Abstract
Background
The prevalence of diastolic dysfunction has not been systematically evaluated in a large population of survivors of childhood cancer using established guidelines and standards.
Objectives
This study sought to assess the prevalence and progression of diastolic dysfunction in adult survivors of childhood cancer exposed to cardiotoxic therapy.
Methods
Comprehensive, longitudinal echocardiographic examinations of adult survivors of childhood cancer ≥18 years of age and ≥10 years from diagnosis in SJLIFE (St. Jude Lifetime Cohort Study) were performed. Diastolic dysfunction was defined based on 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines.
Results
Among 3,342 survivors, the median (25th-75th percentiles [quartile (Q)1-Q3]) age at diagnosis was 8.1 years (Q1-Q3: 3.6-13.7 years), 30.1 years (Q1-Q3: 24.4-37.0 years) at the baseline echocardiography evaluation (Echo 1), and 36.6 years (Q1-Q3: 30.8-43.6 years) at the last follow-up echocardiography evaluation (1,435 survivors) (Echo 2). The proportion of diastolic dysfunction was 15.2% (95% CI: 14.0%-16.4%) at Echo 1 and 15.7% (95% CI: 13.9%-17.7%) at Echo 2, largely attributable to concurrent systolic dysfunction. Less than 5% of survivors with preserved ejection fraction had diastolic dysfunction (2.2% at Echo 1, 3.7% at Echo 2). Using global longitudinal strain assessment in adult survivors with preserved ejection fraction (defined with a cutpoint worse than −15.9%), the proportion of diastolic dysfunction increased to 9.2% at baseline and 9.0% at follow-up.
Conclusions
The prevalence of isolated diastolic dysfunction is low among adults who received cardiotoxic therapies for childhood cancer. The inclusion of left ventricular global longitudinal strain significantly increased the identification of diastolic dysfunction.
Key Words: adult survivors of childhood cancer, anthracycline chemotherapy, chest-directed radiotherapy, diastolic dysfunction, echocardiography
Central Illustration
Despite a significant improvement in outcomes for survivors of childhood cancer, cardiotoxicity resulting from cancer treatment exposure remains a leading cause of early mortality.1,2 Anthracycline chemotherapies and chest-directed radiation therapy are associated with a dose-related increased risk of congestive heart failure.3,4 The biological mechanisms responsible for anthracycline-induced cardiotoxicity are multifaceted, including oxidative stress from accumulation of free radical formation, transcriptional alterations in intracellular adenosine triphosphate production, and mitochondrial dysfunction.5 In addition, a higher lifetime cumulative dose of anthracycline exposure, female sex, younger age of initiation of cancer therapies, radiation exposing the heart, and pre-existing cardiovascular disease are contributors to cardiotoxicity risk among survivors of childhood cancer.6 Although most echocardiographic evaluations of cardiotoxicity have focused on left ventricular (LV) systolic dysfunction, the role of diastolic dysfunction in cardiotoxicity is being increasingly studied. Diastolic dysfunction is described as an integral component in the progression of heart failure in the general population where it has been associated with an increased risk for all-cause mortality.7,8 Hence, tools capable of earlier detection of cardiotoxicity, including more precise assessment of diastolic injury, may improve the identification of patients with dysfunction who may benefit from intervention to preserve cardiac function.
Diastolic function can be assessed noninvasively by echocardiography. Although diastolic dysfunction and its characterization after childhood cancer therapies have been the focus of a number of studies, the reported prevalence remains equivocal because few studies have comprehensively assessed the full spectrum of diastolic variables, often basing the prevalence on only 1 or 2 indicators.4,9, 10, 11 Moreover, most reports are based on the evaluation of populations of limited sample size selected for specific cancer diagnoses.12, 13, 14, 15 The current study aimed to systematically evaluate the prevalence and progression of diastolic dysfunction by echocardiography based on the hierarchical diastolic algorithm provided by the 2016 American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) guidelines in a large population of survivors of childhood cancer.16
Methods
The current analysis includes adult survivors ≥18 years of age and ≥10 years from the diagnosis of childhood cancer diagnosed and treated at St. Jude Children’s Research Hospital and participating in SJLIFE (St. Jude Lifetime Cohort Study). Details of eligibility, recruitment methods, and study design have been published previously.17 At the inception of SJLIFE in 2007, echocardiography was risk based and limited to participants exposed to anthracycline chemotherapy or chest-directed radiotherapy. Subsequently, in 2015, the study was modified to systematically assess all survivors regardless of exposure status. A total of 448 cases were excluded from the current analysis because of significant mitral annular calcification (n = 6) or they were missing echocardiographic parameters necessary for diastolic assessment (n = 442), resulting in 3,342 survivors available for echocardiographic evaluation. This study was approved by the St. Jude Institutional Review Board, and survivors provided informed consent for participation.
Echocardiographic assessment
Standard transthoracic echocardiograms were performed using Vivid 7 (GE Medical Systems) and since 2010 E9 (GE Medical Systems) platforms at the baseline and follow-up visits. Echocardiographic assessment included both standard 2-dimensional and 3-dimensional left ventricular ejection fraction (LVEF) quantification based on the 2016 ASE guidelines; abnormal LVEF was reported as <52% for males and <54% for females.18 Mitral inflow velocities were measured at leaflet tips by pulsed wave Doppler. Tissue Doppler velocities were measured from the apical 4-chamber view at both the medial and lateral mitral annulus. The left atrial (LA) maximum volumes were captured at end-ventricular systole and indexed to participants’ body surface area. The peak tricuspid systolic velocities were recorded and quantified using the simplified Bernoulli equation. Global longitudinal strain (GLS) was obtained using endocardial contours from the apical 4-chamber, apical 2-chamber, and apical 3-chamber views, respectively, timed at 1 complete RR interval.
Decreased LV GLS, which was defined as a value worse than −15.9%, was used as an additional parameter to evaluate diastolic dysfunction.19 The 2016 ASE/EACVI guidelines highlight that although this approach has not been widely tested, it may have specific significance in patients with preserved ejection fraction (EF) and equivocal data after evaluating diastolic parameters.16 Although no specific reference ranges or cutoff values were suggested, our study used the lowest value (LV GLS = −15.9%) reported in the most recent meta-analysis of normal GLS reference ranges.19
Outcome definition for diastolic dysfunction
Diastolic assessment following the hierarchical algorithm was applied for each subject included in this analysis.16 Any participant with echocardiography identified to have overt myocardial disease (reduced LVEF [<52% in men and <54% in women], regional wall motion abnormalities, or left ventricular hypertrophy) was automatically evaluated based on tier B of the 2016 ASE algorithm for the assessment of diastolic dysfunction in subjects with depressed EF.16,18 Subjects with preserved LVEF were assessed using tier A of the hierarchical diagram of the guidelines.16 The following variables were used to assign diastolic function classification: average E/e’ (E velocity divided by mitral annular e’ velocity) >14, septal e’ velocity <7 cm/s, lateral e’ (mitral annular e’ velocity) velocity <10 cm/s, tricuspid regurgitation (TR) velocity >2.8 m/s, and LA volume index >34 mL/m2. As per the “majority rules” guidelines, in the event that 1 of 4 (when 4 were available) or 1 of 3 (when 3 were available) was positive, subjects were classified as having normal diastolic function. When 2 of 4 were positive, the classification was indeterminate. In addition, in subjects with preserved LVEF, the implementation of LV GLS (cutoff = −15.9) was used. Finally, in instances in which 3 of 4 or 2 of 3 variables were positive, the subject was classified as diastolic dysfunction present, and then the application of tier B categorization followed for grading.16
Demographic and exposure variables
Survivor characteristics and the cumulative anthracycline dose were abstracted from the medical record consistent with previous studies.20 Chest-directed radiation therapy dose reconstruction was calculated using anthropomorphic phantoms constructed of tissue-equivalent material as previously described.21 Comorbidities including abnormal glucose metabolism, hypertension, and chronic kidney disease were graded based on system-based chronic and late-onset medical event severity grading in SJLIFE.22
Statistical analysis
Categoric data are presented as the frequency with percentage, and the comparison between groups was performed using the chi-square or Fisher exact test. Continuous variables are expressed as the median with 25th and 75th percentiles (quartile [Q]1-Q3), and the Mann-Whitney U test was used to compare differences between 2 groups. Comparisons among 3 or more groups were performed with 1-way analysis of variance or the Kruskal-Wallis test. Linear mixed-effects models were used to assess the change of echocardiographic parameters over time (at the baseline echocardiography evaluation [Echo 1] and at the follow-up echocardiography evaluation [Echo 2]). The fixed effect included age and time, and the random effect included random intercept and slope, allowing intercept and slopes of the model to vary across subjects. An autoregressive correlation structure was assumed for repeated measurements within individuals. Given the very low proportion of abnormal diastolic function, the grade change over time was not assessed. Furthermore, complete case analysis among the 1,435 survivors with at least 2 echocardiography visits was performed as a sensitivity analysis to assess the robustness of results given that not all survivors had follow-up echocardiography.23 Statistical analyses were performed using R software, version 4.0.3 (The R Foundation).
Results
Population characteristics
The median (Q1-Q3) age at diagnosis was 8.1 years (Q1-Q3: 3.6-13.7 years) (Table 1). The median age at Echo 1 was 30.1 years (Q1-Q3: 24.4-37.0 years) and 36.6 years (Q1-Q3: 30.8-43.6 years) at Echo 2. At baseline, survivors were more likely male (52.7%); 1,645 (49.2%) were treated with chest-directed radiation and anthracycline chemotherapy, 228 (6.8%) with chest-directed radiation but no anthracyclines, 1,259 (37.7%) with anthracycline chemotherapy but no chest radiation, and 210 (6.3%) without anthracycline chemotherapy or chest-directed radiation therapy (Table 1).
Table 1.
Demographic, Primary Cancer Diagnosis, Treatment Characteristics, and Echocardiographic Outcomes
| Echo 1 (n = 3,342) | Echo 2 (n = 1,435) | |
|---|---|---|
| Age at echocardiography, y | 30.1 (24.4- 37.0) | 36.6 (30.8-43.6) |
| Age at diagnosis | 8.1 (3.6-13.7) | |
| BSA | 1.89 ± 0.29 | 1.93 ± 0.30 |
| Female, % | 1,582 (47.3) | 682 (47.5) |
| Race/ethnicity | ||
| Non-Hispanic White | 2,697 (80.7) | 1,177 (82.0) |
| Non-Hispanic Black | 490 (14.7) | 192 (13.4) |
| Hispanic | 97 (2.9) | 43 (3.0) |
| Other | 62 (1.7) | 23 (1.6) |
| Primary cancer diagnosis | ||
| Leukemia | ||
| Acute lymphoblastic leukemia | 1,038 (31.1) | 490 (34.2) |
| Acute myeloid leukemia | 136 (4.1) | 63 (4.3) |
| Other leukemia | 3 (0.09) | 0 (0) |
| Lymphoma | ||
| Non-Hodgkin lymphoma | 239 (7.2) | 125 (8.7) |
| Hodgkin lymphoma | 428 (12.8) | 228 (15.9) |
| CNS tumor | 390 (11.7) | 92 (6.4) |
| Bone tumor | ||
| Ewing sarcoma | 107 (3.2) | 55 (3.8) |
| Osteosarcoma | 138 (4.1) | 79 (5.5) |
| Soft tissue sarcoma | ||
| Rhabdomyosarcoma | 107 (3.1) | 41 (2.9) |
| Nonrhabdomyosarcoma | 85 (2.5) | 21 (1.5) |
| Other malignancies | ||
| Germ cell tumor | 62 (1.9) | 13 (0.9) |
| Melanoma | 12 (0.4) | 1 (0.1) |
| Neuroblastoma | 153 (4.6) | 64 (4.5) |
| Retinoblastoma | 88 (2.6) | 11 (0.8) |
| Wilms tumor | 221 (6.6) | 114 (7.9) |
| Treatment | ||
| Chemotherapy alone | 1,259 (37.7) | 524 (36.5) |
| Radiation alone | 228 (6.8) | 80 (5.6) |
| Chemotherapy and Radiation | 1,645 (49.2) | 803 (56.0) |
| None | 210 (6.3) | 28 (1.9) |
| Anthracycline cumulative dose, mg/m2 | ||
| None | 1,210 (36.2) | 345 (24.0) |
| 1-100 | 751 (22.5) | 375 (26.1) |
| 101-250 | 889 (26.6) | 451 (31.4) |
| >250 | 483 (14.5) | 261 (18.2) |
| Chest-directed RT, Gy | ||
| None | 1,475 (44.1) | 555 (38.7) |
| 1-20 | 423 (12.7) | 210 (14.6) |
| 20-35 | 131 (3.9) | 70 (4.9) |
| >35 | 883 (26.4) | 546 (38.0) |
| Comorbiditiesa | ||
| Abnormal glucose metabolism | ||
| Grade 0 | 2,631 (78.7) | 965 (67.2) |
| Grade 1 | 408 (12.2) | 272 (18.9) |
| Grade 2 | 124 (3.7) | 113 (7.9) |
| Grade 3 | 171 (5.1) | 85 (5.9) |
| Hypertension | ||
| Grade 0 | 1,500 (44.9) | 395 (27.5) |
| Grade 1 | 1,100 (32.9) | 563 (39.2) |
| Grade 2 | 554 (16.6) | 347 (24.2) |
| Grade 3 | 182 (5.5) | 130 (9.1) |
| Chronic kidney disease | ||
| Grade 0 | 2,889 (86.5) | 1,350 (94.1) |
| Grade 1 | 29 (0.9) | 24 (1.7) |
| Grade 2 | 44 (1.3) | 34 (2.4) |
| Grade 3 | 25 (0.7) | 17 (1.2) |
| Medical therapy, % | ||
| β-blocker | 168 (5.0) | 132 (9.2) |
| ACE inhibitor | 184 (5.5) | 133 (9.2) |
| ARB | 75 (2.2) | 53 (3.7) |
| MRA | 12 (0.4) | 12 (0.8) |
Values are median (Q1-Q3), mean ± SD, or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; BSA = body surface area; CNS = central nervous system; Echo 1 = baseline echocardiography evaluation; Echo 2 = follow-up echocardiography evaluation; MRA = Mineralocorticoid receptor antagonist; RT = radiation therapy.
Based on system-based chronic and late- onset medical event severity grading in SJLIFE (St. Jude Lifetime Cohort Study).22
Diastolic dysfunction in the cohort
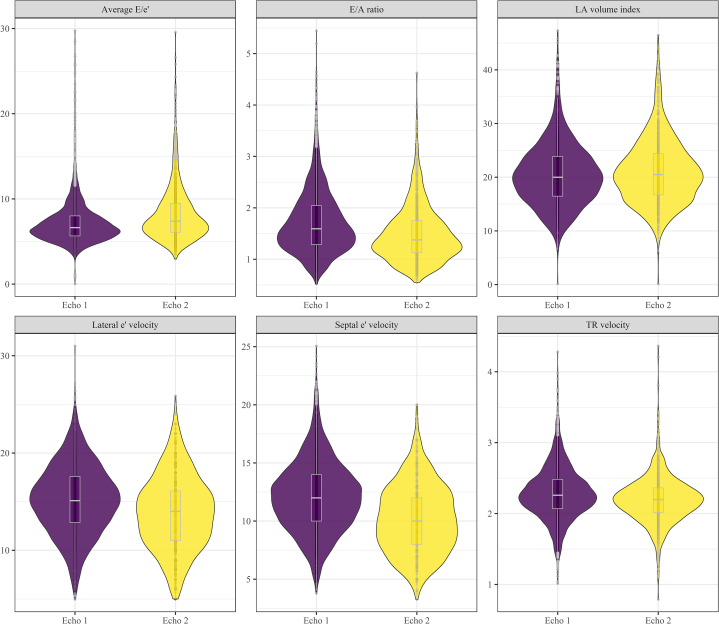
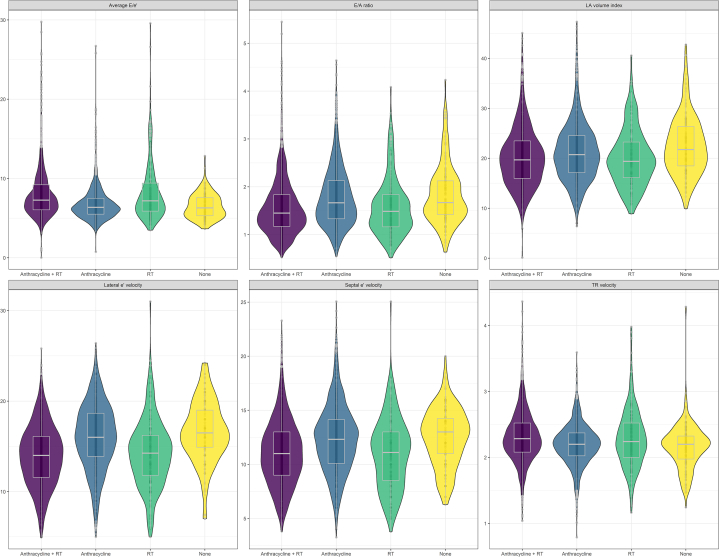
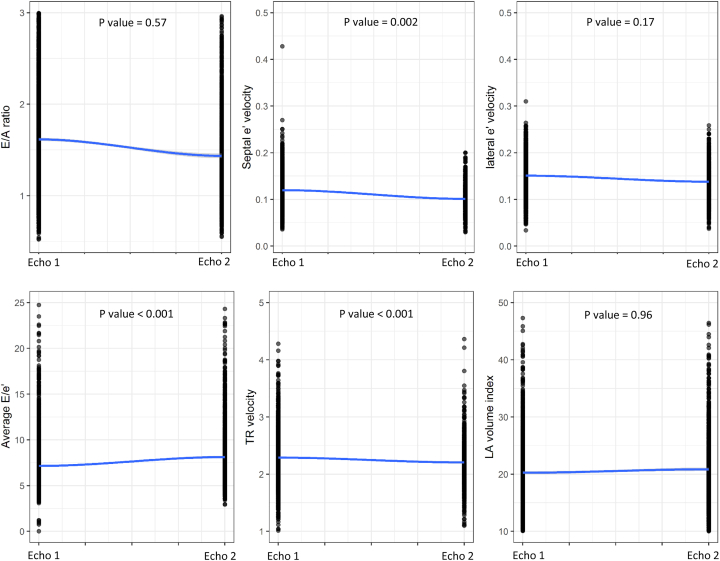
Overall, the proportion of diastolic dysfunction was 15.2% (95% CI: 14.0%-16.4%) at baseline among 3,342 survivors and 15.7% (95% CI: 13.9%-17.7%) at follow-up among 1,435 survivors. Table 2 provides the prevalence of diastolic dysfunction by grade along with the prevalence of individual measures of cardiac function. Notably, most survivors with diastolic dysfunction had grade I dysfunction. Figure 1 illustrates the distribution of the specific echocardiographic variables used to assess diastolic function at Echo 1 and Echo 2. Echocardiographic characteristics and the prevalence of abnormal diastolic dysfunction stratified by time interval (10-year mark) from cancer diagnosis to the first echocardiogram are presented in Supplemental Table 1. Regarding the evaluation of diastolic dysfunction based on therapeutic exposures, no significant difference in the proportion of survivors with various grades of diastolic dysfunction with therapy (anthracycline therapy alone, chest-directed radiotherapy alone, or combined therapy) was identified (P = 0.19) (Table 3, Figure 2). When comparing by treatment exposure, although certain individual echocardiographic variables were statistically significantly different, none represented clinically meaningful differences. When comparing baseline to follow-up echocardiography measures, septal e’ velocity (P = 0.002), average E/e’ (P < 0.001), and TR velocity (P < 0.001) significantly changed over time but not the E/A (E velocity divided by A-wave velocity) ratio (P = 0.57), lateral e’ velocity (P = 0.17), or LA volume index (P = 0.96) (Figure 3). The echocardiographic parameters used to assess diastolic dysfunction are provided, stratified by sex, in Supplemental Table 2.
Table 2.
Echocardiographic Characteristics and Prevalence of Abnormal Diastolic Dysfunction in Survivors of Childhood Cancer Evaluated Longitudinally
| Echo 1 (n = 3,342) | Echo 2 (n = 1,435) | P Valuea | |
|---|---|---|---|
| E/A ratio | 1.56 (1.26-2.01) | 1.36 (1.10-1.74) | 0.57 |
| Septal e’ velocity, cm/s | 12.0 (10.0-14.0) | 10.0 (8.0-12.0) | 0.002 |
| Lateral e’ velocity, cm/s | 15.0 (12.8-17.5) | 14.0 (11.0-16.0) | 0.17 |
| Average E/e’ | 6.64 (5.63-8.01) | 7.38 (6.12-9.38) | < 0.001 |
| TR velocity, m/s | 2.23 (1.99-2.46) | 2.11 (1.69-2.32) | < 0.001 |
| LA volume, mL/m2 | 19.6 (16.0-23.5) | 20.1 (16.4 -24.0) | 0.96 |
| IVSd, mm | 8.7 (7.8-9.8) | 8.8 (7.8-9.8) | <0.001 |
| LVIDd, mm | 44.9 (41.3-48.7) | 44.8 (41.4-48.5) | <0.001 |
| PWd, mm | 8.5 (7.5-9.5) | 8.4 (7.5-9.4) | <0.001 |
| LVEDV, mL | 94.8 (76.9-115.0) | 84.5 (67.7-104.0) | <0.001 |
| LVEDV index, mL/m2 | 51.2 (42.5-59.9) | 44.8 (36.2-53.4) | <0.001 |
| LVESV, mL | 35.8 (28.3-44.8) | 31.6 (25.1-40.9) | <0.001 |
| LVESV index, mL/m2 | 19.2 (15.7-23.2) | 16.6 (13.5-20.9) | <0.001 |
| SV, mL | 57.8 (46.8-71.3) | 51.7 (40.7-63.7) | <0.001 |
| SV index, mL/m2 | 31.2 (25.9-37.2) | 27.4 (21.9-33.0) | <0.001 |
| LV mass, g/m2 | 67.0 (56.5-79.0) | 65.4 (55.7-76.1) | <0.001 |
| LVEF, % | 61.4 (57.2-65.3) | 61.8 (57.0-65.3) | <0.001 |
| GLS, %) | −18.7 ± 3.4 | −18.4 ± 3.7 | 0.80 |
| Diastolic function, % | |||
| Normal | 2,835 (84.8) | 1,210 (84.3) | |
| Grade 1 | 291 (8.7) | 137 (9.6) | |
| Grade 2 | 17 (0.5) | 20 (1.4) | |
| Grade 3 | 88 (2.6) | 19 (1.3) | |
| Indeterminate | 50 (1.5) | 43 (3.0) | |
| Not determined | 61 (1.8) | 6 (0.4) |
Values are median (Q1-Q3) or n (%).
E/A = E velocity divided by A velocity; e’ = mitral annular e’ velocity; E/e’ = E velocity divided by mitral annular e’ velocity; EF = ejection fraction; GLS = global longitudinal strain; IVSd = interventricular septal end diastole; LA = left atrium; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; LVIDd = left ventricular internal diameter end-diastole; PWd = posterior wall end diastole; SV = stroke volume; TR = tricuspid regurgitation; other abbreviations as in Table 1.
The P values are from the age-adjusted linear mixed-effects models.
Figure 1.
Distribution of Echocardiographic Variables
A violin plot illustrating the distribution of echocardiographic variables used to assess diastolic function over time. The shaded area describes probability density; the white box plot denotes the median and IQR. E/A = E velocity divided by A velocity; e’ = mitral annular e’ velocity; E/e’ = E velocity divided by mitral annular e’ velocity; LA = left atrial; TR = tricuspid regurgitation.
Table 3.
Diastolic Echocardiographic Parameters Stratified by Therapy at Baseline Echocardiography Evaluation
| Chemotherapy Alone (n = 1,259) | Radiation Alone (n = 228) | Chemotherapy and Radiation (n = 1645) | P Valuea | |
|---|---|---|---|---|
| E/A ratio | 1.68 (1.34-2.15) | 1.53 (1.21-1.88) | 1.48 (1.21-1.89) | <0.001 |
| Septal e’ velocity, cm/s | 12.6 (10.5-14.4) | 12.0 (10.0-13.8) | 11.3 (9.5-13.3) | 0.10 |
| Lateral e’ velocity, cm/s | 16.1 (13.9-18.6) | 14.8 (12.8-17.0) | 14.2 (12.0-16.4) | <0.001 |
| Average E/e’ | 6.30 (5.45-7.41) | 6.62 (5.49-8.50) | 7.05 (5.93-8.50) | <0.001 |
| TR velocity, m/s | 2.19 (1.95-2.38) | 2.21 (1.90-2.52) | 2.29 (2.06-2.57) | <0.001 |
| LA volume, mL/m2 | 20.0 (16.4-23.8) | 19.3 (15.3-22.3) | 19.0 (15.1-22.8) | 0.002 |
| Diastolic function, % | 0.19 | |||
| Normal | 1,094 (86.9) | 197 (86.4) | 1,346 (81.8) | |
| Grade I | 105 (8.3) | 17 (7.5) | 161 (9.8) | |
| Grade II | 3 (0.2) | 1 (0.4) | 13 (0.8) | |
| Grade III | 32 (2.6) | 5 (2.2) | 48 (2.9) | |
| Indeterminate | 7 (0.6) | 6 (2.6) | 36 (2.2) | |
| Not determined | 18 (1.4) | 2 (0.9) | 41 (2.5) |
Values are median (Q1-Q3) or n (%).
Abbreviations as in Table 2.
The P values are from the Kruskal-Wallis test.
Figure 2.
Distribution of Specific Echocardiographic Variables Stratified by Type of Cardiotoxic Therapy
A violin plot demonstrating the distribution of specific echocardiographic variables used to assess diastolic function stratified by type of cardiotoxic therapy exposure at baseline echocardiography evaluation. The shaded area describes probability density; the white boxplot denotes the median and IQR. RT = therapy; other abbreviations as in Figure 1.
Figure 3.
Changes in Left Ventricular Diastolic Function Over Time Following Childhood Cancer Therapies
Septal velocity, average E/e’, and TR velocity significantly changed over time. Conversely, the E/A ratio, lateral e’ velocity, and LA volume index did not change. The P values are from the age-adjusted linear mixed-effects model. Echo 1 = baseline echocardiography evaluation; Echo 2 = follow-up echocardiography evaluation; other abbreviations as in Figure 1.
Diastolic function in patients at baseline and follow-up with preserved and reduced EF
Overall, the majority of patients with preserved EF had normal diastolic function (97.8% at Echo 1, 96.3% at Echo 2) (Table 4). However, when a GLS of −15.9% was applied as a cutpoint, the proportion with diastolic dysfunction increased to 9.2% at baseline and 9.0% at follow-up. In addition, septal velocity significantly decreased over time (P = 0.002). Similarly, the average E/e’ significantly increased during follow-up (P < 0.001). Conversely, the lateral e’ velocity, E/A ratio, and LA volume index did not change (P > 0.05 for all).
Table 4.
Diastolic Echocardiographic Parameters in the Survivors With Preserved LVEF Group
| Preserved LVEF |
|||
|---|---|---|---|
| Echo 1 (n = 2,899) | Echo 2 (n = 1,205) | P Valuea | |
| E/A ratio | 1.58 (1.28-2.02) | 1.38 (1.11-1.74) | 0.44 |
| E/A ≤0.8, % | 56 (1.9) | 52 (4.3) | |
| 0.8 < E/A <2, % | 2,075 (71.6) | 964 (80.0) | |
| E/A ≥2, % | 756 (26.1) | 181 (15.0) | |
| Septal e’ velocity, cm/s | 12.0 (10.0-14.0) | 10.2 (7.9-12.1) | 0.003 |
| Lateral e’ velocity, cm/s | 15.2 (13.0-17.7) | 13.9 (11.8-16.1) | 0.10 |
| Abnormal septal or lateral e’ velocity, % | 205 (7.1) | 185 (15.4) | |
| Average E/e’ | 6.58 (5.61-7.92) | 7.22 (6.08-9.07) | <0.001 |
| Abnormal average E/e’ >14, % | 43 (1.5) | 54 (4.5) | |
| TR velocity, m/s | 2.23 (1.99-2.46) | 2.10 (1.65-2.31) | <0.001 |
| Abnormal TR velocity >2.8, % | 203 (7.0) | 33 (2.7) | |
| LA volume, mL/m2 | 19.6 (16.0-23.5) | 20.1 (16.3-23.8) | 0.38 |
| Abnormal LA volume >34, % | 36 (1.2) | 28 (2.3) | |
| Diastolic function, % | |||
| Normal | 2,835 (97.8) | 1,161 (96.3) | |
| Grade I | 0 (0) | 0 (0) | |
| Grade II | 11 (0.4) | 10 (0.8) | |
| Grade III | 3 (0.1) | 2 (0.2) | |
| Indeterminate | 50 (1.7) | 32 (2.7) | |
Values are median (Q1-Q3) or n (%).
LVEF = left ventricular ejection fraction; other abbreviations as in Table 2.
The P values are from the age-adjusted linear mixed-effects model; the stratification into preserved LVEF and reduced LVEF for grading diastolic function based on the first echocardiography.
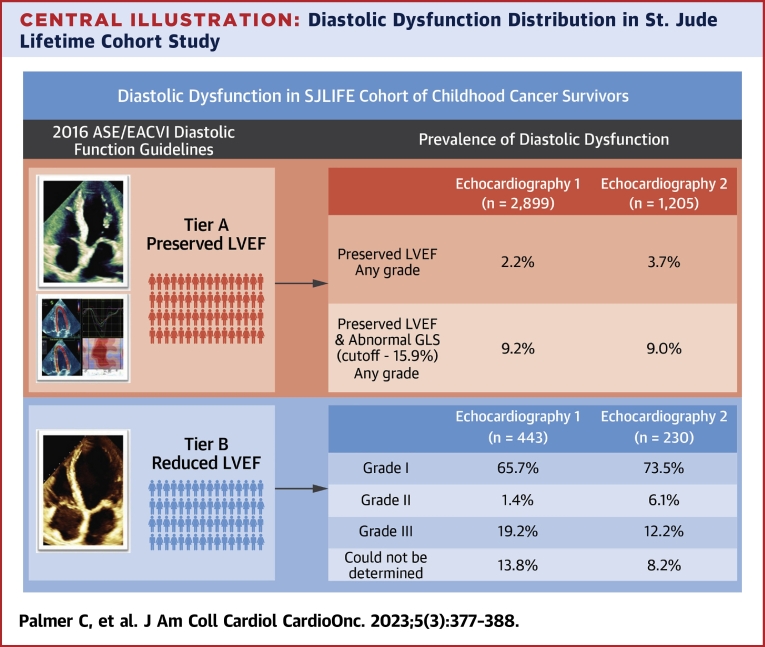
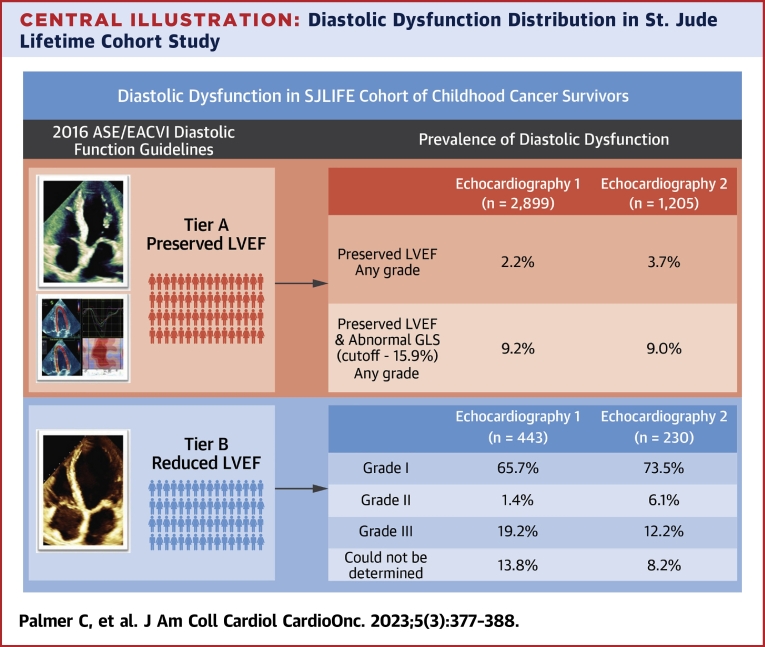
The proportion of the cohort with reduced EF was 13.3% at Echo 1 and 16.0% at Echo 2 (Table 5). At Echo 1, 65.7% of survivors with reduced EF were classified as grade I diastolic dysfunction, 1.4% as grade II, 19.2% as grade III, and grade could not be determined in 13.8% (Table 5). At Echo 2, 73.5% of survivors with reduced EF were classified as grade I diastolic dysfunction, 6.1% as grade II, 12.2% as grade III, and grade could not be determined in 8.2% (Central Illustration). In the population with reduced EF, echocardiographic parameters for assessing diastolic dysfunction including septal e’ velocity and TR velocity significantly changed over time. Conversely, the E/A ratio, lateral e’ velocity, E/e’, and LA volume index did not change during the follow-up.
Table 5.
Diastolic Echocardiographic Parameters in the Reduced LVEF Group
| Reduced LVEF |
|||
|---|---|---|---|
| Echo 1 (n = 443) | Echo 2 (n = 230) | P Valuea | |
| E/A ratio | 1.46 (1.16-1.93) | 1.29 (0.98-1.53) | 0.08 |
| E/A ≤0.8, % | 24 (5.4) | 27 (11.7) | |
| 0.8 < E/A <2, % | 321 (72.5) | 182 (79.1) | |
| E/A ≥2, % | 90 (20.3) | 21 (9.1) | |
| Septal e’ velocity, cm/s | 10.4 (8.4-12.1) | 8.3 (6.2-8.8) | <0.001 |
| Lateral e’ velocity, cm/s | 13.9 (11.2-16.1) | 11.2 (9.1-14.2) | 0.32 |
| Abnormal septal or lateral e’ velocity, % | 87 (19.6) | 95 (41.3) | |
| Average E/e’ | 7.10 (6.01-9.22) | 9.17 (6.85-12.53) | 0.10 |
| Abnormal average E/e’, % | 31 (7.0) | 48 (20.9) | |
| TR velocity, m/s | 2.25 (1.99-2.49) | 2.10 (1.89-2.32) | < 0.001 |
| Abnormal TR velocity, % | 37 (8.4) | 19 (8.3) | |
| LA volume, mL/m2 | 19.6 (15.3-23.7) | 22.1 (18.1-27.5) | 0.15 |
| Abnormal LA volume, % | 3 (0.7) | 13 (7.2) | |
| Diastolic function, % | |||
| Grade I | 291 (65.7) | 169 (73.5) | |
| Grade II | 6 (1.4) | 14 (6.1) | |
| Grade III | 85 (19.2) | 28 (12.2) | |
| Not determined | 61 (13.8) | 19 (8.2) | |
Values are median (Q1-Q3) or median and quartile 1-quartile 3 for non-normal distribution. Categoric variables are presented as n (%).
The P values are from the age-adjusted linear mixed-effects model; the stratification into preserved LVEF and reduced LVEF for grading diastolic function based on the first echocardiography.
Central Illustration.
Diastolic Dysfunction Distribution in St. Jude Lifetime Cohort Study
Tier A displays adult survivors with preserved ejection fraction that could be assigned diastolic grading based on 4 key echocardiographic diastolic variables followed by global longitudinal strain worse than −15.9%. Tier B displays adult survivors with reduced ejection fraction that could be assigned diastolic grading. ASE = American Society of Echocardiography; EACVI = European Association of Cardiovascular Imaging; GLS = global longitudinal strain; LVEF = left ventricular ejection fraction; SJLIFE = St. Jude Lifetime Cohort Study.
Sensitivity analyses
Results based on a complete case analysis (N = 1,435 at Echo 1 and Echo 2) found a similar proportion of diastolic dysfunction using all available data (Supplemental Tables 3 to 6).
Discussion
To our knowledge, our study included the largest population to date of adult survivors of childhood cancer evaluated for the prevalence of diastolic dysfunction with systematic application of ASE/EACVI guidelines. Overall, we determined that diastolic dysfunction in survivors, of whom 12.2% to 19.2% were grade III, is largely attributable to concurrent systolic function. In contrast, only 2.2% of survivors at baseline and 4.6% with preserved EF at follow-up had evidence of diastolic dysfunction. Furthermore, LV GLS significantly improved the identification of diastolic dysfunction in adult survivors with preserved EF largely treated with cardiotoxic therapy.
The historical discordance in the definition and grading of diastolic dysfunction created formidable complexity for the clinician. Because of this, the updated 2016 ASE/EACVI diastolic recommendations were developed to provide a streamlined stepwise hierological assessment using key variables in the absence of myocardial disease (E/A ratio, e′ velocities, E/e′ ratio, TR velocity, and LA volume index).24 Feasible implementation into everyday clinical practice with improved interobserver reliability across a broad range of observer experience was the primary driver in the updated recommendations.24 These refinements have mitigated discordance between variables such that classification is now simplified to majority rules, meaning for positive classification at least 3 of 5 available variables when all are available or 2 of 3 available variables when only 3 are available. These majority rules may explain the lower prevalence of diastolic dysfunction observed in the current study of adult survivors of childhood cancer compared with that previously reported in studies of smaller populations that often limited evaluation to 1 or 2 parameters to diagnose dysfunction.25,26 In addition, the authors believe these refinements to be the explanation of a more modest classification of diastolic dysfunction prevalence within a previous study of a large population of adult cancer survivors.20
Although LV GLS and LA longitudinal strain were also proposed in the 2016 ASE/EACVI guidelines to further assess myocardial function, these approaches had not been widely tested.16 The additional use of LV GLS within our cohort of adult survivors significantly increased the prevalence of diastolic dysfunction. The lack of a recommended LV GLS cutoff value may lead to reluctance to draw conclusions. However, the current study used the most conservative cutoff value for LV GLS reported in the most recent meta-analysis of normal GLS reference ranges.19 Because the SJLIFE echocardiography evaluation did not analyze LA longitudinal strain, no association between this metric and diastolic dysfunction could be reported.
Additionally, adult survivors with reduced EF were more likely to have grade I diastolic dysfunction rather than higher-grade dysfunction. The appropriate grading of diastolic function carries significant prognostic implications; advanced stages of diastolic dysfunction irrespective of LVEF imply worse outcomes in the general population.27,28 Hence, the observation of predominantly grade I diastolic dysfunction when the EF was reduced was quite remarkable. The observation of a low rate of diastolic dysfunction in a large cohort of prospectively followed adult survivors of childhood cancer is significant. Future research using guideline-based assessment of diastolic dysfunction in the interim between cancer diagnosis and treatments and that of long-term survivorship is pivotal.
Study limitations
There are several study limitations to be considered. First, although this the largest cohort to date of adult survivors of childhood cancers, it only includes individuals >10 years from the initial cancer diagnosis and does not capture early changes after therapy. Although late-occurring cardiotoxicity may not become clinically evident until 10 to 20 years after the initial cancer treatment, diastolic dysfunction could have occurred during or immediately after therapy and subsequently improved.28, 29, 30 In the current study, there were no data available to assess diastolic dysfunction at the short-term follow-up proximal to therapy (1-2 years post–cancer therapy). Second, diastolic function in our study was not validated by invasive direct measurement of LV filling pressures or circulating biomarkers, and the lack of this validated benchmark may lead to a type II error. Third, specific echocardiographic variables can be vastly underestimated secondary to inherent limitations of echocardiography. TR velocity is susceptible to underestimation when a complete jet envelope is not available; agitated saline administration was not routinely used within our study to enhance TR velocity and may explain the low prevalence of TR jet velocity >2.8 m/s. In addition, pulmonary vein velocities were not routinely reported within our cohort. Furthermore, given the low proportion of diastolic dysfunction in adult survivors with preserved EF, a multivariable model to identify associations with diastolic dysfunction was not feasible. Multivariable risk factor assessment for survivors with reduced EF has been previously performed by our group and many others.20 Of note, in this current study, interobservability of LV strain analysis was not performed. In addition, this current study could not fully elucidate whether the low proportion of diastolic dysfunction demonstrated was resultant from cancer treatment and/or associated changes seen later in life such as hypertension, diabetes, and obesity. Further consideration should be given to the fact that an optimal cutpoint for abnormal GLS has not been defined; hence, the prevalence of diastolic dysfunction would be varied with different GLS cutpoints. Finally, the presence of missing values, unavoidable in longitudinal studies, may introduce bias. However, a sensitivity analysis using a complete case analysis approach was also performed that confirmed the robustness of the results.23
Conclusions
The use of the hierarchical 2016 ASE/EACVI diastolic algorithm in adult survivors of childhood cancers revealed a low prevalence of diastolic dysfunction after cardiotoxic cancer therapies regardless of therapeutic type. LV GLS with a cutoff of −15.9% significantly improved the identification of diastolic dysfunction within the same cohort.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Application of the hierarchical 2016 ASE/EACVI diastolic algorithm in a large population of adult survivors of childhood cancers after cardiotoxic cancer therapies demonstrated a low prevalence of diastolic dysfunction in the setting of preserved systolic function. Notably, LV GLS significantly improved the identification of diastolic dysfunction.
TRANSLATIONAL OUTLOOK: Future research assessing the role of LV GLS in diastolic function and grading is pivotal in patients after cancer therapies and the risk of cardiovascular events.
Funding Support and Author Disclosures
Support to St. Jude Children’s Research Hospital was provided by National Cancer Institute grant U01 CA195547 (Drs Ness and Hudson [principal investigators]), the Cancer Center Support grant P30 CA21765 (Dr Roberts [principal investigator), and the American Lebanese-Syrian Associated Charities. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Reulen R.C., Winter D.L., Frobisher C., et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong G.T., Liu Q., Yasui Y., et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dalen E.C., van der Pal H.J., Kok W.E., Caron H.N., Kremer L.C. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer C.A., Postma A., Vonk J.M., et al. Systolic and diastolic dysfunction in long-term adult survivors of childhood cancer. Eur J Cancer. 2011;47:2453–2462. doi: 10.1016/j.ejca.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Yeh E.T., Bickford C.L. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Bansal N., Amdani S., Lipshultz E.R., Lipshultz S.E. Chemotherapy-induced cardiotoxicity in children. Expert Opin Drug Metab Toxicol. 2017;13:817–832. doi: 10.1080/17425255.2017.1351547. [DOI] [PubMed] [Google Scholar]
- 7.Shah A.M., Claggett B., Sweitzer N.K., et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus W.J., Tschope C., Sanderson J.E., et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 9.Wolf C.M., Reiner B., Kuhn A., et al. Subclinical cardiac dysfunction in childhood cancer survivors on 10-years follow-up correlates with cumulative anthracycline dose and is best detected by cardiopulmonary exercise testing, circulating serum biomarker, speckle tracking echocardiography, and tissue Doppler imaging. Front Pediatr. 2020;8:123. doi: 10.3389/fped.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shigemitsu S., Takahashi K., Yazaki K., et al. New insight into the intraventricular pressure gradient as a sensitive indicator of diastolic cardiac dysfunction in patients with childhood cancer after anthracycline therapy. Heart Vessels. 2019;34:992–1001. doi: 10.1007/s00380-018-01332-7. [DOI] [PubMed] [Google Scholar]
- 11.Kapusta L., Thijssen J.M., Groot-Loonen J., Antonius T., Mulder J., Daniels O. Tissue Doppler imaging in detection of myocardial dysfunction in survivors of childhood cancer treated with anthracyclines. Ultrasound Med Biol. 2000;26:1099–1108. doi: 10.1016/s0301-5629(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 12.Upshaw J.N., Finkelman B., Hubbard R.A., et al. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. J Am Coll Cardiol Img. 2020;13:198–210. [Google Scholar]
- 13.Christiansen J.R., Hamre H., Massey R., et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483–490. doi: 10.1016/j.amjcard.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 14.Klein Hesselink M.S., Bocca G., Hummel Y.M., et al. Diastolic dysfunction is common in survivors of pediatric differentiated thyroid carcinoma. Thyroid. 2017;27:1481–1489. doi: 10.1089/thy.2017.0383. [DOI] [PubMed] [Google Scholar]
- 15.Rajapreyar P., Lorenzana A., Prabhu A., Szpunar S., Anne P. Tissue Doppler imaging and focal, late-onset anthracycline-induced cardiovascular disease in long term survivors of childhood cancer: a research article. J Clin Diagn Res. 2016;10:SC01–SC04. doi: 10.7860/JCDR/2016/19652.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Howell C.R., Bjornard K.L., Ness K.K., et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50:39–49. doi: 10.1093/ije/dyaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Yingchoncharoen T., Agarwal S., Popovic Z.B., Marwick T.H. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong G.T., Joshi V.M., Ness K.K., et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stovall M., Weathers R., Kasper C., et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 22.Hudson M.M., Ehrhardt M.J., Bhakta N., et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakobsen J.C., Gluud C., Wetterslev J., Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad S.B., Holland D.J., Atherton J.J., Whalley G. New diastology guidelines: evolution, validation and impact on clinical practice. Heart Lung Circ. 2019;28:1411–1420. doi: 10.1016/j.hlc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Border W.L., Sachdeva R., Stratton K.L., et al. Longitudinal changes in echocardiographic parameters of cardiac function in pediatric cancer survivors. J Am Coll Cardiol CardioOnc. 2020;2:26–37. doi: 10.1016/j.jaccao.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryerson A.B., Border W.L., Wasilewski-Masker K., et al. Assessing anthracycline-treated childhood cancer survivors with advanced stress echocardiography. Pediatr Blood Cancer. 2015;62:502–508. doi: 10.1002/pbc.25328. [DOI] [PubMed] [Google Scholar]
- 27.Almeida J.G., Fontes-Carvalho R., Sampaio F., et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging. 2018;19:380–386. doi: 10.1093/ehjci/jex252. [DOI] [PubMed] [Google Scholar]
- 28.Kuznetsova T., Thijs L., Knez J., Herbots L., Zhang Z., Staessen J.A. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoodley P.W., Richards D.A., Boyd A., et al. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur Heart J Cardiovasc Imaging. 2013;14:228–234. doi: 10.1093/ehjci/jes139. [DOI] [PubMed] [Google Scholar]
- 30.Ganame J., Claus P., Eyskens B., et al. Acute cardiac functional and morphological changes after anthracycline infusions in children. Am J Cardiol. 2007;99:974–977. doi: 10.1016/j.amjcard.2006.10.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.