Abstract
Background
Remote ischemic conditioning (RIC) has been beneficial in laboratory studies of anthracycline cardiotoxicity, but its effects in patients is not established.
Objectives
The authors studied the effect of RIC on cardiac biomarkers and function during and after anthracycline chemotherapy.
Methods
The ERIC-Onc study (Effect of Remote Ischaemic Conditioning in Oncology Patients; NCT02471885) was a randomized, single-blind, sham-controlled study of RIC at each chemotherapy cycle. The primary endpoint was troponin T (TnT) during chemotherapy and up to 1 year. Secondary outcomes included cardiac function, major adverse cardiovascular events (MACE), and MACE or cancer death. Cardiac myosin-binding-protein C (cMyC) was investigated in parallel with TnT.
Results
The study was prematurely halted after the evaluation of 55 patients (RIC n = 28, sham n = 27). Biomarkers increased from baseline to cycle 6 of chemotherapy for all patients (median TnT 6 [IQR: 4-9] ng/L to 33 [IQR: 16-36)] ng/L; P ≤ 0.001; cMyC 3 (IQR: 2-5) ng/L to 47 (IQR: 18-49) ng/L; P ≤ 0.001). Mixed-effects regression analysis for repeated measures showed no difference in TnT between the 2 groups (RIC vs sham, mean difference 3.15 ng/L; 95% CI: −0.04 to 6.33; P = 0.053), or cMyC (RIC vs sham, mean difference 4.17 ng/L; 95% CI: −0.12 to 8.45; P = 0.056). There were more MACE and cancer deaths in the RIC group (11 vs 3; HR: 0.25; 95% CI: 0.07-0.90; P = 0.034), with more cancer deaths (8 vs 1; HR: 0.21; 95% CI: 0.04-0.95; P = 0.043) at 1 year.
Conclusions
TnT and cMyC significantly increased during anthracycline chemotherapy with 81% having a TnT ≥14 ng/L at cycle 6. RIC did not affect the rise in biomarkers, but there was a small increase in early cancer deaths, possibly related to the greater proportion of patients with metastatic disease randomized to the RIC group (54%vs 37%). (Effect of Remote Ischaemic Conditioning in Oncology Patients [ERIC-ONC]; NCT02471885)
Key Words: anthracycline, cardiac function, cardioprotection, cardiotoxicity, remote ischemic conditioning, troponin
Central Illustration
Anthracyclines remain a cornerstone therapy for various cancers; however, cardiotoxicity (when defined as a 10% drop in left ventricular [LV] ejection fraction [LVEF] to <50%) occurs in up to 9%1 of cases. The mechanism of injury is multifactorial,2,3 and cardioprotective strategies have shown variable results.3,4 Dexrazoxane is currently the only approved therapy in patients receiving high-dose anthracyclines; however, its use has not been widespread.5,6 Remote ischemic conditioning (RIC) is a noninvasive nonpharmacological cardioprotective intervention that has been repeatedly shown in laboratory and clinical proof-of-concept studies to protect against ischemia-reperfusion injury (IRI),7,8 including the mitigation of cardiac biomarker release in some clinical studies.9 Anthracycline cardiotoxicity and IRI share common pathophysiological mechanisms with doxorubicin enhancing damage from IRI,10 and iron chelators, such as dexrazoxane, inhibiting ferroptosis that is implicated in both pathologies.11 Thus, RIC appears to be a potentially useful intervention to explore. Experiments in animals have shown direct benefit of RIC in anthracycline cardiotoxicity in terms of cell death,12 survival,13 troponin14 release, and LVEF.14,15 In this study, we investigated for the first time in cancer patients, the effect of RIC in patients receiving anthracycline chemotherapy (Effect of Remote Ischaemic Conditioning in Oncology Patients [ERIC-ONC] trial; NCT02471885).16
Methods
Patient population
This was a randomized, single-blind, sham-controlled phase II trial performed at University College London Hospitals (UCLH) in accordance with the Declaration of Helsinki and received ethics approval from the local National Health Service Health Research Authority.16 Patients aged 16 to 80 years, with any cancer, who were designated to receive anthracycline-containing chemotherapy, were recruited. Exclusion criteria were reported previously16 and summarized in Supplemental Table 1; chemotherapy is described in Supplemental Table 2.
Randomization
Randomization was performed 1:1 into a RIC or sham group using randomization software (MinimPy Version 0.3) with minimization for coronary artery disease, treated hypertension, and diabetes.
Study protocol
The study protocol is shown in Figure 1.
Figure 1.
Study Protocol
Blood tests were taken before the remote ischemic conditioning (RIC) intervention that was completed immediately before commencement of anthracyclines. A second blood test 3 to 24 hours post-chemotherapy was performed for troponin T (TnT) and cardiac myosin binding protein C (cMyC). Anthracyclines were administered either as a single bolus injection over 10 to 20 minutes or as a 46-hour infusion at each chemotherapy cycle, typically 3 weeks apart. At their penultimate or ultimate cycle, a 14-day electrocardiogram (ECG) monitor patch was applied. Follow-up was performed at 4 time points as shown. A 5-year survival outcome was added at the request of the ethics committee; this is ongoing. BP = blood pressure; M = months; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Procedures
Intervention protocols
At each chemotherapy session, a blood pressure cuff was applied onto a patient’s arm and connected to an automated RIC machine17 that, before anthracycline dosing, inflated the cuff to a pressure of 200 mm Hg (or 30 mm Hg above systolic if not tolerated or if platelets were between 50 × 109/L and 100 × 109/L) for 5 minutes followed by 5 minutes of reperfusion for a total of 4 cycles. If platelets were <50 × 109/L on the day, then the RIC was omitted on that occasion. For the sham protocol, the cuff was inflated to 10 mm Hg for 5 minutes, then deflated for 4 cycles. The study clinicians (M.M., R.C., A.K.G., and J.M.W.) were blinded to the RIC intervention, but it is acknowledged that patient blinding to RIC vs sham is difficult to achieve and thus not assumed. Data were analyzed blinded to the treatment group.
Cardiac biomarkers
Blood samples for high-sensitivity troponin T (TnT), and cardiac myosin-binding protein C (cMyC) were collected immediately before each RIC application and between 3 to 24 hours after the end of each anthracycline infusion. TnT was measured by a standardized high-sensitivity assay, Elecsys (Roche) with a 10% coefficient of variation, upper limit of normal <14 ng/L, lower limit of detection of 5 ng/L, measurement range 3 to 10,000 ng/L.18 cMyC was assayed by a dedicated laboratory,19,20 utilizing the EMD Millipore on the Erenna platform, which has a lower limit of detection of 0.4 ng/L and a lower limit of quantification (20% coefficient of variation) of 1.2 ng/L.
Echocardiography
Transthoracic echocardiography was performed (GE E6) using a standardized cardio-oncology protocol. LVEF was measured using 4-dimensional volumetric assessment and/or biplane Simpson’s method where possible, or by visual estimate otherwise. Global longitudinal strain (GLS), was measured via GE EchoPac software. Scan acquisition and analysis were undertaken by sonographers and reviewed by clinicians (M.M. and R.C.) blinded to the treatment protocol; the UCLH echo department and sonographers are European Association of Cardiovascular Imaging accredited.
Arrhythmia monitoring
Extended electrocardiogram (ECG) monitoring between cycles 5 and 6 was performed for up to 14 days, using a 1-lead ambulatory electrocardiogram (Zio XT patch, iRhythm Technologies).
Primary outcome
The primary outcome was a comparison between the 2 groups of TnT up to 12 months post-chemotherapy, analyzed both as a continuous and binary (positive [>14 ng/L] vs negative) variable.
The acute effect of anthracyclines and possible effect of RIC on TnT was investigated by comparison of pre- and immediate post-chemotherapy samples, for each cycle of anthracycline treatment (Supplemental Figure 1, Supplemental Table 3).
Secondary outcomes
Cardiac myosin binding protein C
cMyC is released rapidly after acute myocardial injury, and has not been studied in patients receiving anthracyclines. We hypothesized that cMyC might be more sensitive and detected earlier than troponin. Following a study protocol ethics amendment, 22 patients consented to have cMyC analysis. Using pre-chemotherapy samples, the ratio of peak to baseline concentration as well as the ratio at each cycle were compared for each group.
Echocardiographic parameters
The change in LVEF (ΔLVEF) from baseline to 3 and 12 months (3M and 12M, respectively), and the change in GLS (ΔGLS and relative percentage change in GLS) from baseline to 3M and 12M post-chemotherapy was compared between the 2 groups.
NT-proBNP
The change in N-terminal pro–B-type natriuretic peptide (NT-proBNP; ΔNT-proBNP) from baseline to 3M post-chemotherapy was compared between the 2 groups.
Clinical outcomes
Major adverse cardiovascular events (MACE) from enrollment to 12M follow-up were defined as follows: myocardial infarction, heart failure (HF) or asymptomatic LV dysfunction needing hospitalization or initiation of HF medications, life-threatening tachyarrhythmia needing treatment or bradyarrhythmia requiring pacing, and cardiac death. Deaths from any cause, and serious adverse events defined as any infection, venous thromboembolic event, anemia requiring transfusion, gastroesophageal reflux disease, epistaxis, or back pain were recorded (Supplemental Table 4).
Events were adjudicated by study clinicians (J.M.W., A.K.G., and R.C.) blinded to the treatment groups and reviewed by an independent data monitoring committee (DMC).
Arrhythmia incidence
The incidence of arrhythmia during chemotherapy was assessed using the 14-day ECG and defined according to published guidelines.21
Sample size
Sample-size calculations for the study were described before16 and assumed a treatment effect of 35% with 80% power at the 5% significance level, giving a sample size of 128 (n = 64 in each arm of the study). This calculation was based on the (TnT) cardiotoxicity data in cancer patients receiving chemotherapy that was available at the time. The categorical effect of RIC on TnT was hypothesized based on studies in the context of cardiac surgery, elective stenting, and ST-segment elevation myocardial infarction, with event rate reductions between 16% and 49%.22, 23, 24
Statistical analysis
Statistical analysis and graphical representation were performed with the SPSS statistical software (IBM SPSS Statistics, Version 28). Statistical significance was considered at the 5% significance level. For the primary outcome, comparison was performed with a mixed-effects model for repeated measures and presented as the least squares mean difference with 95% CI. The mixed-effects model for repeated measures included treatment arm and time point of TnT sampling as fixed-effect covariates and TnT value as the dependent-effect covariate (with the intercept line as a random-effect covariate). Normality was assessed with Q:Q plots and Shapiro-Wilk tests for each time point of TnT sampling and each group. Summary statistics were described as mean ± SD and median with 25th and 75th percentiles (IQR) for continuous variables, and as counts and percentages for categorical variables. Between-group comparisons were made using the independent samples t-test and Mann-Whitney test for continuous data, and multinomial logistic regression for categorical data. Within-group comparisons were undertaken using the paired samples t-test and Wilcoxon signed rank test for continuous data. Time-to-event data are presented as counts and by using Kaplan-Meier plots. Kaplan-Meier estimates were compared using the log-rank test, and Cox proportional hazards models were used to estimate the HR with 95% CI. Clinical adverse events are presented as counts and percentages.
Results
Patient recruitment
From February 2016 to February 2020, 60 patients were recruited, with 31 allocated to the RIC group and 29 to the sham group. Details of patients excluded are given in Supplemental Figure 1.
Of the 31 in the RIC group, 1 withdrew consent after randomization before chemotherapy, 1 withdrew consent after cycle 1. One patient has not been included in analyses as they were withdrawn at cycle 1 due to the precautionary halting of all clinical trial activity, including nonessential laboratory analyses (eg, nonclinical TnT) not directly related to COVID at UCLH. Of the 29 in the sham group, 2 withdrew consent after randomization but before chemotherapy. One further patient from the sham group withdrew at the 3M follow-up and, with permission, is included in the analysis. Enrollment flow chart shown in Supplemental Figure 2.
At the beginning of the global COVID-19 pandemic in 2020, all non-COVID research was halted at our institution. Simultaneously, a planned interim analysis by the data monitoring committee (DMC) recommended a halt in recruitment for the following reasons: 1) no difference seen in TnT between the 2 groups, despite a higher than predicted (81% vs 49%) incidence of TnT events, suggesting that the study was unlikely to meet its primary endpoint; and 2) there was a signal toward more adverse events identified in the RIC group.
Baseline characteristics
Mean age was 49 ± 16 years with 40% female. Forty-five (82%) had sarcoma, 6 (11%) lymphoma, and 4 (7%) breast cancer; the distribution of cancer types did not differ between the RIC or sham groups. Twenty-five (46%) had metastatic disease, 54% in RIC and 37% in sham. The mean cumulative dose of doxorubicin given during this cycle of chemotherapy was 317 ± 95 mg/m2 (Table 1). Baseline mean LVEF was 61% ± 4%, and baseline TnT was 6 ± 5 ng/L (full echocardiographic and laboratory baseline data are shown into Supplemental Tables 5 to 7).
Table 1.
Baseline Characteristics
| All (N = 55) | Group 1 (RIC) (n = 28) |
Group 2 (Sham) (n = 27) |
|
|---|---|---|---|
| Patient baseline details | |||
| Age, y | 49 ± 16 | 49 ± 17 | 49 ± 16 |
| Gender | |||
| Male | 33 (60) | 18 (64) | 15 (56) |
| Female | 22 (40) | 10 (36) | 12 (44) |
| Medical comorbidities | |||
| Hypertension | 5 (9) | 2 (7) | 3 (11) |
| Diabetes | 2 (4) | 1 (4) | 1 (4) |
| High cholesterol | 9 (16) | 4 (14) | 5 (19) |
| Othera | 21 (38) | 11 (39) | 10 (37) |
| Smoking status | |||
| Current smoker | 13 (24) | 7 (25) | 6 (22) |
| Former smoker | 15 (27) | 7 (25) | 8 (30) |
| Never smoked | 27 (49) | 14 (50) | 13 (48) |
| Family history of ischemic heart disease | |||
| Yes | 6 (11) | 3 (11) | 3 (11) |
| No | 43 (78) | 21 (75) | 22 (82) |
| Unknown | 6 (11) | 4 (14) | 2 (7) |
| Baseline medications | |||
| Beta-blockers | 1 (2) | 0 | 1 (4) |
| ACE inhibitors | 3 (6) | 2 (7) | 1 (4) |
| ARBs | 1 (2) | 0 | 1 (4) |
| CCB | 3 (6) | 2 (7) | 1 (4) |
| Thiazides | 3 (6) | 0 | 3 (11) |
| Statins | 5 (9) | 2 (7) | 3 (11) |
| Anticoagulants | 1 (2) | 1 (4) | 0 |
| Antidiabetic agents | 2 (4) | 1 (4) | 1 (4) |
| PPI | 10 (18) | 5 (18) | 5 (19) |
| Analgesics | 15 (27) | 10 (36) | 5 (19) |
| Steroid inhalers | 5 (9) | 2 (7) | 3 (11) |
| Steroids | 1 (2) | 0 | 1 (4) |
| Other | 21 (38) | 9 (32) | 12 (44) |
| Cancer and chemotherapy baseline details | |||
| Cancer type | |||
| Sarcoma | 45 (82) | 23 (82) | 22 (82) |
| Breast | 4 (7) | 2 (7) | 2 (7) |
| Lymphoma | 6 (11) | 3 (11) | 3 (11) |
| Metastatic | |||
| Yes | 25 (46) | 15 (54) | 10 (37) |
| No | 30 (55) | 13 (46) | 17 (63) |
| Cancer diagnosis type | |||
| New | 43 (78) | 21 (75) | 22 (82) |
| Relapse | 12 (22) | 7 (25) | 5 (19) |
| Anthracycline type | |||
| Doxorubicin | 51 (93) | 26 (93) | 25 (93) |
| Epirubicin | 4 (7) | 2 (7) | 2 (7) |
| Chemotherapy regimen | |||
| Dox | 11 (20) | 7 (25) | 4 (15) |
| D-Ifos | 12 (22) | 7 (25) | 5 (19) |
| FEC-PC | 1 (2) | 0 | 1 (4) |
| D-Cis | 9 (16) | 4 (14) | 5 (19) |
| D-Ola | 2 (4) | 0 | 2 (7) |
| RCHOP | 3 (6) | 1 (4) | 2 (7) |
| MAP | 3 (6) | 1 (4) | 2 (7) |
| VI-Dox | 1 (2) | 0 | 1 (4) |
| FEC-DT | 2 (4) | 1 (4) | 1 (4) |
| CHOEP | 1 (2) | 1 (4) | 0 |
| CHOP | 1 (2) | 1 (4) | 0 |
| IVA-Dox | 1 (2) | 1 (4) | 0 |
| RCHOP-Mtx | 1 (2) | 0 | 1 (4) |
| VIDE | 4 (7) | 2 (7) | 2 (7) |
| VDCIE | 2 (4) | 1 (4) | 1 (4) |
| FEC-D | 1 (2) | 1 (4) | 0 |
| ECOG WHO performance status | |||
| 0 | 30 (55) | 14 (50) | 16 (59) |
| 1 | 18 (33) | 10 (35) | 8 (30) |
| 2 | 2 (4) | 1 (4) | 1 (4) |
| 4 | 1 (2) | 1 (4) | 0 |
| Unknown | 4 (7) | 2 (7) | 2 (7) |
| Total chemotherapy cycles received | |||
| 2 | 2 (4) | 1 (4) | 1 (4) |
| 3 | 12 (22) | 7 (25) | 5 (19) |
| 4 | 9 (16) | 7 (25) | 2 (7) |
| 5 | 4 (7) | 2 (7) | 2 (7) |
| 6 | 28 (51) | 11 (39) | 17 (63) |
| Total cumulative anthracycline dose received, mg/m2b | 317 ± 95 | 301 ± 94 | 333 ± 95 |
| Method of administration | |||
| Slow bolus | 24 (44) | 13 (46) | 11 (41) |
| 46-h infusion | 24 (44) | 12 (43) | 12 (44) |
| Both | 7 (13) | 3 (11) | 4 (15) |
| Intervention details | |||
| RIC/sham full protocol receivedc | |||
| Yes | 50 (91) | 26 (93) | 24 (89) |
| No | 5 (9) | 2 (7) | 3 (11) |
| Total number of RIC/sham cycles, nd | 257 | 125 | 132 |
| LV function parameters | |||
| LVEF, % | 61 ± 4 | 62 ± 5 | 60 ± 3 |
| GLS, % | −18.8 ± 2.6 (n = 45) | −18.9 ± 2.3 (n = 25) | −18.7 ± 2.9 (n = 20) |
| Cardiac biomarkers | |||
| Troponin T, ng/Le | 6 [4-9] (n = 54) | 6 [4-9] (n = 27) | 6 [3-9] |
| NT-proBNP, ng/Lf | 58 [49-89] (n = 51) | 50 [49-76] (n = 24) | 60 [49-95] |
Values are mean ± SD, n (%), or median [IQR], except as noted.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CCB = calcium channel blocker; CHOP = cyclophosphamide-doxorubicin-vincristine-prednisolone; CHOEP = cyclophosphamide-doxorubicin-vincristine-etoposide-prednisolone; Dox = doxorubicin; D-Cis = doxorubicin-cisplatin; D-Ola = doxorubicin-olaratumab; D-Ifos = doxorubicin-ifosfamide; ECOG WHO = Eastern Cooperative Cancer Oncology Group World Health Organization; FEC-D = fluorouracil-epirubicin-cyclophosphamide-docetaxel; FEC-DT = fluorouracil-epirubicin-cyclophosphamide-docetaxel-trastuzumab; FEC-PC = fluorouracil-epirubicin-cyclophosphamide-paclitaxel-carboplatin; GLS = global longitudinal strain; IVA-Dox = ifosfamide-vincristine-dactinomycin-doxorubicin; LV = left ventricular; LVEF = left ventricular ejection fraction; MAP = methotrexate-doxorubicin-cisplatin; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PPI = protein pump inhibitor; RCHOP = rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone; RCHOP-Mtx = rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone-methotrexate; RIC = remote ischemic conditioning; VDCIE = vincristine-doxorubicin-cyclophosphamide-ifosfamide-etoposide; VIDE = vincristine-ifosfamide-doxorubicin-etoposide; VI-Dox = vincristine-ifosfamide-doxorubicin.
Other comorbidities included: chronic obstructive pulmonary disease/airways disease (n = 3), asthma (n = 4), thyroid goiter (n = 1), osteoarthritis (n = 2), spinal stenosis (n = 1), hydronephrosis (n = 1), lipoma (n = 1), hypothyroidism (n = 1), psoriatic arthritis (n = 1), biliary stones (n = 1), chronic hepatitis B (n = 1), Crohn’s disease (n = 1), migraines (n = 2), trigeminal neuralgia (n = 1), ovarian cyst (n = 2), thyroid cyst (n = 1), gout (n = 1), depression (n = 2), rheumatoid arthritis (n = 1), irritable bowel syndrome (n = 1), diverticulosis (1), B12 deficiency with anemia (n = 1) (NB: some patients had more than 1 comorbidity).
For patients receiving epirubicin, the equivalent doxorubicin dose was calculated by multiplying by 0.67.
Four RIC/sham inflations/deflations before each chemotherapy cycle.
One cycle = 4 inflations/deflations performed.
Normal range: 0 to 14 ng/L.
Normal <400 ng/L.
Primary outcome
Effect of RIC on pre-chemotherapy TnT
Baseline pre-chemotherapy TnT was normal (ie, ≤14 ng/L) in 90% of all participants (median 6 [IQR: 4-9] ng/L), but by cycle 4 of chemotherapy, 49% had TnT ≥14 ng/L (median: 14 [IQR: 9-21] ng/L). This rose to 81% by cycle 6 (median: 33 [IQR: 16-36] ng/L) and remained elevated 1 month after anthracycline chemotherapy in 76% (median 24 [IQR: 15-59] ng/L) (Supplemental Tables 8 and 9). Figure 2 shows the effect of RIC on TnT concentrations with no difference between the 2 groups in TnT. Mixed-effects regression, which includes all time points, showed no difference in TnT (RIC vs sham, mean difference 3.15 ng/L; 95% CI: −0.04 to 6.33; P = 0.053).
Figure 2.
Effect of RIC on TnT During Chemotherapy and Follow-Up
The figure shows the effect of RIC on TnT presented as a continuous (A) and as a binary (B) variable. In A, mean TnT concentration rose for both groups and peaked by the end of chemotherapy. Mixed-effects regression analysis showed no difference in the TnT between the 2 groups during chemotherapy and follow-up, with RIC having on average a concentration of 3.15 ng/L higher than sham (95% CI: −0.04 to 6.33 ng/L; P = 0.053). In B, most patients in both groups had a positive (>14 ng/L) TnT by cycle 6 with no significant difference in the number of positive TnT samples between the 2 groups (multinomial logistic regression; P = 0.19). Abbreviations as in Figure 1.
Pre- and immediately post-chemotherapy TnT
There was no difference between TnT in blood drawn immediately before each anthracycline exposure or the sample taken immediately after the end of infusions (mean difference 1.6 ng/L; 95% CI: −0.39 to 3.59; P = 0.12) at any stage in the chemotherapy regimen (Supplemental Figure 2, Supplemental Table 8).
Post-chemotherapy TnT samples were collected at a median time of 134 (IQR: 59.5-222.5) minutes from the end of the doxorubicin injection/infusion (range 13 to 120 minutes). This was a deviation from the planned study protocol for post-dosing blood test sampling (blood test >3 hours up to 24 hours postchemotherapy) and reflected unwillingness of patients to remain in the hospital for >3 hours.
All results for TnT used in the analysis of RIC were derived from the blood sample taken immediately before each cycle of chemotherapy.
Secondary outcomes
cMyC and NT-proBNP
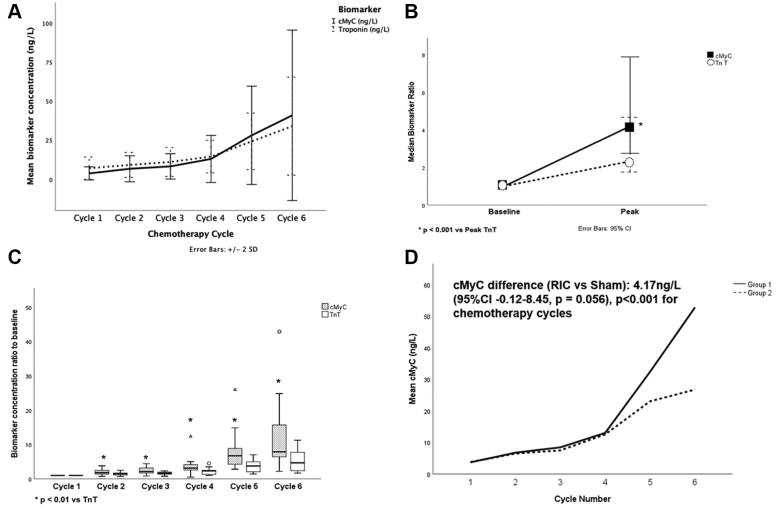
Pre- and post-chemotherapy cMyC concentrations followed a similar pattern to that seen for TnT (Supplemental Figure 3). Figure 3 and Supplemental Tables 10 and 11 illustrate the lack of effect of RIC on cMyC levels.
Figure 3.
cMyC and RIC
The figure shows a comparison of TnT and cMyC (A to C) and the effect of RIC on cMyC (D). In A and B, cMyC follows a similar pattern of rise as TnT during chemotherapy with a statistically significant higher proportional increase from baseline for cMyC compared with TnT at each chemotherapy cycle. In C, the peak concentration for each biomarker was identified and the peak:baseline concentration ratio compared. There was a 4-fold median increase from baseline to peak concentration for cMyC vs a 2-fold median increase for TnT (mean 4-fold), which was significant (Wilcoxon signed rank; P < 0.001). In D, mixed-effect regression analysis showed cMyC levels were on average 4.17 ng/L higher in RIC compared with sham, which did not reach statistical significance (95% CI: −0.12 to 8.45 ng/L; P = 0.056). Abbreviations as in Figure 1.
There was a significant increase in the NT-proBNP from baseline to 3M (58 ± 40 ng/L to 112 ± 269 ng/L; P < 0.001), but no difference between the absolute BNP or ΔBNP between the 2 groups (P = 0.46; P = 0.69, respectively) (Supplemental Table 13).
Echocardiography
There was no significant difference between the mean LVEF or change in (ΔLVEF) at 3M and 12M between the 2 groups (3M mean LVEF difference −0.8%; 95% CI: −4.4% to 2.8%; P = 0.65, 12M mean LVEF difference 0.3%; 95% CI: −3.5% to 4.1%; P = 0.86; 3M ΔLVEF difference 2.3%; 95% CI: −1.4% to 6.1%; P = 0.22, 12M ΔLVEF difference −0.2%; 95% CI: −4.3% to 3.9%; P = 0.93) (Supplemental Table 12).
However, in 3 patients (6% of group; 2 RIC) who developed clinical HF during chemotherapy, changes in LVEF were noted: with a 12% decline in LVEF in 1 patient and 2 with a 7% LVEF decline to a level <50%. An additional 6 patients (4 RIC) developed asymptomatic declines in LVEF of >10% in the follow-up period.
There were no significant differences between the 2 groups in the absolute GLS, ΔGLS, and relative change in GLS from baseline at 3M or 12M postchemotherapy (3M P = 0.79; P = 0.38; P = 0.44, respectively; 12M P = 0.10; P = 0.22; P = 0.27, respectively).
Clinical events
Kaplan-Meier curves of cardiovascular events and cancer deaths are shown in Figure 4 and summarized in Table 2. The combined endpoint of MACE or cancer deaths was more common in the RIC group at 1 year (11 vs 3; HR: 0.25; 95% CI: 0.07-0.90; P = 0.034). Deaths were notified by the treating oncologists and flagged on the electronic medical record. There were 9 deaths, attributed to progression of disease and classified as “expected”; there were no non–cancer-related deaths. Adjudication of events was undertaken by trial clinicians (J.M.W., M.M., and R.C.) from the electronic medical record and subsequently reviewed by the DMC. Clinical serious adverse events are shown in Supplemental Table 14. The most common adverse event was infection; this occurred in 35 patients with 21 (75%) in RIC and 14 (52%) in sham group.
Figure 4.
Clinical Events Outcomes
The figure shows Kaplan-Meier curves for major adverse cardiovascular events (MACE) or cancer death (A), MACE (B), and cancer deaths (C) in RIC vs sham. There were 14 events up to 1 year (11 vs 3 events, RIC vs sham) that reached statistical significance (HR: 0.25; 95% CI: 0.07-0.899; P = 0.034). RIC = remote ischemic conditioning.
Table 2.
MACE or Cancer Deaths Up to 1 Year
| All Patients (N = 55) | RIC (n = 28) | Sham (n = 27) | P Value RIC vs Sham | |
|---|---|---|---|---|
| All events | 14 (25) | 11 (39) | 3 (19) | 0.034 |
| MACE | 5 (9) | 3 (11) | 2 (7) | 0.44 |
| Arrhythmias | 2 (4) | 1 (4) | 1 (4) | |
| Heart failure | 3 (6) | 2 (7) | 1 (4) | |
| Cancer deaths | 9 (16) | 8 (29) | 1 (4) | 0.043 |
Values are n (%).
P value obtained from Cox regression analysis.
MACE = major adverse cardiovascular events; RIC = remote ischemic conditioning.
Arrhythmias
Forty-four patients had ECG monitoring (21 vs 23, RIC vs sham); there were no significant differences between the 2 groups in arrhythmia incidence (Supplemental Table 15).
Twelve patients had nonsustained ventricular tachycardia (5 RIC vs 7 sham), 2 of whom required treatment with beta-blockers.
Discussion
This was the first study to investigate RIC in adult cancer patients receiving anthracycline chemotherapy using blood biomarker changes as a marker of cardiac injury. The study was terminated early due to the global COVID-19 pandemic. The intervention was successfully applied to patients (Table 1), but no difference in the extent of biomarker rise between the RIC and sham treatments was detected (Central Illustration), nor were there any differences in cardiac function. Only 3 patients had an acute, severe clinical cardiac toxicity event with HF, limiting the possible interpretation of the potential for RIC in this scenario.
Central Illustration.
Anthracyclines and Myocardial Injury Biomarkers: The Effect of Remote Ischemic Conditioning
Anthracycline chemotherapy led to a significant rise in cardiac biomarkers with both high sensitivity troponin T (TnT) and the novel biomarker cardiac myosin binding protein C (cMyC) rising early during first few cycles of chemotherapy. In this randomized, single blind, sham-control trial, remote ischemic conditioning (RIC), delivered via a blood pressure cuff on the arm during chemotherapy, had no effect on the levels of biomarkers as markers of cardiotoxicity. SD = standard deviation; SE = standard error.
Nevertheless, reliable insights into the pattern of contemporary cardiac biomarkers during exposure to anthracyclines has been gained, with 76% patients having a positive TnT 1-month post-chemotherapy; previous earlier studies showed only 30% positivity at that point.25 It can be reliably stated from this study that RIC does not appear to attenuate the TnT or cMyC rise with chemotherapy. This admittedly small study did not stratify for cancer severity at randomization, but RIC was associated with a tendency toward earlier cancer deaths and a signal (not significant) for increased infection risk. The premature, forced termination of the study and the imbalance of cancer severity at randomization preclude a robust interpretation of these clinical events.
Are blood cardiac biomarkers the correct tool for the detection of cardiotoxicity in cancer treatment? Cardiac biomarkers TnT and cMyC rose after the third or fourth cycle of doxorubicin, peaking at the last cycle, and remaining elevated 4 weeks after completing chemotherapy. There were no acute changes in the biomarkers in the hours after anthracycline therapy. Although cMyC is a sensitive biomarker,26 it did not add significantly to the ability to discriminate between treatment arms. Biomarker increases are generally associated with worse late outcomes for patients,27 and biomarkers were a reasonable surrogate assessed in our study.
Our study was underpowered, but other factors may have contributed to our results. In animal studies, the myocardial injury is likely to be large and acute; and these studies used higher doses of anthracyclines12, 13, 14 or intracoronary administration15 in an experimental model originally developed to study HF.28 In our study of cancer patients, cardiac injury is more likely to be gradual and progressive. Under such circumstances, the cardiac injury may have been too small to detect any effect from RIC. The extraordinary sensitivity of current biomarkers (a rise of 3.9 ng/L and 41 ng/L per μg of injured myocardium for TnT and cMyC, respectively29) suggests that in this study, the increases in cardiac biomarkers documented would result in the amount of myocardium injured to be too small to detect functional changes and too small to allow an intervention, such as RIC, to be clinically detectable. An analogous situation was encountered in the recently published study of RIC in acute infarction, where a neutral result was attributed to the very low incidence of cardiac events.30,31 Thus, blood cardiac biomarkers may be indicators of a risk for the development of cardiac damage but are likely to be insufficient on their own to define clinically relevant cardiotoxicity, at least in the short term.
The trial included any patient undergoing anthracycline chemotherapy with a balanced distribution of cancer types and dosing (>300 mg/m2) across both groups. However, 54% of RIC patients had metastatic disease compared with 37% in the sham group, producing an imbalance in severity of disease at randomization. This imbalance may account for more cancer deaths in the RIC group up to 12 months post-chemotherapy. The cancer deaths were associated with progression of disease. Ongoing analysis, beyond 12 months, has revealed equalization of the number of deaths. The relationship, if any, of RIC to early deaths seen in this small study is not established, but such theoretical risks have been previously raised.32 Clinical adverse events were similar between the 2 groups, and of those, the majority were due to admissions with fever or infection (Supplemental Table 14). Nevertheless, these data imply caution in the future investigation of potentially cardioprotective interventions in the context of cancer therapy, where inadvertent cancer cell protection or interference with general responses to injury or infection may have unexpected clinical consequences.
Study limitations
This phase II study was underpowered to demonstrate an effect of RIC on classically defined cardiotoxicity, or HF, which occurred in only 6% of the total cohort. Study enrolment was halted due to the COVID-19 pandemic, and the DMC at the time of halting the trial adjudicated that the primary endpoint of a difference in TnT leak between the 2 groups was unlikely to be achieved and, combined with a signal for early complications, recommended cessation of the trial. A signal for early cancer deaths in the RIC group is worthy of note, but the study was unbalanced with regard to cancer severity at randomization, limiting the interpretation of this observation. No consistent changes in LVEF were documented in the majority of our patients, but our study relied upon echocardiography undertaken in the clinical service department and with the acknowledged measurement variability that this approach might produce, small changes in LVEF may have been missed.
Conclusions
Our observation of the almost universal, progressive rise in cardiac biomarkers with increasing doses of anthracyclines, adds to our understanding of the effects chemotherapy, but confirms the difficulty in using blood biomarkers for clinical definitions of cardiotoxicity. This supports the potential approach of including imaging parameters in the definition of cardiotoxicity.33
Remote ischemic conditioning did not reduce the progressive rise in cardiac blood biomarkers during high-dose anthracycline chemotherapy in these patients with severe cancers. Its potential role in severe acute cardiotoxicity cannot be inferred from our data. Larger studies combining results from biomarkers and more sensitive measures of cardiac function over longer periods of time post-chemotherapy are going to be needed to resolve the significance of TnT increases during therapy and the predictive “safe” upper limits for the biomarkers. In this regard, we await with interest the RESILIENCE clinical study (Remote Ischemic Conditioning in Lymphoma Patients Receiving Anthracyclines; NCT05223413), which will involve a larger group of patients with a single cancer type, followed with multimodality cardiac assessments over a longer period.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients receiving anthracycline chemotherapy, there is an early rise in biomarkers of myocardial injury that starts with chemotherapy and persists during follow-up. In this proof-of-concept, phase II, single-blind, randomized controlled trial, RIC did not attenuate a rise in markers of injury, nor were there any changes in cardiac function overall. RIC, although a promising experimental tool for cardioprotection, should be considered with caution in the context of cancer therapy, and any contribution to worse early outcomes needs to be more fully understood.
TRANSLATIONAL OUTLOOK: Future studies should concentrate on delineating the characteristics and mechanisms of the increase in biomarkers, and their role in predicting cardiotoxicity, as well as identifying new strategies of cardioprotection, which should include consideration of late manifestations of cardiac injury, while maintaining the anticancer efficacy of chemotherapy.
Funding Support and Author Disclosures
Funding for this study was from the University College Hospital Charity (Reg No. 1165398), via the McClean Greenbaum Legacy (Fund 1425) and from University College Hospital National Institute for Health Research Biomedical Research Centre (NIHR BRC). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to acknowledge the help and contribution made to this study by their medical oncology colleagues including Prof Daniel Hochhauser, Dr Sandra Strauss, Prof Jeremy Whelan, Dr Jonathan Lambert, Dr Kirit Adeshna, and their teams at UCLH. The authors would also like to thank the patients who agreed to participate. The authors thank Prof Michael Marber, and Dr Bashir Alaour from Kings College, London, for their advice and help with the cardiac myosin-binding protein C analyses.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures and tables, please see the online version of this paper.
Contributor Information
Derek M. Yellon, Email: d.yellon@ucl.ac.uk.
J. Malcolm Walker, Email: malcolm.walker@ucl.ac.uk.
Appendix
References
- 1.Cardinale D., Colombo A., Bacchiani G., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 2.Totzeck M., Schuler M., Stuschke M., et al. Cardio-oncology strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale D., Colombo A., Sandri M.T., et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 4.Gulati G., Heck S.L., Ree A.H., et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tebbi C.K., London W.B., Friedman D., et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25(5):493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh F., Dupuis L.L., Alexander S., Gupta A., Mertens L., Nathan P.C. Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2015;108(4):djv357. doi: 10.1093/jnci/djv357. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G., Botker H.E., Przyklenk, et al. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candilio L., Malik A., Hausenloy D.J. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown) 2013;14(3):193–205. doi: 10.2459/JCM.0b013e328359dd7b. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13(4):193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 10.Gharanei M., Hussain A., Janneh O., et al. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: a mitochondrial division/mitophagy inhibitor. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang X., Wang H., Han D., et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maulik A., Davidson S.M., Piotrowska I., et al. Ischaemic preconditioning protects cardiomyocytes from anthracycline-induced toxicity via the PI3K pathway. Cardiovasc Drugs Ther. 2018;32(3):245–253. doi: 10.1007/s10557-018-6793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertz Z.M., Cain C., Kraskauskas D., et al. Remote ischemic pre-conditioning attenuates adverse cardiac remodeling and mortality following doxorubicin administration in mice. J Am Coll Cardiol CardioOnc. 2019;1(2):221–234. doi: 10.1016/j.jaccao.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q., Wang F., Ryan T.D., Chalasani M., et al. Repeated remote ischemic conditioning reduces doxorubicin-induced cardiotoxicity. J Am Coll Cardiol CardioOnc. 2020;2(1):41–52. doi: 10.1016/j.jaccao.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galán-Arriola C., Villena-Gutiérrez R., Higuero-Verdejo M.I., et al. Remote ischemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc Res. 2021;117(4):1132–1143. doi: 10.1093/cvr/cvaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung R., Maulik A., Hamarneh A., et al. Effect of remote ischaemic conditioning in oncology patients undergoing chemotherapy: rationale and design of the ERIC-ONC study - a single-center, blinded, randomized controlled trial. Clin Cardiol. 2016;39(2):72–82. doi: 10.1002/clc.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell R.M., Rear R., Cunningham J., et al. Effect of remote ischaemic conditioning on contrast-induced nephropathy in patients undergoing elective coronary angiography (ERICCIN): rationale and study design of a randomized single-centre, double-blind placebo-controlled trial. Clin Res Cardiol. 2014;103(3):203–209. doi: 10.1007/s00392-013-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apple F.S., Sandoval Y., Jaffe A.S., et al. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63(1):73–81. doi: 10.1373/clinchem.2016.255109. [DOI] [PubMed] [Google Scholar]
- 19.Marjot J., Liebetrau C., Goodson R.J., et al. The development and application of a high-sensitivity immunoassay for cardiac myosin-binding protein C. Transl Res. 2016;170:17–25.e5. doi: 10.1016/j.trsl.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaier T.E., Twerenbold R., Puelacher C., et al. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation. 2017;136(16):1495–1508. doi: 10.1161/CIRCULATIONAHA.117.028084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnar D.O., Mairesse G.H., Boriani G., et al. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS) Europace. 2019;21(6):844–845. doi: 10.1093/europace/euz046. [DOI] [PubMed] [Google Scholar]
- 22.Hausenloy D.J., Mwamure P.K., Venugopal V., et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370(9587):575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 23.Hoole S.P., Heck P.M., Sharples L., et al. Cardiac remote ischemic preconditioning in coronary stenting (CRISP stent) study. A prospective, randomized control trial. Circulation. 2009;119(6):820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 24.Bøtker H.E., Kharbanda R., Schmidt M.R., et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 25.Cardinale D., Sandri M.T., Colombo A., et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 26.Kaier T.E., Alaour B., Marber M. Cardiac myosin-binding protein C: how a novel biomarker could transform chest pain triage. Biomark Med. 2018;12(8):823–826. doi: 10.2217/bmm-2018-0176. [DOI] [PubMed] [Google Scholar]
- 27.Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen S., Perez-Bouza A., Schälte G., et al. Selective left ventricular adriamycin-induced cardiomyopathy in the pig. J Heart Lung Transplant. 2008;27(1):86–92. doi: 10.1016/j.healun.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Marjot J., Kaier T.E., Martin E.D., et al. Quantifying the release of biomarkers of myocardial necrosis from cardiac myocytes and intact myocardium. Clin Chem. 2017;63(5):990–996. doi: 10.1373/clinchem.2016.264648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausenloy D.J., Kharbanda R.K., Møller U.K., et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet. 2019;394(10207):1415–1424. doi: 10.1016/S0140-6736(19)32039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hausenloy D.J., Ntsekhe M., Yellon D.M. A future for remote ischaemic conditioning in high-risk patients. Basic Res Cardiol. 2020;115(3):1–4. doi: 10.1007/s00395-020-0794-2. [DOI] [PubMed] [Google Scholar]
- 32.Heusch G., Rassaf T. Protection from cardiotoxicity of cancer chemotherapy: a novel target for remote ischaemic conditioning? Cardiovasc Res. 2021;117(4):985–986. doi: 10.1093/cvr/cvaa199. [DOI] [PubMed] [Google Scholar]
- 33.Eschenhagen T., Force T., Ewer M.S., et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13(1):1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.