Whitney U test) (Fig. 4).
Fig. 4.
Comparison of severe postoperative deficits. Forest plot for pooled comparison of postoperative severe deficits between DTI and ISM group. A DTI-navigated group vs. B ISM-assisted group. Comparison of severe postoperative effects reveals that DTI group has lower ratio of severe postoperative deficits, and there is no statistically significant difference between groups. Estimates of relative risk are presented by blue boxes, the area of which is proportional to the number of cases in each study. Ninety-five percent confidence intervals are indicated by a red diamond and horizontal lines
Survival analysis
A total of 5 studies (4 for the DTI group and 1 for the ISM group) documented patient survival [28, 30, 33, 35, 38]. However, it is difficult to systematically compare survival as various survival indicators (including median survival time, progression-free survival, overall survival, and 1/3/5/8-year overall survival rate.) were used in different studies (Appendix 4).
Publication bias detection
Data on late postoperative neurological deficits in the DTI group may be affected by publication bias due to the low number of included publications. As shown in the table of Appendix 5, the P values showed comparison results in other groups were greater than 0.05, there were no publication bias.
Discussion
In the current study, a systematic review and meta-analysis of relevant literature was conducted to compare surgical outcomes in glioma resection assisted by DTI or ISM. Interestingly, DTI enabled a higher rate of GTR than the ISM, but no statistical difference was found between the two groups in the functional outcome analysis. Previous studies showed that ISM could better localize subcortical fiber relative to DTI-assisted neuronavigation [7, 16, 41, 42]. These lines of evidence suggest that ISM could help surgeons better delineate the surgical limit and achieve maximal resection. However, in our meta-analysis, the DTI group achieved a higher ratio of GTR [17, 43, 44]. In general, surgeons prefer DTI to detect potential contact between fiber tracts and tumors in almost all supratentorial lesions to determine the need for subcortical mapping. As a result, gliomas in the DTI group likely had a greater mean distance to adjacent fiber tracts, resulting in a higher mean GTR rate. Moreover, all resections aided by ISM continued until eloquent pathways were identified around the surgical cavity to preserve intratumoral functional components. This approach may have contributed to a relatively lower rate (32% and 30.1%) of GTR [31, 32].
Because most studies did not strictly limit the tumor’s location, some operations were possibly performed under general anesthesia. As a result, surgical removal would mainly rely on the clinical experience of neurosurgeons rather than a subjective assessment [35, 42]. Additionally, various institutions, study duration, and assistive surgical techniques might also be responsible for the percentage of GTR difference. Even so, the advent of DTI made functional protection without awake anesthesia a reality.
Due to the limited sample size of each study, calculating the extent of resection (EOR) was challenging. In this meta-analysis, multiple studies were included, and EOR was measured at separate levels, namely gross total resection, subtotal resection, and partial resection. Consequently, the extent of lesion resection was quantified as the percentage of gross total resection (GTR) [17, 28-36]. Only one of the 14 studies calculated the specific EOR [30]. With the assistance of DTI neuronavigation, the study showed that GTR (residual = 0 cm3) was achieved in 20 of 27 patients. Additionally, the tumor resection rate varied from 89 to 94% in 6 patients and was unclear in the remaining patient. Each study only indicated the approximate area of the tumor location, including the frontal, parietal, and temporal lobes. Furthermore, most of the studies did not indicate whether lesions were in eloquent areas. And none of the studies conducted so far have performed a systematic analysis to compare the neurological status after surgery for different tumor grades. Due to these confounding factors, it was hard to accurately assess the difference between varying eloquent areas and tumor grades, an apt focus for future research.
Functional preservation plays a critical role in the resection of gliomas. Although DTI can identify subcortical tracts using advanced algorithms and biomathematical models, it only provides anatomical information, not functional data. The gold standard for providing functional data when operating near eloquent tracts is ISM [16, 42, 45, 46]. Previous studies have shown that ISM allows neurosurgeons to precisely map and localize subcortical white matter by applying electrical stimulation signals [16, 42, 45, 46]. Furthermore, with inhibitory and excitatory stimulation signals, neurosurgeons can observe the functional response in the surgical process [47-50]. Theoretically, ISM should provide a superior functional prognosis relative to DTI. To validate this hypothesis, we thoroughly analyzed the prevalence of functional outcomes, including early and late neurological impairment and their severity in patients treated using DTI or ISM during glioma surgery. In contrast to this hypothesis, the functional prognosis in patients treated with DTI was similar to those treated using ISM without any significant result.
Glioma resection can be improved by establishing a surgical plan using navigation software (such as Brainlab®) based on DTI data and with the assistance of intraoperative corrections such as probes, intraoperative magnetic resonance imaging, intraoperative ultrasound, and other real-time techniques [17, 51]. Previous studies have reported that intraoperative brain shift and tumor decompression released from the cerebrospinal fluid can cause fiber tract movement, making it harder for neurosurgeons to distinguish the boundary and allow for efficient resection [17]. In daily neurological routine, DTI was commonly used to rebuild a straight and robust subcortical pathway like cortical spinal tracts rather than slender and curved fibers, such as those observed in the optic tract or arcuate fasciculus. Apart from that, the complexity of the central nerve system inhibited the identification of fibers facilitating language function. Therefore, DTI only offered a limited amount of information due to the inherent constraints of the algorithm. The current study included patients with glioma in speech, motor, and optic radiation areas [17, 31, 32, 34, 36-39]. It is possible that numerous cases of glioma located in the motor area were involved in the DTI group, which did not occur in the ISM group. In tumor surgery, subcortical stimulation was necessary for motor or language mapping. With rapid advances in sequence design and image post-processing techniques, cross-fiber identification has become possible, and the automated generation of fiber tracts can be enriched without the need to set up regions of interest. Previously, cerebral edema severely affected the tracking of surrounding fiber tracts; however, this issue is gradually being resolved. Structural connectivity based on diffusion data presents global information or potential neural plasticity beyond functional details. While ISM can help neurosurgeons avoid eloquent areas during surgery, it can also lead to postoperative neurological deficits, such as seizures [43, 45]. In addition, ISM is often performed with awake craniotomy and functional tasks. A recent review indicates that awake craniotomy requires higher basic conditions of patients and may cause more postoperative neurological dysfunction (e.g., stress, anxiety, depression) [52]. Different definitions of neurological status result in a higher heterogeneity of data, potentially confounding the ability to compare neurological statuses [25]. While some patients experienced postoperative early neurological deficits, their long-term neurological outcomes were generally satisfactory. In the case of gliomas located in non-eloquent areas, ISM was not deemed necessary, and its advantages were less evident.
Whether DTI can replace ISM in glioma surgery has been a contentious and much-debated topic. Combined with our clinical diagnosis, treatment and surgical experience, we recommend using DTI independently only under the following conditions: (1) in patients with high-grade gliomas with significant contrast enhancement; (2) in patients with severe preoperative neurological deficits; (3) in cases where the lesions are located further from subcortical white matter in eloquent areas; (4) and in cases where DTI neuronavigation is better suited for stereotactic biopsy of lesions, such as those in deep areas.
The combined application of DTI and ISM can assist surgeons in preoperative assessment of surgical planning and intraoperative real-time functional status evaluation. Additionally, a combination approach uses the advantages of the two techniques and is complementary to each method’s disadvantages. Indeed, some researchers have combined DTI and ISM in the resection of glioma and observed improved patient outcomes [8, 16, 18, 42, 51]. For example, in studies conducted by Ostrý S et al. [18] and Zhu FP et al. [42], patients achieved a higher rate of GTR (68% and 69%) with a combinational approach. These studies suggest that applying DTI and ISM can enhance surgical performance and safety, assisting surgeons in achieving an efficient volumetric glioma resection. We believe that the combined application of ISM and DTI can correct brain shifts in real-time and assist with observing neurological function in patients with particular conditions (e.g., eloquent areas and fiber tract infiltration).
Limitations
The present study had several limitations. Firstly, heterogeneity factors, such as the small number of investigations included and the lack of high-quality RCTs, may affect the results and conclusions. Most of the included studies were observational and retrospective. Hence, conclusions drawn from this meta-analysis must be interpreted with this in mind. Second, differences in technology and equipment over the 22 years (2000–2022) in which the studies were selected might bias the accuracy of the statistics. Third, a lack of preoperative neurological status might lead to a short analysis of neurological function in the perioperative period. Fourth, the lack of a subgroup analysis for low- and high-grade gliomas may result in more heterogeneity in the analysis results. Finally, many of the included studies did not accurately identify the location of the glioma, which might reduce the value of the findings.
Conclusion
Gliomas can be safely resected using either ISM or DTI. This meta-analysis showed that both techniques could be used independently in the surgical removal of glioma. Compared with the ISM group, the DTI group achieved a higher rate of GTR. The postoperative neurological function between groups was similar. These data suggest that DTI is a satisfactory substitute for ISM in glioma surgery. Moreover, the combination of ISM and DTI is theoretically superior to using either method alone.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the supported work by Institute for Intelligent Healthcare, Tsinghua University.
Abbreviations
- DTI
Diffusion tensor imaging
- ISM
Intraoperative subcortical mapping
- EOR
Extent of resection
- GTR
Gross total resection
- NOS
Newcastle-Ottawa Scale
- RCT
Randomized controlled trials
Author contributions
Conception and study design: Yiming Li and Jiahe Guo. Literature search: Yiming Li, Jiahe Guo and Kai Zhang. Data acquisition: Huijie Wei, Jikang Fan, and Tao Li. Data analysis and interpretation: Yiming Li, Jiahe Guo, and Kai Zhang. Manuscript review: Tao Li, Shengping Yu, and Xuejun Yang. Confirmed the final verison of manuscript: Xuejun Yang.
Funding
The study is funded by the Precision Medicine Research Program of Tsinghua University (No. 2022ZLB007), Tianjin Science and Technology Project (No. 21JCYBJC00800), and Natural Science Foundation of Tianjin (No.20JCYBJC00430).
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
None.
Consent to participate
None.
Consent for publication
There are no human research participants provided for publication of the image in figures.
Competing interests
The authors declare no competing interests.
Footnotes
Yiming Li and Jiahe Guo contributed equally to this work.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tao Li, Email: litao@tmu.edu.cn.
Xuejun Yang, Email: ydenny@126.com.
References
- 1.Ostrom QT, et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Śledzińska P et al (2021) Prognostic and predictive biomarkers in gliomas. Int J Mol Sci 22(19) [DOI] [PMC free article] [PubMed]
- 3.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269–282. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico RS, et al. Extent of resection in glioma-a review of the cutting edge. World Neurosurg. 2017;103:538–549. doi: 10.1016/j.wneu.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Camacho A et al (2022) Glioblastoma treatment: state-of-the-art and future perspectives. Int J Mol Sci 23(13) [DOI] [PMC free article] [PubMed]
- 7.Bello L, et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations. Neurosurg Focus. 2010;28(2):E6. doi: 10.3171/2009.12.FOCUS09240. [DOI] [PubMed] [Google Scholar]
- 8.Ohue S et al (2012) Accuracy of diffusion tensor magnetic resonance imaging-based tractography for surgery of gliomas near the pyramidal tract: a significant correlation between subcortical electrical stimulation and postoperative tractography. Neurosurgery 70(2):283–93; discussion 294 [DOI] [PubMed]
- 9.Leclercq D, et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg. 2010;112(3):503–511. doi: 10.3171/2009.8.JNS09558. [DOI] [PubMed] [Google Scholar]
- 10.Young JS, Lee AT, Chang EF. A review of cortical and subcortical stimulation mapping for language. Neurosurgery. 2021;89(3):331–342. doi: 10.1093/neuros/nyaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greisman JD, et al. Subcortical stimulation in brain tumor surgery: a closer look beneath the surface. World Neurosurg. 2022;161:55–63. doi: 10.1016/j.wneu.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Duffau H. Intraoperative cortico-subcortical stimulations in surgery of low-grade gliomas. Expert Rev Neurother. 2005;5(4):473–485. doi: 10.1586/14737175.5.4.473. [DOI] [PubMed] [Google Scholar]
- 13.Vanderweyen DC, et al. The role of diffusion tractography in refining glial tumor resection. Brain Struct Funct. 2020;225(4):1413–1436. doi: 10.1007/s00429-020-02056-z. [DOI] [PubMed] [Google Scholar]
- 14.Potgieser AR, et al. The role of diffusion tensor imaging in brain tumor surgery: a review of the literature. Clin Neurol Neurosurg. 2014;124:51–58. doi: 10.1016/j.clineuro.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Dimou S et al (2013) A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg Rev 36(2):205–14; discussion 214 [DOI] [PubMed]
- 16.Bello L, et al. Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39(1):369–382. doi: 10.1016/j.neuroimage.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Javadi SA, et al. Evaluation of diffusion tensor imaging-based tractography of the corticospinal tract: a correlative study with intraoperative magnetic resonance imaging and direct electrical subcortical stimulation. Neurosurgery. 2017;80(2):287–299. doi: 10.1227/NEU.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 18.Ostrý S et al (2013) Is intraoperative diffusion tensor imaging at 3.0T comparable to subcortical corticospinal tract mapping? Neurosurgery 73(5):797–807; discussion 806–7 [DOI] [PubMed]
- 19.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McInnes MDF, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng X, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 23.Stummer W et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery, 62(3):564–76; discussion 564–76 [DOI] [PubMed]
- 24.McGirt MJ, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 25.De Witt Hamer PC, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 26.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 28.Aibar-Durán J, et al. Intraoperative use and benefits of tractography in awake surgery patients. World Neurosurg. 2020;137:e347–e353. doi: 10.1016/j.wneu.2020.01.210. [DOI] [PubMed] [Google Scholar]
- 29.Cho JM, et al. Clinical use of diffusion tensor image-merged functional neuronavigation for brain tumor surgeries: review of preoperative, intraoperative, and postoperative data for 123 cases. Yonsei Med J. 2014;55(5):1303–1309. doi: 10.3349/ymj.2014.55.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Andrea G, et al. Safe resection of gliomas of the dominant angular gyrus availing of preoperative FMRI and intraoperative DTI: preliminary series and surgical technique. World Neurosurg. 2016;87:627–639. doi: 10.1016/j.wneu.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 31.Duffau H, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98(4):764–778. doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- 32.Duffau H, et al. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109(3):461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- 33.Ohue S et al (2015) Surgical results of tumor resection using tractography-integrated navigation-guided fence-post catheter techniques and motor-evoked potentials for preservation of motor function in patients with glioblastomas near the pyramidal tracts. Neurosurg Rev 38(2):293–306; discussion 306–7 [DOI] [PubMed]
- 34.Raabe A, et al. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014;120(5):1015–1024. doi: 10.3171/2014.1.JNS13909. [DOI] [PubMed] [Google Scholar]
- 35.Wu JS et al (2007) Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 61(5): p. 935–948; discussion 948–9 [DOI] [PubMed]
- 36.Bello L et al (2007) Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 60(1):67–80; discussion 80–2 [DOI] [PubMed]
- 37.Han SJ, et al. Subcortical stimulation mapping of descending motor pathways for perirolandic gliomas: assessment of morbidity and functional outcome in 702 cases. J Neurosurg. 2018;131(1):201–208. doi: 10.3171/2018.3.JNS172494. [DOI] [PubMed] [Google Scholar]
- 38.Ius T et al (2012) Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg 117(6):1039–1052 [DOI] [PubMed]
- 39.Keles GE, et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100(3):369–375. doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- 40.Castellano A, et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol. 2012;14(2):192–202. doi: 10.1093/neuonc/nor188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman JI, et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107(3):488–494. doi: 10.3171/JNS-07/09/0488. [DOI] [PubMed] [Google Scholar]
- 42.Zhu FP et al (2012) Clinical application of motor pathway mapping using diffusion tensor imaging tractography and intraoperative direct subcortical stimulation in cerebral glioma surgery: a prospective cohort study. Neurosurgery 71(6):1170–83; discussion 1183–4 [DOI] [PubMed]
- 43.Szelényi A, et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28(2):E7. doi: 10.3171/2009.12.FOCUS09237. [DOI] [PubMed] [Google Scholar]
- 44.Kombos T, Süss O. Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: a review. Neurosurg Focus. 2009;27(4):E3. doi: 10.3171/2009.8.FOCUS09141. [DOI] [PubMed] [Google Scholar]
- 45.Duffau H. Contribution of cortical and subcortical electrostimulation in brain glioma surgery: methodological and functional considerations. Neurophysiol Clin. 2007;37(6):373–382. doi: 10.1016/j.neucli.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Zolal A et al (2012) The use of diffusion tensor images of the corticospinal tract in intrinsic brain tumor surgery: a comparison with direct subcortical stimulation. Neurosurgery 71(2):331–40; discussion 340 [DOI] [PubMed]
- 47.Asimakidou E et al (2021) Motor evoked potential warning criteria in supratentorial surgery: a scoping review. Cancers (Basel) 13(11) [DOI] [PMC free article] [PubMed]
- 48.Duffau H et al (2004) Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir (Wien) 146(3):265–9; discussion 269–70 [DOI] [PubMed]
- 49.Ghimire P, et al. Intraoperative mapping of pre-central motor cortex and subcortex: a proposal for supplemental cortical and novel subcortical maps to Penfield’s motor homunculus. Brain Struct Funct. 2021;226(5):1601–1611. doi: 10.1007/s00429-021-02274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.You H, Qiao H. Intraoperative neuromonitoring during resection of gliomas involving eloquent areas. Front Neurol. 2021;12:658680. doi: 10.3389/fneur.2021.658680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vassal F, Schneider F, Nuti C. Intraoperative use of diffusion tensor imaging-based tractography for resection of gliomas located near the pyramidal tract: comparison with subcortical stimulation mapping and contribution to surgical outcomes. Br J Neurosurg. 2013;27(5):668–675. doi: 10.3109/02688697.2013.771730. [DOI] [PubMed] [Google Scholar]
- 52.Mofatteh M, et al. Stress, anxiety, and depression associated with awake craniotomy: a systematic review. Neurosurgery. 2023;92(2):225–240. doi: 10.1227/neu.0000000000002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.