Abstract
Background
Sodium glucose cotransporter-2 inhibitors (SGLT2is) are hypothesized to reduce the risk of anthracycline-associated cardiotoxicity.
Objectives
This study sought to determine the association between SGLT2is and cardiovascular disease (CVD) after anthracycline-containing chemotherapy.
Methods
Using administrative data sets, we conducted a population-based cohort study of people >65 years of age with treated diabetes and no prior heart failure (HF) who received anthracyclines between January 1, 2016, and December 31, 2019. After estimating propensity scores for SGLT2i use, the average treatment effects for the treated weights were used to reduce baseline differences between SGLT2i-exposed and -unexposed controls. The outcomes were hospitalization for HF, incident HF diagnoses (in- or out-of-hospital), and documentation of any CVD in future hospitalizations. Death was treated as a competing risk. Cause-specific HRs for each outcome were determined for SGLT2i-treated people relative to unexposed controls.
Results
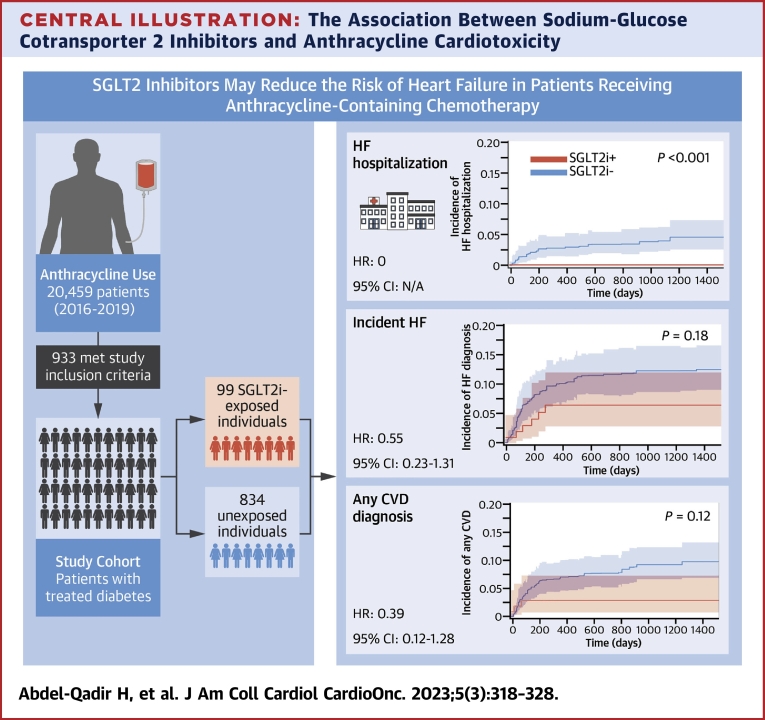
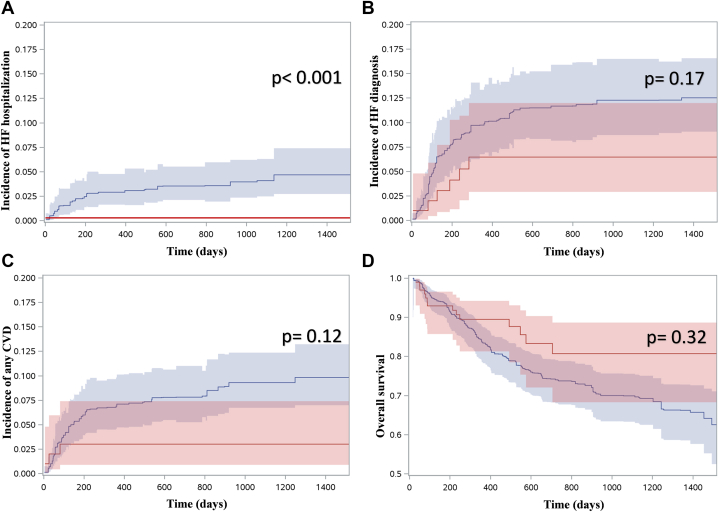
We studied 933 patients (median age 71.0 years, 62.2% female), 99 of whom were SGLT2i treated. During a median follow-up of 1.6 years, there were 31 hospitalizations for HF (0 in the SGLT2i group), 93 new HF diagnoses, and 74 hospitalizations with documented CVD. Relative to controls, SGLT2i exposure was associated with HR of 0 for HF hospitalization (P < 0.001) but no significant difference in incident HF diagnosis (HR: 0.55; 95% CI: 0.23-1.31; P = 0.18) or CVD diagnosis (HR: 0.39; 95% CI: 0.12-1.28; P = 0.12). There was no significant difference in mortality (HR: 0.63; 95% CI: 0.36-1.11; P = 0.11).
Conclusions
SGLT2is may reduce the rate of HF hospitalization after anthracycline-containing chemotherapy. This hypothesis warrants further testing in randomized controlled trials.
Key Words: anthracyclines, cardio-oncology, cardiotoxicity, heart failure, sodium-glucose transport protein 2
Central Illustration
Anthracyclines are important chemotherapeutic agents for several malignancies but increase the risk of heart failure (HF).1 Sodium-glucose cotransporter 2 inhibitors (SGLT2is) reduce cardiovascular disease (CVD) in adults with type 2 diabetes,2,3 reduce adverse outcomes in people with HF,4 and preserve renal function in people with chronic kidney disease.5 These observations, along with intriguing data from in vitro studies and mice models, have raised the hypothesis that SGLT2is can reduce the risk of anthracycline-associated cardiotoxicity.6,7 SGLT2is are generally well tolerated but are associated with rare but important complications. Cancer patients undergoing chemotherapy may be at increased risk for SGLT2i-associated ketoacidosis given the potential for gastrointestinal side effects, decreased oral intake, and fluid perturbations.8, 9, 10, 11, 12 Furthermore, SGLT2is have been postulated to have antitumor properties that may improve cancer-specific outcomes.13, 14, 15, 16, 17, 18
We conducted a population-based cohort study of people ≥65 years of age with treated diabetes and without prior HF who received anthracycline-based chemotherapy for cancer. Our objective was to study the association of SGLT2i exposure with the development of HF. We also studied the association of SGLT2i use with the risk of diabetes-specific adverse outcomes as well as overall mortality, which is expected to be driven by cancer-related deaths. We hypothesized that SGLT2i-treated individuals would have a lower rate of incident HF and mortality with a low rate of diabetes-related adverse outcomes.
Methods
Data sources
This was a population-based cohort study of patients from Ontario, Canada’s largest and most racially diverse province. Health care for long-term residents is provided by the provincial government through the Ontario Health Insurance Plan (OHIP). This allows us to leverage several administrative data sets for analysis. These data sets were linked using unique encoded identifiers and analyzed at ICES (formerly the Institute for Clinical Evaluative Sciences).19 The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Patients with cancer were identified using the Ontario Cancer Registry, which records data on all patients who are diagnosed with malignancy in the province (excluding nonmelanoma skin cancers).20 The Activity Level Reporting database records data on systemic therapy at regional cancer centers, and the New Drug Funding Program records exposure to higher-cost intravenous systemic therapies. These 2 data sets were used to identify patients who received anthracycline-containing chemotherapy. Medical diagnoses were determined using validated algorithms leveraging several administrative data sets.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 The Canadian Institute of Health Information Discharge Abstract Dataset stores data on hospitalized patients, whereas the National Ambulatory Care Reporting System records data on emergency department visits and hospital-based ambulatory care. The OHIP physician claims database records data on physician services. The Ontario Drug Benefit program covers prescription medications for residents ≥65 years of age,38 enabling the determination of exposure to prescription medications. Dates of birth and death were obtained from the Ontario Registered Persons Database. Pretreatment levels of glycated hemoglobin (HbA1c) and creatinine were obtained from the Ontario Laboratory Information System.39 Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.40
Cohort creation
Using these data sets, we identified all patients ≥65 years of age who were treated between January 1, 2016, and December 31, 2019, with anthracycline-containing chemotherapy that began within 1 year of being diagnosed with cancer. These dates were chosen because SGLT2is became available in Ontario in 2015, and we wanted to minimize follow-up after the onset of the coronavirus disease-2019 pandemic. The index date was that of anthracycline initiation. We excluded nonresidents of Ontario, people with inaccurate/missing key data (age, sex, or death dates), people eligible for OHIP coverage <1 year, and long-term care residents. We also excluded patients with prior inpatient or outpatient diagnoses of HF to allow us to focus on the role of SGLT2is on primary prevention. Furthermore, we excluded patients who did not have a pre-existing diagnosis of diabetes and those who were not receiving pharmacologic therapies for diabetes. This left us with a cohort of people ≥65 years of age without prior HF who were receiving medications for diabetes and who received anthracycline-based chemotherapy for cancer.
The primary exposure was treatment with an SGLT2i on the date of chemotherapy initiation. We identified all prescriptions for SGLT2is in the 365 days preceding the chemotherapy start date. Patients were classified as being exposed to SGLT2is if they dispensed 2 prescriptions for an SGLT2i (dapagliflozin, empagliflozin, or canagliflozin) where the second prescription was dispensed within 150% of the number of days supplied by the first prescription, and the period covered by 1 of the 2 prescriptions included the chemotherapy start date. Patients who were dispensed a prescription for an SGLT2i but did not fulfill these criteria were excluded from the analysis because we could not determine if they were taking an SGLT2i or not. Patients who were not dispensed an SGLT2i in the 365 days preceding the index date were considered nonexposed and included in the comparator group.
We studied 3 separate efficacy outcomes a priori: 1) hospitalization with a most responsible (primary) diagnosis of HF (International Classification of Diseases-10th Revision code I50); 2) a new diagnosis of HF in- or out-of-hospital (hospital admission for HF or 2 claims for HF in the National Ambulatory Care Reporting System and/or OHIP within 365 days of each other);21 and 3) hospitalizations that included CVD within any of the discharge diagnostic fields (International Classification of Diseases-10th Revision codes I00-I78) (Supplemental Table 1).36 We also conducted a post hoc analysis in which the outcome encompassed inpatient HF diagnoses in any of the diagnostic fields because new HF diagnoses made as inpatients may be important contributors to hospitalization for patients with cancer even if not listed as the primary diagnoses. Death from any cause was treated as a competing risk for these outcomes. We also studied death from any cause as a competing risk for the aforementioned outcomes. Moreover, we explored diabetes-related complications by identifying diagnoses of hypoglycemia, a composite of ketoacidosis and hyperglycemia as captured in hospitalizations within the Discharge Abstract Dataset, as well as hospitalizations with a most responsible diagnosis related to diabetes.
Statistical analysis
Baseline characteristics of participants were compared based on SGLT2i exposure at baseline. Continuous variables were summarized using the median (with 25th/75th percentiles [quartile 1 (Q1)-quartile (Q3)]), and the Wilcoxon rank sum test was used to determine the statistical significance of differences. Categoric variables were summarized with counts/percentages, and the statistical significance of differences was assessed using the chi-square test. Event rates are presented per 100 person-years with 95% CIs.
Missing values of HbA1c and eGFR were filled in as previously described41 using multiple imputation. The imputation model used the following variables: SGLT2i exposure status; age; sex; year of chemotherapy; cancer category (breast, lymphoma, or other); median neighborhood income quintile; rural residence; diabetes duration; hypertension; ischemic heart disease; atrial fibrillation; chronic obstructive pulmonary disease; Johns Hopkins ACG System Aggregated Diagnosis Groups risk score42; and the use of metformin, insulin, statins, angiotensin antagonists, and beta-blockers. We also used available values of HbA1c and eGFR for calculation of the alternate missing variable. Each imputation model also included the outcome used in that analysis (eg, incident HF in the analysis of the primary outcome). The number of imputed samples was set to twice the percentage of missing observations (18 complete samples were created).
In each of the 18 imputed (ie, complete) data sets, we determined propensity scores (PSs) to reduce differences in measured baseline covariates between SGLT2i-treated and unexposed individuals. Logistic regression was used to model the logit of receiving an SGLT2i conditional on the baseline characteristics used in the imputation model, with age modeled using restricted cubic splines. Given the minimal overlap in the distribution of the PS between SGLT2i-treated and unexposed individuals, we used the PS to calculate the average treatment effect for the treated (ATT) weights. These are defined as follows: w = Z + ([PS[1 − Z]/[1 − PS]), where Z = 1 for SGLT2i-treated and Z = 0 for unexposed individuals.
After the application of ATT weights, standardized differences were used to assess for residual differences in baseline characteristics in the weighted sample, with values <0.1 taken to be indicative of good balance.43 We then used the Aalen-Johansen estimate of the cumulative incidence function to determine the absolute incidence of CVD while treating death as a competing risk. The statistical significance of the difference between groups was estimated using a weighted univariable Fine-Gray regression model with a robust variance estimator. Weighted Kaplan-Meier survival curves were used to estimate differences in overall mortality while using the weighted log-rank test to determine the statistical significance of differences relative to SGLT2i exposure status. Univariable cause-specific hazard regression models were used to estimate the cause-specific HR of each outcome associated with SGLT2i exposure, treating death as a competing risk for nonmortality outcomes. A robust variance estimator was used to account for the within-person homogeneity induced by weighting.44 These analyses were conducted in each of the imputed samples. The mean values of baseline continuous variables, prevalence of baseline categoric variables, regression coefficients, and cumulative incidence function curves were pooled across imputed samples using Rubin’s rules.
All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc) in a Unix environment. The Johns Hopkins ACG System Version 10.0 was used to determine aggregated diagnosis groups risk scores. Cells with <6 individuals were suppressed to reduce reidentification risk as per ICES policies. Statistical significance was defined as a 2-tailed P < 0.05.
Results
Baseline characteristics
We identified 991 Ontarians 18 to 105 years of age who were treated with anthracyclines between 2016 and 2019, were receiving medications for diabetes, and had no prior history of HF (Figure 1). There were 157 patients who dispensed prescriptions for SGLT2is in the year before starting chemotherapy. We excluded 58 patients who dispensed an SGLT2i but whose most recent medication supply did not cover the chemotherapy start date, leaving 99 SGLT2i-treated patients to be included in the analysis along with 834 unexposed patients. Of these, 90 SGLT2i-treated individuals (90.9%) dispensed SGLT2is in the 180 days after the index date, whereas 16 controls (1.9%) dispensed SGLT2is during that period.
Figure 1.
Cohort Flow Diagram
This figure lists the number of participants who met inclusion criteria for the study and the numbers excluded at each step to generate the final study cohort. OHIP = Ontario Health Insurance Plan; SGLT2 = sodium-glucose transport protein 2.
The median age for the study cohort of 933 patients was 71.0 years (Q1-Q3: 68.0-76.0), and 580 (62.2%) were women. Baseline characteristics ae summarized in Table 1. The most used anthracyclines were doxorubicin (n = 723, 77.5%) followed by epirubicin (n = 131, 14.0%). Compared with unexposed patients, SGLT2i-treated patients were younger, with more recent cancer diagnoses, greater frequency of breast cancer, longer diabetes duration, higher HbA1c levels, higher eGFR, and more frequent use of statins. A detailed breakdown of malignancies is provided in Supplemental Table 2. HbA1c values were missing for 7 (7.1%) SGLT2i-treated individuals and 65 (7.8%) controls, whereas creatinine was missing for <6 SGLT2i-treated individuals and 16 (1.9%) controls. After the application of ATT weights derived from the PS, the weighted sample was well-balanced on measured baseline characteristics, with standardized differences <0.1 for each variable in each of the 18 imputed samples for each outcome studied (Table 2, Supplemental Tables 3 to 5).
Table 1.
Baseline Characteristics of Study Participants Stratified by SGLT2i Exposure Status
| SGLT2i Exposed (n = 99) | Unexposed to SGLT2i (n = 834) | P Value | |
|---|---|---|---|
| Age, y | 70 (67-73) | 71 (68-76) | <.001 |
| Male | 35 (35.4) | 318 (38.1) | 0.59 |
| Year of cohort entry | |||
| 2016 | 14 (14.1) | 235 (28.2) | <.001 |
| 2017 | 18 (18.2) | 219 (26.3) | |
| 2018 | 26 (26.3) | 200 (24.0) | |
| 2019 | 41 (41.4) | 180 (21.6) | |
| Rural residence | 13 (13.1) | 93 (11.2) | 0.8 |
| Median neighborhood income quintile | |||
| 1 | 19 (19.2) | 163 (19.5) | 0.85 |
| 2 | 22 (22.2) | 203 (24.3) | |
| 3 | 19 (19.2) | 168 (20.1) | |
| 4 | 22 (22.2) | 140 (16.8) | |
| 5 | 17 (17.2) | 159 (19.1) | |
| Cancer site of origin | |||
| Breast | 49 (49.5) | 273 (32.7) | 0.02 |
| Lymph nodes | 24 (24.2) | 239 (28.7) | |
| Other | 26 (26.3) | 322 (38.6) | |
| Diabetes duration | 15.9 (10.7-20.2) | 12.6 (7.9-17.7) | <.001 |
| Johns Hopkins ADG score | 14.0 (11.0-17.0) | 14.0 (12.0-17.0) | 0.32 |
| Glycated hemoglobin | 7.2 (6.9-7.7) | 6.8 (6.2-7.5) | <.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 80 (65-89) | 73 (58-87) | 0.03 |
| Hypertension | <6 | 80 (9.6) | 0.07 |
| Ischemic heart disease | 12 (12.1) | 80 (9.6) | 0.42 |
| Atrial fibrillation | 6 (6.1) | 71 (8.5) | 0.4 |
| Chronic obstructive pulmonary disease | 7 (7.1) | 45 (5.4) | 0.49 |
| Medication use in past 6 months | |||
| Insulin | 26 (26.3) | 175 (21.0) | 0.23 |
| Metformin | 90 (90.9) | 710 (85.1) | 0.12 |
| Angiotensin antagonist | 78 (78.8) | 599 (71.8) | 0.14 |
| Beta-blockers | 27 (27.3) | 215 (25.8) | 0.75 |
| Statin | 88 (88.9) | 625 (74.9) | 0.002 |
| Sulfonylureas | 39 (39.4) | 207 (24.8) | <.001 |
| Dipeptidyl peptidase-4 inhibitors | 68 (68.7) | 318 (38.1) | <.001 |
Values are median (quartile 1-quartile 3) or n (%).
ADG = aggregated diagnosis group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Table 2.
Baseline Characteristics of Weighted Samples Used for the Outcome of Hospitalization for Heart Failure
| Crude Data |
Weighted Data |
|||||
|---|---|---|---|---|---|---|
| SGLT2i Exposed | SGLT2i Unexposed | SD | SGLT2i Exposed | SGLT2i Unexposed | SD | |
| Age | 72.5 | 70.3 | 0.44 | 70.3 | 70.3 | <0.01 |
| Diabetes duration, y | 13.1 | 15.7 | 0.38 | 15.6 | 15.7 | <0.01 |
| Total ADG score | 14.2 | 13.8 | 0.11 | 13.6 | 13.8 | 0.05 |
| Glycated hemoglobin | 7.0 | 7.6 | 0.43 | 7.5 | 7.6 | 0.03 |
| Estimated GFR | 71.1 | 76.0 | 0.28 | 75.9 | 76.0 | 0.01 |
| Treated in 2016, % | 28.2 | 14.1 | 0.35 | 15.1 | 14.1 | 0.03 |
| Treated in 2017, % | 26.3 | 18.2 | 0.20 | 18.8 | 18.2 | 0.02 |
| Treated in 2018, % | 24.0 | 26.3 | 0.05 | 26.0 | 26.3 | 0.01 |
| Treated in 2019, % | 21.6 | 41.4 | 0.44 | 40.1 | 41.4 | 0.03 |
| Female, % | 61.9 | 64.7 | 0.06 | 65.6 | 64.7 | 0.02 |
| Male, % | 38.1 | 35.4 | 0.06 | 34.4 | 35.4 | 0.02 |
| Breast cancer, % | 32.7 | 49.5 | 0.35 | 50.0 | 49.5 | 0.01 |
| Lymphoma, % | 28.7 | 24.2 | 0.10 | 25.0 | 24.2 | 0.02 |
| Other malignancy, % | 37.9 | 26.3 | 0.25 | 24.6 | 26.3 | 0.04 |
| Insulin use, % | 21.0 | 26.3 | 0.13 | 27.3 | 26.3 | 0.02 |
| Income quintile 1, % | 19.5 | 19.2 | 0.01 | 17.1 | 19.2 | 0.06 |
| Income quintile 2, % | 24.3 | 22.2 | 0.05 | 21.9 | 22.2 | 0.01 |
| Income quintile 3, % | 20.1 | 19.2 | 0.02 | 20.9 | 19.2 | 0.04 |
| Income quintile 4, % | 16.8 | 22.2 | 0.14 | 21.7 | 22.2 | 0.01 |
| Income quintile 5, % | 19.1 | 17.2 | 0.05 | 18.5 | 17.2 | 0.03 |
| Rural residence, % | 11.2 | 13.1 | 0.06 | 12.8 | 13.1 | 0.01 |
| Urban residence, % | 88.7 | 86.9 | 0.06 | 87.1 | 86.9 | 0.01 |
| Metformin, % | 85.1 | 90.9 | 0.18 | 90.0 | 90.9 | 0.03 |
| Statin, % | 74.9 | 88.9 | 0.37 | 87.8 | 88.9 | 0.03 |
| Angiotensin antagonist, % | 71.8 | 78.8 | 0.16 | 80.4 | 78.8 | 0.04 |
| Hypertension, % | 9.6 | <6 | — | 3.5 | <6 | — |
| Ischemic heart disease, % | 9.6 | 12.1 | 0.08 | 12.6 | 12.1 | 0.01 |
| Atrial fibrillation, % | 8.5 | 6.1 | 0.09 | 6.6 | 6.1 | 0.02 |
| COPD, % | 5.4 | 7.1 | 0.07 | 6.5 | 7.1 | 0.02 |
| Beta-blockers, % | 25.8 | 27.3 | 0.03 | 26.7 | 27.3 | 0.01 |
The table presents pooled mean estimates for continuous variables and pooled prevalence for categoric variables. Columns 2 to 4 present unweighted samples and the SD, whereas columns 5 to 7 present weighted samples and SD. The weighted samples were used for association analysis.
ADG = aggregated diagnosis group; COPD = chronic obstructive pulmonary disease; GFR = glomerular filtration rate.
Outcomes
There were 269 deaths (28.8%) in the study cohort during a median available follow-up of 1.6 years (Q1-Q3: 0.8-2.9 years). There were 168 deaths before 2018 for which the cause of death could be assigned, among which 137 (81.5%) were attributed to malignancy and 8 (4.8%) to CVD. There were 31 (3.3%) hospitalizations with a most responsible diagnosis of HF (0 in the SGLT2i group), 93 (10%) new HF diagnoses (6 in the SGLT2i group), and 74 (7.9%) hospitalizations with CVD coded as 1 of the diagnoses (<6 in the SGLT2i group). When inpatient HF diagnoses were expanded to include all diagnostic categories, we identified a total of 57 (6.1%) events (<6 in the SGLT2i group). The rates of HF hospitalization were 0 per 100 person-years in SGLT2i-treated people and 2.1 (1.4-2.9) per 100 person-years in controls. For any HF diagnosis, rates were 3.9 (1.8-8.8) per 100 person-years in SGLT2i-treated people and 6.1 (4.9-7.5) per 100 person-years in controls. The rate of in-hospital HF diagnosis in any diagnostic field was 1.3 (0.3-5.2) per 100 person-years in SGLT2i-treated individuals and 3.7 (2.8-4.8) per 100 person-years in controls, and the rate of diagnosis of any CVD was 1.9 (0.6-6.0) per 100 person-years in the SGLT2i-treated group and 4.9 (3.8-6.1) per 100 person-years in controls. The mortality rate was 8.9 (5.3-15.1) per 100 person-years in SGLT2i-treated people and 16.6 (14.7-18.8) per 100 person-years in controls.
After accounting for baseline differences in the weighted sample, there was a numerically lower risk estimate for all adverse CVD outcomes in the SGLT2i-treated group, as illustrated in the pooled cumulative incidence function curves in Figure 2. Because there were no hospitalizations with HF as the most responsible diagnosis in the SGLT2i group, the HR was 0 (P < 0.001). When considering incident diagnoses of HF (inpatient or outpatient), there was no statistically significant difference in the hazard of new HF diagnosis, with a cause-specific HR of 0.55 (95% CI: 0.23-1.31; P = 0.18) derived from the univariable analysis of the PS-weighted sample. When considering all diagnostic fields for people hospitalized after anthracycline initiation, there was no statistically significant difference in the hazard of HF (HR: 0.32; 95% CI: 0.08-1.38; P = 0.13) or any CVD (HR: 0.39; 95% CI: 0.12-1.28; P = 0.12). There was no statistically significant difference in the hazard of CVD within any of the diagnostic fields in hospitalizations after anthracycline initiation (HR: 0.29; 95% CI: 0.07-1.24; P = 0.09). There was also no statistically significant difference in the risk of death from any cause (HR: 0.63; 95% CI: 0.36-1.11; P = 0.11).
Figure 2.
Risk of Adverse Outcomes in the Weighted Sample
(A to C) Pooled cumulative incidence function curves for the outcomes of interest from the 18 imputed weighted samples, with pooled P values derived from weighted univariable Fine-Gray regression models. (A) Hospitalizations with a most responsible diagnosis of heart failure (HF) (0 events in sodium glucose cotransporter-2 inhibitors [SGLT2i]-exposed group). (B) New diagnoses of HF. (C) Hospitalizations with cardiovascular disease (CVD) among any diagnostic field. (D) Pooled Kaplan-Meier estimates for overall survival from the 18 imputed weighted samples, with P values from the weighted log-rank test. In all figures, the x-axis represents time since chemotherapy initiation (in days). The curves are depicted in red for SGLT2i-exposed individuals and blue for unexposed individuals.
There were no documented episodes of diabetic ketoacidosis, hyperosmolar hyperglycemic state, or hyperglycemia in subsequent hospitalizations in the SGLT2i group compared with 15 (1.8%) in the nonexposed patients (HR: 0). There were <6 documented hypoglycemia events compared with 22 (2.6%) in controls and <6 hospitalizations with a most responsible diagnosis related to diabetes in SGLT2i-treated patients compared with 14 (1.7%). Given the low number of events, further analyses for diabetes outcomes were not pursued.
Discussion
We conducted a population-based cohort study to determine the association of SGLT2i use with HF and other adverse outcomes after anthracycline use in patients with cancer and pre-existing diabetes but no prior HF. We present event rates for important efficacy and safety outcomes that can be useful for planning of future randomized controlled trials (RCTs). PS methods were used to reduce differences in measured baseline covariates. There were no hospitalizations with a most responsible diagnosis of HF or another CVD in the SGLT2i group, translating to an HR of 0 (P < 0.001). SGLT2i use was not associated with a statistically significant reduction in the risk of inpatient or outpatient diagnoses of incident HF (HR: 0.55; 95% CI: 0.23-1.31 in the weighted sample). The study is summarized in the Central Illustration.
Central Illustration.
The Association Between Sodium-Glucose Cotransporter 2 Inhibitors and Anthracycline Cardiotoxicity
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) were associated with reduced rate of hospitalization for heart failure (HF) in patients receiving anthracycline-containing chemotherapy, but there were no significant differences in the rates of other adverse outcomes studied. SGLT2i-treated and unexposed individuals were compared using 18 imputed (ie, complete) data sets and propensity scores to reduce differences in baseline covariates. Univariable cause-specific hazard regression models were employed to estimate the cause-specific HR of each outcome associated with SGLT2i exposure, treating death as a competing risk. Cumulative incidence function determined the absolute incidence of cardiovascular disease (CVD), treating death as a competing risk. SGLT2 = sodium-glucose transport protein 2.
There are limited data on the association of SGLT2is with outcomes in patients treated for cancer. A recent case-control study by Gongora et al45 matched 32 SGLT2i-treated people to 96 controls on age, sex, and anthracycline start date, all of whom were treated with anthracyclines and had pre-existing diabetes. The primary outcome was a composite of newly incident HF, HF admissions, clinically significant arrhythmias, or a >10% absolute decline in left ventricular ejection fraction to a final value <53%. This primary outcome occurred in only 22 people, precluding statistical adjustment for differences in characteristics of SGLT2i-treated and unexposed patients. The incidence was lower in SGLT2i-treated patients (3% vs 22% in controls; P = 0.015), as was unadjusted mortality (9% vs 43%; P < 0.001). Our analysis extends these findings by accounting for an extensive list of baseline covariates. We also studied a higher-risk group of patients and focused on HF as the most plausible outcome to be affected by SGLT2is. Furthermore, we explored the risk of diabetes-related complications, which is germane for older patients undergoing chemotherapy for cancer. Our findings are also concordant with the results of the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) trial in patients without cancer but with HF with preserved ejection fraction.46 In that trial, the use of the SGLT2i empagliflozin was primarily associated with a significant reduction in HF hospitalization with an HR of 0.73 (95% CI: 0.61-0.88; P < 0.001).
The mechanism by which SGLT2i can reduce HF in patients receiving anthracycline therapy is unclear. Quagliarliello et al7 exposed HL-1 adult cardiomyocytes to subclinical concentrations of doxorubicin and trastuzumab and observed that dapagliflozin coadministration increased myocyte viability as measured by mitochondrial dehydrogenase activity, lipid peroxidation, and intracellular calcium homeostasis. Dapagliflozin coadministration also reduced cardiomyocyte expression of inflammatory markers associated with cardiotoxicity development and downregulated the expression of signaling pathways associated with cardiomyocyte apoptosis. Oh et al6 used a C57BL/6J mouse model injected with intraperitoneal doxorubicin to investigate the impact of a diet supplemented with 0.03% empagliflozin on cardiotoxicity. Using cardiac magnetic resonance, they showed that empagliflozin-fed mice had less hypertrophy, less perivascular and interstitial fibrosis, and improved fractional shortening relative to mice fed a control diet. They observed that Sglt2 gene expression was very low in mice hearts, suggesting that direct sodium-glucose transport protein 2 inhibition at the cardiac myocyte level was unlikely to be the underlying mechanism. However, SGLT2i use increased circulating levels of beta-hydroxybutyrate. Coincubation of cardiac myocytes with beta-hydroxybutyrate led to decreased doxorubicin cardiotoxicity, possibly mediated by reducing reactive oxygen species production and improving mitochondrial function.
We also observed a 37% reduction in the hazard of death in the SGT2i-treated patients that was not statistically significant. This mirrors the observations in the age- and sex-matched analysis by Gongora et al.45 There are several in vitro studies14, 15, 16 suggesting that SGLT2i inhibition can reduce tumor growth and induce malignant cell death, potentially providing a synergistic approach to other cancer therapies. Some of the proposed mechanisms13, 14, 15, 16, 17, 18 include decreased glucose availability to metabolically active cancer cells through counteracting 1 of the adaptive approaches of cancer cells to support proliferation by upregulating sodium glucose cotransporter expression as a mechanism to increase glucose influx.18
We must be careful in the interpretation of the findings from our observational study and not overstate our conclusions. The lower risk of adverse outcomes in the SGLT2i group may be explained by the healthy user effect47, 48, 49 wherein healthier people are more likely to start medications than those with worse health. This was demonstrated in the cardio-oncology setting for statin use in which observational data in breast cancer suggested a protective effect against HF that was not borne out in RCTs.41,50, 51, 52 However, SGLT2i-exposed people in our study had longer diabetes duration and higher HbA1c than controls. There may be other unmeasured confounders related to SGLT2i access; we have recently demonstrated that lower socioeconomic status is associated with disparities in access to some services in Ontario despite the absence of substantial financial barriers to health care access.53 It is also plausible that the diuretic effect of SGLT2i may reduce the risk of HF secondary to steroid use or fluid challenges that can accompany the use of anthracyclines. Thus, our data should be interpreted as raising support for RCTs to evaluate the potential cardioprotective effect of SGLT2i, with utilization of the observed event rates and HRs in their planning. One such trial has been registered—the EMPACT (Empagliflozin in the Prevention of Cardiotoxicity in Cancer Patients Undergoing Chemotherapy Based on Anthracyclines; (NCT05271162).
Study limitations
Our administrative data sets do not provide data on clinical variables such as blood pressure, obesity, or smoking. We did not have access to data on ejection fraction, so we could not distinguish HF with reduced vs preserved ejection fraction. We also could not determine which participants have type 1 diabetes, but this is expected to affect <10% of patients.54 We did not have sufficient cause-specific mortality data to explore potential mechanisms of mortality reduction. The small sample size and need to impute missing creatinine/HbA1c values led to wide CIs despite a large effect size. There were no hospitalizations for HF in the SGLT2i group, precluding the ability to estimate CIs for the effect size of a potential protective effect on this outcome.
Conclusions
This population-based cohort study supports the hypothesis that SGLT2is may reduce the risk of HF in patients receiving anthracycline-containing chemotherapy. We did not observe important safety concerns with concurrent exposure to SGLT2is and chemotherapy. Rather, we observed a lower rate of death associated with SGLT2i use that was not statistically significant. These data lend further credence to the need for RCTs of SGLT2i in cancer patients.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: People with diabetes using SGLT2is before starting anthracycline-associated chemotherapy may have a lower risk of HF without an increase in cardiovascular- or diabetes-related adverse events.
TRANSLATIONAL OUTLOOK: The findings from this observational study warrant consideration of RCTs to formally test the hypothesis that SGLT2i use in patients undergoing anthracycline-containing chemotherapy may reduce the risk of subsequent HF.
Funding Support and Author Disclosures
Analysis of this study was funded by the Ted Rogers Centre for Heart Research (to Dr Thavendiranathan). Dr Abdel-Qadir is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. Dr Thavendiranathan is supported by a Tier II Canada Research Chair in CardioOncology. The funding sources had no role in the conduct of the study, the decision to publish or the preparation of the manuscript. Dr Abdel-Qadir has received honoraria from Amgen, AstraZeneca, and Jazz Pharmaceuticals. Dr Thavendiranathan has received speaker honoraria from Amgen, Boehringer Ingelheim, and Takeda. This study was supported by ICES, an independent, nonprofit research institute funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and/or information compiled and provided by Ontario Health, the Ontario Ministry of Health, and the Canadian Institute of Health Information (CIHI). Parts of this report are based on Ontario Registrar General information on deaths, the original source of which is Service Ontario. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources (ICES, CIHI, Ontario Health, ORG, or the Ontario MOH/ MLTC); no endorsement is intended or should be inferred. Dr Neilan has been a consultant to and received fees from Bristol Myers Squibb, Genentech, CRC Oncology, Roche, Sanofi and Parexel Imaging Pharmaceuticals, outside of the current work. Dr Neilan also reports grant funding from Astra Zeneca and Bristol Myers Squibb outside of the current work. Dr Neilan is supported by a gift from A. Curt Greer and Pamela Kohlberg and from Christina and Paul Kazilionis, the Michael and Kathryn Park Endowed Chair in Cardiology, a Hassenfeld Scholar Award, and has additional grant funding from the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL137562, K24HL150238). All other authors have reported that they have no relationships to the contents of this paper to disclose.
Acknowledgments
The authors thank IQVIA Solutions Canada Inc for use of their Drug Information File.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Contributor Information
Husam Abdel-Qadir, Email: h.abdel.qadir@utoronto.ca.
Paaladinesh Thavendiranathan, Email: dinesh.thavendiranathan@uhn.ca.
Appendix
References
- 1.Thavendiranathan P., Abdel-Qadir H., Fischer H.D., et al. Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;34(19):2239–2246. doi: 10.1200/JCO.2015.65.1505. [DOI] [PubMed] [Google Scholar]
- 2.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 3.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink H.J., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 6.Oh C.-M., Cho S., Jang J.-Y., et al. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J. 2019;49(12):1183–1195. doi: 10.4070/kcj.2019.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quagliariello V., De Laurentiis M., Rea D., et al. SGLT2 inhibitor dapagliflozin against anthracycline and trastuzumab-induced cardiotoxicity: the role of MYD88, NLRP3, leukotrienes/interleukin 6 axis and mTORC1/Fox01/3a mediated apoptosis. Eur Heart J. 2020;41(suppl 2) ehaa946.3253. [Google Scholar]
- 8.Thiruvenkatarajan V., Meyer E.J., Nanjappa N., Van Wijk R.M., Jesudason D. Perioperative diabetic ketoacidosis associated with sodium-glucose co-transporter-2 inhibitors: a systematic review. Br J Anaesth. 2019;123(1):27–36. doi: 10.1016/j.bja.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Peters A.L., Henry R.R., Thakkar P., Tong C., Alba M. Diabetic ketoacidosis with canagliflozin, a sodium–glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39(4):532–538. doi: 10.2337/dc15-1995. [DOI] [PubMed] [Google Scholar]
- 10.Peters A.L., Buschur E.O., Buse J.B., et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douros A., Lix L.M., Fralick M., et al. Sodium–glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis: a multicenter cohort study. Ann Intern Med. 2020;173(6):417–425. doi: 10.7326/M20-0289. [DOI] [PubMed] [Google Scholar]
- 12.Bonora B.M., Avogaro A., Fadini G.P. Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: an updated review of the literature. Diabetes Obes Metab. 2018;20(1):25–33. doi: 10.1111/dom.13012. [DOI] [PubMed] [Google Scholar]
- 13.Koepsell H. The Na+-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol Ther. 2017;170:148–165. doi: 10.1016/j.pharmthera.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J., Zhu J., Yu S.-J., et al. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110821. [DOI] [PubMed] [Google Scholar]
- 15.Kaji K., Nishimura N., Seki K., et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142(8):1712–1722. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 16.Kuang H., Liao L., Chen H., et al. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit. 2017;23:3737. doi: 10.12659/MSM.902530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau K.T., Ng L., Wong J.W., et al. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment–a review. Rev Endocr Metab Disord. 2021;22(4):1121–1136. doi: 10.1007/s11154-021-09675-9. [DOI] [PubMed] [Google Scholar]
- 18.Scafoglio C., Hirayama B.A., Kepe V., et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112(30):E4111–E4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schull M.J., Azimaee M., Marra M., et al. ICES: data, discovery, better health. Int J Popul Data Sci. 2019;4(2):1135. doi: 10.23889/ijpds.v4i2.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner D.R., Tammemagi M.C., Bull S.B., Pinnaduwaje D., Andrulis I.L. Using cancer registry data: agreement in cause-of-death data between the Ontario Cancer Registry and a longitudinal study of breast cancer patients. Chronic Dis Can. 2009;30(1):16–19. [PubMed] [Google Scholar]
- 21.Schultz S.E., Rothwell D.M., Chen Z., Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160–166. [PubMed] [Google Scholar]
- 22.Lee D.S., Donovan L., Austin P.C., et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen M.J., Tu J.V., Schull M.J. ICD-10 adaptations of the Ontario acute myocardial infarction mortality prediction rules performed as well as the original versions. J Clin Epidemiol. 2007;60(9):971–974. doi: 10.1016/j.jclinepi.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Tu K., Mitiku T., Lee D.S., Guo H., Tu J.V. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD) Can J Cardiol. 2010;26(7):e225–e228. doi: 10.1016/s0828-282x(10)70412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hux J.E., Ivis F., Flintoft V., Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 26.Lipscombe L.L., Hwee J., Webster L., et al. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res. 2018;18(1):316. doi: 10.1186/s12913-018-3148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu K., Wang M., Young J., et al. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol. 2013;29(11):1388–1394. doi: 10.1016/j.cjca.2013.07.676. [DOI] [PubMed] [Google Scholar]
- 28.Tu K., Nieuwlaat R., Cheng S.Y., et al. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;32(12):1561–1565. doi: 10.1016/j.cjca.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Tu K., Campbell N.R., Chen Z.L., Cauch-Dudek K.J., McAlister F.A. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 30.Gershon A.S., Warner L., Cascagnette P., Victor J.C., To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378(9795):991–996. doi: 10.1016/S0140-6736(11)60990-2. [DOI] [PubMed] [Google Scholar]
- 31.Fleet J.L., Dixon S.N., Shariff S.Z., et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. doi: 10.1186/1471-2369-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaakkimainen R.L., Bronskill S.E., Tierney M.C., et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians' electronic medical records. J Alzheimers Dis. 2016;54(1):337–349. doi: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 33.Robles S.C., Marrett L.D., Clarke E.A., Risch H.A. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495–501. doi: 10.1016/0895-4356(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert T., Neuburger J., Kraindler J., et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapointe-Shaw L., Georgie F., Carlone D., et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0201120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu J.V., Chu A., Donovan L.R., et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8(2):204–212. doi: 10.1161/CIRCOUTCOMES.114.001416. [DOI] [PubMed] [Google Scholar]
- 37.Gomes T., Mamdani M.M., Holbrook A.M., et al. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ. 2013;185:E121–E127. doi: 10.1503/cmaj.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy A.R., O’Brien B.J., Sellors C., Grootendorst P., Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- 39.Campitelli M.A., Kumar M., Greenberg A. Integrating population-wide laboratory testing data with audit and feedback reports for Ontario physicians. Healthc Q. 2018;21(2):6–9. doi: 10.12927/hcq.2018.25630. [DOI] [PubMed] [Google Scholar]
- 40.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel-Qadir H., Bobrowski D., Zhou L., et al. Statin exposure and risk of heart failure after anthracycline- or trastuzumab-based chemotherapy for early breast cancer: a propensity score–matched cohort study. J Am Heart Assoc. 2021;10(2) doi: 10.1161/JAHA.119.018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austin P.C., Walraven C. The mortality risk score and the ADG score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):940–947. doi: 10.1097/MLR.0b013e318229360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austin P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gongora C.A., Drobni Z.D., Silva T.Q.A.C., et al. Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. J Am Coll Cardiol HF. 2022;10(8):559–567. doi: 10.1016/j.jchf.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 47.Brookhart M.A., Patrick A.R., Dormuth C., et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 48.LaFleur J., Nelson R.E., Sauer B.C., Nebeker J.R. Overestimation of the effects of adherence on outcomes: a case study in healthy user bias and hypertension. Heart. 2011;97(22):1862–1869. doi: 10.1136/hrt.2011.223289. [DOI] [PubMed] [Google Scholar]
- 49.Shrank W.H., Patrick A.R., Alan Brookhart M. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546–550. doi: 10.1007/s11606-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hundley W.G., D’Agostino R., Crotts T., et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2022;1(9) doi: 10.1056/evidoa2200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seicean S., Seicean A., Plana J.C., Budd G.T., Marwick T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60(23):2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 52.Acar Z., Kale A., Turgut M., et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2011;58(9):988–989. doi: 10.1016/j.jacc.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 53.Abdel-Qadir H., Akioyamen L.E., Fang J., et al. Association of neighborhood-level material deprivation with atrial fibrillation care in a single-payer health care system: a population-based cohort study. Circulation. 2022;146(3):159–171. doi: 10.1161/CIRCULATIONAHA.122.058949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diabetes Canada Diabetes in Ontario: backgrounder. https://www.diabetes.ca/DiabetesCanadaWebsite/media/Advocacy-and-Policy/Backgrounder/2022_Backgrounder_Ontario_English.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.