Abstract
Curcumin, a natural phytochemical isolated from tumeric roots, represents a candidate for prevention and therapy of colorectal cancer/CRC. However, the exact mechanism of action and the downstream mediators of curcumin’s tumor suppressive effects have remained largely unknown. Here we used a genetic approach to determine the role of the p53/miR-34 pathway as mediator of the effects of curcumin. Three isogenic CRC cell lines rendered deficient for the p53, miR-34a and/or miR-34b/c genes were exposed to curcumin and subjected to cell biological analyses. siRNA-mediated inhibition and ectopic expression of NRF2, as well as Western blot, qPCR and qChIP analyses of its target genes were performed. CRC cells were i.v. injected into NOD/SCID mice and lung-metastases formation was determined by longitudinal, non-invasive imaging. In CRC cells curcumin induced apoptosis and senescence, and suppressed migration and invasion in a p53-independent manner. Curcumin activated the KEAP1/NRF2/ARE pathway by inducing ROS. Notably, curcumin induced miR-34a and miR-34b/c expression in a ROS/NRF2-dependent and p53-independent manner. NRF2 directly induced miR-34a and miR-34b/c via occupying multiple ARE motifs in their promoter regions. Curcumin reverted repression of miR-34a and miR-34b/c induced by IL6 and hypoxia. Deletion of miR-34a and miR-34b/c significantly reduced curcumin-induced apoptosis and senescence, and prevented the inhibition of migration and invasion by curcumin or ectopic NRF2. In CRC cells curcumin induced MET and prevented the formation of lung-metastases in mice in a miR-34a-dependent manner. In addition, we found that curcumin may enhance the therapeutic effects of 5-FU on CRC cells deficient for p53 and miR-34a/b/c. Activation of the KEAP1/NRF2/miR-34a/b/c axis mediates the tumor suppressive activity of curcumin and suggests a new approach for activating miR-34 genes in tumors for therapeutic purposes.
Subject terms: Tumour-suppressor proteins, Translational research
Introduction
Colorectal cancer (CRC) is the 2nd most lethal cancer causing more than 900,000 deaths world-wide every year [1]. It has been estimated that over half of the Western population develops benign adenomatous polyps during its lifetime, and that 10% of these tumors proceed to malignant colorectal carcinomas [2]. Unfortunately, despite recent advances in currently available therapies, the 5-year survival rate of patients with metastatic CRC remains poor (<15%) [3]. Therefore, new approaches and compounds for improved prevention and therapy of CRC are clearly needed.
Curcumin is a polyphenol derived from the rhizome of the turmeric plant (Curcuma longa) and has been a popular food additive in Eastern cuisine. In addition, it has been used for centuries in traditional Chinese and ayurvedic medicine. Notably, curcumin has potential as a preventive and therapeutic agent for CRC, as it suppresses many hallmarks of cancer cells and exhibited promising effects in preclinical and clinical studies [4–6]. For example, the addition of daily oral curcumin to FOLFOX chemotherapy (folic acid/5-fluorouracil/oxaliplatin) significantly prolonged the progression-free survival and overall survival) of patients with metastatic CRC [7]. Moreover, curcumin showed improved erytrocyte sedimentation rate and C-reactive protein/CRP serum levels of in stage 3 CRC patients and improved their quality of life [8]. Daily oral curcumin given to patients with advanced colorectal cancer refractory to standard chemotherapy, resulted in stable disease in 5 of 15 individuals within 4 months of follow-up evaluation [9]. When curcumin was given in combination with mesalamine it resulted in remissions of patients with ulcerative colitis/UC [10, 11]. Furthermore, in familial adenomatous polyposis/FAP patients a combination of curcumin and quercetin reduced the number and size of ileal and rectal adenomas without appreciable toxicity [12]. As the clinical studies only included small numbers of patients, larger, targeted and prospective clinical trials are required to establish curcumin in clinical practice.
Curcumin was shown to affect the expression of non-coding RNAs in CRC cells [13]. Also miR-34a, a p53-inducible microRNA with tumor-suppressive capacities [14, 15], was induced by exposure to curcumin. However, it remained unclear whether this effect of curcumin was mediated by p53 activation. The miR-34a and miR-34b/c genes are down-regulated by p53 inactivation, epigenetic silencing or activation of HIF1A and STAT3, which directly suppress the miR-34a and miR-34b/c genes in the majority of CRCs [16–19]. Loss of miR-34a promotes colitis-associated colon cancer by activating the IL6-STAT3 pathway [20]. Furthermore, concomitant loss of p53 and miR-34a enhances colorectal cancer formation in mouse model of sporadic CRC and is associated with poor survival of CRC patients [21]. In addition, deletion of miR-34a in the ApcMin/+ mouse model of inherited colon cancer enhances the number and size of adenomas and shortens the life span of mice [22]. Therefore, down-regulation of miR-34a/b/c in CRCs does not only commonly occur during intestinal carcinogenesis, but is also causally involved in CRC formation. Taken together, these observations suggest that miR-34a and miR-34b/c have tumor suppressive properties and would therefore represent attractive mediators of tumor prevention and/or suppression elicited by curcumin.
Here we show that curcumin induces the expression of miR-34a and miR-34b/c independent of p53 by activating the KEAP1/NRF2/ARE pathway via the generation of reactive oxygen species/ROS. Furthermore, we demonstrate that the miR-34a/b/c genes are important mediators of the pro-apoptotic, senescence-inducing and metastasis-inhibiting effects of curcumin. Importantly, these effects of curcumin are independent of p53 and dominant over signals from the micro-environment that repress miR-34 expression, such as hypoxia. Therefore, the identified mechanisms provide an opportunity to activate miR-34a and miR-34b/c genes in tumor cells that display inactivation/down-regulation of the p53/miR-34 pathway.
Results
p53-independent effects of curcumin on CRC cells
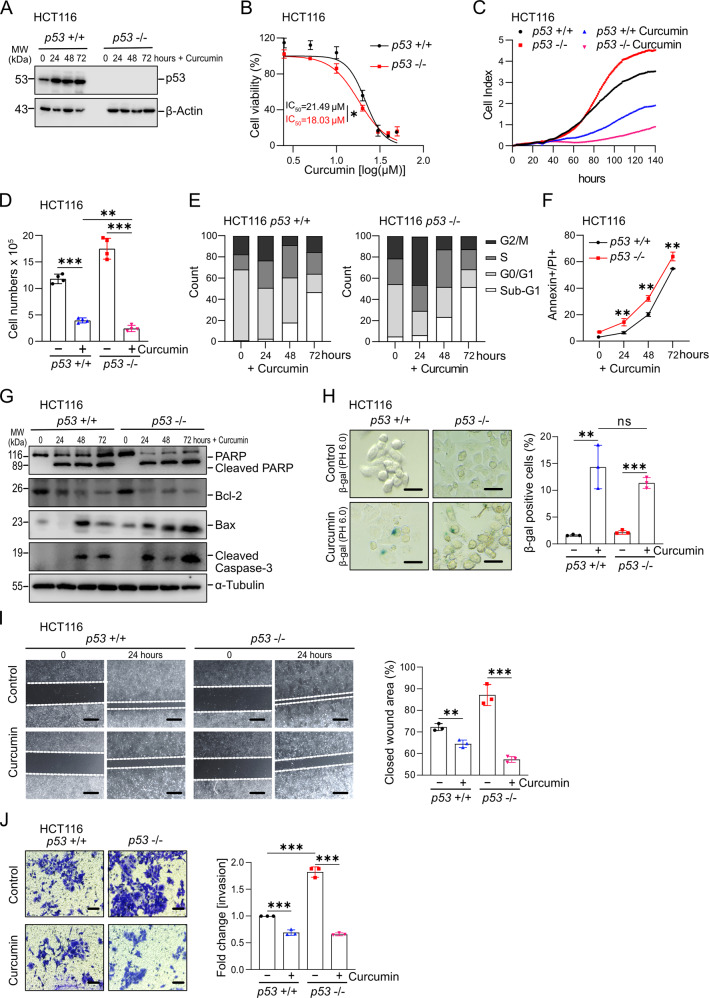
As shown previously [23], the amount of p53 protein increased after treatment of HCT116 cells with curcumin (Fig. 1A). In order to determine the relevance of p53 for the effects of curcumin on CRC cells, we treated HCT116 with wild-type p53 and isogenic HCT116 cells with homozygous deletion of p53 with increasing concentrations of curcumin for 48 hours. P53-proficient cells displayed an IC50 of 21.49 μM, whereas p53-deficient cells had an IC50 of 18.03 μM curcumin (Fig. 1B). Similar effects were observed in p53-proficient or p53-deficient RKO and SW48 CRC cell lines (Figs. S1A and 1D). A concentration of 15 μM curcumin, slightly below the IC50 value of p53-deficient cells, was chosen for the following experiments. Exposure to curcumin strongly reduced the proliferation of both p53-deficient and p53-proficient HCT116 cells as determined by impedance measurements (Fig. 1C) and cell number alterations were confirmed at the final time point (Fig. 1D). Similar p53-independent effects of curcumin were observed in the CRC cell lines RKO and SW48 (Figs. S1B, 1C, 1E, and 1F). Interestingly, the curcumin-induced decrease in viability and proliferation was more pronounced in p53-deficient cells. Therefore, these effects of curcumin on CRC cells are independent of p53, although p53 accumulates after treatment with curcumin.
Fig. 1. p53-independent effects of curcumin on CRC cells.
A Detection of p53 protein by Western blot analysis after treatment with curcumin. β-Actin served as a loading control. B Cell viability of HCT116 cells exposed to different concentrations of curcumin for 48 hours was determined by MTT assays. C Impedance of HCT116 cells treated with curcumin. D Determination of cell number at the final time point of the experiment shown in (C). E Cell cycle analysis using propidium iodide (PI) staining. F Analysis of apoptosis in HCT116 cells treated with curcumin determined by Annexin V FITC and propidium iodide staining. G The level of cleaved PARP, Bcl-2, Bax, and cleaved caspase-3 was analyzed by Western blot analysis after being treated with curcumin for the indicated periods in HCT116 cells. α-Tubulin served as a loading control. H Detection of senescent cells after exposure to curcumin for 48 hours determined by pH 6 β-gal staining. Scale bars: 100 μm. I Wound healing assay of HCT116 cells treated with curcumin for 24 hours (left panel). Results represent the mean (%) of wound closure (right panel). J Determination of invasion in a modified Boyden-chamber assay. Relative invasion of HCT116 cells treated with curcumin for 48 hours. Scale bars: 100 μm. In panels (B), (C), (F), (H), (I), and (J) (n = 3), and (D) (n = 4) mean values ± SD are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we analysed which processes may underlie the curcumin-mediated suppression of proliferation. Curcumin resulted in an increase of cells with sub-G1 DNA content, indicating increased apoptosis, irrespective of the p53 status (Fig. 1E). p53-deficient cells displayed more G2/M-arrest 24 hours after curcumin treatment, whereas cells accumulated more in G0/G1 in WT p53 cells. Annexin V/PI detection revealed a more pronounced increase in apoptosis in p53-deficient when compared to p53-proficient cells (Figs. 1F and S1G). These results were confirmed by detection of cleaved-PARP, cleaved Caspase-3, Bcl-2, and Bax proteins (Figs. 1G, S1H, and S1I): an increase in BAX and cleaved Caspase 3 was detected by 24 hours in p53-deficient cells, whereas p53-proficient cells showed a delayed induction of these proteins by 48 hours. In addition, curcumin induced senescence in HCT116 cells to a similar degree in p53-proficient and p53-deficient HCT116 cells as determined by detection of β-gal pH 6 (Fig. 1H). Finally, curcumin suppressed migration and invasion in p53-proficient and p53-deficient HCT116 cells, with the latter displaying increased basal levels of migration and invasion (Fig. 1I, J). Taken together, curcumin suppressed cell viability, proliferation, migration and invasion, whereas it induced apoptosis and senescence of CRC cells in a p53-independent manner.
Curcumin activates NRF2 via ROS in CRC cells
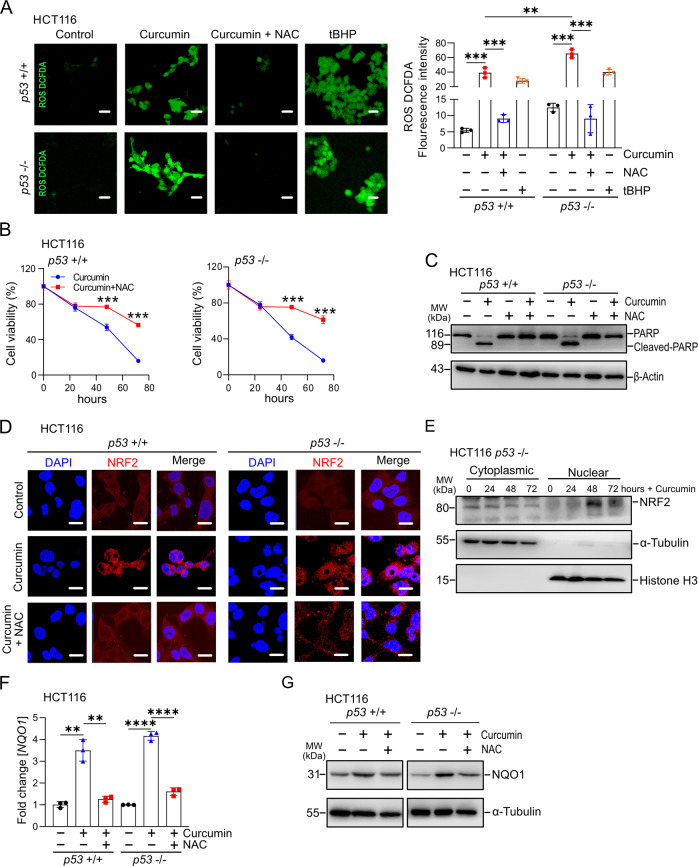
Next, we aimed to determine the mechanism mediating the effects of curcumin on CRC cells. Although curcumin is mainly known for its anti-oxidative effects, it has also been shown to increase the production of reactive oxygen species/ROS [24]. Indeed, ROS levels of HCT116 cells were significantly increased when exposed to curcumin for 48 hours (Fig. 2A). The ROS-inducer tert-butyl hydroperoxide (tBHP) was used as a positive control. The effect of curcumin was suppressed by concomitant treatment with the antioxidant N-acetylcysteine (NAC) (Fig. 2A, left panel). Similar results were observed in RKO and SW48 cells (Figs. S2A and 2B, left panel). Interestingly, the levels of ROS were higher in p53-deficient HCT116 cells than in p53-proficient HCT116 cells, suggesting that p53 suppresses the production of ROS by curcumin (Fig. 2A, right panel). Similar results were obtained in RKO and SW48 cells (Figs. S2A and 2B, right panel). Notably, the negative effect of curcumin on cell viability was partially reversed by NAC in p53-proficient and p53-deficient HCT116 cells (Fig. 2B). Similar results were observed in RKO and SW48 cells (Figs. S2C and 2D). NAC completely suppressed cleavage of PARP and therefore apoptosis induced by curcumin (Fig. 2C). Similar results were observed in RKO and SW48 cells (Fig. S3B).
Fig. 2. Curcumin activates NRF2 via inducing ROS in CRC cells.
A Analysis of ROS formation in HCT116 cells treated as indicated, Left panel: representative IF pictures. Scale bar: 100 µM. Right panel: Quantification of fluorescence intensity. B Cell viability of HCT116 cells after treatment with curcumin and NAC for indicated periods was determined by MTT assays. C Apoptosis of HCT116 cells treated with curcumin and/or NAC for 48 h was determined by Western blot analysis of cleaved PARP protein levels. β-actin served as a loading control. D Immunofluorescence detection of NRF2 protein after treatment with DMSO, 15 μM curcumin, or 15 μM curcumin and 5 mM NAC for 48 hours. Nuclear DNA was detected by DAPI. Scale bar represents 20 µm. E Western blot analysis of NRF2 protein levels in cytoplasmic and nuclear cellular fractions after curcumin treatment. F qPCR analysis of NQO1 expression in HCT116 cells after treatment with DMSO, 15 μM curcumin, or 15 μM curcumin with 5 mM NAC for 48 hours. G Western blot analysis of NQO1 protein levels after treatment with DMSO, 15 μM curcumin, or 15 μM curcumin with 5 mM NAC for 48 hours. In panels A, B, and F mean values ± SD are shown (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The KEAP1/NRF2-pathway is a central mediator of the cellular response to oxidative stress [25]. Upon exposure to ROS the transcription factor NRF2 is released from Keap1 and translocates from the cytoplasm to the nucleus to activate the transcription of its target genes [26]. Indeed, curcumin induced translocation of NRF2 to the nucleus in p53-proficient and p53-deficient HCT116 cells (Fig. 2D). Similar results were observed in RKO and SW48 cells (Fig. S3A). After exposure to curcumin, the NRF2 protein was increased in the nuclear fraction of HCT116 cells, whereas it decreased in the cytoplasmic fraction (Fig. 2E). NRF2 mRNA expression was not affected by curcumin (Fig. S3C). NQO1 is a conserved target gene of NRF2 that is commonly used to monitor the activity of the NRF2 pathway [27]. Consistent with an activation of NRF2, expression of NQO1 mRNA and protein was increased upon curcumin treatment (Figs. 2F, G). Similar results were observed in RKO and SW48 cells (Fig. S3B). Importantly, the induction of nuclear translocation and activation of NRF2 was largely reversed by the inhibition of ROS with NAC (Figs. 2D, F, G and S3A).
Curcumin up-regulates miR-34a and miR-34b/c independent of p53
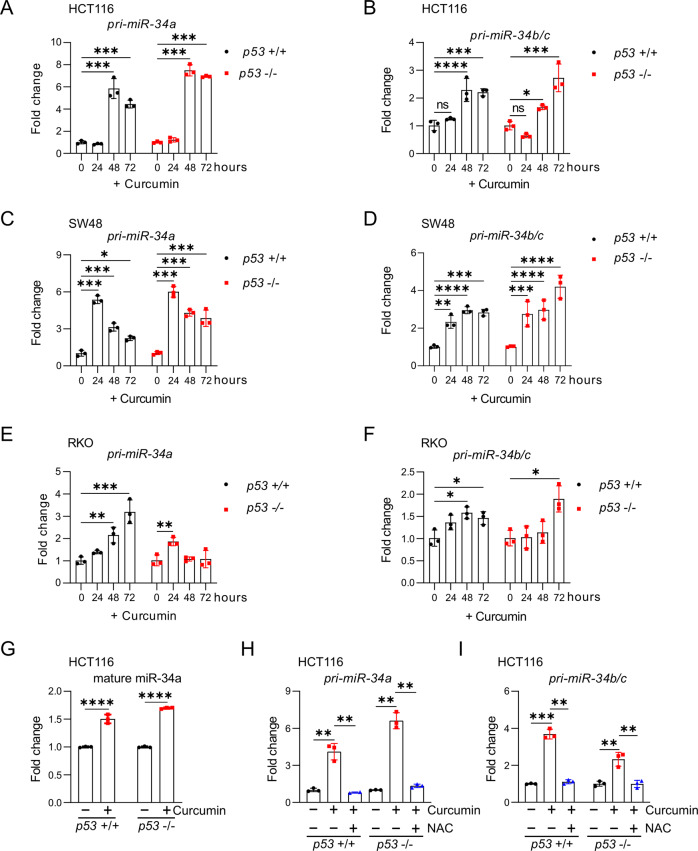
As indicated in the introduction, previously published observations indicating that miR-34a is induced by curcumin sparked our interest in curcumin. Here we determined whether p53 is required for the induction of miR-34 genes by curcumin in a set of p53-deficient, isogenic CRC cell lines. Interestingly, curcumin induced the expression of the primary pri-miR-34a and pri-miR-34b/c transcripts in HCT116 cells independent of their p53 status (Figs. 3A, B, and S4A, B). Similar results were obtained in the CRC cell lines SW48 and RKO (Figs. 3C–F, and S4C-F). The levels of mature miR-34a were also up-regulated by curcumin in p53-proficient and p53-deficient HCT116 cells (Figs. 3G and S4G). Since the induction of pri-miR-34a and pri-miR34b/c by curcumin was prevented by treatment with NAC, it was mediated by ROS (Fig. 3H, I). Taken together, these results showed that curcumin induces miR-34a and miR-34b/c expression in a p53-independent and ROS-dependent manner in CRC cell lines.
Fig. 3. Curcumin up-regulates miR-34a and miR-34b/c independent of p53.
A–F qPCR analysis of pri-miR-34a and pri-miR-34b/c expression after treatment of the indicated cells with 15 μM curcumin for the indicated periods. G qPCR analysis of mature miR-34a expression in HCT116 cells after treatment with 15 μM curcumin for 48 hours. H, I qPCR analyses of pri-miR-34a and pri-miR-34b/c expression in HCT116 cells after treatment with curcumin and/or NAC for 48 hours. In (A)–(I) mean values ± SD are shown (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001.
Curcumin-induced NRF2 directly activates miR-34a and miR-34b/c
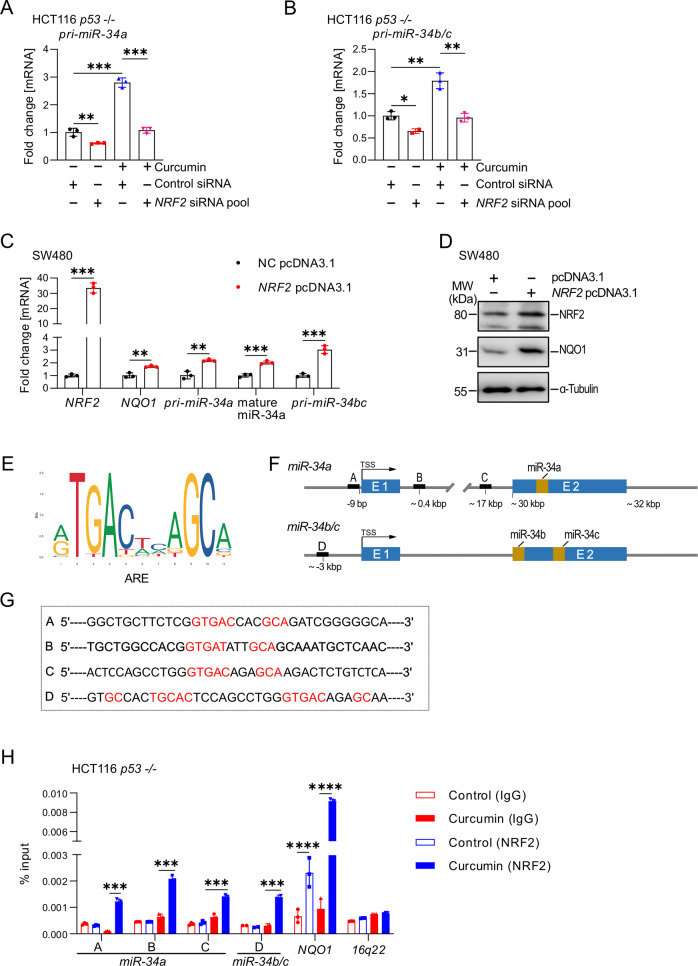
Next, we hypothesized that NRF2, which is activated by curcumin-induced ROS as shown above, may represent a direct inducer of miR-34a and miR-34b/c expression. Indeed, suppression of NRF2 by a pool of 4 different siRNAs prevented the induction of pri-miR-34a and pri-miR-34b/c expression by curcumin in p53-deficient HCT116 cells (Figs. 4A, B, S4A, and S4B). Conversely, ectopic NRF2 expression increased pri-miR-34a and pri-miR-34b/c expression, as well as mature miR-34a, NQO-1 mRNA and protein expression in SW480 cells, which harbour mutant p53 (Figs. 4C, D). By examining the genomic sequence of the miR-34a and miR-34b/c promoter regions, we identified three potential NRF2 binding sites (TGAG/CnnnGC), so-called ARE (Antioxidant Response Elements), in the miR-34a locus and one potential NRF2 binding site in the miR-34b/c locus (Figs. 4E–G). A qChIP assays confirmed enhanced NRF2 occupancy at the four ARE sites within the miR-34a and miR-34b/c promoters after curcumin treatment for 48 hours (Fig. 4H). Therefore, we concluded that curcumin induces the expression of the miR-34a and miR-34b/c genes by activating NRF2, which directly binds to the miR-34a and miR-34b/c promoters and induces their transcription. Notably, this mode of miR-34 activation is p53-independent.
Fig. 4. Curcumin-induced NRF2 directly transactivates miR-34a and miR-34b/c.
A, B qPCR analysis of A. pri-miR-34a and B. pri-miR-34b/c expression in HCT116 cells treated as indicated for 48 hours. C qPCR analysis of indicated mRNAs in SW480 cells after transfection with empty pcDNA3.1 or NRF2 pcDNA3.1 vectors for 72 hours. D Western blot analysis of NRF2 and NQO1 proteins in SW480 cells after transfection with pcDNA3.1 and NRF2 pcDNA3.1 for 72 hours. E ARE consensus sequence defined as 5ʹ-A/G TGA C/G NNNGC A/G-3ʹ, where “N” represents any nucleotide according to jaspar.genereg.net (MA0150.1). F Map of human miR-34a and miR-34b/c genomic regions with indicated NRF2 binding sites. G Sequences of NRF2 binding sites within the miR-34a and miR-34b/c genomic regions. H qChIP analysis of NRF2 occupancy at the human miR-34a and miR-34b/c genomic regions in HCT116 p53 −/− cells after treatment with curcumin or DMSO for 48 hours. NQO1 and 16q22 served as positive and negative controls, respectively. In (A)–(C), and (H) mean values ± SD are shown (n = 3). **P < 0.01, ***P < 0.001.
miR-34a and miR-34b/c mediate the effects of curcumin on CRC cells
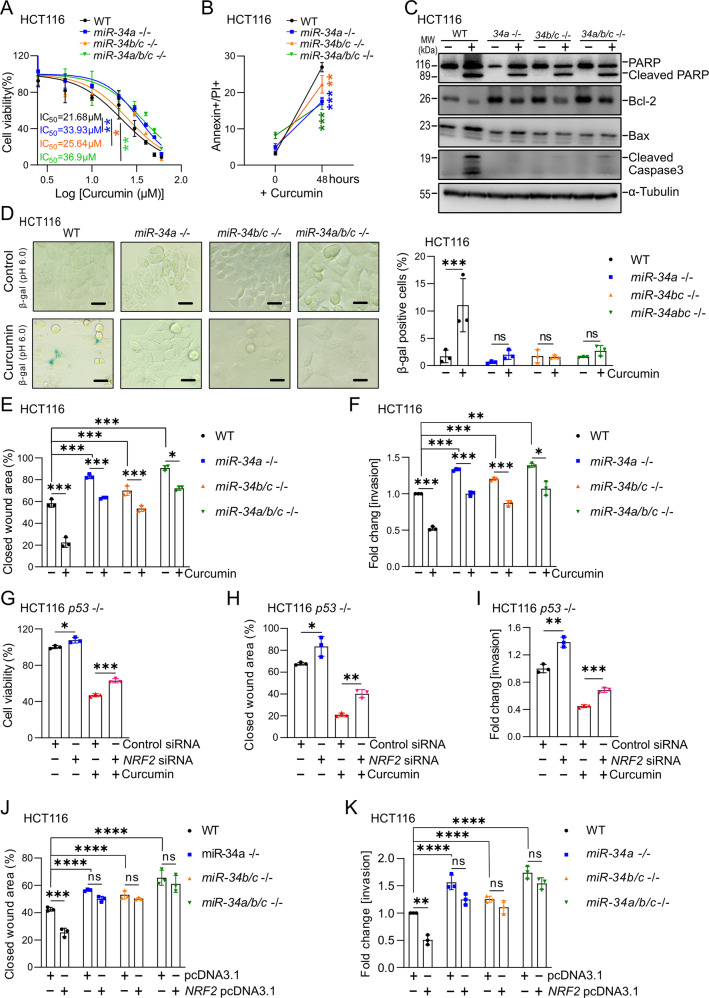
To determine whether the induction of miR-34a and miR-34b/c expression is required for the effects of curcumin, we employed isogenic HCT116 cells that were rendered deficient for miR-34a and/or miR-34b/c genes using a CRSPR/CAS9 approach (their generation will be described elsewhere). The viability of miR-34a- or miR-34a/b/c-deficient HCT116 cells was significantly higher than miR-34-proficient HCT116 cells after treatment with curcumin (Fig. 5A). MiR-34b/c-deficient cells showed an intermediate gain of viability when compared to miR-34a/b/c-proficient HCT116 cells. In agreement, miR-34a/b/c-deficiency resulted in a decreased enhancement of apoptosis by curcumin when compared to miR-34a/b/c-proficient HCT116 cells (Figs. 5B and S6A). In miR-34a/b/c-proficient HCT116 cells, curcumin treatment induced strong cleavage of caspase 3, whereas cleaved caspase 3 was not detectable in miR-34-deficient cells (Fig. 5C). In miR-34a/b/c-proficient HCT116 cells, curcumin suppressed the expression of the anti-apoptotic protein Bcl-2, a known target of miR-34 [28], and induced the expression of the pro-apoptotic protein BAX (Fig. 5C). HCT116 cells with single or combined deletion of miR-34a and miR-34b/c displayed elevated expression of Bcl-2 and after treatment with curcumin the expression of Bcl-2 exceeded the levels detected in untreated WT cells. This effect was most pronounced in the miR-34a/b/c-deficient HCT116 cells. Furthermore, the induction of BAX by curcumin was diminished in HCT116 cells with miR-34a/b/c-deficiency (Fig. 5C). Therefore, it is conceivable that the repression of Bcl-2 by miR-34a/b/c contributes to the induction of apoptosis by curcumin.
Fig. 5. miR-34a and miR-34-b/c mediate the effects of curcumin on CRC cells.
A The indicated cell lines were treated with increasing concentrations of curcumin for 48 hours. IC50 was determined by MTT assays. B Analysis of apoptosis after treatment with 15 μM curcumin for 48 hours determined by Annexin V-FITC and PI staining. C The expression of cleaved PARP, Bcl-2, Bax, and cleaved caspase 3 after treatment with 15 μM curcumin for 48 hours was determined by Western blot analysis. D Detection of senescent cells after curcumin treatment for 48 hours by pH 6 β-gal staining. E Evaluation of migration by wound healing assay 24 hours after treatment with curcumin. F Analysis of invasion using Boyden chamber assays 48 hours after treatment with curcumin or DMSO. G. Analysis of cell viability in HCT116 p53-deficient cells by MTT assay after transfection NRF2 siRNA pool or control siRNA pool 24 hours with curcumin for 48 hours. H Wound healing assay in HCT116 p53-deficient cells treated with curcumin for 24 h after transfection with NRF2-specific siRNA pool or control siRNA pool. I Invasion assay of HCT116 p53-deficient cells exposed to curcumin for 48 hours after transfection with NRF2-specific siRNA pool or control siRNA pool. J Evaluation of migration by wound healing assay 24 hours after transfection with pcDNA3.1 or NRF2 pcDNA3.1. K Analysis of invasion using Boyden chamber assays after transfection with pcDNA3.1 or NRF2 pcDNA3.1. In panels A, B, and D–H mean values ± SD (n = 3) are shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
miR-34a, miR-34b/c, and miR-34a/b/c-deficient cells exhibited no increase in senescence after curcumin treatment as determined by detection of β-galactosidase activity at pH 6.0, whereas WT HCT116 cells showed a signficiant increase in senescence (Fig. 5D). Finally, the curcumin-induced suppression of migration and invasion was significantly lower in miR-34a-, miR-34b/c-, and miR-34a/b/c-deficient cells than in miR-34-proficient HCT116 cells (Figs. 5E, F, S6B, and S6C). In all these assays cells with combined inactivation of miR-34a and miR-34b/c showed the strongest resistance to curcumin. Knockdown of NRF2 by siRNAs partially prevented the curcumin-induced suppression of cell viability (Fig. 5G), migration (Figs. 5H and S7A), and invasion (Figs. 5I and S7B). Furthermore, ectopic expression of NRF2 repressed the migration and invasion of miR-34-proficient HCT116 cells (Figs. 5J, K, S7C, and S7D) in the absence of treatment with curcumin. However, in miR-34a/b/c-deficient HCT116 cells ectopic NRF2 had no effect on migration and invasion, demonstrating that miR-34a and miR-34b/c are required mediators of NRF2 function. Taken together, our results demonstrate that the NRF2-mediated activation of miR-34a and miR-34b/c genes is a required mediator for the effects on curcumin on apoptosis, senescence, migration and invasion.
The induction of miR-34a/b/c by H2O2 and tBHP is mediated by NRF2
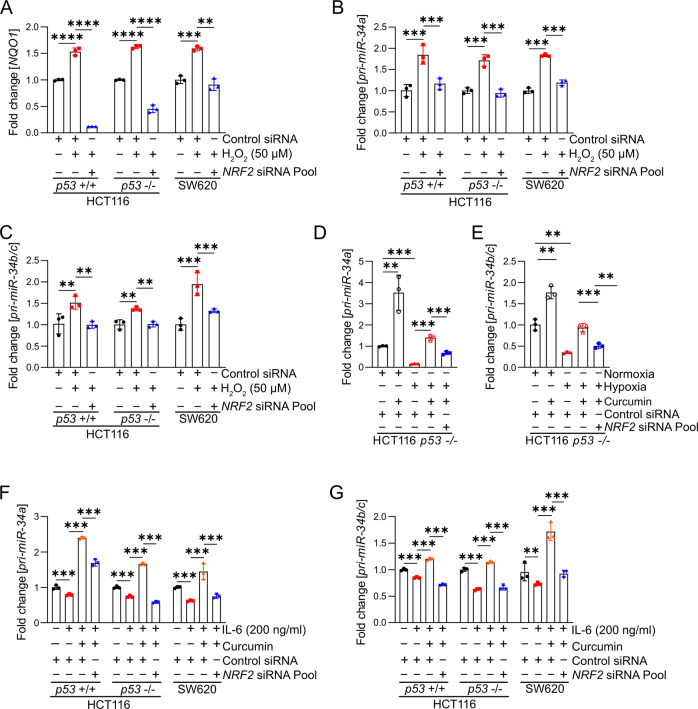
It has been previously shown, that the ROS induced by treatment with H2O2 induces the expression of miR-34a [29]. Here, we determined whether this regulation is mediated by NRF2. First, we analysed cell viability of CRC cell lines after treatment with H2O2 and determined IC50 values of 92.61, 48.35, and 61.52 µM for p53-proficient HCT116 cells, p53-deficient HCT116 cells, and SW620 cells, which express mutant p53, respectively (Figs. S8A and S8B). Treatment of p53-proficient and p53-deficient HCT116 cells as well as SW620 cells with H2O2 or tBHP resulted in the induction of the NRF2 target gene NQO1, indicating that H2O2 and tBHP activate NRF2 (Figs. 6A and S8C). Importantly, H2O2 and tBHP induced the expression of miR-34a/b/c, which was prevented by siRNA-mediated suppression of NRF2 (Figs. 6B, C, S8D, and S8E).
Fig. 6. Effects of the NRF2 axis on regulation of miR-34a/b/c by H2O2, curcumin, hypoxia and IL-6.
A–C Expression of NQO1 (A), pri-miR-34a (B), and pri-miR-34b/c (C) after treatment with H202 and transfection with control or NRF2 siRNA for 24 hours. D, E Expression of pri-miR-34a (D) and pri-miR-34b/c (E) in p53-deficient HCT116 cells after the indicated transfections/treatments. F, G Expression of pri-miR-34a (F) and pri-miR-34b/c (G) in indicated cells after transfection with control or NRF2 siRNA and treatment with curcumin and/or IL-6 (200 ng/ml) for 48 hours. In (A)–(G) mean values ± SD are shown (n = 3)*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The suppression of miR-34a/b/c by hypoxia or IL-6 is reversed by curcumin-induced NRF2
We have previously demonstrated that hypoxia [20] and IL-6-mediated STAT3 activation [18] repress miR-34a/b/c expression in p53-deficient CRC cells. To determine whether curcumin could reverse the hypoxia-mediated suppression of miR-34a/b/c, we cultured p53-deficient HCT116 cells under hypoxia and treated them with curcumin. Indeed, the hypoxia-mediated suppression of miR-34a/b/c expression was completely reversed by curcumin (Figs. 6D, E). However, this reversion was significantly weaker when NRF2 was suppressed by siRNAs (Fig. 6D, E), indicating that this effect of curcumin is largely mediated by NRF2 activation. Previous studies showed that IL-6/STAT3 signalling suppresses miR-34a expression via direct binding of STAT3 to the miR-34a promoter region [20]. Notably, the IL-6-mediated suppression of miR-34a and also that of miR-34b/c was completely reversed by treatment with curcumin (Fig. 6F, G). However, this was not the case when NRF2 was suppressed by siRNA (Fig. 6F, G), indicating that this effect of curcumin was mediated by NRF2.
Curcumin induces MET and inhibits lung-metastases formation via inducing miR-34a
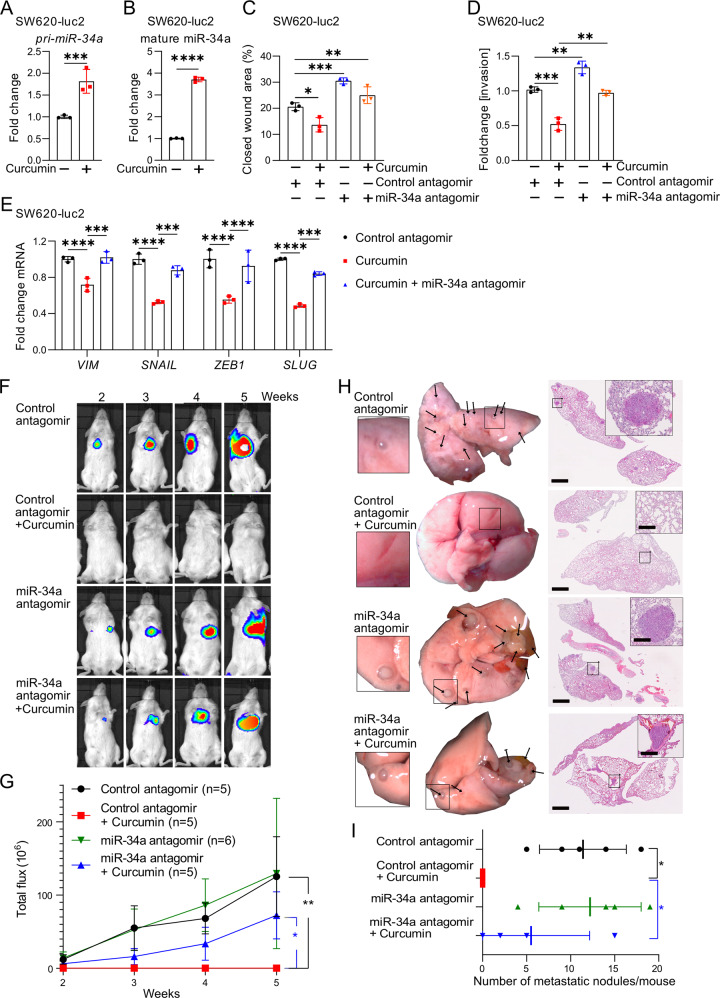
Next, we characterized the effect of curcumin on SW620-Luc2 cells, which stably express luciferase, since we intended to study these cells after transplantation into mice. Curcumin inhibited the viability of SW620-Luc2 cells in a dose-dependent manner (Fig. S9A). The IC50 was 14.49 μM as determined by an MTT assay. This concentration of curcumin was used subsequently. The expression of pri-miR-34a and mature miR-34a was upregulated after exposure of SW620-Luc2 cells to curcumin for 48 hours (Fig. 7A, B). Mature miR-34b and miR-34c were expressed at very low levels in SW620-Luc2 cells when compared to mature miR-34a, also after exposure to curcumin (Fig. S9B). Therefore, we focussed on the analysis of the role of miR-34a for the effects of curcumin on SW620-Luc2 cells. Our previous studies had shown that miR-34a critically contributes to p53-induced mesenchymal-epithelial-transition (MET) in CRC cells [17, 30]. Therefore, we asked whether curcumin induced miR-34a may mediate MET. Indeed, treatment of SW620-Luc2 cells with curcumin resulted in repression of the mesenchymal markers Vimentin (VIM), SNAIL, SLUG, and ZEB1 (Fig. 7E). Also this inhibitory effect of curcumin was abolished by miR-34a-specific antagomirs. Curcumin also suppressed invasion and migration, which presumably is a functional consequence of MET, in SW620-Luc2 cells in a miR-34a-dependent manner, as determined by silencing of miR-34a using antagomirs (Figs. 7C, D, S9C, and 9D). Finally, we performed mouse xenograft experiments to determine whether curcumin affects the capacity of CRC cells to form lung metastases. Therefore, we treated SW620-Luc2 cells ex vivo with curcumin or/and miR-34a-specific antagomirs for 48 hours. Subsequently, these cells were injected into the tail veins of NOD/SCID mice to assess the formation of lung metastasis. Longitudinal, non-invasive imaging showed that the treatment of SW620-Luc2 cells with curcumin completely abrogated metastasis formation within 5 weeks after injection (Fig. 7F, G). However, concomitant inhibition of miR-34a by antagomirs partially restored metastasis formation after curcumin treatment (Fig. 7F, G). Five weeks after injection, resected lungs were devoid of macroscopically visible metastases in mice injected with curcumin-treated SW620-Luc2 cells (Fig. 7H). Haematoxylin and eosin (H&E) staining confirmed the absence of metastatic nodules in mice injected with curcumin-treated cells (Fig. 7H, I). However, SW620-luc2 cells concomitantly treated with miR-34a-antagomirs and curcumin, formed lung-metastases (Fig. 7H, I). In summary, these results show that curcumin inhibits metastases formation via inducing miR-34a.
Fig. 7. Curcumin induces MET and inhibits lung-metastases formation via inducing miR-34a.
A, B Expression of pri-miR-34a (A) and mature miR-34a (B) in SW620-luc2 cells treated with curcumin or DMSO for 48 hours. C Analysis of SW620-luc2 cell migration after transfection with miR-34 antagomirs and/or curcumin for 72 hours. D Analysis of SW620-luc2 cell invasion after transfection with miR-34 antagomirs and/or curcumin for 72 h. E qPCR analyses of EMT-related genes in SW620-luc2 cells 72 hours after the indicated treatments/transfections. F–I Formation of lung metastases by SW620-Luc2 cells, which were treated as indicated for 48 hours and then injected into tail-vein of NOD/SCID mice. Representative images of luciferase signals at the indicated time points after xenografting (F) and the quantification of total photon flux (G). H right: representative lungs 5 weeks after tail vein injection. Arrows indicate metastatic tumor nodules. Left: representative images of H&E-stained resected lungs. Scale bar: 500 μm; 50 μm (insert). I. Quantification of metastatic nodules in the lungs of indicated mice. In (A)–(E) mean values ± SD (n = 3) are shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
p53 and miR-34a/b/c modulate sensitivity towards curcumin and/or 5-FU
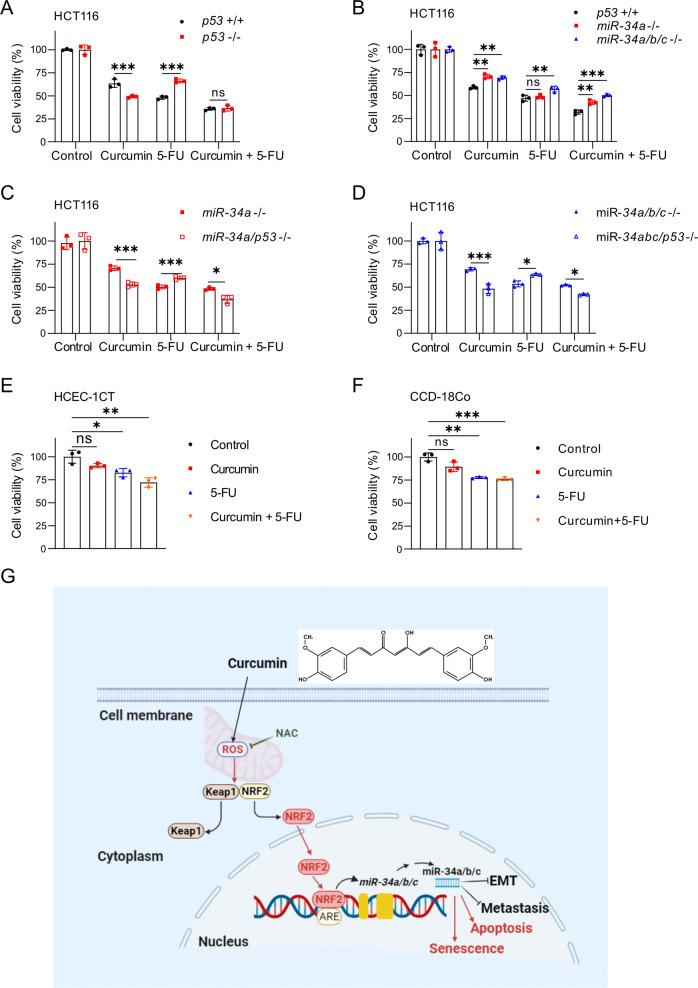
Finally, we investigated the effect of curcumin on CRC cell viability in combination with the chemotherapeutic drug 5-fluorouracil (5-FU), which is widely used for the treatment of CRC. For this we used clinically relevant and tolerable concentrations of 5-FU (2 mg/L) and curcumin (15 µM) [31, 32]. Combined treatment of HCT116 cells with curcumin and 5-FU showed a stronger suppression of cell viability when compared to single treatment with either compound (Fig. 8A). Compared to p53-proficient cells, p53-deficient cells were more sensitive to curcumin but more resistant to 5-FU. However, no difference was observed between p53-deficient and p53-proficient cells when treated with curcumin and 5-FU (Fig. 8A). Compared to wt cells, miR-34a- and miR-34a/b/c-deficient cells were more resistant to curcumin, but only marginally more resistant to 5-FU. However, miR-34a- and miR-34a/b/c-deficiency resulted in a markedly higher resistance to the combination of curcumin and 5-FU (Fig. 8B). Additional deletion of p53 in miR-34a- or miR-34a/b/c-deficient cells increased their resistance to 5-FU. Interestingly, inactivation of p53 reversed the resistance of miR-34a- or miR-34a/b/c-deficient cells to curcumin and the combination of curcumin and 5-FU (Fig. 8C, D). Human colonic epithelial cells (HCEC-1CT) and human intestinal fibroblasts (CCD-18Co cells) were less sensitive to curcumin and/or 5-FU than HCT116 cells (Fig. 8E, F). Taken together, these results suggest that curcumin may enhance the therapeutic effects of 5-FU on CRC cells. Interestingly, this effect is most pronounced in cells lacking p53 and miR-34a/b/c, which are also known to give rise to CRCs with the poorest prognosis [21].
Fig. 8. p53 and miR-34a/b/c modulate sensitivity towards curcumin and/or 5-FU.
A–D HCT116 cells with the indicated genotypes were exposed to curcumin (15 μM), 5-FU (2 mg/L), or curcumin combined with 5-FU. After 48 hours cell viability was determined by MTT assays. E, F The viability of HCEC-1CT and CCD-18Co cells was determined by MTT assay after the indicated treatments for 48 hours. G. Schematic model of the findings obtained in this study.
Discussion
Here we identified a new regulatory connection that explains how curcumin up-regulates the expression of miR-34a and miR-34b/c in a p53-independent manner (for a summary see Fig. 8G). Furthermore, we show that miR-34a and miR-34b/c mediate senescence and apoptosis, and inhibition of migration, invasion and metastasis of CRC cells after exposure to curcumin. Interestingly, the induction of miR-34a and miR-34b/c by curcumin was dominant over their repression by exposure to IL-6 or hypoxia. Therefore, curcumin may re-activate miR-34 expression, which is repressed due to signals generated by the tumor microenvironment. In addition, inhibition of miR-34a function in CRC cells prevented the curcumin-mediated suppression of lung metastases formation of CRC cells transplanted into immune-incompetent mice. Taken together, these results establish a central role of the ROS/KEAP1/NRF2/miR-34a/b/c axis in mediating the tumor suppressive effects of curcumin.
Here, exposure of CRC cells to curcumin induced ROS, which activated the KEAP1/NRF2-pathway. Under normal conditions, NRF2 is rapidly degraded and sequestered in the cytoplasm by a KEAP1-CUL3-RBX1 complex [25]. In the presence of oxidative stress, electrophiles and ROS react with cysteine residues within the KEAP1 protein, which alters the conformation of KEAP1 and results in the release of NRF2 from the KEAP1-CUL3-RBX1 complex. Subsequently, NRF2 translocates to the nucleus, where it binds to ARE motifs in the vicinity of promoters of genes that encode mediators of the antioxidant response and activates these. Here, miR-34a/b/c were not only induced by curcumin, but also by H2O2 and tBHP in an NRF2-dependent manner. Therefore, our results imply that the miR-34a and miR-34b/c genes represent an integral part of the oxidative stress response program controlled by NRF2. The different processes regulated by ROS-induced miR-34, such as cell cycle progression, apoptosis, senescence, autophagy, EMT and migration, may allow cells to appropriately react to oxidative stress. The loss of miR-34a/b/c in cancer cells may allow proliferation and survival under the adverse conditions present in the tumor microenvironment, which includes high levels of ROS. Here, the antioxidant N-acetylcysteine suppressed the induction of apoptosis by curcumin. Therefore, the accumulation of ROS presumably mediates curcumin-induced apoptosis. The diketone group of curcumin conjugates with glutathione-SH, leading to the depletion of the glutathione pool and exhaustion of the antioxidant defense system in cells [33]. Curcumin has both antioxidative and pro-oxidative properties depending on the dose and cell types. At very low concentrations ( ≤ 1 μM) curcumin functions as an antioxidant in non-cancerous cells [34]. However, in cancer cells higher concentrations (5-10 μM) of curcumin induce autophagy and ROS production [35, 36]. In addition, concentrations of curcumin beyond 5.8 ± 1.6 µM induce endoplasmic reticulum (ER) membrane destabilization [37, 34] and inhibit Ca2 + -ATPase (SERCA) [38], thereby leading to release of Ca2+ causing mitochondrial destabilization and thereby additional release of ROS [39]. Interestingly, the amount of ROS induced by curcumin was higher in p53-deficient CRC cells than in p53-proficient cells, indicating that p53 suppresses the generation of ROS. p53 was shown to induce the expression of factors, which may mediate this anti-oxidative effect, such as GLS2 [40], TIGAR [41], and p53R2 [42]. GLS2 is a regulator of glutathione (GSH) synthesis, and may thereby facilitate glutamine metabolism lowering intracellular ROS levels after p53 activation [43, 44]. The p53-induced TIGAR protein represses the expression of fructose-2,6-bisphosphate levels in cells, resulting in an inhibition of glycolysis and an overall decrease in intracellular ROS levels [41, 45]. These regulations may explain why curcumin generated more ROS in p53-deficient cells when compared to p53-proficient HCT116 cells. Importantly, loss of p53 function in tumor cells may sensitize to curcumin-induced apoptosis via allowing an increased generation of ROS. Interestingly, p53-deficient cells were more sensitive to curcumin than p53-proficient cells. This suggests that CRCs, which display a high frequency of p53 inactivation by mutation, may be especially sensitive to treatment with curcumin. The mild induction of ROS by curcumin presumably contributes to its hormetic effects [46], which may according to our results, at least in part, be mediated activation of miR-34a and miR-34b/c.
It was reported that miR-34a expression is induced by curcumin in p53-deficient cells, but not in p53-proficient cells [47]. However, the mechanism remained unknown. Furthermore, another study reported a p53-dependent activation of miR-34a by curcumin [48]. Our results provide clear evidence that miR-34a and miR-34b/c are activated by curcumin in a p53-independent manner via the ROS/KEAP1/NRF2/ARE pathway and represent direct targets of NRF2. Besides p53, which activates miR-34a, several transcription factors that repress miR-34a expression have been described: HIF1α and STAT3 repress miR-34 genes under conditions of hypoxia and inflammation, which are hallmarks of the tumor micro-environment [18, 20]. The repression of miR-34a has been shown to contribute to the invasion and metastasis of CRCs [21]. Since curcumin was able to antagonize the repression of miR-34a and miR-34b/c by hypoxia or IL6 exposure, a therapeutic treatment with curcumin may therefore reactivate miR-34 expression in primary CRC and metastases, and thereby suppress progression of CRCs.
We identified miR-34a and miR-34b/c as central effectors of the ROS/NRF2 pathway which were activated by curcumin as well as by ROS-generating substances, such as H2O2 and tBHP. Therefore, miR-34a and miR-34b/c not only mediate the cellular response to activation of the p53 pathway, but also represent integral components of the response to oxidative stress. Furthermore, our results provide a plausible mechanism for the effects that have been ascribed to curcumin in the prevention and therapy of colorectal cancer and other malignancies. The members of the miR-34 family are frequently silenced in colorectal tumors by DNA methylation [16]. There is evidence that curcumin can reactivate CpG methylated genes [49]. Therefore, CpG-methylation of miR-34a/b/c is presumably not an obstacle for treatment of CRC with curcumin. Originally this study was intended to determine the mode of action of curcumin during tumor prevention. In that scenario, miR-34a/b/c should not be silenced by CpG-methylation. We showed that the anti-tumor effects of curcumin are less pronounced in miR-34-deficient cells. Therefore, it will be important to investigate the in vivo effects of curcumin on CRC treatment and prevention with respect to miR-34 expression in the future. For example, future experiments should include the treatment of wt and miR-34 knockout mouse models of CRC with curcumin and/or chemotherapy. Howells et al. [7] showed in a phase IIa clinical study that the addition of curcumin to FOLFOX treatment significantly improved the progression free and overall survival. The addition of curcumin to cancer therapy is of great interest, since a phase I clinical study showed that the addition of curcumin to FOLFOX treatment is safe and tolerable in patients with metastatic CRC at doses up to 2 grams daily [50]. Moreover, oral consumption of up to 3600 mg curcumin leads to curcumin concentrations in human colorectal mucosa which are in the range of the concentration used in this study [51]. In the future, the findings presented here may be exploited for the development of therapeutic approaches that aim at restoring the tumor suppressive function of the p53/miR-34 pathway.
Materials and Methods
Cell culture and treatments
HCT116, RKO, SW48, CCD-18Co, and SW620-Luc2 cell lines were cultured in McCoy’s 5 A medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Invitrogen) containing 100 units/ml penicillin and 0.1 mg/ml streptomycin. HCEC-1CT immortalized human colonic epithelial cells (Evercyte GmbH) were maintained in DMEM supplemented with 2% FBS, 1× N2 Supplement (contains insulin, apo-transferin, and sodium-selenite; Thermo Fisher Scientific), 20 ng/mL epidermal growth factor (EGF; AF-100–15, PeproTech, part of Thermo Fisher Scientific), 1 μg/mL hydrocortisone (Sigma, Merck), and 1× penicillin/streptomycin (Gibco, Thermo Fisher Scientific). For the 5-FU experiment dialyzed FBS (A3382001; Thermo Fisher Scientific). All cells were incubated at 20% O2, 5% CO2, and 37°C incubator. Curcumin (Sigma-Aldrich, #C1386) was dissolved in DMSO (stock concentration 50 mM) and the working concentration was 15 μM. NAC (Sigma-Aldrich, #A0737) was dissolved in water (stock concentration 100 mM) and the working concentration was 5 mM. IL-6 (Immunotools) was dissolved in water and used at a final concentration of 200 ng/ml. 5-FU (Sigma-Aldrich, # 343922) was dissolved in DMSO (stock concentration 5 mg/ml) and the working concentration was 2 µg/ml. siGENOME Human NRF2 siRNA SMARTPool (Horizon Discovery, USA) and siGENOME Non-Targeting siRNA Control Pools (Horizon Discovery, USA), miRNA antagomir (hsa-miR-34a-5p inhibitor, Invitrogen, MH11030), and respective negative controls were transfected at a concentration of 10 nM using HiPerFect transfection reagent (Qiagen, Hilden, Germany). Hypoxia (0.5% O2) was achieved using a CD210 incubator (Binder, Tuttlingen, Germany).
MTT assay
Cell viability was analyzed by MTT assays. CRC cells were seeded at a density of 3000 cells per well in 96-well plates. After 24 hours, cells were treated with curcumin at the indicated concentrations for 48 hours, followed by addition of 10 μl MTT solution (5 mg/ml) per well and incubation for 4 hours. Cells were lysed with 100 μl DMSO per well for 15 min to release the resulting formazan according to Twentyman et al. [52], and analyzed by measuring the absorbance at 570 nm by a Varioscan system (Thermo Fisher).
RNA isolation and qPCR analysis
Total cellular RNA was isolated and purified from CRC cell lines according to manufacturer’s instruction (High Pure RNA Isolation Kit; Roche). 1ug of total RNA was used for reverse transcription with Verso cDNA Synthesis Kit (Termo Fisher Scientifc, Waltham, MA, USA). Quantitative real-time polymerase chain reaction (qPCR) was performed using Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA) on a LightCycler 480 (Roche) system. qRT-PCR data was normalized to GAPDH or β-actin and analyzed with the ΔΔCt method [53]. Primer sequences information are provided in Table S1.
Immunofluorescence analysis by confocal laser-scanning microscopy
Cells were cultured and treated with curcumin on glass cover slides, fixed in 4% paraformaldehyde/PBS for 15 min, permeabilized in 0.2% Triton X-100 for 5 min, and then blocked in 1% BSA/PBS for 1 h at room temperature. Next, cells were incubated with NRF2 primary antibody (D1Z9C, #12721, Cell Signaling Technology) for 1 h at room temperature, washed 3 time with PBS-Tween and then incubated with cy3-labelled secondary antibody (ab6939, Abcam). Chromatin was stained with DAPI (Roth). The images were acquired by confocal laser scanning microscopy (CLSM) using the LSM700 microscope with a Plan Apochromat 20×/0.8 M27 objective and ZEN 2009 software (Zeiss).
Chromatin immunoprecipitation
HCT116 p53-/- were treated with curcumin for 48 hours to activate NRF2 before cross-linking. ChIP experiments were performed using the iDeal ChIP-qPCR kit (Diagenode, Belgium) according to manufacturer’s instructions. The sequences of qChIP primers are provided in Table S2.
Detection of cellular ROS
Reactive oxygen species (ROS) were detected by DCFDA/H2DCFDA staining. In brief, HCT116 cells were seeded at a density of 104 cells per well in 96 well plates. After 24 hours, curcumin or DMSO was added for 48 hours. The positive control tBHP was added to HCT116 cells for 4 hours before staining. After removal of medium 1X Buffer (100 µl/well) was added. After removal of the 1X Buffer, cells were stained with DCFDA solution (100 µl/well) for 45 min at 37 °C in the dark. After removal of the DCFDA solution, plates were analyzed immediately on a fluorescence plate reader at Ex/Em = 485/535 nm. Image J software was used to analyze the images and compared the control group.
Metastases formation in a xenograft mouse model
SW620 cells stably expressing Luc2 were described previously [30]. Cells were injected into the lateral tail vein of NOD/SCID mice using 25-gauge needles (4 × 106 cells /0.2 ml) in HBSS. From the second week onwards, mice were injected i.p. with D-luciferin (150 mg/kg) and imaged for 5 minutes using the IVIS Illumina System (Caliper Life Sciences) in weekly intervals. Five weeks after tail vein injection, mice were sacrificed and examined for lung metastases using H&E staining. Animal experimentations and analyses were approved by the Government of Upper Bavaria, Germany (55.2-2532.vet_02-18-57).
Statistical analysis
The statistical differences between two groups were calculated using a Student’s t test (two-tailed; unpaired). When more than 2 groups were compared, the one-way analysis of variance (ANOVA) with the Tukey multiple comparison post-test was used. with p < 0.05 considered significant. Asterisks generally indicate: *p < 0.05, **p < 0.01 and ***p < 0.001, n.s. = not significant. Additional method descriptions can be found as supplementary information.
Supplementary information
Author contributions
HH conceived, planned and supervised the project; HH, CL, and MR designed experiments; CL performed experiments and analyzed results; MR performed and analyzed mouse experiments. ZH generated miR-34a/b/c KO cells. HH CL and MR wrote the manuscript. All authors read and approved the final manuscript.
Funding
C.L and Z.H. are recipients of Chinese Scholarship Council fellowships. H.H. received grants from the Rudolf Bartling-Stiftung. Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data, analytic methods, and study materials will be made available to other researchers upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics
Animal experimentations and analyses were approved by the Government of Upper Bavaria, Germany (55.2-2532.vet_02-18-57).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01178-1.
References
- 1.GLOBOCAN Global Cancer Observatory (GCO). https://gco.iarc.fr (accessed on 18 April 2023).
- 2.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/S0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020;9:361–73. doi: 10.1002/cam4.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng W, Goel A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73–86. doi: 10.1016/j.semcancer.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Zhang X, Tong Y, Chen S, Liang J. Curcumin against gastrointestinal cancer: A review of the pharmacological mechanisms underlying its antitumor activity. Front Pharmacol. 2022;13:990475. doi: 10.3389/fphar.2022.990475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojo OA, Adeyemo TR, Rotimi D, Batiha GE, Mostafa-Hedeab G, Iyobhebhe ME, et al. Anticancer properties of curcumin against colorectal cancer: A review. Front Oncol. 2022;12:881641. doi: 10.3389/fonc.2022.881641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149:1133–9. doi: 10.1093/jn/nxz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panahi Y, Saberi-Karimian M, Valizadeh O, Behnam B, Saadat A, Jamialahmadi T, et al. Effects of curcuminoids on systemic inflammation and quality of life in patients with colorectal cancer undergoing chemotherapy: a randomized controlled trial. Adv Exp Med Biol. 2021;1328:1–9. doi: 10.1007/978-3-030-73234-9_1. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [PubMed] [Google Scholar]
- 10.Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444–9 e1. doi: 10.1016/j.cgh.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017;152:398–414 e6. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–8. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Chai R, Chen Y, Zhao S, Bian Y, Wang X. Curcumin targeting non-coding RNAs in colorectal cancer: therapeutic and biomarker implications. Biomolecules. 2022;12:1339. doi: 10.3390/biom12101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–8. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012;12:613–26. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 16.Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–22. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 17.Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–71. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and Hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153:505–20. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–30. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 20.Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–67. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oner MG, Rokavec M, Kaller M, Bouznad N, Horst D, Kirchner T, et al. Combined inactivation of TP53 and MIR34A promotes colorectal cancer development and progression in mice via increasing levels of IL6R and PAI1. Gastroenterology. 2018;155:1868–82. doi: 10.1053/j.gastro.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Jiang L, Hermeking H. miR-34a and miR-34b/c suppress intestinal tumorigenesis. Cancer Res. 2017;77:2746–58. doi: 10.1158/0008-5472.CAN-16-2183. [DOI] [PubMed] [Google Scholar]
- 23.Patino-Morales CC, Soto-Reyes E, Arechaga-Ocampo E, Ortiz-Sanchez E, Antonio-Vejar V, Pedraza-Chaverri J, et al. Curcumin stabilizes p53 by interaction with NAD(P)H:quinone oxidoreductase 1 in tumor-derived cell lines. Redox Biol. 2020;28:101320. doi: 10.1016/j.redox.2019.101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Fang S, Shao X, Li Y, Tong Q, Kong B, et al. Curcumin reverses NNMT-induced 5-fluorouracil resistance via increasing ROS and cell cycle arrest in colorectal cancer cells. Biomolecules. 2021;11:1295. doi: 10.3390/biom11091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020;40:e00099–20. doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA. 2004;101:6379–84. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutter FE, Park BK, Copple IM. Value of monitoring Nrf2 activity for the detection of chemical and oxidative stress. Biochem Soc Trans. 2015;43:657–62. doi: 10.1042/BST20150044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Wang X. miR-34a targets BCL-2 to suppress the migration and invasion of sinonasal squamous cell carcinoma. Oncol Lett. 2018;16:6566–72. doi: 10.3892/ol.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker JR, Vuppusetty C, Colley T, Papaioannou AI, Fenwick P, Donnelly L, et al. Oxidative stress dependent microRNA-34a activation via PI3Kalpha reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci Rep. 2016;6:35871. doi: 10.1038/srep35871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn S, Jackstadt R, Siemens H, Hunten S, Hermeking H. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J. 2013;32:3079–95. doi: 10.1038/emboj.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JJ, Beumer JH, Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol. 2016;78:447–64. doi: 10.1007/s00280-016-3054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 1996;77:441–51. doi: 10.1002/(SICI)1097-0142(19960201)77:3<441::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE, Jr., et al. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem Biol Interact. 2000;128:19–38. doi: 10.1016/S0009-2797(00)00185-X. [DOI] [PubMed] [Google Scholar]
- 34.Rainey NE, Moustapha A, Petit PX. Curcumin, a multifaceted hormetic agent, mediates an intricate crosstalk between mitochondrial turnover, autophagy, and apoptosis. Oxid Med Cell Longev. 2020;2020:3656419. doi: 10.1155/2020/3656419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng Y, Du Q, Zhang Z, Ma J, Han L, Wang Y, et al. Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch Biochem Biophys. 2020;694:108613. doi: 10.1016/j.abb.2020.108613. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Song X, Shang M, Zou W, Zhang M, Wei H, et al. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019;15:1243–53. doi: 10.2217/fon-2018-0708. [DOI] [PubMed] [Google Scholar]
- 37.Wootton LL, Michelangeli F. The effects of the phenylalanine 256 to valine mutation on the sensitivity of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) Ca2+ pump isoforms 1, 2, and 3 to thapsigargin and other inhibitors. J Biol Chem. 2006;281:6970–6. doi: 10.1074/jbc.M510978200. [DOI] [PubMed] [Google Scholar]
- 38.Sala de Oyanguren FJ, Rainey NE, Moustapha A, Saric A, Sureau F, O’Connor JE, et al. Highlighting curcumin-induced crosstalk between autophagy and apoptosis as supported by its specific subcellular localization. Cells. 2020;9:361. doi: 10.3390/cells9020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koiram PR, Veerapur VP, Kunwar A, Mishra B, Barik A, Priyadarsini IK, et al. Effect of curcumin and curcumin copper complex (1:1) on radiation-induced changes of anti-oxidant enzymes levels in the livers of Swiss albino mice. J Radiat Res. 2007;48:241–5. doi: 10.1269/jrr.06103. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 42.Kang MY, Kim HB, Piao C, Lee KH, Hyun JW, Chang IY, et al. The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death Differ. 2013;20:117–29. doi: 10.1038/cdd.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:7461–6. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci USA. 2010;107:7455–60. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci USA. 2012;109:20491–6. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A. Hormetic effects of curcumin: What is the evidence? J Cell Physiol. 2019;234:10060–71. doi: 10.1002/jcp.27880. [DOI] [PubMed] [Google Scholar]
- 47.Roy S, Levi E, Majumdar AP, Sarkar FH. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J Hematol Oncol. 2012;5:58. doi: 10.1186/1756-8722-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toden S, Okugawa Y, Buhrmann C, Nattamai D, Anguiano E, Baldwin N, et al. Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev Res (Phila) 2015;8:431–43. doi: 10.1158/1940-6207.CAPR-14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Link A, Balaguer F, Shen Y, Lozano JJ, Leung HC, Boland CR, et al. Curcumin modulates DNA methylation in colorectal cancer cells. PLoS One. 2013;8:e57709. doi: 10.1371/journal.pone.0057709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James MI, Iwuji C, Irving G, Karmokar A, Higgins JA, Griffin-Teal N, et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015;364:135–41. doi: 10.1016/j.canlet.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–5. doi: 10.1158/1055-9965.120.14.1. [DOI] [PubMed] [Google Scholar]
- 52.Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56:279–85. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, analytic methods, and study materials will be made available to other researchers upon reasonable request.