Abstract
Parkinson's disease (PD) is the most common neurodegenerative movement disorder in the elderly. As the pathogenesis of PD is still not fully understood, medications with the capacity of halting the disease progression are currently unavailable. The discovery of genes that are causative for, or increase susceptibility to PD is pivotal for the development of novel therapeutic approaches, as they are critical for the onset of PD and the molecular pathways underlying its pathogenesis. By reviewing relevant data, we discuss causative genes, and those associated with PD susceptibility and quantitative traits. Through Gene Ontology database and STRING analysis, we emphasize the roles of inorganic cation transmembrane transport pathways and hypothalamic pituitary thyroid axis, in addition to the established roles of inflammation/oxidative stress and mitochondrial dysfunction in the pathogenesis of PD. It is hoped these insights 1) untangle the clinical complex presentations of PD, 2) reveal the interwoven molecular network leading to PD, and 3) identify critical molecular targets to facilitate novel PD drug discovery, with a view to providing improved consultation and personalized medicine for patients with PD in the future.
Keywords: Drug discovery, Genetics, Molecular function, Parkinson's disease, Quantitative traits
Introduction
Parkinson's disease (PD) is the second most common age-related neurodegenerative disease worldwide, affecting over ten million people in the world. The prevalence of PD increases with age, affecting 0.1%–0.2% of the population across all ages, 1% of those over 60 years, and the disease costs over 51.9 billion dollars annually.1,2 An increased understanding of this disease is critically important, particularly in those countries with an ageing population. Many recent studies have identified genetic variants associated with PD. This review will focus on the common PD risk genetic loci and single nucleotide polymorphisms (SNPs) identified in European Caucasian and Asian populations and their impacts on the precision medicine of PD.
Although the pathogenesis of PD is not yet fully understood, striatal dopamine deficiency due to the degeneration of dopaminergic neurons of the substantia nigra pars compacta has been recognized as a PD hallmark.3,4 The substantia nigra appears depigmented macroscopically due to the death of neuromelanin containing dopaminergic neurons, and there are two distinct microscopic features for the pathological diagnosis of PD: intracellular α-synuclein aggregations and dopaminergic cell degeneration.5,6 α-Synuclein can exist as a small soluble monomer which can form oligomers or larger protein aggregates, that are components of Lewy bodies and Lewy neurites in dopaminergic neurons.6,7 Due to the dopaminergic neuronal dysfunction and cell death, there is insufficient dopamine in the striatum, which affects the initiation of movement,4,5 that in turn accounts for the movement symptoms displayed by patients. Therefore, replacing striatal dopamine through medications such as l-dopa is an effective symptomatic treatment,3,5 but all existing symptomatic therapies for PD (including l-dopa) do not target the underlying molecular mechanisms of the disease, and have little to no impact on disease progression.
The clinical presentations of PD are asymmetrical and develop progressively.5 Although the common motor symptoms are bradykinesia, resting tremor, muscle rigidity and postural instability, there are a variety of non-motor presentations including neuropsychiatric symptoms (e.g., depression, anxiety, sleep disorders), autonomic dysfunction (e.g., gastrointestinal symptoms of constipation), as well as sensory symptoms (e.g., olfactory dysfunction), which may present earlier than motor symptoms.8 Other symptoms such as pain, fatigue, weight changes, and dementia can also occur, usually in late stages of PD.7,9 Age is the major risk factor for PD, but risk is also attributed to environmental factors that include dairy products, pesticides, methamphetamine, and brain trauma.10 The genetic component of PD has received more interest following GWAS studies that implicate familial PD genes as risk loci for sporadic PD.11 This builds the foundation of identifying the SNPs and associated genes to advance our understanding of the molecular mechanisms that confer increased risk of PD.
PD causative genes
The first PD causative mutation was discovered in the SNCA gene in Italian and Greek kindreds in 1997.12 Subsequently, many other PD causative genes were identified from linkage analysis by segregating genes from monogenetic PD-affected families (Table 1). PD causative genes follow either autosomal dominant or recessive inherited patterns, mainly reflecting gain or loss of its correspondent molecular functions respectively.
Table 1.
PD causative genes.
| PARK | Gene | Loci | Protein cellular distribution and function | Mutations | Inheritance | Clinical Phenotypes |
|---|---|---|---|---|---|---|
| PARK1 | SNCA | 4q21-23 | α-synuclein17: presynaptic signaling and membrane trafficking.93 | A53T, A30P,17,94 A18T, A29S, G46L, H50G, G51A95 | AD | Young-onset and late-onset hereditary Lewy body PD96 |
| PARK2 | PRKN | 6q25.2-q27 | Parkin RBR E3 ubiquitin protein ligase: regulate the autophagic degradation of mitochondria.54 | Deletions of exons 1, 4 and 5, and P113Xfs, R275W, G430D and R33X97 | AR | Young-onset PD (mean onset of PD at 32 y.o.)98 |
| PARK3(putative) | Unknown | 2p13 | – | Not identified | AD | Late onset idiopathic Lewy body PD.99 |
| PARK4 | SNCA | 4p22.1 | α-synuclein17: presynaptic signaling and membrane trafficking.93 | duplication and triplication.18,19 | AD | Young-onset and late-onset hereditary Lewy body PD96 |
| PARK5(putative) | UCHL1 | 4p13 | Ubiquitin C-Terminal Hydrolase L1: processing ubiquitinated proteins and ubiquitin precursors.100 | Unconfirmed | AD | Late onset PD(mean onset of PD at 50 y.o.)100 |
| PARK6 | PINK1 | 1p36.12 | PTEN induced kinase 1: mitochondria degradation44 | Deletions in exon 1, 5, and 751,97, g.16378G > A, c.1488 + 1G > A51 and W90Xfs97 | AR | Young-onset (mean age at 31.6 y.o.), slowly progressive levodopa-responsive PD.51 |
| PARK7 | DJ-1 | 1p36.23 | Parkinsonism associated deglycase: cell protection from toxic stresses.101 | M26I, G78G, R98Q, R98R, D149A, A167A and InsA+120.102 | AR | Young-onset PD.102 |
| PARK8 | LRRK2 | 12q12 | Dardarin: GTPase and kinase.103 | G2019S,104 R1441G, R1441 C/H, Y1699C, R1628P, G2385R and I2020T.105 | AD | Mean onset of PD at 58·1 y.o, hereditary (mutations present in 10% patients) and idiopathic (mutations present in 4% of patients) Lewy body PD.7,33 |
| PARK9 (parkinsonism causative) | ATP13A2 | 1p36.13 | Lysosomal type 5 ATPase: maintain intracellular cation homeostasis and neuronal integrity106 | Deletion in exon 26, 22-bp duplication (1632_1653dup22), 2-bp insertion (1103insGA),107 1306+5G-A,108 G504R,109 M810R,110 G877R.111 |
AR | Kufor-Rakeb syndrome: early onset idiopathic Parkinsonism associated with mask-like face, rigidity and bradykinesia, spasticity, supranuclear gaze palsy and dementia.112 |
| PARK10(putative) | Unknown | 1p32 | – | Unconfirmed | AD | Late onset PD (mean onset of PD at 65.8 y.o.)113 |
| PARK11(putative) | GIGYF2(confronted) | 2q37.1 | GRB10-interacting GYF protein 2: repressing translation initiation114 | Deletion L1230_Q1237del, N478T, H1992R115 | AD | Late onset idiopathic PD116 |
| PARK12(putative) | Unknown | Xq21-q25 | – | Unconfirmed | X-linked | Late onset PD117 |
| PARK13(putative) | HTRA2(confronted) | 2p13.1 | HtrA Serine Peptidase 2: proteolytic activity and promotes apoptosis118 | G399S and A141S119 | AD | Late onset PD (mean onset of PD at 57.3 y.o.) in German population |
| PARK14 (parkinsonism causative) | PLA2G6 | 22q13.1 | Phospholipase A2 Group VI: phospholipid remodeling for cellular membrane homeostasis120 | R741Q and R747W121 | AR | Parkinsonism, dystonia and cognitive decline121 |
| PARK15 (parkinsonism causative) | FBXO7 | 22q12.3 | F-box only protein 7: mediating the ubiquitination and proteasomal degradation122 | A498Stop, T22M, and splice-site IVS7 + 1G/T123 | AR | Early onset levodopa-responsive Parkinsonian-pyramidal syndrome124 |
| PARK16(putative) | Unknown | 1q32 | – | unconfirmed | unconfirmed | unconfirmed |
| PARK17 | VPS35 | 16q11.2 | VPS35 retromer complex component: vesicle transport and membrane-protein recycling.125,126 | A620A127 and P316S126 | AD | Late onset, hereditary PD (mean onset of PD at 53 y.o.).127 |
| PARK18 | EIF4G1 | 3q26-q28 | Eukaryotic translation initiation factor 4 gamma 1: involved in mRNA translation.128 | A1205H, A502V, G686C, S1164A and A1197T.129 | AD | Late onset PD (mean onset of PD at 52–64 y.o. for each mutation). |
| PARK19 | DNAJC6 | 1p31.3 | DnaJ Heat Shock Protein Family (Hsp40) Member C6: clathrin-mediated endocytosis (by similarity) | Q734X130 Q789X131 and R927G132 | AR | Juvenile onset or early adult-onset PD132,133 |
| PARK20 | SYNJ1 | 21q22.11 | Synaptojanin-1: clathrin-mediated endocytosis (by similarity) | R258Q,134,135 R459P136 | AR | Early onset PD in an Iranian and an Italian population134 |
| PARK21 | DNAJC13 | 3q22.1 | DnaJ heat shock protein family (Hsp40) member C13: endosomal membrane regulation.137 | A855S138 | AD | Late onset PD (mean onset of PD at 67 y.o.)138 |
| PARK22 | CHCHD2 | 7p11.2 | Coiled-coil-helix-coiled-coil-helix domain containing 2: mitochondrial respiration139 | A32T, P34L, and I80V139 | AD | Early onset PD139 |
| PARK23 | VPS13C | 15q22.2 | Vacuolar protein sorting-associated protein 13C: Maintaining mitochondrial transmembrane potential.131 | Truncating mutations131 | AR | Rapidly progressive PD in Turkish and French population131 |
PD causative genes with autosomal dominant inherence
Although multiple genes have been shown causative for PD (Table 1), two important PD causative genes identified so far are SNCA and LRRK2. SNCA encodes α-synuclein, a major component of pathological hallmark of Lewy bodies in PD,13 while mutations in LRRK2 are the most common indicators of inherited PD.14,15 Over time, more PD causative genes with autosomal dominant inherence were identified (Table 1), but none has overtaken the importance of SNCA or LRRK2 from pathological or genetic perspective, as outlined below.
α-Synuclein is a 140 amino acid presynaptic protein with multiple conformations and exists in many oligomeric states in a dynamic equilibrium.16 Mutant α-synuclein changes its conformation making it prone to form aggregates and Lewy bodies. Amongst the SNCA mutations, the missense mutation A53T was hypothesized as disrupting the α helix and extending the β sheet structure.12 In addition to the Greek pedigree, A53T and other missense mutations in SNCA such as A30P and E46K have been identified in over 12 Mediterranean PD families.11 These SNCA missense mutations lead to structural changes in α-synuclein,17 in which A30P and A53T mutations form annular and pore-like protofibrils, and annular and tubular prefibrillar oligomers correspondingly, under electron microscopy, analytical ultracentrifugation and scanning transmission electron microscopy.17 Apart from point mutations, multiplications of the SNCA region lead to correspondingly elevated expression of α-synuclein, and hence cause typical and atypical PD.11,18 The SNCA genomic multiplications occur due to unequal cross-over during either intra-allelic or inter-allelic recombination or both.19 The multiplications appear to associate with early onset of PD,11 e.g., SNCA triplication has been identified as causing dominant early-onset PD.20 The dosage of SNCA multiplications impacts on the severity of PD and dementia presentations19 due to SNCA over-expression, that can increase α-synuclein aggregation and fibril formation.
The addition of recombinant α-synuclein fibrils to primary neurons led to the selective decreases in synaptic proteins, progressive impairments in neuronal connectivity and eventually neuron death.21 In addition, inoculation of recombinant α-synuclein fibrils in the striatum of mice led to pathological cell to cell α-synuclein transmission eventually resulting in dopaminergic neuronal loss in the substantia nigra accompanied by motor deficit.22 Thus, pathologic levels of α-synuclein can induce neuronal toxicity.
The N-terminal 32 amino acids of human α-synuclein contain cryptic mitochondrial targeting signal, which is important for α-synuclein binding to mitochondrial membrane and allows α-synuclein to be imported into mitochondria.23,24 Mitochondrial accumulation of α-synuclein potentially affects respiratory complex I activity, increases oxidative stress, and leads to neuronal toxicity.23,24 The C-terminus of α-synuclein interacts with the microtubule binding domain of tau, particularly when tau is hyperphosphorylated,25 and facilitates the formation of neuropathological intraneuronal filamentous inclusinons.26,27 Molecular functions of α-synuclein provide further pathophysiological evidence about the critical role of α-synuclein in the pathogenesis of PD.
LRRK2 encodes a large 51-exon multi-domain protein of over 2500 amino acids.28 Its multiple roles include participation in vesicle sorting by mediating the endosomal-autophagic pathway and late endosomal membrane trafficking.29, 30, 31 Over 40 missense mutations in LRRK2 have been identified,11 all of them displaying an autosomal dominant PD pattern, of which G2385R variant (rs34778348) confers a risk for people to develop PD in Asia.32 Studies have found the frequency and penetrance of LRRK2 mutations vary significantly among different ethnicities.28 LRRK2 mutations are found in 2% of patients with PD.14 Gly2019Ser is the most common LRRK2 mutation, and is present at high frequencies mainly in amongst North African and Arabs idiopathic and hereditary PD patients at 39% and 36% correspondingly, as well as in Caucasian PD patients,33 but Gly2019Ser is a rare mutation in Asian populations.11 Other studies have also shown that the frequency and penetrance of LRRK2 mutations vary significantly amongst different ethnicities.28
LRRK2-coded protein contains a GTPase core and kinase domain. The GTPase catalytic core regulates its kinase domain.34 LRRK2 phosphorylates endophilin A at S75 which regulates synaptic vesicle endocytosis and EndoA-dependent membrane tubulation.35 LRRK2 also phosphorylates eukaryotic initiation factor 4E-binding protein (4E-BP), which modulates the eIF4E/4E-BP pathway and stimulates eIF4E-mediated protein translation, resulting in attenuation of resistance to oxidative stress and survival of dopaminergic neuron.36 PD associated mutations in LRRK2 increase its kinase activity on endophilin-A leading to initiation of endocytosis,37 and they can also affect protein synthesis, mitochondrial quality control and further influence neuronal viability.38,39 LRRK2 facilitates α-synuclein inclusion formation.40, 41, 42 Either mutant α-synuclein or mutant LRRK2 can block or disrupt mitophagy or delay autophagosome trafficking.43 Convergent mechanisms of LRRK2 and α-synuclein can act on different targets within the autophagy-lysosomal system, thus leading to PD pathogenesis.
PD causative genes with AR inherence
PD causative genes with AR inherence often occur in PD patients with early onset. Among them, the most common mutations are in PRKN (previously known as PARK2), followed by PINK1,44 and DJ-1,45 accounting for about 18%, 15% and 0.2% of early onset PD respectively.46, 47, 48, 49 Deletions and mutations in PRKN gene are associated with degeneration of pigmented neurons in the substantia nigra, similar to that seen in PD, but without Lewy bodies on brain autopsy.50,51 However, the compound heterozygous PRKN or PINK1 mutations had dopaminergic neuron loss in substantia nigra and the presence of Lewy bodies.50,51 The pathological differences lie in the complete or partial depletion of its molecular functions caused by mutations. PRKN encodes the E3 ubiquitin ligase parkin, and glycosylated α-synuclein is one of the substrates normally ubiquitinated by parkin.52 Therefore, the parkin mutations inhibits the degradation of α-synuclein. Moreover, parkin deficient mice do not show exacerbated α-synuclein aggregation when crossed with A53T mice,53 suggesting strong dominant effects of SNCA. Parkin contributes to mitochondrial degradation along with PINK1 and DJ-1.54 Mutations in these PD-AR genes have been associated with dysfunction in PRKN and PARK1 mediated mitochondrial quality control through processes including mitophagy, transport, biogenesis, fission and fusion.55, 56, 57
Genes associated with PD susceptibility
Since 2009, genome wide association studies (GWAS) have opened a new era to identify PD susceptibility genes via comparison between PD and controls. The first European PD GWAS analysis identified two genetic risk loci with 1713 PD cases and 3978 controls and replicated with 3361 cases and 4573 controls.58 The risk loci identified were SNCA and MAPT, containing risk SNPs rs2736990 and rs393152, respectively.58 This study also replicated PARK16 and the SNPs rs823128 as one of the SNPs previously identified in a Japanese cohort.58 However, some genetic regions e.g., MAPT have genetic heterogeneity in different ethnicities, and the PD association with MAPT gene was not replicated in a Japanese population, according to the GWAS study in 2009.59
The meta-analysis performed by the International Parkinson Disease Genomics Consortium in 2011, involved 5 American and European cohorts and over 7 million SNPs, identified up to 11 risk loci and the most significant SNP in each locus.60 Referring to the genes closest to a SNP, these SNPs were: chr1:154,105,678 (SYT11), rs6710823 (ACMSD), rs2102808 (STK39), rs11711441 (MCCC1/LAMP3), chr4:911,311 (GAK), rs11724635 (BST1), rs356219 (SNCA), chr6:32,588,205 (HLA-DRB5), rs1491942 (LRRK2), rs12817488 (CCDC62/HIP1R) and rs2942168 (MAPT).60
Nalls et al's meta-analysis carried in 2014 initiated an expansion in the discovery of PD associated SNPs by involving all the up-to-date PD GWAS data in European population.61 The study involved over 7 million variants from 1000 Genomes Project in over 13,000 cases and over 95,000 controls. There were 26 independent risk genetic loci identified by primary meta-analysis with the GWAS summary statistics.61 In the replication test in a separate sample set using NeuroX genotyping array that includes over 264,000 variants, 22 out of the 26 genetic loci were replicated and 6 novel loci were identified: SIPA1L2, INPP5F, MIR4697, GCH1, VPS13C and DDRGK1.61 A total of 28 independent risk variants (SNPs) for PD across 24 loci were identified in that study.61 There is evidence suggesting interactions between risk loci eg.rs199347 associates with increased expression of NUPL2 and decreased methylation of GPNMB, and rs823118 increases RAB7L1 expression and decreases BUCKS1 expression.61
In 2017, Chang et al's GWAS identified 12 risk loci, one of them being the novel locus: rs9468199.62 They then performed meta-analysis with their GWAS data and recent GWAS data, and identified 35 novel risk loci of which 17 loci could be replicated.62 On the other hand, the GWAS analysis included six East Asian regions, including mainland China, Hong Kong, Taiwan, Singapore, Malaysia, and Korea, also confirming SNCA and LRRK2 as the most significant risk loci, as well as MCCC1, and 14 other loci reported in European studies.63 This finding suggested mutations in SNCA and LRRK2 significantly change corresponding protein functions causing PD, while their non-coding genetic variants lead to subtle changes in protein functions, conferring risk to develop PD. While MAPT is reported to be a PD risk gene in Asian populations, it appears there are different genetic risk variants of MAPT in Asian populations compared to Caucasians.63,64
The 2019 meta-analysis Nalls et al performed included 17 recent GWAS datasets in European populations, involving over 37,000 cases, over 18,000 PD family cases and 1.4 million controls.65 Ninety PD risk SNPs involved in 78 risk loci were identified.65 On the other hand, the meta-analysis of recent GWAS data conducted in 2020 from mainland China, Hong Kong, Taiwan, Singapore, Malaysia and South Korea populations, identified 11 risk loci, of which 9 were previously identified in a European population: PARK16, ITPKB, MCCC1, SNCA, FAM47E-SCARB2, DLG2, LRRK2, RIT2 and FYN.66 There were novel SNPs rs246814 and rs9638616 associated with SV2C and WBSCR17 (GALNT17) genes respectively, in which the SV2C intronic SNP was subsequently replicated in the European cohort, but the WBSCR17 associated variant did not increase PD risk in European populations.66 Thus, these recent studies in Asia show population genetic heterogeneity in certain PD risk genes. However, GWAS studies of PD with Asian populations are still at a relatively early stage, with a limited number of studies. Future studies are needed to explore the genetic risk factors of PD in different ethnic groups and obtain a better understanding of any common or population-specific genetic variants amongst different ancestries. These aims match those of the recently established Global Parkinson's Genetic program (GP2) which seeks to genotype >150,000 volunteers from Africa, Asia, Europe, and the American continent (https://parkinsonsroadmap.org/gp2/).
So far, there have been over 90 independent PD risk SNPs identified in European populations, and these could explain 16–36% of the heritable risk of PD depending on prevalence.65 The GWAS PD susceptibility studies have been summarized (Table 2), and replication in different populations is an essential step for susceptibility gene confirmation. However, the high heterogeneity of different genomic constructs in human ethnic groups and low effect of the SNPs could potentially result in them failing to be replicated.61 This resolution of genetic factors could be improved by increasing the sample size.61 PD risk SNPs are typically associated with a small individual risk, but they occur more frequently in the population compared to PD causative mutations, and have substantial cumulative risk.67 These SNPs are only associated with a small PD risk and are not useful independently in making prognosis of an individual under risk to develop PD. A polygenic risk score (PRS) was therefore introduced, which is calculated by accumulating each risk SNPs as parameters.68,69 This allows each individual to receive a PRS and understand their PD genetic susceptibility. Based on the currently identified risk SNPs, the PRS model could predict PD with a sensitivity of 0.628 and a specificity of 0.686.65 Therein, PRS combining information on additional numbers of PD risk SNPs to assess the risk for developing PD is likely the future direction of genetics of PD.
Table 2.
PD susceptibility loci via GWAS or Meta-GWAS studies.
| Timeline | Number of risk loci | Number of SNP (rs) | Number of cases vs. control |
Population | References | |
|---|---|---|---|---|---|---|
| Discovery stage | Replication stage | |||||
| 2009 | 3 | 3 | 1713 vs. 3978 | 3361 vs. 4573 | Caucasian | GWAS58 |
| 2009 | 4 | 23 | 1078 vs. 2628 | 612 vs. 14,139 321 vs 1614 |
Japanese | GWAS59 |
| 2011 | 11 | 11 | 5333 vs. 12,019 | 7053 vs. 9007 | Caucasian | Meta-GWAS60 |
| 2014 | 22 | 28 | 13,708 vs. 95,282 | 5353 vs. 5551 | Caucasian | Meta-GWAS61 |
| 2017 | 35 | 44 | GWAS: 6476 vs. 302,042 Meta-analysis: 13,000+ vs. 95,000+ |
5851 vs. 5866 | Caucasian | GWAS and meta-GWAS62 |
| 2017 | 73 | 90 | 779 vs. 13,227 | 5125 vs. 17,604 | Asian | GWAS63 |
| 2019 | 78 | 90 | 30,271 vs. 1,014,601 | 26,035 vs 403,190 | Caucasian | Meta-GWAS65 |
| 2020 | 11 | 11 | 6724 vs. 24,851 | 58,533 vs 1,871,337 | Asian | GWAS and meta-GWAS66 |
Genes associated with PD quantitative traits
Compared to PD genetic susceptibility studies aimed at identifying people at risk of developing PD, genetic studies on PD quantitative traits represent another important stream to identify genetic contributions to the disease process and to further distinguish PD risk from variants affecting PD progression, as slowing/stopping progression is a major goal. PD quantitative traits include continuous variables that include onset age, motor and non-motor severity measures. Patients carrying mutant genes with AR inheritance often have a benign disease course, whereas patients carrying SNCA triplication often have more severe disease course compared to patients with SNCA duplication.19 Heterozygous mutations in GBA accounts for 2.3%–17.9% patients with PD, although GBA is usually not considered as a PD gene due to the incomplete penetrance of GBA mutations, and thus GBA mutations are instead frequently viewed as a strong risk factor for PD.70 GBA gene mutations have also been associated with PD symptoms severity, rate of disease progression, and age of onset.70
Some of the PD risk loci have been shown to be associated with the age at onset71 and the progression of PD in Caucasian and in Asian populations.64,72 Furthermore, PRS also indicates contribution to the prognosis for PD progression, which is proven to associate with the motor and cognitive functional decline among PD patients.69 There is evidence suggesting a higher PRS is associated with early PD onset, however, PRS was not shown to associate with amount of α-synuclein in CSF, which might suggest more SNPs are need to be identified and included in the PRS calculation.73 In addition, a GWAS association study with PD progression was first attempted this year to evaluate genomic contribution to the motor and non-motor progression of PD,74 and a genome-wide survival study this year identified a novel synaptic locus increasing the polygenic score of cognitive progression in PD.75 However, apart from large longitudinal prospective PD cohorts required, the input clinical quantitative measures and the algorithm to reflect the clinical progression of PD are also challenges in conducting such GWAS studies to truly reveal genetic factors associated with the progression of PD.
Molecular pathways related to PD genetic factors
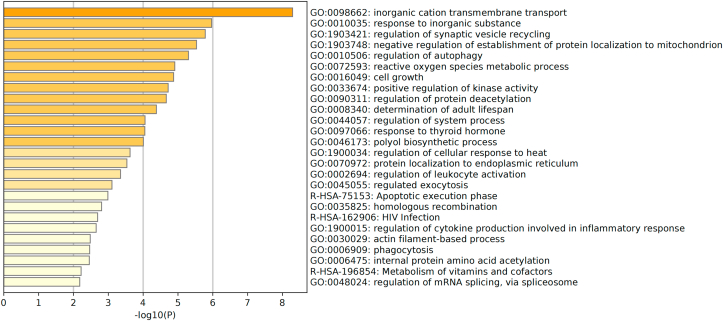
The genes associated with PD risk are predominantly expressed in neurons,65 with some exceptions, e.g., Coetzee et al studied 4 risk loci containing risk SNPs which were shown in non-neuronal cells,26,67 encouraging future studies to investigate the function of SNPs associated with the etiology of PD. Majority of the associated genes of these SNPs are protein coding genes that shared the same biological pathways. There have been 10 biological pathways identified as enriched in these encoded proteins, 4 of which are associated with vacuolar functions, and three involving a known pharmaceutical target, e.g., kinase signaling and calcium transporters.65 According to the Gene Ontology (GO) database,76 the biological pathways of currently identified SNPs were listed and their associated genes belonging to different categories as demonstrated in Figure 1.
Figure 1.
Bar chart of different categories of the protein functions. The proteins are encoded by the PD risk genes and their functions base on Gene Ontology (GO) database.76 The bar chart is stimulated using Metascape.140 The top 25 of the function categories were included in this chart.
Figure 1 shows that the pathway with the highest P-value for enrichment of PD genes is inorganic cation transmembrane transport, a process whereby inorganic cations are transported across membrane by means of a transporter or pore. This is related to 712 genes, and 17 of them are related to PD, such as LRRK2, RIMS1, etc.76 In the PD risk gene products involved in inorganic cation transmembrane transport, 8 are associated with calcium ion transmembrane transport, which is important for regulation of mitochondrial function. Mitochondrial dysfunction and redox metals, i.e., inorganic substance with 12 genes involved, can cause oxidative stress, which has been shown to contribute to the etiology of PD.77 A recent study showed over-expression of α-synucleins increased Voltage Dependent Ion Channel 3 (VDIC3) permeability for calcium ions resulting in a net influx.78 Another cation, magnesium is also related to PD. Long-term magnesium deficiency leads to loss of dopaminergic neurons, and epidemiological studies show a higher incidence of PD in patients with low magnesium concentrations.79 These studies highlight the importance of inorganic cation imbalance to PD etiology, and as a potential target for therapeutic intervention.
By STRING analysis (Fig. 2), PD risk genetic products network shows 105 nodes, of which 32 are hub nodes with connection degrees much greater than the average edge degree of the network which is 2.11.80 Some hub nodes demonstrate prominently abundant connections with other nodes, such as SNCA, LRRK2, MAPT, GBA, VPS13C, DGKQ and NOD2, which are all protein coding genes. Some of these are discussed above (Table 1). DGKQ encodes for diacylglycerol kinase θ protein, and is mainly expressed in the brain, mediating lipid and protein interaction in signal-transducing complexes,81 modulating calcium signalling and synaptic vesicles trafficking at nerve terminals.82 NOD2 encodes nucleotide-binding oligomerization domain-containing protein 2, which are intracellular signalling proteins mediating NF-κB activation and apoptosis.83 Inflammation-derived oxidative stress accelerates the neurodegeneration in nigrostriatal pathway in PD.83
Figure 2.
Network diagram demonstrates interactions between the risk gene products. The PD risk genetic products network shows 105 nodes and 111 edges. The different colours in the figure indicate different biological processes in which the highlighted genes are involved. Red represents “regulation of peroxidase activity”; Dark blue represents “activated T cell proliferation”; Light green represents “glycosylceramide catabolic process”; Light yellow represents “negative regulation of protein targeting to mitochondrion”; Pink represents “negative regulation of establishment of protein localization to mitochondrion”; Dark green represents “positive regulation of nitric-oxide synthase biosynthetic process”; Light blue represents “negative regulation of amine transport”; Dark yellow represents “response to thyroid hormone”; Purple represents “negative regulation of response to drug”; Brown represents “regulation of cytokine production involved in inflammatory response”.76 The graphic is made using STRING v11.0.
There are 111 edges in this network which is much greater than the expected number of 42, suggesting more interactions than random connections. Each edge represents a common pathway in which the genes products are involved. It demonstrates enrichment in certain biological process networks, such as regulation of peroxidase activity involving α-synuclein and LRRK2. These reflect the molecular pathways relevant to these genes involved in PD. The top 10 pathways with high strength of enrichment are colored in Figure 2, e.g., peroxidase regulation and activated T-cell proliferation. α-Synuclein and LRRK2 are involved in the peroxidase regulation pathway. Glutathione peroxidase has been shown to be protective against oxidative stress in the progression of PD.84 The activated T-cell proliferation pathway involves FYN and SATB1. The expanded terminal effector CD8+ and cytotoxic CD4+ peripheral T cells in PD patients85 suggest T-cells could be a therapeutic target to lessen neurodegeneration in PD.
Figure 2 reflects the molecular pathways relevant to these genetic products involved in PD, where reactive oxygen species induced inflammation/oxidative stress and mitochondrial dysfunction have also been shown in GO database analysis (Fig. 1). Interestingly, both analyses show that response to thyroid hormone is related to PD (Figure 1, Figure 2), which indicates that hypothalamic pituitary thyroid axis may play an important role in PD pathogenesis. Research has shown that regulation of thyroid-stimulating hormone and thyroid hormones correlates with the severity of PD.86
These identified pathways correlate with other databases including Reactome, KEGG, BIOCARTA, Pathway Interaction Database, Matrisome project, Signalling Gateway, Sigma Aldrich and SuperArray SABiosciences.87 These databases also suggest pathways such as lipid metabolism, immune response, synaptic transmission, endosomal–lysosomal dysfunction and apoptosis mediated by initiator and executioner caspases.87 Moreover, adaptive and innate immune response, vesicular-mediated transport, and lipid metabolism affected by signalling mechanisms were all associated with PD.87 Recent studies showed that LRRK2 phosphorylates SYNJ1 and DNAJC6 for vesicle endocytosis and recycling.37,88 Other PARK genes encoded proteins such as Parkin, involve AMPA-type glutamate receptor (AMPAR) trafficking.89 Mutations lead to AMPAR trafficking defects which affect synaptic plasticity, and this impacts on information processing leading to PD psychiatric symptoms.8,90 Further studies are required to identify other potential pathways and search for any link with the biological hallmarks of PD.
Genetic implications for PD therapy
There have been several PD risk genes identified as therapeutic targets. The GBA target treatment focus on its encoded protein glucocerebrosidase, and glucosylceramide synthase inhibitor and ambroxol hydrochloride have already been used in clinical trials, with the latter therapy displaying promising indications.91 Early stage of clinical trials (BIIB094 and DNL201) targeting LRRK2 expression or its kinase activity are underway (NCT03976349 & NCT03710707). There is also evidence suggesting deep brain stimulation therapy is effective in certain monogenic PD patients such as those with LRRK2 p.G2019S or PRKN mutations.87,92 This was not effective in patients with mutations such as SNCA and GBA, possibly due to their associated rapid disease progression.92
The identified genetic factors also contribute to the need to adjust the appropriate dosage of levodopa medication for PD patients. The mutations within genes involved in levodopa metabolism (DDC and COMT), dopamine transportation (DAT) and dopamine signaling (DRD2 and DRD3) greatly affect the required dosage of these medications.87 Unfortunately, we still do not have sufficient insights as to what mechanisms to target in individual patients as we lack a full understanding of the pathways associated with PD. Therefore, identifying the genes with their biological pathway involved in current PD treatment regime is important in providing patients with personalized practice.
Conclusion
Since the late 20th century, studies have been investigating genetic associations with PD. To date, there have been over 70 genes and their specific SNPs identified to increase PD risk. These genetic risk factors also associate with the type and severity of PD clinical manifestations, age of onset and PD progression. However, those identified so far only represent a small proportion of PD risk genetic factors. Future studies should continue exploring novel loci using advanced genotyping arrays in larger sample sizes. The heterogeneous genomic construct among different populations warrants validation and confirmation for PD susceptibility genes. In addition, characterizing the genomic contributions to the progression and subtypes of PD represents a medical advance poised to facilitate clinical practice in the real world of PD management. This would further increase the accuracy of disease treatment and provide a better management plan for PD patients, to achieve evidence-based, high-quality medicine.
Author contributions
YH designed the project and critically revised the manuscript; JW drafted the manuscript; MM and AC co-supervised JW and actively participated in the manuscript revision.
Conflict of interests
The authors declare no conflict of interests.
Funding
This work is supported by National Natural Science Foundation of China (No. NSFC 82071417, YH). AC received grant funding from the Australian government.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Tysnes O.B., Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna) 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 2.Yang W., Hamilton J.L., Kopil C., et al. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis. 2020;6:15. doi: 10.1038/s41531-020-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poewe W., Seppi K., Tanner C.M., et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 4.Kalia L.V., Lang A.E. Parkinson disease in 2015:evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol. 2016;12(2):65–66. doi: 10.1038/nrneurol.2015.249. [DOI] [PubMed] [Google Scholar]
- 5.Farrer M.J. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7(4):306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 6.Mor D.E., Ischiropoulos H. The convergence of dopamine and α-synuclein: implications for Parkinson's disease. J Exp Neurosci. 2018;12 doi: 10.1177/1179069518761360. 1179069518761360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia L.V., Kalia S.K., McLean P.J., et al. Α-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73(2):155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussein A., Guevara C., Valle P., et al. Non-motor symptoms of Parkinson’s disease: the neurobiology of early psychiatric and cognitive dysfunction. Neuroscientist. 2021;10738584211011979 doi: 10.1177/10738584211011979. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhuri K.R., Healy D.G., Schapira A.H. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 10.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 11.Lesage S., Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 12.Polymeropoulos M.H., Lavedan C., Leroy E., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 13.Spillantini M.G., Schmidt M.L., Lee V.M., et al. Alpha-synuclein in lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 14.Ross O.A., Toft M., Whittle A.J., et al. Lrrk2 and Lewy body disease. Ann Neurol. 2006;59(2):388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- 15.Giasson B.I., Covy J.P., Bonini N.M., et al. Biochemical and pathological characterization of Lrrk2. Ann Neurol. 2006;59(2):315–322. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 16.Dehay B., Bourdenx M., Gorry P., et al. Targeting α-synuclein for treatment of Parkinson's disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14(8):855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lashuel H.A., Petre B.M., Wall J., et al. α-Synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322(5):1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 18.Singleton A.B., Farrer M., Johnson J., et al. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 19.Ross O.A., Braithwaite A.T., Skipper L.M., et al. Genomic investigation of alpha-synuclein multiplication and Parkinsonism. Ann Neurol. 2008;63(6):743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mata I.F., Shi M., Agarwal P., et al. SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch Neurol. 2010;67(11):1350–1356. doi: 10.1001/archneurol.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpicelli-Daley L.A., Luk K.C., Patel T.P., et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk K.C., Kehm V., Carroll J., et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devi L., Raghavendran V., Prabhu B.M., et al. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283(14):9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G., Zhang C., Yin J., et al. Alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett. 2009;454(3):187–192. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 25.Haggerty T., Credle J., Rodriguez O., et al. Hyperphosphorylated Tau in an α-synuclein-overexpressing transgenic model of Parkinson's disease. Eur J Neurosci. 2011;33(9):1598–1610. doi: 10.1111/j.1460-9568.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alim M.A., Hossain M.S., Arima K., et al. Tubulin seeds alpha-synuclein fibril formation. J Biol Chem. 2002;277(3):2112–2117. doi: 10.1074/jbc.M102981200. [DOI] [PubMed] [Google Scholar]
- 27.Giasson B.I., Forman M.S., Higuchi M., et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 28.Paisán-Ruíz C., Nath P., Washecka N., et al. Comprehensive analysis of LRRK2 in publicly available Parkinson's disease cases and neurologically normal controls. Hum Mutat. 2008;29(4):485–490. doi: 10.1002/humu.20668. [DOI] [PubMed] [Google Scholar]
- 29.Alegre-Abarrategui J., Christian H., Lufino M.M., et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18(21):4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodson M.W., Zhang T., Jiang C., et al. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2011;21(6):1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steger M., Tonelli F., Ito G., et al. Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife. 2016;5 doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrer M.J., Stone J.T., Lin C.H., et al. Lrrk2 G2385R is an ancestral risk factor for Parkinson's disease in Asia. Park Relat Disord. 2007;13(2):89–92. doi: 10.1016/j.parkreldis.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Healy D.G., Falchi M., O'Sullivan S.S., et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webber P.J., Smith A.D., Sen S., et al. Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J Mol Biol. 2011;412(1):94–110. doi: 10.1016/j.jmb.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matta S., van Kolen K., da Cunha R., et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75(6):1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Imai Y., Gehrke S., Wang H.Q., et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27(18):2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao M., Wu Y., Ashrafi G., et al. Parkinson sac domain mutation in synaptojanin 1 impairs clathrin uncoating at synapses and triggers dystrophic changes in dopaminergic axons. Neuron. 2017;93(4):882–896. doi: 10.1016/j.neuron.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin I., Kim J.W., Lee B.D., et al. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson's disease. Cell. 2014;157(2):472–485. doi: 10.1016/j.cell.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J.W., Yin X., Jhaldiyal A., et al. Defects in mRNA translation in LRRK2-mutant hiPSC-derived dopaminergic neurons lead to dysregulated calcium homeostasis. Cell Stem Cell. 2020;27(4):633–645. doi: 10.1016/j.stem.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y., Song Y.J.C., Murphy K., et al. LRRK2 and parkin immunoreactivity in multiple system atrophy inclusions. Acta Neuropathol. 2008;116(6):639–646. doi: 10.1007/s00401-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 41.Guerreiro P.S., Huang Y., Gysbers A., et al. LRRK2 interactions with α-synuclein in Parkinson's disease brains and in cell models. J Mol Med (Berl) 2013;91(4):513–522. doi: 10.1007/s00109-012-0984-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volpicelli-Daley L.A., Abdelmotilib H., Liu Z., et al. G2019S-LRRK2 expression augments α-synuclein sequestration into inclusions in neurons. J Neurosci. 2016;36(28):7415–7427. doi: 10.1523/JNEUROSCI.3642-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hara D.M., Pawar G., Kalia S.K., et al. LRRK2 and α-synuclein: distinct or synergistic players in Parkinson's disease? Front Neurosci. 2020;14:577. doi: 10.3389/fnins.2020.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawajiri S., Saiki S., Sato S., et al. Genetic mutations and functions of PINK1. Trends Pharmacol Sci. 2011;32(10):573–580. doi: 10.1016/j.tips.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Hedrich K., Djarmati A., Schäfer N., et al. DJ-1 (PARK7) mutations are less frequent than Parkin (PARK2) mutations in early-onset Parkinson disease. Neurology. 2004;62(3):389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- 46.Kock N., Müller B., Vieregge P., et al. Role of SCA2 mutations in early- and late-onset dopa-responsive Parkinsonism. Ann Neurol. 2002;52(2):257–258. doi: 10.1002/ana.10270. [DOI] [PubMed] [Google Scholar]
- 47.Kitada T., Asakawa S., Hattori N., et al. Mutations in the parkin gene cause autosomal recessive juvenile Parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara-Paul L., Hulihan M.M., Kachergus J., et al. PINK1 mutations and Parkinsonism. Neurology. 2008;71(12):896–902. doi: 10.1212/01.wnl.0000323812.40708.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcalay R.N., Caccappolo E., Mejia-Santana H., et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67(9):1116–1122. doi: 10.1001/archneurol.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrer M., Chan P., Chen R., et al. Lewy bodies and Parkinsonism in families with parkin mutations. Ann Neurol. 2001;50(3):293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 51.Samaranch L., Lorenzo-Betancor O., Arbelo J.M., et al. PINK1-linked Parkinsonism is associated with Lewy body pathology. Brain. 2010;133(Pt 4):1128–1142. doi: 10.1093/brain/awq051. [DOI] [PubMed] [Google Scholar]
- 52.Shimura H., Schlossmacher M.G., Hattori N., et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293(5528):263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 53.von Coelln R., Thomas B., Andrabi S.A., et al. Inclusion body formation and neurodegeneration are parkin independent in a mouse model of alpha-synucleinopathy. J Neurosci. 2006;26(14):3685–3696. doi: 10.1523/JNEUROSCI.0414-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narendra D., Walker J.E., Youle R. Mitochondrial quality control mediated by PINK1 and Parkin: links to Parkinsonism. Cold Spring Harbor Perspect Biol. 2012;4(11):a011338. doi: 10.1101/cshperspect.a011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J., Liu W., Li R., et al. Mitophagy in Parkinson's disease: from pathogenesis to treatment. Cells. 2019;8(7):712. doi: 10.3390/cells8070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarffe L.A., Stevens D.A., Dawson V.L., et al. Parkin and PINK1:much more than mitophagy. Trends Neurosci. 2014;37(6):315–324. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin S.M., Youle R.J. PINK1- and parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125(Pt 4):795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simón-Sánchez J., Schulte C., Bras J.M., et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satake W., Nakabayashi Y., Mizuta I., et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41(12):1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 60.Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nalls M.A., Pankratz N., Lill C.M., et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang D., Nalls M.A., Hallgrímsdóttir I.B., et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet. 2017;49(10):1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foo J.N., Tan L.C., Irwan I.D., et al. Genome-wide association study of Parkinson's disease in East Asians. Hum Mol Genet. 2017;26(1):226–232. doi: 10.1093/hmg/ddw379. [DOI] [PubMed] [Google Scholar]
- 64.Wang G., Huang Y., Chen W., et al. Variants in the SNCA gene associate with motor progression while variants in the MAPT gene associate with the severity of Parkinson's disease. Park Relat Disord. 2016;24:89–94. doi: 10.1016/j.parkreldis.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Nalls M.A., Blauwendraat C., Vallerga C.L., et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091–1102. doi: 10.1016/S1474-4422(19)30320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foo J.N., Chew E.G.Y., Chung S.J., et al. Identification of risk loci for Parkinson disease in asians and comparison of risk between asians and Europeans: a genome-wide association study. JAMA Neurol. 2020;77(6):746–754. doi: 10.1001/jamaneurol.2020.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coetzee S.G., Pierce S., Brundin P., et al. Enrichment of risk SNPs in regulatory regions implicate diverse tissues in Parkinson's disease etiology. Sci Rep. 2016;6:30509. doi: 10.1038/srep30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Euesden J., Lewis C.M., O'Reilly P.F. PRSice: polygenic risk score software. Bioinformatics. 2015;31(9):1466–1468. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul K.C., Schulz J., Bronstein J.M., et al. Association of polygenic risk score with cognitive decline and motor progression in Parkinson disease. JAMA Neurol. 2018;75(3):360–366. doi: 10.1001/jamaneurol.2017.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alcalay R.N., Levy O.A., Waters C.C., et al. Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations. Brain. 2015;138(Pt 9):2648–2658. doi: 10.1093/brain/awv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Y., Wang G., Rowe D., et al. SNCA gene, but not MAPT, influences onset age of Parkinson's disease in Chinese and australians. BioMed Res Int. 2015;2015:135674. doi: 10.1155/2015/135674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y., Rowe D.B., Halliday G.M. Interaction between α-synuclein and tau genotypes and the progression of Parkinson's disease. J Parkinsons Dis. 2011;1(3):271–276. doi: 10.3233/JPD-2011-11027. [DOI] [PubMed] [Google Scholar]
- 73.Ibanez L., Dube U., Saef B., et al. Parkinson disease polygenic risk score is associated with Parkinson disease status and age at onset but not with alpha-synuclein cerebrospinal fluid levels. BMC Neurol. 2017;17(1):198. doi: 10.1186/s12883-017-0978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan M.M.X., Lawton M.A., Jabbari E., et al. Genome-wide association studies of cognitive and motor progression in Parkinson's disease. Mov Disord. 2021;36(2):424–433. doi: 10.1002/mds.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu G., Peng J., Liao Z., et al. Genome-wide survival study identifies a novel synaptic locus and polygenic score for cognitive progression in Parkinson's disease. Nat Genet. 2021;53(6):787–793. doi: 10.1038/s41588-021-00847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Consortium G.O. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32(suppl_1):D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lan A.P., Chen J., Chai Z.F., et al. The neurotoxicity of iron, copper and cobalt in Parkinson's disease through ROS-mediated mechanisms. Biometals. 2016;29(4):665–678. doi: 10.1007/s10534-016-9942-4. [DOI] [PubMed] [Google Scholar]
- 78.Rosencrans W.M., Aguilella V.M., Rostovtseva T.K., et al. αSynuclein regulates mitochondrial calcium transport through the voltage dependent anion channel. Biophys J. 2021;120(3):194a. [Google Scholar]
- 79.Yamanaka R., Shindo Y., Oka K. Magnesium is a key player in neuronal maturation and neuropathology. Int J Mol Sci. 2019;20(14):3439. doi: 10.3390/ijms20143439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das S., Meher P.K., Rai A., et al. Statistical approaches for gene selection, hub gene identification and module interaction in gene co-expression network analysis: an application to aluminum stress in soybean (Glycine max L.) PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houssa B., Schaap D., van der Wal J., et al. Cloning of a novel human diacylglycerol kinase (DGKtheta) containing three cysteine-rich domains, a proline-rich region, and a pleckstrin homology domain with an overlapping Ras-associating domain. J Biol Chem. 1997;272(16):10422–10428. doi: 10.1074/jbc.272.16.10422. [DOI] [PubMed] [Google Scholar]
- 82.Redenšek S., Trošt M., Dolžan V. Genetic determinants of Parkinson's disease: can they help to stratify the patients based on the underlying molecular defect? Front Aging Neurosci. 2017;9:20. doi: 10.3389/fnagi.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Q., An X., Li Z., et al. P268S in NOD2 associates with susceptibility to Parkinson's disease in Chinese population. Behav Brain Funct. 2013;9:19. doi: 10.1186/1744-9081-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gökçe Çokal B., Yurtdaş M., Keskin Güler S., et al. Serum glutathione peroxidase, xanthine oxidase, and superoxide dismutase activities and malondialdehyde levels in patients with Parkinson's disease. Neurol Sci. 2017;38(3):425–431. doi: 10.1007/s10072-016-2782-8. [DOI] [PubMed] [Google Scholar]
- 85.Wang P., Yao L., Luo M., et al. Single-cell transcriptome and TCR profiling reveal activated and expanded T cell populations in Parkinson's disease. Cell Discov. 2021;7(1):52. doi: 10.1038/s41421-021-00280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohammadi S., Dolatshahi M., Rahmani F. Shedding light on thyroid hormone disorders and Parkinson disease pathology: mechanisms and risk factors. J Endocrinol Invest. 2021;44(1):1–13. doi: 10.1007/s40618-020-01314-5. [DOI] [PubMed] [Google Scholar]
- 87.Bandres-Ciga S., Saez-Atienzar S., Kim J.J., et al. Large-scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol. 2020;140(3):341–358. doi: 10.1007/s00401-020-02181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen M., Krainc D. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson's disease. Proc Natl Acad Sci Unit States Am. 2018;115(21):5576–5581. doi: 10.1073/pnas.1717590115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cortese G.P., Zhu M., Williams D., et al. Parkin deficiency reduces hippocampal glutamatergic neurotransmission by impairing AMPA receptor endocytosis. J Neurosci. 2016;36(48):12243–12258. doi: 10.1523/JNEUROSCI.1473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kessels H.W., Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mullin S., Smith L., Lee K., et al. Ambroxol for the treatment of patients with Parkinson disease with and without glucocerebrosidase gene mutations: a nonrandomized, noncontrolled trial. JAMA Neurol. 2020;77(4):427–434. doi: 10.1001/jamaneurol.2019.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuusimäki T., Korpela J., Pekkonen E., et al. Deep brain stimulation for monogenic Parkinson's disease: a systematic review. J Neurol. 2020;267(4):883–897. doi: 10.1007/s00415-019-09181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Logan T., Bendor J., Toupin C., et al. Α-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci. 2017;20(5):681–689. doi: 10.1038/nn.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krüger R., Kuhn W., Müller T., et al. AlaSOPro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 95.Konno T., Ross O.A., Puschmann A., Dickson D.W., Wszolek Z.K. Autosomal dominant Parkinson's disease caused by SNCA duplications. Park Relat Disord. 2016;22(Suppl 1):S1–S6. doi: 10.1016/j.parkreldis.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mueller J.C., Fuchs J., Hofer A., et al. Multiple regions of alpha-synuclein are associated with Parkinson's disease. Ann Neurol. 2005;57(4):535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- 97.Tan M.M.X., Malek N., Lawton M.A., et al. Genetic analysis of Mendelian mutations in a large UK population-based Parkinson's disease study. Brain. 2019;142(9):2828–2844. doi: 10.1093/brain/awz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan K., Hu P., Song C., et al. Novel compound heterozygous PRKN variants in a Han-Chinese family with early-onset Parkinson's disease. Parkinsons Dis. 2019;2019:9024894. doi: 10.1155/2019/9024894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gasser T., Müller-Myhsok B., Wszolek Z.K., et al. A susceptibility locus for Parkinson's disease maps to chromosome 2p13. Nat Genet. 1998;18(3):262–265. doi: 10.1038/ng0398-262. [DOI] [PubMed] [Google Scholar]
- 100.Leroy E., Boyer R., Auburger G., et al. The ubiquitin pathway in Parkinson's disease. Nature. 1998;395(6701):451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 101.Clements C.M., McNally R.S., Conti B.J., et al. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci Unit States Am. 2006;103(41):15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abou-Sleiman P.M., Healy D.G., Quinn N., et al. The role of pathogenic DJ-1 mutations in Parkinson's disease. Ann Neurol. 2003;54(3):283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- 103.Cookson M.R. Cellular effects of LRRK2 mutations. Biochem Soc Trans. 2012;40(5):1070–1073. doi: 10.1042/BST20120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lill C.M. Genetics of Parkinson's disease. Mol Cell Probes. 2016;30(6):386–396. doi: 10.1016/j.mcp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Rui Q., Ni H., Li D., et al. The role of LRRK2 in neurodegeneration of Parkinson disease. Curr Neuropharmacol. 2018;16(9):1348–1357. doi: 10.2174/1570159X16666180222165418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schultheis P.J., Hagen T.T., O'Toole K.K., et al. Characterization of the P5 subfamily of P-type transport ATPases in mice. Biochem Biophys Res Commun. 2004;323(3):731–738. doi: 10.1016/j.bbrc.2004.08.156. [DOI] [PubMed] [Google Scholar]
- 107.Schneider S.A., Paisan-Ruiz C., Quinn N.P., et al. ATP13A2 mutations (PARK9) cause neurodegeneration with brain iron accumulation. Mov Disord. 2010;25(8):979–984. doi: 10.1002/mds.22947. [DOI] [PubMed] [Google Scholar]
- 108.Ramirez A., Heimbach A., Gründemann J., et al. Hereditary Parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 109.di Fonzo A., Chien H.F., Socal M., et al. ATP13A2 missense mutations in juvenile Parkinsonism and young onset Parkinson disease. Neurology. 2007;68(19):1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 110.Bras J., Verloes A., Schneider S.A., et al. Mutation of the Parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet. 2012;21(12):2646–2650. doi: 10.1093/hmg/dds089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santoro L., Breedveld G.J., Manganelli F., et al. Novel ATP13A2 (PARK9) homozygous mutation in a family with marked phenotype variability. Neurogenetics. 2011;12(1):33–39. doi: 10.1007/s10048-010-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Najim al-Din A.S., Wriekat A., Mubaidin A., et al. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: kufor-rakeb syndrome. Acta Neurol Scand. 1994;89(5):347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 113.Hicks A.A., Pétursson H., Jónsson T., et al. A susceptibility gene for late-onset idiopathic Parkinson's disease. Ann Neurol. 2002;52(5):549–555. doi: 10.1002/ana.10324. [DOI] [PubMed] [Google Scholar]
- 114.Morita M., Ler L.W., Fabian M.R., et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol Cell Biol. 2012;32(17):3585–3593. doi: 10.1128/MCB.00455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bras J., Simón-Sánchez J., Federoff M., et al. Lack of replication of association between GIGYF2 variants and Parkinson disease. Hum Mol Genet. 2009;18(2):341–346. doi: 10.1093/hmg/ddn340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lautier C., Goldwurm S., Dürr A., et al. Mutations in the GIGYF2 (TNRC15) gene at the PARK11 locus in familial Parkinson disease. Am J Hum Genet. 2008;82(4):822–833. doi: 10.1016/j.ajhg.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pankratz N., Nichols W.C., Uniacke S.K., et al. Genome screen to identify susceptibility genes for Parkinson disease in a sample without parkin mutations. Am J Hum Genet. 2002;71(1):124–135. doi: 10.1086/341282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balakrishnan M.P., Cilenti L., Mashak Z., et al. THAP5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/HtrA2 protease during cell death. Am J Physiol Heart Circ Physiol. 2009;297(2):H643–H653. doi: 10.1152/ajpheart.00234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strauss K.M., Martins L.M., Plun-Favreau H., et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet. 2005;14(15):2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 120.Larsson P.K., Claesson H.E., Kennedy B.P. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J Biol Chem. 1998;273(1):207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 121.Paisan-Ruiz C., Bhatia K.P., Li A., et al. Characterization of PLA2G6 as a locus for dystonia-Parkinsonism. Ann Neurol. 2009;65(1):19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang Y.F., Cheng C.M., Chang L.K., et al. The F-box protein Fbxo7 interacts with human inhibitor of apoptosis protein cIAP1 and promotes cIAP1 ubiquitination. Biochem Biophys Res Commun. 2006;342(4):1022–1026. doi: 10.1016/j.bbrc.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 123.di Fonzo A., Dekker M.C.J., Montagna P., et al. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology. 2009;72(3):240–245. doi: 10.1212/01.wnl.0000338144.10967.2b. [DOI] [PubMed] [Google Scholar]
- 124.Shojaee S., Sina F., Banihosseini S.S., et al. Genome-wide linkage analysis of a Parkinsonian-pyramidal syndrome pedigree by 500 K SNP arrays. Am J Hum Genet. 2008;82(6):1375–1384. doi: 10.1016/j.ajhg.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braschi E., Goyon V., Zunino R., et al. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol. 2010;20(14):1310–1315. doi: 10.1016/j.cub.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 126.Vilariño-Güell C., Wider C., Ross O.A., et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zimprich A., Benet-Pagès A., Struhal W., et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adjibade P., Grenier St-Sauveur V., et al. DDX3 regulates endoplasmic reticulum stress-induced ATF4 expression. Sci Rep. 2017;7(1):13832. doi: 10.1038/s41598-017-14262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chartier-Harlin M.C., Dachsel J.C., Vilariño-Güell C., et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89(3):398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Köroğlu Ç., Baysal L., Cetinkaya M., et al. DNAJC6 is responsible for juvenile Parkinsonism with phenotypic variability. Park Relat Disord. 2013;19(3):320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 131.Elsayed L.E., Drouet V., Usenko T., et al. A novel nonsense mutation in DNAJC6 expands the phenotype of autosomal-recessive juvenile-onset Parkinson's disease. Ann Neurol. 2016;79(2):335–337. doi: 10.1002/ana.24591. [DOI] [PubMed] [Google Scholar]
- 132.Olgiati S., Quadri M., Fang M., et al. DNAJC6 mutations associated with early-onset Parkinson's disease. Ann Neurol. 2016;79(2):244–256. doi: 10.1002/ana.24553. [DOI] [PubMed] [Google Scholar]
- 133.Edvardson S., Cinnamon Y., Ta-Shma A., et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile Parkinsonism. PLoS One. 2012;7(5):e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krebs C.E., Karkheiran S., Powell J.C., et al. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat. 2013;34(9):1200–1207. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quadri M., Fang M., Picillo M., et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat. 2013;34(9):1208–1215. doi: 10.1002/humu.22373. [DOI] [PubMed] [Google Scholar]
- 136.Kirola L., Behari M., Shishir C., et al. Identification of a novel homozygous mutation Arg459Pro in SYNJ1 gene of an Indian family with autosomal recessive juvenile Parkinsonism. Park Relat Disord. 2016;31:124–128. doi: 10.1016/j.parkreldis.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 137.Freeman C.L., Hesketh G., Seaman M.N. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci. 2014;127(Pt 9):2053–2070. doi: 10.1242/jcs.144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vilariño-Güell C., Rajput A., Milnerwood A.J., et al. DNAJC13 mutations in Parkinson disease. Hum Mol Genet. 2014;23(7):1794–1801. doi: 10.1093/hmg/ddt570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jansen I.E., Bras J.M., Lesage S., et al. CHCHD2 and Parkinson's disease. Lancet Neurol. 2015;14(7):678–679. doi: 10.1016/S1474-4422(15)00094-0. [DOI] [PubMed] [Google Scholar]
- 140.Zhou Y., Zhou B., Pache L., et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]