Abstract
Background
Anthracycline cardiotoxicity is a concern in survivors of childhood cancers. Recent evidence suggests that remote ischemic conditioning (RIC) may offer myocardial protection.
Objectives
This randomized sham-controlled single-blind study tested the hypothesis that RIC may reduce myocardial injury in pediatric cancer patients receiving anthracycline chemotherapy.
Methods
We performed a phase 2 sham-controlled single-blind randomized controlled trial to determine the impact of RIC on myocardial injury in pediatric cancer patients receiving anthracycline-based chemotherapy. Patients were randomized to receive RIC (3 cycles of 5-minute inflation of a blood pressure cuff placed over 1 limb to 15 mm Hg above systolic pressure) or sham intervention. The intervention was applied within 60 minutes before initiation of the first dose and before up to 4 cycles of anthracycline therapy. The primary outcome was the plasma high-sensitivity cardiac troponin T (hs-cTnT) level. The secondary outcome measures included echocardiographic indexes of left ventricular systolic and diastolic function and the occurrence of cardiovascular events.
Results
A total of 68 children 10.9 ± 3.9 years of age were randomized to receive RIC (n = 34) or sham (n = 34) intervention. Plasma levels of hs-cTnT showed a progressive increase across time points in the RIC (P < 0.001) and sham (P < 0.001) groups. At each of the time points, there were no significant differences in hs-cTnT levels or LV tissue Doppler and strain parameters between the 2 groups (all P > 0.05). None of the patients developed heart failure or cardiac arrhythmias.
Conclusions
RIC did not exhibit cardioprotective effects in childhood cancer patients receiving anthracycline-based chemotherapy. (Remote Ischaemic Preconditioning in Childhood Cancer [RIPC]; NCT03166813)
Key Words: anthracycline, pediatric cancer, remote ischemic conditioning
Central Illustration
In children with solid tumors and hematologic malignancies, survival rates have improved significantly from <50% in the 1970s to 80% in the present era with the introduction of anthracyclines into chemotherapy protocols.1 Nonetheless, the cardiotoxic side effects of anthracycline remain an issue of concern.2,3 Modification of infusion strategies has not been shown to have significant cardioprotective effects,4,5 whereas data on the benefits of liposomal anthracyclines in children are lacking. Dexrazoxane has shown promise in attenuating the cardiac effects of anthracycline in children with acute lymphoblastic leukemia, lymphoma, and solid tumors.6, 7, 8, 9
Remote ischemic conditioning (RIC) is the phenomenon wherein brief episodes of reversible ischemia and reperfusion applied to 1 vascular bed confer global protection and render resistance to ischemic reperfusion injury of remote tissues and organs.10 Preclinical data provide relatively consistent evidence among different species that RIC confers protection against ischemic reperfusion injury.11 Although the exact mechanistic nature of signal transduction remains to be defined, neuronal pathways12 and humoral communications,13 with putative contributors including nitric oxide,14 stromal-derived factor 1α,15 and microRNA,16 may be involved.
Accumulating human study data support the potential clinical translational potential of RIC, which can be achieved through repeated cycles of inflation and deflation of a blood pressure cuff placed over 1 limb to induce transient ischemia and reperfusion, respectively.17 Protective effects of RIC have been reported in children undergoing cardiac surgery requiring cardiopulmonary bypass.17, 18, 19, 20 Importantly, anthracycline cardiotoxicity and myocardial reperfusion injury share similar pathways, including liposomal peroxidation, reactive oxidation species, and calcium overload.21 Therefore, it is tempting to speculate that RIC may possibly confer cardioprotection against anthracycline therapy. Recent studies in rodent22,23 and porcine24 models of anthracycline-induced cardiotoxicity have provided evidence of potential cardioprotective effects of RIC. Whether the application of RIC before anthracycline therapy can ameliorate anthracycline-induced cardiotoxicity in children with cancer has not been explored.
This phase 2 randomized sham-controlled single-blind study tested the hypothesis that RIC may reduce myocardial injury in pediatric cancer patients receiving anthracycline chemotherapy. We determined the effects of RIC on: 1) the changes in plasma levels of high-sensitivity cardiac troponin T (hs-cTnT); 2) the parameters of left ventricular (LV) systolic and diastolic function; and 3) the occurrence of clinical cardiovascular events in childhood cancer patients undergoing anthracycline-based chemotherapy.
Methods
Patient recruitment
Pediatric patients newly diagnosed with solid tumors and hematologic malignancies and who required anthracycline-based chemotherapy were recruited from the pediatric oncology ward over a 4-year period. The inclusion criteria included: 1) patients 4 to 18 years of age; 2) newly diagnosed patients with hematologic malignancies or solid tumors referred for anthracycline-based chemotherapy; and 3) no history of being treated with anthracycline-based regimens in the past. The exclusion criteria included: 1) the existence of congenital or acquired heart disease; 2) the presence of syndromic disorders; 3) an abnormal baseline echocardiographic assessment; 4) peripheral vascular disease that renders RIC impossible; and 5) a platelet count <30,000/μL. The body weight and height were measured, and the body mass index and body surface area were calculated accordingly. The following clinical information was collected from the medical notes: demographic factors, type of cancer, anthracycline dosages, blood counts, and renal and liver test results. All parents of minors gave written informed consent to participate in this study, which was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster and the Hong Kong Children’s Hospital. This trial is registered at clinicaltrials.gov (NCT03166813).
Randomization
The recruited patients were randomly allocated in a 1:1 ratio to either the RIC intervention or the sham group before the first dose of anthracycline. This was performed using a computer-generated number by an unblinded staff member not involved in the data collection or analyses. Stratified randomization with minimization was based on 2 age groups (4-10 years and 11-18 years) and 3 diagnostic categories (leukemias, lymphomas, and solid tumors) to ensure an approximate balance in the number of participants. Study personnel who applied the intervention and trial participants were not blinded. On the other hand, clinicians and coinvestigators involved in data collection and/or analyses were blinded to patient characteristics and treatment allocation.
RIC intervention
The intervention RIC protocol was induced by 3 cycles of inflation of a blood pressure cuff placed over the upper or lower limb to 15 mm Hg above the systolic blood pressure for 5 minutes followed by 5 minutes of cuff deflation to 0 mm Hg.17,25 Although many pediatric studies have used 4 cycles of cuff inflation and deflation,17,20 3 cycles of RIC have also been reported to offer cardioprotection in animal models26 and in children undergoing cardiac surgery.18, 19, 20 Given the concern of skin petechiae and bruising in our patients with possible thrombocytopenia, we used 3 cycles to induce RIC. The sham protocol involved only the placement of a blood pressure cuff but without inflation for 30 minutes. The intervention and sham protocols were applied at baseline within 60 minutes before initiation of the first dose of anthracycline and within 60 minutes before each subsequent dose of anthracycline. The RIC intervention or sham protocol was applied at most 4 times to accommodate for the differences in anthracycline regimens among different types of childhood malignancies (Figure 1).
Figure 1.
Study Protocol
Newly diagnosed pediatric cancer patients between 2018 and 2021 were screened for eligibility to be recruited into the study. hs-cTnT = high-sensitivity cardiac troponin T; RIC = remote ischemic conditioning.
Study outcomes
The primary outcome measure was plasma hs-cTnT, which was measured at 7 time points (TPs): 1) at baseline (TP1); 2) within 24 hours after each of the 4 cycles of anthracycline (TP2-TP5); 3) within 1 week after completion of all anthracycline treatments (TP6); and 4) at 3 months after completion of all anthracycline treatment (TP7). Blood samples were centrifuged immediately after collection, and the plasma was stored at −80 °C until assay. The levels of plasma hs-cTnT were measured using a highly sensitive cardiac troponin T assay on an automated platform (Elecysys 2010 Troponin T hs, Roche Diagnostics), which has a lower detection limit of 5 ng/L and an analytical coefficient of variation of 2.93%.
The secondary outcome measures included echocardiographic indexes of LV systolic and diastolic function and the occurrence of cardiovascular events. Echocardiographic assessment of LV function was performed at TP1, TP6, and TP7. All assessments were performed using the Vivid i portable ultrasound machine (GE Medical System) and analyzed off-line by the Echopac (version 201, GE Medical System). The average values of the echocardiographic indexes from 3 cardiac cycles were obtained. The following cardiac functional parameters were determined as reported previously27: 1) transmitral early (E) and late (A) diastolic inflow velocities, E deceleration time, and E/A ratio; 2) peak systolic and early (e) and late (a) diastolic myocardial tissue velocities, e/a ratio, E/e ratio, and LV free wall myocardial acceleration during isovolumic contraction at the basal LV free wall–mitral annular junction; 3) LV systolic and end-diastolic dimensions, shortening fraction, and ejection fraction; and 4) global LV longitudinal systolic strain and systolic and diastolic strain rates by speckle tracking echocardiography performed from the 4-chamber view given the potential difficulties of optimizing 3 apical views and ensuring a relatively consistent heart rate during acquisition in distressed childhood cancer patients and the reported good correlation between global longitudinal strain values based on 4-chamber and 3 apical views.28 We and others have reported on the high reproducibility of strain measurements in children during and after chemotherapy and have summarized the reproducibility measurements in our recent systematic review.29 For the measurements of global LV longitudinal systolic strain, systolic strain rate, and early and late diastolic strain rates, the intraobserver variability was 4.3%, 5.1%, 5.4%, and 5.7%, respectively, whereas the interobserver variability was 6.9%, 5.4%, 6.0%, and 7.0%, respectively. Clinical cardiovascular events included the development of heart failure and cardiac arrhythmias, the need to institute cardiac medications, and cardiac death.
Sample size calculation
Sample size calculations were based on the predicted difference in the primary outcome of plasma levels of hs-cTnT with RIC within 1 week after completion of all anthracycline treatments at TP6. We have previously found in children with leukemia undergoing anthracycline-based treatment an hs-cTnT level of 2.90 ± 5.23 ng/L, which increased to 15.13 ± 8.96 ng/L within 1 week after completion of all anthracycline treatments.30 For an estimated treatment effect of 50% reduction of the post-treatment hs-cTnT level,11 the recruitment of 48 patients (24 patients in each arm) would achieve a statistical power of 80%. Given the possible attrition of cancer patients and the possibility of a lower than predicted effect size, we have included 10 more patients in each arm for a target total of 68 patients.
Statistical analysis
Results were analyzed in accordance with the intention-to-treat principle. Missing data were replaced using group mean values to reduce bias toward the lower or upper extreme. The demographic variables, echocardiographic parameters, and natural log-transformed levels of plasma hs-cTnT at baseline are presented using mean ± SD for normally distributed continuous variables and counts for categoric variables overall and by randomized group. A value of 1 ng/L was assigned when the hs-cTnT level was below the limit of detection for the purpose of analysis. Non-normal continuous data are presented as median (IQR). Longitudinal changes in the plasma hs-cTnT levels and echocardiographic parameters during the course of anthracycline therapy were compared using generalized linear models with generalized estimating equations. Repeated measurements of the plasma hs-cTnT levels and echocardiographic parameters were adjusted for the age and sex of patients, the TP of assessment, and the interaction between the TP and the randomized group with post hoc analyses using the Dunnett test. The model results are presented as the least square mean with the 95% CI. Patients in each group at each of the TPs were further dichotomized into those with hs-cTnT >8 ng/L, which has been reported to be a better cutoff for children 6 to 14 years of age,31 and those with hs-cTnT ≤8 ng/L. The proportion of patients with hs-cTnT above the cutoff in the 2 groups was compared using the same model as described earlier. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute) and IBM SPSS Statistics version 26 (SPSS, Inc).
Results
Study participants
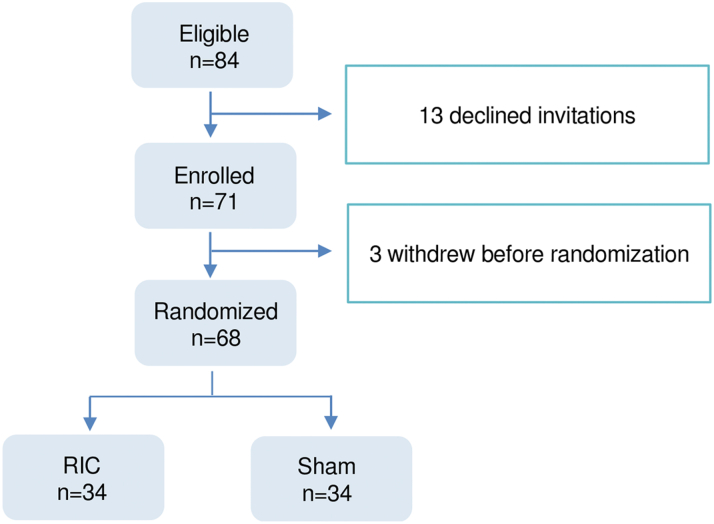
From January 2018 to December 2021, a total of 84 eligible patients were invited to participate in the study. Thirteen patients declined, and 3 withdrew before randomization; the remaining 68 were randomized to receive the RPIC or sham protocol (Figure 2). There were no protocol deviations. One patient in the sham group died of neutropenic sepsis after TP5. None of the patients developed petechial or other skin lesions after repeated cycles of inflation of a blood pressure cuff. In the RIC group, there were no dropouts. In the sham group, 2 patients withdrew after TP2, 1 died after TP5 of neutropenic sepsis as mentioned previously, 1 died after TP7 of progressive malignancy, and 2 were lost to follow-up after TP5 and TP6.
Figure 2.
Recruitment and Flow of Study Participants
From January 2018 to December 2021, a total of 84 eligible patients were identified to be eligible for inclusion. Thirteen patients declined, and 3 withdrew before randomization; the remaining 68 were randomized to receive remote ischemic conditioning (RIC) or sham protocol.
Table 1 shows the demographic parameters, clinical characteristics, and baseline parameters of the RIC and sham groups. The majority of the patients (71% in both the RIC and sham groups) had hematologic malignancies. The cumulative dose of anthracyclines was similar between the 2 groups (P = 0.95).
Table 1.
Demographic and Clinical Parameters
| RIPC (n = 34) | Sham (n = 34) | |
|---|---|---|
| Demographic data | ||
| Age, y | 11.6 ± 3.8 | 10.3 ± 4.0 |
| Male:female | 20:14 | 23:11 |
| Body weight, kg | 39.9 ± 16.6 | 40.1 ± 21.2 |
| Body height, m | 1.4 ± 0.2 | 1.4 ± 0.2 |
| Body mass index, kg/m2 | 18.3 ± 3.9 | 18.0 ± 4.9 |
| Diagnosis | ||
| Acute lymphoblastic leukemia | 12 | 14 |
| Acute myeloid leukemia | 5 | 4 |
| Hodgkin lymphoma | 3 | 4 |
| Non-Hodgkin lymphoma | 4 | 2 |
| Osteosarcoma | 5 | 5 |
| Ewing sarcoma | 2 | 2 |
| Other solid tumors | 3 | 3 |
| Blood investigation at baseline | ||
| Hemoglobin, g/dL | 10.6 ± 2.0 | 10.4 ± 3.0 |
| White blood cell, ×109/L | 7.6 (3.1-10.6) | 7.1 (3.2-9.5) |
| Platelet, ×109/L | 233.5 ± 155.3 | 257.9 ± 211.6 |
| Urea, mmol/L | 3.6 ± 1.2 | 3.9 ± 1.9 |
| Creatinine, μmol/L | 40.5 (32.0-52.0) | 38.5 (29.0-49.0) |
| Alanine aminotransferase, IU/L | 24.0 (16.0-45.5) | 21.0 (17.0-28.0) |
| Aspartate aminotransferase, U/L | 24.0 (19.0-35.0) | 24.0 (17.0-48.0) |
| Gamma-glutamyl transferase, IU/L | 28.0 (17.0-40.0) | 17.0 (16.0-23.0) |
| Bilirubin, μmol/L | 8.0 (5.0-11.5) | 7.5 (5.5-11.5) |
| Albumin, g/L | 35.9 ± 4.3 | 36.3 ± 4.9 |
| Globulin, g/L | 33.7 ± 5.5 | 34.7 ± 5.8 |
| Anthracycline dose, mg/m2 | 156.6 ± 96.6 | 154.5 ± 105.5 |
Values are mean ± SD or median (IQR).
Primary outcome
The plasma levels of hs-cTnT showed a progressive increase across TPs in both the RIC group (P < 0.001) and the sham group (P < 0.001) (Figure 3). Post hoc analyses revealed that in both groups the levels of hs-cTnT were significantly higher than the baseline level from TP4 through TP7 (all P < 0.01). However, at each of the time points, there were no significant differences between the 2 groups (all P > 0.05). Table 2 shows the changes in the logarithmically transformed levels of hs-cTnT across TPs and the proportion of patients with an absolute hs-cTnT level >8 ng/L. A progressive increase in the proportion of patients with levels exceeding 8 ng/L was evident in both groups, but at each of the TPs, there were no significant differences between the 2 groups (all P > 0.05).
Figure 3.
Longitudinal Changes in Natural Log Transformed hs-cTnT Levels
Natural log-transformed plasma levels of high-sensitivity cardiac troponin T (hs-cTnT) showed a progressive increase across the time points (TPs) in the remote ischemic conditioning group (denoted in red) and the sham group (denoted in blue). At each of the TPs, there were no significant differences between the 2 groups. The error bars represent least square mean ± 95% CI.
Table 2.
hs-cTnT Levels in the RIC and Sham Groups
| ln hs-cTnT Level (ng/L) |
Percentage of Subjects With hs-cTnT >8 ng/L |
|||||
|---|---|---|---|---|---|---|
| RIC | Sham | P Value | RIC (%) | Sham (%) | P Value | |
| TP1 | 0.19 (0.02-0.42) | 0.47 (0.10-0.83) | — | 5.9 | 14.7 | — |
| TP2 | 0.28 (0.04-0.52) | 0.40 (0.12-0.68) | 0.56 | 2.9 | 2.9 | 0.54 |
| TP3 | 0.40 (0.10-0.69) | 0.68 (0.36-1.00) | 0.68 | 11.8 | 5.9 | 0.16 |
| TP4 | 1.48 (1.04-1.93) | 1.66 (1.29-2.04) | 0.29 | 47.1 | 35.3 | 0.13 |
| TP5 | 2.03 (1.70-2.36) | 1.85 (1.47-2.24) | 0.18 | 67.6 | 64.7 | 0.27 |
| TP6 | 2.05 (1.59-2.52) | 2.48 (2.06-2.90) | 0.36 | 52.9 | 70.6 | 0.84 |
| TP7 | 1.93 (1.55-2.31) | 1.98 (1.59-2.36) | 0.24 | 61.8 | 70.6 | 0.57 |
Values are least square means (95% CI) unless otherwise indicated.
hs-cTnT = high-sensitivity cardiac troponin T; ln = natural logarithmic transformation; RIC = remote ischemic conditioning; TP = time point.
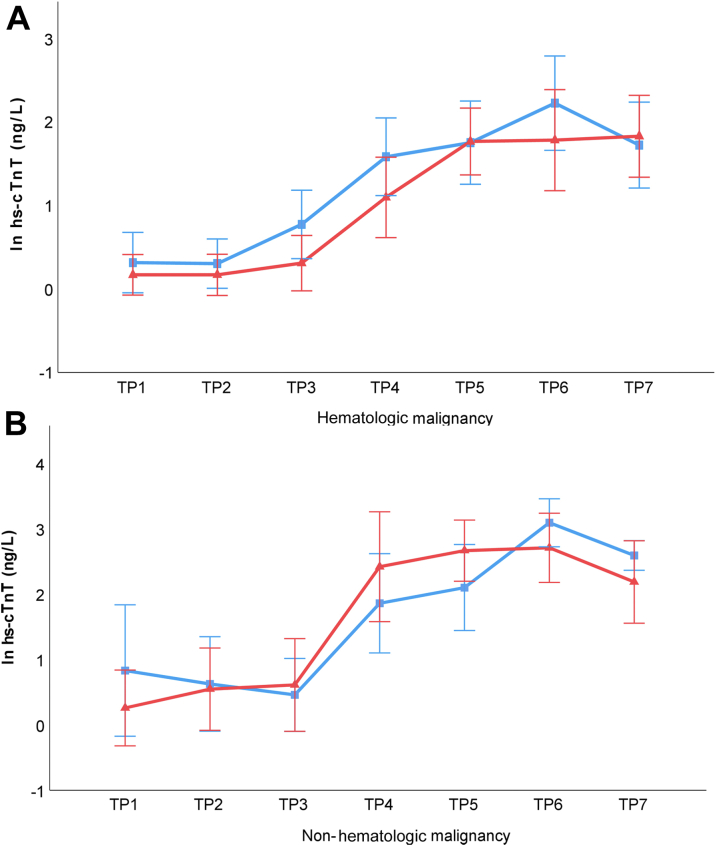
Further subgroup analysis was performed with patients stratified by hematologic and nonhematologic malignancies (Figures 4A and 4B). Similarly, at each of the TPs, there were no significant differences between RIC and sham intervention (all P > 0.05).
Figure 4.
Longitudinal Changes in Natural Log-Transformed hs-cTnT Levels in Hematologic and Nonhematologic Malignancies
The natural log-transformed plasma levels of hs-cTnT in (A) patients with hematologic malignancies and (B) patients with nonhematologic malignancies showed a progressive increase across the time points in both the remote ischemic conditioning group (denoted in red) and the sham group (denoted in blue). At each of the TPs, there were no significant differences in hs-cTnT between the 2 interventions in both patient groups. The error bars represent least square mean ± 95% CI. Abbreviations as in Figure 3.
Secondary outcomes
Table 3 summarizes the echocardiographic parameters at TP1, TP6, and TP7. There were no statistically significant differences between the RIC group and the sham group at any of these TPs for LV dimensions, transmitral Doppler parameters, mitral annular systolic and early diastolic velocities, and indexes of LV systolic and diastolic myocardial deformation. At TP7, the early diastolic mitral annular velocities were statistically higher in the sham than the control group (P = 0.045). In both groups, there was a tendency toward worsening of mitral systolic and early diastolic annular velocities, LV ejection fraction, and LV systolic strain across the 3 TPs (all P < 0.05). None of the patients in the 2 groups had LV ejection fraction <50% at any of the TPs.
Table 3.
Echocardiographic Indexes of LV Function in the RIC and Sham Groups
| Baseline (TP1) |
Within 1 Week of Completion of Anthracycline Treatment (TP6) |
At 3 months After Completion of Anthracycline of Treatment (TP7) |
||||||
|---|---|---|---|---|---|---|---|---|
| RIC | Sham | RIC | Sham | P Value | RIC | Sham | P Value | |
| Transmitral Doppler indexes | ||||||||
| E, cm/s | 101.5 (95.6-107.4) | 102.2 (95.6–108.7) | 92.8 (84.7-100.7) | 93.5 (87.8-99.2)a | 0.82 | 96.3 (89.6-102.9) | 98.9 (93.5-104.3) | 0.60 |
| A, cm/s | 54.5 (49.8-59.3) | 51.7 (47.9-55.4) | 58.7 (53.2-64.2) | 55.5 (50.8-60.3) | 0.98 | 51.2 (46.5-55.9) | 52.7 (48.8-56.7) | 0.26 |
| E deceleration time, ms | 102.2 (87.9-116.3) | 114.8 (102.1-127.4) | 108.1 (96.1-120.1) | 104.2 (96.0-112.4) | 0.14 | 109.2 (99.6-109.2) | 107.1 (97.9-116.3) | 0.17 |
| E/A ratio | 1.9 (1.8-2.1) | 2.1 (1.9-2.2) | 1.7 (1.5-1.8)a | 1.8 (1.6-1.9)a | 0.72 | 2.0 (1.8-2.2)a | 2.0 (1.9-2.1)a | 0.16 |
| Mitral annular tissue velocities | ||||||||
| s', cm/s | 7.6 (7.1-8.2) | 7.6 (6.9-8.4) | 6.8 (6.4-7.3)a | 6.0 (5.2-6.7)a | 0.081 | 6.7 (5.9-7.5)a | 5.7 (4.8-6.6)a | 0.12 |
| e', cm/s | 12.0 (11.1-12.8) | 11.6 (10.7-12.5) | 10.8 (9.8-11.7)a | 9.5 (8.8-10.3)a | 0.12 | 10.8 (10.1-11.6)a | 11.2 (10.6-11.8) | 0.32 |
| a', cm/s | 5.0 (4.5-5.5) | 4.7 (4.2-5.2) | 4.5 (4.2-4.8) | 4.2 (3.9-4.6) | 0.93 | 4.5 (4.1-5.0) | 5.1 (4.8-5.3) | 0.045b |
| IVA, m/s2 | 1.3 (1.2-1.4) | 1.2 (1.1-1.4) | 1.1 (0.9-1.2) | 1.1 (0.9-1.3) | 0.30 | 1.0 (0.8-1.1) | 1.1 (1.0-1.2) | 0.069 |
| e'/a’ ratio | 2.7 (2.3-3.1) | 2.7 (2.3-3.1) | 2.6 (2.2-2.9) | 2.3 (2.2-2.5) | 0.39 | 2.4 (2.1-2.6) | 2.3 (2.1-2.5) | 0.76 |
| E/e’ ratio | 8.9 (8.0-9.8) | 9.1 (8.3-9.9) | 9.2 (8.1-10.3) | 10.2 (9.3-11.1)a | 0.35 | 9.1 (7.8-10.4) | 9.0 (8.4-9.5) | 0.72 |
| LV dimensions and ejection fraction | ||||||||
| EDD, cm | 4.2 (4.0-4.3) | 4.0 (3.7-4.3) | 4.2 (4.0-4.4)a | 4.0 (3.7-4.2)a | 0.64 | 4.2 (4.1-4.4)a | 4.0 (3.8-4.4)a | 0.93 |
| ESD, cm | 2.9 (2.8-3.1) | 2.8 (2.6-3.0)a | 3.0 (2.8-3.1) | 2.8 (2.6-3.0) | 0.41 | 3.1 (2.9-3.2)a | 2.9 (2.6-3.1)a | 0.56 |
| EF, % | 65.3 (64.6-66.8) | 66.8 (65.5-68.5) | 63.9 (62.4-65.4)a | 66.4 (64.7-68.6)a | 0.20 | 62.2 (56.3-64.2)a | 65.6 (63.5-67.7)a | 0.071 |
| Global longitudinal myocardial deformation | ||||||||
| Systolic strain, % | 16.3 (15.5-17.1) | 16.1 (15.0-17.3) | 14.9 (14.2-15.6)a | 14.5 (14.0-15.0)a | 0.75 | 14.7 (14.1-15.4)a | 15.5 (15.1-15.9)a | 0.22 |
| SRs, /s | 1.0 (0.9-1.0) | 1.0 (1.0-1.1) | 0.9 (0.8-1.0) | 0.9 (0.9-1.0)a | 0.42 | 0.9 (0.8-0.9)a | 0.9 (0.8-0.9)a | 0.42 |
| SRe, /s | 1.6 (1.5-1.7) | 1.6 (1.5-1.7) | 1.5 (1.4-1.6) | 1.4 (1.3-1.5) | 0.28 | 1.5 (1.3-1.7) | 1.6 (1.5-1.7) | 0.80 |
| SRa, /s | 0.5 (0.5-0.6) | 0.5 (0.5-0.6) | 0.5 (0.5-0.6) | 0.5 (0.5-0.6) | 0.63 | 0.5 (0.4-0.5) | 0.6 (0.5-0.6) | 0.22 |
Values are least square means (95% CI).
A = atrioventricular late diastolic inflow velocity; a’ = late diastolic annular myocardial tissue velocity; E = atrioventricular early diastolic inflow velocity; e’ = early diastolic annular myocardial tissue velocity; EDD = end-diastolic dimension; EF = ejection fraction; ESD = end-systolic dimension; IVA = myocardial acceleration during isovolumic contraction; LV = left ventricular; RIC = remote ischemic conditioning; s’ = systolic annular velocity; SRa = global late diastolic strain rate; SRe = global early diastolic strain rate; SRs = global systolic strain rate; TP = time point.
Statistically significant by post hoc Dunnett test compared with baseline for each of the groups.
Statistically significant different between RIC and sham group,
None of the patients developed heart failure or cardiac arrhythmias. However, at the time of prehydration and posthydration during chemotherapy, diuretic agents including furosemide, spironolactone, hydrochlorothiazide, and/or amiloride were prescribed to maintain fluid balance. The use of diuretic agents was protocol driven, and diuretic agents were prescribed when fluid balance exceeded a specific threshold. Overall, diuretic agents were prescribed in 79.4% (27/34) and 58.8% (20/34) of the RIC and sham groups, respectively (P = 0.11). None of the patients were prescribed diuretic agents for clinical heart failure. Antihypertensive agents including angiotensin-converting enzyme inhibitors, beta-blockers, and/or calcium-channel blockers were prescribed when blood pressure of the patients was above the 99th percentile for age, sex, and height. Transient hypertension was noted in 8 patients during the course of chemotherapy from TP2 to TP5, the cause being related to the use of a corticosteroid or retinoic acid as part of the treatment regimen in 4 patients, pain after operation for osteosarcoma in 2 patients, a renal tumor in 1, and nephrolithiasis with transient hydronephrosis in 1. Overall, antihypertensive medications were prescribed in 17.6% (6/34) and 5.9% (2/34) of the RIC and sham groups, respectively (P = 0.26). None of the patients required long-term antihypertensive therapy.
Discussion
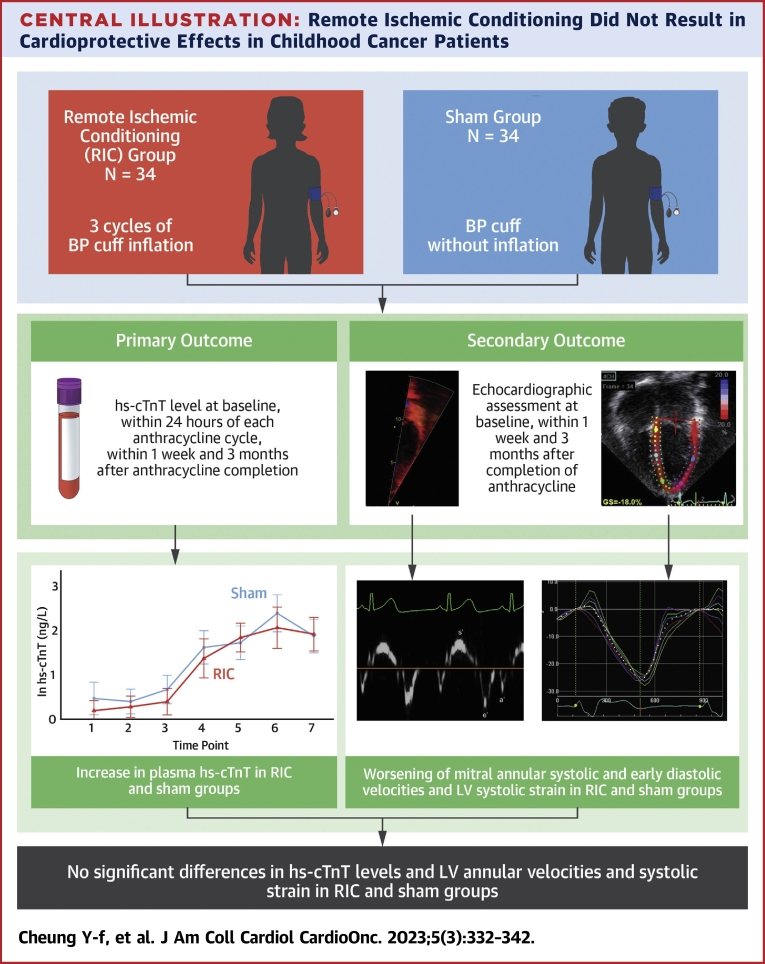
This sham-controlled single-blind randomized controlled study assessed the cardioprotective role of RIC in pediatric cancer patients receiving anthracycline-based chemotherapy. Our findings demonstrate that RIC did not have significant effects on hs-cTnT plasma levels in childhood cancer patients undergoing anthracycline-based chemotherapy (Central Illustration). Parameters of LV systolic and diastolic function and the occurrence of cardiovascular events were also similar between the RIC and sham groups at different TPs. Further subgroup analyses did not reveal differences in hs-cTnT levels between patients with hematologic malignancies and those with nonhematologic malignancies.
Central Illustration.
Remote Ischemic Conditioning Did Not Result in Cardioprotective Effects in Childhood Cancer Patients
Remote ischemic conditioning (RIC) did not have significant effects on the plasma levels of high-sensitivity cardiac troponin T (hs-cTnT), the parameters of left ventricular (LV) systolic and diastolic function, or the occurrence of cardiovascular events in childhood cancer patients undergoing anthracycline-based chemotherapy followed up until 3 months after completion of anthracycline therapy. BP = blood pressure.
Pediatric application of RIC
The noninvasive nature and the low cost render RIC a potentially useful cardioprotective strategy in children. Previous pediatric studies have focused on congenital heart disease patients undergoing cardiac surgery that requires cardiopulmonary bypass.17, 18, 19,32, 33, 34, 35, 36 Nonetheless, the randomized controlled trials17, 18, 19,32, 33, 34, 35, 36 and meta-analyses20,37 of RIC in children undergoing cardiac surgery with cardiopulmonary bypass have shown inconsistent results, which may be related to variability in age, the presence of hypoxemia in cyanotic cardiac patients, the type of anesthesia received, and varying degrees of surgical insult.35 Notwithstanding, interest is increasing on the potential application of RIC as a cardioprotective intervention to limit anthracycline-induced myocardial damage.22, 23, 24,38 Given the release of putative factors into the systemic circulation during RIC that target oxidative stress, apoptosis, inflammation, and mitochondrial dysfunction11,39 and similar pathways involved in anthracycline cardiotoxicity and myocardial reperfusion,21 the cardioprotective role of RIC has recently been explored in animal models of anthracycline-induced cardiotoxicity.
Animal models of RIC on anthracycline-induced cardiotoxicity
Preclinical RIC studies have been performed in rodent22,23 and porcine24 models of anthracycline-induced cardiotoxicity. Gertz et al22 demonstrated in a mouse model that RIC applied at 1 hour before doxorubicin administration attenuated cardiac fibrosis and apoptosis. He et al23 applied a repeated RIC protocol at 30 minutes before and every day for 5 days after doxorubicin injections in a mouse model and found attenuation of doxorubicin-induced cardiomyocyte apoptosis, reduced inflammation, increased autophagy signaling, and in vivo myocardial protection with reduced doxorubicin-induced troponin I elevations and better cardiac function. Using a porcine model, Galán-Arriola et al24 reported that RIC before intracoronary doxorubicin injections resulted in higher LV ejection fraction and less cardiac fibrosis. These preclinical studies have provided in vitro evidence of potential cardioprotective effects of RIC against anthracycline-induced cardiotoxicity.
Clinical trials of RIC in cancer patients
The present study is the first to explore the potential role of RIC in protection against anthracycline-induced cardiotoxicity. However, our findings show that RIC did not offer myocardial protection during treatment and up to 3 months after completion of anthracycline therapy. Because this is the only completed pediatric trial to date, no other data for comparison exist. The ERIC-ONC (Effect of Remote Ischemic Conditioning in Oncology; NCT02471885) trial,38 which is a single-center blinded randomized controlled RIC trial in adult oncology patients, has also been completed recently.
Our pediatric patients received a relatively low cumulative anthracycline dose with a mean of about 150 mg/m2. Nonetheless, studies have shown that no dose of anthracycline is without risk.40 Blanco et al41 reported odds ratios of 1.65 and 3.85 of cardiomyopathy risk in survivors of childhood cancer when exposed to a cumulative anthracycline dose of 101 to 150 mg/m2 and 151 to 200 mg/m2, respectively. Our findings of a progressive increase in hs-cTnT levels and worsening of echocardiographic indexes of LV systolic and diastolic function attest to the presence of subclinical myocardial damage, which permits the interrogation of potential cardioprotective effects of RIC.
The use of diuretic agents and antihypertensive agents may alter preload and afterload and confound the assessment of load-dependent indexes of LV systolic function, in particular LV ejection fraction. Although there seemed to be a numerically greater proportion of patients in the RIC than the sham group requiring diuretic agents and antihypertensive agents, this was not statistically significant, and the explanation for this is unknown.
Although the exact reasons for the failure to demonstrate findings anticipated based on previous experimental studies are not clear, differences between experimental conditions and the complex in vivo milieu in pediatric cancer patients are obvious. The experimental setting differs from the clinical treatment in terms of the administration of usually a single dose of anthracycline,22,23 infusion of the drug through an unusual intracoronary route,24 and the need for anesthesia during RIC.22, 23, 24 On the other hand, the complex milieu in pediatric cancer patients may influence or interact with the effects of RIC caused by age; genotype42; and the different cancer treatment protocols that include multiple doses of anthracyclines, radiation therapy, and other types of cytotoxic chemotherapy or newer molecularly targeted agents.40 Additionally, during the course of chemotherapy, alteration of the cardiac load and function may occur during episodes of sepsis and after prehydration before the administration of chemotherapy. To date, there are no data on the potential effects of the coexistence of cancer cells and their destruction on RIC efficacy; future studies in this regard are warranted.
Study limitations
Several limitations to this study warrant comment. First, the patient population is heterogeneous in terms of the underlying cancer diagnosis and hence the treatment protocols. Whether RIC shows similar protective effects after cardiac irradiation or other types of cytotoxic chemotherapy or newer molecularly targeted agents has not been explored. Second, although we have further grouped the patients into those having hematologic and those with nonhematologic malignancies, the power of the study may not be adequate to detect significant differences. Because about 70% of patients had hematologic malignancies, it could be of interest in future studies to explore the effects of RIC in children having solid tumors. Third, the imbalance between the RIC and sham groups in terms of exposure to diuretic agents and antihypertensive agents, albeit statistically insignificant, may potentially alter preload and afterload and confound the assessment of LV systolic function. Fourth, it is unclear what constitutes optimal RIC in children. Whether repeated RIC, as described by He et al,23 would exert a more obvious effect in our patients is unclear. Fifth, it remains possible that the peak level of hs-cTnT at TP6 might have been missed given that blood sampling was performed within 1 week after completion of all anthracycline treatments. Nonetheless, even with the determination of hs-cTnT levels within 24 hours in TP2 to TP5, no significant differences were found between the RIC and the sham group. Sixth, the follow-up duration of the patients was relatively short. It is not possible based on the present study design to determine whether RIC protects against the development of long-term cardiotoxicity. Given the progressive increase in hs-cTnT levels across different TPs, further studies to explore differences in long-term cardiac function between the RIC and the sham group are warranted. Finally, the assessment of apoptosis, autophagy, fibrosis, and inflammation of the myocardium is not possible. As shown by Gertz et al,22 the findings in the rodent model of attenuated cardiac fibrosis and apoptosis with RIC may not be translated into changes in LV ejection fraction or strain. It remains possible, albeit speculative, that these subclinical effects may still benefit cardiac recovery in the long-term after chemotherapy.
Conclusions
We have shown in this study that RIC did not exhibit cardioprotective effects in childhood cancer patients receiving anthracycline-based chemotherapy followed up until 3 months after the completion of anthracycline therapy. The complex clinical milieu, including patient factors and treatment regimens, might have precluded a demonstrable cardioprotective effect of RIC in pediatric cancer patients.
Perspectives.
COMPETENCY IN CLINICAL KNOWLEDGE: Anthracycline cardiotoxicity is a concern in survivors of childhood cancers. Heart failure is a major cause of morbidity and mortality in long-term childhood cancer survivors. RIC did not exhibit cardioprotective effects in childhood cancer patients receiving anthracycline-based chemotherapy followed up until 3 months after the completion of anthracycline therapy.
TRANSLATIONAL OUTLOOK: The exploration of noninvasive remote ischemic conditioning as a modality of myocardial protection is a novel concept in the field of pediatric cardio-oncology. Further studies and close collaboration between pediatric oncologists and cardiologists to assess the long-term impact of this and other noninvasive strategies in selected high-risk pediatric oncology patients may be informative.
Funding Support and Author Disclosures
This study was supported by the Health and Health Services Research Fund, Food and Health Bureau, Hong Kong SAR Government (grant 05161506). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Gatta G., Capocaccia R., Coleman M.P., Ries L.A., Berrino F. Childhood cancer survival in Europe and the United States. Cancer. 2002;95:1767–1772. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- 2.Kremer L.C., van Dalen E.C., Offringa M., Ottenkamp J., Voûte P.A. Anthracyline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19:191–196. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz S.E., Lipsitz S.R., Sallan S.E., et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz S.E., Sallan S.E., Giantris A.L., Lipsitz S.R., Dalton V., Colan S.D. 48 h continuous doxorubicin infusion is not cardioprotective in children assessed 18 months later: the DFCI 91001 ALL protocol. Proc Am Soc Clin Oncol. 1998;17:528a. [Google Scholar]
- 5.Lipshultz S.E., Giantris A.L., Lipsitz S.R., et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20:1677–1682. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 6.Lipshultz S.E., Scully R.E., Lipsitz S.R., et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomized, multicenter trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow E.J., Doody D.R., Amenian S.H., et al. Effect of dexrazoxane on heart function among long-term survivors of childhood leukemia and lymphoma: a report from the children’s oncology group (COG) Blood. 2016;128:696. [Google Scholar]
- 8.Choi H.S., Park E.S., Kang H.J., et al. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J Korean Med Sci. 2010;25:1336–1342. doi: 10.3346/jkms.2010.25.9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh F., Dupuis L.L., Alexander S., Gupta A., Mertens L., Nathan P.C. Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2015;108:djv357. doi: 10.1093/jnci/djv357. pii. [DOI] [PubMed] [Google Scholar]
- 10.Przyklenk K., Kauer B., Ovize M., Kloner R.A., Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G., Bøtker H.E., Przyklenk K., Redington A., Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gho B.C., Schoemaker R.G., van den Doel M.A., Duncker D.J., Verdouw P.D. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinov I.E., Li J., Cheung M.M., et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 14.Rassaf T., Totzeck M., Hendgen-Cotta U.B., Shiva S., Heusch G., Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 15.Davidson S.M., Selvaraj P., He D., et al. Remote ischemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Rohailla S., Gelber N., et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 17.Cheung M.M.H., Kharbanda R.K., Konstantinov J.E., et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 18.Wenwu Z., Debing Z., Renwei C., et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31:22–29. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q., Wang T., Chen S., et al. Cardiac protective effects of remote ischemic preconditioning in children undergoing tetralogy of Fallot repair surgery: a randomized controlled trial. Eur Heart J. 2017;21:2503–2513. doi: 10.1093/eurheartj/ehx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan W., Zhang C., Liu J., Li X., Chen Y., Miao Q. Remote ischemic preconditioning has a cardioprotective effect in children in the early postoperative phase: a meta-analysis of randomized controlled trials. Pediatr Cardiol. 2018;39:617–626. doi: 10.1007/s00246-017-1802-7. [DOI] [PubMed] [Google Scholar]
- 21.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 22.Gertz Z.M., Cain C., Kraskauskas D., et al. Remote ischemic pre-conditioning attenuates adverse cardiac remodeling and mortality following doxorubicin administration in mice. J Am Coll Cardiol CardioOnc. 2019;1:221–234. doi: 10.1016/j.jaccao.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Q., Wang F., Ryan T.D., Chalasani M., Redington A.N. Repeated remote ischemic conditioning reduces doxorubicin-induced cardiotoxicity. J Am Coll Cardiol CardioOnc. 2020;2:41–52. doi: 10.1016/j.jaccao.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galán-Arriola C., Villena-Gutiérrez R., Higuero-Verdejo M.I., et al. Remote ischemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc Res. 2021;117:1132–1143. doi: 10.1093/cvr/cvaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharbanda R.K., Nielsen T.T., Redington A.N. Translation of remote ischemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 26.Kharbanda R.K., Mortensen U.M., White P.A., et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 27.Cheung Y.F., Hong W.J., Chan G.C., Wong S.J., Ha S.Y. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010;96:1137–1141. doi: 10.1136/hrt.2010.194118. [DOI] [PubMed] [Google Scholar]
- 28.Thavendiranathan P., Negishi T., Coté M.A., et al. Single versus standard multiview assessment of global longitudinal strain for the diagnosis of cardiotoxicity during cancer therapy. J Am Coll Cardiol Img. 2018;11:1109–1118. doi: 10.1016/j.jcmg.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Li V.W., So E.K., Wong W.H., Cheung Y.F. Myocardial deformation imaging by speckle-tracking echocardiography for assessment of cardiotoxicity in children during and after chemotherapy: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2022;35:629–656. doi: 10.1016/j.echo.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Cheung Y.F., Li V.W., Lai C.T., et al. Circulating high-sensitivity troponin T and microRNAs as markers of myocardial damage during childhood leukaemia treatment. Pediatr Res. 2021;89:1245–1252. doi: 10.1038/s41390-020-1049-5. [DOI] [PubMed] [Google Scholar]
- 31.Guo Q., Yang D., Zhou Y., et al. Establishment of the reference interval for high-sensitivity cardiac troponin T in healthy children of Chongqing Nan’an district. Scand J Clin Lab Invest. 2021;81:579–584. doi: 10.1080/00365513.2021.1979245. [DOI] [PubMed] [Google Scholar]
- 32.Jones B.O., Pepe S., Sheeran F.L., et al. Remote ischemic preconditioning in cyanosed neonates undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg. 2013;146:1334–1340. doi: 10.1016/j.jtcvs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Pavione M.A., Carmona F., de Castro M., Carlotti A.P. Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg. 2012;144:178–183. doi: 10.1016/j.jtcvs.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Pepe S., Liaw N.Y., Hepponstall M., et al. Effect of remote ischemic preconditioning on phosphorylated protein signaling in children undergoing tetralogy of Fallot repair: a randomized controlled trial. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCrindle B.W., Clarizia N.A., Khaikin S., et al. Remote ischemic preconditioning in children undergoing cardiac surgery with cardiopulmonary bypass: a single-center double-blinded randomized trial. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez M.C.V., Spenceley N., Ilina M., Danton M.H.D. A prospective randomized blinded trial of remote ischemic preconditioning in children undergoing cardiac surgery. Semin Thorac Cardiovasc Surg. 2020;32:313–322. doi: 10.1053/j.semtcvs.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Tie H.T., Luo M.Z., Li Z.H., et al. Remote ischemic preconditioning fails to benefit pediatric patients undergoing congenital cardiac surgery: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung R., Maulik A., Hamarneh A., et al. Effect of remote ischemic conditioning in oncology patients undergoing chemotherapy: rationale and design of the ERIC-ONC Study--a single-center, blinded, randomized controlled trial. Clin Cardiol. 2016;39:72–82. doi: 10.1002/clc.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivaraman V., Pickard J.M.J., Hausenloy D.J. Remote ischemic conditioning: cardiac protection from afar. Anaesthesia. 2015;70:732–748. doi: 10.1111/anae.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipshultz S.E., Adams M.J., Colan S.D., et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 41.Blanco J.G., Sun C.L., Landier W., et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatia S. Genetics of anthracycline cardiomyopathy in cancer survivors: JACC: CardioOncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2020;2:539–552. doi: 10.1016/j.jaccao.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]