Abstract
Two separate bodies of literature point to the link between family bereavement and cardiovascular health and between sleep quality and cardiovascular outcomes. However, less is known about the joint influence of family bereavement and sleep quality on cardiovascular functioning. The aims of this study were to examine the relationships between experiencing the death of a family member and heart rate variability (HRV) and to further explore whether these associations differ by sleep quality. Using data from the Midlife in the United States (MIDUS) Biomarker Project, the sample for this study included respondents who experienced the death of an immediate family member – father, mother, spouse, sibling, or child – within a year before the Biomarker project and those who did not experience any deaths (N = 962). We used two measures of HRV and sleep quality was measured using the Pittsburgh Sleep Quality Index. Results showed that experiencing the death of a family member was associated with worse HRV only among those with poor sleep quality and not for those with good sleep quality. These results suggest that poor sleep quality may indicate psychophysiological vulnerability for those who experienced the death of a family member. Interventions to improve sleep quality could be effective in enhancing cardiovascular health of bereaved individuals.
Keywords: family death, cardiovascular health, sleep, psychosocial, physiological
Introduction
The death of a family member is one of the major life events that individuals typically experience during adulthood. For example, studies on the prevalence of family bereavement find that 75% of adults are likely to experience the deaths of both parents by the age of 62 (Hooyman & Kramer, 2006) and that 12% of older men and 34% of women aged 65 and older are widowed (Roberts et al., 2018). Given the increased importance of family relationships in adulthood (Carstensen, 1992), experiencing the death of a close family member has significant health implications for adults. In particular, many studies confirm the health-damaging effects of family bereavement on cardiovascular outcomes including an elevated risk of cardiovascular disease (Bartrop et al., 2016; Buckley, Sunari, et al., 2012; Stroebe et al., 2007). For example, an extensive review of meta-analyses and multiple cohort studies (Kivimäki & Steptoe, 2018) shows that family bereavement is associated with two-times higher risk of experiencing cardiac events such as myocardial infarction and stroke.
As a potential physiological mechanism that contributes to an increased risk of cardiac events among the bereaved, heart rate variability (HRV) has begun to receive attention (Bartrop et al., 2016; Buckley et al., 2010). HRV refers to the degree of variation in the time intervals of heartbeats, and it provides information about cardiovascular autonomic control and the body’s capacity to respond to external stressors. Reduced HRV suggests autonomic dysfunction and is found to be one of significant correlates of cardiovascular disease such as coronary heart disease and stroke (Haensel et al., 2008; Thayer et al., 2010; Thayer & Lane, 2007). To our knowledge, there are only three studies to date that have examined the association between bereavement and HRV in adults, and findings are mixed. While one study (O’Connor et al., 2002) reports no significant differences in HRV among the groups of bereaved, depressed, and healthy (control) individuals, two other studies report that recently bereaved individuals (within 6 months of the death) have lower levels of HRV compared to the non-bereaved group (Buckley, Stannard, et al., 2012; Fagundes et al., 2018).
Sleep quality is a potential moderator of the association between family bereavement and HRV, considering that sleep is both a physiological and psychological process. Sleep as a physiological process is related to the autonomic nervous system (ANS) that also modulates cardiovascular functioning (Tobaldini et al., 2013). Studies consistently find that poor sleep quality as measured by disrupted sleep and sleep problems is significantly linked to autonomic imbalance with lower levels of HRV (Castro-Diehl et al., 2016; Jackowska et al., 2012; Meerlo et al., 2008). Sleep is also closely related to psychological stress (Lee et al., 2017; Sin et al., 2017), where poor sleep and psychological stress jointly contribute to the risk of illness including cardiovascular disease (Benham, 2010). Because sleep functions as a restorative process that helps one’s body to cope with stressors, poor sleep quality may interfere with effective stress responses and make individuals more vulnerable to the health costs of experiencing intense stressors such as family bereavement (Bodnar & Kiecolt-Glaser, 1994; Hamilton et al., 2007). Therefore, the stress of bereavement coupled with poor sleep is likely to have a more detrimental impact on HRV compared to bereaved individuals with good sleep.
While sleep quality has been widely examined in the bereavement literature, this research has been largely limited to exploring changes in sleep quality as a consequence of bereavement (Buckley et al., 2010; Buckley, Sunari, et al., 2012; Lancel et al., 2020). It is equally important to examine the role sleep quality as a specific condition under which experiencing a family member’s death is linked to cardiovascular functioning, as this can provide insights in identifying individuals who may be particularly vulnerable to the adverse health consequences of family bereavement and further developing tailored intervention strategies that aim to protect the health of bereaved individuals.
As to better understand the underlying processes through which family bereavement and sleep quality are jointly related to cardiovascular functioning, we examined the associations between experiencing the death of a family member and HRV and further explored whether these associations differed by sleep quality. In particular, we focused on a recent death (within 12 months since bereavement) of an immediate family member. By using HRV as a physiological marker of cardiovascular functioning, findings of this study can shed light on identifying individuals who may have a greater risk of experiencing cardiovascular events following the death of a family member.
Methods
Sample
Sample participants were drawn from the Biomarker Project of the Midlife in the United States (MIDUS) study. MIDUS is a national study of health and well-being of US adults, and the Biomarker Project that followed the main MIDUS survey involved comprehensive biological assessments on a subsample of MIDUS participants. Participants of the Biomarker Project visited one of three participating General Clinical Research Centers (UCLA, Georgetown, and the University of Wisconsin, Madison) and completed two-day data collection protocol and physical health assessments. Further details regarding the study protocol and the variables measured are available elsewhere (Dienberg Love et al., 2010). This study combined the original MIDUS respondents and the MIDUS Refresher which was added to replenish the original MIDUS cohort.
Of the 2,118 respondents who participated in the Biomarker project (1,255 from the original MIDUS cohort and 863 from the Refresher), 1,086 participants did not experience any deaths between their participation in the main survey and the Biomarker Project and 1,032 individuals reported experiencing a death of a close person. Of those who experienced death events, we selected 121 individuals who had an immediate family member (i.e., parent, spouse, sibling, child) die within 12 months before the Biomarker Project. We limited time since death to one year because studies found the risk of health problems to be the highest during the first year after experiencing a family member’s death (N. J. Johnson et al., 2000; Stroebe et al., 2007). Among the 1,207 participants (121 who experienced a family member’s death and 1,086 who did not experience any death events), 245 individuals who were missing on at least one of the study variables were dropped from the analyses. Those who were excluded from the study sample were older and more likely to be men and non-White. There were no significant differences in terms of education, marital status, bereavement group membership, and sleep quality. The final sample for this study was 962 adults (nfamily death = 94, nno death = 868) aged between 26 and 85. Flow chart for sample selection is provided in Supplementary Figure 1. Comparison of the sample descriptives between the bereaved and non-bereaved groups is presented in Table 1.
Table 1.
Sample Descriptives and Tests of Group Differences Between Those Who Experienced a Family Member’s Death and Those Who Did Not Experience Any Family Member Deaths (N = 962a)
| Variable | Experienced a Family Member’s Death (n = 94) |
No Family Member Death (n = 868) |
Tests of Differences |
|---|---|---|---|
| Percentage | p-valueb | ||
|
| |||
| Women | 52.1 % | 55.7 % | .515 |
| Married | 55.3 % | 64.4 % | .082 |
| White | 71.3 % | 81.7 % | .015 |
| Currently smoking | 12.8 % | 13.3 % | .895 |
| Taking hypertension medication | 35.1 % | 24.3 % | .022 |
| Have cardiovascular disease | 9.6 % | 6.6 % | .273 |
| Engage in physical activity | 69.2 % | 75.7 % | .164 |
| Good sleep quality | 46.8 % | 54.5 % | .156 |
|
| |||
| M (SD) | p-valuec | ||
|
| |||
| Age | 57.8 (11.3) | 53.8 (12.3) | .0014 |
| Level of educationd | 7.6 (2.8) | 8.0 (2.5) | .07 |
| BMI | 30.0 (7.0) | 29.3 (6.7) | .172 |
| RMSSD ln(ms) | 2.8 (0.7) | 3.0 (0.6) | .007 |
| High-Frequency HRV ln(ms2) | 4.7 (1.4) | 5.0 (1.3) | .005 |
Notes. SD = standard deviation; BMI = body mass index; Ln = natural log; Ms = milliseconds.
N derived from listwise deletion of study variables.
Chi-square test of difference.
Independent sample t-test of difference.
Level of education was coded as 1 = Grade school or below ~ 12 = Doctoral or professional degree.
Measures
Family bereavement.
Experiencing the death of a family member was measured using the question “Has anyone close to you, a close friend or relative, passed away since the main survey interview?” from the MIDUS Biomarker Project. If yes, respondents provided detailed information about up to five deaths they experienced including the gender of the decedent, the month and year of the death, and the relationship with the decedent. Because the relationship with the decedent was an open-ended question, two coders categorized the responses based on the type of relationship reported. Consensus between the two coders regarding the categorization of responses was high, with inter-rater reliability of κ = 0.99. Based on the 16 categories created (e.g., friend, relative, in-law), we selected respondents who experienced the death of an immediate family member including father, mother, spouse, brother, sister, and child within the 12 months of their participation in the Biomarker Project. Of the 94 individuals who experienced the death of a family member, 55 (58.5%) reported the death of a parent, 1 experienced death of a spouse (1.1%), 32 reported the death of a sibling (34.1%), and 6 reported the death of their child (6.4%). For this study, we created a dichotomous variable that indicated family bereavement experience, with 1 = experienced the death of an immediate family member and 0 = did not experience any death events.
Heart rate variability.
HRV is operationalized as variability in the intervals between consecutive R waves. This study used two indices of HRV, which are root mean squared successive differences (RMSSD; measured in msec) and high frequency HRV (0.15–0.40 Hz; HF-HRV; measured in msec2). Both RMSSD and HF-HRV are frequently used measures of vagal tone (Laborde et al., 2017; Shaffer & Ginsberg, 2017). HRV measures were obtained from electrocardiograph (ECG) records collected during an 11-minute seated resting period. Analog ECG signals were digitized at 500 Hz by a 16-bit National Instruments analog-to-digital board using a microcomputer. To identify R waves, ECG waveforms were submitted to proprietary event detection software and were visually inspected based on established procedures to correct for any software errors (Berntson et al., 1990). The resulting RR intervals were submitted to Fourier-based spectral analysis to compute HF-HRV. All HRV measures were calculated as a mean of two baseline 300-second (5-minute) epochs and were natural log-transformed to normalize the distributions. Higher values indicate better functioning HRV. Five-minute epochs are conventionally referred to as “short-term” assessments of HRV and are reliable for these measures (Shaffer & Ginsberg, 2017). Detailed information regarding the assessment of HRV measures is available elsewhere (Weinstein et al., 2019).
Sleep quality.
Sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI). PSQI is a well-validated measure of subjective sleep quality over the past month (Buysse et al., 1989) and is known to have good internal consistency reliability and construct validity (Carpenter & Andrykowski, 1998). The questionnaire is consisted of 19 items that measure seven components of sleep, which include: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medications, and daytime dysfunction. For the last three components, respondents were asked to report the frequency of sleep-related problems, disturbances in daily functioning, and taking medicines to help sleep on a 4-point scale, ranging from 0 = not during the past month to 3 = three or more times in a week. Responses for subjective sleep quality ranged from 0 = very good sleep to 3 = very bad sleep. Sleep latency, duration, and habitual sleep efficiency were scored based on the respondents’ reported timing and duration of their sleep periods. Each of the seven components weighted equally from 0–3 points, and were summed together to calculate a global sleep score (ranging from 0 to 21). Higher scores indicated worse sleep quality.
We dichotomized the global sleep score using a suggested cut-off score of five (Buysse et al., 2008). Specifically, respondents with a global sleep score of five or lower were categorized as having a good sleep quality (coded as 0) and those who scored higher than five were categorized as having a poor sleep quality (coded as 1). We used this dichotomous measure of sleep quality because the clinical cut-off (PSQI > 5) has been widely used in clinics and research to identify individuals with overall poor sleep (Buysse et al., 2008; Dzierzewski et al., 2015; Lee et al., 2020). In our sample, 46.3% of the respondents (53.2% of those who experienced a family member’s death and 45.5% of those who did not experience any death events) were categorized as having a poor sleep quality.
Covariates.
We controlled for demographic and health-related covariates that may confound the associations of interest, including age, gender, race, education, marital status, body mass index (BMI), smoking status, hypertension medication, engagement in physical activity, and physician-diagnosed heart disease. Age was calculated by subtracting the respondent’s date of birth from their date of participation in the Biomarker project. Gender was coded as 0 = men and 1 = women, and race was coded 0 = non-White and 1 = White. Education was measured based on the highest level of education completed. Responses ranged from 1 = No school or some grade school, 2 = Junior high school, 3 = Some high school … 11 = Master’s degree, and 12 = Doctoral or other professional degree. Marital status was coded as 0 = not married (i.e., divorced, separated, widowed, never married) and 1 = married. BMI was calculated based on the respondent’s weight and height measured during the clinic visit. Smoking status was coded as 0 = never smoked regularly, 1 = smoked regularly in the past, and 2 = currently smoking regularly. Hypertension medication was coded as 0 = not currently taking hypertension medication and 1 = currently taking hypertension medication. Engagement in physical activity was measured with a question “Do you engage in regular exercise, or activity, of any type for 20 minutes or more at least 3 times/week?” Responses were coded as 0 = no and 1 = yes. Heart disease was coded 0 = no heart disease and 1 = ever had a heart disease diagnosed by a physician.
Plan of Analyses
For each HRV indices, this study performed hierarchical linear regression analyses using STATA 16.1 (StataCorp, 2019). In the first analysis model, we examined the main effect of experiencing the death of a family member on HRV, after controlling for covariates. In the subsequent model, we added an interaction term between family member’s death and sleep quality to examine whether the associations between family bereavement and HRV differed by sleep quality. With significant interaction effects, we performed simple slope tests and plotted the effects to better visualize the results (Dawson, 2014). All continuous variables were centered at their sample means to better interpret the intercept values and interaction plots.
Results
Descriptive Statistics
Descriptive statistics and tests of difference comparing those who experienced the death of a family member and those who did not experience any deaths are presented in Table 1. There were no significant differences between the two groups based on gender, marital status, and sleep quality. For race, those who experienced a family member’s death consisted of a smaller proportion of White respondents (71.3%) compared to those who did not experience any death events (81.7%). The bereavement group was also significantly older (Mage = 57.8 for bereaved group; Mage = 53.8 for non-bereaved group) and had lower (i.e., worse) levels of HRV (MRMSSD = 2.8 and MHF-HRV = 4.7 for bereaved group; MRMSSD = 3.0 and MHF-HRV = 5.0 for non-bereaved group). The two groups did not differ in their levels of education and BMI.
Experiencing the Death of a Family Member, HRV, and Sleep Quality
Table 2 presents regression results on the associations between experiencing the death of a family member and HRV and moderation by sleep quality. Model 1 tested for the main effect of experiencing a family member’s death and Model 2 examined the interaction effect between family member’s death and sleep quality. Results from Model 1 showed that there were marginally significant associations between experiencing the death of a family member and HRV indices. Specifically, those who experienced family bereavement had lower (i.e., worse) levels of RMSSD (B = −.127, p = .050) and HF-HRV (B = −.254, p = .057) compared to those who did not experience any death events. Results also showed that having poor sleep quality was associated with lower levels of RMSSD (B = −.081, p = .039) and HF-HRV (B = −.182, p = .025).
Table 2.
Linear Regression Results on the Association Between Experiencing the Death of a Family Member and HRV and Interaction Effects of Sleep Quality (N = 962)
| RMSSD ln (ms) | HF-HRV ln (ms2) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | B (95% CI) | p-value | |
| Constant | 3.12 (2.976; 3.265) |
< .001 | 3.097 (2.952; 3.242) |
< .001 | 5.184 (4.887; 5.482) |
< .001 | 5.145 (4.847; 5.444) |
< .001 |
| Experienced a family member’s death | −.127 (−.253; .0002) |
.050 | .059 (−.124; .242) |
.529 | −.254 (−.516; .007) |
.057 | .051 (−.327; .429) |
.793 |
| Poor sleep quality | −.081 (−.158; −.004) |
.039 | −.045 (−.126; .036) |
.274 | −.182 (−.341; −.023) |
.025 | −.123 (−.29; .044) |
.149 |
| Family member’s death x Sleep quality | - | - | −.352 (−.604; −.101) |
.006 | - | - | −.58 (−1.10; −0.06) |
.029 |
| Age | −.014 (−.018; −.011) |
< .001 | −.015 (−.018; −.011) |
< .001 | −.035 (−.042; −.028) |
< .001 | −.035 (−.042; −.028) |
< .001 |
| Women | .024 (−.053; .101) |
.546 | .022 (−.055; .099) |
.576 | .182 (.023; .341) |
.025 | .179 (.021; 0.338) |
.027 |
| Education | −.006 (−.022; .011) |
.498 | −.005 (−.022; .011) |
.507 | −.011 (−.045; .022) |
.508 | −.011 (−.044; .022) |
.516 |
| White | −.191 (−.295; −.086) |
< .001 | −.188 (−.293; −.084) |
< .001 | −.351 (−.567; −.134) |
.002 | −.35 (−.563; −.131) |
.002 |
| Married | −.019 (−.103; .065) |
.660 | −.018 (−.102; .065) |
.665 | −.062 (−.235; .111) |
.483 | −.061 (−.234; .111) |
.486 |
| BMI | −.006 (−.012; −.001) |
.033 | −.007 (−.012; −.001) |
.028 | −.015 (−.027; −.003) |
.016 | −.015 (−.027; −.003) |
.014 |
| Former smoker | .015 (−.074; 0.104) |
.745 | .015 (−.074; .103) |
.747 | .072 (−.111; .255) |
.444 | .071 (−.112; .254) |
.445 |
| Current smoker | .112 (−.01; .233) |
.072 | .105 (−.016; .227) |
.088 | .215 (−.036; .466) |
.093 | .205 (−.046; .456) |
.109 |
| Taking hypertension medication | .039 (−.056; .133) |
.424 | .043 (−.051; .138) |
.367 | .069 (−.127; .265) |
.489 | .077 (−.119; .272) |
.440 |
| Heart disease diagnosis | .12 (−.032; .273) |
.122 | .129 (−.023; .281) |
.097 | .149 (−.166; .463) |
.354 | .162 (−.152; .477) |
.311 |
| Engage in physical activity | .025 (−.064; 0.115) |
.579 | .032 (−.057; .121) |
.477 | .064 (−.12; .249) |
.493 | .076 (−.108; .26) |
.419 |
Notes. RMSSD = root mean squared successive differences; HF-HRV = high frequency HRV; Ln = natural logged; Ms = milliseconds; BMI = body mass index; B = unstandardized beta coefficient. All continuous variables were centered at their mean; All HRV measures were natural log-transformed.
Model 2 that tested for the interaction effect of sleep quality showed a significant moderation by sleep quality for both indices of HRV (B = −.352, p = .006 for RMSSD; B = −.58, p = .029 for HF-HRV). To further probe the significant interaction effects, we performed simple slope tests and plotted the effects. The results are presented in Figure 1.
Figure 1.
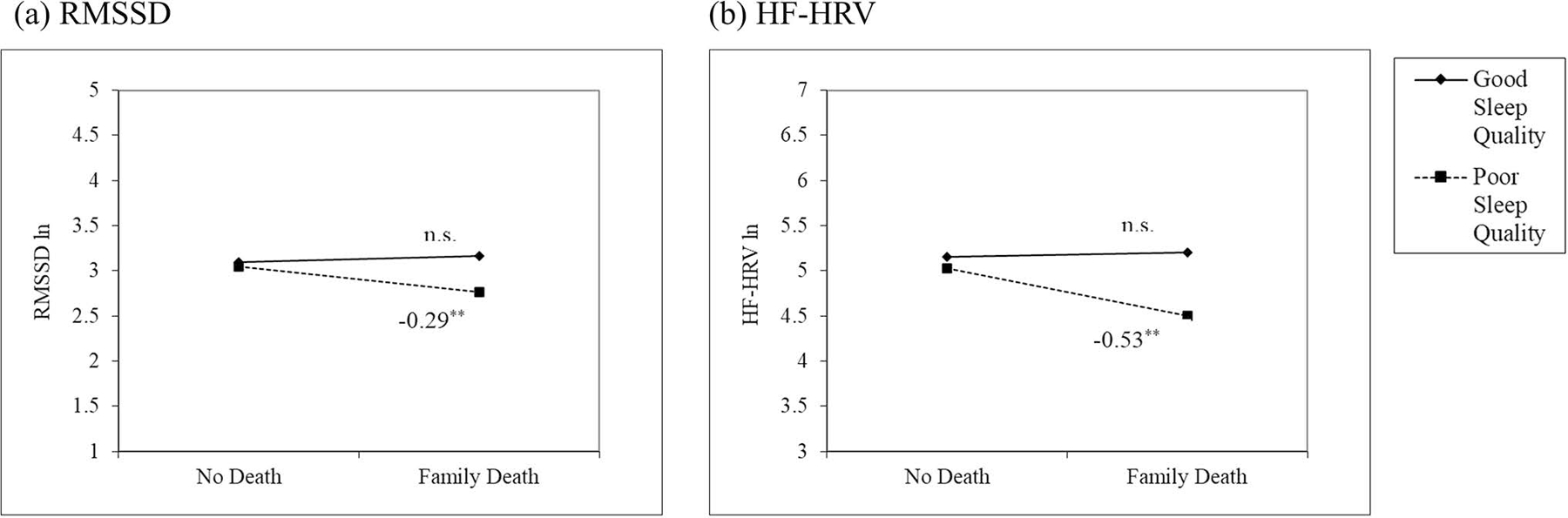
Interaction effects between experiencing the death of a family member and sleep quality on (a) RMSSD and (b) HF-HRV. Solid line indicates individuals with good sleep quality and dotted line indicates individuals with poor sleep quality. All HRV values were natural log-transformed. RMSSD = root mean squared successive differences; HF-HRV = high frequency HRV; n.s. = not significant. **p < .01.
Figure 1 shows that for both RMSSD and HF-HRV, experiencing the death of a family member was associated with lower levels of HRV only among those who reported poor sleep quality (B = −.294, p = .001 for RMSSD; B = −.529, p = .004 for HF-HRV). The associations were not significant for those who had good sleep quality. For example, results from RMSSD (Figure 1-a) showed that among individuals who reported poor sleep quality, those who experienced the death of a family member had 25.2% lower RMSSD compared to those who did not experience any deaths.
Supplementary Analyses
Due to the skewed distribution of sleep quality score in the study sample, using a binary measure of sleep quality was preferable compared to using a continuous measure of sleep quality in this study. As a supplementary analysis, we log-transformed continuous sleep quality score to normalize the distribution and tested whether our results would differ with a continuous measure of sleep quality. Results showed similar interaction patterns, where the interaction effect between experiencing a family member’s death and sleep quality was B = −.233, p = .022 for RMSSD and B = −.399, p = .058 for HF-HRV. In addition, among individuals who experienced a family member’s death, we also examined whether the association between sleep quality and HRV differed by time since death (in months). Results showed no significant differences by time since death.
Discussion
Two separate and growing literatures are exploring the link between bereavement and cardiovascular health (Buckley, Stannard, et al., 2012; Fagundes et al., 2018) and between sleep quality and cardiovascular outcomes (Castro-Diehl et al., 2016; Jackowska et al., 2012) in adulthood. While prior studies point to the role of bereavement and sleep as significant determinants of cardiovascular health, research failed to consider the joint dynamics of these two factors on cardiovascular outcomes. Also, previous findings on bereavement and cardiovascular health mainly focused on the early stages of bereavement, within 6 months since death (Buckley, Stannard, et al., 2012; Fagundes et al., 2018). This study uniquely extends prior studies by examining the interplay between family bereavement and sleep quality using a national sample of US adults. In addition, because the health consequences of experiencing the death of a family member can be long-lasting beyond the earlier stages of bereavement (N. J. Johnson et al., 2000; Stroebe et al., 2007), we included bereaved individuals who were within 12 months since the death of a family member. This study used heart rate variability (HRV) as a physiological marker of cardiovascular functioning, as this was found to be a significant correlate of future cardiovascular events (Bartrop et al., 2016; Haensel et al., 2008). We first explored the associations between experiencing the death of a family member and HRV (i.e., RMSSD and HF-HRV) and further tested whether these associations differed by sleep quality.
Findings showed significant interaction effects for both indices of HRV, such that experiencing the death of a family member was associated with lower (i.e., worse) levels of HRV only among individuals with poor sleep quality and not for those with good sleep quality. These results suggest that individuals with worse sleep may be more vulnerable to the detrimental cardiovascular health costs of stressful events such as experiencing the death of a family member. Such findings add to the prior literature on stress, sleep, and health by showing that the joint effects of family bereavement and sleep quality extends to a physiological measure of cardiovascular functioning.
Among those with poor sleep quality, our findings showed that experiencing the death of a family member was associated with lower levels of HRV. HRV is considered to be an indirect measure of vagal tone, or the amount of influence that the vagus nerve of the parasympathetic nervous system (PNS) has on cardiac functioning. While one’s body normally maintains homeostasis in the parasympathetic and sympathetic branches of the autonomic nervous system, long-term or intense stress can induce autonomic imbalance that involves prolonged sympathetic activation and parasympathetic underactivity (Schneider & Schwerdtfeger, 2020; Schubert et al., 2009). Such dysfunction results in decreased levels of HRV, which may lead to a higher risk of cardiovascular disease (Bartrop et al., 2016; Haensel et al., 2008). Considering that the death of a family member involves intense stress responses such as physiological arousal, emotional disturbance, and grief (Mayer et al., 2013; Parkes & Prigerson, 2010), the distress of losing a loved one could trigger autonomic dysregulation that lowers HRV. This may also explain the popular term “broken heart syndrome”, which refers to a cardiovascular dysfunction following an acute emotional or physical stress such as bereavement (Virani et al., 2007). While this finding is consistent with previous studies which found that bereaved individuals had lower levels of HRV compared to the nonbereaved group (Buckley, Stannard, et al., 2012; Fagundes et al., 2018), these were limited to examining recent bereavement experiences within 6 months since death. This study extends previous research by finding that the cardiovascular health impact of experiencing the death of a family member may be long-lasting beyond the early stages of bereavement.
For HRV, poor sleep quality may exacerbate the impact of experiencing the death of a family member on cardiovascular autonomic functioning due to the role of sleep as a restorative process that influences the body’s ability to cope with stressors (Hamilton et al., 2007). Sleep is considered as an adaptive process that helps individuals to adequately respond to external stressors, and lack of good sleep may disturb effective stress responses. One of the major stress responses involves autonomic nervous system that modulates HRV. Studies on sleep and autonomic functioning find that while night-time sleep is associated with parasympathetic dominance with a decrease in sympathetic activity, low-quality sleep is linked to higher sympathetic activity and lower parasympathetic vagal tone (Castro-Diehl et al., 2016; Tobaldini et al., 2017). The latter is an indication of activated stress systems, and prolonged activation of stress systems from chronic sleep problems can result in a dysregulation of the autonomic cardiovascular control and decreased HRV (Meerlo et al., 2008).
Ability to cope with stressors is particularly important for those who experienced the death of a family member. Not only is the death itself a traumatic event, but bereavement also comes with secondary stressors that may arise as a consequence of losing the role that the deceased family member played. This may involve having to learn how to do household chores, manage finances, live alone, and find new source of support (Stroebe & Schut, 1999). Experiencing such stressors may be more taxing for individuals when it is combined with poor sleep because low-quality sleep inhibits adaptive response to stressors. Therefore, having poor sleep quality may render bereaved individuals more vulnerable to the psychophysiological costs of experiencing the death of a family member, as indicated by lower (i.e., worse) levels of HRV.
This study has several strengths, including the intensive assessment of HRV in a national sample of US adults. Also, findings from this study broadens our understanding of family bereavement and HRV by examining the moderating role of sleep quality. There are, however, some limitations of this study to note. First, we focused on the role of perceived sleep quality based on a well-validated scale that incorporates multiple components of sleep (Buysse et al., 1989). Future studies that incorporate both subjective and objective measures of sleep (e.g., actigraphy, polysomnography) can provide a more enriched understanding of how different sleep characteristics influence the associations between family death experiences and cardiovascular functioning. It should be also noted that because resting HRV is influenced by a number of other factors such as age and fitness (Laborde et al., 2017), interpreting the role of sleep quality in HRV as a distinct measure of autonomic functioning warrants some caution. Future studies that use long-term measurement of HRV (e.g., ambulatory assessments of HRV) along with objective measures of sleep are needed in order to better understand the link between sleep quality and HRV.
Also, we were not able to control for respondents’ clinically diagnosed sleep disorders such as sleep apnea or insomnia because we did not have the information available. Given the strong associations between sleep disorders and cardiovascular diseases (Malhotra & Loscalzo, 2009), the combined health implications of experiencing a family member’s death and sleep quality may differ between clinical and non-clinical samples.
Third, this study did not explore mediating mechanisms that may link family bereavement and HRV such as complicated grief (CG). Previous studies show that individuals with CG in reaction to bereavement had attenuated physiological reactivity (LeBlanc et al., 2016), which suggest CG as a potential mediator between bereavement and HRV. Examining these mechanisms could broaden our understanding of the specific pathways through which bereavement influences HRV outcomes. Fourth, this study used cross-sectional data. While the Biomarker Project provided HRV measures of already-bereaved individuals, measures of HRV taken before the death experience was not available. Considering that the death of a family member is a critical event that is followed by an abrupt change in health outcomes (Wilson et al., 2020), having longitudinal measurements of HRV pre- and post-family death would allow for a more precise test of causal relationships between family bereavement and HRV.
In addition, this study did not account for the context of the family member’s death. The impact of family member’s death on cardiovascular functioning may differ depending on the type and quality of the relationship involved. For example, experiencing the death of a child is often considered as the most disruptive of all deaths that adults experience (Parkes & Prigerson, 2010). Studies that compared across different types of family death found that being bereaved of a child was associated with worse health outcomes such as depression and psychiatric illness compared to the death of a parent, a sibling, or a spouse (Guldin et al., 2017; Stroebe et al., 2007). In our study sample, the number of respondents for each type of family death were not sufficient to make comparisons (e.g., 1 spousal bereavement, 6 parental bereavement). Future studies could provide a more nuanced understanding of the relation between family bereavement and cardiovascular health by specifying the type and quality of relationship with the deceased.
Lastly, the vast majority of MIDUS participants were white (80.7%), which limits the generalizability of the study findings to other racial and ethnic groups. Experiences of a family member’s death may differ by race (Umberson, 2017), and race is also a significant determinant of sleep patterns and cardiovascular health (Egan et al., 2017; Grandner et al., 2016; D. A. Johnson et al., 2019). Future studies need examine whether our findings are replicated in a racially more diverse sample and also compare how these associations differ by race and ethnicity.
Despite the limitations, this study adds to the literature on bereavement and health by examining the joint effects of family bereavement and sleep quality on cardiovascular functioning. Results suggest that those with poor sleep quality may be more vulnerable to the negative cardiovascular health consequences of experiencing the death of a family member. These findings provide evidence needed to ground interventions that aim to improve sleep quality among the bereaved individuals and reduce the health toll of experiencing the death of a close family member.
Supplementary Material
Funding.
The original Midlife in the United States (MIDUS) study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Longitudinal follow-up of the original MIDUS study was supported by grants from the National Institute on Aging (P01-AG020166, U19-AG051426). The biomarker data collection was further supported from the National Institutes of Health National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) program (UL1TR001409 for Georgetown, UL1TR001881 for UCLA, and 1UL1RR025011 for UW). Support also came from Population Research Center at The University of Texas at Austin which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P2CHD042849) and Center on Aging and Population Sciences at The University of Texas at Austin which is supported by the National Institute on Aging (P30AG066614).
Footnotes
Conflicts of interest. The authors declare no conflicts of interest.
Declarations
Ethics approval. Data collection for both MIDUS II and MIDUS Refresher Biomarker projects were approved by IRB at the University of Wisconsin-Madison.
Consent to participate. All participants provided written consent prior to participation.
Consent for publication. Not applicable.
Availability of data and material. MIDUS data is publicly available for download via ICPSR website, at https://www.icpsr.umich.edu
References
- Bartrop R, Buckley T, & Tofler G (2016). Bereavement and the risk of cardiovascular disease. In Alvarenga ME & Byrne D (Eds.), Handbook of Psychocardiology (pp. 229–246). Springer; Singapore. 10.1007/978-981-287-206-7_18 [DOI] [Google Scholar]
- Benham G (2010). Sleep: An important factor in stress-health models. Stress and Health, 26(3), 204–214. 10.1002/smi.1304 [DOI] [Google Scholar]
- Berntson GG, Quigley KS, Jang JF, & Boysen ST (1990). An approach to artifact identification: Application to heart period data. Psychophysiology, 27(5), 586–598. 10.1111/j.1469-8986.1990.tb01982.x [DOI] [PubMed] [Google Scholar]
- Bodnar JC, & Kiecolt-Glaser JK (1994). Caregiver depression after bereavement: Chronic stress isn’t over when it’s over. Psychology and Aging, 9(3), 372–380. 10.1037/0882-7974.9.3.372 [DOI] [PubMed] [Google Scholar]
- Buckley T, McKinley S, Tofler G, & Bartrop R (2010). Cardiovascular risk in early bereavement: A literature review and proposed mechanisms. International Journal of Nursing Studiethays, 47(2), 229–238. 10.1016/j.ijnurstu.2009.06.010 [DOI] [PubMed] [Google Scholar]
- Buckley T, Stannard A, Bartrop R, McKinley S, Ward C, Mihailidou AS, Morel-Kopp M-C, Spinaze M, & Tofler G (2012). Effect of early bereavement on heart rate and heart rate variability. The American Journal of Cardiology, 110(9), 1378–1383. 10.1016/j.amjcard.2012.06.045 [DOI] [PubMed] [Google Scholar]
- Buckley T, Sunari D, Marshall A, Bartrop R, McKinley S, & Tofler G (2012). Physiological correlates of bereavement and the impact of bereavement interventions. Dialogues in Clinical Neuroscience, 14(2), 129–139. 10.31887/DCNS.2012.14.2/tbuckley [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TK, Owens J, Lee L, Reis SE, & Matthews KA (2008). Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine, 04(06), 563–571. 10.5664/jcsm.27351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the pittsburgh sleep quality index. Journal of Psychosomatic Research, 45(1), 5–13. 10.1016/S0022-3999(97)00298-5 [DOI] [PubMed] [Google Scholar]
- Carstensen LL (1992). Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging, 7(3), 331–338. 10.1037//0882-7974.7.3.331 [DOI] [PubMed] [Google Scholar]
- Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, & Shea S (2016). Sleep duration and quality in relation to autonomic nervous system measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep, 39(11), 1927–1940. 10.5665/sleep.6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JF (2014). Moderation in management research: What, why, when, and how. Journal of Business and Psychology, 29(1), 1–19. 10.1007/s10869-013-9308-7 [DOI] [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, & Ryff CD (2010). Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22(8), 1059–1080. 10.1177/0898264310374355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzewski JM, Mitchell M, Rodriguez JC, Fung CH, Jouldjian S, Alessi CA, & Martin JL (2015). Patterns and predictors of sleep quality before, during, and after hospitalization in older adults. Journal of Clinical Sleep Medicine, 11(1), 45–51. 10.5664/jcsm.4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KJ, Knutson KL, Pereira AC, & von Schantz M (2017). The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Medicine Reviews, 33, 70–78. 10.1016/j.smrv.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Murdock KW, LeRoy A, Baameur F, Thayer JF, & Heijnen C (2018). Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: Mechanisms underlying cardiovascular risk? Psychoneuroendocrinology, 93, 65–71. 10.1016/j.psyneuen.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Grandner MA, Williams NJ, Knutson KL, Roberts D, & Jean-Louis G (2016). Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Medicine, 18, 7–18. 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldin M-B, Ina Siegismund Kjaersgaard M, Fenger-Grøn M, Thorlund Parner E, Li J, Prior A, & Vestergaard M (2017). Risk of suicide, deliberate self-harm and psychiatric illness after the loss of a close relative: A nationwide cohort study. World Psychiatry, 16(2), 193–199. 10.1002/wps.20422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel A, Mills PJ, Nelesen RA, Ziegler MG, & Dimsdale JE (2008). The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology, 33(10), 1305–1312. 10.1016/j.psyneuen.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NA, Catley D, & Karlson C (2007). Sleep and the affective response to stress and pain. Health Psychology, 26(3), 288–295. 10.1037/0278-6133.26.3.288 [DOI] [PubMed] [Google Scholar]
- Hooyman NR, & Kramer BJ (2006). Living through loss: Interventions across the life span Columbia University Press. [Google Scholar]
- Jackowska M, Dockray S, Endrighi R, Hendrickx H, & Steptoe A (2012). Sleep problems and heart rate variability over the working day. Journal of Sleep Research, 21(4), 434–440. 10.1111/j.1365-2869.2012.00996.x [DOI] [PubMed] [Google Scholar]
- Johnson DA, Jackson CL, Williams NJ, & Alcántara C (2019). Are sleep patterns influenced by race/ethnicity - a marker of relative advantage or disadvantage? Evidence to date. Nature and Science of Sleep, 11, 79–95. 10.2147/NSS.S169312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NJ, Backlund E, Sorlie PD, & Loveless CA (2000). Marital status and mortality: The national longitudinal mortality study. Annals of Epidemiology, 10(4), 224–238. 10.1016/S1047-2797(99)00052-6 [DOI] [PubMed] [Google Scholar]
- Kivimäki M, & Steptoe A (2018). Effects of stress on the development and progression of cardiovascular disease. Nature Reviews Cardiology, 15(4), 215–229. 10.1038/nrcardio.2017.189 [DOI] [PubMed] [Google Scholar]
- Laborde S, Mosley E, & Thayer JF (2017). Heart rate variability and cardiac vagal tone in psychophysiological research – Recommendations for experiment planning, data analysis, and data reporting. In Frontiers in Psychology (Vol. 8, p. 213). 10.3389/fpsyg.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel M, Stroebe M, & Eisma MC (2020). Sleep disturbances in bereavement: A systematic review. Sleep Medicine Reviews, 53, 101331. 10.1016/j.smrv.2020.101331 [DOI] [PubMed] [Google Scholar]
- LeBlanc NJ, Unger LD, & McNally RJ (2016). Emotional and physiological reactivity in Complicated Grief. Journal of Affective Disorders, 194, 98–104. 10.1016/j.jad.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Lee S, Crain TL, McHale SM, Almeida DM, & Buxton OM (2017). Daily antecedents and consequences of nightly sleep. Journal of Sleep Research, 26(4), 498–509. 10.1111/jsr.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Stone KL, Engeland CG, Lane NE, & Buxton OM (2020). Arthritis, sleep health, and systemic inflammation in older men. Arthritis care & research, 72(7), 965–973. 10.1002/acr.23923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D. “Dale” M., Rosenfeld AG, & Gilbert K (2013). Lives forever changed: Family bereavement experiences after sudden cardiac death. Applied Nursing Research, 26(4), 168–173. 10.1016/j.apnr.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Malhotra A, & Loscalzo J (2009). Sleep and cardiovascular disease: An overview. Progress in Cardiovascular Diseases, 51(4), 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, & Suchecki D (2008). Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Medicine Reviews, 12(3), 197–210. 10.1016/j.smrv.2007.07.007 [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Allen JJB, & Kaszniak AW (2002). Autonomic and emotion regulation in bereavement and depression. Journal of Psychosomatic Research, 52(4), 183–185. 10.1016/S0022-3999(02)00292-1 [DOI] [PubMed] [Google Scholar]
- Parkes CM, & Prigerson HG (2010). Bereavement: Studies of Grief in Adult Life (4th Ed.). Routledge. [Google Scholar]
- Roberts AW, Ogunwole SU, Blakeslee L, & Rabe MA (2018). The population 65 years and older in the United States: 2016 [Google Scholar]
- Schneider M, & Schwerdtfeger A (2020). Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: a meta-analysis. Psychological Medicine, 50(12), 1937–1948. 10.1017/S003329172000207X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Lambertz M, Nelesen RA, Bardwell W, Choi J-B, & Dimsdale JE (2009). Effects of stress on heart rate complexity—A comparison between short-term and chronic stress. Biological Psychology, 80(3), 325–332. 10.1016/j.biopsycho.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, & Ginsberg JP (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, & Buxton OM (2017). Bidirectional, temporal associations of sleep with positive events, affect, and stressors in daily life across a week. Annals of Behavioral Medicine, 51(3), 402–415. 10.1007/s12160-016-9864-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. (2019). Stata Statistical Software: Release 16 StataCorp LLC. [Google Scholar]
- Stroebe M, & Schut H (1999). The dual process model of coping with bereavement: Rationale and description. Death Studies, 23(3), 197–224. 10.1080/074811899201046 [DOI] [PubMed] [Google Scholar]
- Stroebe M, Schut H, & Stroebe W (2007). Health outcomes of bereavement. The Lancet, 370(9603), 1960–1973. 10.1016/S0140-6736(07)61816-9 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2007). The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology, 74(2), 224–242. 10.1016/j.biopsycho.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, & Brosschot JF (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L, & Montano N (2017). Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neuroscience & Biobehavioral Reviews, 74, 321–329. 10.1016/j.neubiorev.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Nobili L, Strada S, Casali K, Braghiroli A, & Montano N (2013). Heart rate variability in normal and pathological sleep. Frontiers in Physiology, 4, 294. 10.3389/fphys.2013.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D (2017). Black deaths matter: Race, relationship loss, and effects on survivors. Journal of Health and Social Behavior, 58(4), 405–420. 10.1177/0022146517739317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Khan AN, Mendoza CE, Ferreira AC, & de Marchena E (2007). Takotsubo cardiomyopathy, or broken-heart syndrome. Texas Heart Institute Journal, 34(1), 76–79. https://pubmed.ncbi.nlm.nih.gov/17420797 [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Ryff CD, & Seeman TE (2019). Midlife in the United States (MIDUS Refresher 1): Biomarker Project, 2012–2016 Inter-university Consortium for Political and Social Research [distributor]. 10.3886/ICPSR36901.v6 [DOI] [Google Scholar]
- Wilson SJ, Padin AC, Bailey BE, Laskowski B, Andridge R, Malarkey WB, & Kiecolt-Glaser JK (2020). Spousal bereavement after dementia caregiving: A turning point for immune health. Psychoneuroendocrinology, 118, 104717. 10.1016/j.psyneuen.2020.104717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


