Abstract
The inhibitory effect of di-imine-SB namely ((N1Z, N4E)-N1, N4-bis (4 (dimethylamino) benzylidene) butane 1,4-diamine) on X65-steel in 1 M HCl has been investigated experimentally and theoretically. The electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP), and weight loss outcomes display the anticorrosion properties of “di-imine- SB”. The inhibitory efficiency exceeds 90% at the optimal concentration of 1 × 10–3 M “di-imine- SB”. The metal surface was examined further using scanning electron microscope (SEM) and energy dispersive X-ray (EDX). The effectiveness of the di-imine-SB is returned into its adsorption on X65-steel surface and found in agreement with Langmuir adsorption isotherm. According to the standard Gibbs free energy of adsorption , di-imine-SB adsorption tends to be chemical rather than physical, it increases the activation energy () of metal dissolution reaction and makes it hard to occur. The PDP data suggested anodic and cathodic type of the di-imine-SB inhibitor. Meanwhile, increasing the resistance of X65-steel to 301 Ω cm2 after adding 1 mM of di-imine-SB confirms its protective effect. Whereas, the positive value of the fraction of electron transference (ΔN, 0.746), confirms the affinity of di-imine-SB to share electrons to the partially filed 3d-orbital of Fe forming strong protective film over X65-steel surface. Aided by Monte Carlo (MC) simulation, the calculated adsorption energy (Eads) suggests excessive adsorption affinity of di-imine-SB on metal surface over the corrosive chlorides and hydronium ions. A good correlation between the theoretical hypothesis and the experimental inhibition efficiency has been achieved. The comparative study showed the superior of the di-imine-SB as potential corrosion inhibitor compared with those reported before. Finally, global reactivity descriptors; electron affinity (A), ionization potential (I), electronegativity (χ), dipole moment (µ), global hardness (), electrophilicity index and, Fukui indices were also calculated and found well correlated to the reactivity of di-imine-SB.
Subject terms: Chemistry, Electrochemistry
Introduction
Carbon steel is thought to be vulnerable to corrosion in aggressive acidic media, like chloride and sulphate ions1,2 which makes it favorable economically and industrially. Corrosion inhibitors, the corresponding safeguards, are therefore deemed essential to either regulate/control or minimize/reduce corrosion rate, particularly in HCl media3–6. Organic Schiff bases are considered effective “corrosion inhibitors” of metals/alloys, they have hetero atoms (such as nitrogen, oxygen, sulphur, and/or phosphorous) and π bond(s)7–10. Nevertheless, Schiff bases are typically made from inexpensive precursors via the condensation–NH2 moiety with either ketone or an aldehyde11–13, thus became popular. The imine (–C=N–) functional group(s) and the available electron densities (ED) are essential for Schiff bases to cling/adhere to both metal and/or alloy surfaces. Such adsorption declare the formation of a fluffy/persistent film on steel surfaces, which causes a sever retardation of anodic and/or cathodic reaction(s), that slows down the corrosion rate consequently14,15. The innovation of imine metal chelates including benzylidene derivatives have demonstrated excellent efficacy in hindering the corrosion of copper- mild-, carbon- and stainless-steel in HCl, HNO3 and H2SO4 media16–21. To effectively apply for X65-steel corrosion inhibitor, a Schiff base with two benzylidene ring(s) and four nitrogen hetero atom(s) has been synthesized in the current study. Employing literature review, the di-imine-SB named ((N1Z, N4E)-N1, N4-bis (4-(dimethyl amino) benzylidene) butane-1,4-diamine), was neither theoretically nor electrochemically considered as a corrosion inhibitor for carbon steel. As a result, it was synthesized and subsequently examined herein as a potential inhibitor of the corrosion of X65-steel in 1 M HCl at various concentrations and temperatures. We have used weight loss, PDP, and EIS approaches to achieve high inhibition efficiency (IE%) on the X65-steel surface. The surface morphology of X65-steel before and after being immersed in 1 M HCl was examined using SEM and EDX methods. Additionally, we have used Materials Studio 6.0 software with the methods of Density Function Theory (DFT) and Monte Carlo (MC) adsorption locator modules to simulate the adsorption of di-imine-SB/X65-steel. The molecular and electronic structures were refined/optimized, and the calculation of EHOMO, ELUMO, and Egap, allows estimating the inhibition probability of di-imine-SB. It is worth mentioning that, “di-imine-SB” surfactants were found effective anticorrosion in our recent studies22 and its chelate efficiency with various metals could be one of our future objectives.
Experimental
Chemicals and Schiff base
Chemicals of high purity grade were used as supplied from Alpha Chemical Company including, 37% HCl, Ethanol (≥ 95%). The candidate inhibitor "di-imine-SB" (N1Z, N4E)-N1,N4-bis (4(dimethyl-amino)benzylidene)butane1,4-diamine), Supplementary Fig. S1, has been already prepared and fruitfully characterized using elemental analysis, infrared-, Raman- and NMR- spectroscopic measurements by our group23.
Medium test and Steel specimen
The following aqueous solutions were prepared using bi-distilled water at 298 ± 1 K. Then a stock solution of 1 × 10–2 M of di-imine-SB was prepared HCl (1 M), followed by additional “di-imine-SB” concentrations (1 × 10–5–1 × 10–3 M) in HCl (1 M). The elemental analysis of X65-steel in weight percentages (wt.%) are, “98.569 (Fe), 0.210 (Si), 0.103 (C), 0.024 (P), 0.012 (Ni), 1.05 (Mn) and 0.032 (Co)”.
Weight loss measurements
The weight loss (WL) technique is commonly used for assessing the inhibitor efficiency (IE%)24. Several concentrations of di-imine-SB (1 × 10–5–1 × 10–3 M) in HCl (1 M) were prepared to account for its inhibition efficiency towards X65-steel surface. The WL was carried out in a water bath equipped with a thermostat from 298 to 328 K. The X65-steel coupons were cut into the appropriate dimensions of (4.7 × 1.8 × 0.14) cm3 for length, width, and thickness, respectively. After that, it was polished with emery paper (grades 600–2500), rinsed with bi-distilled water, dipped in acetone to check for the complete removal of any oxide coatings, and then dried. The weight of these coupons is weighted up to four decimal places before being immersed in closed glass vessels filled by 100 mL of 1 M HCl (blank solution), and the above-mentioned sequential concentrations of di-imine-SB for 6 h at 98 ± 1 K. To eliminate the corrosion product(s), steel coupons were cleaned, rinsed with bi-distilled water, dried and finally weighed. Each testing was triplicated to measure the average weight loss.
Electrochemical measurements (ECM)
A 100 mL jacketed glass cell with a platinum sheet “an auxiliary electrode”, Ag/AgCl (3 M KCl) “a reference electrode”, and X65 steel “a working electrode”, all coupled to an electrochemical system that is injected into a capillary tube. A computerised Auto-lab (PGSTAT-128N) potentiostat/galvanostat was used to perform the ECM at first. The working electrode was initially cut into a cylindrical shape, insulated by epoxy resin, and left with a surface area of 1 cm2 in contact with solution. Like weight loss coupons, the electrode surface is abraded firmly up to mirror image, rinsed with bi-distilled water and dried. To account for the potential inhibitory behaviour of “di-imine-SB”, the ECM were initially performed on an X65-steel electrode bathed in HCl (1 M) in absence and presence of different concentration of di-imine-SB for 30 min, this period is found enough to reach a steady state of the Eocp (open circuit potential)25, see Supplementary Fig. S2. The EIS and PDP measurements were carried out, Consequently, the EIS was performed using frequency ranged from (100–0.0001) kHz with peak-to-peak amplitude of 0.01 V. We have assembled the anodic/cathodic polarization curves within a potential range of ± 400 mV around the Eocp employing 0.001 V/s (scan rate) using NOVA 2.1.4 software and the PDP data outcomes. The weight loss and electrochemical tests were repeated three times, the mean values and standard deviations were given in Supplementary Table S1.
Surface characterization
QUANTA FEG-250, Field Emission Gun Scanning Electron Microscope (FE-SEM) attached with Energy Dispersive X-ray (EDX) unit has been used for surface characterizations of X65-steel specified slides (1 × 1 × 0.1) cm3 in absence (blank) and presence of di-imine-SB at optimum concentration (Cinh, opt: 1 × 10–3 M) after being immersed for 6 h (WL conditions).
Computational procedure
As the corrosion process investigated herein in acidic media, the examined di-imine-SB molecules in the gaseous- (isolated molecule in vacuum), solvated-, (aqueous, dielectric constant of water is 78.56) and protonated- (HCl) states were optimized and their quantum chemical indices were estimated using DFT utilizing BIOVIA Materials Studio 6.0 (17.1.0.48) software26, https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-materials-studio. The DMol3 module has been created using the Generalized Gradient Approximation (GGA) and group functional basis designed to take into consideration its chemical reactivity using medium quality tolerance parameters, a Becke One Parameter (BOP) and Double Numerical plus Polarization (DNP-3.5). The energies (EHOMO and ELOMO) of the Highest Occupied Molecular Orbital (HOMO) and that of the Lowest Unoccupied Molecular Orbital (LUMO), energy gap in eV (ΔE = ELUMO − EHOMO), ionization potential (I), dipole moment (µ), electron affinity (A), electronegativity (χ) and fraction of the electron transferred (∆N) were computed. Aided by MCs computations using the adsorption locator module in vacuum/solvent, simulating the adsorption and interaction between di-imine-SB and X65-steel “Fe (1 1 0)” surface with excellent surface stability27. Using the same surface cleavage, the adsorption energy (Eads) is simulated in a box with dimensions (49.6 × 49.6 × 24.05) Å3 with periodic boundary conditions extending the cleavage plane to a (15 × 15) super cell. After that, 15 Å vacuum slab built over Fe (1 1 0) plane to eliminate the periodic boundary effect. The MCs annealing was processed for di-imine-SB over Fe (1 1 0) in gas in which (200H2O + 5H3O+ + 5Cl−) acidic solution was simulated through 10 cycles (15,000 steps) for each run, and the Etot of the system has been estimated at equilibrium. The force field was set to COMPASS (Condensed-phase Optimized Molecular Potentials for Atomistic Simulation Studies) with fine quality of the energy calculation28.
Results and discussion
Weight loss measurements, effect of concentration and temperature
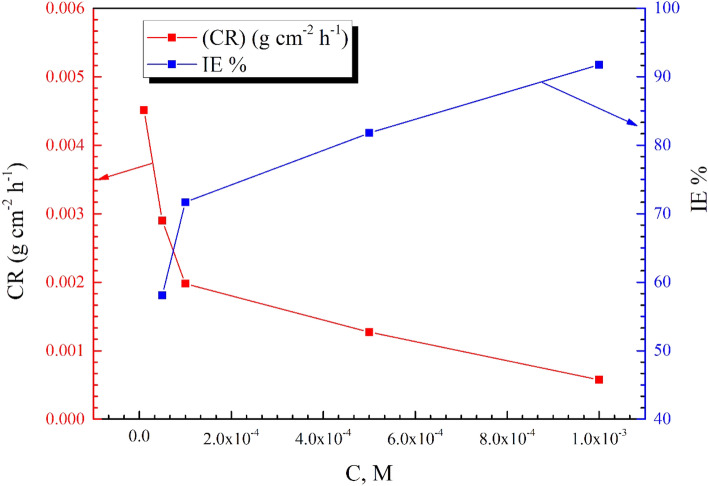
The weight loss (WL) output parameters of X65-steel in the absence/presence of di-imine-SB/HCl (1 M) at 298 ± 1 K are displayed in Fig. 1 and Table 1 implementing different concentrations of an inhibitor. The corrosion rate (CR) in g cm−2 h−1 were calculated by implementing the formula: , where (S) is the surface area of the steel coupons (cm2), (d) is the density of iron (7.85 g cm−3) and (t) is the immersion time (6 h). Whereas, the degree of surface coverage and inhibition efficiency () values can be calculated from: , where, ΔW is average weight loss (), W0 and W are the weight loss of X65-steel coupons in the absence and presence of di-imine-SB inhibitor, respectively. Due to the adsorption of inhibitor molecules on coupons surface29, IE% rises and the corrosion rate (CR) falls upon increasing Cinh (Fig. 1 and Table 1). Maximum IE of 92% is achieved with 1 × 10–3 M Schiff base at 298 ± 1 K owing to the molecular interactions beside the inductive effect (+ I) of −(Me)2 groups which enhances the ED at nitrogen’s leading to effective adsorption30.
Figure 1.
Influence of the inhibitor dose on the rate of corrosion (CR) and the inhibition performance (IE%) of the prepared di-imine-SB inhibitor at 298 ± 1 K.
Table 1.
Corrosion parameters obtained from weight loss (WL) measurements for X65-steel coupons after 6 h immersions in 1 M HCl adding different concentrations of di-imine-SB and without adding it at different temperatures.
| Temperature ± 1 K | C (M) | 1 M HCl Medium | ||||
|---|---|---|---|---|---|---|
| ΔW (g) | CR | Ɵ | IE (%) | |||
| (g cm−2 h−1) | (g m−2 d−1) | |||||
| 298 | Blank | 0.7873 | 0.0069 | 1656 | – | – |
| 1 × 10–5 | 0.4286 | 0.0038 | 912 | 0.4556 | 45.56 | |
| 5 × 10–5 | 0.3300 | 0.0029 | 696 | 0.5808 | 58.08 | |
| 1 × 10–4 | 0.2229 | 0.0019 | 456 | 0.7168 | 71.68 | |
| 5 × 10–4 | 0.1435 | 0.0012 | 288 | 0.8177 | 81.77 | |
| 1 × 10–3 | 0.0649 | 0.0005 | 120 | 0.9175 | 91.75 | |
| 308 | Blank | 1.0718 | 0.0095 | 2280 | – | – |
| 1 × 10–5 | 0.5982 | 0.0053 | 1272 | 0.4419 | 44.19 | |
| 5 × 10–5 | 0.4689 | 0.0041 | 984 | 0.5625 | 56.25 | |
| 1 × 10–4 | 0.3181 | 0.0028 | 672 | 0.7032 | 70.32 | |
| 5 × 10–4 | 0.2260 | 0.0020 | 480 | 0.7891 | 78.91 | |
| 1 × 10–3 | 0.1659 | 0.0014 | 336 | 0.8452 | 84.52 | |
| 318 | Blank | 1.1868 | 0.0105 | 2520 | – | – |
| 1 × 10–5 | 0.6693 | 0.0059 | 1416 | 0.436 | 43.60 | |
| 5 × 10–5 | 0.5435 | 0.0048 | 1152 | 0.542 | 54.20 | |
| 1 × 10–4 | 0.3599 | 0.0031 | 744 | 0.6967 | 69.67 | |
| 5 × 10–4 | 0.2691 | 0.0023 | 552 | 0.7733 | 77.33 | |
| 1 × 10–3 | 0.2119 | 0.0018 | 432 | 0.8215 | 82.15 | |
| 328 | Blank | 1.2423 | 0.0110 | 2640 | – | – |
| 1 × 10–5 | 0.711 | 0.0063 | 1512 | 0.4277 | 42.77 | |
| 5 × 10–5 | 0.5839 | 0.0051 | 1224 | 0.5299 | 53.00 | |
| 1 × 10–4 | 0.3921 | 0.0034 | 816 | 0.6844 | 68.44 | |
| 5 × 10–4 | 0.3021 | 0.0026 | 624 | 0.7568 | 75.68 | |
| 1 × 10–3 | 0.2448 | 0.0021 | 504 | 0.8029 | 80.29 | |
WL measurements were performed at temperatures of 298, 308, 318, and 328 (± 1 K) for X65-steel reaction in HCl (1 M) in the absence and/or presence of various di-imine-SB concentrations (Table 1, Cinh) to assess the impact of temperature on the IE% of an “inhibitor” candidate and to determine kinetic and thermodynamic parameters. At 1 × 10–3 M (Cinh, opt), the IE% decreased to 80.29% at 328 ± 1 K, the corrosion rate (CR) increases at elevated temperatures, owing to the desorption of more di-imine-SB molecules from the surface of X65-steel coupons, lowering the IE%. This behavior is demonstrated experimentally by the evolution of H2(g) bubbles on the surface of X65 steel, accompanied by substantial weakening of molecular interactions among di-imine-SB molecules that covers X65-steel surface supporting “physical adsorption”31,32.
Adsorption isotherm
Di-imine-SB's adsorption on X65-steel has a considerable inhibitive effect on HCl offensive action. Therefore, we have considered herein various models of adsorption isotherms “Langmuir, Temkin, Frumkin, Al-Awady, Flory–Huggins and Freundlich”33. Nevertheless, the adsorption process depends on metal type, temperature, θ “the degree of surface coverage” and the corrosion potential at metal/solution interface34. The estimated values of surface coverage were evaluated based on WL data and the correlation coefficient (R2) at different Cinh from 298 to 328 K, in which the adsorption fits Langmuir adsorption isotherm model rather than other models (Table 2). As per the equation35,36: , where ( is the adsorption equilibrium constant, () is the inhibitor molar concentration “di-imine-SB ” and , attained from the WL as mentioned before37. The plot of from 298–328 ± 1 K produces a straight line and values were determined from the intercept (Fig. 2, Table 3) in which both R2 and slope are close to unity which favors Langmuir’s adsorption isotherm. As the temperature exceeded 328 K, the rate of inhibitor desorption was higher than the rate of its adsorption. At 318 K, water adsorption outpaced water desorption, indicating that di-imine-SB molecules were strongly adsorbed to the surface of X65 steel38. Moreover, 39, is the standard Gibbs free energy of adsorption, R is the universal gas constant (8.314 J mol−1 K), T is the absolute temperature (± 1 K), and 55.5 represents the molar concentration of water in solution. The calculated values of for di-imine-SB within 298–328 K are recorded in Table 3, all has negative numerical values which indicates a spontaneous adsorption process40. When ≤ − 20 kJ mol−1, favoring dual electrostatic interaction between inhibitor molecules and metal surface “physical adsorption”, while the values nearby − 40 kJ mol−1 or higher suggests NLP donation/interactions of the “inhibitor” to metal surface a “chemisorption adsorption”41. Thus, − 40 kJ mol−1 > > − 20 kJ mol−1 indicate physicochemical adsorption but the chemisorption process is dominant41,42. Similarly, the temperatures declare the enhanced the numerical values of − upon rising it, favoring the adsorption of di-imine-SB inhibitor molecule on X65-steel surface (Table 3).
Table 2.
Output isotherm parameters for di-imine-SB adsorption on X65-steel/HCl(1 M) surface interface using different models at 298 K.
| Isotherm model | Parameters | R2 | Kads (M−1) | ΔG°ads (kJ mol−1) |
|---|---|---|---|---|
| Langmuir | Slope, 1.078 | 0.999 | 31.40 | − 35.605 |
| Freundlich | 1/n, 0.149 | 0.971 | 0.379 | − 7.548 |
| Temkin | A, 0.228 | 0.979 | 0.843 | − 9.530 |
| Flowry-Huggins | X, 1.998 | 0.907 | 0.695 | − 9.049 |
| El-Awady | 1/y, 1.867 | 0.948 | 4.729 | − 12.253 |
| Frumkin | Z, − 1.941 | 0.866 | 0.394 | − 7.644 |
Figure 2.
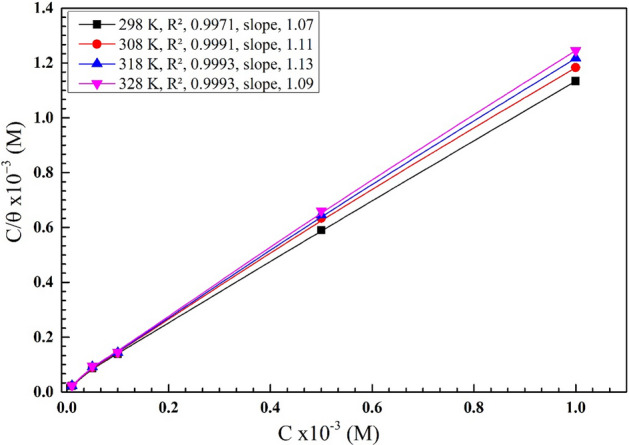
The Langmuir isotherm adsorption plot for X65-steel in 1 M HCl containing different concentrations of the synthesized di-imine-SB compound at different temperatures.
Table 3.
Adsorption thermodynamic parameters of the X56-steel surface in 1 M HCl solution containing different concentrations of the synthesized di-imine-SB at various temperatures in kelvin.
| Temp. ± 1 K | Slope | R2 | Kads (M−1) × 103 | ΔG°ads (kJ mol−1) | ΔH°ads (kJ mol−1) | ΔS°ads (kJ mol−1 K−1) |
|---|---|---|---|---|---|---|
| 298 | 1.07 | 0.9990 | 31.40 | − 35.605 | − 12.6657 | 119.439 |
| 308 | 1.11 | 0.9991 | 38.28 | − 37.307 | 121.128 | |
| 318 | 1.13 | 0.9993 | 39.19 | − 38.581 | 121.324 | |
| 328 | 1.09 | 0.9993 | 38.88 | − 39.772 | 121.256 |
Thermodynamic parameters
Adsorption Thermodynamic parameters
Aided by Van’t Hoff equation43: ; we can determine thermodynamic parameters that controls the adsorption of di-imine-SB “inhibitor” on the surface of X65-steel in 1 M HCl solution. Figure 3 illustrates the plot of (M−1) versus 1/T (K−1), the heat of adsorption ) can be obtained from the slope . Whereas, the standard adsorption entropy can be inferred from: 44, the calculated and values are listed together in Table 3. The calculated < 0, thus either “exothermic adsorption” physisorption or chemisorption process, otherwise both sorption’s materialize with different extent. Also, are numerically positive (Table 3) causing disorder increases which are the driving force for effective adsorption of di-imine-SB on X65-steel surface in acidic solution and the replacement of adsorbed water molecules by inhibitor molecules45.
Figure 3.
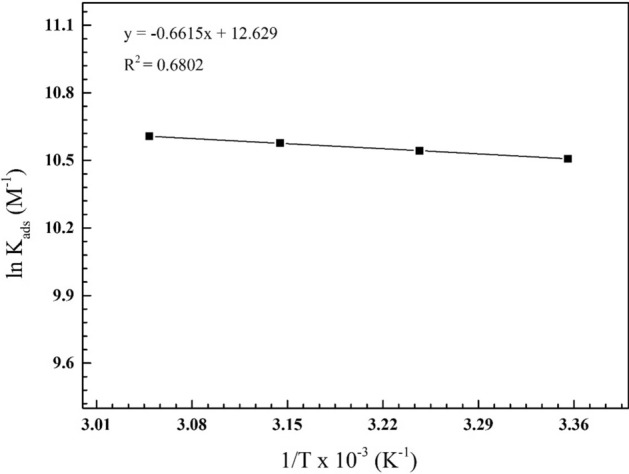
The relation between ln Kads and 1/T for the X65-steel in 1 M HCl medium containing different concentrations of the di-imine-SB inhibitor that applied at different temperature values.
Activation thermodynamic parameters
The mechanism of corrosion inhibition and the properties of the inhibitor under experimental settings must be clarified using the kinetic model. Based on the values of corrosion rate () derived from WL measurements (Section "Adsorption isotherm"), the activation energy (), enthalpy- (), and entropy- of activation () were estimated from Arrhenius and transition state equation46: , where is the Arrhenius constant. could be obtained from the slope (/) of linear relationship (ln vis 1/T) in 1 M HCl without (blank) and with different concentrations “di-imine-SB” inhibitor (Fig. 4). When compared to lower levels of the blank (untreated solution), it is evident that Ea is directly proportional to [di-imine-SB], which is attributed to the development/thickening of an electric double layer47. Moreover, the metal dissolution reaction requires higher activation energies, hence the entire adsorption process is activation-controlled (Table 4) which is confirmed by a slight decrease in upon increasing the temperature, such behavior suggests physical adsorption48. From the slope and intercept, () and () were calculated, respectively by plotting of ln (CR/T) vis 1/T out of the following transition state equation, and were calculated, where is Avogadro's number and is Planck’s constant, (Fig. 5, Table 4). Endothermic dissolution process of X65-steel is expected when () > 0, endorsing difficult dissolution49 of X65-steel in presence of di-imine-SB “inhibitor”. The large and imaginary values of activation entropies () in both inhibited/uninhibited aqueous di-imine-SB (Table 4), favor associated- rather than dissociated- activated complex (the rate-determining step), owing to disordering decrease takes place from reactants50.
Figure 4.

Arrhenius plots, variation of ln CR against 1/T, for X65-steel in 1 M HCl devoid of and containing various concentration of di-imine-SB compound.
Table 4.
Activation thermodynamic parameters for corrosion of X65-steel in 1 M HCl in absence and presence of different concentration di-imine-SB at different temperatures.
| Conc. (M) | Ea (kJ mol−1) | ΔHa (kJ mol−1) | ΔSa (kJ mol−1 K−1) |
|---|---|---|---|
| Blank | 12.0777 | 9.4801 | − 253.793 |
| 1 × 10–5 | 13.3866 | 10.7890 | − 254.432 |
| 5 × 10–5 | 15.2480 | 12.6504 | − 250.369 |
| 1 × 10–4 | 14.9046 | 12.3070 | − 254.774 |
| 5 × 10–4 | 19.7318 | 17.1342 | − 242.104 |
| 1 × 10–3 | 34.7488 | 32.1512 | − 197.291 |
Figure 5.
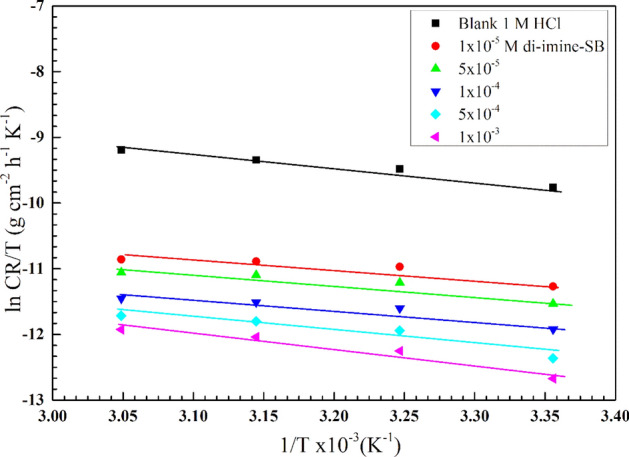
Transition state relation of ln (CR/T) against 1/T, for X65-steel in 1 M HCl devoid of and containing various concentration of di-imine-SB compound.
Potentiodynamic polarization measurements (PDP)
In order to determine the cathodic and anodic polarization curves for X65-steel corrosion in 1 M hydrochloric acid with/without di-imine-SB at 298 K, the PDP approach was used after holding the electrodes at Eopc for 30 min. Nevertheless, the PDP findings support the WL acquired information. Meanwhile, we can account for corrosion potential (), specific corrosion current density (), polarization resistance (), anodic (βa) and cathodic (βa) Tafel slopes by extrapolating anodic/cathodic Tafel lines. The and IE% values, were derived from: and 51,52, where, symbolize the values of specific corrosion current density with/without di-imine-SB “inhibitor”, respectively (Table 5). While was estimated from “Stern–Geary” equation: 53,54. The effect of increasing Cinh molecules on the anodic/cathodic Tafel lines (E vs. I) of X65-steel in 1 M HCl at 298 K is displayed in Fig. 6. Due to the anodic/cathodic polarization curves being shifted to more noble orientations in comparison to the blank, the presence of "di-imine-SB" results in a drop in both icorr and CR (Fig. 6). Note that, the variability in Tafel slopes (βa, βc) wasn’t tangible, so the addition of di-imine-SB has no effect on the reaction mechanism. Adding di-imine-SB" to the anodic and cathodic areas within ± 85 mV results in no discernible change in Ecorr compared to those of the blank one, indicating a mixed-type inhibitor55,56. The minor shift in ΔE values supports the geometry blocking active sites paradigm57 inhibitory effect of di-imine-SB in agreement with potential time graphs (Supplementary Fig. S2) for X65-steel in the absence/presence di-imine-SB inhibitor, in which a steady state potential attained after 30 min of immersion. For polarization resistance, increasing the values of were returned to the adsorption of di-imine-SB molecules through the dissociation of X65-steel owing to their blocking active centers effect. At Cinh, opt (1 × 10–3 M), 90% IE values intended from the icorr (Table 5), thus “di-imine-SB” inhibitor is found effective to reduce the surface corrosion of X65-steel in 1 M HCl by its adsorption via active sites otherwise by covering/coating the electrode surface57.These results agree with WL measurements, that dominates "di-imine-SB" over other known inhibitors under the same conditions58–62 (Table 6).
Table 5.
Tafel parameters for X65-steel in 1 M HCl in absence and presence of di-imine-SB employing different concentrations at 298 K.
| Conc. (M) | − Ecorr, (V) | icorr | βa (mV dec−1) | − βc (mV dec−) | CR (mpy) | RP (Ω) | θ | IE % | |
|---|---|---|---|---|---|---|---|---|---|
| (µA cm−2) | (A m−2) | ||||||||
| Di-imine-SB Inhibitor | |||||||||
| Blank | 0.439 | 800.45 | 8.0045 | 111.21 | 186.07 | 366.39 | 37.76 | – | – |
| 1 × 10–5 |
0.457 0.433 |
533.20 | 5.3320 | 101.50 | 165.09 | 244.06 | 51.19 | 0.3338 | 33.38 |
| 5 × 10–5 | 271.00 | 2.7100 | 85.40 | 156.30 | 124.04 | 88.49 | 0.6614 | 66.14 | |
| 1 × 10–4 | 0.437 | 248.53 | 2.4853 | 109.61 | 150.70 | 113.76 | 110.89 | 0.6895 | 68.95 |
| 5 × 10–4 | 0.438 | 156.46 | 1.5646 | 89.83 | 146.23 | 71.61 | 154.46 | 0.8045 | 80.45 |
| 1 × 10–3 | 0.441 | 79.83 | 0.7983 | 90.12 | 142.40 | 36.54 | 300.20 | 0.9002 | 90.02 |
Figure 6.

Potentiodynamic polarization curves for X65-steel in 1 M HCl in absence and presence of different concentration of di-imine-SB at 298 K.
Table 6.
Comparison between the corrosion inhibition efficiency of the prepared di-imine-SB inhibitor and some other reported inhibitor for Fe-alloys in acidic media (1 M HCl) using PDP.
Electrochemical impedance spectroscopic study (EIS)
Figures 7 and 8 respectively represent Nyquist and Bode plots for X65-steel corrosion in 1 M HCl solution without/with several Cinh “di-imine-SB” after 30 min. immersion at 298 ± 1 K. Inspecting Fig. 7 we can conclude that, the center of the Nyquist impedance curves didn't appear to be a perfect semicircle; instead, it was depressed. This can be endorsed to the frequency dispersion phenomenon owing to the roughness and heterogeneity of the X65-steel surface63,64. The Nyquist plots include a single capacitive loop with a single time constant in the Bode-phase plot, hence the reaction of X65-steel in 1 M HCl is controlled by charge transfer process65. Additionally, as the number of "di-imine-SB" molecules in the capacitive loop rises (Cinh), the width of the loop also grows, resulting the CR to drop due to surface coverage of the X65-steel, by the adsorbed di-imine-SB layer66. Adding di-imine-SB inhibitor resulting layered adsorption on the X65-steel surface shift the impedance modulus/Z/to higher values and phase angle toward − 90° employing lower and intermediate frequency regions67, refer to Bode-phase angle plot (Fig. 8).
Figure 7.
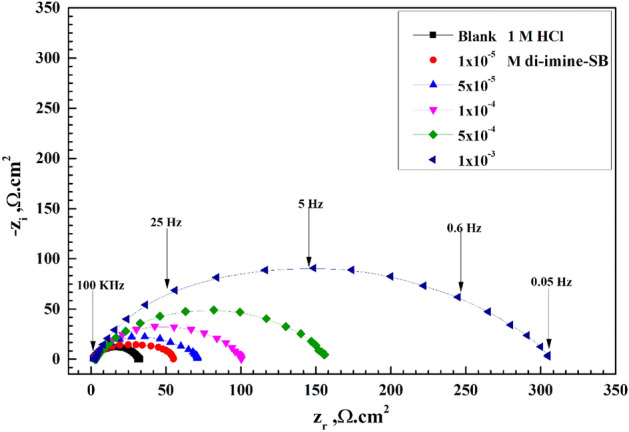
Nyquist diagram for X65-steel in 1 M HCl in absence and presence of different concentration of di-imine-SB at 298 K.
Figure 8.
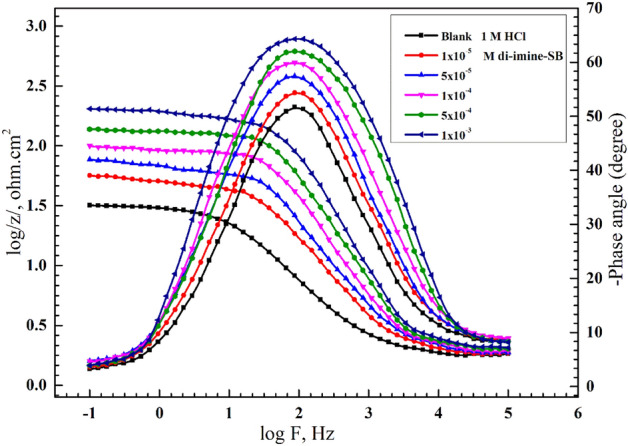
Bode-phase degree diagram for X65-steel in 1 M HCl in absence and presence of different concentration of di-imine-SB at 298 K.
The X65-steel reaction mechanism is unaffected by the presence of di-imine-SB since the shape of EIS spectra (Nyquist and Bode) does not alter. The inset of Fig. 9 suggests an outstanding simulation of "the best equivalent circuit" that fits the EIS data in the absence/presence of "di-imine-SB" at the optimum concentration (Cinh, opt) of 1 × 10–3 M including solution resistance (, constant phase element (CPE) and polarization resistance 68–70. The ideal CPE can replace the frequency dispersion-prone CPE (Cdl) to produce simulated data that is better suited71. With the aid of EIS spectral data fits, the electrochemical parameters and the IE% values were derived from the equation: 72, where, and are the polarization resistances of X65-steel without/with “di-imine-SB”, respectively. Whereas, the double-layer capacitance is derived from: 73, where, (), is the frequency at maximum imaginary resistance (Table 7). Moreover, the values of () is directly proportional to the added di-imine-SB compared to the blank, while () values show an opposite trend (Table 7). The measured value (52.03 cm2) at 1 × 10–5 M compared to blank (30.31 cm2) reaches a maximum at 301 cm2 with 1 × 10–3 M di-imine-SB inhibitor, representing the greatest increase in surface coverage. The adsorption of “di-imine-SB” molecules on X65-steel surface causes desorption of water molecules, thus the protective layered “di-imine-SB” diminishes the amount of aqueous HCl being in contact with the surface, lowering the steel corrosion rate74. The IE% of di-imine-SB verses X65-steel in 1 M HCl exceeds 90% which was supported by WL and PDP measurements. This is connected to the –C=N group, hetero atoms, benzene ring and electron donating groups (CH3) in the di-imine-SB structure, in which the methyl moiety increases the ED on the nitrogen’s, ensuing high IE%75.
Figure 9.
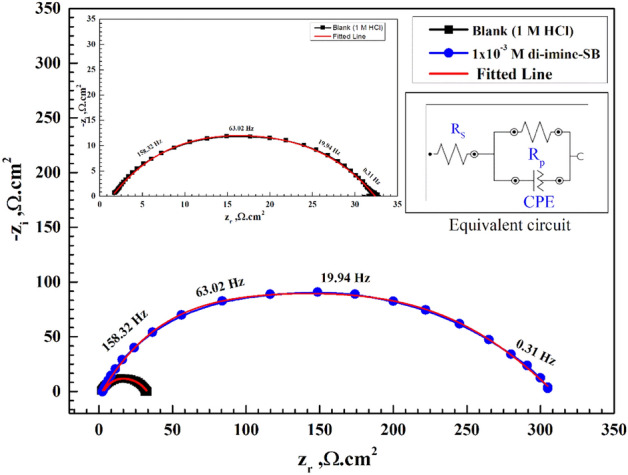
Nyquist plots for X65-steel in 1 M HCl in absence and presence of optimized, 1 × 10–3 M, concentration of di-imine-SB (representative one) using the proposed equivalent circuit.
Table 7.
Electrochemical impedance spectroscopy parameters for X65-steel in 1 M HCl in absence and presence of different concentrations of di-imine-SB at 298 K.
| Inhibitor | Conc. (M) | Rs (Ω cm2) | Rct (Ω cm2) | n | Cdl (F cm−2) × 10–5 | θ | IE % |
|---|---|---|---|---|---|---|---|
| Di-imine-SB | Blank | 1.549 | 30.31 | 0.803 | 4.177 | – | – |
| 1 × 10–5 | 1.946 | 52.03 | 0.772 | 3.853 | 0.4176 | 41.76 | |
| 5 × 10–5 | 1.634 | 69.21 | 0.803 | 3.533 | 0.5622 | 56.22 | |
| 1 × 10–4 | 1.416 | 98.45 | 0.783 | 1.284 | 0.6922 | 69.22 | |
| 5 × 10–4 | 1.071 | 152.03 | 0.764 | 5.248 | 0.8006 | 80.06 | |
| 1 × 10–3 | 1.587 | 301.00 | 0.791 | 1.672 | 0.8993 | 89.93 |
Computational part
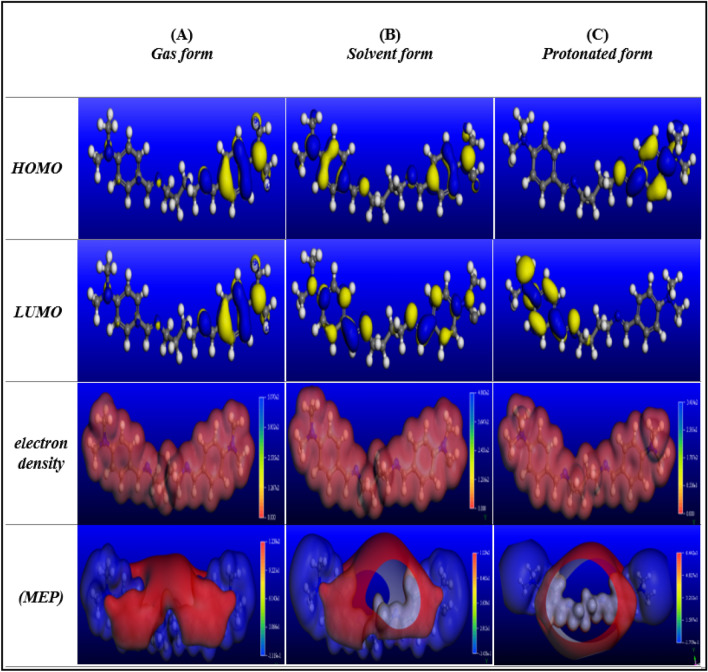
DFT is acknowledged to be a powerful approach for predicting the chemical/structural reactivity of an inhibitor candidates28. Aided by Koopman’s theorem has been used to target, , and ΔEgap = − in eV. The frontier orbital energies can be correlated with various quantum parameters, e.g., ionization potential (), electron affinity (), global hardness (), and electronegativity () as per the following equations76,77: ; ; ; , (Table 8). After successful geometry optimization of the examined di-imine-SB, we carried out HOMO, LUMO, and electron density (ED) calculations in addition to molecular electrostatic potential (MEP) mapping distribution for gaseous, solvated H2O, and protonated forms (Fig. 10). The HOMO and the LUMO are located above “active centers” of high ED (imine groups, benzylidene rings and the terminal tertiary amines) which enhance the probability of adsorbing “di-imine-SB” molecules on steel surface. Nevertheless, the ED regions can be detected from MEP map (Fig. 10), the extra negative charge “red region” includes NLP and the benzylidene ring's π-electrons (i.e., nucleophiles), promotes di-imine-SB's adsorption on metal surfaces in addition to the d-orbitals of Fe78 resulting its adsorption, causing corrosion inhibition. Nevertheless, the aggressive chloride anions are driven away from the metal surface by such adsorption, creating a protective layer that reduces metal corrosion and raises the IE%. An additional local reactivity descriptor is the Fukui function/indices that has been widely applied to either account or understand the interaction/reactivity of an inhibitor towards steel corrosion79. The Fukui indices are active sites for nucleophilic and electrophilic attack regarding local reactivity are calculated using the finite mathematical difference approximations80: and , where is the charge at atomic center k. While atomic charges can be obtained through different approaches, Hirshfeld charges are the most accurate since it is corrected based on the bond order between atoms. Table.S-2 lists the Fukui indices for di-imine-SB in the gaseous and aqueous phases calculated using the Hirshfeld charges obtained from Materials Studio 6.0 (17.1.0.48) software according to DMol3 module. Observing ƒ+ and ƒ− Fukui values (Table S2), the highly positive (ƒ+) favor nucleophilic attack in that region/atom, therefore the inhibitor candidate accepts electrons from steel surface in easy manner. On the other hand, the high negative (ƒ−) suggest an electrophilic attack in which the inhibitor donate electrons to steel surface81. The highest ƒ+ located on N1, N6, C7, C9 and C17; so steel back donation can occur at these sites and those estimated for ƒ− are located on N14, N24, C18 and C20 centers providing steel with electrons including N1 and N6 atoms, hereby mutually involved in both donating and accepting electrons. Meanwhile, the chemical reactivity an inhibitor candidate's often increases with smaller (ΔEgap) due to the low excitation energy needed for an electron to be promoted (HOMO → LUMO). Such values were in accordance with those reported by Obot et al.82, the computed ΔEgap of 3.01, 2.84, and 0.40 eV for gaseous-, aqueous-, and protonated phases, respectively (Table 8). Moreover, the inhibitor outermost electrons are more polarizable towards d-orbitals of iron. Therefore, the di-imine-SB molecules can accept electrons (ELUMO will be reduced) from iron d-orbitals based on back-donation concept “synergetic effect” reflecting high adsorption efficiency. On the other hand, ΔEgap is directly proportional to the hardness (), the harder the molecule the larger is ΔEgap. Nevertheless, the lesser ΔEgap, the investigated molecule is more likely soft, accordingly soft molecules are frequently more reactive than hard one83. The hardness () values were decreasing in the order: protonated < aqueous < gas, respectively and the EHOMO of the protonated di-imine-SB is found higher than those estimated for the solvated and neutral molecules (Table 8). Thus, the protonated form has the highest propensity to donate electrons which favors strong adsorption binds on the surface of X65-steel. Moreover, di-imine-SB (H+) has the highest chemical reactivity owing to lowest values of ΔE, and values, therefore it is more feasible to bind effectively on the surface of X65-steel than other forms82. The fraction of electron transference (ΔN) displays the reactivity di-imine-SB is derived from ΔN = (φFe − compd.)/[2 (ηFe + ηcompd.)], where the work function (φ) of Fe (1 1 0) plan is 4.82 Ev, refer to Table 884. According to Lukovits’s findings, when ΔN is less than 3.685, the adsorption probability features of the adsorbent increases with growing ΔN value. Also, the positive values of ΔN, indicates the ability of electron(s) transfer from HOMO of di-imine-SB to partially filed Fe 3d-orbitals. Whereas the imaginary back donation energy (Eback) represent the required energy to control the back donation process from 3d-orbitals (Fe) to di-imine-SB86. Moreover, the higher the inhibition efficiency (IE%) the higher is the dipole moment (µ) as a result of strong dipole–dipole interaction, causing corrosion diminishes on X65-steel surface41, refer to Table 8.
Table 8.
Quantum chemical calculated parameters of the investigated di-imine-SB inhibitor.
| Phase | EHOMO (eV) | ELUMO (eV) | Δ E (eV) | A (eV) | I (eV) | X (eV) | Ƞ (eV) | ΔN | µ (Debye) | Eback | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas | − 4.077 | − 1.066 | 3.011 | 1.066 | 4.077 | 2.571 | 1.505 | 0.746 | 0.805 | − 0.376 | ||
| Water | − 4.312 | − 1.471 | 2.840 | 1.471 | 4.312 | 2.892 | 1.420 | 0.678 | 0.426 | − 0.355 | ||
| Protonated | − 1.167 | − 0.760 | 0.407 | 0.760 | 1.167 | 0.963 | 0.203 | 9.464 | 5.402 | − 0.051 | ||
Figure 10.
The (HOMO), (LUMO) total occupation, total electron density and molecular electrostatic potential (MEP) mapping distribution for the investigated di-imine-SB inhibitor in (A) gas, (B) solvent, (C) protonated form. Structures were extracted from Dassault Systems Materials Studio Tutorials, BIOVIA, 5005 Wateridge Vista Drive, San Diego, CA 92121, USA, 2017.
Monte Carlo simulation (MCs)
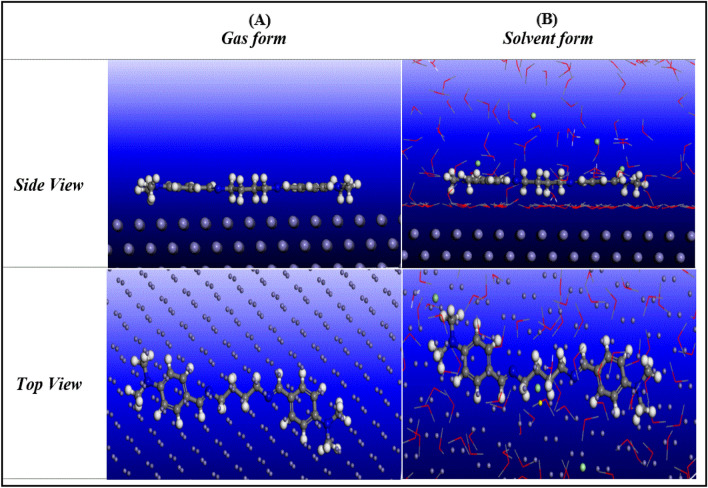
Monte Carlo simulation (MCs) is a powerful theoretical tool that can mimic inhibitors molecular interactions with iron metal surfaces, the current computations is similar to that suggested by Singh et al.87. Snapshots for the optimized di-imine-SB in gas phase on Fe (1 1 0) cleaved surface are presented in Fig. 11A in addition to that simulated in acidic solution (200 H2O molecules, 5 H3O+ and 5 Cl− ions) to mimic the corrosion media (Fig. 11B) until reaching an equilibrium. The optimized structure side view (snapshots) reveals a strong interactions between flat parallel orientation of the di-imine-SB and the Fe (1 1 0) surface which lead to iron steel surface protection73. On the other hand, top views show di-imine-SB molecules covering large surface area of X65-steel surface. Energy of adsorption (Eads) is the energy released upon inhibitor binding “relaxed” on metal surface while the rigid adsorption energy (Erigid), is the released/required energy for the unrelaxed adsorbate “an inhibitor” to be adsorbed on the adsorbent “Metal surface”, whereas the deformation energy (Edef) is the released energy from the relaxation of the adsorbed molecules on steel surface (Eads = Erigid + Edef)88. The total Energy (Etot) of an inhibitor, is the sum of internal energy and adsorption energy (Eads). In fact, the least values of Eads (Table 9) reveal a spontaneous physical adsorption of gaseous- and solvated- di-imine-SB on the X65-steel surface, i.e., more adsorbent molecules are located on the surface in agreement with earlier interpretations89. Consistently, Eads of di-imine-SB “inhibitor” is found too low compared to that of H2O, H3O+ and Cl− ions (Table 9). Therefore, it has propensity to progressively replace the adsorbed water molecules to form a thermodynamically stable adsorption layered film on Fe (1 1 0) surface. While Eads show further decrease in presence of water molecules which is associated with intermolecular HB interactions between di-imine-SB and water molecules that enhance its adsorption on the steel surface90. Both DFT and MCs calculations favors the feasible application of di-imine-SB as a potential corrosion inhibitor.
Figure 11.
Side and top views of the adsorption mode of di-imine-SB inhibitor molecule in (A) gas and -(B) simulated acid solution on Fe (1 1 0) substrate, extracted from Dassault Systems Materials Studio Tutorials, BIOVIA, 5005 Wateridge Vista Drive, San Diego, CA 92121, USA, 2017.
Table 9.
The outputs energies calculated by Monte Carlo simulation for di-imine-SB inhibitor in gas and simulated acid solution phases on Fe (1 1 0).
| Compound | ET (KJ mol−1) | Eads (KJ mol−1) | Erig.ads (KJ mol−1) | Edef. (KJ mol−1) | 3D | 3D H2O | 3D H3O+ | 3D Cl− |
|---|---|---|---|---|---|---|---|---|
| Di-imine-SB | − 276.72 | − 226.52 | − 211.42 | − 15.11 | − 226.53 | – | – | – |
| Di-imine-SB + H2O + 5HCl | − 3462.49 | − 4621.12 | − 3410.05 | − 1211.07 | − 132.53 | − 6.64 | − 147.05 | − 99.34 |
SEM and EDX
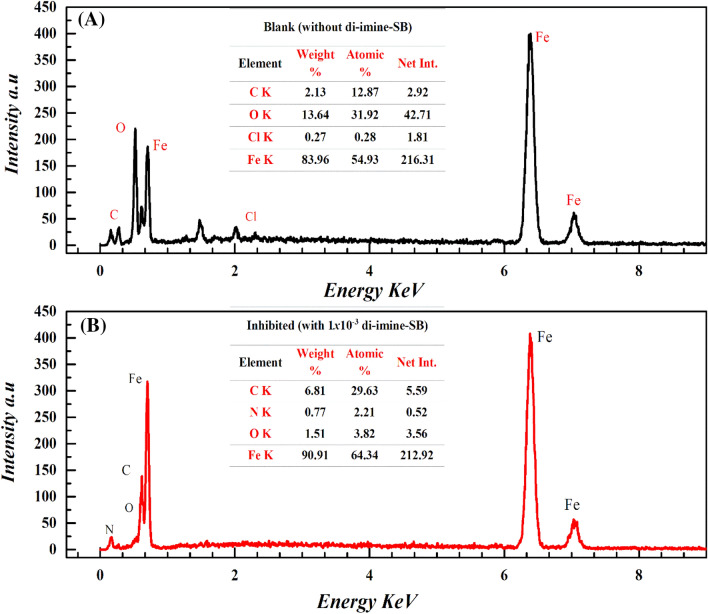
The displayed SEM (Fig. 12) and EDX (Fig. 13) measurements for X65-steel sheets (1 × 1 × 0.1) cm3 immersed for 6 h in 1 M HCl (blank) and those treated with 1 × 10–3 M di-imine-SB “inhibitor” at the same conditions. Analyzing the SEM images in the absence of di-imine-SB “inhibitor”, an irregular rough damaged X65-steel surface is observed owing to steel dissolution in 1 M HCl corrosive media (Fig. 12a). On the other hand, the lumpiness was absent, and a smooth X65-steel surface was evidenced after being treated with 1 mM of di-imine-SB which provides good protection against corrosion and lowering its rate (Fig. 12b). The EDX spectra Fig. 13a,b configure the elemental and aqueous components on the outer layer surface of X65-steel before and after being in contact with di-imine-SB (1 mM). The appearance of the nitrogen atom (N) peak and increasing the C-content ensures the adsorption of di-imine-SB on the steel surface forming a protected area of the adsorbed inhibitor, which reduces the corrosion. These outcomes are also supported by the decrease of chlorine and oxygen peak intensities. increasing the intensity of Fe-peak confirms the reduction of metal oxide formation due to the HCl severity as well91. EDX study showed in Fig. 13a,b confirms the SEM observations and found consistent with WL, PDP and EIS results.
Figure 12.
SEM for the X65-steel surface: (A) sample immersed in 1 M HCl without di-imine-SB inhibitor, (B) sample immersed in (1) M HCl with (1 × 10–3 M) of di-imine-SB inhibitor.
Figure 13.
EDX for the X65-steel surface: (A) sample immersed in 1 M HCl acid solution without di-imine-SB inhibitor, (B) sample immersed in 1 M HCl acid solution with (1 × 10–3 M) of di-imine-SB inhibitor.
The proposed di-imine-SB inhibition mechanism
The di-imine-SB "Schiff base" investigated herein is structurally featured with imine (–CH = N–) functionals, delocalized aromatic π electrons in addition to –N(Me)2 moieties that may interact with X65-steel surface. Firstly, few nucleophiles active centers (N+) get protonated into the acidic medium (1 M HCl) and electrostatically interact with the accumulated chloride (Cl−) ions nearby the metal-solution interface, therefore represent physical adsorption. After partial metal dissolution, the unoccupied d-orbitals that accept(s) lone-pair electrons; thus, gets coordinated with nitrogen through imine functional group(s), suggests chemical adsorption. Nevertheless, the expected back donation results from π electrons delocalization’s/interaction’s with X65-steel. The current results support mixed physical/chemical adsorption (Fig. 14) which found consistent with other molecules containing benzylidene and imine groups in addition to hetero-atoms92–94.
Figure 14.
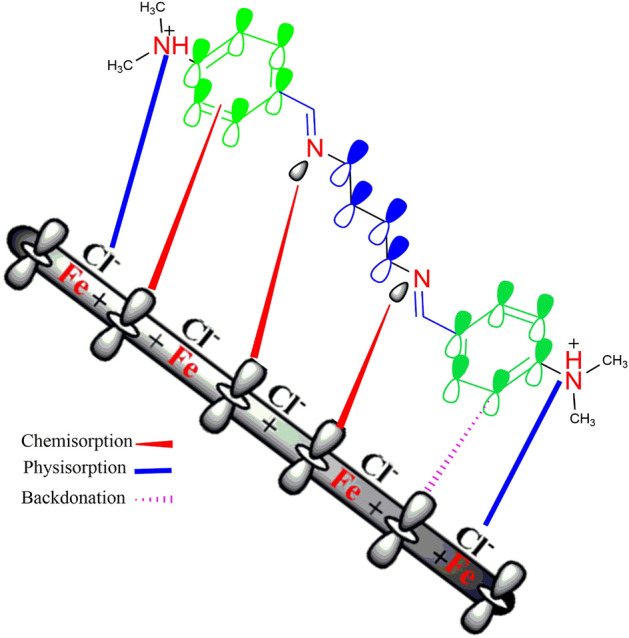
Suggested adsorption model of the prepared di-imine-SB inhibitor over the X-65 steel.
Conclusion
The di-imine-SB compound has enriched with electron donating groups that support its role as X65-steel corrosion inhibitor. It decreases the weight loss of X65-steel to 0.0649 g with inhibition efficiency 91.75% at 298 K. Adsorption behavior of di-imine-SB followed Langmuir adsorption model. value (− 35.605 kJ mol−1) indicated the physicochemical adsorption of di-imine-SB on the X65-steel surface; however, chemisorption process is a bit dominant. di-imine-SB dropped the Icorr of X65-steel from 800.45 µA cm−2 to 79.83 µA cm−2 as it shielded the active centers of X65-steel away from HCl offensive action. Also, di-imine-SB compound decreased the capacitance of the double layer at the HCl/X65-steel interface to 0.1672 µF cm−2 due to the higher resistance of protective layer formed at HCl/X65-steel interface. Thermodynamic parameters of X65-steel reaction confirmed the protective effect of di-imine-SB compound. SEM analysis indicated the smoothness appearance of X65-steel surface after adding 1 mM of di-imine-SB, and this in agreement with the experimental data. The experimental results were supported by DFT and MC simulation calculations, which also provided deep insight into the corrosion inhibition process.
Supplementary Information
Acknowledgements
The authors are grateful to the agreement of STDF and EKB with Springer Nature to fund open access publications.
Author contributions
A.N.: Investigation, Methodology, Data curation, Writing Original Draft; N.M.E.B.: Supervision, Investigation, Methodology, Data curation, Formal Analysis, writing original draft; M.A.M.: Supervision, Investigation, Validation, Writing-review; H.M.A.: Supervision, Validation, Data curation, initial writing; T.A.M.: Supervision, Investigation, Methodology, Validation, Formal Analysis, Data curation, Writing-review & editing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analyzed during this study are included in this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
N. M. EL Basiony, Email: n.elbasiony@skku.edu, Email: nasser_elbasuny@epri.sci.eg
Tarek A. Mohamed, Email: tarek_ama@hotmail.com, Email: tarek_ama@azhar.edu.eg
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-37321-8.
References
- 1.Ahamad I, Prasad R, Quraishi M. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010;52:1472–1481. doi: 10.1016/j.corsci.2010.01.015. [DOI] [Google Scholar]
- 2.Chauhan L, Gunasekaran G. Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros. Sci. 2007;49:1143–1161. doi: 10.1016/j.corsci.2006.08.012. [DOI] [Google Scholar]
- 3.Solmaz R. Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5 M HCl solution. Corros. Sci. 2014;81:75–84. doi: 10.1016/j.corsci.2013.12.006. [DOI] [Google Scholar]
- 4.Popova A, Sokolova E, Raicheva S, Christov M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003;45:33–58. doi: 10.1016/S0010-938X(02)00072-0. [DOI] [Google Scholar]
- 5.Kumar S, Kumar M, Handa A. Combating hot corrosion of boiler tubes: A study. Eng. Fail. Anal. 2018;94:379–395. doi: 10.1016/j.engfailanal.2018.08.004. [DOI] [Google Scholar]
- 6.Saha SK, Murmu M, Murmu NC, Banerjee P. Benzothiazolylhydrazine azomethine derivatives for efficient corrosion inhibition of mild steel in acidic environment: Integrated experimental and density functional theory cum molecular dynamics simulation approach. J. Mol. Liq. 2022;364:120033. doi: 10.1016/j.molliq.2022.120033. [DOI] [Google Scholar]
- 7.Abiola OK, Otaigbe J, Kio O. Gossipium hirsutum L. extracts as green corrosion inhibitor for aluminum in NaOH solution. Corros. Sci. 2009;51:1879–1881. doi: 10.1016/j.corsci.2009.04.016. [DOI] [Google Scholar]
- 8.Abboud Y, et al. The inhibition of mild steel corrosion in acidic medium by 2, 2′-bis (benzimidazole) Appl. Surf. Sci. 2006;252:8178–8184. doi: 10.1016/j.apsusc.2005.10.060. [DOI] [Google Scholar]
- 9.Gopiraman M, Selvakumaran N, Kesavan D, Karvembu R. Adsorption and corrosion inhibition behaviour of N-(phenylcarbamothioyl) benzamide on mild steel in acidic medium. Prog. Org. Coat. 2012;73:104–111. doi: 10.1016/j.porgcoat.2011.09.006. [DOI] [Google Scholar]
- 10.Lashgari M, Arshadi M-R, Miandari S. The enhancing power of iodide on corrosion prevention of mild steel in the presence of a synthetic-soluble Schiff-base: Electrochemical and surface analyses. Electrochim. Acta. 2010;55:6058–6063. doi: 10.1016/j.electacta.2010.05.066. [DOI] [Google Scholar]
- 11.Issaadi S, et al. Novel thiophene symmetrical Schiff base compounds as corrosion inhibitor for mild steel in acidic media. Corros. Sci. 2011;53:1484–1488. doi: 10.1016/j.corsci.2011.01.022. [DOI] [Google Scholar]
- 12.Elsaeed S, et al. Corrosion and hydrogen evolution rate control for X-65 carbon steel based on chitosan polymeric ionic liquids: Experimental and quantum chemical studies. RSC Adv. 2018;8:37891–37904. doi: 10.1039/C8RA05444D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha SK, Murmu M, Murmu NC, Banerjee P. Synthesis, characterization and theoretical exploration of pyrene based Schiff base molecules as corrosion inhibitor. J. Mol. Struct. 2021;1245:131098. doi: 10.1016/j.molstruc.2021.131098. [DOI] [Google Scholar]
- 14.Mohamed H, Farag A, Badran B. Friendly to environment heterocyclic adducts as corrosion inhibitors for steel in water-borne paints. J. Appl. Polym. Sci. 2010;117:1270–1278. [Google Scholar]
- 15.Şafak S, Duran B, Yurt A, Türkoğlu G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012;54:251–259. doi: 10.1016/j.corsci.2011.09.026. [DOI] [Google Scholar]
- 16.Shabani-Nooshabadi M, Behpour M, Razavi FS, Hamadanian M, Nejadshafiee V. Study of N-benzylidene derivatives synthesized as corrosion inhibitors for copper in HCl solution. RSC Adv. 2015;5:23357–23366. doi: 10.1039/C5RA00561B. [DOI] [Google Scholar]
- 17.Al-Taweel S, Al-Janabi K, Luaibi H, Al-Amiery A, Gaaz T. Evaluation and characterization of the symbiotic effect of benzylidene derivative with titanium dioxide nanoparticles on the inhibition of the chemical corrosion of mild steel. Int. J. Corros. Scale Inhib. 2019;8:1149–1169. [Google Scholar]
- 18.Al-Bghdadi, S. B., Hanoon, M. M., Odah, J. F., Shaker, L. M. & Al-Amiery, A. A. Multidisciplinary Digital Publishing Institute Proceedings 27.
- 19.Belghiti M, et al. Understanding the adsorption of newly Benzylidene-aniline derivatives as a corrosion inhibitor for carbon steel in hydrochloric acid solution: Experimental, DFT and molecular dynamic simulation studies. Arab. J. Chem. 2020;13:1499–1519. doi: 10.1016/j.arabjc.2017.12.003. [DOI] [Google Scholar]
- 20.Nandini S, Ronald N, Adimule SP, Krishnamurthy P. Anticorrosive effects of derivatives of 4-{[4-(dimethylamino) benzylidene] amino}-1, 2, 4-triazole on 316 stainless steel in HCl medium: Experimental and computational study. J. Fail. Anal. Prev. 2021;21:1057–1076. doi: 10.1007/s11668-021-01149-z. [DOI] [Google Scholar]
- 21.El-Lateef HMA, El-Dabea T, Khalaf MM, Abu-Dief AM. Innovation of imine metal chelates as corrosion inhibitors at different media: A collective study. Int. J. Mol. Sci. 2022;23:9360. doi: 10.3390/ijms23169360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd El-Lateef HM, Tantawy AH. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016;6:8681–8700. doi: 10.1039/C5RA21626E. [DOI] [Google Scholar]
- 23.Mohamed TA, Abdu AN, Migahed M. Raman and infrared spectral analysis, normal coordinate analysis, DFT calculations of novel Schiff base containing di-imine moieties. Egypt. J. Chem. 2022;2022:7143. [Google Scholar]
- 24.Obot I, Obi-Egbedi N. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2010;52:198–204. doi: 10.1016/j.corsci.2009.09.002. [DOI] [Google Scholar]
- 25.El-Tabei A, El-Tabey AE, El Basiony N. Newly imine-azo dicationic amphiphilic for corrosion and sulfate-reducing bacteria inhibition in petroleum processes: Laboratory and theoretical studies. Appl. Surf. Sci. 2021;573:151531. doi: 10.1016/j.apsusc.2021.151531. [DOI] [Google Scholar]
- 26.Basiony NE, Elgendy A, Nady H, Migahed M, Zaki E. Adsorption characteristics and inhibition effect of two Schiff base compounds on corrosion of mild steel in 0.5 M HCl solution: experimental, DFT studies, and Monte Carlo simulation. RSC Adv. 2019;9(9):10473–10485. doi: 10.1039/C9RA00397E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedir AG, Abd El-raouf M, Abdel-Mawgoud S, Negm NA, El Basiony NM. Corrosion inhibition of carbon steel in hydrochloric acid solution using ethoxylated nonionic surfactants based on Schiff base: Electrochemical and computational investigations. ACS Omega. 2021;6:4300–4312. doi: 10.1021/acsomega.0c05476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Basiony N, Badr EE, Baker SA, El-Tabei A. Experimental and theoretical (DFT&MC) studies for the adsorption of the synthesized Gemini cationic surfactant based on hydrazide moiety as X-65 steel acid corrosion inhibitor. Appl. Surf. Sci. 2021;539:148246. doi: 10.1016/j.apsusc.2020.148246. [DOI] [Google Scholar]
- 29.Radwan AB, Sliem MH, Okonkwo PC, Shibl MF, Abdullah AM. Corrosion inhibition of API X120 steel in a highly aggressive medium using stearamidopropyl dimethylamine. J. Mol. Liq. 2017;236:220–231. doi: 10.1016/j.molliq.2017.03.116. [DOI] [Google Scholar]
- 30.Benali O, Larabi L, Traisnel M, Gengembre L, Harek Y. Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption on carbon steel in 1 M HClO4. Appl. Surf. Sci. 2007;253:6130–6139. doi: 10.1016/j.apsusc.2007.01.075. [DOI] [Google Scholar]
- 31.Schorr M, Yahalom J. The significance of the energy of activation for the dissolution reaction of metal in acids. Corros. Sci. 1972;12:867–868. doi: 10.1016/S0010-938X(72)80015-5. [DOI] [Google Scholar]
- 32.Abboud Y, et al. A novel azo dye, 8-quinolinol-5-azoantipyrine as corrosion inhibitor for mild steel in acidic media. Desalination. 2009;237:175–189. doi: 10.1016/j.desal.2007.12.031. [DOI] [Google Scholar]
- 33.Lipkowski, J. & Ross, P. N. Adsorption of Molecules at Metal Electrodes. (VCH New York, 1992).
- 34.Bentrah H, Rahali Y, Chala A. Gum Arabic as an eco-friendly inhibitor for API 5L X42 pipeline steel in HCl medium. Corros. Sci. 2014;82:426–431. doi: 10.1016/j.corsci.2013.12.018. [DOI] [Google Scholar]
- 35.Saha SK, Dutta A, Ghosh P, Sukul D, Banerjee P. Adsorption and corrosion inhibition effect of Schiff base molecules on the mild steel surface in 1 M HCl medium: A combined experimental and theoretical approach. Phys. Chem. Chem. Phys. 2015;17:5679–5690. doi: 10.1039/C4CP05614K. [DOI] [PubMed] [Google Scholar]
- 36.Saha SK, Dutta A, Ghosh P, Sukul D, Banerjee P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach. Phys. Chem. Chem. Phys. 2016;18:17898–17911. doi: 10.1039/C6CP01993E. [DOI] [PubMed] [Google Scholar]
- 37.Bentiss F, et al. Understanding the adsorption of 4H–1, 2, 4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl. Surf. Sci. 2007;253:3696–3704. doi: 10.1016/j.apsusc.2006.08.001. [DOI] [Google Scholar]
- 38.Zhang S, Tao Z, Liao S, Wu F. Substitutional adsorption isotherms and corrosion inhibitive properties of some oxadiazol-triazole derivative in acidic solution. Corros. Sci. 2010;52:3126–3132. doi: 10.1016/j.corsci.2010.05.035. [DOI] [Google Scholar]
- 39.Ahamad I, Prasad R, Quraishi M. Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros. Sci. 2010;52:933–942. doi: 10.1016/j.corsci.2009.11.016. [DOI] [Google Scholar]
- 40.Quraishi M, Sharma HK. 4-Amino-3-butyl-5-mercapto-1, 2, 4-triazole: A new corrosion inhibitor for mild steel in sulphuric acid. Mater. Chem. Phys. 2003;78:18–21. doi: 10.1016/S0254-0584(02)00313-9. [DOI] [Google Scholar]
- 41.Migahed M, Nasser A, Elfeky H, El-Rabiei M. The synthesis and characterization of benzotriazole-based cationic surfactants and the evaluation of their corrosion inhibition efficiency on copper in seawater. RSC Adv. 2019;9:27069–27082. doi: 10.1039/C9RA04461B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannan P, Karthikeyan J, Murugan P, Rao TS, Rajendran N. Corrosion inhibition effect of novel methyl benzimidazolium ionic liquid for carbon steel in HCl medium. J. Mol. Liq. 2016;221:368–380. doi: 10.1016/j.molliq.2016.04.130. [DOI] [Google Scholar]
- 43.Li X, Deng S, Fu H. Benzyltrimethylammonium iodide as a corrosion inhibitor for steel in phosphoric acid produced by dihydrate wet method process. Corros. Sci. 2011;53:664–670. doi: 10.1016/j.corsci.2010.10.013. [DOI] [Google Scholar]
- 44.Moretti G, Guidi F, Fabris F. Corrosion inhibition of the mild steel in 0.5 M HCl by 2-butyl-hexahydropyrrolo [1, 2-b][1, 2] oxazole. Corros. Sci. 2013;76:206–218. doi: 10.1016/j.corsci.2013.06.044. [DOI] [Google Scholar]
- 45.Hegazy M, Rashwan S, Kamel M, El Kotb M. Synthesis, surface properties and inhibition behavior of novel cationic gemini surfactant for corrosion of carbon steel tubes in acidic solution. J. Mol. Liq. 2015;211:126–134. doi: 10.1016/j.molliq.2015.06.051. [DOI] [Google Scholar]
- 46.Abd El Wanees S, Alahmdi MI, Abd El Azzem M, Ahmed HE. 4, 6-Dimethyl-2-oxo-1, 2-dihydropyridine-3-carboxylic acid as an inhibitor towards the corrosion of C-steel in acetic acid. Int. J. Electrochem. Sci. 2016;11:e3466. [Google Scholar]
- 47.Musa AY, et al. A comparative study of the corrosion inhibition of mild steel in sulphuric acid by 4, 4-dimethyloxazolidine-2-thione. Corros. Sci. 2009;51:2393–2399. doi: 10.1016/j.corsci.2009.06.024. [DOI] [Google Scholar]
- 48.Hegazy M, Samy R, Labena A, Wadaan MA, Hozzein WN. 4, 4′-(((1E, 5E)-pentane-1, 5-diylidene) bis (azanylylidene)) bis (1-dodecylpyridin-1-ium) bromide as a novel corrosion inhibitor in an acidic solution (part I) Mater. Sci. Eng. C. 2020;110:110673. doi: 10.1016/j.msec.2020.110673. [DOI] [PubMed] [Google Scholar]
- 49.Abdallah M, Eltass HM, Hegazy M, Ahmed H. Adsorption and inhibition effect of novel cationic surfactant for pipelines carbon steel in acidic solution. Prot. Met. Phys. Chem. Surf. 2016;52:721–730. doi: 10.1134/S207020511604002X. [DOI] [Google Scholar]
- 50.Bouklah M, Hammouti B, Lagrenee M, Bentiss F. Thermodynamic properties of 2, 5-bis (4-methoxyphenyl)-1, 3, 4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros. Sci. 2006;48:2831–2842. doi: 10.1016/j.corsci.2005.08.019. [DOI] [Google Scholar]
- 51.Bayol E, Gürten T, Gürten AA, Erbil MJ. Interactions of some Schiff base compounds with mild steel surface in hydrochloric acid solution. Mater. Chem. Phys. 2008;112:624–630. doi: 10.1016/j.matchemphys.2008.06.012. [DOI] [Google Scholar]
- 52.Zhang Q, Hua Y. Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim. Acta. 2009;54:1881–1887. doi: 10.1016/j.electacta.2008.10.025. [DOI] [Google Scholar]
- 53.Stern M, Geary AL. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957;104:56. doi: 10.1149/1.2428496. [DOI] [Google Scholar]
- 54.Matthews DB. The Stern-Geary and related methods for determining corrosion rates. Aust. J. Chem. 1975;28:243–251. doi: 10.1071/CH9750243. [DOI] [Google Scholar]
- 55.Migahed M, Attia A, Habib R. Study on the efficiency of some amine derivatives as corrosion and scale inhibitors in cooling water systems. RSC Adv. 2015;5:57254–57262. doi: 10.1039/C5RA11082C. [DOI] [Google Scholar]
- 56.Lalitha A, Ramesh S, Rajeswari S. Surface protection of copper in acid medium by azoles and surfactants. Electrochim. Acta. 2005;51:47–55. doi: 10.1016/j.electacta.2005.04.003. [DOI] [Google Scholar]
- 57.Cao P, Gu R, Tian Z. Electrochemical and surface-enhanced Raman spectroscopy studies on inhibition of iron corrosion by benzotriazole. ACS Publ. 2002;18:7609–7615. [Google Scholar]
- 58.Hashim NZN, Kassim K, Zaki HM, Alharthi AI, Embong Z. XPS and DFT investigations of corrosion inhibition of substituted benzylidene Schiff bases on mild steel in hydrochloric acid. Appl. Surf. Sci. 2019;476:861–877. doi: 10.1016/j.apsusc.2019.01.149. [DOI] [Google Scholar]
- 59.Bedair M, et al. Benzidine-based Schiff base compounds for employing as corrosion inhibitors for carbon steel in 1.0 M HCl aqueous media by chemical, electrochemical and computational methods. J. Mol. Liq. 2020;317:114015. doi: 10.1016/j.molliq.2020.114015. [DOI] [Google Scholar]
- 60.Meng Y, et al. Inhibition of mild steel corrosion in hydrochloric acid using two novel pyridine Schiff base derivatives: A comparative study of experimental and theoretical results. RSC Adv. 2017;7:43014–43029. doi: 10.1039/C7RA08170G. [DOI] [Google Scholar]
- 61.AltunbaşŞahin E, Tezcan F, Solmaz R, Kardaş GJ. Inhibitive effect of 4-amino-N-benzylidene-benzamide Schiff base on mild steel corrosion in HCl solution. J. Adhes. Sci. Technol. 2020;34:135–152. doi: 10.1080/01694243.2019.1662202. [DOI] [Google Scholar]
- 62.Liang C, et al. Synthesis of 2-aminofluorene bis-Schiff base and corrosion inhibition performance for carbon steel in HCl. J. Mol. Liq. 2019;277:330–340. doi: 10.1016/j.molliq.2018.12.095. [DOI] [Google Scholar]
- 63.Mourya P, Banerjee S, Singh M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014;85:352–363. doi: 10.1016/j.corsci.2014.04.036. [DOI] [Google Scholar]
- 64.Saha SK, Park YJ, Kim JW, Cho SO. Self-organized honeycomb-like nanoporous oxide layer for corrosion protection of type 304 stainless steel in an artificial seawater medium. J. Mol. Liq. 2019;296:111823. doi: 10.1016/j.molliq.2019.111823. [DOI] [Google Scholar]
- 65.Yang Z, et al. Structure of a novel Benzyl Quinolinium Chloride derivative and its effective corrosion inhibition in 15 wt.% hydrochloric acid. Corros. Sci. 2015;99:281–294. doi: 10.1016/j.corsci.2015.07.023. [DOI] [Google Scholar]
- 66.Labjar N, et al. Corrosion inhibition of carbon steel and antibacterial properties of aminotris-(methylenephosphonic) acid. Mater. Chem. Phys. 2010;119:330–336. doi: 10.1016/j.matchemphys.2009.09.006. [DOI] [Google Scholar]
- 67.Yadav M, Sharma U, Yadav PJ. Isatin compounds as corrosion inhibitors for N80 steel in 15% HCl. Egypt. J. Pet. 2013;22:335–344. doi: 10.1016/j.ejpe.2013.10.001. [DOI] [Google Scholar]
- 68.Özcan M, Dehri İ, Erbil M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004;236:155–164. doi: 10.1016/j.apsusc.2004.04.017. [DOI] [Google Scholar]
- 69.Solmaz R, Kardaş G, Çulha M, Yazıcı B, Erbil M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta. 2008;53:5941–5952. doi: 10.1016/j.electacta.2008.03.055. [DOI] [Google Scholar]
- 70.Solmaz R. Investigation of the inhibition effect of 5-((E)-4-phenylbuta-1, 3-dienylideneamino)-1, 3, 4-thiadiazole-2-thiol Schiff base on mild steel corrosion in hydrochloric acid. Corros. Sci. 2010;52:3321–3330. doi: 10.1016/j.corsci.2010.06.001. [DOI] [Google Scholar]
- 71.Abd El-Lateef HM, Abu-Dief AM, Mohamed MA. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017;1130:522–542. doi: 10.1016/j.molstruc.2016.10.078. [DOI] [Google Scholar]
- 72.Wang X, Yang H, Wang F. cationic gemini-surfactant as effective inhibitor for mild steel in HCl solutions. Corros. Sci. 2010;52:1268–1276. doi: 10.1016/j.corsci.2009.12.018. [DOI] [Google Scholar]
- 73.El Basiony N, et al. Synthesis, characterization, experimental and theoretical calculations (DFT and MC) of ethoxylated aminothiazole as inhibitor for X65 steel corrosion in highly aggressive acidic media. J. Mol. Liq. 2020;297:111940. doi: 10.1016/j.molliq.2019.111940. [DOI] [Google Scholar]
- 74.Abd-Elaal AA, Elbasiony N, Shaban SM, Zaki E. Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J. Mol. Liq. 2018;249:304–317. doi: 10.1016/j.molliq.2017.11.052. [DOI] [Google Scholar]
- 75.Li S, et al. Some aspects of quantum chemical calculations for the study of Schiff base corrosion inhibitors on copper in NaCl solutions. Corros. Sci. 1999;41:1769–1782. doi: 10.1016/S0010-938X(99)00014-1. [DOI] [Google Scholar]
- 76.Xia G, et al. Synergic effect of methyl acrylate and N-cetylpyridinium bromide in N-cetyl-3-(2-methoxycarbonylvinyl) pyridinium bromide molecule for X70 steel protection. Corros. Sci. 2015;94:224–236. doi: 10.1016/j.corsci.2015.02.005. [DOI] [Google Scholar]
- 77.Saha SK, Murmu M, Murmu NC, Obot I, Banerjee P. Molecular level insights for the corrosion inhibition effectiveness of three amine derivatives on the carbon steel surface in the adverse medium: A combined density functional theory and molecular dynamics simulation study. Surf .Interfaces. 2018;10:65–73. doi: 10.1016/j.surfin.2017.11.007. [DOI] [Google Scholar]
- 78.Khalil N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta. 2003;48:2635–2640. doi: 10.1016/S0013-4686(03)00307-4. [DOI] [Google Scholar]
- 79.Saha SK, Ghosh P, Hens A, Murmu NC, Banerjee P. Density functional theory and molecular dynamics simulation study on corrosion inhibition performance of mild steel by mercapto-quinoline Schiff base corrosion inhibitor. Physica E. 2015;66:332–341. doi: 10.1016/j.physe.2014.10.035. [DOI] [Google Scholar]
- 80.El Adnani Z, et al. DFT theoretical study of 7-R-3methylquinoxalin-2 (1H)-thiones (RH; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci. 2013;68:223–230. doi: 10.1016/j.corsci.2012.11.020. [DOI] [Google Scholar]
- 81.Bedair M, Fouda A, Ismail M, Mostafa A. Inhibitive effect of bithiophene carbonitrile derivatives on carbon steel corrosion in 1 M HCl solution: experimental and theoretical approaches. Ionics. 2019;25:2913–2933. doi: 10.1007/s11581-018-2811-0. [DOI] [Google Scholar]
- 82.Obot I, Obi-Egbedi N, Ebenso E, Afolabi A, Oguzie E. Experimental, quantum chemical calculations, and molecular dynamic simulations insight into the corrosion inhibition properties of 2-(6-methylpyridin-2-yl) oxazolo [5, 4-f][1, 10] phenanthroline on mild steel. Res. Chem. Intermed. 2013;39:1927–1948. doi: 10.1007/s11164-012-0726-3. [DOI] [Google Scholar]
- 83.Khaled K. Monte Carlo simulations of corrosion inhibition of mild steel in 0.5 M sulphuric acid by some green corrosion inhibitors. J. Solid State Electrochem. 2009;13:1743–1756. doi: 10.1007/s10008-009-0845-y. [DOI] [Google Scholar]
- 84.Salim M, Azab M, Abo-Riya MA, Abd-El-Raouf M, Basiony NE. Controlling C-steel dissolution in 1 M HCl solution using newly synthesized ρ-substituted imine derivatives: Theoretical (DFT and MCs) and experimental investigations. J. Mol. Struct. 2023;1274:134357. doi: 10.1016/j.molstruc.2022.134357. [DOI] [Google Scholar]
- 85.Verma C, Quraishi M, Rhee KY. Electronic effect vs Molecular size effect: Experimental and computational based designing of potential corrosion inhibitors. Chem. Eng. J. 2022;430:132645. doi: 10.1016/j.cej.2021.132645. [DOI] [Google Scholar]
- 86.El Basiony N, Tawfik EH, El-raouf MA, Fadda AA, Waly MM. Synthesis, characterization, theoretical calculations (DFT and MC), and experimental of different substituted pyridine derivatives as corrosion mitigation for X-65 steel corrosion in 1M HCl. J. Mol. Struct. 2021;1231:129999. doi: 10.1016/j.molstruc.2021.129999. [DOI] [Google Scholar]
- 87.Singh A, Ansari K, Quraishi M, Lgaz H, Lin Y. Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: Theoretical and experimental approaches. J. Alloy. Compd. 2018;762:347–362. doi: 10.1016/j.jallcom.2018.05.236. [DOI] [Google Scholar]
- 88.Zeino A, Abdulazeez I, Khaled M, Jawich MW, Obot IB. Mechanistic study of polyaspartic acid (PASP) as eco-friendly corrosion inhibitor on mild steel in 3% NaCl aerated solution. J. Mol. Liq. 2018;250:50–62. doi: 10.1016/j.molliq.2017.11.160. [DOI] [Google Scholar]
- 89.Olasunkanmi LO, Obot IB, Ebenso EE. Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4, 5-dihydropyrazol-3-yl] phenyl} methanesulfonamides on mild steel in 1 M HCl: Experimental and theoretical studies. RSC Adv. 2016;6:86782–86797. doi: 10.1039/C6RA11373G. [DOI] [Google Scholar]
- 90.Obot IB, Ebenso EE, Kabanda MM. Metronidazole as environmentally safe corrosion inhibitor for mild steel in 0.5 M HCl: experimental and theoretical investigation. J. Environ. Chem. Eng. 2013;1:431–439. doi: 10.1016/j.jece.2013.06.007. [DOI] [Google Scholar]
- 91.Bedir AG, Abd El-raouf M, Abdel-Mawgoud S, Negm NA, El Basiony N. Corrosion inhibition of carbon steel in hydrochloric acid solution using ethoxylated nonionic surfactants based on Schiff base: Electrochemical and computational investigations. ACS Omega. 2021;6:4300–4312. doi: 10.1021/acsomega.0c05476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh A, et al. An impending inhibitor useful for the oil and gas production industry: Weight loss, electrochemical, surface and quantum chemical calculation. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-017-13877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bedair MA, Abuelela AM, Zoghaib WM, Mohamed TA. Molecular structure, tautomer's, reactivity and inhibition studies on 6-Methyl-2-thiouracil for mild steel corrosion in aqueous HCl (1.00 M): Experimental and Theoretical Studies. J. Mol. Struct. 2021;1244:130927. doi: 10.1016/j.molstruc.2021.130927. [DOI] [Google Scholar]
- 94.Abuelela AM, Bedair MA, Zoghaib WM, Wilson LD, Mohamed TA. Molecular structure and mild steel/HCl corrosion inhibition of 4, 5-Dicyanoimidazole: Vibrational, electrochemical and quantum mechanical calculations. J. Mol. Struct. 2021;1230:129647. doi: 10.1016/j.molstruc.2020.129647. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.